Abstract
Background
Limited data are available on the clinical impact of healthy lifestyle behaviors on the risk of dementia in patients with new‐onset atrial fibrillation (AF). Here, we aimed to evaluate the association between a combination of healthy lifestyle behaviors and the risk of incident dementia in patients with AF.
Methods and Results
Using the Korean National Health Insurance database between 2009 and 2016, we identified 199 952 adult patients who were newly diagnosed as AF without dementia. Patients were categorized into 4 groups by healthy lifestyle behavior score (HLS) with 1 point each being assigned for no current smoking, alcohol abstinence, and regular exercise. The HLS 0, 1, 2, and 3 groups included 4.4%, 17.4%, 53.4%, and 24.8% of the patients, respectively. We performed an inverse probability of treatment weighting to balance covariates between HLS groups. The HLS 1, 2, and 3 groups were associated with a lower risk of dementia compared with the HLS 0 group (hazard ratio [HR], 0.769; 95% CI, 0.704–0.842 for HLS 1; HR, 0.770; 95% CI, 0.709–0.836 for HLS 2; and HR, 0.622; 95% CI, 0.569–0.679 for HLS 3). The risk of dementia showed a tendency to decrease with an increase in HLS (P‐for‐trend <0.001).
Conclusions
A clustering of healthy lifestyle behaviors was associated with a significantly lower risk of dementia in patients with new‐onset AF. These findings support the promotion of a healthy lifestyle within an integrated care approach to AF patient management.
Keywords: atrial fibrillation, dementia, healthy lifestyle behaviors
Subject Categories: Atrial Fibrillation, Cognitive Impairment, Lifestyle, Risk Factors, Exercise
Nonstandard Abbreviations and Acronyms
- HLS
healthy lifestyle behavior score
- IPTW
inverse probability of treatment weighting
- NHIS
National Health Insurance Service
- OAC
oral anticoagulant
Clinical Perspective
What Is New?
A clustering of healthy lifestyle behaviors (including not smoking, alcohol abstinence, and regular physical activity) was associated with a significantly lower risk of all dementia, Alzheimer dementia, and vascular dementia in patients with new‐onset atrial fibrillation.
There was a trend for lower risk of dementia as the number of healthy lifestyle behaviors increased.
The beneficial effect of a healthy lifestyle behavior cluster on the risk of dementia was consistently and more prominently observed in the low‐risk subgroups.
What Are the Clinical Implications?
This study has an implication to extend the previous findings, which lifestyle behavior prevents incident dementia, to patients with new‐onset atrial fibrillation.
The results of our study support the promotion of a healthy lifestyle within an integrated care approach to atrial fibrillation patient management.
Dementia is emerging as one of the greatest health problems of an aging society. 1 Given that dementia causes patients to lose multiple abilities such as memory, language, problem‐solving, and cognitive skills that are essential for independent living, its development makes caring for such patients difficult. 2 , 3
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia, increases the risk of stroke, congestive heart failure, and hospitalization causing an increase in mortality and morbidity. 4 , 5 , 6 Moreover, AF is an independent risk factor for all forms of dementia. 7 , 8 , 9 , 10 Although the pathophysiological mechanisms of AF‐induced dementia are not fully established, there are some explanations for this association, including stroke, silent stroke, cerebral hypoperfusion attributable to beat‐to‐beat heart rate variability, and vascular inflammation. 11 , 12
As dementia and AF are both age‐related diseases, they have common risk factors. In patients with AF, oral anticoagulant (OAC) therapy is associated with a low risk of dementia. 10 , 13 , 14 , 15 Although previous reports have described an association between individual components of healthy lifestyle behaviors 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 or the combination of such lifestyle factors and the risk of dementia in the general population, 30 , 31 data remain limited on the relationship between the risk of dementia and combination of healthy lifestyle behaviors amongst patients with new‐onset AF.
In this study, using a Korean nationwide population‐based cohort, we examined the association between the combination of healthy lifestyle behaviors (including not smoking, alcohol abstinence, and regular physical activity) and risk of incident dementia in patients with new‐onset AF.
Methods
All data and materials have been made publicly available at the National Health Insurance Sharing Service and can be assessed at https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do.
Data Source and Study Population
For this analysis, we used data from the Korean National Health Insurance Service (NHIS) database. 32 , 33 The Korean NHIS is a mandatory health insurance program administered by the Korean government, and it covers almost the entire Korean population (≈50 million people). The Korean NHIS established the comprehensive claims database on enrollees’ medical usage to review and reimburse the medical expense. This database contains enrollees’ sociodemographic information, and all the medical services uses both at outpatient clinics and at admissions, including diagnostic codes using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes, examinations, prescription records, procedures, and surgeries. Except for deaths or rare loss of qualifications, data on medical use are considered to have no missing values in the NHIS system. We also used the national health screening examination data that linked with the NHIS database. All Korean adults are recommended to receive a national health examination every 1 or 2 years provided by the Korean National Insurance Cooperation. Physical examinations, regular blood tests, and self‐reported questionnaires on their lifestyle behaviors are administered. The participation rate of the national health screening examinations was 74.8% in 2014. 32
We screened patients newly diagnosed with non‐valvular AF between January 1, 2009 and December 31, 2016 (n=576 077) and included patients who underwent a national health screening examination within 2 years after receiving the AF diagnosis (n=209 880) (Figure 1). We excluded patients aged <20 years (n=29), those for whom values were missing from the health screening examinations (n=1189), and those with dementia (n=8713). A total of 199 952 patients were included in this study. This study was exempted from review by the Seoul National University Hospital Institutional Review Board (E‐2105‐173‐1221).
Figure 1. Flowchart of the study population and follow‐up.
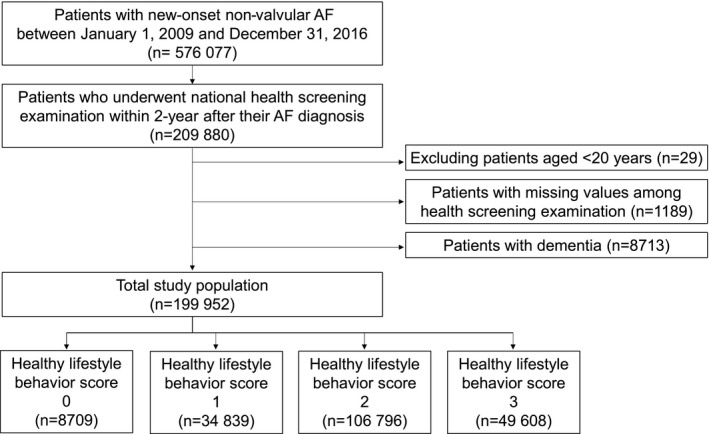
Healthy lifestyle behavior score was calculated by assigning 1 point each for no current smoking, abstaining from alcohol, and performing regular exercise. AF indicates atrial fibrillation.
Definition of Healthy Lifestyle Behavior Score
During each national health screening period, the participants of the NHIS cohort respond to a series of self‐reported questionnaires on lifestyle behaviors. We investigated 3 lifestyle behaviors, including smoking status, alcohol consumption, and physical activity, based on this questionnaire. Smoking status was classified as non‐smoker, ex‐smoker, or current smoker. Alcohol consumption was classified as non‐drinker and current drinker. Finally, physical activity intensity and frequency were recorded. Light physical activity was defined as walking slowly or sweeping carpets for >30 minutes per day, moderate physical activity was defined as brisk walking, dancing, or gardening for >30 minutes per day, and vigorous physical activity was defined as running fast, cycling, or aerobics >20 minutes per day. 34 , 35 , 36 Physical activity was divided into regular exercise, which was defined as moderate physical activity performed ≥5 times per week or vigorous physical activity performed ≥3 times per week, or a lack of regular exercise. 35 , 36
The healthy lifestyle behavior score (HLS), ranging from 0 to 3, was calculated by assigning 1 point each to no current smoking, alcohol abstinence, and regular exercise. Patients were categorized into 4 groups according to HLS.
Covariates
We investigated age, sex, and comorbidities (hypertension, diabetes, dyslipidemia, heart failure, prior ischemic stroke, prior myocardial infarction, peripheral artery disease, chronic obstructive pulmonary disease, cancer, and chronic kidney disease) which were based on the diagnoses defined in the medical claim and health screening examination databases. Table S1 shows the detailed definitions of these comorbidities.
The CHA2DS2‐VASc score was calculated based on the patients’ demographic information and comorbidities. 5 Body mass index was calculated as body weight in kilograms divided by the square of height in meters (kg/m2). The status of medication, including OACs (warfarin or non‐vitamin K antagonist OACs), antiplatelet agents, and statins, was obtained. Low income was defined as having a lower 25% of income of the entire Korean population.
Study Outcomes
The primary outcome was the first occurrence of dementia, including Alzheimer dementia, vascular dementia, and other forms. Dementia was defined using the diagnostic codes (F00, F01, F02, F03, G30, or G31) and prescription of medication for dementia such as rivastigmine, galantamine, memantine, or donepezil. 10 , 37 , 38 , 39 Secondary outcomes were defined as individual components of dementia (Alzheimer dementia [F00 or G30] and vascular dementia [F01]). When both codes for 2 types of dementia were claimed, we followed the primary diagnosis, and if both were claimed as additional diagnoses up to the secondary diagnoses, the patient was classified as other forms of dementia. Patients were followed up starting from the index date (at the health screening examination) until the occurrence of the study outcomes, death, or December 31, 2017, whichever came first.
Statistical Analysis
The baseline characteristics are presented as mean±SD for continuous variables and number (percentage) for categorical variables. The incidence rate of dementia was calculated by dividing the total number of dementia diagnoses by the total person‐years during the follow‐up period (per 1000 person‐years).
Before comparing the risk of dementia among HLS groups, since the baseline characteristics of each group stratified by HLS were significantly different, we performed an inverse probability of treatment weighting (IPTW) to balance covariates between HLS groups. 40 The propensity score used in the IPTW was calculated using age, sex, hypertension, diabetes, dyslipidemia, heart failure, prior ischemic stroke, prior myocardial infarction, peripheral artery disease, chronic obstructive pulmonary disease, cancer, chronic kidney disease, CHA2DS2‐VASc score, use of OAC, antiplatelet agents, and statins, body mass index, and low income. An imbalance in a covariate after IPTW was noted when the maximum absolute standardized difference of the covariates exceeded 0.1 (10%). 41
The association between the risk of dementia and HLS was analyzed using a weighted Cox proportional hazards regression model with IPTW and presented as the hazard ratio (HR) and 95% CIs for the primary and secondary outcomes. Each HLS group was analyzed using the HLS 0 group as the reference group. P‐for‐trend were calculated including the ordinal HLS variable as a continuous variable in the Cox proportional hazards regression model to show trend for lower risk of dementia according to increase in HLS. Weighted incidence rates were calculated as the weighted number of clinical events per 1000 person‐years at risk. Kaplan‒Meier method was used to present weighted cumulative incidence curves and outcomes were evaluated using a log rank test.
As a sensitivity analysis, a Cox proportional hazards regression model was used to compare the HLS groups for the risk of clinical outcomes. The Cox models were as follows: model 1 was unadjusted; model 2 was adjusted for age and sex; and model 3 was further adjusted for all variables used in the propensity score calculation.
As dementia does not occur immediately after exposure to unhealthy lifestyle or risk factors, we additionally analyzed the incidence of dementia among patients who had at least 1‐year follow‐up duration. Namely, we excluded patients with <1 year from the index period to the occurrence of dementia or end of follow‐up. As dementia is a cognitive disorder that is affected by incident stroke, we further performed analyses after censoring patients with incident stroke during follow‐up.
Since smoking is a well‐known risk factor for stroke in patients with AF and cognitive dysfunction/dementia in the general population, 42 , 43 , 44 we conducted a sensitivity analysis using modified HLS score excluding smoking factor and including exercise and alcohol consumption. We evaluated the association between these modified HLS scores and the risk of dementia both in non‐current smokers and current smokers.
According to a recent study, early rhythm control could affect the risk of stroke in patients with newly diagnosed AF. 45 Thus, we conducted a sensitivity analysis to adjust rhythm control treatment on the final multivariable Cox regression model.
Subgroup analyses were performed for age (<65, 65–75, and ≥75 years), sex, CHA2DS2‐VASc score ([men <2 and women <3], [men ≥2 and women ≥3]), presence of prior history of ischemic stroke, and use of OACs. We performed a multivariable Cox proportional hazards regression model for subgroup analyses. For the multivariable adjustment, variables including propensity score calculation were included in the model.
In the main analysis, the reference group was HLS 0 group. However, there is a possibility that HLS 0 group tended to care less about their health and have more other unhealthy lifestyle behaviors compared with other HLS groups. The association of dementia between the HLS 0 group and HLS 1, 2, or 3 groups could be mainly attributed to these characteristics of HLS 0. To address the impact of the clustering HLS on the risk of dementia, we additionally performed exploratory analyses to provide the HRs for dementia of the group pooled HLS 2 and 3 groups compared with HLS 1 group. Also, we added the HRs for dementia of HLS 2 and 3 groups compared with HLS 1 group.
To evaluate the association between exercise dose or intensity and the risk of dementia, we conducted an exploratory analysis. Exercise doses or intensity were defined as follow: (1) moderate‐intensity physical activity for 3 to 7 days per week and vigorous‐intensity physical activity for 3 to 7 days per week, (2) moderate‐intensity physical activity for 5 to 7 days per week and vigorous‐intensity physical activity for 5 to 7 days per week, (3) moderate to vigorous physical activity for 1 to 2 days per week, 3 to 4 days per week, 5 to 6 days per week, and ≥7 days per week, 46 and (4) calculating metabolic equivalent of task (MET)‐min per week, and categorized into 0 to <500 MET‐min per week, 500 to 999 MET‐min per week, 1000 to 1499 MET‐min per week, and ≥1500 MET‐min per week. 47 The non‐regular exercise group was the reference group.
All analyses were 2‐tailed, and statistical significance was defined as values of P<0.05. Statistical analyses were conducted using SAS (version 9.4; ASA Institute Inc., Cary, NC, USA).
Patient and Public Involvement
As the study was conducted using deidentified nationwide data, the authors had no direct contact information of the individual study participants. No patients were involved in establishing the research question or the outcome measures, nor were they involved in developing plans for recruitment, design, or implementation of the study. No patients were asked to advise on interpretation or writing of the results. There is no plan for dissemination of the study results to participants and linked communities.
Results
A total of 199 952 patients (mean age, 63.2 [SD, 12.6] years; 60.2% men) were included in this analysis (Figure 1). The study population was distributed by HLS as follows: 8709 (4.4%) patients in the HLS 0 group; 34 839 (17.4%) in HLS 1 group; 106 796 (53.4%) in HLS 2 group; and 49 608 (24.8%) in HLS 3 group. The median duration between the AF diagnosis and national health screening examination was 318 (interquartile range, 164–496) days. The baseline characteristics by the HLS group are presented in Table 1.
Table 1.
Baseline Characteristics of the Study Population
| Pre‐IPTW | Maximum ASD | Post‐IPTW | Maximum ASD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy lifestyle behavior score | Healthy lifestyle behavior score | |||||||||
| 0 (n=8709) | 1 (n=34 839) | 2 (n=106 796) | 3 (n=49 608) | 0 (n=8136) | 1 (n=34 109) | 2 (n=107 374) | 3 (n=49 324) | |||
| Age, y | ||||||||||
| Mean±SD | 56.2±12.4 | 59.3±13.0 | 64.9±12.4 | 63.5±11.7 | 0.70 | 61.7±12.1 | 62.5±12.7 | 63.0±13.0 | 63.4±12.0 | 0.08 |
| <65 | 74.8 | 63.3 | 45.2 | 49.5 | 58.0 | 53.8 | 51.5 | 48.7 | ||
| 65 to <75 | 19.0 | 25.4 | 32.7 | 34.6 | 25.5 | 28.8 | 30.2 | 34.4 | ||
| ≥75 | 6.15 | 11.3 | 22.1 | 15.9 | 16.5 | 17.4 | 18.4 | 16.9 | ||
| Sex (men) | 94.7 | 86.1 | 53.6 | 50.4 | 1.14 | 64.3 | 62.2 | 60.6 | 60.2 | 0.08 |
| Low income | 19.8 | 18.5 | 17.8 | 16.7 | 0.08 | 19.2 | 18.4 | 17.7 | 17.9 | 0.04 |
| CHA2DS2‐VASc | ||||||||||
| Mean±SD | 2.31±1.57 | 2.65±1.71 | 3.55±1.97 | 3.35±1.82 | 0.69 | 3.09±1.74 | 3.2±1.84 | 3.26±1.96 | 3.32±1.83 | 0.07 |
| 0 | 8.6 | 6.3 | 3.0 | 2.6 | 0.26 | 3.9 | 3.6 | 4.2 | 3.0 | 0.06 |
| 1 | 27.4 | 23.4 | 13.6 | 13.6 | 17.4 | 16.7 | 16.8 | 14.2 | ||
| 2 | 25.3 | 23.2 | 17.8 | 20.0 | 21.6 | 20.5 | 19.4 | 19.7 | ||
| ≥3 | 38.7 | 47.0 | 65.7 | 63.8 | 57.1 | 59.2 | 59.6 | 63.1 | ||
| Comorbidities | ||||||||||
| Hypertension | 82.8 | 83.6 | 85.7 | 83.1 | 0.08 | 83.0 | 83.8 | 84.4 | 84.7 | 0.05 |
| Diabetes | 24.4 | 22.9 | 23.9 | 22.2 | 0.05 | 20.9 | 22.4 | 23.2 | 23.6 | 0.06 |
| Dyslipidemia | 38.8 | 40.9 | 46.4 | 47.1 | 0.17 | 43.5 | 44.7 | 45.1 | 45.4 | 0.04 |
| Heart failure | 26.1 | 28.1 | 34.7 | 31.4 | 0.19 | 30.7 | 32.3 | 32.1 | 32.8 | 0.05 |
| Prior ischemic stroke | 15.5 | 18.6 | 25.9 | 24.8 | 0.26 | 22.3 | 23.3 | 23.6 | 24.2 | 0.04 |
| Prior MI | 9.7 | 10.6 | 11.8 | 11.6 | 0.07 | 10.9 | 11.4 | 11.3 | 11.6 | 0.02 |
| PAD | 18.1 | 19.5 | 22.7 | 20.7 | 0.11 | 20.1 | 21.2 | 21.3 | 21.7 | 0.04 |
| COPD | 16.1 | 17.6 | 20.8 | 19.5 | 0.12 | 19.7 | 19.5 | 19.6 | 19.8 | 0.01 |
| Cancer | 2.2 | 3.5 | 5.8 | 8.0 | 0.27 | 6.1 | 6.1 | 5.7 | 5.9 | 0.01 |
| CKD | 7.8 | 11.2 | 18.4 | 16.5 | 0.32 | 15.3 | 16.0 | 16.1 | 16.6 | 0.04 |
| Health examination | ||||||||||
| BMI (kg/m2) | ||||||||||
| Mean±SD | 22.2±3.5 | 24.6±3.4 | 24.5±3.4 | 24.4±3.2 | 0.10 | 24.4±3.5 | 24.5±3.4 | 24.5±3.4 | 24.5±3.2 | 0.04 |
| ≥25 | 39.8 | 43.7 | 42.9 | 40.6 | 0.07 | 41.1 | 42.6 | 42.6 | 41.5 | 0.03 |
| Antithrombotic treatment | ||||||||||
| Oral anticoagulants | 20.4 | 22.9 | 27.1 | 27.8 | 0.17 | 25.2 | 26.4 | 26.1 | 26.7 | 0.03 |
| Warfarin | 15.2 | 16.5 | 18.6 | 18.9 | 0.11 | 17.9 | 18.0 | 18.2 | 18.3 | 0.01 |
| NOAC | 5.2 | 6.4 | 8.6 | 8.9 | 0.14 | 7.3 | 8.4 | 7.9 | 8.4 | 0.04 |
| Antiplatelet agent | 24.6 | 26.3 | 26.9 | 24.3 | 0.06 | 24.2 | 25.8 | 26.0 | 26.2 | 0.05 |
| Aspirin | 21.7 | 22.8 | 22.9 | 20.5 | 0.06 | 20.4 | 22.0 | 22.2 | 22.2 | 0.05 |
| P2Y12 inhibitor | 5.9 | 6.5 | 7.5 | 6.9 | 0.06 | 7.0 | 6.7 | 7.1 | 7.5 | 0.03 |
| Statin | 14.1 | 15.4 | 18.7 | 18.6 | 0.12 | 15.8 | 17.7 | 17.8 | 18.0 | 0.06 |
Categorical variables were presented as a percentage and continuous variables were presented as mean and SD.
Healthy lifestyle behavior score was calculated by assigning 1 point each for no current smoking, abstaining from alcohol, and performing regular exercise. ASD indicates absolute standardized difference; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; IPTW, inverse probability of treatment weighting; MI, myocardial infarction; NOAC, non‐vitamin K antagonist oral anticoagulant; PAD, peripheral artery disease; and SBP, systolic blood pressure.
Patients in the HLS 0 group who had a cluster of 3 unhealthy lifestyle behaviors (current smoking, current drinking, and lack of regular exercise) were younger, were more likely to be men, had lower CHA2DS2‐VASc scores, and had a lower prevalence of comorbidities compared with those in the HLS 3 group with 3 healthy lifestyle behaviors. The HLS 0 group showed a lower body mass index and higher prevalence of low income than did the HLS 3 group. The proportion of patients receiving OACs and statins was higher in the HLS 3 group than in the HLS 0 group.
Risk of Dementia by HLS
During a median 3.4 (IQR, 1.7–5.5) years of follow‐up, dementia occurred in 327 (9.99/1000 person‐years), 1425 (11.01/1000 person‐years), 7625 (19.80/1000 person‐years), and 2444 (13.52 per 1000 person‐years) patients in the HLS 0, 1, 2, and 3 groups, respectively. Table 1 shows that baseline characteristics were well balanced among the different HLS groups after IPTW. The weighted incidence rates of dementia were 21.8, 16.8, 16.8, and 13.6/1000 person‐years in the HLS 0, 1, 2, and 3 groups, respectively.
The weighted cumulative incidence curves for the HLS groups are shown in Figure 2. After IPTW, the HLS 1, 2, and 3 groups were associated with a lower risk of dementia compared with the HLS 0 group (HR, 0.769 [95% CI, 0.704–0.842] for HLS 1; HR, 0.770 [95% CI, 0.709–0.836] for HLS 2; and HR, 0.622 [95% CI, 0.569–0.679] for HLS 3). The beneficial effect of the combination of healthy lifestyle behaviors was consistently observed for the risk of both Alzheimer dementia and vascular dementia (Figure 3). P‐for‐trends for risk of dementia, Alzheimer dementia, and vascular dementia according to HLS were <0.001 (Figure 3).
Figure 2. Weighted Kaplan‒Meier curves for incident dementia by healthy lifestyle behavior score.
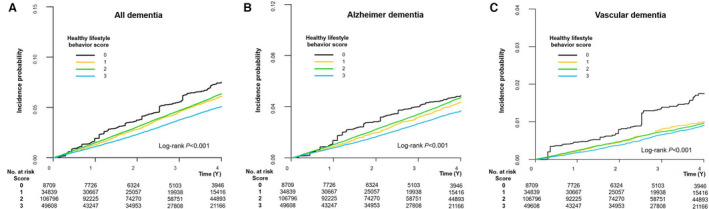
The healthy lifestyle behavior score groups 1, 2, and 3 showed a lower incidence rate of dementia than the healthy lifestyle behavior score 0 group. (A) All dementia. (B) Alzheimer dementia. (C) Vascular dementia. HLS indicates healthy lifestyle behavior score.
Figure 3. Risk of primary and secondary outcomes by healthy lifestyle behavior score: an inverse probability of treatment weighting analysis.
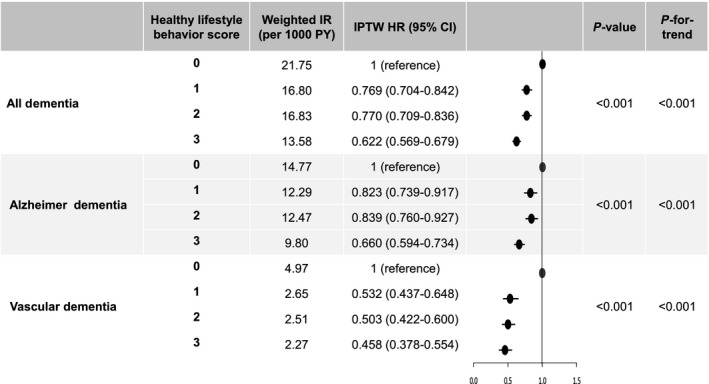
Healthy lifestyle behavior score was calculated by assigning 1 point each for no current smoking, abstaining from alcohol, and performing regular exercise. Lines indicate 95% confidential interval; bars, indication of hazard ratio 1.0; and dots, hazard ratio. HR indicates hazard ratio; IPTW, inverse probability of treatment weighting; IR, incidence rate; and PY, person‐years.
Sensitivity Analyses
After multivariable adjustment using model 3, the results of the competing risk analysis of the HLS 1, 2, and 3 groups compared with the HLS 0 group were in line with those of the main analysis (HR, 0.753 [95% CI, 0.667–0.849] for HLS 1; HR, 0.728 [95% CI, 0.650–0.815] for HLS 2; and HR, 0.622 [95% CI, 0.553–0.699] for HLS 3). Consistent results were observed for the risk of Alzheimer dementia and vascular dementia. Table S2 shows the unadjusted and adjusted HR values for the primary and secondary outcomes by HLS group.
In the sensitivity analyses including patients who had at least 1‐year follow‐up duration from index period, and censoring patients with incident stroke during the follow‐up, the results were largely consistent with those of the main analysis (Table 2).
Table 2.
Sensitivity Analyses
| Healthy lifestyle behavior score | No. | Event | IR per 1000 person‐years |
Adjusted HR (95% CI) |
P value |
|---|---|---|---|---|---|
| Sensitivity analysis I: excluding patients with <1 y follow‐up duration from index period to the occurrence of dementia or end of follow‐up | |||||
| All dementia | |||||
| 0 | 7718 | 263 | 10.72 | 1 (reference) | <0.001 |
| 1 | 30 647 | 1146 | 11.86 | 0.758 (0.662–0.867) | |
| 2 | 92 171 | 5847 | 20.50 | 0.714 (0.629–0.811) | |
| 3 | 43 221 | 1924 | 14.33 | 0.618 (0.542–0.705) | |
| Alzheimer dementia | |||||
| 0 | 7718 | 162 | 6.66 | 1 (reference) | <0.001 |
| 1 | 30 647 | 829 | 8.58 | 0.869 (0.734–1.029) | |
| 2 | 92 171 | 4426 | 15.52 | 0.833 (0.709–0.978) | |
| 3 | 43 221 | 1396 | 10.40 | 0.702 (0.595–0.828) | |
| Vascular dementia | |||||
| 0 | 7718 | 75 | 3.06 | 1 (reference) | <0.001 |
| 1 | 30 647 | 187 | 1.94 | 0.468 (0.358–0.613) | |
| 2 | 92 171 | 834 | 2.92 | 0.425 (0.332–0.544) | |
| 3 | 43 221 | 325 | 2.42 | 0.410 (0.317–0.532) | |
| Sensitivity analysis II: censoring incident stroke during follow‐up | |||||
| All dementia | |||||
| 0 | 8709 | 255 | 7.98 | 1 (reference) | <0.001 |
| 1 | 34 839 | 1185 | 9.38 | 0.796 (0.695–0.912) | |
| 2 | 106 796 | 6741 | 17.88 | 0.794 (0.699–0.902) | |
| 3 | 49 608 | 2147 | 12.10 | 0.682 (0.598–0.778) | |
| Alzheimer dementia | |||||
| 0 | 8709 | 177 | 5.54 | 1 (reference) | <0.001 |
| 1 | 34 839 | 903 | 7.14 | 0.854 (0.727–1.004) | |
| 2 | 106 796 | 5155 | 13.67 | 0.834 (0.715–0.971) | |
| 3 | 49 608 | 1618 | 9.12 | 0.717 (0.612–0.839) | |
| Vascular dementia | |||||
| 0 | 8709 | 52 | 1.63 | 1 (reference) | <0.001 |
| 1 | 34 839 | 148 | 1.17 | 0.535 (0.390–0.735) | |
| 2 | 106 796 | 843 | 2.24 | 0.594 (0.445–0.793) | |
| 3 | 49 608 | 289 | 1.63 | 0.514 (0.380–0.695) | |
Adjusted for age, sex, hypertension, diabetes, dyslipidemia, heart failure, prior ischemic stroke, prior myocardial infarction, peripheral artery disease, chronic obstructive pulmonary disease, cancer, chronic kidney disease, CHA2DS2‐VASc score, use of oral anticoagulant, antiplatelet agent, and statin, body mass index (BMI), and low income. Healthy lifestyle behavior score was calculated by assigning 1 point each for no current smoking, abstaining from alcohol, and performing regular exercise. HR indicates hazard ratio; and IR, incidence rate.
The results of sensitivity analyses that evaluated the association between HLS scores and the risk of dementia in non‐current smokers and current smokers using modified HLS scores, including exercise and alcohol consumption, are presented in Table S3. In non‐current smokers, although the risk reduction was attenuated in higher HLS scores compared with main results, the trends of lower risk of dementia in the higher modified HLS compared with modified HLS 0 group was shown. Since smoking cessation has a strong preventive effect on dementia, the beneficial effects of alcohol abstinence or regular physical exercise were attenuated in non‐smokers. However, an increased number of healthy lifestyles above HLS 2 was still associated with a lower risk of dementia. Among current smokers, higher HLS scores were consistently associated with a lower risk of dementia compared with HLS score 0 group (P=0.007). From these sensitivity analyses, although the amplitude of risk reduction in higher HLS scores composed of exercise and alcohol consumption was slightly different according to smoking status, HLS scores excluding smoking also showed an inverse correlation with the risk of dementia.
Table S4 showed the proportion of patients receiving rhythm control from AF diagnosis to index health examination. In the total study population, 25.1% of patients received rhythm control treatment for a median of 318 days (interquartile range, 164–496 days) after AF diagnosis. There was a higher proportion of patients receiving rhythm control in the HLS 3 group than other HLS groups. After adjusting rhythm control treatment, the HRs of HLS 1, 2, and 3 compared with HLS 0 for dementia, Alzheimer dementia, and vascular dementia were consistent with the main results (Table S5).
Subgroup Analyses
There was consistency in the results of the subgroup analyses stratified by age, sex, CHA2DS2‐VASc score, prior stroke history, and OAC use. The trends of lower risk of dementia in the HLS 1, 2, and 3 groups compared with the risk of dementia in the HLS 0 group were not significantly different among the different subgroups (Table 3). The beneficial effect of higher HLS was more prominent in younger patients (age <65 years), lower CHA2DS2‐VASc scores (men <2 and women <3), and those without a prior history of ischemic stroke (Table 3). There were no significant interactions between subgroups and the impact of HLS score on the risks of Alzheimer dementia and vascular dementia (Tables S6 and S7).
Table 3.
Subgroup Analyses
| Subgroup | HLS | No. | Events | IR per 1000 person‐years |
Adjusted HR (95% CI) |
P value | P‐for‐interaction |
|---|---|---|---|---|---|---|---|
| Age (y) | |||||||
| <65 | 0 | 6515 | 88 | 3.50 | 1 (reference) | <0.001 | 0.215 |
| 1 | 22 040 | 233 | 2.72 | 0.705 (0.552–0.902) | |||
| 2 | 48 275 | 621 | 3.26 | 0.623 (0.494–0.785) | |||
| 3 | 24 539 | 250 | 2.59 | 0.479 (0.372–0.616) | |||
| 65 to <75 | 0 | 1658 | 142 | 23.51 | 1 (reference) | <0.001 | |
| 1 | 8860 | 597 | 18.58 | 0.750 (0.625–0.901) | |||
| 2 | 34 956 | 2852 | 22.36 | 0.727 (0.612–0.864) | |||
| 3 | 17 180 | 1085 | 17.56 | 0.609 (0.509–0.728) | |||
| ≥75 | 0 | 536 | 97 | 61.94 | 1 (reference) | <0.001 | |
| 1 | 3939 | 595 | 50.77 | 0.791 (0.638–0.981) | |||
| 2 | 23 565 | 4152 | 62.11 | 0.789 (0.643–0.968) | |||
| 3 | 7889 | 1109 | 48.93 | 0.684 (0.555–0.843) | |||
| Sex | |||||||
| Men | 0 | 8249 | 304 | 9.77 | 1 (reference) | <0.001 | 0.280 |
| 1 | 29 994 | 1166 | 10.47 | 0.755 (0.665–0.857) | |||
| 2 | 57 185 | 2963 | 14.51 | 0.745 (0.661–0.839) | |||
| 3 | 24 989 | 1048 | 11.85 | 0.639 (0.562–0.727) | |||
| Women | 0 | 460 | 23 | 14.38 | 1 (reference) | <0.001 | |
| 1 | 4845 | 259 | 14.32 | 0.632 (0.413–0.969) | |||
| 2 | 49 611 | 4662 | 25.77 | 0.565 (0.374–0.851) | |||
| 3 | 24 619 | 1396 | 15.12 | 0.408 (0.318–0.725) | |||
| CHA2DS2‐VASc score | |||||||
| Men <2 and women <3 | 0 | 3268 | 28 | 2.09 | 1 (reference) | 0.006 | 0.531 |
| 1 | 11 581 | 75 | 1.58 | 0.612 (0.396–0.946) | |||
| 2 | 24 679 | 230 | 2.25 | 0.633 (0.422–0.951) | |||
| 3 | 12 749 | 94 | 1.80 | 0.475 (0.306–0.737) | |||
| Men ≥2 and women ≥3 | 0 | 5441 | 299 | 15.47 | 1 (reference) | <0.001 | |
| 1 | 23 258 | 1350 | 16.49 | 0.767 (0.677–0.87) | |||
| 2 | 82 117 | 7395 | 26.15 | 0.740 (0.657–0.833) | |||
| 3 | 36 859 | 2350 | 18.29 | 0.632 (0.559–0.715) | |||
| Prior ischemic stroke | |||||||
| No | 0 | 7361 | 197 | 7.02 | 1 (reference) | <0.001 | 0.085 |
| 1 | 28 359 | 849 | 7.9 | 0.727 (0.622–0.849) | |||
| 2 | 79 163 | 4108 | 13.95 | 0.674 (0.582–0.782) | |||
| 3 | 37 320 | 1323 | 9.5 | 0.570 (0.489–0.664) | |||
| Yes | 0 | 1348 | 130 | 27.83 | 1 (reference) | <0.001 | |
| 1 | 6480 | 576 | 26.25 | 0.769 (0.635–0.930) | |||
| 2 | 27 633 | 3517 | 38.80 | 0.783 (0.655–0.936) | |||
| 3 | 12 288 | 1121 | 26.97 | 0.671 (0.559–0.807) | |||
| OAC | |||||||
| No | 0 | 6932 | 260 | 9.65 | 1 (reference) | <0.001 | 0.609 |
| 1 | 26 877 | 1073 | 10.29 | 0.733 (0.639–0.839) | |||
| 2 | 77 822 | 5528 | 18.57 | 0.691 (0.608–0.786) | |||
| 3 | 35 812 | 1754 | 12.68 | 0.595 (0.521–0.680) | |||
| Yes | 0 | 1777 | 67 | 11.60 | 1 (reference) | <0.001 | |
| 1 | 7962 | 352 | 14.00 | 0.840 (0.646–1.091) | |||
| 2 | 28 974 | 2097 | 23.98 | 0.867 (0.677–1.110) | |||
| 3 | 13 796 | 690 | 16.25 | 0.724 (0.562–0.933) | |||
Adjusted for age, sex, hypertension, diabetes, dyslipidemia, heart failure, prior ischemic stroke, prior myocardial infarction, peripheral artery disease, chronic obstructive pulmonary disease, cancer, chronic kidney disease, CHA2DS2‐VASc score, use of oral anticoagulant, antiplatelet agent, and statin, body mass index, and low income. HLS indicates healthy lifestyle behavior score; HR, hazard ratio; IR, incidence rate; and OAC, oral anticoagulant.
Exploratory Analyses
The results of exploratory analyses to compare the risk of dementia for patients having HLS 1 to those having HLS 2 or 3 are presented in Tables S8 and S9. Among patients with at least 1 healthy lifestyle behavior, HLS 2 or 3 groups were associated with a lower risk of all dementia compared with HLS 1 group (P=0.010). When the risk of dementia for HLS 2 and 3 was compared with HLS 1, respectively, HLS 3 was significantly associated with a lower risk of all dementia (HR, 0.826 [95% CI, 0.773–0.884]), whereas HLS 2 tends to show a lower risk (HR, 0.968 [95% CI, 0.912–1.027]). The risk of dementia showed a tendency to decrease with an increase in HLS (P‐for‐trend <0.001). From these exploratory analyses, clustering of healthy lifestyle behaviors was associated with a lower risk of dementia compared with single healthy lifestyle behavior.
The association between exercise dose or intensity and the risk of dementia are presented in Table S10. First, the vigorous‐intensity exercise showed lower HRs than moderate‐intensity exercise when performing those exercises for the same ranges of days per week. When the exercise dose was defined using the frequency of moderate to vigorous exercise per week, 5 to 6 per week moderate to vigorous exercise showed the lowest HRs compared with non‐regular exercise group. When calculating the exercise dose as metabolic equivalent of task (MET)‐min per week, there was an inverse dose‐response relationship between MET‐min per week and the risk of dementia. Patients with 1000 to 1499 MET‐min per week and ≥1500 MET‐min per week showed a significantly lower risk of dementia compared with non‐regular exercise group, those with <500 MET‐min per week and those with 500 to 999 MET‐min per week.
Discussion
In this analysis, based on a large‐scale nationwide population‐based cohort, we investigated whether a combination of healthy lifestyle behaviors was associated with a low risk of dementia in patients with new‐onset AF. Our principal findings are as follows: (1) a substantial number and proportion of patients continued to engage in some of their unhealthy lifestyle behaviors after their diagnosis of AF; (2) patients with clustering of healthy lifestyle behaviors had a significantly lower risk of all dementia, Alzheimer dementia, and vascular dementia than those of patients with a combination of unhealthy lifestyle behaviors; and (3) the beneficial effect of a healthy lifestyle behavior cluster on the risk of dementia was consistently and more prominently observed in the low‐risk subgroups, including patients with younger age (age <65 years), lower CHA2DS2‐VASc score (men <2 and women <3), and those without a prior history of ischemic stroke, than in high‐risk subgroups.
As the prevalence of dementia is increasing in our increasingly aging population, dementia is gaining attention as a major clinical outcome of patients with AF. OAC treatment according to patient stroke risk profile was associated with a low risk of dementia in patients with AF. 10 , 13 , 14 , 15 Catheter ablation as a rhythm control strategy was also associated with a low risk of dementia in patients with AF. 48 , 49
The recent European Society of Cardiology AF management guidelines highlighted the Atrial Fibrillation Better Care Pathway for the integrated care management of AF: “A” Anticoagulation/Avoid stroke; “B” Better symptom management with a patient‐centered symptom‐directed rate or rhythm control; and “C” Cardiovascular risk factor and comorbidity optimization. 5 There is sporadic evidence of “A” and “B” preventing dementia as shown above, and compliance with the “ABC” pathway is associated with a low risk of dementia. 50
Although many studies have elucidated that healthy lifestyle behaviors are associated with reducing the AF burden and risks of adverse clinical outcomes in observational studies 51 , 52 , 53 , 54 , 55 and well‐designed randomized controlled trials, 56 there are limited data on whether healthy lifestyle behaviors could decrease the risk of dementia in patients with AF. Indeed, healthy lifestyle behaviors could effectively prevent dementia in patients with AF since they can simultaneously help maintain one’s cognitive reserve and reduce neuropathological damage by decreasing the AF burden. From this perspective, our study supports the importance of the “C” criterion in the “ABC” pathway for the prevention of dementia in patients with AF.
Lifestyle modification has been traditionally emphasized for the prevention of dementia, and the effect of healthy lifestyle behaviors has been well established. 3 The association between smoking status and dementia was also examined. 16 , 17 , 18 Although a smoker may die of another cause before developing dementia, the risk of smoking on incident dementia is evident. 19 Studies examining the long‐term effects of alcohol consumption on dementia have produced conflicting results according to alcohol intake amount. 20 , 21 , 22 , 23 Even though the association between heavy alcohol use and an increased risk of dementia was consistent in numerous observational studies, light to moderate alcohol use was associated with a decreased risk. 21 , 23 Nevertheless, a French hospital cohort study 24 revealed that alcohol use disorders were the strongest modifiable risk factor for dementia onset as reflected in the 2020 report of the Lancet Commission. 3
Regular exercise is also associated with a reduced risk of dementia. 25 , 26 , 27 Although randomized controlled trials did not show that exercise prevents cognitive decline or dementia, 28 , 29 this was perhaps because of small sample sizes, short intervention periods, limited follow‐up durations, and differences in cohorts and protocols. As many long‐term follow‐up observational studies reported an inverse relationship between exercise and the risk of dementia, the association has been well established. 25 , 26 , 27 In addition, physical exercise prevents dementia in older people by improving balance, reducing falls, improving mood and function. 57
The studies mentioned above focused on an isolated component of lifestyle behaviors and the prevention of dementia. However, lifestyle factors are not isolated; rather, they often occur in clusters with other healthy lifestyle factors. 35 , 58 , 59 In this context, 1 study showed that the increased number of the American Heart Association’s Life’s Simple 7 metrics (non‐smoking; body mass index <25; regular physical activity; eating fish, fruits, and vegetables; untreated cholesterol <200 mg/dL; untreated fasting glucose <100 mg/dL; and untreated blood pressure <120/80 mm Hg) was associated with a reduced risk of dementia. 30 Another study also showed that healthy lifestyle factors (no current smoking, regular physical activity, healthy diet, and moderate alcohol consumption) were statistically independent of genetic risk factors for dementia and the association between the combination of healthy lifestyle behaviors and the risk of dementia, consistent with our study findings. 31
Although some studies reported that a combination of healthy lifestyle behaviors was associated with a decreased risk of dementia in the general population, 30 , 60 , 61 we are unaware of any previous study in patients with AF. In this large study, the HLS 1, 2, and 3 groups were associated with a substantially lower risk of dementia, Alzheimer dementia, and vascular dementia compared with the HLS 0 group. According to our results, the risk reduction of vascular dementia was more prominent than that of Alzheimer dementia in patients with a healthier lifestyle. In recent studies from our group, adoption of healthy lifestyle changes after AF diagnosis, such as alcohol abstinence, quitting smoking, and performing regular exercise, were associated with a lower risk of stroke. 10 , 43 , 47 , 62 Since vascular dementia could be regarded as a manifestation of subclinical stroke, the close association of a healthy lifestyle with stroke risk reduction could be a plausible mechanism of the risk reduction of vascular dementia through clustering healthy lifestyles. In patients with AF, vascular dementia, and Alzheimer dementia risks are known to increase. 10 Although the mechanism of Alzheimer dementia has not been fully elucidated, several studies have reported that cerebrovascular disease, including cerebral infarct, affects the onset and progression of Alzheimer dementia. 63 , 64 , 65 Healthy lifestyle behavior might lower the risk of Alzheimer dementia by lowering the general risk burden of cardiovascular disease.
Interestingly, the IPTW HR for all dementia types was similar between HLS 1 and 2 groups, whereas the HLS 3 group showed lower IPTW HR for all dementia types than HLS 1 and 2 groups. This trend was shown in the weighted cumulative incidence curves and was consistent with IPTW HR for Alzheimer dementia. On the other hand, if patients had at least 1 healthy lifestyle behavior, there was no significant difference in reducing vascular dementia according to the number of healthy lifestyle behaviors. Based on these results, at least 1 healthy lifestyle behavior is associated with as low a risk of dementia as 2 healthy lifestyle behaviors in patients with AF. Thus, health care professionals should motivate patients to correct their unhealthy lifestyle behaviors.
Although we did not directly compare the impact of each factor on the risk of dementia in the main analysis, according to the results of complementary analysis (Table S11), current smoking was associated with a higher risk of dementia by 24% compared with non‐smokers. Regular physical activity was associated with a lower risk of dementia by 20% compared with non‐regular exercise group. Thus, among lifestyle factors, both smoking and exercise were significantly associated with the risk of dementia. Moderate to heavy alcohol consumption tended to be associated with a higher risk of dementia. Alcohol consumption per se, as a single factor, did not show a significant increase in dementia risk. More importantly, our study results suggested that there was a trend for a lower risk of dementia as the number of healthy lifestyle behaviors increased. The impact of bad or good lifestyle habit combinations might reflect the synergistic effect and affect the dose or intensity of each component. In our model, oral anticoagulation did not show a significant association with the risk of dementia.
In subgroup analysis, the beneficial effect of a healthy lifestyle behavior cluster on the risk of dementia was consistently and more prominently observed in patients with younger age (age <65 years), lower CHA2DS2‐VASc score (men <2 and women <3), and those without a prior history of ischemic stroke, which were considered to be the low‐risk subgroups for incident dementia, than in high‐risk subgroups. In addition, the benefits of a healthy lifestyle behavior cluster were consistently observed regardless of whether the patients were treated with OAC. Of note, patients with unhealthy lifestyle behaviors in this population tended to have relatively low‐risk baseline characteristics. Therefore, it would be beneficial to encourage that even low‐risk patients modify their unhealthy lifestyle behaviors to prevent dementia.
Limitations
This study had several limitations. First, because of the inherent limitations of the study design, the associations between the combination of healthy lifestyle behaviors and the risk of dementia could not be directly interpreted as a causal relationship. Second, we collected information on the healthy lifestyle behaviors of patients using self‐reported questionnaires, which could have resulted in recall bias. Third, although we balanced the baseline characteristics of the different groups using IPTW and performed a multivariate adjustment, we could not exclude the possibility of unmeasured confounding factors. Fourth, changes in baseline variables or lifestyle behaviors during follow‐up, including lifestyle factors and medication use, were not considered in this analysis. Fifth, HLS could be associated with the individual’s interest in their health and the actual treatment adherence (or compliance). Although we assessed the prescription records and balanced the medications used, including oral anticoagulation therapy among different HLS groups, we could not fully elucidate the difference and impact of the implementation of guideline adherent therapy and the actual treatment adherence in different HLS score groups in this data set. Sixth, we included patients with new‐onset AF from the entire Korean population. To evaluate the impact of healthy lifestyle behaviors, we only included patients who received a national health examination within 2‐year after AF diagnosis. Patients whose lifestyle behaviors information indicated patients who received the national health checkup provided by the government and there might be the possibility of a selection bias. Patients without lifestyle behavior information were older, more likely to be women and had more prevalent comorbidities than those with lifestyle behavior information (Table S12). These results should be evaluated and applied to the general population with caution. Finally, as we used diagnostic codes to define comorbidities and clinical outcomes in the claims database, errors may have arisen from coding inaccuracies.
Conclusions
A clustering of healthy lifestyle behaviors was associated with a significantly lower risk of dementia in patients with new‐onset AF. These findings support the promotion of a healthy lifestyle within an integrated care approach to AF patient management.
Sources of Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea, the Ministry of Food and Drug Safety) (Project Number: 202013B14), and by the Korea National Research Foundation funded by the Ministry of Education, Science and Technology (grant 2020R1F1A106740).
Disclosures
E.‐K.C. have received research grants or speaking fees from Bayer, BMS/Pfizer, Biosense Webster, Chong Kun Dang, Daiichi‐Sankyo, Dreamtech Co., Ltd., Medtronic, Samjinpharm, Sanofi‐Aventis, Seers Technology, Skylabs, and Yuhan; G.Y.H.L. is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi‐Sankyo, but no fees are received personally; no other relationships or activities that could appear to have influenced the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S12
Acknowledgments
We used data from the Korean NHIS database. The authors would like to thank the NHIS for cooperation.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023739
For Sources of Funding and Disclosures, see page 12.
Contributor Information
Sang‐Hyeon Park, Email: walecang@gmail.com.
So‐Ryoung Lee, Email: minerva1368@gmail.com.
Eue‐Keun Choi, Email: choiek17@snu.ac.kr.
References
- 1. Wu YT, Beiser AS, Breteler MMB, Fratiglioni L, Helmer C, Hendrie HC, Honda H, Ikram MA, Langa KM, Lobo A, et al. The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurol. 2017;13:327–339. doi: 10.1038/nrneurol.2017.63 [DOI] [PubMed] [Google Scholar]
- 2.2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020 Mar 10 [epub ahead of print]. [DOI] [PubMed]
- 3. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen‐Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y‐H, McAnulty JH, Zheng Z‐J, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 6. Kim D, Yang P‐S, Jang E, Yu HT, Kim T‐H, Uhm J‐S, Kim J‐Y, Pak H‐N, Lee M‐H, Joung B, et al. 10‐year nationwide trends of the incidence, prevalence, and adverse outcomes of non‐valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018;202:20–26. doi: 10.1016/j.ahj.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 7. Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population‐based study. The Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.STR.28.2.316 [DOI] [PubMed] [Google Scholar]
- 8. Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm. 2010;7:433–437. doi: 10.1016/j.hrthm.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 9. Dublin S, Anderson ML, Haneuse SJ, Heckbert SR, Crane PK, Breitner JCS, McCormick W, Bowen JD, Teri L, McCurry SM, et al. Atrial fibrillation and risk of dementia: a prospective cohort study. J Am Geriatr Soc. 2011;59:1369–1375. doi: 10.1111/j.1532-5415.2011.03508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim D, Yang P‐S, Yu HT, Kim T‐H, Jang E, Sung J‐H, Pak H‐N, Lee M‐Y, Lee M‐H, Lip GYH, et al. Risk of dementia in stroke‐free patients diagnosed with atrial fibrillation: data from a population‐based cohort. Eur Heart J. 2019;40:2313–2323. doi: 10.1093/eurheartj/ehz386 [DOI] [PubMed] [Google Scholar]
- 11. Chopard R, Piazza G, Gale SA, Campia U, Albertsen IE, Kim J, Goldhaber SZ. Dementia and atrial fibrillation: pathophysiological mechanisms and therapeutic implications. Am J Med. 2018;131:1408–1417. doi: 10.1016/j.amjmed.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 12. Bunch TJ. Atrial fibrillation and dementia. Circulation. 2020;142:618–620. doi: 10.1161/CIRCULATIONAHA.120.045866 [DOI] [PubMed] [Google Scholar]
- 13. Mongkhon P, Fanning L, Lau WCY, Tse G, Lau KK, Wei L, Kongkaew C, Wong ICK. Oral anticoagulant and reduced risk of dementia in patients with atrial fibrillation: a population‐based cohort study. Heart Rhythm. 2020;17:706–713. doi: 10.1016/j.hrthm.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 14. Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J. 2018;39:453–460. doi: 10.1093/eurheartj/ehx579 [DOI] [PubMed] [Google Scholar]
- 15. Friberg L, Andersson T, Rosenqvist M. Less dementia and stroke in low‐risk patients with atrial fibrillation taking oral anticoagulation. Eur Heart J. 2019;40:2327–2335. doi: 10.1093/eurheartj/ehz304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohara T, Ninomiya T, Hata J, Ozawa M, Yoshida D, Mukai N, Nagata M, Iwaki T, Kitazono T, Kanba S, et al. Midlife and late‐life smoking and risk of dementia in the community: the Hisayama study. J Am Geriatr Soc. 2015;63:2332–2339. doi: 10.1111/jgs.13794 [DOI] [PubMed] [Google Scholar]
- 17. Reitz C, den Heijer T, van Duijn C, Hofman A, Breteler MM. Relation between smoking and risk of dementia and Alzheimer disease: the Rotterdam study. Neurology. 2007;69:998–1005. doi: 10.1212/01.wnl.0000271395.29695.9a [DOI] [PubMed] [Google Scholar]
- 18. Juan D, Zhou DH, Li J, Wang JY, Gao C, Chen M. A 2‐year follow‐up study of cigarette smoking and risk of dementia. Eur J Neurol. 2004;11:277–282. doi: 10.1046/j.1468-1331.2003.00779.x [DOI] [PubMed] [Google Scholar]
- 19. Chang CC, Zhao Y, Lee CW, Ganguli M. Smoking, death, and Alzheimer disease: a case of competing risks. Alzheimer Dis Assoc Disord. 2012;26:300–306. doi: 10.1097/WAD.0b013e3182420b6e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langballe EM, Ask H, Holmen J, Stordal E, Saltvedt I, Selbæk G, Fikseaunet A, Bergh S, Nafstad P, Tambs K. Alcohol consumption and risk of dementia up to 27 years later in a large, population‐based sample: the HUNT study, Norway. Eur J Epidemiol. 2015;30:1049–1056. doi: 10.1007/s10654-015-0029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Handing EP, Andel R, Kadlecova P, Gatz M, Pedersen NL. Midlife alcohol consumption and risk of dementia over 43 years of follow‐up: a population‐based study from the Swedish Twin Registry. J Gerontol A Biol Sci Med Sci. 2015;70:1248–1254. doi: 10.1093/gerona/glv038 [DOI] [PubMed] [Google Scholar]
- 22. Ruitenberg A, van Swieten JC, Witteman JC, Mehta KM, van Duijn CM, Hofman A, Breteler MM. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–286. doi: 10.1016/S0140-6736(02)07493-7 [DOI] [PubMed] [Google Scholar]
- 23. Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289:1405–1413. doi: 10.1001/jama.289.11.1405 [DOI] [PubMed] [Google Scholar]
- 24. Schwarzinger M, Pollock BG, Hasan OSM, Dufouil C, Rehm J, Baillot S, Guibert Q, Planchet F, Luchini S. Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study. Lancet Public Health. 2018;3:e124–e132. doi: 10.1016/S2468-2667(18)30022-7 [DOI] [PubMed] [Google Scholar]
- 25. Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498 [DOI] [PubMed] [Google Scholar]
- 26. Rovio S, Kåreholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure‐time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8 [DOI] [PubMed] [Google Scholar]
- 27. Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004 [DOI] [PubMed] [Google Scholar]
- 28. Sink KM, Espeland MA, Castro CM, Church T, Cohen R, Dodson JA, Guralnik J, Hendrie HC, Jennings J, Katula J, et al. Effect of a 24‐month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the life randomized trial. JAMA. 2015;314:781–790. doi: 10.1001/jama.2015.9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D, Dosanjh S, Slowther AM, Khan I, Petrou S, et al. Dementia and physical activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ. 2018;361:k1675. doi: 10.1136/bmj.k1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Samieri C, Perier MC, Gaye B, Proust‐Lima C, Helmer C, Dartigues JF, Berr C, Tzourio C, Empana JP. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320:657–664. doi: 10.1001/jama.2018.11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hyppönen E, Kuzma E, Llewellyn DJ. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322:430–437. doi: 10.1001/jama.2019.9879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi EK. Cardiovascular research using the Korean national health information database. Korean Circ J. 2020;50:754–772. doi: 10.4070/kcj.2020.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jeong HG, Kim DY, Kang DW, Kim BJ, Kim CK, Kim Y, Yang W, Park ES, Lee SH. Physical activity frequency and the risk of stroke: a nationwide cohort study in Korea. J Am Heart Assoc. 2017;6:e005671. doi: 10.1161/JAHA.117.005671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee SR, Choi EK, Ahn HJ, Han KD, Oh S, Lip GYH. Association between clustering of unhealthy lifestyle factors and risk of new‐onset atrial fibrillation: a nationwide population‐based study. Sci Rep. 2020;10:19224. doi: 10.1038/s41598-020-75822-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim MK, Han K, Park YM, Kwon HS, Kang G, Yoon KH, Lee SH. Associations of variability in blood pressure, glucose and cholesterol concentrations, and body mass index with mortality and cardiovascular outcomes in the general population. Circulation. 2018;138:2627–2637. doi: 10.1161/CIRCULATIONAHA.118.034978 [DOI] [PubMed] [Google Scholar]
- 37. Lee SH, Han K, Cho H, Park YM, Kwon HS, Kang G, Yoon KH, Kim MK. Variability in metabolic parameters and risk of dementia: a nationwide population‐based study. Alzheimers Res Ther. 2018;10:110. doi: 10.1186/s13195-018-0442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee SR, Choi EK, Park SH, Jung JH, Han KD, Oh S, Lip GYH. Comparing warfarin and 4 direct oral anticoagulants for the risk of dementia in patients with atrial fibrillation. Stroke. 2021;52:3459–3468. doi: 10.1161/STROKEAHA.120.033338 [DOI] [PubMed] [Google Scholar]
- 39. Lim J, Lee SR, Choi EK, Han KD, Jung JH, Ahn HJ, Yun JP, Kwon S, Oh S, Lip GYH. Exercise and the risk of dementia in patients with newly diagnosed atrial fibrillation: a nationwide population‐based study. J Clin Med. 2021;10. doi: 10.3390/jcm10143126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731–2738. doi: 10.1161/STROKEAHA.110.590257 [DOI] [PubMed] [Google Scholar]
- 43. Lee SR, Choi EK, Jung JH, Han KD, Oh S, Lip GYH. Smoking cessation after diagnosis of new‐onset atrial fibrillation and the risk of stroke and death. J Clin Med. 2021;10. doi: 10.3390/jcm10112238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. doi: 10.1186/1471-2318-8-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. Early rhythm‐control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 46. Kim K, Choi S, Hwang SE, Son JS, Lee JK, Oh J, Park SM. Changes in exercise frequency and cardiovascular outcomes in older adults. Eur Heart J. 2020;41:1490–1499. doi: 10.1093/eurheartj/ehz768 [DOI] [PubMed] [Google Scholar]
- 47. Ahn HJ, Lee SR, Choi EK, Han KD, Jung JH, Lim JH, Yun JP, Kwon S, Oh S, Lip GYH. Association between exercise habits and stroke, heart failure, and mortality in Korean patients with incident atrial fibrillation: a nationwide population‐based cohort study. PLoS Medicine. 2021;18:e1003659. doi: 10.1371/journal.pmed.1003659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim D, Yang P‐S, Sung J‐H, Jang E, Yu HT, Kim T‐H, Uhm J‐S, Kim J‐Y, Pak H‐N, Lee M‐H, et al. Less dementia after catheter ablation for atrial fibrillation: a nationwide cohort study. Eur Heart J. 2020;41:4483–4493. doi: 10.1093/eurheartj/ehaa726 [DOI] [PubMed] [Google Scholar]
- 49. Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, et al. Patients treated with catheter ablation for atrial fibrillation have long‐term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:839–845. doi: 10.1111/j.1540-8167.2011.02035.x [DOI] [PubMed] [Google Scholar]
- 50. Yang P‐S, Sung J‐H, Jang E, Yu HT, Kim T‐H, Uhm J‐S, Kim J‐Y, Pak H‐N, Lee M‐H, Lip GYH, et al. The effect of integrated care management on dementia in atrial fibrillation. J Clin Med. 2020;9:1696. doi: 10.3390/jcm9061696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Albertsen IE, Rasmussen LH, Lane DA, Overvad TF, Skjøth F, Overvad K, Lip GYH, Larsen TB. The impact of smoking on thromboembolism and mortality in patients with incident atrial fibrillation. Chest. 2014;145:559–566. doi: 10.1378/chest.13-1740 [DOI] [PubMed] [Google Scholar]
- 52. Lim C, Kim T‐H, Yu HT, Lee S‐R, Cha M‐J, Lee J‐M, Park J, Park J‐K, Kang K‐W, Shim J, et al. Effect of alcohol consumption on the risk of adverse events in atrial fibrillation: from the comparison study of drugs for symptom control and complication prevention of atrial fibrillation (CODE‐AF) registry. Europace. 2021;23:548–556. doi: 10.1093/europace/euaa340 [DOI] [PubMed] [Google Scholar]
- 53. Proietti M, Boriani G, Laroche C, Diemberger I, Popescu MI, Rasmussen LH, Sinagra G, Dan G‐A, Maggioni AP, Tavazzi L, et al. Self‐reported physical activity and major adverse events in patients with atrial fibrillation: a report from the EURObservational research programme pilot survey on atrial fibrillation (EORP‐AF) general registry. Europace. 2016:euw150. doi: 10.1093/europace/euw150 [DOI] [PubMed] [Google Scholar]
- 54. Garnvik LE, Malmo V, Janszky I, Ellekjær H, Wisløff U, Loennechen JP, Nes BM. Physical activity, cardiorespiratory fitness, and cardiovascular outcomes in individuals with atrial fibrillation: the HUNT study. Eur Heart J. 2020;41:1467–1475. doi: 10.1093/eurheartj/ehaa032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Choi S, Chang J, Kim K, Kim SM, Koo H‐Y, Cho MH, Cho IY, Lee H, Son JS, Park SM, et al. Association of smoking cessation after atrial fibrillation diagnosis on the risk of cardiovascular disease: a cohort study of South Korean men. BMC Public Health. 2020;20:168. doi: 10.1186/s12889-020-8275-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, Prabhu S, Stub D, Azzopardi S, Vizi D, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20–28. doi: 10.1056/NEJMoa1817591 [DOI] [PubMed] [Google Scholar]
- 57. de Labra C, Guimaraes‐Pinheiro C, Maseda A, Lorenzo T, Millán‐Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr. 2015;15:154. doi: 10.1186/s12877-015-0155-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schuit AJ, van Loon AJ, Tijhuis M, Ocke M. Clustering of lifestyle risk factors in a general adult population. Prev Med. 2002;35:219–224. doi: 10.1006/pmed.2002.1064 [DOI] [PubMed] [Google Scholar]
- 59. Poortinga W. The prevalence and clustering of four major lifestyle risk factors in an English adult population. Prev Med. 2007;44:124–128. doi: 10.1016/j.ypmed.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 60. Sabia S, Fayosse A, Dumurgier J, Schnitzler A, Empana J‐P, Ebmeier KP, Dugravot A, Kivimäki M, Singh‐Manoux A. Association of ideal cardiovascular health at age 50 with incidence of dementia: 25 year follow‐up of Whitehall II cohort study. BMJ. 2019:l4414. doi: 10.1136/bmj.l4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC. Healthy lifestyle and the risk of Alzheimer dementia: findings from 2 longitudinal studies. Neurology. 2020;95:e374–e383. doi: 10.1212/WNL.0000000000009816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee SR, Choi EK, Jung JH, Han KD, Oh S, Lip GYH. Lower risk of stroke after alcohol abstinence in patients with incident atrial fibrillation: a nationwide population‐based cohort study. Eur Heart J. 2021;42:4759–4768. doi: 10.1093/eurheartj/ehab315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Han F, Ali Raie A, Shioda N, Qin ZH, Fukunaga K. Accumulation of beta‐amyloid in the brain microvessels accompanies increased hyperphosphorylated tau proteins following microsphere embolism in aged rats. Neuroscience. 2008;153:414–427. doi: 10.1016/j.neuroscience.2008.02.044 [DOI] [PubMed] [Google Scholar]
- 64. de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0 [DOI] [PubMed] [Google Scholar]
- 65. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population‐based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S12


