Abstract
Hypertensive disorders of pregnancy are among the most serious conditions that pregnancy care providers face; however, little attention has been paid to the concept of tailoring clinical care to reduce associated adverse maternal and perinatal outcomes based on the underlying disease pathogenesis. This narrative review discusses the integration of phenotype‐based clinical strategies in the management of high‐risk pregnant patients that are currently not common clinical practice: real‐time placental growth factor testing at Mount Sinai Hospital, Toronto and noninvasive hemodynamic monitoring to guide antihypertensive therapy at the University of Washington Medical Center, Seattle. Future work should focus on promoting more widespread integration of these novel strategies into obstetric care to improve outcomes of pregnancies at high risk of adverse maternal‐fetal outcomes from these complications of pregnancy.
Keywords: antihypertensive agents; female sex; hemodynamic monitoring; humans; hypertension, pregnancy‐induced; placenta growth factor; pregnancy
Subject Categories: Growth Factors/Cytokines, Hemodynamics, High Blood Pressure, Preeclampsia, Pregnancy
Nonstandard Abbreviations and Acronyms
- CO
cardiac output
- HR
heart rate
- PlGF
placental growth factor
- sFlt‐1
soluble fms‐like tyrosine kinase‐1
- SV
stroke volume
- TPR
total peripheral resistance
The hypertensive disorders of pregnancy collectively account for 14% of all maternal deaths worldwide and are among the most serious conditions that pregnancy care providers face. 1 Considerable progress has been achieved in the past 10 years toward a better understanding of the pathogenesis of preeclampsia, the most severe form of hypertension that develops during pregnancy, and its associated conditions. 2 Hypertensive disorders of pregnancy have significant impacts on long‐term maternal and perinatal health; for example, a diagnosis of early‐onset preeclampsia increases a patient’s risk of lifelong cardiovascular disease‐related mortality by approximately 75% in comparison with patients who remained normotensive. 3 , 4 , 5
Distinct pathways leading to hypertension in pregnancy have previously been identified, are multifactorial, and include preexisting maternal health, systemic inflammation, and abnormal placentation, in the pathogenesis of preeclampsia. 6 Systemic hemodynamics and circulating biomarker characteristics, including peripheral vascular resistance, circulating PlGF (placental growth factor) levels, and cardiac output (CO), have been found to be superior to clinical characteristics such as body mass index, family history, and maternal age in distinguishing the major phenotypes of hypertension in pregnancy. 6 Assessment of local uteroplacental hemodynamics, represented by the mean uterine artery pulsatility index determined by pulsed‐wave Doppler ultrasonography, is an additional tool available to clinicians to differentiate hypertensive phenotypes. 6 , 7 This ultrasound modality identifies pregnancies at risk of developing the most common placental disease associated with early‐onset preeclampsia, namely maternal‐vascular malperfusion. 8 , 9 Although uterine artery Doppler is a key component of first trimester screening and intervention to reduce the incidence of early‐onset preeclampsia, 10 it has not been widely adopted into North American clinical practice because of cost implications and lack of evidence for its effectiveness at later stages of pregnancy.
A recent investigation identified unique subgroups among preeclampsia patients based on maternal blood pressure trajectories, providing insight into unique maternal subphenotypes and associated pregnancy outcomes. 11 Integration of a consolidated assessment of clinical characteristics, maternal hemodynamics, and placental function may become a useful strategy to develop pathway‐specific treatment programs that tailor clinical management to the underlying maternal phenotype.
Importantly, the development of effective multimodal screening for preeclampsia provides the opportunity to identify patients at increased risk of hypertensive disorders of pregnancy in the late first and early second trimesters. Although multimodal screening effectively identifies patients at risk of early‐onset preeclampsia, these strategies have not yet been widely adopted into clinical practice. 10 , 12 , 13 , 14 Low‐dose aspirin (150–162 mg/day depending on available drug formulation) prophylaxis initiated between 11 to 14 weeks of gestation significantly reduces the incidence of early‐onset preeclampsia in patients identified as being at high risk for early‐onset preeclampsia, with no effects on the incidence of late‐onset preeclampsia or gestational hypertension. 10 However, beyond low‐dose aspirin prophylaxis, little attention has been paid to the concept of tailoring clinical strategies to specific patients who develop hypertension in pregnancy to reduce associated adverse maternal and perinatal outcomes.
In this narrative review we focus on 2 strategies that we believe could allow clinicians to deliver more effective maternal‐fetal care in the context of hypertensive disorders of pregnancy. The first tool is the provision of real‐time maternal serum PlGF testing, which has been successfully integrated into the management of high‐risk pregnancies at Sinai Health System in Toronto, Canada. 9 The second is the use of noninvasive hemodynamic profiling to guide antihypertensive therapy in pregnant patients, which has become standard clinical practice at the University of Washington Medical Center in Seattle, WA. Based on our combined experiences, we propose a pragmatic phenotype‐based clinical approach that clinicians may adopt to deliver improved maternal and perinatal outcomes to hypertensive pregnant patients. Clinical case vignettes are included to illustrate how these concepts have been incorporated into high‐risk pregnancy care at our institutions.
Pathogenesis and Clinical Presentation of Preeclampsia
Preeclampsia is a potentially severe hypertensive disorder of pregnancy characterized by new‐onset maternal hypertension diagnosed after 20 weeks of gestation with associated end‐organ damage involving 1 or more organ systems. 15 , 16 The clinical presentation may vary from asymptomatic hypertension to critical hypertensive emergencies with organ injury and circulatory compromise. Across this spectrum, patients can be broadly classified by disease severity into an early‐ and a late‐onset manifestation of preeclampsia. 17 , 18 Patients who present early in pregnancy (<34 weeks of gestation) typically exhibit a more severe clinical phenotype that involves a higher degree of placental dysfunction and associated fetal growth restriction (FGR). 19 , 20 , 21 , 22 , 23 Abnormal placentation is hypothesized to be one of the main pathways that underlies the pathogenesis of early‐onset preeclampsia, mediating the elevation of antiangiogenic sFlt‐1 (soluble fms‐like tyrosine kinase‐1) protein and the suppression of proangiogenic PlGF protein. These endocrine changes interfere with systemic maternal vascular function and mediate transient renal injury with proteinuria. 23 , 24 , 25 Patients who present with preeclampsia later in pregnancy (≥34 weeks of gestation) typically exhibit a less severe clinical phenotype and mild placental involvement that is undetectable prenatally. The development of late‐onset preeclampsia is by contrast predominantly associated with underlying maternal constitutional factors, including preexisting abnormalities of cardiovascular function. 19 , 20 , 21 In broad terms, the pragmatic clinical division between early‐ and late‐onset preeclampsia based on a gestational age cutoff of 34 weeks distinguishes a minority of patients with severe placental dysfunction and growth restricted fetuses from the majority of patients with generally adequate placental function who have appropriately grown healthy fetuses. 26 Optimization of maternal prepregnancy hemodynamics through exercise or other strategies has been suggested as a potentially effective intervention to prevent preeclampsia and improve pregnancy outcomes. 27 , 28
It would be of great clinical interest to develop a more thorough understanding of the relative importance of prepregnancy cardiovascular function in comparison with factors arising during pregnancy, especially the importance of placental diseases, and adverse pregnancy outcomes. Previous research has suggested that an altered prepregnancy hemodynamic phenotype in healthy women aspiring to conceive is associated with the development of preeclampsia and FGR in the subsequent pregnancy. 29
Although these tend to be the classic presentations, the timing of preeclampsia diagnosis is not always synonymous with clinical severity or typical maternal and fetal phenotypes. Common maternal risk factors, including moderate obesity, chronic hypertension, and renal disease, may exert important influences on prepregnancy maternal cardiovascular and metabolic health and therefore also affect the clinical presentation, severity, and pregnancy outcomes of women destined to develop preeclampsia. 30
Placental Growth Factor—Improving the Prediction and Diagnosis of Preeclampsia
PlGF is a proangiogenic protein released by the placental syncytiotrophoblast surface covering the floating placental villi into the maternal circulation as an endocrine signal to promote normal systemic endothelial function. In normal pregnancy, maternal PlGF levels rise to a peak at 28 to 30 weeks of gestation, declining beyond 36 weeks when normal fetal growth velocity decelerates. 31 , 32 The increase in circulating PlGF levels in normal pregnancy mediates the adaptation of the maternal vasculature to pregnancy, characterized by a significant decline in systemic vascular resistance as pregnancy advances. 33 , 34 , 35 These adaptations facilitate the maintenance of normal blood pressure as maternal blood volume and CO increase to accommodate the metabolic needs of the growing fetus. 36
Patients who subsequently develop early‐onset preeclampsia, which is commonly associated with FGR, exhibit depressed circulating PlGF levels as early as late first or early second trimester. 31 , 32 Previous research has determined that the measurement of circulating PlGF levels is an effective strategy for the prediction and diagnosis of preeclampsia and is being steadily adopted in some countries. 37 , 38 Our group at Sinai Health System in Toronto integrated PlGF testing for pregnant patients with suspected placental dysfunction, hypertensive disorders of pregnancy, and/or FGR between 200 weeks and 35 6 weeks of gestation in March 2017. Based on clinical recommendations and previous research, we adopted a pragmatic gestational age‐independent PlGF cutoff of 100 pg/mL; pregnant patients with PlGF levels <100 pg/mL are defined as having abnormal test results. 39 Between March 2017 and December 2019, 979 patients completed PlGF testing and delivered at Sinai Health System. We recently reported our findings, confirming that pregnant patients with low PlGF levels were at strikingly increased risk of developing early‐onset preeclampsia (adjusted odds ratio, 58.6; 95% CI, 52.3–65.8), relative to at‐risk patients with normal PlGF levels. 9 Importantly, patients with low PlGF levels exhibited increased rates of preterm birth regardless of delivery indication, with over 60% delivering preterm and within a month of PlGF testing. 9 Low PlGF levels were also associated with increased risk of stillbirth (adjusted odds ratio, 16.1; 95% CI, 7.7–34.0), which was mostly associated with underlying placental diseases, principally maternal vascular malperfusion. A minority of stillbirths with normal maternal PlGF levels had a range of rare fetal causes.
The integration of PlGF testing has the potential to have profound practical effects on clinical decision‐making and patient safety managing high‐risk pregnancies at Sinai Health System (Figure 1). Clinical knowledge of PlGF levels and underlying placental function in high‐risk pregnant patients supplements standard assessments of placental anatomy and provides an opportunity to both (1) identify normotensive, asymptomatic patients at the highest risk prior of developing placental‐mediated complications, hypertensive disorders of pregnancy and/or FGR; and (2) allow for the accurate and timely diagnosis of placental‐mediated complications, hypertensive disorders of pregnancy, and/or FGR in symptomatic patients presenting to obstetric clinics or triage. Research by our group and others has determined that abnormal PlGF levels typically indicate severe maternal and fetal phenotypes in high‐risk pregnant patients. 9 , 31 , 32 The integration of PlGF testing provides a clinical advantage to tailor the management of these patients based on maternal phenotype and potentially affect maternal and fetal outcomes. Future work will focus on the impacts of PlGF testing on severe maternal outcomes, including eclampsia; renal, hepatic, or cardiac failure; and intensive care unit admission. Since the integration of PlGF testing at our center, potentially avoidable stillbirth in this high‐risk population occurred at a rate of 2 per 1000 over the 2.5 year period, which is half of the reported risk of stillbirth in the unselected and unmonitored Canadian population of 7.8 per 1000 total deliveries. 40
Figure 1. Integration of placental growth factor (PlGF) testing into clinical care of high‐risk pregnant patients: key points.

The prevailing view of clinicians working in our high volume (7600 births/year) center is that the integration of PlGF testing has greatly improved their confidence in the assessment and management of patients at risk of placental dysfunction, hypertensive disorders of pregnancy, and/or FGR.
Our group is also highly interested in evaluating the additional utility of sFlt‐1 testing in the clinical management of high‐risk patients. There is strong evidence to support sFlt‐1 testing as a prognostic and diagnostic tool for preeclampsia, either presented alone or more commonly as a sFlt‐1:PlGF ratio. 31 , 38 , 41 Because of cost limitations and encouraging data regarding diagnostic test precision of PlGF alone, 37 , 42 our center proceeded with PlGF testing as a single biomarker. This decision was also based on the natural trajectories of PlGF and sFlt‐1 levels in normotensive and preeclamptic pregnancies, with PlGF levels typically differentiating much earlier than sFlt‐1 levels between affected and unaffected women. 31 , 43
Placental Growth Factor Testing Key Points
Recent data from our group at Sinai Health System determined that low PlGF status (<100 pg/mL) in high‐risk pregnant patients between 200 and 356 weeks of gestation was associated with substantially increased rates of imminent preterm delivery and stillbirth associated with early‐onset preeclampsia 9
Based on clinical experiences from our group at Sinai Health System, the integration of PlGF testing into obstetrical care of high‐risk pregnant patients represents a powerful, innovative tool that provides a unique insight into the maternal phenotype
The integration of PlGF testing in our center allows pregnant patients with normal PlGF levels to avoid unnecessary surveillance and interventions, and patients with low PlGF levels to receive appropriate maternal‐fetal care, including admission for clinical monitoring and timely iatrogenic preterm birth
Clinical Case 1: Preeclampsia With Low PlGF Levels and Subclinical Cardiac Failure on Labetalol
A healthy 28‐year‐old G1P0 White patient with no clinical risk factors for preeclampsia was found to be hypertensive by her family physician at 30 weeks of gestation. A diagnosis of preeclampsia was established in the obstetrical triage unit by serial blood pressure recordings (averaging 145/95) and the detection of an elevated urine protein/creatinine ratio. Additional blood testing excluded hemolysis, elevated liver enzymes and low platelets syndrome. The patient was asymptomatic and reported normal fetal movements. The patient had a reactive nonstress test and fundal height was appropriate for gestational age. She was sent home on labetalol by mouth but returned 2 days later feeling progressively breathless. She denied any of the typical symptoms of preeclampsia. Her blood pressures continued to remain mild range. Her repeat laboratory evaluation was unremarkable. Labetalol was up‐titrated and she was discharged. She returned again within 24 hours continuing to complain of worsening shortness of breath. Her blood pressure remained mild range. Maternal‐fetal medicine was consulted, noting that the maternal heart rate values from her current and previous triage blood pressure assessments were low (65–70 bpm) and she was hemoconcentrated (hemoglobin 140 g/L). Her lung bases elicited some crackles on auscultation. A PlGF test (62 pg/mL) was very low, <2.5th centile for gestational age. 44 A troponin was slightly elevated (32 units; normal <10) and her brain natriuretic peptide was substantially elevated (401 pg/mL; normal <10). An ECG showed sinus rhythm. A bedside echocardiogram showed no valvular dysfunction but a significantly reduced CO for gestational age (3.1 L/min; normal 7 L/min—Figure 2A). A diagnosis of vasoconstrictive early‐onset preeclampsia was made, with mild cardiac impairment. Labetalol was discontinued and the patient was started on nifedipine XL 30 mg twice daily, with subsequent improvements in blood pressure and respiratory symptoms. Her brain natriuretic peptide declined to 205 pg/mL. Ultrasound demonstrated features of FGR, with bilateral abnormal uterine artery Dopplers, a small placenta, and abnormal umbilical artery Doppler. The patient was admitted for observation, antenatal corticosteroids, and fetal monitoring. Ultimately, she had a planned cesarean delivery 10 days later at 31 + 3, owing to further deterioration in umbilical artery Dopplers accompanied by abnormal fetal Doppler waveforms of the ductus venosus. The placenta exhibited multiple features of severe maternal‐vascular malperfusion pathology.
Figure 2.

Nomograms generated by the University of Washington Medical Center team to interpret the maternal hemodynamics.
A, Longitudinal changes in cardiac output (mean±1 SD) in normotensive healthy pregnant patients, as estimated by UltraCom method at the University of Washington, Seattle. Blue line=mean cardiac output; red lines=1 SD. B, Relationship between mean arterial pressure and cardiac output. Isometric lines of total peripheral resistance (TPR) are included in order to display all parameters on 1 graph.
Clinical Case 2: Gestational Hypertension With Normal PlGF Levels Treated With Labetalol
A 38‐year‐old G3P2 patient of Filipino descent was found to have asymptomatic hypertension at her 35‐week antenatal visit. Her body mass index was 32 and she had diet‐controlled gestational diabetes. An ultrasound examination demonstrated a normally grown fetus in the cephalic presentation. She was induced between 35 to 36 weeks of gestation secondary to hypertensive disorders of pregnancy in her 2 previous pregnancies in a community hospital. She was evaluated in triage by maternal‐fetal medicine and her blood pressure averaged 140/90, with heart rate 85 to 90 bpm. Her bloodwork was normal, including a hemoglobin of 103 g/L and a urine protein/creatinine ratio was normal. A PlGF test was normal (142 pg/mL). A diagnosis of gestational hypertension was made. She was given labetalol 200 mg 3 times a day. Three days later, she felt well. Her blood pressure averaged 132/78 and her heart rate averaged 72 to 76 bpm. She remained stable through weekly appointments, leading to spontaneous labor and birth at 39 weeks, with discharge home on postpartum day 1 on a reduced 100 mg 3 times a day dose of labetalol for the following 7 days. Thereafter, she remained normotensive.
Noninvasive Hemodynamics—Tailoring Antihypertensive Therapy for Pregnant Patients
Pregnant patients exert significant cardiovascular adaptations to support the demands of exponential fetal growth. These changes have been classically described as “a state of physiologic adaption to a protracted volume overloaded state with preserved intrinsic myocardial contractility/relaxation.” 36 Significant decreases in systolic and diastolic blood pressure are observed in the first trimester relative to nonpregnant patients, with subsequent normalization from the second trimester to term. 36 , 45 Heart rate (HR), stroke volume (SV), and CO are significantly increased from the first trimester throughout gestation. 36 , 45 Progressive increases in left ventricular mass are also observed in healthy pregnant patients, with adaptive changes to systolic and diastolic function associated with increased SV. 36
Patients who develop either early‐ or late‐onset preeclampsia exhibit contrasting hemodynamic and cardiovascular phenotypes, though both are initially recognized clinically by the detection of hypertension. Relative to normotensive pregnant patients, those with late‐onset preeclampsia typically exhibit a high cardiac output, low total peripheral resistance hemodynamic profile (referred to throughout the remainder of this article as “high output”), which is characterized by increased HR, increased SV, increased CO, and decreased total peripheral resistance (TPR); the increased cardiac workload may contribute to mild cardiovascular dysfunction. 17 , 46 , 47 , 48 , 49 In contrast, patients who develop early‐onset preeclampsia typically exhibit a low cardiac output, high total peripheral resistance hemodynamic profile (referred to throughout the remainder of this article as “high resistance”), which is characterized by increased TPR with decreased HR, decreased SV, decreased CO. 17 , 46 , 47 , 49 Cardiovascular impairment is more severe in patients with early‐onset preeclampsia, including biventricular systolic dysfunction that may lead to left ventricular hypertrophy. 47
Although these are the classic hemodynamic profiles associated with late‐ and early‐onset preeclampsia, additional hemodynamic profiles and patterns in pregnant patients with preeclampsia have been characterized. For example, hemodynamic crossover from a high output state to a high resistance state has been observed in patients with clinical preeclampsia; interestingly, patients with hemodynamic crossover exhibit high rates of intrauterine fetal demise. 50 , 51 A hemodynamic phenotype characterized by high resistance has also been strongly linked to the development of FGR. 51 , 52 , 53 , 54 , 55 , 56
Understanding the underlying maternal hemodynamics affords the opportunity to improve blood pressure control in hypertensive pregnancies by providing a physiologic basis for the appropriate selection of antihypertensive therapies. Refocusing on a new concept of normalizing hemodynamics as the primary goal of antihypertensive therapy could, in theory, prevent or at least mitigate progressive organ ischemia due to widespread systemic vasoconstriction and depressed CO. Promising research from a small number of trials suggests that antihypertensive therapy for pregnant patients guided by hemodynamics is more effective for the prevention of adverse pregnancy outcomes, such as severe hypertension and preterm birth, in comparison with generic prescribing of familiar drugs that have widely varying actions (reviewed in more detail here 57 ). A recent case study also emphasized the potential effectiveness of optimizing antihypertensive medications based on underlying maternal hemodynamic dysfunction in a pregnant patient diagnosed with FGR in a hypertensive crisis. 58 A recent prospective cohort study determined that the risk of recurrent preeclampsia in a high‐risk pregnant population could be significantly reduced through the tailored, phenotype‐based intervention with antihypertensive medications to normalize maternal hemodynamic imbalance before the development of hypertension, relative to standard clinical care. 59 Importantly, this tailored approach did not have adverse effects on fetal growth. A phenotype‐based approach has been successfully implemented in nonpregnant patients and has been shown to achieve superior blood pressure control than clinical judgment alone. 60 Unfortunately, there are no phenotype‐specific recommendations within existing clinical practice guidelines to guide antihypertensive therapy in pregnant patients.
The University of Washington Medical Center in Seattle has integrated noninvasive hemodynamic monitoring into standard clinical care since the late 1980s. By using noninvasive monitoring technology, the group is aware of the evolving underlying hemodynamics to guide the threshold and type of antihypertensive medication selection. The UltraCOM Cardiac Output Monitor (Lawrence Medical, Redmond, WA), an operator‐dependent Doppler echocardiography device that obtains the velocity time integral of blood flow velocity in the ascending aorta is used. 61 Hemodynamic measurements are made in the left lateral recumbent position after at least 5 minutes of rest. Blood pressure is measured in the left arm by an automated sphygmomanometer. The aortic diameter is measured with A‐mode ultrasound just above the sinuses of Valsalva and is used to calculate cross‐sectional area. SV is the product of cross‐sectional area and velocity time integral. CO is the product of HR and SV. The mean arterial pressure is calculated using the standard formula: mean arterial pressure=(2*diastolic blood pressur+systolic blood pressure)/3, and TPR is calculated using the formula TPR=80*mean arterial pressure/CO. Before full integration of noninvasive hemodynamic monitoring into routine clinical care at the University of Washington Medical Center, multiple studies were performed to validate the technology in pregnant 61 , 62 , 63 subjects and to characterize hemodynamic trajectories in normal and hypertensive pregnancies. 45 , 64 , 65 , 66 This methodology was then used to evaluate hemodynamic effects of antihypertensive medications in pregnancy with varying mechanisms of action, including atenolol, hydralazine, clonidine, and furosemide. The accumulated experience evolved into a clinical guide on timing and choice of antihypertensive medications to be used to manage hypertensive disorders in pregnancy. 67 , 68 , 69
In the current model of care at the University of Washington Medical Center, patients at high risk of developing preeclampsia are referred to the Hypertension Consultation Program for both preconception and antepartum consultation. Blood pressure is taken manually and automatically on at least 2 separate occasions from which mean arterial pressure is calculated. An UltraCOM hemodynamic assessment is performed to determine HR, CO, SV, and TPR. Each patient’s hemodynamic data are plotted on nomograms to interpret the CO and TPR (Figure 2). Treatment response in the context of the patient’s hemodynamic profile is assessed longitudinally throughout pregnancy. In this way, the overall response to treatment may be viewed, in addition to blood pressure control. Blood pressure targets are a systolic pressure <135 mm Hg and a diastolic pressure <85 mm Hg, as tight control of blood pressure is associated with fewer episodes of severe hypertension without negatively affecting neonatal birthweight. 70 We use a similar target for nonpregnant patients who present for preconception care and strongly recommend strict blood pressure management before pregnancy, as excellent blood pressure control optimizes endovascular conditions and may reduce future risks of preeclampsia as well as early pregnancy loss (please see Data S1 and Figure S1 for a representative case of preconception management of chronic hypertension). 71
The medication management strategy is then tailored to the underlying hemodynamic phenotype of each patient (Table, 72 , 73 , 74 ). Hypertensive patients with elevated CO (typically with low TPR) for gestational age receive beta‐blockade (atenolol or labetalol), whereas those with elevated TPR (typically with low CO) are initially treated with vasodilation (nifedipine XL or hydralazine). In patients with an elevated SV, persistent hypertension despite hemodynamic‐tailored blood pressure therapy, and clinical signs of volume overload, a diuretic (furosemide) is considered. Patients who are prescribed furosemide are also given a prescription for potassium and routine assessment of the amniotic fluid volume is performed. Nonselective beta‐blockade use in circumstances with low CO not surprisingly has been associated with FGR. 75 , 76 , 77 We therefore carefully monitor the CO response and adjust treatment accordingly, because an excessive drop in CO or increase in TPR is associated with poor fetal growth. 78 Patients with elevated TPR are carefully monitored to evaluate for normalization of TPR and a consequent increase in CO. The addition of low dose beta‐blockade is considered for these patients in order to maintain blood pressure control in the setting of compensatory tachycardia. Finally, the association between low CO and FGR has implications for Doppler assessments, becaus the choice of antihypertensive agent may affect umbilical Doppler waveforms. 79 A logical selection and titration of drug treatment will likely mitigate these potential fetal risks. 78 , 80 , 81
Table 1.
Mechanism of Action of Standardly Used Medications
| Drug name | Usual oral starting dose (Maximum dose) | Mechanism of action | Appropriate hemodynamic profile for therapy use |
|---|---|---|---|
| Atenolol |
12.5–25 mg twice a day (75 mg 3 times a day) |
Competitively blocks response to beta‐adrenergic stimulation, selectively blocks beta1‐receptors with little or no effect on beta2‐receptors except at high doses | High CO |
| Labetalol |
200 mg twice a day (400 mg 3 times a day) |
High CO | |
| Hydralazine |
5 mg 3 times a day (20 mg 4 times a day) |
|
High TPR |
| Nifedipine XL |
30 mg daily (60 mg twice a day) |
Inhibits calcium ion entry into the “slow channels” or select voltage‐sensitive areas of vascular smooth muscle and myocardium during depolarization, producing a relaxation of coronary vascular smooth muscle and coronary vasodilation; reduces peripheral vascular resistance, producing a reduction in arterial blood pressure | High TPR |
CO indicates cardiac output; and TPR, total peripheral resistance.
Noninvasive Hemodynamic Testing Key Points
Classic phenotypes of hypertension include high output (increased HR, SV, and CO with decreased TRP) and high resistance (increased TRP with decreased HR, SV, and CO).
The high CO phenotype is more commonly associated with late‐onset preeclampsia, normal fetal growth, and more favorable maternal and perinatal outcomes.
The high resistance phenotype is more commonly associated with early‐onset preeclampsia, FGR, and worse maternal and perinatal outcomes often in the setting of iatrogenic preterm birth.
Antihypertensive treatment that aims to normalize underlying hemodynamic abnormalities leads to superior blood pressure control and likely mitigates maternal and fetal risks.
Clinical Case 3: High Output Hypertension Despite Beta Blockade
A 35‐year‐old G5P3 presented to the Hypertension Consultation Clinic at 15 weeks of gestation with chronic hypertension and a history of preeclampsia in each of her 3 prior pregnancies. During this pregnancy, her blood pressures at 8 and 11 weeks were 120/86 and 154/94. At her initial clinical visit at 15 weeks, on no antihypertensive medications, her blood pressures were 120/78, 133/79, and 127/76, with a markedly elevated CO of 12.6, a TPR of 592, and an HR of 80 (Figure 3A and 3B). Given her high output state, she was started on atenolol 25 mg twice a day and returned at 28 weeks. In the interim, she was diagnosed with gestational diabetes and started on insulin. A growth ultrasound performed at 27 weeks demonstrated a normally grown fetus with an abdominal circumference approximately 2 weeks ahead. Her blood pressures at 28 weeks were 118/70, 120/74, and 125/74, with a CO of 10.8, a TPR of 674, and an HR of 73. Given her persistent elevation in CO, her atenolol was increased to 25 mg 3 times a day. A growth ultrasound at 31 weeks demonstrated continued normal fetal growth with an abdominal circumference that continued to measure 2 weeks ahead. She was seen for a final visit at 34 weeks. On atenolol 25 mg 3 times a day, her blood pressures were 122/67, 122/73, and 123/74, with a CO that remained elevated at 11.5, a TPR of 628, and an HR of 75. No medication changes were made at that time given reassuring blood pressures and adequate beta‐blockade. She ultimately developed superimposed preeclampsia without severe features at 37+2 weeks with newly detected proteinuria on a 24‐hour collection. She underwent a repeat cesarean delivery of a 3420 g (86th centile) infant. She did well postpartum and was discharged to home on atenolol 25 mg twice a day.
Figure 3. Persistent high output state with chronic hypertension and delivery at 37 weeks.
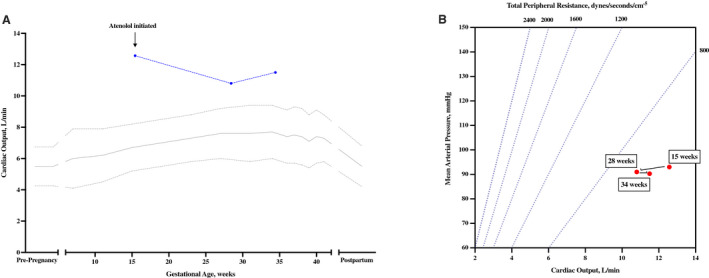
Serial hemodynamic assessments demonstrated high cardiac output. Treatment with atenolol at 15 weeks reduced cardiac output toward normal, but in all stages of pregnancy the patient was in a hyperdynamic state. A, Serial cardiac output measurements. B, Relationship between mean arterial pressure and cardiac output with total peripheral resistance (TPR) plotted as isometric lines.
Clinical Case 4: Hemodynamically Directed Change in Medication
A 31‐year‐old G2P1 presented to the Hypertension Consultation Clinic at 16 weeks of gestation with a history of severe preeclampsia requiring delivery at 31 weeks. During this pregnancy, her blood pressures at 7 and 12 weeks were 119/83 and 132/83. Her blood pressures at her initial clinical visits at 16 and 22 weeks, on no antihypertensive medications, were between 104/64 and 118/71 with overall normal hemodynamic parameters (Figure 4A and 4B). No medications were initiated at those visits; however, her referring provider was advised to initiate atenolol 12.5 mg twice a day with blood pressures persistently greater than 135/85. At 26 weeks an ultrasound demonstrated fetal growth at the 11th centile. She returned at 30 weeks having been started on atenolol 25 mg once a day in the morning and 12.5 mg once a day in the evening by her primary provider for elevated blood pressures at home. At this assessment, her blood pressures were 114/68, 115/70, and 101/69, with a CO of 8.8, a TPR of 724, and an HR of 82. She had a follow‐up growth ultrasound on that day that showed stable fetal growth with an estimated fetal weight again in the 11%. Given her increasing CO and reported elevated blood pressures at home, her atenolol was increased to 25 mg twice a day. By 33 weeks her blood pressures were normal at 102/70, 114/74, and 122/71, but her CO had dropped to 6.2 and her TPR had increased to 1135. A growth ultrasound demonstrated an estimated fetal weight in the 8th centile but now accompanied by an elevated umbilical artery Doppler pulsatility index. Given this change, she was observed on labor and delivery for fetal monitoring and medication adjustment. Her atenolol was decreased to 12.5 mg twice a day and she was started on nifedipine 30 mg XL daily to provide vasodilation and increase CO. Preeclampsia laboratory results at that time indicated that urine protein/creatinine ratio was normal. She was discharged home and returned for a final visit before delivery at 36 weeks. At that visit, on atenolol 12.5 mg twice a day and nifedipine 30 mg XL daily, her blood pressures were 124/64, 112/68, and 110/67. Her CO had increased to 7.0, her TPR had decreased to 929, and her HR was 72, which were interpreted as normal. Follow‐up umbilical artery Doppler remained abnormal, but diastolic flow had improved. She ultimately delivered by repeat cesarean delivery at 37 weeks for suspected FGR with abnormal umbilical artery Doppler studies. Birthweight was 2389 g (15th centile). The baby did well, and she was discharged home on atenolol 12.5 mg twice a day with normal blood pressures.
Figure 4. Hemodynamically directed change in antihypertensive medications resulting in a 37‐week delivery in the setting of suspected growth restriction.

A, Serial cardiac output measurements. B, Relationship between mean arterial pressure and cardiac output with total peripheral resistance (TPR) plotted as isometric lines.
Clinical Case 5: High Resistance Treated With Hydralazine
A 33‐year‐old G1P0 patient at 32 weeks of gestation presented to the Hypertension Consultation Clinic because of new‐onset hypertension. In early pregnancy her blood pressure was 130/80 and decreased in the second trimester. By 28 weeks, her blood pressure was 135/86 and then at 32 weeks was found to be 154/109. Preeclampsia laboratory results at 32 weeks were normal, including a 24‐hour urine that had no detectable proteinuria. A growth ultrasound demonstrated an estimated fetal weight in the 15th centile. At her initial assessment, her blood pressures were 140/92 and 131/78, with a CO of 4.2, a TPR of 1822, and an HR of 67 (Figure 5A and 5B). She was started on hydralazine 5 mg twice a day given her high resistance state. One week later her blood pressures had reduced to 125/79 and 124/73, with a CO that had increased slightly to 5.5, a TPR of 1309, and an HR of 64. The hydralazine was increased to 10 mg twice a day given the persistently elevated TPR. At 35 weeks, on hydralazine 10 mg twice a day, her blood pressures were 126/72 and 116/73, with a CO of 5.7, a TPR of 1218, and an HR of 65. Her preeclampsia laboratory results remained normal. Her hydralazine was further increased to 10 mg 3 times a day and she was induced at 37 weeks’ 4 days in the setting of gestational hypertension. Birthweight was 2722 g (28%). She continued hydralazine 10 mg 3 times a day upon discharge with stable blood pressures.
Figure 5. High resistance state noted at 32 weeks requiring increasing doses of hydralazine with ultimate delivery at 37 weeks.

A, Serial cardiac output measurements. B, Relationship between mean arterial pressure and cardiac output with total peripheral resistance (TPR) plotted as isometric lines.
Proposed Recommendations for Phenotype‐Driven Clinical Care of Hypertensive Pregnant Patients
Understanding the maternal phenotype provides a window of opportunity to tailor clinical management and optimize decision‐making for high‐risk patients, complementing the current standard‐of‐care approach to high‐risk pregnancy management (Figure 6).
Figure 6. Proposed recommendations for phenotype‐driven clinical care of hypertensive pregnant patients.

LMWH indicates low molecular weight heparin; PlGF, placental growth factor; and sFlt1, soluble fms‐like tyrosine kinase.
Prepregnancy period
Clinicians are recommended to assess the patients’ obstetrical history and clinical risk factors to estimate the risk of preeclampsia in a subsequent pregnancy.
In patients at high risk of developing preeclampsia and/or with existing hypertension, noninvasive hemodynamic assessment may be considered, and the patient should be counseled to start low‐dose aspirin prophylaxis by 12 weeks of gestation.
Knowledge of patients’ pre‐pregnancy phenotype is valuable to optimize maternal health before pregnancy and provide a baseline to monitor hemodynamic changes during pregnancy.
First Trimester
Multimodal screening of all pregnant patients is recommended to identify a screen‐positive group of patients at high risk for preeclampsia who benefit from low‐dose aspirin prophylaxis. Such models use family history, maternal clinical factors, blood‐based biomarkers, mean arterial pressure, and uterine‐artery pulsatility index to assess preeclampsia risk. 10 , 12 , 13
PlGF testing in the first trimester may be incorporated into first trimester screening to identify pregnant patients at high risk of early‐onset preeclampsia. 10 , 32
Initiation of low‐dose aspirin prophylaxis may be recommended in patients identified as high risk either based on clinical risk factors alone or from multimodal screening in the first trimester.
Noninvasive hemodynamic assessment at the end of the first trimester for pregnant patients with existing hypertension or increased risk of developing hypertension during pregnancy; optimization of underlying maternal hemodynamics based on phenotype is recommended.
Second Trimester
Doppler assessment of placental function (uterine arteries) and fetal growth (umbilical arteries) is recommended. Screening pregnant patients to identify those at increased risk of preeclampsia continues to be recommended in the second trimester. 24
Initiation of low‐dose aspirin prophylaxis continues to be recommended in high‐risk pregnant patients, ideally with therapy beginning before 16 weeks of gestation.
Based on our clinical experience at Sinai Health System, PlGF testing is recommended following 20 weeks of gestation when clinicians are concerned about the risk of placental dysfunction, hypertensive disorders of pregnancy, and/or FGR 9 ; the Society of Obstetricians and Gynaecologists of Canada recommends considering prophylactic doses of low‐molecular weight heparin in patients at the highest risk of placental dysfunction, for example in those with low PlGF levels despite low‐dose aspirin prophylaxis.
Based on our clinical experience at the University of Washington Medical Center, serial noninvasive assessment of maternal hemodynamics is recommended with continued medication titration based on hemodynamics.
Third Trimester
Continued Doppler assessment of fetal growth and well‐being is recommended, especially in the presence of impaired fetal growth and/or abnormal uterine artery Doppler.
Consideration of PlGF testing is recommended up to 356 weeks of gestation to assess the risk of imminent preterm birth due to early‐onset preeclampsia or the risk of stillbirth due to associated FGR. 9
Continued serial noninvasive assessment of maternal hemodynamics and optimization of underlying maternal hemodynamics based on phenotype are recommended.
The integration of PlGF testing and noninvasive hemodynamic monitoring into standard clinical care at our respective institutions has significantly improved our ability to deliver phenotype‐driven, tailored clinical care to high‐risk pregnant patients. Our respective clinical experiences compliment recent evidence that the clinical management of hypertensive disorders of pregnancy could benefit from a tailored approach. 59 , 82 Pregnant patients with an abnormal phenotype require higher‐level maternal‐fetal care that may include steroid administration, admission for intensive monitoring and planned iatrogenic preterm birth. The integration of these innovative strategies into clinical care is equally as important for patients who exhibit normal maternal phenotypes, who can then avoid unnecessary monitoring and interventions. In addition to individualized therapy, both institutions have a systematic, team‐based approach to coordinating care of hypertensive pregnant patients, which likely further improves outcomes.
The incorporation of a measure of placental function and noninvasive assessments of hemodynamic performance permit clinicians to construct a more comprehensive picture of the pathogenesis of underlying placental disease and hemodynamics, which in turn informs clinical decision‐making and has the potential to deliver improved maternal and perinatal outcomes. Relative to nonpregnant hypertensive patients, treatment of hypertension in pregnancy is more time sensitive and initial clinical decisions regarding management can have a significant impact on pregnancy outcomes. Understanding the underlying maternal phenotype can provide clinicians with a critical information to allow them to address the pathophysiology of each patient without a lengthy clinical workup, ideally preventing or minimizing disease progression.
Conclusions
In summary, a coordinated hypertension in pregnancy consultation program that has integrated PlGF testing for the screening and diagnosis of preeclampsia and noninvasive hemodynamic monitoring for optimization of antihypertensive therapy has delivered significant advances in the clinical management of pregnant patients with hypertensive disorders at our respective institutions. The integration of biomarkers for risk stratification and noninvasive hemodynamics to guide therapy may be able to (1) better identify the pathogenesis of maternal hypertension, (2) direct appropriate therapy that is safe for the fetus, and (3) ultimately prevent adverse maternal and perinatal outcomes. Future work should focus on promoting more widespread integration of these novel strategies into obstetric care to improve maternal and fetal pregnancy outcomes of high‐risk pregnancies.
Sources of Funding
J.C. Kingdom is supported by the Alva Foundation and Canadian Institutes of Health Research.
Disclosures
J.C. Kingdom has given talks on PlGF testing to high‐risk pregnancy groups across Canada on behalf of Roche Diagnostics. J.C. Kingdom is in receipt of a pilot grant from Roche Diagnostics to evaluate the role of PlGF screening to deliver virtual antenatal care. The remaining authors have no disclosures to report.
Supporting information
Data S1. Supplemental Clinical Case
Figure S1
Acknowledgments
The figures were created with https://biorender.com/.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023694
For Sources of Funding and Disclosures, see page 12.
References
- 1. Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, Gulmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 2. Chappell LC, Cluver CA, Kingdom J, Tong S. Pre‐eclampsia. Lancet. 2021;398:341–354. doi: 10.1016/S0140-6736(20)32335-7 [DOI] [PubMed] [Google Scholar]
- 3. Shen M, Smith GN, Rodger M, White RR, Walker MC, Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre‐eclampsia. PLoS One. 2017;12:e0175914. doi: 10.1371/journal.pone.0175914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K, et al. Cardiovascular disease‐related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139:1069–1079. doi: 10.1161/CIRCULATIONAHA.118.036748 [DOI] [PubMed] [Google Scholar]
- 5. Leon LJ, McCarthy FP, Direk K, Gonzalez‐Izquierdo A, Prieto‐Merino D, Casas JP, Chappell L. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: a CALIBER study. Circulation. 2019;140:1050–1060. doi: 10.1161/CIRCULATIONAHA.118.038080 [DOI] [PubMed] [Google Scholar]
- 6. McLaughlin K, Zhang J, Lye SJ, Parker JD, Kingdom JC. Phenotypes of pregnant women who subsequently develop hypertension in pregnancy. J Am Heart Assoc. 2018;7. doi: 10.1161/JAHA.118.009595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flo K, Wilsgaard T, Vårtun A, Acharya G. A longitudinal study of the relationship between maternal cardiac output measured by impedance cardiography and uterine artery blood flow in the second half of pregnancy. BJOG. 2010;117:837–844. doi: 10.1111/j.1471-0528.2010.02548.x [DOI] [PubMed] [Google Scholar]
- 8. Wright E, Audette MC, Ye XY, Keating S, Hoffman B, Lye SJ, Shah PS, Kingdom JC. Maternal vascular malperfusion and adverse perinatal outcomes in low‐risk nulliparous women. Obstet Gynecol. 2017;130:1112–1120. doi: 10.1097/AOG.0000000000002264 [DOI] [PubMed] [Google Scholar]
- 9. McLaughlin K, Snelgrove JW, Audette MC, Syed A, Hobson SR, Windrim RC, Melamed N, Carmona S, Kingdom JC. PlGF (Placental Growth Factor) testing in clinical practice: evidence from a Canadian tertiary maternity referral center. Hypertension. 2021;77:2057–2065. doi: 10.1161/HYPERTENSIONAHA.121.17047 [DOI] [PubMed] [Google Scholar]
- 10. Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622. doi: 10.1056/NEJMoa1704559 [DOI] [PubMed] [Google Scholar]
- 11. Roell KR, Harmon QE, Klungsøyr K, Bauer AE, Magnus P, Engel SM. Clustering longitudinal blood pressure trajectories to examine heterogeneity in outcomes among preeclampsia cases and controls. Hypertension. 2021;77:2034–2044. doi: 10.1161/HYPERTENSIONAHA.120.16239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guy GP, Leslie K, Diaz Gomez D, Forenc K, Buck E, Khalil A, Thilaganathan B. Implementation of routine first trimester combined screening for pre‐eclampsia: a clinical effectiveness study. BJOG. 2021;128:149–156. doi: 10.1111/1471-0528.16361 [DOI] [PubMed] [Google Scholar]
- 13. North RA, McCowan LME, Dekker GA, Poston L, Chan EHY, Stewart AW, Black MA, Taylor RS, Walker JJ, Baker PN, et al. Clinical risk prediction for pre‐eclampsia in nulliparous women: development of model in international prospective cohort. BMJ. 2011;342:d1875. doi: 10.1136/bmj.d1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allotey J, Snell KIE, Smuk M, Hooper R, Chan CL, Ahmed A, Chappell LC, von Dadelszen P, Dodds J, Green M, et al. Validation and development of models using clinical, biochemical and ultrasound markers for predicting pre‐eclampsia: an individual participant data meta‐analysis. Health Technol Assess. 2020;24:1–252. doi: 10.3310/hta24720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, Magee LA, Audibert F, Bujold E, Côté A‐M, Douglas MJ, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obst Gynaecol Canada. 2014;36:416–441. doi: 10.1016/S1701-2163(15)30588-0 [DOI] [PubMed] [Google Scholar]
- 16. ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25. [DOI] [PubMed] [Google Scholar]
- 17. Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 1979;2008:873–880. doi: 10.1161/HYPERTENSIONAHA.108.117358 [DOI] [PubMed] [Google Scholar]
- 18. Vasapollo B, Novelli GP, Valensise H. Total vascular resistance and left ventricular morphology as screening tools for complications in pregnancy. Hypertension. 1979;2008:1020–1026. doi: 10.1161/HYPERTENSIONAHA.107.105858 [DOI] [PubMed] [Google Scholar]
- 19. Crispi F, Llurba E, Dominguez C, Martin‐Gallan P, Cabero L, Gratacos E. Predictive value of angiogenic factors and uterine artery Doppler for early‐ versus late‐onset pre‐eclampsia and intrauterine growth restriction. Ultrasound Obst Gynecol. 2008;31:303–309. doi: 10.1002/uog.5184 [DOI] [PubMed] [Google Scholar]
- 20. Magnussen EB, Vatten LJ, Lund‐Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre‐eclampsia: population based cohort study. BMJ. 2007;335:978. doi: 10.1136/bmj.39366.416817.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogge G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, Kim CJ, Hassan SS. Placental lesions associated with maternal underperfusion are more frequent in early‐onset than in late‐onset preeclampsia. J Perinat Med. 2011;39:641–652. doi: 10.1515/jpm.2011.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levytska K, Higgins M, Keating S, Melamed N, Walker M, Sebire NJ, Kingdom JC. Placental pathology in relation to uterine artery Doppler findings in pregnancies with severe intrauterine growth restriction and abnormal umbilical artery Doppler changes. Am J Perinatol. 2017;34:451–457. [DOI] [PubMed] [Google Scholar]
- 23. Ho AEP, Hutter J, Jackson LH, Seed PT, McCabe L, Al‐Adnani M, Marnerides A, George S, Story L, Hajnal JV, et al. T2* placental magnetic resonance imaging in preterm preeclampsia: an observational cohort study. Hypertension. 1979;2020:1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti‐VEGF antibodies and soluble VEGF receptor 1 (sFlt‐1) induces proteinuria. J Biol Chem. 2003;278:12605–12608. doi: 10.1074/jbc.C300012200 [DOI] [PubMed] [Google Scholar]
- 25. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zur RL, Kingdom JC, Parks WT, Hobson SR. The placental basis of fetal growth restriction. Obstet Gynecol Clin North Am. 2020;47:81–98. doi: 10.1016/j.ogc.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 27. Genest DS, Falcao S, Gutkowska J, Lavoie JL. Impact of exercise training on preeclampsia: potential preventive mechanisms. Hypertension. 1979;2012(60):1104–1109. doi: 10.1161/HYPERTENSIONAHA.112.194050 [DOI] [PubMed] [Google Scholar]
- 28. Reyes LM, Davenport MH. Exercise as a therapeutic intervention to optimize fetal weight. Pharmacol Res. 2018;132:160–167. doi: 10.1016/j.phrs.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 29. Foo FL, Mahendru AA, Masini G, Fraser A, Cacciatore S, MacIntyre DA, McEniery CM, Wilkinson IB, Bennett PR, Lees CC. Association between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertension. 1979;2018:442–450. doi: 10.1161/HYPERTENSIONAHA.118.11092 [DOI] [PubMed] [Google Scholar]
- 30. Oliveira N, Magder LS, Blitzer MG, Baschat AA. First‐trimester prediction of pre‐eclampsia: external validity of algorithms in a prospectively enrolled cohort. Ultrasound Obstetrics Gynecol. 2014;44:279–285. doi: 10.1002/uog.13435 [DOI] [PubMed] [Google Scholar]
- 31. Levine RJ, Maynard SE, Qian C, Lim K‐H, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884 [DOI] [PubMed] [Google Scholar]
- 32. Agrawal S, Shinar S, Cerdeira AS, Redman C, Vatish M. Predictive Predictive performance of PlGF (Placental Growth Factor) for screening preeclampsia in asymptomatic women. Hypertension. 1979;2019:1124–1135. doi: 10.1161/HYPERTENSIONAHA.119.13360 [DOI] [PubMed] [Google Scholar]
- 33. Osol G, Celia G, Gokina N, Barron C, Chien E, Mandala M, Luksha L, Kublickiene K. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1381–H1387. doi: 10.1152/ajpheart.00922.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McLaughlin K, Baczyk D, Potts A, Hladunewich M, Parker JD, Kingdom JC. Low molecular weight heparin improves endothelial function in pregnant women at high risk of preeclampsia. Hypertension. 2017;69:180–188. doi: 10.1161/HYPERTENSIONAHA.116.08298 [DOI] [PubMed] [Google Scholar]
- 35. Ling HZ, Guy GP, Bisquera A, Poon LC, Nicolaides KH, Kametas NA. Maternal hemodynamics in screen‐positive and screen‐negative women of the ASPRE trial. Ultrasound Obst Gynecol. 2019;54:51–57. doi: 10.1002/uog.20125 [DOI] [PubMed] [Google Scholar]
- 36. Melchiorre K, Sharma R, Khalil A, Thilaganathan B. Maternal Cardiovascular function in normal pregnancy: evidence of maladaptation to chronic volume overload. Hypertension. 1979;2016:754–762. doi: 10.1161/HYPERTENSIONAHA.115.06667 [DOI] [PubMed] [Google Scholar]
- 37. Duhig KE, Myers J, Seed PT, Sparkes J, Lowe J, Hunter RM, Shennan AH, Chappell LC, Bahl R, Bambridge G, et al. Placental growth factor testing to assess women with suspected pre‐eclampsia: a multicentre, pragmatic, stepped‐wedge cluster‐randomised controlled trial. Lancet. 2019;393:1807–1818. doi: 10.1016/S0140-6736(18)33212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, et al. Predictive value of the sFlt‐1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838 [DOI] [PubMed] [Google Scholar]
- 39. Hurrell A, Beardmore‐Gray A, Duhig K, Webster L, Chappell LC, Shennan AH. Placental growth factor in suspected preterm pre‐eclampsia: a review of the evidence and practicalities of implementation. BJOG. 2020;127:1590–1597. doi: 10.1111/1471-0528.16425 [DOI] [PubMed] [Google Scholar]
- 40. Centre for Surveillance and Applied Research PHAoC . Perinatal Health Indicators Data Tool. 2020th ed. Public Health Infobase Ottawa, ON: Public Health Agency of Canada; 2020. [Google Scholar]
- 41. Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–919. doi: 10.1161/CIRCULATIONAHA.111.054361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, Simpson N, Waugh J, Anumba D, Kenny LC, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128:2121–2131. doi: 10.1161/CIRCULATIONAHA.113.003215 [DOI] [PubMed] [Google Scholar]
- 43. Chaiyasit N, Sahota DS, Ma R, Choolani M, Wataganara T, Sim WS, Chaemsaithong P, Wah YMI, Hui SYA, Poon LC. Prospective evaluation of international prediction of pregnancy complications collaborative network models for prediction of preeclampsia: role of serum sFlt‐1 at 11–13 weeks’ gestation. Hypertension. 2022;79:314–322. doi: 10.1161/HYPERTENSIONAHA.121.18021 [DOI] [PubMed] [Google Scholar]
- 44. McLaughlin K, Hobson SR, Chandran AR, Agrawal S, Windrim RC, Parks WT, Bowman AW, Sovio U, Smith GC, Kingdom JC. Circulating maternal placental growth factor responses to low‐molecular‐weight heparin in pregnant patients at risk of placental dysfunction. Am J Obstet Gynecol. 2021. doi: 10.1016/j.ajog.2021.08.027 [DOI] [PubMed] [Google Scholar]
- 45. Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol. 1990;76:1061–1069. [PubMed] [Google Scholar]
- 46. Melchiorre K, Sutherland G, Sharma R, Nanni M, Thilaganathan B. Mid‐gestational maternal cardiovascular profile in preterm and term pre‐eclampsia: a prospective study. BJOG. 2013;120:496–504. doi: 10.1111/1471-0528.12068 [DOI] [PubMed] [Google Scholar]
- 47. Melchiorre K, Sutherland GR, Watt‐Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy. 2012;31:454–471. doi: 10.3109/10641955.2012.697951 [DOI] [PubMed] [Google Scholar]
- 48. Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 1979;2011(57):85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321 [DOI] [PubMed] [Google Scholar]
- 49. Doherty A, Carvalho JC, Drewlo S, El‐Khuffash A, Downey K, Dodds M, Kingdom J. Altered hemodynamics and hyperuricemia accompany an elevated sFlt‐1/PlGF ratio before the onset of early severe preeclampsia. J Obst Gynaecol Canada. 2014;36:692–700. doi: 10.1016/S1701-2163(15)30511-9 [DOI] [PubMed] [Google Scholar]
- 50. Bosio PM, McKenna PJ, Conroy R, O'Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94:978–984. [DOI] [PubMed] [Google Scholar]
- 51. Easterling TR, Benedetti TJ, Carlson KC, Brateng DA, Wilson J, Schmucker BS. The effect of maternal hemodynamics on fetal growth in hypertensive pregnancies. Am J Obstet Gynecol. 1991;165:902–906. doi: 10.1016/0002-9378(91)90436-U [DOI] [PubMed] [Google Scholar]
- 52. Oben J, Tomsin K, Mesens T, Staelens A, Molenberghs G, Gyselaers W. Maternal cardiovascular profiling in the first trimester of pregnancies complicated with gestation‐induced hypertension or fetal growth retardation: a pilot study. J Matern‐Fetal Neonatal Med. 2014;27:1646–1651. doi: 10.3109/14767058.2013.871700 [DOI] [PubMed] [Google Scholar]
- 53. Stott D, Papastefanou I, Paraschiv D, Clark K, Kametas NA. Longitudinal maternal hemodynamics in pregnancies affected by fetal growth restriction. Ultrasound Obstet Gynecol. 2017;49:761–768. doi: 10.1002/uog.17340 [DOI] [PubMed] [Google Scholar]
- 54. Roberts LA, Ling HZ, Poon LC, Nicolaides KH, Kametas NA. Maternal hemodynamics, fetal biometry and Doppler indices in pregnancies followed up for suspected fetal growth restriction. Ultrasound in Obstet Gynecol. 2018;52:507–514. doi: 10.1002/uog.19067 [DOI] [PubMed] [Google Scholar]
- 55. Ferrazzi E, Stampalija T, Monasta L, Di Martino D, Vonck S, Gyselaers W. Maternal hemodynamics: a method to classify hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2018;218:124.e1–124.e11. doi: 10.1016/j.ajog.2017.10.226 [DOI] [PubMed] [Google Scholar]
- 56. Perry H, Lehmann H, Mantovani E, Thilaganathan B, Khalil A. Are maternal hemodynamic indices markers of fetal growth restriction in pregnancies with a small‐for‐gestational‐age fetus? Ultrasound in Obstet Gynecol. 2020;55:210–216. doi: 10.1002/uog.20419 [DOI] [PubMed] [Google Scholar]
- 57. McLaughlin K, Scholten RR, Kingdom JC, Floras JS, Parker JD. Should maternal hemodynamics guide antihypertensive therapy in preeclampsia? Hypertension. 1979;2018(71):550–556. doi: 10.1161/HYPERTENSIONAHA.117.10606 [DOI] [PubMed] [Google Scholar]
- 58. Lees C, Ferrazzi E. Relevance of haemodynamics in treating pre‐eclampsia. Curr Hypertens Rep. 2017;19:76. doi: 10.1007/s11906-017-0766-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mulder EG, Ghossein‐Doha C, Cauffman E, Lopes van Balen VA, Schiffer VMMM, Alers R‐J, Oben J, Smits L, van Kuijk SMJ, Spaanderman MEA. Preventing recurrent preeclampsia by tailored treatment of nonphysiologic hemodynamic adjustments to pregnancy. Hypertension. 1979;2021(77):2045–2053. doi: 10.1161/HYPERTENSIONAHA.120.16502 [DOI] [PubMed] [Google Scholar]
- 60. Thilaganathan B, Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension. 1979;2019(73):522–531. doi: 10.1161/HYPERTENSIONAHA.118.11191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Easterling TR, Watts DH, Schmucker BC, Benedetti TJ. Measurement of cardiac output during pregnancy: validation of Doppler technique and clinical observations in preeclampsia. Obstet Gynecol. 1987;69:845–850. [PubMed] [Google Scholar]
- 62. Easterling TR, Carlson KL, Schmucker BC, Brateng DA, Benedetti TJ. Measurement of cardiac output in pregnancy by Doppler technique. Am J Perinatol. 1990;7:220–222. doi: 10.1055/s-2007-999486 [DOI] [PubMed] [Google Scholar]
- 63. Lee W, Rokey R, Cotton DB. Noninvasive maternal stroke volume and cardiac output determinations by pulsed Doppler echocardiography. Am J Obstet Gynecol. 1988;158:505–510. doi: 10.1016/0002-9378(88)90014-2 [DOI] [PubMed] [Google Scholar]
- 64. Chandraratna PA, Nanna M, McKay C, Nimalasuriya A, Swinney R, Elkayam U, Rahimtoola SH. Determination of cardiac output by transcutaneous continuous‐wave ultrasonic Doppler computer. Am J Cardiol. 1984;53:234–237. doi: 10.1016/0002-9149(84)90718-5 [DOI] [PubMed] [Google Scholar]
- 65. Huntsman LL, Stewart DK, Barnes SR, Franklin SB, Colocousis JS, Hessel EA. Noninvasive Doppler determination of cardiac output in man. Clinical validation. Circulation. 1983;67:593–602. doi: 10.1161/01.CIR.67.3.593 [DOI] [PubMed] [Google Scholar]
- 66. Nishimura RA, Callahan MJ, Schaff HV, Ilstrup DM, Miller FA, Tajik AJ. Noninvasive measurement of cardiac output by continuous‐wave Doppler echocardiography: initial experience and review of the literature. Mayo Clin Proc. 1984;59:484–489. doi: 10.1016/S0025-6196(12)60438-8 [DOI] [PubMed] [Google Scholar]
- 67. Easterling TR, Benedetti TJ, Schmucker BC, Carlson KL. Antihypertensive therapy in pregnancy directed by noninvasive hemodynamic monitoring. Am J Perinatol. 1989;6:86–89. doi: 10.1055/s-2007-999553 [DOI] [PubMed] [Google Scholar]
- 68. Rothberger S, Carr D, Brateng D, Hebert M, Easterling TR. Pharmacodynamics of clonidine therapy in pregnancy: a heterogeneous maternal response impacts fetal growth. Am J Hypertens. 2010;23:1234–1240. doi: 10.1038/ajh.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carr DB, Gavrila D, Brateng D, Easterling TR. Maternal hemodynamic changes associated with furosemide treatment. Hypertens Pregnancy. 2007;26:173–178. doi: 10.1080/10641950701204489 [DOI] [PubMed] [Google Scholar]
- 70. Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, et al. Less‐tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–417. doi: 10.1056/NEJMoa1404595 [DOI] [PubMed] [Google Scholar]
- 71. Nobles CJ, Mendola P, Mumford SL, Naimi AI, Yeung EH, Kim K, Park H, Wilcox B, Silver RM, Perkins NJ, et al. Preconception blood pressure levels and reproductive outcomes in a prospective cohort of women attempting pregnancy. Hypertension. 1979;2018(71):904–910. doi: 10.1161/HYPERTENSIONAHA.117.10705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Goa KL, Benfield P, Labetalol SEM. A reappraisal of its pharmacology, pharmacokinetics and therapeutic use in hypertension and ischaemic heart disease. Drugs. 1989;37:583–627. [DOI] [PubMed] [Google Scholar]
- 73. Carvalho TMJP, de Carvalho CR, Cunha SP, de Baraldi CO, Marques MP, Antunes NJ, Godoy ALPC, Lanchote VL. Influence of gestational diabetes mellitus on the stereoselective kinetic disposition and metabolism of labetalol in hypertensive patients. Eur J Clin Pharmacol. 2011;67:55–61. doi: 10.1007/s00228-010-0896-0 [DOI] [PubMed] [Google Scholar]
- 74. McComb MN, Chao JY, Ng TM. Direct vasodilators and sympatholytic agents. J Cardiovasc Pharmacol Ther. 2016;21:3–19. doi: 10.1177/1074248415587969 [DOI] [PubMed] [Google Scholar]
- 75. Bellos I, Pergialiotis V, Papapanagiotou A, Loutradis D, Daskalakis G. Comparative efficacy and safety of oral antihypertensive agents in pregnant women with chronic hypertension: a network metaanalysis. Am J Obstet Gynecol. 2020;223:525–537. doi: 10.1016/j.ajog.2020.03.016 [DOI] [PubMed] [Google Scholar]
- 76. Duan L, Ng A, Chen W, Spencer HT, Lee MS. Beta‐blocker subtypes and risk of low birth weight in newborns. J Clin Hypertens. 2018;20:1603–1609. doi: 10.1111/jch.13397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Meidahl Petersen K, Jimenez‐Solem E, Andersen JT, Petersen M, Brødbæk K, Køber L, Torp‐Pedersen C, Poulsen HE. β‐Blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population‐based cohort study. BMJ Open. 2012;2:e001185. doi: 10.1136/bmjopen-2012-001185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Easterling TR, Carr DB, Brateng D, Diederichs C, Schmucker B. Treatment of hypertension in pregnancy: effect of atenolol on maternal disease, preterm delivery, and fetal growth. Obstet Gynecol. 2001;98:427–433. doi: 10.1097/00006250-200109000-00012 [DOI] [PubMed] [Google Scholar]
- 79. Harper A, Murnaghan GA. Maternal and fetal haemodynamics in hypertensive pregnancies during maternal treatment with intravenous hydralazine or labetalol. Br J Obstet Gynaecol. 1991;98:453–459. doi: 10.1111/j.1471-0528.1991.tb10339.x [DOI] [PubMed] [Google Scholar]
- 80. Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol. 1999;93:725–733. doi: 10.1097/00006250-199905000-00018 [DOI] [PubMed] [Google Scholar]
- 81. Chaffin D, Cottrell J, Cummings K, Jude D. 821: directed antihypertensive therapy improves growth restriction and perinatal mortality in women with chronic hypertension. Am J Obstet Gynecol. 2020;222:S516–S517. doi: 10.1016/j.ajog.2019.11.836 [DOI] [PubMed] [Google Scholar]
- 82. Duhig KE, Myers J, Seed PT, Sparkes J, Lowe J, Hunter RM, Shennan AH, Chappell LC, Bahl R, Bambridge G, et al. Placental growth factor testing to assess women with suspected pre‐eclampsia: a multicentre, pragmatic, stepped‐wedge cluster‐randomised controlled trial. Lancet. 2019;393:1807–1818. doi: 10.1016/S0140-6736(18)33212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Clinical Case
Figure S1


