Abstract
Background
Right ventricular outflow tract (RVOT) stenosis after repair of tetralogy of Fallot has been linked with favorable right ventricular remodeling but adverse outcomes. The aim of our study was to assess the hemodynamic impact and prognostic relevance of right ventricular pressure load in this population.
Methods and Results
A total of 296 patients with repaired tetralogy of Fallot (mean age, 17.8±7.9 years) were included in a prospective cardiovascular magnetic resonance multicenter study. Myocardial strain was quantified by feature tracking technique at study entry. Follow‐up, including the need for pulmonary valve replacement, was assessed. The combined end point consisted of ventricular tachycardia and cardiac death. A higher echocardiographic RVOT peak gradient was significantly associated with smaller right ventricular volumes and less pulmonary regurgitation, but lower biventricular longitudinal strain. During a follow‐up of 10.1 (0.1–12.9) years, the primary end point was reached in 19 of 296 patients (cardiac death, n=6; sustained ventricular tachycardia, n=2; and nonsustained ventricular tachycardia, n=11). A higher RVOT gradient was associated with the combined outcome (hazard ratio [HR], 1.03; 95% CI, 1.00–1.06; P=0.026), and a cutoff gradient of ≥25 mm Hg was predictive for cardiovascular events (HR, 3.69; 95% CI, 1.47–9.27; P=0.005). In patients with pulmonary regurgitation ≥25%, a mild residual RVOT gradient (15–30 mm Hg) was not associated with a lower risk for pulmonary valve replacement.
Conclusions
Higher RVOT gradients were associated with less pulmonary regurgitation and smaller right ventricular dimensions but were related to reduced biventricular strain and emerged as univariate predictors of adverse events. Mild residual pressure gradients did not protect from pulmonary valve replacement. These results may have implications for the indication for RVOT reintervention in this population.
Keywords: magnetic resonance imaging, prognosis, right ventricular pressure overload, strain, tetralogy of Fallot
Subject Categories: Magnetic Resonance Imaging (MRI), Prognosis, Mortality/Survival, Congenital Heart Disease, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- PR
pulmonary regurgitation
- TOF
tetralogy of Fallot
Clinical Perspective
What Is New?
In patients after repair of tetralogy of Fallot, higher right ventricular outflow tract gradients were related to reduced biventricular strain and emerged as univariate predictors of adverse events.
Mild residual pressure gradients did not reduce the risk for later pulmonary valve replacement.
What Are the Clinical Implications?
Our findings may affect the timing and indication of pulmonary valve replacement procedures in patients after repair of tetralogy of Fallot.
Prospective studies will need to determine whether pulmonary valve replacement performed at lower levels of right ventricular pressure load will be associated with favorable outcomes.
Following surgical repair of tetralogy of Fallot (TOF), pulmonary regurgitation (PR) frequently emerges as the predominant residual lesion causing progressive right ventricular (RV) enlargement, functional impairment, and, potentially, sudden cardiac death. 1 Surgical management of patients after TOF repair has therefore shifted toward a pulmonary valve‐sparing repair technique with the aim of preserving RV integrity. 2 Subsequently, some degree of residual pulmonary stenosis is typically accepted after repair and has been shown to have beneficial effects on the described adverse RV remodeling process by reducing PR and RV enlargement without compromising RV function. 3 , 4 , 5 Furthermore, mild residual RV outflow tract (RVOT) gradients may also postpone the need for pulmonary valve replacement (PVR). 6 , 7 In contrast, recent data from several studies demonstrated elevated RV pressure as an independent predictor for adverse outcome. 8 , 9 , 10 These apparently paradoxical findings suggest that the effect of RV pressure load on biventricular remodeling in patients with repaired TOF is not yet sufficiently characterized and that the underlying pathophysiological mechanisms that link increased RV pressures with unfavorable outcomes are unclear. Accordingly, the aim of this study was to assess the hemodynamic impact and prognostic relevance of RV pressure load in a larger cohort of patients with repaired TOF.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
Patients with repaired TOF were enrolled in a prospective nationwide study by the German Competence Network for Congenital Heart Defects between 2003 and 2009 (Follow Up of Post‐Repair Tetralogy of Fallot; https://www.clinicaltrials.gov/; unique identifier, NCT00266188). This multicenter study included patients with repaired TOF, aged >8 years, from 14 German sites undergoing a standardized protocol, including the collection of demographic and clinical data (including echocardiography) and the assessment of cardiopulmonary exercise testing and cardiovascular magnetic resonance (CMR) imaging, as published. 11 , 12
For the current study, patients with cardiopulmonary exercise testing data (peak oxygen uptake and percentage predicted and peak heart rate), echocardiographic assessment of the peak systolic RVOT gradient, and a comprehensive initial CMR examination (including biventricular volumetry and quantification of pulmonary regurgitation fraction using phase‐contrast flow measurements) were considered for further analysis. These examinations were performed within a close time interval at study entrance, and the patients were then prospectively followed up until July 2018. Data on initial postrepair RVOT gradients as well as longitudinal hemodynamic changes were not assessed.
Appropriate 4‐chamber and midventricular short‐axis cine loops of study entry CMR examinations were extracted from the central database of the Competence Network for Congenital Heart Defects. CMR feature tracking analysis (TomTec Imaging Systems, Unterschleissheim, Germany) of both ventricles was performed to assess peak global longitudinal, circumferential, and radial strain values, the early diastolic strain rate, and interventricular synchrony, as described previously. 13
Information on follow‐up data, including the occurrence of predefined adverse events and PVR procedures (both surgical and transcatheter valve implantation), was available through the infrastructure of the Competence Network for Congenital Heart Defects as all patients were also separately enrolled in the national registry database for congenital heart defects. These data include survival information as well as up‐to‐date medical reports obtained regularly from the physicians treating the patients. The study was approved by all ethics committees of the participating centers. Informed written consent was obtained from all patients or their caretakers.
Statistical Analysis
All continuous variables were tested for normality using the D'Agostino‐Pearson omnibus test and are presented as a mean with SD or median and range. Comparisons between the patient groups with versus without adverse events were made with the Student t test, the Mann‐Whitney U test, or the Fisher exact test, as appropriate. The Pearson correlation coefficient was used to analyze simple linear relationships between different variables. Outcome analyses were based on freedom from PVR and the primary composite end point, including cardiac death, successful resuscitation, and documented episodes of ventricular tachycardia (either sustained or nonsustained) that occurred during the follow‐up time from the CMR study. The relationship between demographic, clinical, and CMR variables and outcomes was investigated by univariable and bivariable (including the peak RVOT gradient and one other significant parameter on univariable testing) Cox proportional‐hazard analysis (hazard ratio [HR] with 95% CI). To further assess the prognostic impact of residual RV pressure load, the study population was divided into 2 groups according to a cutoff RVOT gradient of <25 and ≥25 mm Hg. This threshold emerged from previous studies that assessed the relevance of residual RVOT stenosis in patients after repair of TOF. 4 , 5 , 13 The log‐rank (Mantel‐Cox) test was performed to compare the risk for PVR in subgroups with different degrees of RV pressure and/or volume overload. Analysis was performed using GraphPad (San Diego, CA) and SPSS (version 25.0; IBM Inc, Armonk, NY) statistical software packages. For all analyses, a 2‐tailed P<0.05 was used as the criterion for statistical significance.
Results
Patient Characteristics and CMR Data
From the total population of 407 patients with repaired TOF enrolled in the multicenter study, 337 had a suitable data set for the intended analysis that included echocardiographic peak RVOT gradient, a comprehensive CMR analysis, and a completed cardiopulmonary exercise testing examination (Figure 1). A comparison between the analysis sample and those patients excluded is displayed in Table S1.
Figure 1. Graph displaying the study flow with the total number of 407 patients after repair of tetrology of Fallot (TOF) who were originally included in the national TOF multicenter cardiovascular magnetic resonance (CMR) study. A total of 70 patients were excluded because of an incomplete data set.
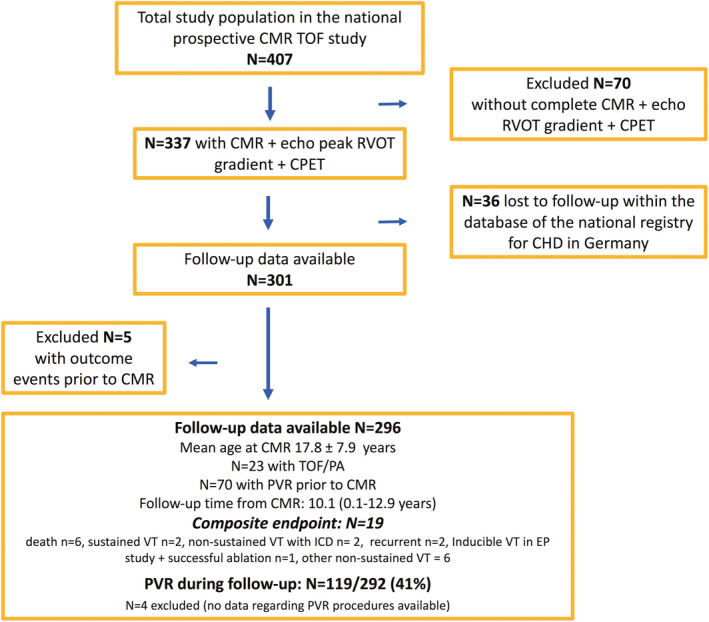
Of the 337 patients, another 36 had to be excluded as no follow‐up data were available within the database of the national registry for congenital heart disease (CHD). Finally, of 296 patients with a median follow‐up time of 10.1 (range, 0.1–12.9) years, 19 experienced adverse events. Note that 5 patients were excluded in the outcome analysis as these patients exhibited adverse events before the CMR study. Pulmonary valve replacement (PVR) procedures were performed in 119 of 292 patients during the follow‐up. CPET indicates cardiopulmonary exercise testing; echo, echocardiographic; EP, electrophysiologic study; ICD, implantable cardioverter‐defibrillator; N, number of patients; RVOT, right ventricular outflow tract; TOF/PA, pulmonary atresia with ventricular septal defect (Fallot type); and VT, ventricular tachycardia.
In 301 of these 337 patients (89%), longitudinal follow‐up data were available (in 36 patients, no follow‐up data could be obtained within the national database of the registry for congenital heart disease in Germany). For the outcome analysis, another 5 patients were excluded as these patients already exhibited one of the predefined end points (nonsustained ventricular tachycardia [VT] in 3 patients and sustained VT in 2 patients) and were subsequently treated with specific antiarrhythmic drug therapy and/or received PVR. Finally, 296 patients who had a CMR study at a mean age of 17.8±7.9 years (range, 7.0–58.0 years) were included in the outcome analysis. The mean peak echocardiographic RVOT gradient was 20.7±14.9 mm Hg (median, 16 mm Hg; range, 2–83 mm Hg). For details, please see Table 1.
Table 1.
Study Population With Initial Demographic, Clinical, and CMR Imaging Data
| Parameter | All patients (N=296) |
Patients without event (N=277) |
Patients with event (N=19) |
|---|---|---|---|
| Demographics | |||
| Diagnosis, n (%) | |||
| Data available | 283 (96) | 265 (96) | 18 (95) |
| TOF/pulmonary stenosis | 260 (92) | 243 (92) | 17 (94) |
| TOF/PA | 23 (8) | 22 (8) | 1 (6) |
| Age at CMR, y | 16.0 (7.0–58.0) | 16.0 (7.0–58.0) | 16.0 (8.0–44.0) |
| Age at corrective surgery, y | 1.0 (0.1–28.0) | 1.0 (0.1–28.0) | 1.0 (0.1–19.0) |
| Type of repair, n (%) | |||
| Data available | 258 (87) | 240 (87) | 18 (95) |
| Without TAP | 143 (55) | 131 (55) | 12 (67) |
| TAP | 62 (24) | 58 (24) | 4 (22) |
| TAP and pulmonary artery plasty | 53 (21) | 51 (21) | 2 (11) |
| Time from repair, y | 14 (6–48) | 14 (6–48) | 15 (8–35) |
| Follow‐up, y | 10.1 (0.1–12.9) | 10.1 (0.1–12.9) | 10.1 (0.6–12.9) |
| PVR during follow‐up, n/total (%) | 119/292 (41) | 105/274 (38) | 14/18 (78) |
| Clinical status and history | |||
| Initial palliation, n/total (%) | 67/282 (24) | 60/264 (24) | 7/18 (39) |
| Previous PVR, n/total (%) | 70/296 (24) | 62/277 (22) | 8/19 (42) |
| NYHA>class I, n/total (%) | 96/296 (32) | 86/277 (31) | 10/19 (52) |
| 12‐Lead ECG: QRS duration, ms | 147±22 | 147±22 | 146±26 |
| Cardiopulmonary exercise test | |||
| Peak VO2 at VAT, mL/min per kg | 23.8±7.5 | 24.0±7.4 | 21.6±9.0 |
| Peak VO2, mL/min per kg | 31.1±8.4 | 31.3±8.2 | 26.4±10.7 |
| Peak heart rate, /min | 172 (79–204) | 172 (79–204) | 170 (89–197) |
| Echocardiography | |||
| Peak RVOT gradient, mm Hg | 20.7±14.9 | 20.0±14.3 | 29.9±19.3 |
| Tricuspid valve regurgitation>moderate, n/total (%) | 22/211 (10) | 19/195 (10) | 3/16 (19) |
| CMR study | |||
| RVEDVi, mL/m2 | 116 (67–242) | 115 (67–242) | 119 (84–224) |
| RVESVi, mL/m2 | 57 (20–186) | 56 (20–161) | 66 (26–186) |
| RVSVI, mL/m2 | 60±16 | 60±16 | 60±13 |
| RVEF, % | 51±9 | 51±9 | 47±12 |
| PR, % | 27 (0–65) | 27 (0–65) | 28 (1–54) |
| RV mass, g/m2 | 33 (10–103) | 33 (10–103) | 35 (24–71) |
| RV mass/volume ratio | 0.29 (0.08–0.74) | 0.22 (0.08–0.74) | 0.30 (0.16–0.66) |
| LVEDVi, mL/m2 | 82 (43–195) | 81 (43–126) | 87 (60–195) |
| LVESVi, mL/m2 | 34 (17–166) | 34 (17–91) | 37 (22–166) |
| LVSVI, mL/m2 | 47 (23–87) | 47 (23–87) | 47 (28–68) |
| LVEF, % | 58 (15–74) | 58 (20–74) | 56 (15–69) |
| LV mass, g/m2 | 54 (30–127) | 54 (30–109) | 55 (33–127) |
| LV mass/volume ratio | 0.69±0.18 | 0.69±0.18 | 0.69±0.20 |
| Feature tracking analysis | |||
| RV‐LS, % | −12.9±4.6 | −13.1±4.5 | −9.9±5.0 |
| RV‐CS, % | −15.2±4.1 | −15.4±4.0 | −13.3±5.1 |
| LV‐LS, % | −13.8±5.0 | −14.0±4.9 | −12.1±6.5 |
| LV‐CS, % | −20.7±4.8 | −21.0±4.3 | −16.9±7.8 |
| LV‐RS, % | 25.3±8.8 | 25.6±8.5 | 21.2±11.4 |
| RV EDSR LS, 1/s | 0.67 (0.22–3.76) | 0.92 (0.22–3.76) | 0.96 (0.49–2.37) |
| RV EDSR CS, 1/s | 0.93 (0.29–2.71) | 0.93 (0.29–2.71) | 0.90 (0.45–1.43) |
| LV EDSR LS, 1/s | 1.08 (0.25–4.03) | 1.07 (0.25–4.03) | 1.22 (0.28–1.81) |
| LV EDSR CS, 1/s | 1.49 (0.41–2.76) | 1.52 (0.46–2.73) | 1.34 (0.41–2.76) |
| Interventricular dyssynchrony LS, ms | 44 (0–607) | 42 (0–607) | 26 (0–235) |
| Interventricular dyssynchrony CS, ms | 46 (0–159) | 46 (0–159) | 48 (0–124) |
Data are displayed as mean with 1 SD or as median (range). CMR indicates cardiovascular magnetic resonance; CS, circumferential strain; EDSR, early diastolic strain rate; LS, longitudinal strain; LV, left ventricular; LVEDVi, indexed left ventricular end‐diastolic volume; LVEF, LV ejection fraction; LVESVi, indexed left ventricular end‐systolic volume; LVSVi, indexed left ventricular stroke volume; NYHA, New York Heart Association; PA, pulmonary atresia; PR, pulmonary regurgitation; PVR, pulmonary valve replacement; RS, radial strain; RV, right ventricular; RVEDVi, indexed right ventricular end‐diastolic volume; RVESVi, indexed right ventricular end‐systolic volume; RVSVi, indexed right ventricular stroke volume; RVEF, RV ejection fraction; RVOT, RV outflow tract; TAP, transannular patch; TOF, tetralogy of Fallot; VAT, ventilator anaerobic threshold; and VO2, oxygen uptake.
Correlations
Peak RVOT gradient was significantly associated with smaller RV volumes (r=−0.16; P=0.004) and less PR (r=−0.12; P=0.026), but lower RV (r=−0.23; P=0.0004) and left ventricular (LV) longitudinal systolic strain (r=−0.15; P=0.016), and lower early diastolic strain rate (r=−0.17 [P=0.01] and r=−0.22 [P=0.0006]; Figure 2). No significant relationships were found between RVOT gradient and RV ejection fraction (RVEF), RV mass, and RV mass/volume ratio. LV dimensions and LV ejection fraction were not related to the RVOT gradient. Although not of statistical significance, a trend toward reduced exercise capacity (both peak oxygen uptake and oxygen uptake at ventilator anaerobic threshold) was observed with increasing RVOT gradients.
Figure 2. Graph displaying several linear correlations between the echocardiographic (echo) peak right ventricular outflow tract (RVOT) gradient and cardiovascular magnetic resonance measures of biventricular dimensions and function as well as results from cardiopulmonary exercise testing.
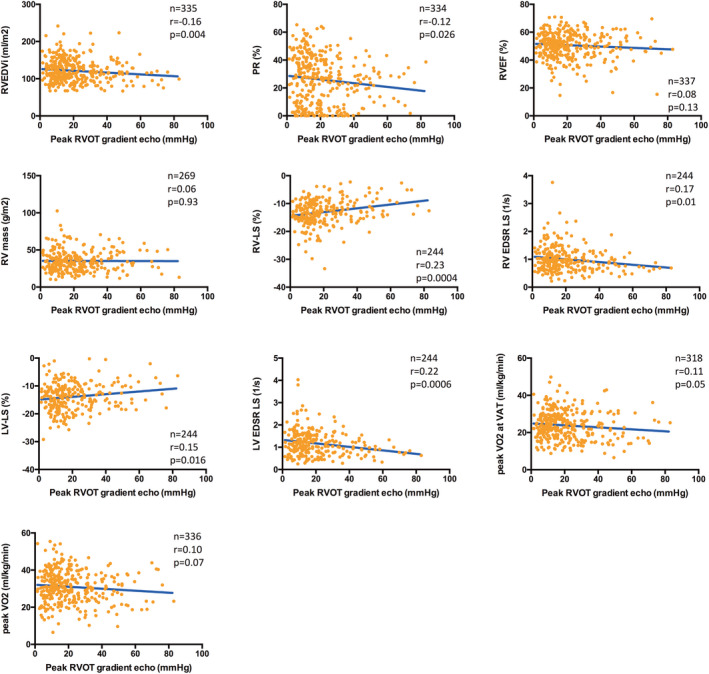
Higher RVOT gradients were significantly associated with smaller right ventricular end‐diastolic volumes (RVEDVI) and less pulmonary regurgitation (PR) but were also related with reduced global right ventricular (RV) and left ventricular (LV) longitudinal systolic (strain) and diastolic (strain rate) deformation. No significant correlation was observed between peak RVOT gradient and exercise capacity (peak oxygen uptake [VO2] and percentage predicted). EDSR indicates early diastolic strain rate; EF, ejection fraction; LS, longitudinal strain; and VAT, ventilator anaerobic threshold.
Prognostic Relevance of RVOT Gradient
During a median follow‐up of 10.1 (range, 0.1–12.9) years, the primary end point was reached in 19 of 296 patients, occurring at a median time of 10.1 (range, 0.6–12.9) years after the CMR study (cardiac death, n=6; sustained VT, n=2; and nonsustained VT, n=11). Using univariable Cox‐regression analysis, a higher RVOT gradient was significantly associated with the combined outcome (HR, 1.03; 95% CI, 1.01–1.06; P=0.006). Other significant predictors were New York Heart Association functional class >1, peak oxygen uptake, PVR during follow‐up, right ventricular end‐systolic volume (RVESVi), left ventricular end‐diastolic volume (LVEDVi), left ventricular end‐systolic volume (LVESVi), RV longitudinal strain, RV circumferential strain, LV circumferential strain, and LV radial strain (Table 2).
Table 2.
Univariable Predictors of the Combined Study End Point of Cardiac Death, Successful Resuscitation, and Sustained or Nonsustained VT
| Parameter | HR | 95% CI | P value |
|---|---|---|---|
| Diagnosis (TOF/PA vs TOF) | 1.10 | 0.14–8.40 | 0.93 |
| Age at CMR (y) | 1.04 | 0.99–1.08 | 0.11 |
| Age at corrective surgery (y) | 1.05 | 0.96–1.15 | 0.27 |
| Type of repair (TAP vs no TAP) | 0.63 | 0.23–1.71 | 0.37 |
| Time from repair (y) | 1.05 | 0.98–1.11 | 0.15 |
| Initial palliation (yes/no) | 2.22 | 0.74–6.63 | 0.15 |
| Previous PVR (yes/no) | 2.37 | 0.94–5.93 | 0.07 |
| PVR during follow‐up (yes/no) | 3.57 | 1.15–11.1 | 0.028* |
| NYHA class >I (yes/no) | 3.42 | 1.34–8.73 | 0.01* |
| QRS (ms) | 0.99 | 0.97–1.01 | 0.34 |
| Peak VO2 at VAT, mL/min per kg | 0.95 | 0.88–1.01 | 0.10 |
| Peak VO2, mL/min per kg | 0.92 | 0.88–0.97 | 0.004* |
| Peak heart rate (/min) | 0.98 | 0.97–1.00 | 0.08 |
| RVOT gradient, mm Hg | 1.03 | 1.01–1.06 | 0.006* |
| Tricuspid valve regurgitation > moderate (yes/no) | 1.19 | 0.32–4.41 | 0.79 |
| RVEDVi, mL/m2 | 1.01 | 1.00–1.03 | 0.10 |
| RVESVi, mL/m2 | 1.02 | 1.00–1.04 | 0.02* |
| RVSVI, mL/m2 | 1.00 | 0.97–1.03 | 0.88 |
| RVEF, % | 0.96 | 0.92–1.01 | 0.13 |
| PR, % | 1.00 | 0.97–1.03 | 0.97 |
| RV mass, g/m2 | 1.01 | 0.98–1.04 | 0.56 |
| RV mass/volume ratio | 1.21 | 0.02–66.52 | 0.93 |
| LVEDVi (mL/m2) | 1.04 | 1.01–1.06 | 0.001* |
| LVESVi, mL/m2 | 1.03 | 1.02–1.05 | <0.001* |
| LVSVI, mL/m2 | 1.00 | 0.95–1.06 | 0.91 |
| LVEF, % | 0.96 | 0.91–1.00 | 0.07 |
| LV mass, g/m2 | 1.01 | 0.98–1.04 | 0.49 |
| LV mass/volume ratio | 0.47 | 0.03–9.16 | 0.62 |
| RV‐LS, % | 1.22 | 1.07–1.39 | 0.004* |
| RV‐CS, % | 1.15 | 1.01–1.31 | 0.03* |
| LV‐LS, % | 1.07 | 0.96–1.19 | 0.24 |
| LV‐CS, % | 1.16 | 1.06–1.27 | 0.002* |
| LV‐RS, % | 0.93 | 0.87–1.00 | 0.04* |
| RV EDSR LS (1/s) | 0.78 | 0.20–3.00 | 0.71 |
| RV EDSR CS (1/s) | 0.26 | 0.05–1.47 | 0.13 |
| LV EDSR LS (1/s) | 0.93 | 0.37–2.34 | 0.88 |
| LV EDSR CS (1/s) | 0.53 | 0.17–1.69 | 0.28 |
| Interventricular dyssynchrony LS, ms | 1.01 | 1.00–1.01 | 0.16 |
| Interventricular dyssynchrony CS, ms | 0.99 | 0.97–1.01 | 0.25 |
CMR indicates cardiovascular magnetic resonance; CS, circumferential strain; EDSR, early diastolic strain rate; HR, hazard ratio; LS, longitudinal strain; LV, left ventricular; LVEDVi, indexed left ventricular end‐diastolic volume; LVEF, LV ejection fraction; LVESVi, indexed left ventricular end‐systolic volume; LVSVi, indexed left ventricular stroke volume; NYHA, New York Heart Association; PA, pulmonary atresia; PR, pulmonary regurgitation; PVR, pulmonary valve replacement; RS, radial strain; RV, right ventricular; RVEDVi, indexed right ventricular end‐diastolic volume; RVESVi, indexed right ventricular end‐systolic volume; RVSVi, indexed right ventricular stroke volume; RVEF, RV ejection fraction; RVOT, RV outflow tract; TAP, transannular patch; TOF, tetralogy of Fallot; VAT, ventilator anaerobic threshold; VO2, oxygen uptake; and VT, ventricular tachycardia.
indicates statistical significance (P<0.05).
A peak RVOT gradient of ≥25 mm Hg was associated with a >3‐fold increase in adverse cardiovascular events (HR, 3.69; 95% CI, 1.47–9.27; P=0.005) (Figure 3).
Figure 3. Diagram showing freedom from the composite end point (cardiac death and sustained and nonsustained ventricular tachycardia) during follow‐up from the cardiovascular magnetic resonance (CMR) study using Kaplan‐Meier curves according to the peak right ventricular outflow tract (RVOT) gradient of ≥25 and <25 mm Hg.
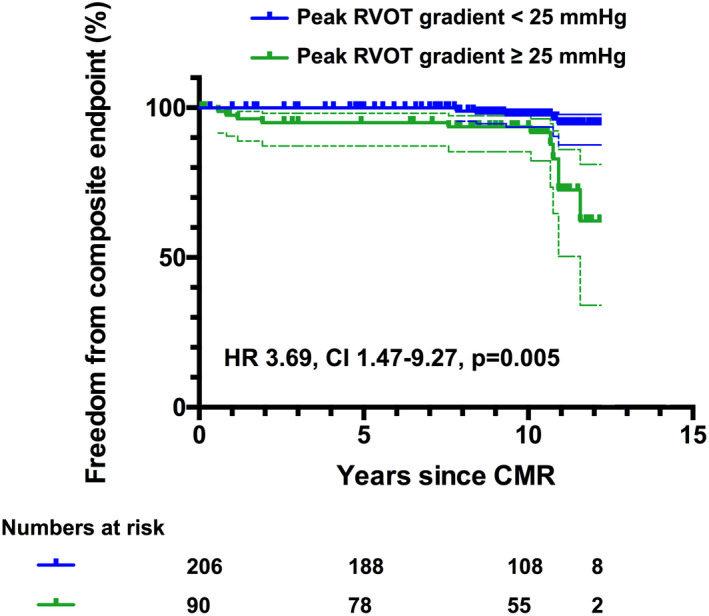
Using Cox proportional hazard analysis, a peak RVOT gradient ≥25 mm Hg was associated with a >3‐fold increased risk for adverse cardiovascular events (hazard ratio [HR], 3.69; 95% CI, 1.47–9.27; P=0.005).
A subgroup analysis according to a peak RVOT gradient of <25 and ≥25 mm Hg and the degree of PR (<25% and ≥25%) is displayed in Figure S1. Patients with moderate PR and a peak RVOT gradient ≥25 mm Hg demonstrated significantly more adverse events than patients with moderate PR and a peak RVOT gradient <25 mm Hg (P<0.001). In patients with severe PR ≥25%, RVOT gradients >25 or <25 mm Hg led to no significant difference (P=0.38).
Instead of multivariable testing that may not yield reliable results given the limited number of adverse events (“overfitting of the model”), bivariable testing was performed to elucidate the relationship between the peak RVOT and other parameters found to be significant on univariate analysis (Table 3). All tested parameters remained independent predictors, except of the paired testing with RV longitudinal strain, where the RVOT gradient reached a level of significance of P=0.05.
Table 3.
Cox Proportional Hazard Analysis Using a Bivariable Model of the Parameters That Reached a Significant Level on Univariable Testing to Identify Their Prognostic Relevance in Conjunction With the Peak RVOT Gradient
| Parameter | HR | 95% CI | P value |
|---|---|---|---|
| RVOT gradient, mm Hg | 1.03 | 1.00–1.05 | 0.025* |
| Peak VO2 at VAT, mL/min per kg | 0.93 | 0.88–0.98 | 0.01* |
| RVOT gradient, mm Hg | 1.03 | 1.00–1.06 | 0.016* |
| LVEDVi, mL/m2 | 1.03 | 1.01–1.05 | 0.003* |
| RVOT gradient, mm Hg | 1.04 | 1.00–1.06 | 0.007* |
| LVESVi, mL/m2 | 1.03 | 1.02–1.05 | <0.001* |
| RVOT gradient, mm Hg | 1.03 | 1.00–1.06 | 0.05 |
| RV‐LS, % | 1.17 | 1.02–1.35 | 0.023* |
| RVOT gradient, mm Hg | 1.03 | 1.00–1.06 | 0.045* |
| LV‐CS (%) | 1.14 | 1.04–1.24 | 0.006* |
CS indicates circumferential strain; HR, hazard ratio; LS, longitudinal strain; LV, left ventricular; LVEDVi, left ventricular end‐diastolic volume; LVESVi, left ventricular end‐systolic volume; RV, right ventricular; RVOT, RV outflow tract; VAT, ventilator anaerobic threshold; and VO2, oxygen uptake.
indicates statistical significance (P<0.05).
A second univariable Cox proportional‐hazard analysis was performed that only considered death and sustained VT as adverse events (N=8) (Table S2). Peak RVOT gradient remained a significant predictor for adverse events (HR, 1.04; 95% CI, 1.00–1.07; P=0.046). Comparison between patients with <25 and ≥25 mm Hg RVOT gradient revealed a significant and much higher risk for patients with higher gradients (HR, 17.9; 95% CI, 2.19–146.2; P=0.007).
Role of Peak RVOT Gradient in the Need for PVR
Data on PVR procedures during follow‐up were available in 292 of the 296 patients (definite information on PVR was not available in 4 patients). PVR was performed in 119 patients (41%) at a median of 3.0 (range, 0.1–12.3) years after the CMR examination (median follow‐up in the entire population, 8.3 years; range, 0.1–12.3 years).
Using univariable Cox‐regression analysis, a higher RVOT gradient was significantly associated with the need for PVR (HR, 1.02; 95% CI, 1.01–1.03; P=0.002). Other significant factors predictive for PVR were initial palliation, poorer New York Heart Association functional class, increased RV volumes and RV mass, PR severity, as well as lower RVEF and LV ejection fraction (Table 3).
To assess the impact of a mild residual RVOT stenosis (peak RVOT gradient 15–30 mm Hg) on the need for PVR, this subgroup was compared with patients with no relevant gradient (<15 mm Hg) and a group of patients with moderate or severe RVOT gradient (>30 mm Hg) (Figure 4 and Table S3). The groups were further separated into patients with <25% PR and a group with ≥25% PR. In patients with ≥25% PR, the need for PVR was similar between the different RVOT stenosis subgroups. Patients with <25% PR and RVOT gradients <30 mm Hg had the lowest risk for PVR (Table 4).
Figure 4. Graph displaying freedom from pulmonary valve replacement (PVR) procedures during follow‐up using Kaplan‐Meier curves (displayed with 95% CIs).
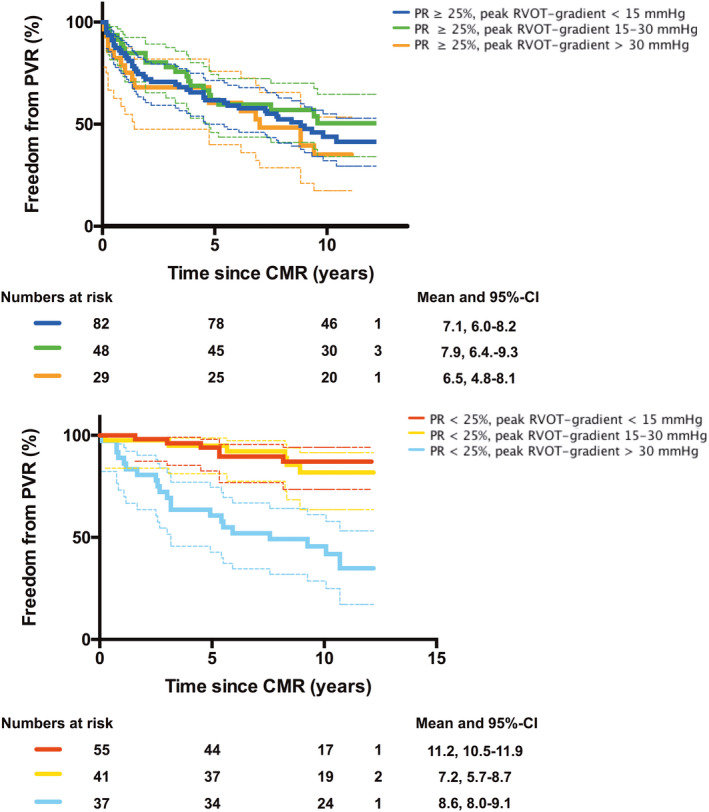
The study population was divided into different subgroups according to the peak right ventricular outflow tract (RVOT) gradient <15, 15 to 30, and >30 mm Hg and the severity of pulmonary regurgitation (PR; <25% and ≥25%). Statistical comparisons between the subgroups were made using the log‐rank (Mantel‐Cox) test (P values are displayed in Table S3). Note that in patients with PR ≥25% (top panel), no significant difference in the need for PVR surgery was observed between the subgroups, suggesting that mild residual RVOT stenosis seems not to protect from PVR. In patients with PR <25% (bottom panel), the subgroup with a peak RVOT gradient >30 mm Hg had a significant higher risk for PVR during follow‐up than those with a gradient <15 and 15 to 30 mm Hg (P<0.001, respectively). CMR indicates cardiovascular magnetic resonance.
Table 4.
Univariable Predictors of the Need for PVR (N=119/292 Patients) During Follow‐Up
| Parameter | HR | 95% CI | P value |
|---|---|---|---|
| Diagnosis (TOF/PA vs TOF) | 0.75 | 0.40–1.39 | 0.36 |
| Age at CMR (y) | 0.98 | 0.96–1.01 | 0.17 |
| Age at corrective surgery (y) | 0.96 | 0.91–1.02 | 0.16 |
| Type of repair (TAP vs no TAP) | 0.61 | 0.41–0.89 | 0.01* |
| Time from repair (y) | 0.99 | 0.96–1.02 | 0.40 |
| Initial palliation (yes/no) | 1.58 | 1.03–2.43 | 0.04* |
| Previous PVR (yes/no) | 1.09 | 0.72–1.66 | 0.69 |
| NYHA class >I (yes/no) | 1.74 | 1.20–2.51 | 0.003* |
| QRS (ms) | 1.01 | 1.00–1.02 | 0.01* |
| Peak VO2 at VAT, mL/min per kg | 0.98 | 0.96–1.01 | 0.18 |
| Peak VO2, mL/min per kg | 0.98 | 0.96–1.00 | 0.08 |
| Peak heart rate (/min) | 0.99 | 0.98–1.00 | 0.02* |
| RVOT gradient, mm Hg | 1.02 | 1.01–1.03 | 0.002* |
| Tricuspid valve regurgitation>moderate (yes/no) | 1.31 | 0.70–2.46 | 0.41 |
| RVEDVi, mL/m2 | 1.01 | 1.00–1.03 | <0.001* |
| RVESVi, mL/m2 | 1.03 | 1.02–1.03 | <0.001* |
| RVSVI, mL/m2 | 1.02 | 1.01–1.04 | <0.001* |
| RVEF, % | 0.96 | 0.95–0.98 | <0.001* |
| PR, % | 1.04 | 1.03–1.05 | <0.001* |
| RV mass, g/m2 | 1.02 | 1.01–1.04 | <0.001* |
| RV mass/volume ratio | 0.46 | 0.08–2.79 | 0.40 |
| LVEDVi, mL/m2 | 1.00 | 0.99–1.01 | 0.86 |
| LVESVi, mL/m2 | 1.01 | 1.00–1.02 | 0.09 |
| LVSVI, mL/m2 | 0.98 | 0.96–1.00 | 0.06 |
| LVEF, % | 0.98 | 0.96–1.00 | 0.02* |
| LV mass, g/m2 | 1.00 | 0.98–1.01 | 0.68 |
| LV mass/volume ratio | 0.70 | 0.22–2.17 | 0.53 |
CMR indicates cardiovascular magnetic resonance; HR, hazard ratio; LV, left ventricular; LVEDVi, indexed left ventricular end‐diastolic volume; LVEF, LV ejection fraction; LVESVi, indexed left ventricular end‐systolic volume; LVSVi, indexed left ventricular stroke volume; NYHA, New York Heart Association; PA, pulmonary atresia; PR, pulmonary regurgitation; PVR, pulmonary valve replacement; RV, right ventricular; RVEDVi, indexed right ventricular end‐diastolic volume; RVESVi, indexed right ventricular end‐systolic volume; RVSVi, indexed right ventricular stroke volume; RVEF, RV ejection fraction; RVOT, RV outflow tract; TAP, transannular patch; TOF, tetralogy of Fallot; VAT, ventilator anaerobic threshold; and VO2, oxygen uptake.
indicates statistical significance (P<0.05).
Discussion
Our study demonstrated that a higher peak RVOT gradient was associated with less PR and smaller RV volumes in patients with repaired TOF, but also negatively affected biventricular systolic and diastolic longitudinal strain at study entry, even in a relatively young study population, and emerged as a univariate predictor for adverse cardiovascular events. More important, peak RVOT pressure gradients of >25 mm Hg were associated with a >3‐fold higher risk for death and ventricular tachyarrhythmia, even in this young cohort. Although it remains unclear whether the peak RVOT gradient will come out as an independent predictor of adverse outcomes in this population, the results of our study underscore the hemodynamic and prognostic relevance of mild to moderate RV pressure load in patients with repaired TOF that may also have implications for the indication and timing of RVOT reinterventions. Furthermore, our study also showed that a mild residual RVOT gradient of 15 to 30 mm Hg did not protect from later PVR in patients with significant PR.
Hemodynamic Impact of RV Pressure Load
Preservation of pulmonary valve competence and biventricular function are primary objectives in the long‐term surveillance of patients with repaired TOF. Mild to moderate residual RVOT gradients are usually well tolerated and are even supposed to have beneficial effects on exercise performance, RV enlargement, and function. 3 , 4 The results of our study support these results in so far as increased RVOT gradients were associated with less PR and smaller RV dimensions. 3 , 4 , 5 , 14 , 15 Other than reported in previous observations, no significant relationship was observed between exercise capacity and RV afterload. 16
Remarkably, although RVEF and LV ejection fraction were not related to residual pressure load, a weak but significant inverse relationship with both RV and LV longitudinal systolic and diastolic myocardial strain/strain rate was observed. Contrary findings were published previously, showing preserved systolic RV strain values in patients with repaired TOF who presented with higher RVOT gradients. 13 The underlying reason for these divergent findings remains speculative, but differences in patient demographics, such as age, surgical techniques, and reinterventions, offer potential explanations.
Although the reported simple linear correlations are not able to detect any causal relationships, it seems worth discussing potential mechanisms linking higher RV afterload with impaired RV performance. RV myocardial fibrosis is now recognized as an adverse marker for clinical outcomes in patients with repaired TOF. 17 Recent CMR studies assessed diffuse myocardial fibrosis and reported lower degrees of fibrosis within the RV myocardium with increasing RVOT gradients. 18 However, in a histopathological study performed at the time of PVR, Kido and colleagues found an elevated amount of RV fibrosis in patients with a higher RV/LV pressure ratio. 19 In fact, the CMR T1 mapping techniques applied cannot differentiate whether the observed expansion in extracellular matrix is caused by a “real” profibrotic process or is attributable to a “relative” increase caused by cardiomyocyte atrophy. Whether increased RV afterload induces reactive fibrotic remodeling of the myocardium in patients with repaired TOF therefore remains unclear, in particular where RV pressures are only mildly or moderately increased.
As a consequence of elevated RV pressure, compensatory myocardial hypertrophy occurs and promotes RV myocardial stiffness that potentially protects from progressive ventricular dilatation. 20 , 21 This assumption is supported by the finding that diastolic RV longitudinal strain rate was associated with higher pressure gradients in our population. Finally, as seen with RV myocardial strain values, a higher RVOT gradient was also negatively associated with LV longitudinal systolic and diastolic strain/strain rate, which highlights the importance of ventricular interactions in patients after repair of TOF. 22
RV Pressure Load as a Predictor of Outcome
In addition to established clinical risk factors, our study demonstrated that elevated RVOT gradients measured any time after repair emerged as univariate predictors of cardiac death and ventricular tachyarrhythmia over a long‐term course. Several previous CMR studies in patients with repaired TOF identified risk factors for adverse cardiovascular events; however, RV pressure load has not been consistently analyzed in all of these studies, and where it has been included in the analysis, its prognostic relevance differs between studies. 8 , 23 , 24
Our findings are in accordance with those of the multicenter INDICATOR (International Multicenter TOF Registry) study that revealed increased RV pressure as an independent risk factor. 8 , 25 In contrast to our population, higher RV mass as well as lower LV ejection fraction were further significant predictors. To explain the different results, several differences between the INDICATOR study and our study group seem worth considering: our cohort was substantially younger (median, 16.0 versus 24.4 years), the follow‐up, including adverse events, was prospectively assessed, nonsustained VT was considered as part of the composite end point, and correlations to initial exercise testing results were possible. Accordingly, our patients may potentially reflect a more contemporary cohort of TOF survivors with smaller RVs and less PR who may be affected by risk factors detected at an earlier stage.
However, RV pressure was not found to be predictive for the occurrence of VT in another CMR multicenter study. 24 Nevertheless, increased RV afterload constitutes an important risk factor after repair of TOF, as recently demonstrated by Egbe and colleagues, who showed that elevated systolic RV pressure, assessed either invasively or noninvasively, was independently associated with death and heart transplantation. 10 Another study by this group further revealed that combined indexes of RV systolic pressure and function (ie, right ventricular end‐systolic volume and RVEF) better correlated with disease severity than volumetric measures alone. This finding demonstrates the importance of linking indexes of RV afterload with that of RV performance, in particular as these variables seem to be related and hold prognostic relevance. As reported in previous studies, RV systolic strain, but not RVEF, appeared to be a significant predictor in univariable testing. 12 , 22 , 26 , 27 More important, when combined in a bivariable model, solely RV longitudinal strain but not peak RVOT gradient remained independently associated with the combined end point. It might therefore be possible that an increased RVOT gradient translates into unfavorable outcomes by its negative impact on RV deformation.
In this study, an RVOT gradient of ≥25 mm Hg was found to be associated with a >3‐fold increased risk for the combined end point in our TOF population. This cutoff appears to be in accordance with echocardiographic systolic RV pressures of >40 mm Hg that were associated with poorer outcome in other studies. 8 , 9 These findings may potentially affect the timing of PVR procedures, whereas no conclusions can be drawn on what type of RVOT gradient is acceptable at the time of repair. In clinical routine, significantly higher RVOT gradients are typically tolerated, as current guidelines recommend PVR for patients with two thirds systolic RV of systemic pressure and/or RVOT interventions with gradients >50 mm Hg. Notably, patients with primary RV pressure load showed superior recovery of RV systolic function after PVR compared with patients with isolated PR. 28 It remains unclear whether earlier RVOT intervention can ultimately result in improved outcomes and whether an “optimal” balance between RVOT stenosis and PR can be found to effectively reduce RV volume overload and preserve RV integrity without impacting long‐term outcomes. These aspects will need to be addressed in future studies. 29 , 30 , 31
Role of Peak RVOT Gradient on the Need for PVR
The need for multiple RVOT reinterventions attributable to RV hemodynamic overload constitutes a significant burden for patients with repaired TOF. Although the optimal timing for PVR has yet to be determined, it is important to consider factors that delay the need for PVR surgery. 7 A previous study by van der Hulst and colleagues reported that a mild residual RVOT gradient of 15 to 30 mm Hg reduced the requirement for PVR during follow‐up compared with patients with <15– and >30–mm Hg gradients. 6 The results of our study, however, do not support these findings. In patients with ≥25% PR, freedom from PVR did not differ significantly between patients with no (<15 mm Hg), mild (15–30 mm Hg), and moderate/severe (>30 mm Hg) RVOT gradients in our population. Furthermore, the need for PVR in patients with <25% PR and an RVOT gradient >30 mm Hg was similar to that in patients with more severe PR. Although the incidence of PVR was comparable within the 2 cohorts (41% and 41.5%), differences in ages, the “era” of surgical correction, inclusion criteria, duration of follow‐up, and criteria for the decision for PVR present factors that may explain these conflicting findings.
Study Limitations
The estimation of RV afterload using the peak systolic RVOT gradient may underestimate RV pressure, considering that elevated “downstream” pulmonary vascular resistance may be present in patients with repaired TOF. 10 Its relevance seems to be overestimated in the presence of a pulmonary PR fraction >25%. 32 Nevertheless, the assessment of RVOT gradient may be a more precise and reliable method compared with measuring the tricuspid valve regurgitation velocity jet, especially in patients with only mild tricuspid valve regurgitation. Serial measurements of the RVOT gradients and PR fractions both before and after CMR examination were not assessed in this study, but could potentially provide insight into the longitudinal evolution and progression of RV pressure load and its prognostic impact. 33 Furthermore, data on changes in peak RVOT gradient and biventricular function during exercise could also add important hemodynamic information. 34
The overall low number of adverse events, and thus the lack of a multivariable outcome analysis together with the inclusion of nonsustained VT as an end point, limits the statistical power of the outcome analysis. However, even when only patients with death and sustained VT were considered in the outcome analysis, a higher RVOT gradient remained of prognostic significance. A total of 27% of patients from the original data set were excluded for lack of long‐term follow‐up data; therefore, a certain selection bias may be present (for details, see Table S1). However, the observed differences to our understanding should not have a critical effect on the results of our analysis and its drawn conclusions as the main parameters, such as age at repair, type of repair, amount of residual stenosis/regurgitation, and exercise tolerance, were not statistically significantly different. As mentioned, the inclusion of patients with Fallot‐type pulmonary atresia (although not identified as a risk factor) as well as patients with previous PVR affects the character of the study population and needs to be considered when comparing our results with those of other studies with cohorts consisting primarily of patients with a “native” RVOT. The potential risk of PVR during follow‐up needs to be considered in view of the significant higher proportion of PVR procedures in the group with an adverse outcome. It remains unclear whether these events were related (at least in some part) to the surgical procedures or may rather reflect disease severity before PVR.
Conclusions
Although higher peak RVOT gradients were associated with less PR and smaller RV dimensions, an inverse relationship with reduced systolic and diastolic biventricular longitudinal strain was present. Increased RVOT gradients also emerged as univariate predictors of adverse cardiovascular events in the long‐term course. Mildly increased pressure gradients had no protective effect on later PVR procedures. These results may have implications for the indication and timing of RVOT reintervention in patients with repaired TOF. An optimal degree of pressure load, which ensures a protective effect on RV remodeling without compromising ventricular function and outcome, still needs to be determined.
Appendix
List of the German Competence Network for Congenital Heart Defects Investigators
Gunter Kerst, Majed Kanaan, Klinik für Kinderkardiologie; Corinna Lebherz, Klinik für Kardiologie, André Rüffer, Herzchirurgie für Kinder und Erwachsene mit angeborenen Herzfehlern, Universitätsklinikum Aachen; Aachen.
Dimitrios Gkalpakiotis, Praxis für Kinderkardiologie; Aachen.
Andrea Schedifka, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Ahrensfelde.
Gernot Buheitel, Joachim Streble, II. Klinik für Kinder und Jugendliche, Universitätsklinikum Augsburg; Augsburg.
Rainer Willing, Klinik für Kinder‐ und Jugendmedizin, Kinderkardiologie, Ubbo‐Emmius‐Klinik; Aurich.
Stephan Schubert, Kai Thorsten Laser, Karl‐Otto Dubowy Kinderherzzentrum/Zentrum für angeborene Herzfehler, Klinik für Kinderkardiologie und angeborene Herzfehler; Eugen Sandica, Kinderherzzentrum/Zentrum für angeborene Herzfehler, Klinik für Kinderherzchirurgie und angeborene Herzfehler, Herz‐ und Diabeteszentrum NRW; Bad Oeynhausen.
Burkhard Trusen, Praxis Kinder‐ und Jugendmedizin, Kinderkardiologe; Bamberg.
Felix Berger, Oliver Miera, Stanislav Ovroutski, Katharina Schmitt, Klinik für angeborene Herzfehler und Kinderkardiologie; Joachim Photiadis, Klinik für die Chirurgie Angeborener Herzfehler/Kinderherzchirurgie, Deutsches Herzzentrum Berlin; Berlin.
Felix Berger, Bernd Opgen‐Rhein, Katja Weiss, Sabine Klaassen, Klinik für Pädiatrie mit Schwerpunkt Kardiologie, Charité ‐ Universitätsmedizin Berlin, Campus Virchow‐Klinikum; Berlin.
Christoph Berns, Praxis für Kinderheilkunde, Jugendmedizin und Kinderkardiologie; Berlin.
Thomas Boeckel, Guido Haverkämper, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Berlin.
Andreas Kästner, Heike Koch, Björn Peters, Gemeinschaftspraxis für Pädiatrische Kardiologie; Berlin.
Florian Schmidt, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Berlin.
Jens Timme, Konstanze Engel, Birgit Franzbach, Gabriela Senft, Facharztpraxis für Kinderkardiologie und Erwachsene mit angeborenem Herzfehler; Berlin.
Frank Beyer, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Bielefeld.
Klaus Winter, Klinik für Kinder‐ und Jugendmedizin, St.‐Agnes‐Hospital; Bocholt.
Johannes Breuer, Martin Schneider, Zentrum für Kinderheilkunde, Abteilung für Kinderkardiologie; Boulos Asfour, Klinik und Poliklinik für Herzchirurgie, Universitätsklinikum Bonn; Bonn.
Jens Bahlmann, Eberhard Griese, Kinderkardiologische Gemeinschaftspraxis; Braunschweig.
Trong Phi Lê, Klinik für strukturelle und angeborene Herzfehler/ Kinderkardiologie, Klinikum Links der Weser; Bremen.
Joachim Hebe, Jan‐Hendrik Nürnberg, Elektrophysiologie Bremen, Zentrum Bremen am Klinikum Links der Weser; Bremen.
Annette Magsaam, Praxis für Kinderkardiologie und Angeborene Herzfehler; Bremen.
Ronald Müller, Praxis für Angeborene Herzfehler/ Kinderkardiologie; Bremen.
Ludger Potthoff, Praxis Celler Centrum für Kinder‐ und Jugendmedizin, Kinderkardiologie; Celle.
Renate Voigt, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Chemnitz.
Tim Krüger, Kinderarzt‐Praxis Ilmenau/Coburg, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Coburg.
Hubert Gerleve, Ulrich Kleideiter, Kinder‐ und Jugendklinik, Christophorus‐Kliniken Coesfeld; Coesfeld.
Dirk Schneider‐Kulla, Klinik für Pädiatrie/Kinder‐ und Jugendheilkunde, Kinderkardiologie; Jürgen Krülls‐Münch, I. Medizinische Klinik, Klinik für Kardiologie, Angiologie und internistische Intensivtherapie, Carl‐Thiem‐Klinikum Cottbus; Cottbus.
Thomas Menke, Kinderkardiologie, Vestische Kinder‐ und Jugendklinik Datteln; Datteln.
Martin Lehn, Praxis für Kinder‐ und Jugendkardiologie und für Erwachsene mit angeborenen Herzfehlbildungen; Dortmund.
Antje Heilmann, Helge Tomczak, Praxis für Kinderkardiologie, Kinderzentrum Dresden‐Friedrichstadt; Dresden.
Gleb Tarusinov, Klinik für Kinderkardiologie ‐ Angeborene Herzfehler; Michael Scheid, Kinderherzchirurgie und Chirurgie für angeborene Herzfehler, Herzzentrum Duisburg; Duisburg.
Ertan Mayatepek, Frank Pillekamp, Klinik für Allgemeine Pädiatrie, Neonatologie und Kinderkardiologie; Artur Lichtenberg, Klinik für Kardiovaskuläre Chirurgie, Universitätsklinikum Düsseldorf; Düsseldorf.
Christiane Terpeluk, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Ehingen.
Bruno Kolterer, Kinderkardiologische Schwerpunktpraxis; Erfurt.
Sven Dittrich, Kinderkardiologische Abteilung; Ulrike Gundlach, Medizinische Klinik 2 ‐Kardiologie und Angiologie; Robert Cesnjevar, Kinderherzchirurgische Abteilung, Universitätsklinikum Erlangen, Friedrich‐Alexander‐Universität Erlangen‐Nürnberg; Erlangen.
Carsten Müntjes, Klinik für Kinderheilkunde III, Abteilung für Pädiatrische Kardiologie Universitätsklinikum Essen; Essen.
Geert Morf, Praxis für Kinder‐ und Jugendmedizin und EMAH, Kinderkardiologie; Flensburg.
Anoosh Esmaeili, Klinik für Kinder‐ und Jugendmedizin, Kinderkardiologie, Universitätsklinikum Frankfurt; Frankfurt.
Stephan Backhoff, Praxis für Kinderkardiologie/angeborene Herzerkrankungen; Frankfurt.
Brigitte Stiller, Zentrum für Kinder‐ und Jugendmedizin, Klinik für Angeborene Herzfehler/Pädiatrische Kardiologie; Friedhelm Beyersdorf, Klinik für Herz‐ und Gefäßchirurgie; Johannes Kroll, Klinik für Herz‐ und Gefäßchirurgie, Sektion Kinderherzchirurgie, Universitäts‐Herzzentrum Freiburg Bad Krozingen; Freiburg.
Nicole Häffner, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Freiburg.
Jannos Siaplaouras, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie, Erwachsene mit angeborenem Herzfehler, Bluttransfusionswesen am Herz‐Jesu‐Krankenhaus; Fulda.
Antje Masri‐Zada, Praxis für Kardiologie; Gera.
Christian Jux, Klinik für Kinderkardiologie und angeborene Herzfehler; Andreas Böning, Hakan Akintürk, Klinik für Herz‐, Kinderherz‐ und Gefäßchirurgie, Universitätsklinikum Gießen und Marburg; Gießen.
Thomas Paul, Matthias Sigler, Klinik für Pädiatrische Kardiologie und Intensivmedizin mit Neonatologie und Pädiatrischer Pneumologie; Theodor Tirilomis, Klinik für Thorax‐, Herzund Gefäßchirurgie – Schwerpunkt Kinderherzchirurgie, Universitätsklinikum Göttingen; Göttingen.
Gabriele Schürer, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Greiz.
Johannes Hartmann, Schwerpunktpraxis für Kinder‐ und Jugendkardiologie; Hagen.
Ralph Grabitz, Uta Liebaug, Universitätsklinik und Poliklinik für Pädiatrische Kardiologie, Universitätsklinikum Halle (Saale); Halle.
Claudius Rotzsch, Kinderkardiologische Praxis; Halle.
Rainer Kozlik‐Feldmann, Carsten Rickers, Thomas Mir, Michael Hübler, Jörg Sachweh, Kinderkardiologie/Herzchirurgie für angeborene Herzfehler, Universitäres Herz‐ und Gefäßzentrum UKE Hamburg; Hamburg.
Stefan Renz, Andreas Schemm, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie und EMAH; Hamburg.
Bernd Friedrich, Otmar Schlobohm, Kinder‐ und Jugendarztpraxis, Kinderkardiologie; Hamburg.
Dietmar Böthig, Burkhard Wermter, Andrea Kelter‐Klöpping, Klinik für Pädiatrische Kardiologie und Intensivmedizin; Alexander Horke, Chirurgie angeborener Herzfehler; Johann Bauersachs, Mechthild Westhoff‐Bleck, Klinik für Kardiologie und Angiologie, Medizinische Hochschule Hannover; Hannover.
Matthias Gorenflo, Zentrum für Kinder‐ und Jugendmedizin Pädiatrische Kardiologie/Angeborene Herzfehler, Matthias Karck, Tsvetomir Loukanov, Klinik für Herzchirurgie, Universitätsklinikum Heidelberg; Heidelberg.
Hermann Schrüfer, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Hettstadt.
Martin Wilken, Kinderarzt‐Praxis Hof/Nail; Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Hof.
Hashim Abdul‐Khaliq, Tanja Rädle‐Hurst, Axel Rentzsch, Klinik für Pädiatrische Kardiologie; Hans‐Joachim Schäfers, Klinik für Thorax‐ und Herz‐Gefäß‐Chirurgie, Universitätsklinikum des Saarlandes; Homburg.
Hagen Reichert, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Reisemedizin, Gelbfieberimpfstelle; Homburg.
Daniel Vilser, Klinik für Kinder‐ und Jugendmedizin, Sektion Kardiologie, Universitätsklinikum Jena; Jena.
Thomas Kriebel, Klinik für Kinder‐ und Jugendmedizin, Kinderkardiologie, Westpfalz‐Klinikum; Kaiserslautern.
Arnulf Boysen, Schwerpunktpraxis für angeborene Herzfehler; Karlsruhe.
Anselm Uebing, Inga Voges, Klinik für angeborene Herzfehler und Kinderkardiologie; Tim Attmann, Joachim Thomas Cremer, Jens Scheewe, Klinik für Herz‐ und Gefäßchirurgie, Universitätsklinikum Schleswig‐Holstein; Kiel.
Regina Buchholz‐Berdau, Peter Möller, Gemeinschaftspraxis der Kinder‐ und Jugendärzte und Kinderkardiologen; Kiel.
Thorsten Horter, Schwerpunktpraxis für Kinder‐ und Jugendkardiologie; Kiel.
Konrad Brockmeier, Klinik und Poliklinik für Kinderkardiologie, Gerardus B. W. EBennink, Klinik für Herz‐ und Thoraxchirurgie, Schwerpunkt Kinderherzchirurgie, Stephan Baldus, Klinik III für Innere Medizin, Herzzentrum Universitätsklinikum Köln; Köln.
Alex Gillor, Praxis für Kinder‐ und Jugendkardiologie; Köln.
Tim Niehues, Wolfgang Lawrenz, Zentrum für Kinder‐ und Jugendmedizin, Kinderkardiologie, HELIOS Klinikum Krefeld; Krefeld.
Steffen Leidig, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Lauf.
Ingo Dähnert, Frank‐Thomas Riede, Universitätsklinik für Kinderkardiologie; Martin Kostelka, Universitätsklinik für Herzchirurgie, Kinderherzchirurgie, Herzzentrum Leipzig; Leipzig.
Liane Kändler, Medizinisches Versorgungszentrum Jessen, Außenstelle Wittenberg und Klinik für Kinder‐ und Jugendmedizin, Paul Gerhardt Diakonie und Pflege GmbH; Lutherstadt Wittenberg.
Martin Bethge, Stefan Köster, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie und EMAH; Lübeck.
Christoph Schröder, Praxis für Kinderkardiologie, Kinderpneumologie, Erwachsene mit angeborenen Herzfehlern; Lüneburg.
Jens Karstedt, Kardiologische Schwerpunkpraxis für Kinder und Jugendliche am Klinikum Magdeburg; Magdeburg.
Uwe Seitz, Praxis für Kinder‐ Jugendmedizin, Kinderkardiologie; Maintal.
Christoph Kampmann, Zentrum für Kinder‐ und Jugendmedizin, Abteilung für Kinderkardiologie, Daniel‐Sebastian Dohle, Klinik und Poliklinik für Herz‐, Thorax‐ und Gefäßchirurgie, Universitätsmedizin der Johannes Gutenberg‐Universität Mainz; Mainz.
Frank Stahl, Praxis für Kinder‐ und Jugendkardiologie, arterielle Hypertonie bei Kindern und Jugendlichen, Erwachsene mit angeborenen Herzfehlern; Mannheim.
Mojtaba Abedini, Praxis für Kinder‐ und Jugendkardiologie am Universitätsklinikum Gießen und Marburg; Marburg.
Joachim Müller‐Scholden, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Marktheidenfeld.
Peter Ewert, Alfred Hager, Michael Huntgeburth, Harald Kaemmerer, Nicole Nagdyman, Jörg Schoetzau, Oktay Tutarel, Klinik für Kinderkardiologie und Angeborene Herzfehler; Rüdiger Lange, Klinik für Herz‐ und Gefäßchirurgie; Jürgen Hörer, Klinik für Chirurgie angeborener Herzfehler und Kinderherzchirurgie Deutsches Herzzentrum München; München.
Nikolaus AHaas, Abteilung Kinderkardiologie und Pädiatrische Intensivmedizin; Jürgen Hörer, Herzchirurgische Klinik und Poliklinik, Sektion Kinderherzchirurgie; Klinikum der Ludwig‐Maximilians‐Universität, Campus Großhadern; München.
Michael Hauser, Praxis für Kinder‐ und Jugendkardiologie und Erwachsene mit angeborenen Herzfehlern; München.
Alexander Roithmaier, Praxis für Kinder‐ und Jugendmedizin, Schwerpunktpraxis für Kinderund Jugendkardiologie; München.
Hans‐Gerd Kehl, Astrid Lammers, Klinik für Kinder‐ und Jugendmedizin – Pädiatrische Kardiologie, Edward Malec, Department für Herz‐ und Thoraxchirurgie, Abteilung Kinderherzchirurgie; Helmut Baumgartner, Gerhard Diller, Klinik und Poliklinik für Erwachsene mit angeborenen (EMAH) und erworbenen Herzfehlern, Universitätsklinikum Münster; Münster.
Roswitha Bahle, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Neubrandenburg.
Gerald Hofner, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Neudrossenfeld.
Stefan Zink, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Nürnberg.
Roland Reif, Helmut Singer; Gemeinschaftspraxis für Kinder‐ und Jugendmedizin, Kinderkardiologie, Allergologie, Asthmatraining, Psychotherapie; Nürnberg.
Christoph Parlasca, Klinik für Kinder‐ und Jugendmedizin, Evangelisches Krankenhaus Oberhausen; Oberhausen.
Matthias WFreund, Michael Schumacher, Universitätsklinik für Kinder‐ und Jugendmedizin, Klinik für Neonatologie, Intensivmedizin und Kinderkardiologie, Klinikum Oldenburg ‐Elisabeth‐Kinderkrankenhaus; Oldenburg.
Oliver Dewald, Universitätsklinik für Herzchirurgie, Klinikum Oldenburg; Oldenburg.
Christine Darrelmann, Gemeinschaftspraxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Oldenburg.
Olaf Willmann, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Osnabrück.
Norbert Schmiedl, Praxis für Kinderkardiologie; Passau.
Peter Quick, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Plauen.
Dirk Hillebrand, Praxis für Kinder‐ und Jugendmedizin, Schwerpunktpraxis Kinderkardiologie, Angeborene Herzfehler; Pinneberg.
Stephan Michele Eiselt, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie und EMAH; Reinbek.
Torsten Nekarda, Klinik für Kinder‐ und Jugendmedizin, Agaplesion Diakonieklinikum Rotenburg; Rotenburg.
Michael Eberhard, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Rottweil.
Georg Baier, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Schwabach.
Frank Uhlemann, Zentrum für angeborene Herzfehler, Olgahospital; Stuttgart.
Ioannis Tzanavaros, Chirurgie für angeborene Herzfehler/Kinderherzchirurgie, Sana Herzchirurgie Stuttgart; Stuttgart.
Alexander Beyer, Gudrun Binz, Steffen Hess, Thomas Teufel, Kinderkardiologische Praxis Stuttgart/EMAH‐Schwerpunktpraxis; Stuttgart.
Ronald‐Peter Handke, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Trier.
Michael Hofbeck, Renate Kaulitz, Ludger Sieverding, Kinderheilkunde II ‐ Kinderkardiologie, Intensivmedizin und Pulmologie; Christian Schlensak, Thorax‐, Herz‐ und Gefäßchirurgie, Migdat Mustafi, Sektion Chirurgie angeborener Herzfehler – Kinderherzchirurgie, Universitätsklinikum Tübingen; Tübingen.
Christian Apitz, Michael Kaestner, Klinik für Kinder‐ und Jugendmedizin, Sektion Pädiatrische Kardiologie, Universitätsklinikum Ulm; Ulm.
Jürgen Holtvogt, Klinik für Kinder‐ und Jugendmedizin, Kinderkardiologie, St. Marienhospital Vechta; Vechta.
Carl‐Friedrich Wippermann, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Walluf.
Sönke Hinz, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie; Weyhe.
Andreas Heusch, Zentrum für Kinder‐ und Jugendmedizin, Abteilung Kinderkardiologie und ‐pneumologie, HELIOS Klinikum Wuppertal; Wuppertal.
Johannes Wirbelauer, Kinderklinik, Kinderkardiologie/ EMAH, Universitätsklinikum Würzburg; Würzburg.
Wolfgang Brosi, Praxis für Kinder‐ und Jugendmedizin, Kinderkardiologie und –pneumologie, Allergologie, Umweltmedizin, Asthma‐, Neurodermitis‐ und Anaphylaxietrainer; Würzburg.
Sources of Funding
The study was supported by the Doris‐Haag Stiftung, Frankfurt am Main, Germany and the Willy‐Robert‐Pitzer Stiftung, Frankfurt am Main, Germany. This work was further supported by the Competence Network for Congenital Heart Defects, funded by the Federal Ministry of Education and Research (FKZ 01G10210 for first funding period; FKZ 01GI0601 for second and third funding periods).
Disclosures
None.
Supporting information
Tables S1–S3
Figure S1
For Sources of Funding and Disclosures, see page 15.
Contributor Information
Heiner Latus, heiner.latus@googlemail.com.
the German Competence Network for Congenital Heart Defects Investigators:
Gunter Kerst, Majed Kanaan, Corinna Lebherz, André Rüffer, Dimitrios Gkalpakiotis, Andrea Schedifka, Gernot Buheitel, Joachim Streble, Rainer Willing, Stephan Schubert, Kai Thorsten Laser, Eugen Sandica, Burkhard Trusen, Felix Berger, Oliver Miera, Stanislav Ovroutski, Katharina Schmitt, Joachim Photiadis, Felix Berger, Bernd Opgen‐Rhein, Katja Weiss, Sabine Klaassen, Christoph Berns, Thomas Boeckel, Guido Haverkämper, Andreas Kästner, Heike Koch, Björn Peters, Florian Schmidt, Jens Timme, Konstanze Engel, Birgit Franzbach, Gabriela Senft, Frank Beyer, Klaus Winter, Johannes Breuer, Martin Schneider, Jens Bahlmann, Eberhard Griese, Trong Phi Lê, Joachim Hebe, Jan‐Hendrik Nürnberg, Annette Magsaam, Ronald Müller, Ludger Potthoff, Renate Voigt, Tim Krüger, Hubert Gerleve, Ulrich Kleideiter, Dirk Schneider‐Kulla, Jürgen Krülls‐Münch, Thomas Menke, Martin Lehn, Antje Heilmann, Helge Tomczak, Gleb Tarusinov, Michael Scheid, Ertan Mayatepek, Frank Pillekamp, Artur Lichtenberg, Christiane Terpeluk, Bruno Kolterer, Sven Dittrich, Ulrike Gundlach, Robert Cesnjevar, Carsten Müntjes, Geert Morf, Anoosh Esmaeili, Stephan Backhoff, Brigitte Stiller, Friedhelm Beyersdorf, Johannes Kroll, Nicole Häffner, Jannos Siaplaouras, Antje Masri‐Zada, Christian Jux, Andreas Böning, Hakan Akintürk, Thomas Paul, Matthias Sigler, Theodor Tirilomis, Gabriele Schürer, Johannes Hartmann, Ralph Grabitz, Uta Liebaug, Claudius Rotzsch, Rainer Kozlik‐Feldmann, Carsten Rickers, Thomas Mir, Michael Hübler, Jörg Sachweh, Stefan Renz, Andreas Schemm, Bernd Friedrich, Otmar Schlobohm, Dietmar Böthig, Burkhard Wermter, Andrea Kelter‐Klöpping, Alexander Horke, Johann Bauersachs, Mechthild Westhoff‐Bleck, Matthias Gorenflo, Matthias Karck, Tsvetomir Loukanov, Hermann Schrüfer, Martin Wilken, Hashim Abdul‐Khaliq, Tanja Rädle‐Hurst, Axel Rentzsch, Hans‐Joachim Schäfers, Hagen Reichert, Daniel Vilser, Thomas Kriebel, Arnulf Boysen, Anselm Uebing, Inga Voges, Tim Attmann, Joachim Thomas Cremer, Jens Scheewe, Regina Buchholz‐Berdau, Peter Möller, Thorsten Horter, Konrad Brockmeier, Gerardus B. W. E Bennink, Stephan Baldus, Alex Gillor, Tim Niehues, Wolfgang Lawrenz, Steffen Leidig, Ingo Dähnert, Frank‐Thomas Riede, Martin Kostelka, Liane Kändler, Martin Bethge, Stefan Köster, Christoph Schröder, Jens Karstedt, Uwe Seitz, Christoph Kampmann, Daniel‐Sebastian Dohle, Frank Stahl, Mojtaba Abedini, Joachim Müller‐Scholden, Peter Ewert, Alfred Hager, Michael Huntgeburth, Harald Kaemmerer, Nicole Nagdyman, Jörg Schoetzau, Oktay Tutarel, Rüdiger Lange, Jürgen Hörer, Nikolaus A Haas, Jürgen Hörer, Michael Hauser, Alexander Roithmaier, Hans‐Gerd Kehl, Astrid Lammers, Edward Malec, Helmut Baumgartner, Gerhard Diller, Roswitha Bahle, Gerald Hofner, Stefan Zink, Roland Reif, Helmut Singer, Christoph Parlasca, Matthias W Freund, Michael Schumacher, Oliver Dewald, Christine Darrelmann, Olaf Willmann, Norbert Schmiedl, Peter Quick, Dirk Hillebrand, Stephan Michele Eiselt, Torsten Nekarda, Michael Eberhard, Georg Baier, Frank Uhlemann, Ioannis Tzanavaros, Alexander Beyer, Gudrun Binz, Steffen Hess, Thomas Teufel, Ronald‐Peter Handke, Michael Hofbeck, Renate Kaulitz, Ludger Sieverding, Christian Schlensak, Christian Apitz, Michael Kaestner, Jürgen Holtvogt, Carl‐Friedrich Wippermann, Sönke Hinz, Andreas Heusch, Johannes Wirbelauer, and Wolfgang Brosi
References
- 1. Davlouros PA, Kilner PJ, Hornung TS, Li W, Francis JM, Moon JC, Smith GC, Tat T, Pennell DJ, Gatzoulis MA. Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: detrimental role of right ventricular outflow aneurysms or akinesia and adverse right‐to‐left ventricular interaction. J Am Coll Cardiol. 2002;40:2044–2052. doi: 10.1016/S0735-1097(02)02566-4 [DOI] [PubMed] [Google Scholar]
- 2. Boni L, García E, Galletti L, Pérez A, Herrera D, Ramos V, Marianeschi SM, Comas JV. Current strategies in tetralogy of Fallot repair: pulmonary valve sparing and evolution of right ventricle/left ventricle pressures ratio. Eur J Cardio‐Thorac Surg. 2009;35:885–889; discussion 889‐890. doi: 10.1016/j.ejcts.2009.01.016 [DOI] [PubMed] [Google Scholar]
- 3. Spiewak M, Biernacka EK, Małek LA, Petryka J, Kowalski M, Miłosz B, Zabicka M, Miśko J, Rużyłło W. Right ventricular outflow tract obstruction as a confounding factor in the assessment of the impact of pulmonary regurgitation on the right ventricular size and function in patients after repair of tetralogy of Fallot. J Magn Reson Imaging: JMRI. 2011;33:1040–1046. doi: 10.1002/jmri.22532 [DOI] [PubMed] [Google Scholar]
- 4. Yoo BW, Kim JO, Kim YJ, Choi JY, Park HK, Park YH, Sul JH. Impact of pressure load caused by right ventricular outflow tract obstruction on right ventricular volume overload in patients with repaired tetralogy of Fallot. J Thorac Cardiovasc Surg. 2012;143:1299–1304. doi: 10.1016/j.jtcvs.2011.12.033 [DOI] [PubMed] [Google Scholar]
- 5. Latus H, Gummel K, Rupp S, Valeske K, Akintuerk H, Jux C, Bauer J, Schranz D, Apitz C. Beneficial effects of residual right ventricular outflow tract obstruction on right ventricular volume and function in patients after repair of tetralogy of Fallot. Pediatr Cardiol. 2013;34:424–430. doi: 10.1007/s00246-012-0476-4 [DOI] [PubMed] [Google Scholar]
- 6. van der Hulst AE, Hylkema MG, Vliegen HW, Delgado V, Hazekamp MG, Rijlaarsdam ME, Holman ER, Blom NA, Roest AA. Mild residual pulmonary stenosis in tetralogy of Fallot reduces risk of pulmonary valve replacement. Ann Thorac Surg. 2012;94:2077–2082. doi: 10.1016/j.athoracsur.2012.06.065 [DOI] [PubMed] [Google Scholar]
- 7. Frigiola A, Hughes M, Turner M, Taylor A, Marek J, Giardini A, Hsia TY, Bull K. Physiological and phenotypic characteristics of late survivors of tetralogy of Fallot repair who are free from pulmonary valve replacement. Circulation. 2013;128:1861–1868. doi: 10.1161/CIRCULATIONAHA.113.001600 [DOI] [PubMed] [Google Scholar]
- 8. Valente AM, Gauvreau K, Assenza GE, Babu‐Narayan SV, Schreier J, Gatzoulis MA, Groenink M, Inuzuka R, Kilner PJ, Koyak Z, et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the indicator cohort. Heart. 2014;100:247–253. doi: 10.1136/heartjnl-2013-304958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shiraishi S, Takahashi M, Sugimoto A, Tsuchida M. Predictors of ventricular tachyarrhythmia occurring late after intracardiac repair of tetralogy of Fallot: combination of QRS duration change rate and tricuspid regurgitation pressure gradient. J Thorac Dis. 2017;9:5112–5119. doi: 10.21037/jtd.2017.11.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egbe AC, Taggart NW, Reddy YNV, Sufian M, Banala K, Vojjini R, Najam M, Osman K, Obokata M, Borlaug BA. Assessment and implications of right ventricular afterload in tetralogy of Fallot. Am J Cardiol. 2019;124:1780–1784. doi: 10.1016/j.amjcard.2019.08.035 [DOI] [PubMed] [Google Scholar]
- 11. Sarikouch S, Boethig D, Peters B, Kropf S, Dubowy KO, Lange P, Kuehne T, Haverich A, Beerbaum P. Poorer right ventricular systolic function and exercise capacity in women after repair of tetralogy of Fallot: a sex comparison of standard deviation scores based on sex‐specific reference values in healthy control subjects. Circ Cardiovasc Imaging. 2013;6:924–933. doi: 10.1161/CIRCIMAGING.112.000195 [DOI] [PubMed] [Google Scholar]
- 12. Orwat S, Diller G‐P, Kempny A, Radke R, Peters B, Kühne T, Boethig D, Gutberlet M, Dubowy K‐O, Beerbaum P, et al. Myocardial deformation parameters predict outcome in patients with repaired tetralogy of Fallot. Heart. 2016;102:209–215. doi: 10.1136/heartjnl-2015-308569 [DOI] [PubMed] [Google Scholar]
- 13. Latus H, Hachmann P, Gummel K, Khalil M, Yerebakan C, Bauer J, Schranz D, Apitz C. Impact of residual right ventricular outflow tract obstruction on biventricular strain and synchrony in patients after repair of tetralogy of Fallot: a cardiac magnetic resonance feature tracking study. Eur J Cardiothorac Surg. 2015;48:83‐90. doi: 10.1055/s-0034-1394034 [DOI] [PubMed] [Google Scholar]
- 14. Chen C‐A, Chen S‐Y, Wang J‐K, Tseng W‐Y, Chiu H‐H, Chang C‐I, Chiu I‐S, Chen Y‐S, Yang M‐C, Lu C‐W, et al. Ventricular geometric characteristics and functional benefit of mild right ventricular outflow tract obstruction in patients with significant pulmonary regurgitation after repair of tetralogy of Fallot. Am Heart J. 2014;167:555–561. doi: 10.1016/j.ahj.2013.12.026 [DOI] [PubMed] [Google Scholar]
- 15. Kilner PJ, Balossino R, Dubini G, Babu‐Narayan SV, Taylor AM, Pennati G, Migliavacca F. Pulmonary regurgitation: the effects of varying pulmonary artery compliance, and of increased resistance proximal or distal to the compliance. Int J Cardiol. 2009;133:157–166. doi: 10.1016/j.ijcard.2008.06.078 [DOI] [PubMed] [Google Scholar]
- 16. Freling HG, Willems TP, van Melle JP, van Slooten YJ, Bartelds B, Berger RM, van Veldhuisen DJ, Pieper PG. Effect of right ventricular outflow tract obstruction on right ventricular volumes and exercise capacity in patients with repaired tetralogy of Fallot. Am J Cardiol. 2014;113:719–723. doi: 10.1016/j.amjcard.2013.10.049 [DOI] [PubMed] [Google Scholar]
- 17. Babu‐Narayan SV, Kilner PJ, Li W, Moon JC, Goktekin O, Davlouros PA, Khan M, Ho SY, Pennell DJ, Gatzoulis MA. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of Fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405–413. doi: 10.1161/CIRCULATIONAHA.105.548727 [DOI] [PubMed] [Google Scholar]
- 18. Chen CA, Dusenbery SM, Valente AM, Powell AJ, Geva T. Myocardial ECV fraction assessed by CMR is associated with type of hemodynamic load and arrhythmia in repaired tetralogy of Fallot. JACC Cardiovasc Imaging. 2016;9:1–10. [DOI] [PubMed] [Google Scholar]
- 19. Kido T, Ueno T, Taira M, Ozawa H, Toda K, Kuratani T, Sawa Y. Clinical predictors of right ventricular myocardial fibrosis in patients with repaired tetralogy of Fallot. Circ J. 2018;82:1149–1154. doi: 10.1253/circj.CJ-17-1088 [DOI] [PubMed] [Google Scholar]
- 20. Bove T, Vandekerckhove K, Bouchez S, Wouters P, Somers P, Van Nooten G. Role of myocardial hypertrophy on acute and chronic right ventricular performance in relation to chronic volume overload in a porcine model: relevance for the surgical management of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2014;147:1956–1965. doi: 10.1016/j.jtcvs.2013.10.026 [DOI] [PubMed] [Google Scholar]
- 21. Gatzoulis MA, Clark AL, Cullen S, Newman CG, Redington AN. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot: restrictive physiology predicts superior exercise performance. Circulation. 1995;91:1775–1781. doi: 10.1161/01.CIR.91.6.1775 [DOI] [PubMed] [Google Scholar]
- 22. Diller G‐P, Kempny A, Liodakis E, Alonso‐Gonzalez R, Inuzuka R, Uebing A, Orwat S, Dimopoulos K, Swan L, Li W, et al. Left ventricular longitudinal function predicts life‐threatening ventricular arrhythmia and death in adults with repaired tetralogy of Fallot. Circulation. 2012;125:2440–2446. doi: 10.1161/CIRCULATIONAHA.111.086983 [DOI] [PubMed] [Google Scholar]
- 23. Bokma JP, de Wilde KC, Vliegen HW, van Dijk AP, van Melle JP, Meijboom FJ, Zwinderman AH, Groenink M, Mulder BJM, Bouma BJ. Value of cardiovascular magnetic resonance imaging in noninvasive risk stratification in tetralogy of Fallot. JAMA Cardiol. 2017;2:678–683. doi: 10.1001/jamacardio.2016.5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beurskens NEG, Hagdorn QAJ, Gorter TM, Berger RMF, Vermeulen KM, van Melle JP, Ebels TE, Lui GK, Ceresnak SR, Chan FP, et al. Risk of cardiac tachyarrhythmia in patients with repaired tetralogy of Fallot: a multicenter cardiac MRI based study. Int J Cardiovasc Imaging. 2019;35:143–151. doi: 10.1007/s10554-018-1435-9 [DOI] [PubMed] [Google Scholar]
- 25. Geva T, Mulder B, Gauvreau K, Babu‐Narayan SV, Wald RM, Hickey K, Powell AJ, Gatzoulis MA, Valente AM. Preoperative predictors of death and sustained ventricular tachycardia after pulmonary valve replacement in patients with repaired tetralogy of Fallot enrolled in the indicator cohort. Circulation. 2018;138:2106–2115. doi: 10.1161/CIRCULATIONAHA.118.034740 [DOI] [PubMed] [Google Scholar]
- 26. Hagdorn QAJ, Vos JDL, Beurskens NEG, Gorter TM, Meyer SL, van Melle JP, Berger RMF, Willems TP. CMR feature tracking left ventricular strain‐rate predicts ventricular tachyarrhythmia, but not deterioration of ventricular function in patients with repaired tetralogy of Fallot. Int J Cardiol. 2019;295:1–6. doi: 10.1016/j.ijcard.2019.07.097 [DOI] [PubMed] [Google Scholar]
- 27. Moon TJ, Choueiter N, Geva T, Valente AM, Gauvreau K, Harrild DM. Relation of biventricular strain and dyssynchrony in repaired tetralogy of Fallot measured by cardiac magnetic resonance to death and sustained ventricular tachycardia. Am J Cardiol. 2015;115:676–680. doi: 10.1016/j.amjcard.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 28. Harrild DM, Marcus E, Hasan B, Alexander ME, Powell AJ, Geva T, McElhinney DB. Impact of transcatheter pulmonary valve replacement on biventricular strain and synchrony assessed by cardiac magnetic resonance feature tracking. Circ Cardiovasc Interv. 2013;6:680–687. doi: 10.1161/CIRCINTERVENTIONS.113.000690 [DOI] [PubMed] [Google Scholar]
- 29. Bokma JP, Geva T, Sleeper LA, Babu Narayan SV, Wald R, Hickey K, Jansen K, Wassall R, Lu M, Gatzoulis MA, et al. A propensity score‐adjusted analysis of clinical outcomes after pulmonary valve replacement in tetralogy of Fallot. Heart. 2018;104:738–744. doi: 10.1136/heartjnl-2017-312048 [DOI] [PubMed] [Google Scholar]
- 30. Harrild DM, Berul CI, Cecchin F, Geva T, Gauvreau K, Pigula F, Walsh EP. Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation. 2009;119:445–451. doi: 10.1161/CIRCULATIONAHA.108.775221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geva T. Tetralogy of Fallot repair: ready for a new paradigm. J Thorac Cardiovasc Surg. 2012;143:1305–1306. doi: 10.1016/j.jtcvs.2012.01.076 [DOI] [PubMed] [Google Scholar]
- 32. Sakaki S, Murakami T, Shiraishi M, Yamamoto M. Impact of pulmonary valve regurgitation on pressure difference of pulmonary valve stenosis in patients with tetralogy of Fallot after repair. Pediatr Cardiol. 2018;39:1663–1668. doi: 10.1007/s00246-018-1947-z [DOI] [PubMed] [Google Scholar]
- 33. Tan C, Soquet J, Brizard CP, d'Udekem Y. Evolution of residual and recurrent right ventricular outflow tract obstruction after tetralogy of Fallot repair. J Thorac Cardiovasc Surg. 2020;159:e275–e277. doi: 10.1016/j.jtcvs.2019.09.190 [DOI] [PubMed] [Google Scholar]
- 34. Hasan BS, Lunze FI, McElhinney DB, Stantcheva E, Brown DW, Rhodes J, Chen MH. Exercise stress echocardiographic assessment of outflow tract and ventricular function in patients with an obstructed right ventricular‐to‐pulmonary artery conduit after repair of conotruncal heart defects. Am J Cardiol. 2012;110:1527–1533. doi: 10.1016/j.amjcard.2012.07.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


