Abstract
Background
A relevant proportion of patients with suspected coronary artery disease undergo invasive coronary angiography showing normal or nonobstructive coronary arteries. However, the prevalence of coronary microvascular disease (CMD) and coronary spasm in patients with nonobstructive coronary artery disease remains to be determined. The objective of this study was to determine the prevalence of coronary CMD and coronary vasospastic angina in patients with no obstructive coronary artery disease.
Methods and Results
A systematic review and meta‐analysis of studies assessing the prevalence of CMD and vasospastic angina in patients with no obstructive coronary artery disease was performed. Random‐effects models were used to determine the prevalence of these 2 disease entities. Fifty‐six studies comprising 14 427 patients were included. The pooled prevalence of CMD was 0.41 (95% CI, 0.36–0.47), epicardial vasospasm 0.40 (95% CI, 0.34–0.46) and microvascular spasm 24% (95% CI, 0.21–0.28). The prevalence of combined CMD and vasospastic angina was 0.23 (95% CI, 0.17–0.31). Female patients had a higher risk of presenting with CMD compared with male patients (risk ratio, 1.45 [95% CI, 1.11–1.90]). CMD prevalence was similar when assessed using noninvasive or invasive diagnostic methods.
Conclusions
In patients with no obstructive coronary artery disease, approximately half of the cases were reported to have CMD and/or coronary spasm. CMD was more prevalent among female patients. Greater awareness among physicians of ischemia with no obstructive coronary arteries is urgently needed for accurate diagnosis and patient‐tailored management.
Keywords: angina with nonobstructive coronary artery disease, ischemia with no obstructive coronary artery disease, vasospastic angina
Subject Categories: Angiography, Functional Magnetic Resonance Imaging (fMRI), Magnetic Resonance Imaging (MRI), Nuclear Cardiology and PET, Ultrasound
Nonstandard Abbreviations and Acronyms
- CFR
coronary flow reserve
- CMD
coronary microvascular disease
- WISE
Women’s Ischemia Syndrome Evaluation
Clinical Perspective
What Is New?
In patients with no obstructive coronary artery disease, approximately half of cases present with underlying disease, either coronary microvascular disease or coronary vasospasm.
Coronary microvascular disease is more prevalent in female patients; nonetheless, male patients are affected in a significant proportion.
Invasive and noninvasive diagnostic methods identified a similar proportion of patients with coronary microvascular disease.
What Are the Clinical Implications?
The large variability of methods, definitions, and thresholds for diagnosing coronary microvascular disease and coronary vasospasm is a call to a refinement and standardization of diagnostic tools.
Greater awareness among physicians of ischemia with no obstructive coronary arteries is urgently needed for proper diagnosis and patient‐tailored management.
Ischemic heart disease is the leading cause of mortality and morbidity globally. 1 However, in clinical practice, a relevant proportion of patients with suspected coronary artery disease (CAD) undergo invasive coronary angiography showing normal or nonobstructive coronary arteries. 2 Although many of these patients are considered as having normal coronary arteries, ischemia with no obstructive CAD has been associated with increased cardiovascular risk and higher rates of repeat coronary angiography. 3 , 4 , 5 Recent guidelines reflect the wide spectrum of etiopathogenesis of ischemic heart disease and chronic coronary syndromes. 6 Not only coronary atherosclerosis, but disorders of microcirculation and vasomotion may be part of the intricate process leading to myocardial ischemia. Coronary microvascular disease (CMD) is increasingly seen as an important contributor to the pathophysiology of ischemic heart disease. The diagnosis of CMD can be ascertained by means of invasive cardiac catheterization or noninvasive imaging techniques (Figure 1). 7 Epicardial spasm, a separate clinical entity, can also lead to myocardial ischemia and myocardial infarction. 8 , 9 The diagnosis of coronary spasm ideally relies on the results of provocation tests performed in the catheterization laboratory. However, the prevalence of CMD and coronary spasm in patients with nonobstructive CAD remains to be determined.
Figure 1. Methods used for evaluation of microvascular disease.
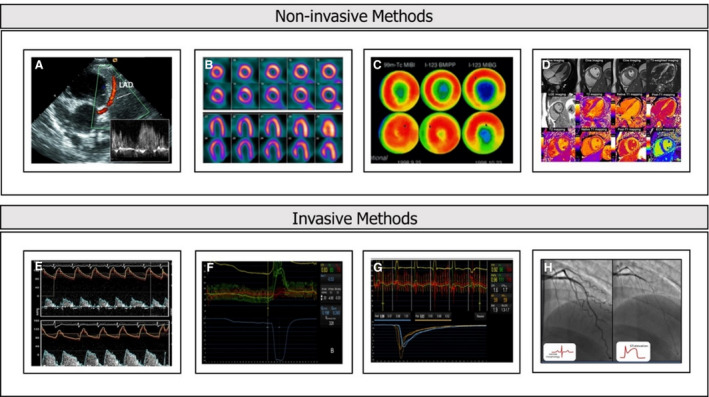
A, Transthoracic echocardiography with Doppler of LAD. B, PET. C, MIBI SPECT. D, CMR. E, Doppler CFR. F, Absolute coronary blood flow measured by thermodilution. G, Thermodilution, CFR and IMR. H, Acetylcholine testing. CFR indicates coronary flow reserve; CMR, cardiac magnetic resonance; IMR, index of microcirculatory resistance. LAD, left anterior descending artery; MIBI SPECT, myocardial perfusion imaging on single photon emission computed tomography; and PET, positron emission tomography.
The aim of the present systematic review and meta‐analysis was to determine the prevalence of CMD and coronary spasm assessed by invasive and noninvasive methods in patients with no obstructive CAD.
Methods
The data that support the findings of this study are available from the first author upon reasonable request.
Search Strategy and Selection Criteria
Studies describing prevalence of coronary microvascular disease and coronary spasm among patients with no obstructive CAD were reviewed. Two reviewers (N.M. and G.M.) systematically searched PubMed and Scopus. The search was conducted in August 2021, starting from inception, and was performed separately for coronary microvascular dysfunction and coronary vasospasm (Table S1). No restrictions were applied for language. Additionally, reference lists of the eligible studies and recent systematic reviews were screened to identify relevant studies. In case of multiple publications with the same population, the latest report was used. The inclusion criteria were: (1) studies comprising patients with suspected CAD, (2) presenting with no obstructive coronary disease, and (3) undergoing a diagnostic test for CMD, spasm, or both with a report of the number of patients testing positive and the total number of patients evaluated. Studies were divided into 2 groups according to the pathophysiology assessed: CMD and coronary vasospasm, respectively. The definition of no obstructive coronary disease and the threshold of diagnostics tests used to define the presence of CMD were based on each individual study. The present systematic review and meta‐analysis is presented in agreement with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses reporting guidelines (Table S2). 10 Quality of included studies was assessed by the Quality Assessment of Diagnostic Accuracy Studies tool. 11 Risk of bias was evaluated across 4 domains: patient selection, index test, reference standard, and flow and timing. This systematic review and meta‐analysis was registered in PROSPERO (International Prospective Register of Systematic Reviews) (CRD42020220077).
Outcomes of Interest
The primary outcome of interest was the prevalence of CMD and/or coronary vasospasm among patients with no obstructive CAD. Patients’ demographic and clinical characteristics, diagnostic methods performed, and number of positive patients were collected. In the present meta‐analysis, definitions of CMD and vasospasm were used according to the ones defined in each study.
Statistical Analysis
Categorical variables are reported as percentages, and continuous variables are reported as mean±SD. To account for heterogeneity between studies, a random‐effects model based on the Der Simonian‐Laird method was used. 12 Weighted events are reported with 95% CIs. Heterogeneity was assessed using the I 2 value. I 2 values of 25%, 50%, and 75% represented mild, moderate, and severe inconsistency, respectively. Random‐effects meta‐regression analyses were used to explore the influence of sex, clinical characteristics, type of diagnostic method, different inclusion, and exclusion criteria on the outcome of interest. Linearity was assessed visually. Pairwise meta‐analysis was performed to compare the risk of CMD between sexes. All analyses were performed using R version 4.0.2 meta and metafor packages (R Foundation for Statistical Computing, Vienna, Austria).
Results
One hundred fifty‐five articles received a complete review, and 56 studies met inclusion criteria and were included in the meta‐analysis (Figure 2). 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 Overall, 14 427 patients were included. The mean age was 59±5 years, 65% were women, and 21% had diabetes. Most of the patients (75%) underwent invasive evaluation. Studies included in the systematic review, methods used, inclusion criteria, and definitions are described in Table S3.
Figure 2. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flowchart.
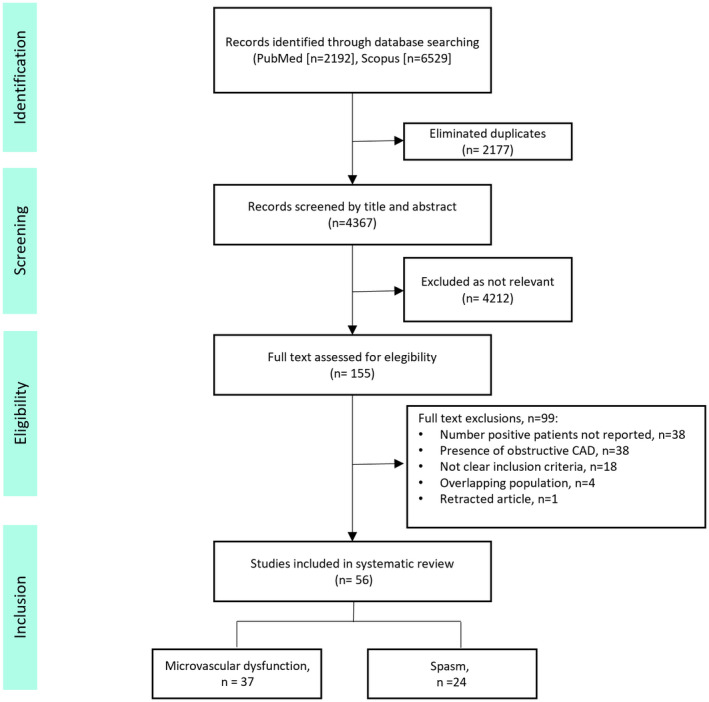
CAD indicates coronary artery disease.
The risk of bias was low on the index test, reference standard, flow, and timing. Nevertheless, in 11% (6/56) of the studies, the risk of bias in the patient selection was considered high because of inclusion of women only (Figure S1). The assessment of the quality of the studies included in the meta‐analysis is presented in (Table S4).
Coronary Microvascular Disease
Thirty‐seven studies reporting rates of CMD in patients with no obstructive CAD were included. They comprised 7212 participants; the mean age was 59±5 years, 61% were women, 66% had hypertension, 22% had diabetes, and 19% were smokers. Twenty‐four studies used invasive methods for diagnosing CMD, whereas 14 used noninvasive methods. Assessment of invasive coronary flow reserve (CFR), either by Doppler or thermodilution techniques, was the most used method (45%), followed by positron emission tomography in 32% of patients (Figure 3). Table 1 shows baseline clinical characteristics of patients undergoing CMD investigations.
Figure 3. Bar plot chart with studies evaluating the prevalence of CMD assessed by invasive (different shades of red) and noninvasive (different shades of blue) methods.

Solid gray line illustrates the 42% pooled prevalence of CMD, and the dashed lines illustrate 95% CIs. CFR indicates invasive measurement of coronary flow reserve, Doppler, and thermodilution method; CMR, cardiac magnetic resonance; IMR, index of microcirculatory resistance; PET, positron emission tomography; and SPECT, myocardial perfusion imaging on single photon emission computed tomography.
Table 1.
Number of Positive Patients and Baseline Clinical Characteristics of the Patients Included in the Studies Investigating the Prevalence of Coronary Microvascular Disease
| Study | Patients included | No. positive, n (%) | Age, y | Women, n (%) | Hypertension, n (%) | Diabetes, n (%) | Dyslipidemia, n (%) | Current smoker, n (%) |
|---|---|---|---|---|---|---|---|---|
| Cassar, 2009 13 | 376 | 170 (45%) | 49±11 | 254 (68%) | 157 (42%) | 36 (10%) | 208 (55%) | NA |
| Godo, 2020 32 | 148 | 91 (62%) | 44±9 | 111 (75%) | 79 (53%) | 11 (7%) | 91 (62%) | 60 (41%) |
| Ford, 2018 33 | 151 | 78 (52%) | 61±10 | 111 (74%) | 125 (81%) | 29 (19.2%) | 120 (79.5%) | 24 (15.9%) |
| Graf, 2006 35 | 58 | 42 (72%) | 58±10 | 39 (67%) | NA | 8 (18%) | NA | 17 (29%) |
| Hasdai, 1998 36 | 203 | 118 (58%) | 51 (17–78) | 158 (78%) | 59 (29%) | 8 (4%) | 88 (43.3%) | 28 (27%) |
| Kobayashi, 2015 39 | 157 | 39 (25%) | 64±12 | 117 (29%) | 77 (49%) | 38 (24%) | 91 (58%) | 47 (30%) |
| Kotecha, 2019 40 | 23 | 16 (70%) | 63±8 | NA | 6 (26%) | NA | NA | NA |
| Lee, 2015 42 | 137 | 38 (28%) | 54±11 | 107 (77%) | 74 (53%) | 32 (23%) | 87 (63%) | 11 (8%) |
| Michelsen, 2018 43 | 919 | 241 (26%) | 62±9 | 919 (100%) | 467 (51%) | 117 (13%) | 580 (63%) | 149 (16%) |
| Murthy, 2014 44 | 1218 | 641 (53%) | 62 (53–69) | 813 (67%) | 894 (73%) | 363 (30%) | 663 (54%) | 121 (10%) |
| Pargaonkar, 2019 47 | 155 | 34 (22%) | 54±13 | 119 (77%) | 68 (44%) | 26 (17%) | 90 (58%) | 23 (15%) |
| Pargaonkar, 2020 48 | 88 | 32 (36%) | NA | 53 (60%%) | NA | NA | NA | NA |
| Pepine, 2010 49 | 152 | 74 (49%) | 55±10 | 189 (100%) | 57 (30%) | 21 (11%) | 50 (26%) | 19 (10%) |
| Quesada, 2020 50 | 150 | 67 (45%) | 54±12 | 36 (24%) | 75 (50%) | 25 (17%) | 90 (60%) | 22 (15%) |
| Sade, 2009 53 | 65 | 27 (40%) | 55±8 | 68 (100%) | 37 (54%) | NA | 35 (52%) | 16 (24%) |
| Safdar, 2020 54 | 124 | 81 (65%) | 51±11 | 91 (73%) | 81 (65%) | 42 (34%) | 53 (43%) | 20 (16%) |
| Sakamoto, 2012 55 | 73 | 12 (16%) | 65±8 | 36 (49%) | 33 (45%) | 6 (8%) | 17 (23%) | 11 (15%) |
| Sara, 2016 56 | 926 | 281 (30%) | 52±13 | 567 (61%) | 371 (40%) | 59 (6%) | 485 (52%) | 111 (12%) |
| Schindler, 2005 58 | 72 | 50 (69%) | 58 _ 8 | 28 (39%) | 50 (69%) | 3 (4%) | 30 (42%) | 18 (25%) |
| Sicari, 2009 61 | 394 | 87 (22%) | 61±10 | 223 (57%) | 238 (60%) | 69 (18%) | NA | 120 (31%) |
| Suda, 2019 63 | 187 | 75 (40%) | 63±12 | 74 (40%) | 100 (54%) | 52 (28%) | 66 (35%) | 52 (28%) |
| Taqueti, 2018 64 | 201 | 108 (54%) | 66 (57–79) | 130 (65%) | 152 (76%) | 129 (64%) | 66 (33%) | 16 (8%) |
| Uemura, 2016 65 | 61 | 16 (26%) | 59±15 | 18 (30%) | 37 (61%) | 15 (25%) | NA | 37 (61%) |
| Verna, 2018 66 | 101 | 45 (45%) | 60±11 | 48 (48%) | 58 (57%) | 9 (9%) | 53 (53%) | 21 (21%) |
| Solberg, 2019 62 | 66 | 11 (17%) | 54±9 | 66 (100%) | 15 (23%) | 2 (3%) | 8 (12%) | 44 (67%) |
| Schroder, 2019 59 | 174 | 49 (28%) | 64±10 | NA | NA | NA | NA | NA |
| Sara, 2019 57 | 129 | 49 (38%) | 50±12 | 61 (47%) | NA | NA | NA | NA |
| Kumar, 2020 41 | 163 | 107 (66%) | 57±12 | 79 (48%) | 118 (72%) | 37 (23%) | 122 (75%) | 30 (18%) |
| De Vita, 2019 34 | 30 | 18 (60%) | 67±10 | 19 (63%) | 19 (63%) | 4 (13%) | 16 (53%) | 15 (50%1) |
| Mygind, 2016 45 | 54 | 20 (37%) | 62±8 | 54 (100%) | 29 (54%) | NA | 34 (63%) | 34 (63%) |
| Panza, 1997 46 | 66 | 13 (20%) | 49±10 | 44 (67%) | NA | Na | NA | NA |
| Schroder, 2018 60 | 97 | 37 (38%) | 62 (31–79) | 97 (100%) | NA | NA | NA | NA |
| Reis, 1999 52 | 48 | 29 (60%) | 54±10 | 48 (100%) | 23 (48%) | 6 (13%) | 24 (49%) | NA |
| Kim, 2013 38 | 40 | 11 (28%) | 53±11 | NA | NA | NA | NA | NA |
| Ishimori, 2011 37 | 18 | 8 (44%) | 41±11 | 18 | NA | NA | NA | NA |
| Rahman, 2019 51 | 85 | 45 (53%) | 57±10 | 66 (78%) | 25 (29%) | 11 (13%) | 23 (27%) | 12 (14%) |
| Konst, 2020 67 | 103 | 38 (37%) | 62±9 | NA | NA | NA | NA | NA |
NA indicates information is not available.
The pooled prevalence of CMD was 0.41 (95% CI, 0.36–0.47; I 2=94%; Figure 4). In 18 studies, CMD prevalence were reported separately for men and women. In the meta‐regression analysis, there was no association between the proportion of women included in each study and prevalence of CMD. However, the risk of testing positive for CMD was 1.45 times greater than for men (Figure 5). The prevalence of CMD derived from invasive and noninvasive diagnostic methods was similar (0.43 [95% CI, 0.33–0.53] for invasive methods versus 0.42 [95% CI, 0.36–0.49] for noninvasive methods (P=0.993; Figure S2). Among noninvasive methods, a higher rate of CMD was found in patients who underwent positron emission tomography examination (0.46 [95% CI, 0.46–0.65]) compared with other noninvasive techniques (0.40 [95% CI, 0.30–0.55]; P=0.019).
Figure 4. Prevalence of coronary microvascular dysfunction.
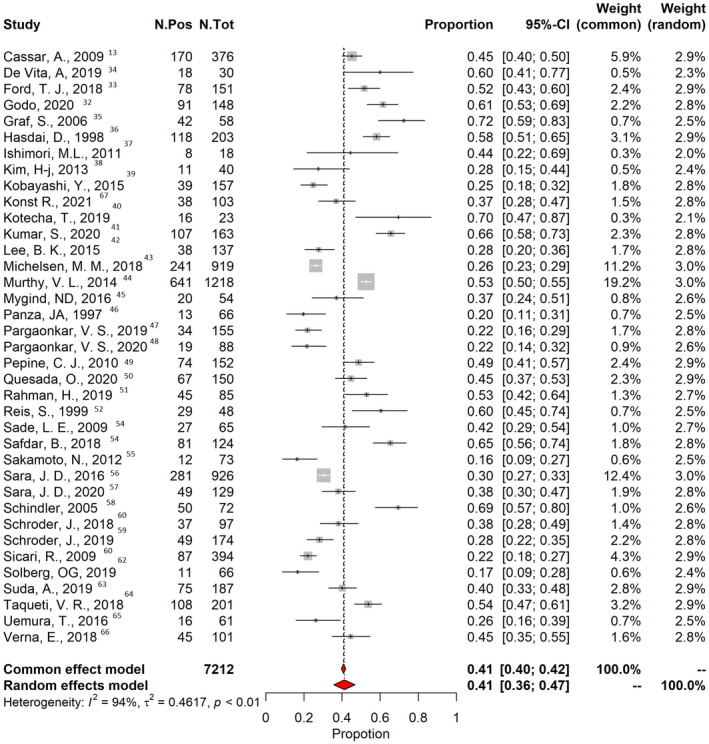
The vertical black line indicates the pooled averaged prevalence rate estimate, and the red diamond represents the overall estimated prevalence with 95% CI in a random‐effects model. Gray squares indicate weighted‐point estimates of incidence for each single study, with gray horizontal lines indicating 95% CI. I 2 indicates Higgins index of heterogeneity. Pos indicates positive; and Tot, total.
Figure 5. Sensitivity analysis of prevalence of microvascular disease according to sex.
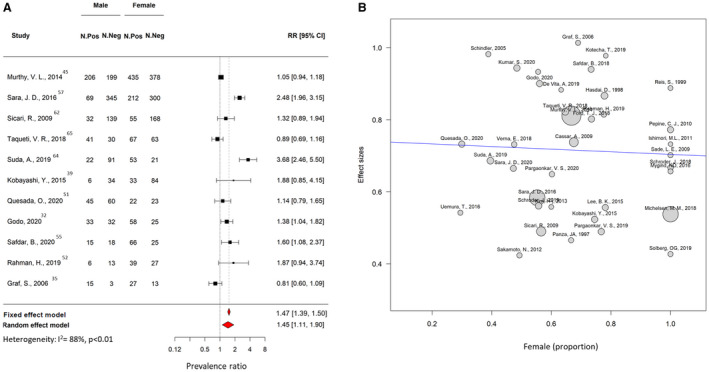
A, Forest plot illustrating the risk ratio (RR) and 95% CI of prevalence of coronary microvascular disease according to sex. B, Metaregression plot showing association between the prevalence of coronary microvascular resistance (y‐axis) and the proportion of women included in each study. The size of the bubble represents the number of patients included in each study. Neg indicates negative; and Pos, positive.
Sensitivity analyses addressing definitions of CMD based on different CFR thresholds (eg, abnormal CFR considered ≤2.5 or ≤2.0) found no significant difference in rate of CMD (0.43 [95% CI, 0.35–0.51] for CFR ≤2.5 versus 0.46 [95% CI, 0.33–0.60] for CFR ≤2.0 (P=0.986; Figure S3). A separate analysis including only studies with at least 200 patients, performed to prevent overestimation bias seen in small studies, found similar prevalence of CMD (0.42 [95% CI, 0.36–0.49]).
Vasospastic Angina
Twenty‐four studies investigating the presence of coronary vasospasm were included. They comprised 6553 patients; the mean age was 60.5±8.0 years, 39% were women, 21% had diabetes and 32% were smokers. Table 2 shows baseline and clinical characteristics of the patients undergoing coronary spasm investigations. Among studies investigating the presence of coronary vasospasm, 21 addressed epicardial spasm only, and 13 also reported the proportion of patients with microvascular spasm. The overall prevalence of coronary epicardial and microvascular spasm was 0.49 (95% CI, 0.43–0.56; I 2=96%; Figure 6). The prevalence of epicardial spasm was 0.40 (95% CI, 0.33–0.47; I 2=96%), whereas the prevalence of microvascular spasm was 0.24 (95% CI, 0.21–0.28; I 2=87%; Figure 7, Figure S4). For most of the patients, acetylcholine was used for the provocation test (98%), 14 , 15 , 16 , 17 , 19 , 20 , 21 , 22 , 23 , 31 , 63 , 66 , 68 and 2 studies used ergonovine. 30 , 34 No significant difference was found considering the type of provocation test and prevalence of spasm 0.49 (95% CI, 0.38–0.55) for acetylcholine versus 0.48 (95% CI, 0.39–0.57) for ergonovine (P=0.935). In 12 studies, coronary spasm prevalence was reported separately for men and woman. The prevalence of coronary spasm was similar between sexes 0.28 (95% CI, 0.22–0.53) in women versus 0.25 (95% CI, 0.18–0.35) in men (Figure 8). From subgroup analyses considering different definitions of epicardial spasm (ie, based on ≥90% or ≥70% coronary vasoconstriction), no significant difference in rate of spasm was detected: 0.47 (95% CI, 0.35–0.50) for ≥90 constriction versus 0.49 (95% CI, 0.42–0.55) for ≥70% constriction (P=0.133).
Table 2.
Number of Positive Patients and Baseline Clinical Characteristics of the Patients Included in the Studies Investigating the Prevalence of Vasospasm
| Study | Patients included | No. positive, n (%) | Age, y | Women, n (%) | Hypertension, n (%) | Diabetes, n (%) | Dyslipidemia, n (%) | Current smoker, n (%) |
|---|---|---|---|---|---|---|---|---|
| Aziz, 2017 14 | 1379 | 813 (59%) | 62±11.9 | 799 (58%) | 970 (70%) | 237 (17%) | 841 (61%) | 502 (36%) |
| Ford, 2018 33 | 151 | 56 (37%) | 61 (53–68) | 111 (74%) | NA | 29 (19%) | 120 (80%) | 24 (16%) |
| Hoshino, 2016 15 | 292 | 90 (30%) | 64±11 | 156 (51.7%) | 114 (39%) | 33 (11%) | 98 (34%) | 130 (45%) |
| Kim, 2018 16 | 328 | 128 (39%) | 58±10.4 | 233 (71%) | 128 (39%) | 31 (9.4%) | 72 (22%) | 39 (12%) |
| Mohri, 1998 17 | 117 | 81 (74%) | 63 (54–68) | 59 (50%) | 56 (48%) | 26 (22%) | 49 (42%) | 50 (43%) |
| Montone, 2018 18 | 80 | 37 (46%) | 63±11 | 40 (50%) | 32 (40%) | 8 (10%) | 19 (24%) | 17 (21%) |
| Montone, 2020 19 | 210 | 118 (56%) | 62±11 | 82 (39%) | 79 (38%) | 13 (6%) | 54 (26%) | 27 (13%) |
| Oh, 2019 20 | 464 | 156 (34%) | 57±11 | 164 (35%) | 60 (13%) | 23 (5%) | 94 (20%) | 48 (10%) |
| Ohba, 2012 21 | 370 | 264 (71%) | 63±11 | 211 (57%) | 197 (53%) | 73 (20%) | 193 (52%) | 107 (29%) |
| Ong, 2014 23 | 847 | 488 (58%) | 62±12 | 485 (57%) | 609 (72%) | 142 (17%) | 460 (54%) | 307 (36%) |
| Ong, 2012 22 | 124 | 77 (53%) | 64±10 | 100 (%) | 102 (71%) | 31 (22%) | 83 (58%) | 22 (15%) |
| Ong, 2014 24 | 137 | 69 (50%) | 63±11 | 93 (68%) | 105 (77%) | 27 (20%) | 73 (53%) | 38 (28%) |
| Pirozzolo, 2020 25 | 96 | 56 (58%) | 65±12 | 49 (51%) | 84 (88%) | 15 (16%) | 84 (88%) | 25 (26%) |
| Quyyumi, 1992 26 | 51 | 5 (10%) | 51±11 | 31 (61%) | 20 (39%) | NA | NA | NA |
| Suda, 2019 63 | 187 | 126 (67%) | 63±12 | 74 (40%) | 100 (54%) | 52 (28%) | 66 (35%) | 52 (28%) |
| Sun, 2002 29 | 55 | 14 (26%) | 60±10 | 23 (42%) | 26 (47%) | 9 (16%) | 26 (47%) | 30 (55%) |
| Sun, 2005 28 | 131 | 101 (79%) | 59±11 | 69 (53%) | 59 (45%) | 30 (13%) | 50 (38%) | 36 (27%) |
| Tsuchida, 2005 30 | 102 | 74 (77%) | 57±11 | 15 (15%) | 43 (42%) | 31 (30%) | NA | 82 (80%) |
| Uemura, 2016 65 | 61 | 15 (28%) | 59±15 | 18 (30%) | 37 (61%) | 15 (25%) | NA | 37 (61%) |
| Verna, 2018 66 | 101 | 57 (57%) | 60±11 | 48 (48%) | 58 (57%) | 9 (9%) | 53 (52%) | 21 (20%) |
| Seitz, 2020 27 | 847 | 283 (33%) | 64±11 | 529 (63%) | 533 (63%) | 129 (15%) | 411 (49%) | 260 (31%) |
| Yamanaga, 2015 31 | 50 | 29 (58%) | 62±13 | 24 (48%) | 28 (56%) | 10 (20%) | 29 (58%) | 10 (20%) |
| Quesada, 2020 50 | 150 | 83 (55%) | 54±12 | 36 (24%) | 75 (50%) | 25 (17%) | 90 (60%) | 22 (15%) |
| Hasdai, 1998 36 | 203 | 59 (29%) | 51 [17–78] | 158 (78%) | 59 (29%) | 8 (4%) | 88 (43%) | 28 (14%) |
NA indicates information is not available.
Figure 6. Prevalence of coronary vasospasm.
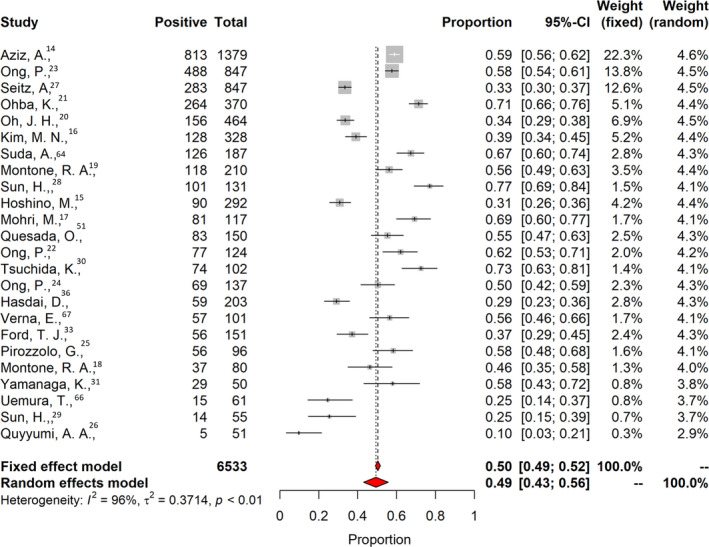
The vertical black line indicates the pooled averaged prevalence rate estimate, and the red diamond represents the overall estimated prevalence with 95% CI in a random‐effects model. Gray squares indicate weighted‐point estimates of incidence for each single study, with gray horizontal lines indicating 95% CI. I 2 indicates Higgins index of heterogeneity.
Figure 7. Prevalence of coronary microvascular spasm.
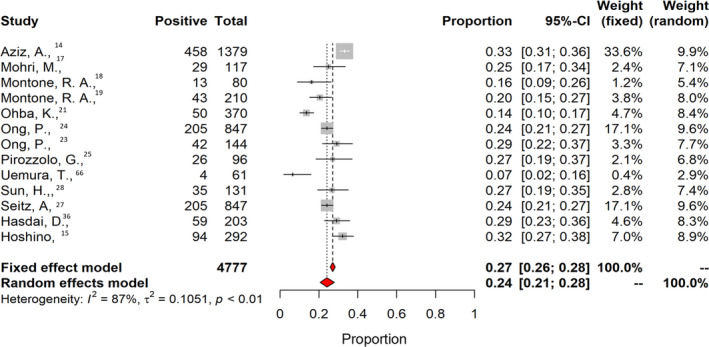
Figure 8. Sensitivity analysis of prevalence of coronary vasospasm according to sex.
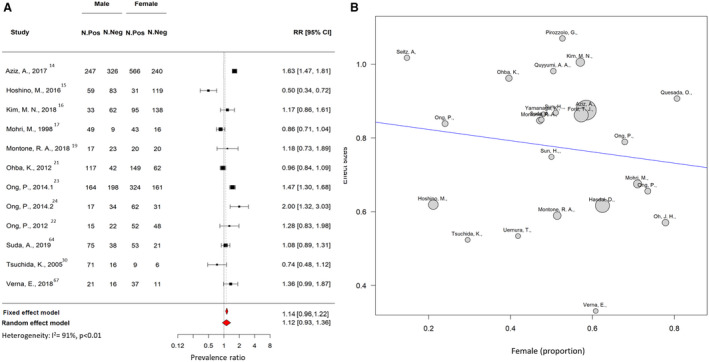
A, Forest plot illustrating the risk ratio (RR) and 95% CI of the prevalence of coronary vasospasm according to sex. B, Metaregression plot showing association between the prevalence of coronary vasospasm (y‐axis) and the proportion of women included in each study. The size of the bubble represents the number of patients included in each study. Neg indicates negative; and Pos, positive.
Combined Prevalence of CMD and Coronary Vasospasm
In 3 of the studies, 33 , 36 , 63 patients underwent evaluation for CMD and spasm. Overall, 541 patients, with a mean age of 58±10.2 years and comprising 63% women, were included. The prevalence of CMD alone was 0.23 (95% CI, 0.10–0.45), coronary spasm alone (either epicardial or microvascular) 0.19 (95% CI, 0.10–0.33), and coexistent CMD and coronary vasospasm in 0.23 (95% CI, 0.17–0.31).
Discussion
The main findings of the present systematic review and meta‐analysis can be summarized as follows: (1) The proportion of patients with no obstructive coronary arteries presenting with CMD was 41%, whereas coronary spasm (epicardial and/or microvascular) was present in 49% of the cases. (2) Women are more likely than men to be affected by CMD. (3) Invasive and noninvasive diagnostic methods identified similar proportions of patients with CMD. (4) There was high heterogeneity between studies in the observed prevalence of CMD and vasospastic angina.
There is an increasing awareness among clinicians of the importance of microvascular function testing in patients with nonobstructive coronary arteries. 7 , 69 Murthy et al reported that even in the absence of obstructive coronary atherosclerosis, 53% of patients who present with chest pain have evidence of inducible myocardial ischemia. Moreover, it was shown that the presence of CMD identifies patients at increased risk of death and myocardial infarction. 44 , 70 The present meta‐analysis found that almost half of patients with no obstructive coronary arteries undergoing evaluation of the coronary microcirculation have CMD. Coronary function testing enables stratifying management of patients from different endotypes of ischemia with no obstructive CAD. Individualized treatment strategies are required, given the different pathophysiological mechanisms underlying these distinct disease endotypes. Objective evidence of the cause of chest pain and stratified therapy positively influence the quality of life of these patients. 33 , 71 Furthermore, identification of CMD or coronary spasm as the cause of symptoms prevents patients from undergoing repeated invasive diagnostic evaluations, which may reduce health care costs and allows for medical therapy optimization according to a specific diagnosis. 72
Coronary microvascular dysfunction has been deceivingly recognized as a women’s disease. 73 The WISE (Women’s Ischemia Syndrome Evaluation) study demonstrated that 39% of women who present with chest pain and no obstructive CAD have evidence of induced myocardial ischemia and coronary vasomotor dysfunction. 49 However, Murthy et al showed, using positron emission tomography, that CMD was highly prevalent in both sexes (51% in men versus 54% in women). 44 The present meta‐analysis found that CMD is highly prevalent in both sexes; however, women are more likely to have CMD. 44 , 49 , 74 An important fact to consider is that a substantial number of the studies did not evaluate men in a similar proportion to women.
Stratified Approach
The prevalence of CMD in patients with angina and no obstructive CAD undergoing invasive angiography depends on the methods and cutoffs applied. Assessment of invasive CFR was found to be the most‐used method for detecting CMD. However, it was derived mainly using a Doppler* or thermodilution technique. 33 , 39 , 42 , 50 In addition, some studies used a cutoff value of ≤2.5, 13 , 36 , 50 , 51 , 52 , 55 , 56 , 66 , 75 whereas others used ≤2.0. 32 , 33 , 39 , 41 , 42 The different methods and cutoffs may partially explain the high between‐study heterogeneity. However, we found that the prevalence of CMD was similar between methods and cutoffs. The recently published consensus document on diagnosis of CMD defined specific thresholds for identification of distinct endotypes of ischemia with no obstructive CAD. 76 Here, CMD is defined as the presence of symptoms of myocardial ischemia, unobstructed coronary arteries (ie, diameter stenosis <50% or fractional flow reserve >0.80), and any of the following: index of microcirculatory resistance >25, CFR ≤2.0, and hyperemic microvascular resistance >1.9. Vasospastic angina, assessed with an acetylcholine provocation test, is considered positive for epicardial spasm when ≥90% diameter stenosis (compared with the angiography performed after nitrate administration) occurs with angina and ischemic ECG changes, whereas microvascular spasm is defined as the presence of angina and ischemic ECG changes without severe epicardial narrowing. 76
Despite the increasing awareness of CMD as a cause of chest pain, diagnostic methods to assess its presence remain underused. 77 There are 2 main barriers to the widespread adoption of these methods in clinical practice. One refers to the limited availability of methods to diagnose CMD, such as positron emission tomography and invasive measurements. The second arises from the lack of effective medical therapies to treat CMD. Therefore, future research should focus on the evaluation of therapies to improve quality of life in patients with CMD. A breakthrough in this field would potentially facilitate the widespread adoption of CMD and vaso‐function testing in clinical practice.
Limitations
The main limitation of the present meta‐analysis is the lack of data on individual patients, which would have allowed for a standardization of CMD and coronary spasm definitions. Moreover, we observed a high level of heterogeneity between studies. The possibility of publication bias cannot be excluded (Figure S5). We were unable to identify specific variables leading to heterogeneity; however, this is most likely related to the inclusion criteria of each individual study and the difference between definitions of CMD and spasm that were used across the studies (Table S2). Another fact that should be accounted for is the possibility of false‐positive cases, especially in the studies with noninvasive imaging. 78 , 79 During the past years, more attention has been drawn to the fact that CFR is unable to define the pathophysiologic substrate for all cases of angina with no obstructive coronary arteries. It has been suggested that assessing the full range coronary pathophysiology requires concepts beyond CFR, such as regional size–severity quantification versus global perfusion and subendocardial perfusion on relative tomographic images. 80
Conclusions
In patients with no obstructive CAD, approximately half of the cases present with underlying disease, either CMD or coronary vasospasm. CMD is more prevalent in women; nonetheless, men are affected in a significant proportion. The large variability of methods, definitions, and thresholds for diagnosing these conditions is a call to a refinement and standardization of diagnostic tools. Greater awareness among physicians of ischemia with no obstructive coronary arteries is urgently needed for accurate diagnosis and patient‐tailored management.
Sources of Funding
None.
Disclosures
Dr Sonck reports research grants provided by the Cardiopath PhD program. Dr Berry is employed by the University of Glasgow, which holds research and/or consultancy agreements with AstraZeneca, Abbott Vascular, Boehringer Ingelheim, GSK, HeartFlow, Opsens, and Novartis. Dr Berry received research funding from the British Heart Foundation (RE/18/6134217). Dr De Bruyne has a consulting relationship with Boston Scientific, Abbott Vascular, CathWorks, Siemens, and Coroventis Research; receives research grants from Abbott Vascular, Coroventis Research, CathWorks, and Boston Scientific; and holds minor equities in Philips, Siemens, GE Healthcare, Edwards Life Sciences, HeartFlow, Opsens, and Celyad. Dr Collet reports receiving research grants from Biosensor, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, and Abbott Vascular; and consultancy fees from HeartFlow, Opsens, Abbott Vascular, and Philips. The remaining authors have no disclosures to report.
Supporting information
Figures S1–S5
For Sources of Funding and Disclosures, see page 11.
Footnotes
REFERENCES
- 1. Khan MAB, Hashim MJ, Mustafa H, Baniyas MY, Al Suwaidi SKBM, AlKatheeri R, Alblooshi FMK, Almatrooshi MEAH, Alzaabi MEH, Al Darmaki RS, et al. Global epidemiology of ischemic heart disease: results from the Global Burden of Disease Study. Cureus. 2020;12:e9349. doi: 10.7759/cureus.9349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, Jørgensen E, Kelbæk H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331 [DOI] [PubMed] [Google Scholar]
- 4. Da Costa A, Isaaz K, Faure E, Mourot S, Cerisier A, Lamaud M. Clinical characteristics, aetiological factors and long‐term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3‐year follow‐up study of 91 patients. Eur Heart J. 2001;22:1459–1465. doi: 10.1053/euhj.2000.2553 [DOI] [PubMed] [Google Scholar]
- 5. Radico F, Zimarino M, Fulgenzi F, Ricci F, Di Nicola M, Jespersen L, Chang SM, Humphries KH, Marzilli M, De Caterina R. Determinants of long‐term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta‐analysis. Eur Heart J. 2018;39:2135–2146. doi: 10.1093/eurheartj/ehy185 [DOI] [PubMed] [Google Scholar]
- 6. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 7. Masi S, Rizzoni D, Taddei S, Widmer RJ, Montezano AC, Lüscher TF, Schiffrin EL, Touyz RM, Paneni F, Lerman A, et al. Assessment and pathophysiology of microvascular disease: recent progress and clinical implications. Eur Heart J. 2021;42:2590–2604. doi: 10.1093/eurheartj/ehaa857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J. 2015;36:475–481. doi: 10.1093/eurheartj/ehu469 [DOI] [PubMed] [Google Scholar]
- 9. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Group ESD . Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2018;40:237–269. doi: 10.1093/eurheartj/ehy462 [DOI] [PubMed] [Google Scholar]
- 10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 11. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 12. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 13. Cassar A, Chareonthaitawee P, Rihal CS, Prasad A, Lennon RJ, Lerman LO, Lerman A. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2:237–244. doi: 10.1161/CIRCINTERVENTIONS.108.841056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aziz A, Hansen HS, Sechtem U, Prescott E, Ong P. Sex‐related differences in vasomotor function in patients with angina and unobstructed coronary arteries. J Am Coll Cardiol. 2017;70:2349–2358. doi: 10.1016/j.jacc.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 15. Hoshino M, Yonetsu T, Mizukami A, Matsuda Y, Yoshioka K, Sudo Y, Ninomiya R, Soeda M, Kuroda S, Ono M, et al. Moderate vasomotor response to acetylcholine provocation test as an indicator of long‐term prognosis. Heart Vessels. 2016;31:1943–1949. doi: 10.1007/s00380-016-0827-9 [DOI] [PubMed] [Google Scholar]
- 16. Kim MN, Kim HL, Park SM, Shin MS, Yu CW, Kim MA, Hong KS, Shim WJ. Association of epicardial adipose tissue with coronary spasm and coronary atherosclerosis in patients with chest pain: analysis of data collated by the KoRean wOmen’S chest pain rEgistry (koROSE). Heart Vessels. 2018;33:17–24. doi: 10.1007/s00380-017-1029-9 [DOI] [PubMed] [Google Scholar]
- 17. Mohri M, Koyanagi M, Egashira K, Tagawa H, Ichiki T, Shimokawa H, Takeshita A. Angina pectoris caused by coronary microvascular spasm. Lancet. 1998;351:1165–1169. doi: 10.1016/S0140-6736(97)07329-7 [DOI] [PubMed] [Google Scholar]
- 18. Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Cammà G, Lanza GA, Crea F. Patients with acute myocardial infarction and non‐obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91–98. doi: 10.1093/eurheartj/ehx667 [DOI] [PubMed] [Google Scholar]
- 19. Montone RA, Niccoli G, Russo M, Giaccari M, Del Buono MG, Meucci MC, Gurguglione F, Vergallo R, D’Amario D, Buffon A, et al. Clinical, angiographic and echocardiographic correlates of epicardial and microvascular spasm in patients with myocardial ischaemia and non‐obstructive coronary arteries. Clin Res Cardiol. 2020;109:435–443. doi: 10.1007/s00392-019-01523-w [DOI] [PubMed] [Google Scholar]
- 20. Oh JH, Song S, Kim C, Ahn J, Park JS, Lee HW, Choi JH, Lee HC, Cha KS, Hong TJ. Effect of intracoronary adenosine on ergonovine‐induced vasoconstricted coronary arteries. Cardiol J. 2019;26:653–660. doi: 10.5603/CJ.a2018.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohba K, Sugiyama S, Sumida H, Nozaki T, Matsubara J, Matsuzawa Y, Konishi M, Akiyama E, Kurokawa H, Maeda H, et al. Microvascular coronary artery spasm presents distinctive clinical features with endothelial dysfunction as nonobstructive coronary artery disease. J Am Heart Assoc. 2012;1:e002485. doi: 10.1161/JAHA.112.002485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. the ACOVA study (abnormal coronary vasomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol. 2012;59:655–662. doi: 10.1016/j.jacc.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 23. Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, Hill S, Schäufele T, Mahrholdt H, Kaski JC, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723–1730. doi: 10.1161/CIRCULATIONAHA.113.004096 [DOI] [PubMed] [Google Scholar]
- 24. Ong P, Athanasiadis A, Hill S, Schäufele T, Mahrholdt H, Sechtem U. Coronary microvascular dysfunction assessed by intracoronary acetylcholine provocation testing is a frequent cause of ischemia and angina in patients with exercise‐induced electrocardiographic changes and unobstructed coronary arteries. Clin Cardiol. 2014;37:462–467. doi: 10.1002/clc.22282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pirozzolo G, Seitz A, Athanasiadis A, Bekeredjian R, Sechtem U, Ong P. Microvascular spasm in non‐ST‐segment elevation myocardial infarction without culprit lesion (MINOCA). Clin Res Cardiol. 2020;109:246–254. doi: 10.1007/s00392-019-01507-w [DOI] [PubMed] [Google Scholar]
- 26. Quyyumi AA, Cannon RO III, Panza JA, Diodati JG, Epstein SE. Endothelial dysfunction in patients with chest pain and normal coronary arteries. Circulation. 1992;86:1864–1871. doi: 10.1161/01.CIR.86.6.1864 [DOI] [PubMed] [Google Scholar]
- 27. Seitz A, Gardezy J, Pirozzolo G, Probst S, Athanasiadis A, Hill S, Mahrholdt H, Bekeredjian R, Sechtem U, Ong P. Long‐term follow‐up in patients with stable angina and unobstructed coronary arteries undergoing intracoronary acetylcholine testing. JACC Cardiovasc Interv. 2020;13:1865–1876. doi: 10.1016/j.jcin.2020.05.009 [DOI] [PubMed] [Google Scholar]
- 28. Sun H, Fukumoto Y, Ito A, Shimokawa H, Sunagawa K. Coronary microvascular dysfunction in patients with microvascular angina: analysis by TIMI frame count. J Cardiovasc Pharmacol. 2005;46:622–626. doi: 10.1097/01.fjc.0000181291.96086.ae [DOI] [PubMed] [Google Scholar]
- 29. Sun H, Mohri M, Shimokawa H, Usui M, Urakami L, Takeshita A. Coronary microvascular spasm causes myocardial ischemia in patients with vasospastic angina. J Am Coll Cardiol. 2002;39:847–851. doi: 10.1016/S0735-1097(02)01690-X [DOI] [PubMed] [Google Scholar]
- 30. Tsuchida K, Hori T, Tanabe N, Makiyama Y, Ozawa T, Saigawa T, Watanabe R, Tanaka T, Nasuno A, Fukunaga H, et al. Relationship between serum lipoprotein(a) concentrations and coronary vasomotion in coronary spastic angina. Circ J. 2005;69:521–525. doi: 10.1253/circj.69.521 [DOI] [PubMed] [Google Scholar]
- 31. Yamanaga K, Tsujita K, Komura N, Kaikita K, Sakamoto K, Miyazaki T, Saito M, Ishii M, Tabata N, Akasaka T, et al. Single‐wire pressure and flow velocity measurement for quantifying microvascular dysfunction in patients with coronary vasospastic angina. Am J Physiol Heart Circ Physiol. 2015;308:H478–H484. doi: 10.1152/ajpheart.00593.2014 [DOI] [PubMed] [Google Scholar]
- 32. Godo S, Corban MT, Toya T, Gulati R, Lerman LO, Lerman A. Association of coronary microvascular endothelial dysfunction with vulnerable plaque characteristics in early coronary atherosclerosis. EuroIntervention. 2020;16:387–394. doi: 10.4244/EIJ-D-19-00265 [DOI] [PubMed] [Google Scholar]
- 33. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72:2841–2855. doi: 10.1016/j.jacc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 34. De Vita A, Manfredonia L, Lamendola P, Villano A, Ravenna SE, Bisignani A, Niccoli G, Lanza GA, Crea F. Coronary microvascular dysfunction in patients with acute coronary syndrome and no obstructive coronary artery disease. Clin Res Cardiol. 2019;108:1364–1370. doi: 10.1007/s00392-019-01472-4 [DOI] [PubMed] [Google Scholar]
- 35. Graf S, Khorsand A, Gwechenberger M, Schutz M, Kletter K, Sochor H, Dudczak R, Maurer G, Pirich C, Porenta G, et al. Myocardial perfusion in patients with typical chest pain and normal angiogram. Eur J Clin Invest. 2006;36:326–332. doi: 10.1111/j.1365-2362.2006.01635.x [DOI] [PubMed] [Google Scholar]
- 36. Hasdai D, Holmes DR Jr, Higano ST, Burnett JC Jr, Lerman A. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc. 1998;73:1133–1140. doi: 10.4065/73.12.1133 [DOI] [PubMed] [Google Scholar]
- 37. Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, Slomka PJ, Thomson LEJ, Schapira J, Yang Y, et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging. 2011;4:27–33. doi: 10.1016/j.jcmg.2010.09.019 [DOI] [PubMed] [Google Scholar]
- 38. Kim HJ, Hong MK, Kim SH, Chung SM, Chung EJ, Han SW, Ryu KH. Evaluation of microvascular angina with timi frame count using nitroprusside induced hyperemia. Microvasc Res. 2013;87:95–99. doi: 10.1016/j.mvr.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 39. Kobayashi Y, Fearon WF, Honda Y, Tanaka S, Pargaonkar V, Fitzgerald PJ, Lee DP, Stefanick M, Yeung AC, Tremmel JA. Effect of sex differences on invasive measures of coronary microvascular dysfunction in patients with angina in the absence of obstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1433–1441. doi: 10.1016/j.jcin.2015.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kotecha T, Martinez‐Naharro A, Boldrini M, Knight D, Hawkins P, Kalra S, Patel D, Coghlan G, Moon J, Plein S, et al. Automated pixel‐wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. JACC Cardiovasc Imaging. 2019;12:1958–1969. doi: 10.1016/j.jcmg.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar S, Mehta PK, Eshtehardi P, Hung OY, Koh J‐S, Kumar A, Al‐Badri A, Rabah R, D'Souza M, Gupta S, et al. Functional coronary angiography in symptomatic patients with no obstructive coronary artery disease. Catheter Cardiovasc Interv. 2020. Sep 9. [epub ahead of print]. doi: 10.1002/ccd.29237 [DOI] [PubMed] [Google Scholar]
- 42. Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michelsen MM, Pena A, Mygind ND, Bech J, Gustafsson I, Kastrup J, Hansen HS, Høst N, Hansen PR, Prescott E. Coronary microvascular dysfunction and myocardial contractile reserve in women with angina and no obstructive coronary artery disease. Echocardiography. 2018;35:196–203. doi: 10.1111/echo.13767 [DOI] [PubMed] [Google Scholar]
- 44. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. doi: 10.1161/CIRCULATIONAHA.113.008507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mygind ND, Michelsen MM, Pena A, Frestad D, Dose N, Aziz A, Faber R, Høst N, Gustafsson I, Hansen PR, et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. J Am Heart Assoc. 2016;5:e003064. doi: 10.1161/JAHA.115.003064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panza JA, Laurienzo JM, Curiel RV, Unger EF, Quyyumi AA, Dilsizian V, Cannon RO III. Investigation of the mechanism of chest pain in patients with angiographically normal coronary arteries using transesophageal dobutamine stress echocardiography. J Am Coll Cardiol. 1997;29:293–301. doi: 10.1016/S0735-1097(96)00481-0 [DOI] [PubMed] [Google Scholar]
- 47. Pargaonkar VS, Kobayashi Y, Kimura T, Schnittger I, Chow EKH, Froelicher VF, Rogers IS, Lee DP, Fearon WF, Yeung AC, et al. Accuracy of non‐invasive stress testing in women and men with angina in the absence of obstructive coronary artery disease. Int J Cardiol. 2019;282:7–15. doi: 10.1016/j.ijcard.2018.10.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pargaonkar VS, Lee JH, Chow EKH, Nishi T, Ball RL, Kobayashi Y, Kimura T, Lee DP, Stefanick ML, Fearon WF, et al. Dose‐response relationship between intracoronary acetylcholine and minimal lumen diameter in coronary endothelial function testing of women and men with angina and no obstructive coronary artery disease. Circ Cardiovasc Interv. 2020;13:e008587. doi: 10.1161/CIRCINTERVENTIONS.119.008587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia. Results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quesada O, AlBadri A, Wei J, Shufelt C, Mehta PK, Maughan J, Suppogu N, Aldiwani H, Cook‐Wiens G, Nelson MD, et al. Design, methodology and baseline characteristics of the Women's Ischemia Syndrome Evaluation–Coronary Vascular Dysfunction (WISE‐CVD). Am Heart J. 2020;220:224–236. doi: 10.1016/j.ahj.2019.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, Scannell C, Clapp B, Marber M, Webb A, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation. 2019;140:1805–1816. doi: 10.1161/CIRCULATIONAHA.119.041595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, Walsh EG, Fuisz AR, Kerensky R, Detre KM, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469–1475. doi: 10.1016/S0735-1097(99)00072-8 [DOI] [PubMed] [Google Scholar]
- 53. Sade LE, Eroglu S, Bozbaş H, Özbiçer S, Hayran M, Haberal A, Müderrisoǧlu H. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009;204:580–585. doi: 10.1016/j.atherosclerosis.2008.09.038 [DOI] [PubMed] [Google Scholar]
- 54. Safdar B, D'Onofrio G, Dziura J, Russell RR, Johnson C, Sinusas AJ. Prevalence and characteristics of coronary microvascular dysfunction among chest pain patients in the emergency department. Eur Heart J Acute Cardiovasc Care. 2020;9:5–13. doi: 10.1177/2048872618764418 [DOI] [PubMed] [Google Scholar]
- 55. Sakamoto N, Iwaya S, Owada T, Nakamura Y, Yamauchi H, Hoshino Y, Mizukami H, Sugimoto K, Yamaki T, Kunii H, et al. A reduction of coronary flow reserve is associated with chronic kidney disease and long‐term cardio‐cerebrovascular events in patients with non‐obstructive coronary artery disease and vasospasm. Fukushima J Med Sci. 2012;58:136–143. doi: 10.5387/fms.58.136 [DOI] [PubMed] [Google Scholar]
- 56. Sara JD, Lennon RJ, Ackerman MJ, Friedman PA, Noseworthy PA, Lerman A. Coronary microvascular dysfunction is associated with baseline qtc prolongation amongst patients with chest pain and non‐obstructive coronary artery disease. J Electrocardiol. 2016;49:87–93. doi: 10.1016/j.jelectrocard.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 57. Sara JD, Taher R, Kolluri N, Vella A, Lerman LO, Lerman A. Coronary microvascular dysfunction is associated with poor glycemic control amongst female diabetics with chest pain and non‐obstructive coronary artery disease. Cardiovasc Diabetol. 2019;18:22. doi: 10.1186/s12933-019-0833-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schindler TH, Nitzsche EU, Schelbert HR, Olschewski M, Sayre J, Mix M, Brink I, Zhang X‐L, Kreissl M, Magosaki N, et al. Positron emission tomography‐measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol. 2005;45:1505–1512. doi: 10.1016/j.jacc.2005.01.040 [DOI] [PubMed] [Google Scholar]
- 59. Schroder J, Mygind ND, Frestad D, Michelsen M, Suhrs HE, Bove KB, Gustafsson I, Kastrup J, Prescott E. Pro‐inflammatory biomarkers in women with non‐obstructive angina pectoris and coronary microvascular dysfunction. Int J Cardiol Heart Vasc. 2019;24:100370. doi: 10.1016/j.ijcha.2019.100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schroder J, Zethner‐Moller R, Bové KB, Mygind ND, Hasbak P, Michelsen MM, Gustafsson I, Kastrup J, Prescott E. Protein biomarkers and coronary microvascular dilatation assessed by rubidium‐82 PET in women with angina pectoris and no obstructive coronary artery disease. Atherosclerosis. 2018;275:319–327. doi: 10.1016/j.atherosclerosis.2018.06.864 [DOI] [PubMed] [Google Scholar]
- 61. Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near‐normal coronary arteries. Am J Cardiol. 2009;103:626–631. doi: 10.1016/j.amjcard.2008.10.033 [DOI] [PubMed] [Google Scholar]
- 62. Solberg OG, Stavem K, Ragnarsson A, Beitnes JO, Skårdal R, Seljeflot I, Ueland T, Aukrust P, Gullestad L, Aaberge L. Index of microvascular resistance to assess the effect of rosuvastatin on microvascular function in women with chest pain and no obstructive coronary artery disease: a double‐blind randomized study. Catheter Cardiovasc Interv. 2019;94:660–668. doi: 10.1002/ccd.28157 [DOI] [PubMed] [Google Scholar]
- 63. Suda A, Takahashi J, Hao K, Kikuchi Y, Shindo T, Ikeda S, Sato K, Sugisawa J, Matsumoto Y, Miyata S, et al. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J Am Coll Cardiol. 2019;74:2350–2360. doi: 10.1016/j.jacc.2019.08.1056 [DOI] [PubMed] [Google Scholar]
- 64. Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. doi: 10.1093/eurheartj/ehx721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Uemura T, Yamamuro M, Kaikita K, Takashio S, Utsunomiya D, Hirakawa K, Nakayama M, Sakamoto K, Yamamoto E, Tsujita K, et al. Late gadolinium enhancement on cardiac magnetic resonance predicts coronary vasomotor abnormality and myocardial lactate production in patients with chronic heart failure. Heart Vessels. 2016;31:1969–1979. doi: 10.1007/s00380-016-0816-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Verna E, Ghiringhelli S, Provasoli S, Scotti S, Salerno‐Uriarte J. Epicardial and microvascular coronary vasomotor dysfunction and its relation to myocardial ischemic burden in patients with non‐obstructive coronary artery disease. J Nucl Cardiol. 2018;25:1760–1769. doi: 10.1007/s12350-017-0871-6 [DOI] [PubMed] [Google Scholar]
- 67. Konst RE, Meeder JG, Wittekoek ME, Maas A, Appelman Y, Piek JJ, van de Hoef TP, Damman P, Elias‐Smale SE. Ischaemia with no obstructive coronary arteries. Neth Heart J. 2020;28:66–72. doi: 10.1007/s12471-020-01451-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Camma G, Lanza GA, Crea F. Patients with acute myocardial infarction and non‐obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91–98. doi: 10.1093/eurheartj/ehx667 [DOI] [PubMed] [Google Scholar]
- 69. Pries AR, Reglin B. Coronary microcirculatory pathophysiology: can we afford it to remain a black box? Eur Heart J. 2017;38:478–488. doi: 10.1093/eurheartj/ehv760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gdowski MA, Murthy VL, Doering M, Monroy‐Gonzalez AG, Slart R, Brown DL. Association of isolated coronary microvascular dysfunction with mortality and major adverse cardiac events: a systematic review and meta‐analysis of aggregate data. J Am Heart Assoc. 2020;9:e014954. doi: 10.1161/JAHA.119.014954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Olson MB, Kelsey SF, Matthews K, Shaw LJ, Sharaf BL, Pohost GM, Cornell CE, McGorray SP, Vido D, Bairey Merz CN. Symptoms, myocardial ischaemia and quality of life in women: results from the NHLBI‐sponsored WISE study. Eur Heart J. 2003;24:1506–1514. doi: 10.1016/S0195-668X(03)00279-3 [DOI] [PubMed] [Google Scholar]
- 72. Rutledge T, Vaccarino V, Johnson BD, Bittner V, Olson MB, Linke SE, Cornell CE, Eteiba W, Sheps DS, Francis J, et al. Depression and cardiovascular health care costs among women with suspected myocardial ischemia: prospective results from the WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2009;53:176–183. doi: 10.1016/j.jacc.2008.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anderson RD, Petersen JW, Mehta PK, Wei J, Johnson BD, Handberg EM, Kar S, Samuels B, Azarbal B, Kothawade K, et al. Prevalence of coronary endothelial and microvascular dysfunction in women with symptoms of ischemia and no obstructive coronary artery disease is confirmed by a new cohort: the NHLBI‐sponsored Women's Ischemia Syndrome Evaluation‐Coronary Vascular Dysfunction (WISE‐CVD). J Interv Cardiol. 2019;2019:7169275. doi: 10.1155/2019/7169275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Corban MT, Prasad A, Gulati R, Lerman LO, Lerman A. Sex‐specific differences in coronary blood flow and flow velocity reserve in symptomatic patients with non‐obstructive disease. EuroIntervention. 2019;16:1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sara JDS, Corban MT, Prasad M, Prasad A, Gulati R, Lerman LO, Lerman A. Prevalence of myocardial bridging associated with coronary endothelial dysfunction in patients with chest pain and non‐obstructive coronary artery disease. EuroIntervention. 2020;15:1262–1268. doi: 10.4244/EIJ-D-18-00920 [DOI] [PubMed] [Google Scholar]
- 76. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, Prescott E, Karam N, Appelman Y, Fraccaro C, et al. An EAPCI expert consensus document on ischaemia with non‐obstructive coronary arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504–3520. doi: 10.1093/eurheartj/ehaa503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, Prescott E, Karam N, Appelman Y, Fraccaro C, et al. An EAPCI expert consensus document on ischaemia with non‐obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention. 2021;16:1049–1069. doi: 10.4244/EIJY20M07_01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Djaïleb L, Riou L, Piliero N, Carabelli A, Vautrin E, Broisat A, Leenhardt J, Machecourt J, Fagret D, Vanzetto G, et al. spect myocardial ischemia in the absence of obstructive CAD: contribution of the invasive assessment of microvascular dysfunction. J Nucl Cardiol. 2018;25:1017–1022. doi: 10.1007/s12350-017-1135-1 [DOI] [PubMed] [Google Scholar]
- 79. Zimarino M, Marano R, Radico F, Curione D, De Caterina R. Coronary computed tomography angiography, ECG stress test and nuclear imaging as sources of false‐positive results in the detection of coronary artery disease. J Cardiovasc Med (Hagerstown). 2018;19(suppl 1):e133–e138. doi: 10.2459/JCM.0000000000000591 [DOI] [PubMed] [Google Scholar]
- 80. Gould KL, Johnson NP. Coronary physiology beyond coronary flow reserve in microvascular angina: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2018;72:2642–2662. doi: 10.1016/j.jacc.2018.07.106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S5


