Abstract
Background
Clinical outcomes of acute myocardial infarction complicated by cardiogenic shock remain poor with high in‐hospital mortality. Veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) has been widely used for patients with acute myocardial infarction complicated by cardiogenic shock refractory to conservative therapy, which is likely fatal without mechanical circulatory support. However, whether additional intra‐aortic balloon pumping (IABP) use during VA‐ECMO support improves clinical outcomes remains controversial. This study sought to investigate prognostic impact of the combined VA‐ECMO plus IABP treatment compared with VA‐ECMO alone.
Methods and Results
From the nationwide Japanese administrative case‐mix Diagnostic Procedure Combination (DPC), the JROAD (Japanese Registry of All Cardiac and Vascular Diseases)–DPC, we identified 3815 patients with acute myocardial infarction complicated by cardiogenic shock who underwent primary percutaneous coronary intervention and managed with VA‐ECMO. Of these, 2964 patients (77.7%) were managed with IABP (VA‐ECMO plus IABP), whereas 851 (22.3%) were managed without IABP (VA‐ECMO alone). We compared in‐hospital, 7‐day, and 30‐day mortality between the VA‐ECMO plus IABP versus the VA‐ECMO alone support. Patients managed with VA‐ECMO plus IABP demonstrated significantly lower in‐hospital, 7‐day, and 30‐day mortality than those managed with VA‐ECMO alone (adjusted odds ratios [95% CI] of 0.47 [95% CI, 0.38–0.59], 0.41 [95% CI, 0.33–0.51], and 0.30 [95% CI, 0.25–0.37], respectively). The findings were consistent in the propensity matching and inverse probability of treatment‐weighting models.
Conclusions
This large‐scale, nationwide study demonstrated that the combination of VA‐ECMO plus IABP support was associated with significantly lower mortality compared with VA‐ECMO support alone in patients presenting with acute myocardial infarction complicated by cardiogenic shock who underwent primary percutaneous coronary intervention.
Keywords: acute myocardial infarction, cardiogenic shock, intra‐aortic balloon pumping, venoarterial extracorporeal membrane oxygenation
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care, Heart Failure
Nonstandard Abbreviations and Acronyms
- AMI‐CS
acute myocardial infarction complicated by cardiogenic shock
- CS
cardiogenic shock
- DPC
Diagnostic Procedure Combination
- IABP
intra‐aortic balloon pumping
- JROAD
Japanese Registry of All Cardiac and Vascular Diseases
- MCS
mechanical circulatory support
- PSM
propensity score matching ; VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation
- VA‐ECMO
veno‐arterial extracorporeal membrane oxygenation
Clinical Perspective
What Is New?
This large‐scale, nationwide study demonstrated that the combination of veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) plus intra‐aortic balloon pumping support was associated with significantly lower mortality compared with VA‐ECMO support alone in patients presenting with acute myocardial infarction complicated by cardiogenic shock who underwent primary percutaneous coronary intervention.
The lower mortality in the VA‐ECMO plus intra‐aortic balloon pumping group was consistent across important subgroups stratified by age, sex, and the presence of cardiac arrest at admission.
The combined VA‐ECMO plus intra‐aortic balloon pumping was not associated with significant increases in cerebral and bleeding events compared with VA‐ECMO alone.
What Are the Clinical Implications?
The results of the present study support active use of intra‐aortic balloon pumping to improve clinical outcomes of patients with acute myocardial infarction complicated by cardiogenic shock when VA‐ECMO is indicated.
Cardiogenic shock (CS) is a life‐threatening condition associated with end‐organ hypoperfusion and hypoxia resulting from reduced cardiac output. CS complicates approximately 5% to 12% of acute myocardial infarction (AMI). 1 Despite recent advances in reperfusion therapy, in particular primary percutaneous coronary intervention (PCI), clinical outcomes of AMI complicated by CS (AMI‐CS) remain poor with high in‐hospital mortality. 2 Conservative management with inotropes and vasopressors is associated with serious limitations, including arrhythmias, increased myocardial oxygen demand, and limited circulatory support to maintain adequate perfusion pressure. To overcome the limitations of the conservative treatment, mechanical circulatory support (MCS) has been being developed to improve hemodynamics and outcome in CS. 3
Veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) can be placed peripherally and quickly and has been widely used for the treatment of patients with AMI‐CS refractory to conservative therapy, which is likely fatal without MCS. Despite the lifesaving technology, the most important concern in VA‐ECMO support is retrograde aortic flow toward the left ventricle (LV), which can cause a significant increase in LV afterload, compromising LV function. In the setting of AMI with severe LV dysfunction, the LV would not be able to tolerate the increased afterload, further impairing its performance. 4 , 5 Intra‐aortic balloon pumping (IABP) may be an option to optimize hemodynamic status during VA‐ECMO by reducing afterload and improving coronary perfusion.
However, whether additional IABP use during VA‐ECMO support improves clinical outcomes remains controversial. 6 This study sought to investigate prognostic impact of the combined VA‐ECMO plus IABP treatment compared with VA‐ECMO alone in patients with AMI who received primary PCI by analyzing a large‐scale, Japanese nationwide registry data.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Population
The present study was a retrospective observational study based on a nationwide Japanese administrative case‐mix Diagnostic Procedure Combination (DPC), the Japanese Registry of All Cardiac and Vascular Diseases (JROAD)–DPC. 7 In brief, the JROAD‐DPC database is DPC‐based payment health insurance claim data about hospitalizations attributed to cardiovascular diseases collected from the 1040 Japanese Circulation Society–certified hospitals between April 2012 and March 2018. Using the International Classification of Diseases, Tenth Revision (ICD‐10) codes of I21.0, I21.1, I21.2, I21.3, I21.4, and I21.9 for AMI hospitalization recorded as “the main diagnosis,” “the admission precipitating diagnosis,” or “the most resource‐consuming diagnosis” in the DPC claim data, 260 543 patients with AMI aged ≥20 years were identified. ICD‐10 code for AMI was validated in the JROAD‐DPC database, as previously described. 8 Of these patients, 28 350 patients were excluded because of missing Killip classifications. The remaining participants were divided into 2 groups based on the SCAI (Society for Cardiovascular Angiography and Interventions) stages of CS. 9 CS, equivalent to the SCAI C/D/E group, was defined as Killip classification 3 or 4 that met at least 1 of the following criteria: (1) MCS use or (2) intravenous administration of catecholamines on admission; other patients were defined as non‐CS, equivalent to the SCAI A/B group. Of the patients with CS, we identified 3815 patients who underwent primary PCI and managed with VA‐ECMO who were included in the present analyses (Figure 1). Of these, 2964 patients (77.7%) were managed with IABP (VA‐ECMO plus IABP), whereas 851 (22.3%) were managed without IABP (VA‐ECMO alone; Figure 1).
Figure 1. Patient selection.
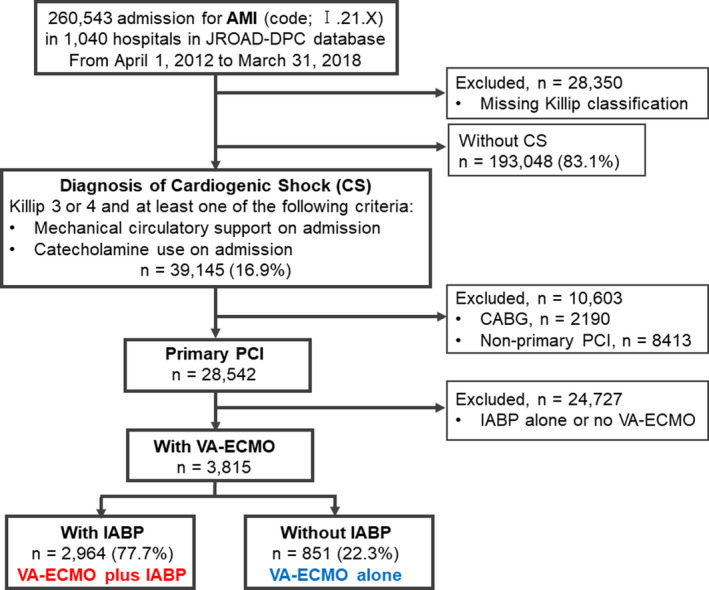
AMI indicates acute myocardial infarction; CABG, coronary artery bypass grafting; CS, cardiogenic shock DPC, Diagnosis Procedure Combination; IABP, intra‐aortic balloon pumping; JROAD, Japanese Registry of All Cardiac and Vascular Diseases; PCI, percutaneous coronary intervention; and VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation.
Ethical Considerations
This study was conducted in accordance with the Declaration of Helsinki and its amendments. The ethics committees at Miyazaki Prefectural Nobeoka Hospital (No. 20200721‐1), Kawasaki Medical School (No. 3928), and Kumamoto University Hospital (No. 2095) approved the study protocol. The study was exempted from the requirement for individual informed consent because of the opt‐out policy.
Outcomes
The primary outcome was in‐hospital all‐cause mortality. The secondary outcomes include 7‐day and 30‐day mortality and the incidence of stroke, major bleeding, intracranial bleeding, and gastrointestinal bleeding during the index hospitalization. We also extracted data on the incidence of mechanical respirator use, hemodialysis, and ventricular assist device implantation.
Statistical Analysis
Data were presented as the median and interquartile range (IQR) for continuous variables and number (percentage) for categorical variables. The incidence of each clinical event was described as per 1000 patient‐days, and the risk ratio of the event between the 2 treatment arms was calculated. To estimate the effect of the 2 treatments on all‐cause mortality, odds ratios (ORs) and 95% CIs were calculated with random effects to account for institution‐related variation with multivariable adjustments to reduce the effect of known possible confounders. In addition, propensity score matching (PSM) was performed between the VA‐ECMO plus IABP group and the VA‐ECMO alone group based on the estimated propensity scores using nearest‐neighbor matching (1:1) within a caliper width of 0.2 standard deviation without replacement. Furthermore, to confirm the robustness of the results, inverse probability of treatment weighting with the same predicted probability used in the PSM was performed as sensitivity analysis for the same outcome. The actuarial survivals were calculated using the Kaplan–Meier method, with the log‐rank test used for the comparison between the 2 groups. A 2‐sided P value of <0.05 was considered to denote the presence of a statistically significant difference. For more details, please see Data S1.
Results
Patient Background
The baseline characteristics of the 2 groups before PSM are shown in Table 1. Before PSM, patients in the VA‐ECMO alone group (n=851) were older, more often women, and more likely to have peripheral vascular disease. In contrast, patients in the VA‐ECMO plus IABP group (n=2964) were more likely to undergo emergency admission; use an ambulance; and have hypertension, dyslipidemia, diabetes, and atrial fibrillation. IABP was more prone to be used in hospitals with higher teaching status, larger numbers of hospital beds and board‐certified cardiologists, a coronary care unit, cardiac surgery, and board‐certified rehabilitation. The percentage of patients who showed cardiac arrest at admission was similar between the 2 groups. After PSM, baseline characteristics were well balanced between the 2 groups (n=846; Table S1). Temporal trends in the usage rate of VA‐ECMO and VA‐ECMO plus IABP and in‐hospital mortality are shown in Figure 2. There was a slight increase in VA‐ECMO use among patients with AMI‐CS; however, the usage rate of IABP among patients on VA‐ECMO support and in‐hospital mortality were seemingly unchanged during the study period.
Table 1.
Patient and Institutional Characteristics
|
VA‐ECMO plus IABP (n=2964) |
VA‐ECMO alone (n=851) |
P value | |
|---|---|---|---|
| Age, y | 66 (68–74) | 70 (61–78) | <0.001 |
| Age categories, y | <0.001 | ||
| ≥20 to <50 | 336 (11.3) | 71 (8.3) | |
| ≥50 to <60 | 532 (18.0) | 126 (14.8) | |
| ≥60 to <70 | 930 (31.4) | 228 (26.8) | |
| ≥70 to <80 | 790 (26.7) | 256 (30.1) | |
| ≥80 to <90 | 361 (12.2) | 155 (18.2) | |
| ≥90 | 15 (0.5) | 15 (1.8) | |
| Male sex | 2514 (84.8) | 664 (78.0) | <0.001 |
| Body mass index | 24.0 (21.7–26.4) | 23.7 (21.6–25.8) | 0.062 |
| Emergency admission* | 2945 (99.6) | 834 (98.0) | <0.001 |
| Ambulance use † | 2632 (89.0) | 729 (85.7) | 0.008 |
| Smoker ‡ | 1165 (39.3) | 288 (33.8) | 0.008 |
| Full score Barthel index at admission | 220 (7.4) | 75 (8.8) | 0.18 |
| Killip classification | 0.83 | ||
| Killip 3 | 175 (5.8) | 48 (5.6) | |
| Killip 4 | 2791 (94.9) | 803 (94.4) | |
| Prior ischemic heart disease | 40 (1.4) | 8 (0.9) | 0.34 |
| Hypertension | 652 (22.0) | 154 (18.1) | 0.014 |
| Dyslipidemia | 462 (15.6) | 92 (10.8) | 0.001 |
| Diabetes | 623 (21.0) | 134 (15.8) | 0.001 |
| Atrial fibrillation | 105 (3.5) | 12 (1.4) | 0.002 |
| Chronic pulmonary disease | 29 (1.0) | 6 (0.7) | 0.46 |
| Peripheral vascular disease | 91 (3.1) | 43 (5.1) | 0.006 |
| Cerebrovascular disease | 105 (3.5) | 27 (3.2) | 0.60 |
| Renal disease | 198 (6.7) | 57 (6.7) | 0.99 |
| Malignancy | 39 (1.3) | 9 (1.1) | 0.55 |
| Cardiac arrest at admission | 518 (17.5) | 140 (16.5) | 0.49 |
| Hospital teaching status § | 0.001 | ||
| A | 2837 (96.9) | 804 (94.5) | |
| B | 90 (3.0) | 44 (5.2) | |
| C | 1 (0.03) | 3 (0.4) | |
| Hospital with the number of hospital beds ≥500 | 1777 (60.0) | 422 (49.6) | <0.001 |
| Number of board‐certified cardiologists per hospital | <0.001 | ||
| 0 to 2 | 220 (7.4) | 99 (11.6) | |
| 3 to 5 | 990 (33.4) | 329 (39.8) | |
| 6 to 9 | 944 (31.9) | 238 (28.0) | |
| ≥10 | 803 (27.1) | 174 (20.5) | |
| Unknown | 7 (0.2) | 1 (0.1) | |
| Hospital with CCU | 2889 (97.5) | 816 (95.9) | 0.015 |
| Hospital with cardiac surgery | 2642 (89.1) | 723 (85.0) | 0.001 |
| Hospitals with board‐certified cardiac rehabilitation | 2511 (84.7) | 656 (77.1) | <0.001 |
| Aging rate | 0.027 | ||
| First quartile | 851 (28.7) | 269 (31.6) | |
| Second quartile | 768 (25.9) | 236 (27.7) | |
| Third quartile | 673 (22.7) | 154 (18.1) | |
| Fourth quartile | 672 (22.7) | 192 (22.6) | |
| Board‐certified hospital density | 0.025 | ||
| First quartile | 698 (23.6) | 172 (20.2) | |
| Second quartile | 627 (21.2) | 159 (18.7) | |
| Third quartile | 965 (32.6) | 300 (35.3) | |
| Fourth quartile | 674 (22.7) | 220 (25.9) |
Values are expressed as median (interquartile range) or number (percentage). CCU indicates coronary care unit; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pumping; VA, veno‐arterial.
Data were not available in 7 and 0 patients for emergency admission in the VA‐ECMO plus IABP group and VA‐ECMO alone group, respectively.
Data were not available in 7 and 0 patients for ambulance use in the VA‐ECMO plus IABP group and VA‐ECMO alone group, respectively.
Data were not available in 776 and 258 patients for smoker in the VA‐ECMO plus IABP group and VA‐ECMO alone group, respectively.
The hospital teaching statuses were categorized as follows: class A, >2 board‐certified cardiologists and 30 cardiovascular beds; class B, >1 board‐certified cardiologists and 15 cardiovascular beds; and class C, none of the above.
Figure 2. Temporal trends in the usage rates of VA‐ECMO and VA‐ECMO plus IABP and in‐hospital mortality in patients with AMI‐CS.
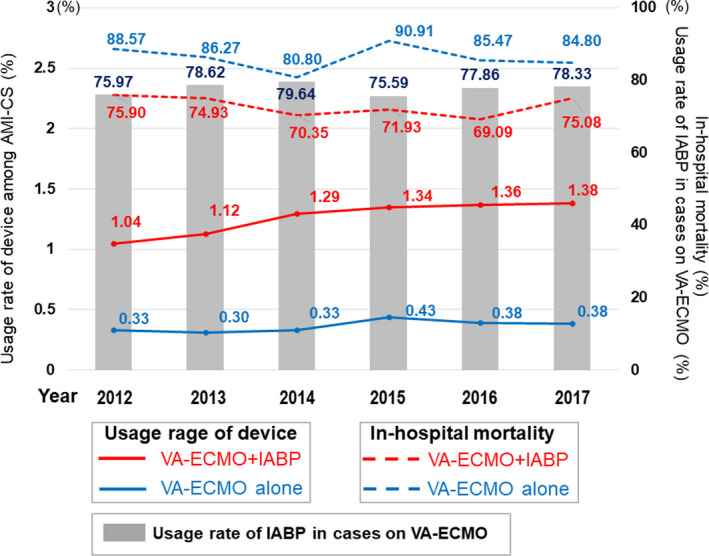
AMI‐CS indicates acute myocardial infarction complicated by cardiogenic shock; IABP, intra‐aortic balloon pumping; and VA‐ECMO, venoarterial extracorporeal membrane oxygenation.
Clinical Outcomes
The in‐hospital, 7‐day, and 30‐day mortality of the VA‐ECMO plus IABP group were significantly lower than the VA‐ECMO alone group (ORs, 0.42 [95% CI, 0.34–0.52], 0.28 [95% CI, 0.23–0.33], and 0.37 [95% CI, 0.30–0.46], respectively; Table 2 and Table S2). These results were consistent in the multivariable analyses and in the PSM and inverse probability of treatment weighting cohorts after adjustment for patient and institutional characteristics (ORs for in‐hospital mortality: 0.47 [95% CI, 0.38–0.59] [multivariable model 1], 0.48 [95% CI, 0.37–0.63] [PSM], and 0.42 [95% CI, 0.34–0.52] [inverse probability of treatment weighting]; Table 2 and Table S2). The Kaplan–Meier curves for overall survival showed a significant separation between the 2 treatment strategies both before and after PSM (Figure 3). The subgroup analyses stratified by the presence of cardiac arrest, sex, and age showed that combined VA‐ECMO plus IABP was consistently associated with lower mortality compared with VA‐ECMO alone in all subgroups, and these analyses especially showed that there was a significant interaction for the treatment effect of additional IABP between the age subgroups favoring age >75 years (P for interaction=0.025; Table S3). Patients in the VA‐ECMO plus IABP group spent a median of 8.5 days (IQR, 3–29 days) in the hospital, whereas patients in the VA‐ECMO alone group spent 2 days (IQR, 1–6 days) in the hospital during the index admission.
Table 2.
Odds Ratios of In‐Hospital, 7‐Day, and 30‐Day Mortality in Patients With AMI‐CS Managed With VA‐ECMO Plus IABP Compared With VA‐ECMO Alone
| In‐hospital mortality | 7‐day mortality | 30‐day mortality | |
|---|---|---|---|
| Univariable | 0.42 (0.34–0.52) | 0.28 (0.23–0.33) | 0.37 (0.30–0.46) |
| Multivariable | |||
| Model 1 | 0.47 (0.38–0.59) | 0.30 (0.25–0.37) | 0.41 (0.33–0.51) |
| Model 2 | 0.47 (0.38–0.59) | 0.30 (0.25–0.37) | 0.41 (0.33–0.51) |
| Model 3 | 0.47 (0.37–0.59) | 0.30 (0.25–0.36) | 0.40 (0.33–0.50) |
| PSM | 0.48 (0.37–0.63) | 0.28 (0.22–0.36) | 0.43 (0.33–0.56) |
| IPTW | 0.42 (0.34–0.52) | 0.28 (0.23–0.33) | 0.37 (0.30–0.46) |
Values are expressed as odds ratio (95% CI). The following variables were used for the multivariable adjustment: Model 1 included age category, sex, full score Barthel index at admission, Killip classification, comorbidities, cardiac arrest at admission, and hospital characteristics; model 2 included age category, sex, full score Barthel index at admission, Killip classification, cardiac arrest at admission, and hospital characteristics; and model 3 included age category, sex, full score Barthel index at admission, Killip classification, comorbidities, and cardiac arrest on admission. AMI‐CS indicates acute myocardial infarction complicated by cardiogenic shock; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pumping; IPTW, inverse probability of treatment weighting; PSM, propensity score matching; and VA, veno‐arterial.
Figure 3. Kaplan–Meier curves for overall survival stratified by treatment strategy.
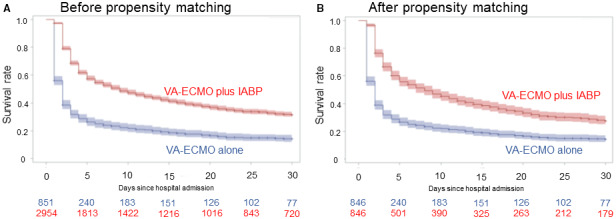
Kaplan–Meier curves are shown for overall survival up to 30 days after the hospital admission in patients treated with venoarterial ECMO plus IABP vs venoarterial ECMO alone (A) before and (B) after propensity score matching. The color areas around the solid lines indicate 95% CIs. IABP indicates intra‐aortic balloon pumping; and VA‐ECMO, venoarterial extracorporeal membrane oxygenation.
The percentage of patients who had full scores Barthel index at discharge was significantly higher in the VA‐ECMO plus IABP group than in the VA‐ECMO alone group (14.9 versus 8.1%, respectively). There were no significant differences in the incidence of stroke (risk ratio after PSM, 0.82 [95% CI, 0.57–1.19]); major bleeding, 0.92 [95% CI, 0.72–1.19]); intracranial bleeding, 1.28 [95% CI, 0.41–4.02]); and gastrointestinal bleeding, 0.74 [95% CI, 0.43–1.30]) during the index hospitalization between the VA‐ECMO plus IABP and the VA‐ECMO alone groups (Table S4). A risk of mechanical ventilator use was lower in patients in the VA‐ECMO plus IABP group, whereas there were no significant differences in hemodialysis use and ventricular assist device implantation between the 2 groups (Table S5).
We explored the effect of the timing of VA‐ECMO and IABP introduction on outcomes in the VA‐ECMO plus IABP group. The in‐hospital mortality was higher in patients who started on IABP with subsequent VA‐ECMO than in those who had IABP and VA‐ECMO on the same day or those who started on VA‐ECMO with subsequent IABP (81.7% versus 72.2%; P=0.030).
Discussion
In this large cohort of patients with AMI undergoing primary PCI complicated by CS requiring VA‐ECMO support, we demonstrated that IABP use combined with VA‐ECMO was associated with a significantly lower mortality compared with VA‐ECMO support alone. The results were consistent after adjustments for confounding factors using multivariable analysis, PSM, and inverse probability of treatment weighting methods.
The role of primary PCI is established as the first‐line acute‐phase treatment of AMI, improving clinical outcomes based on the sound evidence. Therefore, we focused on patients treated with primary PCI in the present study, excluding those treated without it to reduce substantial potential confounding. 10 , 11 However, there are still many uncertainties and controversies regarding the indication of MCS as a part of AMI treatment. In 2012, the IABP‐SHOCK Ⅱ trial showed no benefit of IABP in AMI‐CS 12 ; thereafter, a routine use of IABP was not recommended in patients with AMI‐CS. 10 However, the study included a higher percentage of patients with mild or moderately severe CS complicating AMI, and limited data exist on the effectiveness of simultaneous IABP support for patients with severe AMI‐CS requiring VA‐ECMO.
There have been a few retrospective, observational studies that focused on investigating the effectiveness of additional IABP use in combination with VA‐ECMO on clinical outcomes in patients with AMI‐CS. A single‐center, retrospective, observational study in Korea analyzed 96 consecutive patients with AMI‐CS who were assisted by VA‐ECMO. In that study, the combined use of VA‐ECMO and IABP did not improve in‐hospital mortality (VA‐ECMO with IABP versus VA‐ECMO alone; 51.2% versus 54.5%; P=0.747). 13 In contrast, another retrospective, observational, single‐center study in France enrolled consecutive 106 patients with AMI‐CS with a need for VA‐ECMO and showed that the use of IABP in addition to VA‐ECMO was an independent protective factor for mortality (adjusted hazard ratio [HR], 0.48 [95% CI, 0.28–0.80]; P=0 06). 14 Although these and other single studies showed conflicting results, 15 , 16 the findings from recent meta‐analyses are in favor of active use of IABP in addition to VA‐ECMO for AMI‐CS treatment. In a meta‐analysis by Romeo et al, they compared the effect of VA‐ECMO plus IABP versus IABP in 301 patients with AMI‐CS from 6 observational studies and found a significantly lower in‐hospital mortality (risk reduction=−22%; P=0.008) in the group of patients treated with VA‐ECMO plus IABP compared with VA‐ECMO alone. 17 However, the authors reported that trial sequential analysis could not be performed because of the small number of patients included, suggesting inadequate sample size to conclude it. 17 A recent meta‐analysis by Vallabhajosyula et al included 2023 patients with AMI‐CS from 7 observational studies, applying a more broad definition of AMI than Romeo et al, and found that concomitant IABP with VA‐ECMO was associated with lower mortality than VA‐ECMO alone (50.8% versus 62.4%; risk ratio, 0.56 [95% CI, 0.46–0.67]; P<0.001), although there was no significant survival benefit in the other cohorts such as the postcardiotomy CS cohort or in the total cohort. 18 The present study derived from the Japanese nationwide registry incorporated a much larger population of 3815 patients with AMI‐CS requiring VA‐ECMO, showing a remarkable lower mortality in patients treated with VA‐ECMO plus IABP compared with VA‐ECMO alone without additional increases in the adverse events, including stroke, major bleeding, intracranial bleeding, and gastrointestinal bleeding during the index hospitalization. In addition, there was a higher percentage of patients treated with VA‐ECMO plus IABP discharged with full activities of daily living status as assessed by the Barthel index. These results support the benefit of the simultaneous IABP use during VA‐ECMO in patients with AMI‐CS when indicated.
A previous study from a Japanese inpatient database by Aso et al, 19 which included 1650 patients with CS from July 2010 to March 2013, also investigated the effect of IABP combined with VA‐ECMO on mortality of CS. The results were consistent with our study in that the concomitant IABP plus VA‐ECMO was associated with improved mortality (HR, 0.74 [95% CI, 0.63–0.86]), although they included patients with CS attributed not only to AMI but also to various cardiac disorders in which ischemic heart disease accounted for 43%. Another difference between their study and ours is that they excluded patients with cardiac arrest at admission, whereas 17% of the patients included in the present study had cardiac arrest. In the subgroup analysis stratified by the presence of cardiac arrest at admission, additional IABP support was associated with a lower mortality in patients with AMI‐CS both with and without cardiac arrest without a statistically significant interaction. The other subgroup analyses stratified by sex and age showed that combined VA‐ECMO plus IABP was consistently associated with lower mortality compared with VA‐ECMO alone regardless of sex and age. In addition, there was a significant interaction between the age subgroups favoring age >75 years for unclear reason. These results are reassuring, not raising a concern about the additional IABP therapy to VA‐ECMO even in the elderly population and regardless of sex and the presence of cardiac arrest.
Theoretically, IABP support can offer beneficial effects as an unloading strategy during VA‐ECMO. VA‐ECMO produces a retrograde aortic flow, increasing LV afterload and compromising LV efficiency. 20 This increased LV afterload can result in an increased LV chamber size with a consequent increase in left atrial pressure and pulmonary edema. 20 , 21 IABP improves coronary and peripheral perfusion with an increase in diastolic pressure via diastolic balloon inflation and decrease afterload via systolic balloon deflation, enhancing aortic valve opening and augmenting LV performance. 22 Additional IABP support was shown to decrease myocardial oxygen demand and pulmonary capillary wedge pressure and prevent hydrostatic pulmonary edema in patients managed with VA‐ECMO. 23 , 24 In addition, unlike physiologic pulsatile flow, VA‐ECMO creates a nonpulsatile flow pattern, which is supposed to be disadvantageous at organ perfusion and tissue oxygen exchange. 25 IABP support can establish pulsatile flow during VA‐ECMO. Previous studies reported that the pulsatility created by using an IABP in combination with a nonpulsatile pump, such as peripheral VA‐ECMO as well as roller pump and centrifugal pump, improved organ perfusion and recovery. 26 , 27 , 28 In addition, an animal investigation showed that adjunctive IABP improved myocardial oxygen supply demand balance with both peripherally and centrally cannulated ECMO. 29 These beneficial effects of IABP potentially compensate for the limitations of VA‐ECMO. In the present study, the difference in survival rate between two treatment arms was observed within a few days after the hospitalization as shown in the Kaplan–Meier survival curves. The hemodynamic advantage derived from additional IABP use, unloading LV afterload, augmenting LV performance and organ perfusion, may potentially prevent pulmonary congestion and life‐threatening multiorgan failure in the very acute phase of the treatment, ultimately improving survival of AMI‐CS requiring VA‐ECMO.
The important finding of the present study is that the addition of IABP did not increase the incidence of bleeding complications. IABP is relatively safe with lower rate of complications compared with VA‐ECMO. 30 In the IABP‐SHOCK Ⅱ trial, 12 the rate of major bleeding did not differ between patients with AMI‐CS treated with IABP and those without IABP. When VA‐ECMO and IABP are used simultaneously, the occurrence of complications such as bleeding may more largely depend on the management of VA‐ECMO. Therefore, additional IABP use might not significantly increase bleeding events in such institutions capable of managing VA‐ECMO as observed in the present study. With respect to the sequence of MCS, our exploratory analysis showed that the in‐hospital mortality was higher in patients who started on IABP with subsequent VA‐ECMO than in others, including those who started on VA‐ECMO with subsequent IABP and those who had VA‐ECMO and IABP on the same day. Patients who had early IABP placement followed by escalation to VA‐ECMO might indicate a rapidly worsening patient phenotype portending worse outcomes.
Recently, Impella was introduced as a percutaneous MCS device that can be quickly implanted through peripheral vessels without surgical intervention and produce retrograde, transaortic unloading of the LV, providing superior hemodynamic support than IABP. 5 A previous study showed that adding Impella to VA‐ECMO significantly decreased pulmonary artery systolic and diastolic pressures in patients with refractory CS. 31 Recent retrospective studies from 1 or 2 centers showed that the addition of Impella to VA‐ECMO for patients with refractory CS was associated with lower mortality, lower inotrope use, higher rate of successful bridging to either recovery or further therapy, and comparable safety profiles as compared with VA‐ECMO alone. 32 , 33 A recent meta‐analysis supports the mortality benefit of additional Impella to VA‐ECMO in patients with CS treated with VA‐ECMO. 34 Further accumulation of research on this strategy for AMI‐CS is needed. Nonetheless, considering the limited institutions where Impella is available and its cost, we believe there is still a demand for IABP as an adjunctive treatment for AMI‐CS.
Limitations
First, although we adjusted for known confounders, the data are observational in nature and inherently subject to residual confounding. For example, detailed hemodynamics, angiographic, and echocardiographic data could not be extracted from the data set. Information on burden and complexity of disease (eg, SYNTAX score) and completeness of revascularization was not available. In addition, the length of stay in the group with VA‐ECMO alone is much shorter than in the group with VA‐ECMO plus IABP. This might be partially because of early withdrawal of care for these patients because of expected poor outcomes or family wishes. We were not sure about a variation in management between different centers in this regard. Although we performed analyses with multiple adjustments, these data were not available for which our results were not adjusted. Second, the indication of VA‐ECMO may vary, albeit slightly, between the institutions, which may lead to selection bias. Third, the data lacked granularity to differentiate between cardiovascular versus noncardiovascular mortality. Fourth, we could not investigate the effect of Impella during VA‐ECMO on clinical outcomes because there were very few cases managed with Impella in this study period. Fifth, the dates of the adverse event occurrence other than death were not available in the data set; therefore, we could not perform a time‐to‐event analysis for those events. Sixth, we could not extract information on the detailed timing of the CS, IABP placement, and ECMO placement on an hourly basis. In addition, we could not accurately differentiate patients who had VA‐ECMO and IABP implantation on the same day from those who started on VA‐ECMO with subsequent IABP in this registry. Therefore, the results of the MCS sequence in the present study was just of an exploratory analysis, requiring further investigation with data of higher accuracy. Finally, this registry includes mostly Japanese patients treated under the health care system in Japan. The results may be less generalizable to other populations under the health care systems of other countries.
Conclusions
This large‐scale, nationwide study demonstrated that the combination of VA‐ECMO plus IABP support was associated with significantly lower mortality compared with VA‐ECMO support alone in patients presenting with AMI‐CS who underwent primary PCI without significant increases in cerebral and bleeding events. These results support active use of IABP to improve clinical outcomes of patients with AMI‐CS when VA‐ECMO is indicated.
Sources of Funding
This study was partially supported by a grant from the Japanese Society of Cardiovascular Interventional Therapeutics.
Disclosures
None.
Supporting information
This work was presented as a poster at the American College of Cardiology (ACC) Congress, April 2–4, in Washington, DC.
For Sources of Funding and Disclosures, see page 10.
See Editorial by Bansal et al.
References
- 1. Tehrani BN, Truesdell AG, Psotka MA, Rosner C, Singh R, Sinha SS, Damluji AA, Batchelor WB. A standardized and comprehensive approach to the management of cardiogenic shock. JACC Heart Fail. 2020;8:879–891. doi: 10.1016/j.jchf.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 3. Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36:1223–1230. doi: 10.1093/eurheartj/ehv051 [DOI] [PubMed] [Google Scholar]
- 4. Ostadal P, Mlcek M, Kruger A, Hala P, Lacko S, Mates M, Vondrakova D, Svoboda T, Hrachovina M, Janotka M, et al. Increasing venoarterial extracorporeal membrane oxygenation flow negatively affects left ventricular performance in a porcine model of cardiogenic shock. J Transl Med. 2015;13:266. doi: 10.1186/s12967-015-0634-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meani P, Gelsomino S, Natour E, Johnson DM, Rocca H‐P, Pappalardo F, Bidar E, Makhoul M, Raffa G, Heuts S, et al. Modalities and effects of left ventricle unloading on extracorporeal life support: a review of the current literature. Eur J Heart Fail. 2017;19(suppl 2):84–91. doi: 10.1002/ejhf.850 [DOI] [PubMed] [Google Scholar]
- 6. Shi Y, Wang Y, Sun X, Tang Y, Jiang M, Bai Y, Liu S, Jiang W, Yuan H, Lu Y, et al. Effects of mechanical circulatory support devices in patients with acute myocardial infarction undergoing stent implantation: a systematic review and meta‐analysis of randomised controlled trials. BMJ Open. 2021;11:e044072. doi: 10.1136/bmjopen-2020-044072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uemura S, Okamoto H, Nakai M, Nishimura K, Miyamoto Y, Yasuda S, Tanaka N, Kohsaka S, Kadota K, Saito Y, et al. Primary percutaneous coronary intervention in elderly patients with acute myocardial infarction ‐ an analysis from a Japanese Nationwide Claim‐Based Database. Circ J. 2019;83:1229–1238. doi: 10.1253/circj.CJ-19-0004 [DOI] [PubMed] [Google Scholar]
- 8. Nakai M, Iwanaga Y, Sumita Y, Kanaoka K, Kawakami R, Ishii M, Uchida K, Nagano N, Nakayama T, Nishimura K, et al. Validation of acute myocardial infarction and heart failure diagnoses in hospitalized patients with the Nationwide Claim‐Based JROAD‐DPC Database. Circ Rep. 2021;3:131–136. doi: 10.1253/circrep.CR-21-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 10. O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;2013:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- 11. Yoshida H, Sakakura K, Yamamoto K, Taniguchi Y, Tsukui T, Seguchi M, Jinnouchi H, Wada H, Moriya T, Fujita H. Comparison of in‐hospital death following ST‐elevation myocardial infarction between secondary emergency and tertiary emergency. Cardiovasc Interv Ther. 2021;36:444–451. doi: 10.1007/s12928-020-00698-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thiele H, Zeymer U, Neumann F‐J, Ferenc M, Olbrich H‐G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al.; IABP‐SHOCK II Trial Investigators . Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 13. Park TK, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, Sung K, Lee YT, Gwon HC. Clinical impact of intra‐aortic balloon pump during extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock. BMC Anesthesiol. 2014;14:27. doi: 10.1186/1471-2253-14-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Overtchouk P, Pascal J, Lebreton G, Hulot J‐S, Luyt C‐E, Combes A, Kerneis M, Silvain J, Barthelemy O, Leprince P, et al. Outcome after revascularisation of acute myocardial infarction with cardiogenic shock on extracorporeal life support. EuroIntervention. 2018;13:e2160–e2168. doi: 10.4244/EIJ-D-17-01014 [DOI] [PubMed] [Google Scholar]
- 15. Ro SK, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Extracorporeal life support for cardiogenic shock: influence of concomitant intra‐aortic balloon counterpulsation. Eur J Cardiothorac Surg. 2014;46:186–192; discussion 192. doi: 10.1093/ejcts/ezu005 [DOI] [PubMed] [Google Scholar]
- 16. van den Brink FS, Zivelonghi C, Vossenberg TN, Bleeker GB, Winia VL, Sjauw KD, Ten Berg JM. VA‐ECMO with IABP is associated with better outcome than VA‐ECMO alone in the treatment of cardiogenic shock in ST‐elevation myocardial infarction. J Invasive Cardiol. 2021;33:E387–E392. [DOI] [PubMed] [Google Scholar]
- 17. Romeo F, Acconcia MC, Sergi D, Romeo A, Francioni S, Chiarotti F, Caretta Q. Percutaneous assist devices in acute myocardial infarction with cardiogenic shock: review, meta‐analysis. World J Cardiol. 2016;8:98–111. doi: 10.4330/wjc.v8.i1.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vallabhajosyula S, O’Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Eleid MF, Dunlay SM, Gersh BJ, Rihal CS, et al. Concomitant intra‐aortic balloon pump use in cardiogenic shock requiring veno‐arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv. 2018;11:e006930. doi: 10.1161/CIRCINTERVENTIONS.118.006930 [DOI] [PubMed] [Google Scholar]
- 19. Aso S, Matsui H, Fushimi K, Yasunaga H. The effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: an analysis using a nationwide inpatient database. Crit Care Med. 2016;44:1974–1979. doi: 10.1097/CCM.0000000000001828 [DOI] [PubMed] [Google Scholar]
- 20. Yannopoulos D, Bartos JA, Raveendran G, Conterato M, Frascone RJ, Trembley A, John R, Connett J, Benditt DG, Lurie KG, et al. Coronary artery disease in patients with out‐of‐hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70:1109–1117. doi: 10.1016/j.jacc.2017.06.059 [DOI] [PubMed] [Google Scholar]
- 21. Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117:686–697. doi: 10.1161/CIRCULATIONAHA.106.613596 [DOI] [PubMed] [Google Scholar]
- 22. Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail. 2018;11:e004905. doi: 10.1161/CIRCHEARTFAILURE.118.004905 [DOI] [PubMed] [Google Scholar]
- 23. Petroni T, Harrois A, Amour J, Lebreton G, Brechot N, Tanaka S, Luyt C‐E, Trouillet J‐L, Chastre J, Leprince P, et al. Intra‐aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation*. Crit Care Med. 2014;42:2075–2082. doi: 10.1097/CCM.0000000000000410 [DOI] [PubMed] [Google Scholar]
- 24. Bréchot N, Demondion P, Santi F, Lebreton G, Pham T, Dalakidis A, Gambotti L, Luyt C‐E, Schmidt M, Hekimian G, et al. Intra‐aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial‐extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care. 2018;7:62–69. doi: 10.1177/2048872617711169 [DOI] [PubMed] [Google Scholar]
- 25. Madershahian N, Liakopoulos OJ, Wippermann J, Salehi‐Gilani S, Wittwer T, Choi YH, Naraghi H, Wahlers T. The impact of intraaortic balloon counterpulsation on bypass graft flow in patients with peripheral ECMO. J Card Surg. 2009;24:265–268. doi: 10.1111/j.1540-8191.2009.00807.x [DOI] [PubMed] [Google Scholar]
- 26. Onorati F, Esposito A, Comi MC, Impiombato B, Cristodoro L, Mastroroberto P, Renzulli A. Intra‐aortic balloon pump‐induced pulsatile flow reduces coagulative and fibrinolytic response to cardiopulmonary bypass. Artif Organs. 2008;32:433–441. doi: 10.1111/j.1525-1594.2008.00563.x [DOI] [PubMed] [Google Scholar]
- 27. Onorati F, Presta P, Fuiano G, Mastroroberto P, Comi N, Pezzo F, Tozzo C, Renzulli A. A randomized trial of pulsatile perfusion using an intra‐aortic balloon pump versus nonpulsatile perfusion on short‐term changes in kidney function during cardiopulmonary bypass during myocardial reperfusion. Am J Kidney Dis. 2007;50:229–238. doi: 10.1053/j.ajkd.2007.05.017 [DOI] [PubMed] [Google Scholar]
- 28. Onorati F, Cristodoro L, Bilotta M, Impiombato B, Pezzo F, Mastroroberto P, di Virgilio A, Renzulli A. Intraaortic balloon pumping during cardioplegic arrest preserves lung function in patients with chronic obstructive pulmonary disease. Ann Thorac Surg. 2006;82:35–43. doi: 10.1016/j.athoracsur.2006.02.045 [DOI] [PubMed] [Google Scholar]
- 29. Sauren LD, Reesink KD, Selder JL, Beghi C, van der Veen FH, Maessen JG. The acute effect of intra‐aortic balloon counterpulsation during extracorporeal life support: an experimental study. Artif Organs. 2007;31:31–38. doi: 10.1111/j.1525-1594.2007.00337.x [DOI] [PubMed] [Google Scholar]
- 30. Subramaniam AV, Barsness GW, Vallabhajosyula S, Vallabhajosyula S. Complications of temporary percutaneous mechanical circulatory support for cardiogenic shock: an appraisal of contemporary literature. Cardiol Ther. 2019;8:211–228. doi: 10.1007/s40119-019-00152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akanni OJ, Takeda K, Truby LK, Kurlansky PA, Chiuzan C, Han J, Topkara VK, Yuzefpolskaya M, Colombo PC, Karmpaliotis D, et al. EC‐VAD: combined use of extracorporeal membrane oxygenation and percutaneous microaxial pump left ventricular assist device. ASAIO J. 2019;65:219–226. doi: 10.1097/MAT.0000000000000804 [DOI] [PubMed] [Google Scholar]
- 32. Patel SM, Lipinski J, Al‐Kindi SG, Patel T, Saric P, Li J, Nadeem F, Ladas T, Alaiti A, Phillips A, et al. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with Impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J. 2019;65:21–28. doi: 10.1097/MAT.0000000000000767 [DOI] [PubMed] [Google Scholar]
- 33. Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Müllerleile K, Colombo A, et al. Concomitant implantation of Impella® on top of veno‐arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19:404–412. doi: 10.1002/ejhf.668 [DOI] [PubMed] [Google Scholar]
- 34. Russo JJ, Aleksova N, Pitcher I, Couture E, Parlow S, Faraz M, Visintini S, Simard T, Di Santo P, Mathew R, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73:654–662. doi: 10.1016/j.jacc.2018.10.085 [DOI] [PubMed] [Google Scholar]
- 35. Cabinet Office . Annual Report on the Ageing Society: 2018 (Summary). Available at: https://www8.cao.go.jp/kourei/english/annualreport/2018/2018pdf_e.html
- 36.e‐Stat: Portal Site of Official Statistics of Japan. Available at: https://www.e‐stat.go.jp/en/stat‐search?page=1
- 37. Austin PC. A tutorial on multilevel survival analysis: methods, methods, models and applications. Int Stat Rev. 2017;85:185–203. doi: 10.1111/insr.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


