Abstract
Background
The aim of this study was to investigate the association between night‐to‐night adherence to continuous positive airway pressure (CPAP) therapy and both home blood pressure (BP) level on the following day and seasonal variation in home BP in patients with obstructive sleep apnea.
Methods and Results
We analyzed 105 participants who had been diagnosed with obstructive sleep apnea (average apnea‐hypopnea index, 49.7±18.4 per hour) and who were already receiving CPAP therapy. Home BP (twice every morning and evening) and CPAP adherence data were automatically transmitted to a server for 1 year. A mixed‐effects model for repeated measures analysis was used to examine associations of night‐to‐night good CPAP adherence with day‐to‐day home BP within the same patient after adjusting for covariates. The average number of days in which patients achieved both CPAP adherence and morning or evening home BP measurement was 206.6±122.7 days (21 487 readings) and 191.2±126.3 days (20 170 readings), respectively. Good CPAP adherence (>4 hours per night of use) was achieved on the evening or morning before home BP measurements (86.8% and 86.9%, respectively). After adjustment for confounders, good CPAP adherence was negatively associated with morning home systolic BP (β, −0.663; P=0.004) and diastolic BP (β, −0.829; P<0.001). Morning home systolic BP in winter in the individuals with good CPAP adherence was significantly lower than that in individuals without such adherence (P<0.05). These associations were not found in evening home BP.
Conclusions
Good adherence to CPAP therapy was negatively associated with morning home BP on the following day in patients with obstructive sleep apnea. The association was remarkable in the winter season.
Keywords: adherence, continuous positive airway pressure, home blood pressure monitoring, obstructive sleep apnea
Subject Categories: Hypertension
Nonstandard Abbreviations and Acronyms
- DBP
diastolic blood pressure
- HBPM
home blood pressure monitoring
- SBP
systolic blood pressure
- SDB
sleep disordered breathing
Clinical Perspective
What Is New?
In 105 participants who had been diagnosed with obstructive sleep apnea and who were already receiving continuous positive airway pressure therapy, good adherence to continuous positive airway pressure therapy was negatively associated with morning home blood pressure on the following day; the association was remarkable in the winter season.
The current study shows that night‐to‐night good adherence to continuous positive airway pressure therapy (>4 hours per night) is negatively associated with home blood pressure on the following morning, but not to evening home blood pressure in patients with obstructive sleep apnea.
The individuals with good adherence to continuous positive airway pressure therapy showed a negative association in seasonal variation of morning home blood pressure compared with those without.
What Are the Clinical Implications?
The results of the present study suggest that good adherence to continuous positive airway pressure therapy is negatively associated with morning home blood pressure, and thereby contributes to the prevention of cardiovascular events throughout the year in patients with obstructive sleep apnea.
The strong linkage between obstructive sleep apnea (OSA) and hypertension has been well acknowledged in both cross‐sectional and longitudinal studies, 1 , 2 and OSA has been recognized as one of the causes of secondary hypertension. 3 , 4 , 5 Continuous positive airway pressure (CPAP) therapy has been recommended as the first‐choice treatment for severe OSA. Several previous studies have reported that CPAP therapy reduces blood pressure (BP) in patients with OSA and hypertension, but this effect is partly dependent on adherence to CPAP use. 6 , 7 , 8
Recent international guidelines recommend the use of home BP monitoring (HBPM) for the management of hypertension, 3 , 4 , 5 because HBPM, especially home BP readings in the morning period, has greater predictive power for cardiovascular events compared with office BP. 9 , 10 Hence, the identification of factors that are determinant of morning BP is clinically important for the control of morning BP, and thereby for a reduction of cardiovascular events. OSA has been considered to be an important factor affecting morning hypertension. 11 , 12 Although chronically poor CPAP adherence is already known to worsen BP control, clinical evidence for daily home BP control related to nightly CPAP adherence is lacking. To the best of our knowledge, there has been no study about the daily association between CPAP adherence and morning BP over a long‐term follow‐up period. Moreover, previous studies have demonstrated a significant seasonal variation in home BP, with higher home BP in winter than summer. 13 , 14 However, this association was observed only for morning home BP and not for evening home BP. 15 Therefore, we further hypothesized that night‐to‐night adherence to CPAP use may affect the seasonal variation in morning home BP.
The aim of this study was thus to investigate whether night‐to‐night adherence to CPAP is associated with home BP on the successive days during long‐term follow‐up or the seasonal variation in home BP in patients with OSA.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patients
We enrolled study participants who were diagnosed with OSA according to the American Academy of Sleep Medicine guidelines, 16 namely, all participants had an apnea‐hypopnea index of ≥20 on overnight polysomnography or that of ≥40 on polygraphy. We performed CPAP therapy according to the recommendations of the Japanese health insurance system. The details of the OSA diagnosis and CPAP therapy are given in Data S1. The participants were recruited from 4 institutes. The institutional review board of Jichi Medical University approved the study. All participants provided written informed consent. Demographic information was collected by physicians at each participating institute. Diagnosed hypertension, dyslipidemia, and diabetes were defined as a self‐reported physician's diagnosis of each disease or current use of correspondent medication. Body mass index (BMI) was calculated from measured weight and height. The adherence to pharmacotherapy at baseline was evaluated by a Morisky score. 17 Office BP measurements were obtained at local medical centers using any validated cuff oscillometric device. All participants included in this study used a SLEEPMATE9 or SLEEPMATE10 CPAP device (TEIJIN, Tokyo, Japan). CPAP titration was exclusively performed by using these CPAP devices, which provide an autotitrating function, or partly by using these devices and also conventional polysomnography, depending on the individual institute. These CPAP devices record daily CPAP usage data and automatically transmit the data to a server. CPAP adherence was measured using the internal clocks in the CPAP devices. Good CPAP adherence was defined as >4 hours per night according to a previous study. 18 According to the guidelines of the health insurance system in Japan, all participants performing CPAP therapy were required to visit the clinic or hospital once within 3 months to receive education on OSA and its treatment by their physician, in addition to updating their antihypertensive drug information.
Home BP Measurement
Self‐measured home BP values were obtained according to the current guidelines. 5 The participants measured their BP twice every morning and twice every evening, with the measurements taken in a seated position in both the morning (within 1 hour of waking and before taking antihypertensive medication) and evening (before going to bed) for 1 consecutive year. The averages of morning and evening BP per 1 day were calculated separately. Data of HBPM in all participants were collected during June 2016 and March 2018. Home BP and heart rate were measured using an HEM‐7252G‐HP automatic device (Omron Healthcare, Kyoto, Japan). 19 All data of home BP and pulse rate were automatically transmitted to the server (https://www2.med‐link.jp/ht/pro/index.php).
Statistical Analysis
A total of 110 patients with OSA undergoing CPAP therapy initially consented to participate in this study. Two of them later withdrew consent, and 3 other participants lacked sufficient HBPM data. Therefore, our final sample comprised 105 participants. Of these 105 participants, 1 participant failed to perform HBPM in the morning, and therefore the analysis related to morning home BP was performed in only 104 participants. Categorical variables and mean (SD) for continuous variables were used to describe the study population. Each of the morning and evening home BP measurements was matched with the duration of CPAP use taken the previous night during 1 year from the start of home BP measurement. A mixed‐effects model for repeated measures analysis was used to examine associations of night‐to‐night good CPAP adherence with day‐to‐day home BP within each patient at the inter‐ and intraindividual level after adjusting for age, sex, BMI, drinking, smoking, prevalent diabetes and cardiovascular disease (CVD), and the use of an antihypertensive drug. We also performed stratified analysis according to these covariates. Moreover, to examine the monthly association of good night‐to‐night CPAP adherence with day‐to‐day home BP within the same person, we used a mixed‐effects model of repeated measures analysis that included CPAP adherence, time points (month), and the interaction between CPAP adherence and time points as fixed effects, and age, sex, BMI, drinking, smoking, prevalent diabetes and CVD, and the use of an antihypertensive drug as covariates. We conducted an additional sensitivity analysis using a previous study’s definition of good CPAP adherence as >3 hours per night. 20 A 2‐sided P value <0.05 was accepted as significant. All statistical analyses were performed with SAS version 9.4 software (SAS Institute, Cary, NC).
Results
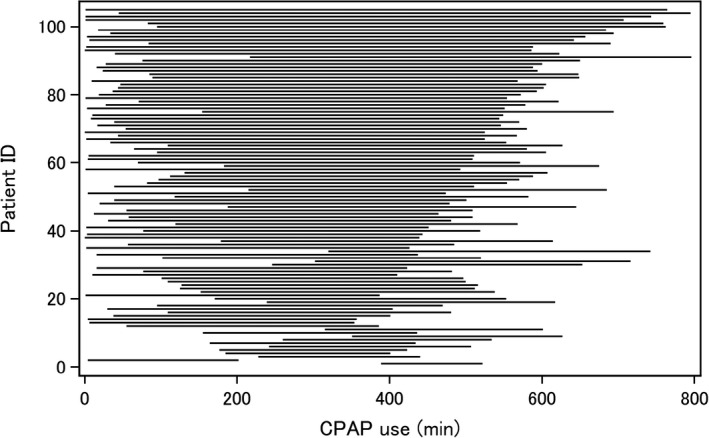
Baseline characteristics of the 105 participants and the groups of lower and higher CPAP adherence using the median value of the percentage (77.7%) of good CPAP adherence during study period are shown in Table 1. When confirming the diagnosis for OSAS, the average apnea‐hypopnea index was 49.7±18.4 per hour. The average duration of CPAP use before the study was 40.9 months (range, 0.5–178.3 months). The average number of days with morning and evening home BP measurement per patient was 206.6±122.7 days and 191.2±126.3 days, respectively. In total, 21 487 readings for morning home BP and 20 170 readings for evening home BP were analyzed for night‐to‐night CPAP adherence. There were no significant differences in baseline characteristics between the group with lower and higher CPAP adherence except for age and morning pulse rate. Figure 1 shows the distribution of the hours of CPAP use per night in each individual during the study. The prevalence of good CPAP adherence was 86.8% (18 661/21 487) and 86.9% (17 530/20 170) in the morning home BP and evening home BP matched with the duration of CPAP use taken the previous night, respectively. The mean morning and evening home systolic BP (SBP)/diastolic BP (DBP) values were 131.8±13.3/82.6±10.1 mm Hg and 127.1±16.0/77.0±9.8 mm Hg, respectively.
Table 1.
Baseline Characteristics
| Characteristic | Value |
|---|---|
| No. of patients | 105 |
| Age, y | 61.0±13.2 |
| Men, % | 82.9 |
| Body mass index, kg/m2 | 29.6±5.5 |
| Habitual drinking, % | 30.5 |
| Current smoking, % | 14.3 |
| Diabetes, % | 27.6 |
| Dyslipidemia, % | 50.5 |
| Atrial fibrillation, % | 8.6 |
| Prevalent cardiovascular disease, % | 16.2 |
| Antihypertensive drug use, % | 81.9 |
| Calcium antagonist, % | 58.1 |
| Angiotensin II receptor blocker, % | 56.2 |
| ACE inhibitor, % | 1.9 |
| β‐Blocker, % | 17.1 |
| α‐Blocker, % | 11.4 |
| Diuretics, % | 24.8 |
| Office SBP, mm Hg | 138.0±17.5 |
| Office DBP, mm Hg | 81.5±12.9 |
| Office PR, bpm | 74.0±11.0 |
| Morning SBP, mm Hg | 131.8±13.3 |
| Morning DBP, mm Hg | 82.6±10.1 |
| Morning PR, bpm | 68.5±9.5 |
| Evening SBP, mm Hg | 127.1±16.0 |
| Evening DBP, mm Hg | 77.0±9.8 |
| Evening PR, bpm | 72.8±10.3 |
| Parameters of sleep apnea | |
| AHI, events/h | 49.6±18.4 |
| Obstructive apnea index, events/h | 28.7±18.0 |
| Mixed apnea index, events/h | 4.6±7.4 |
| Central apnea index, events/h | 2.0±3.4 |
| Hypopnea index, events/h | 20.7±15.9 |
| Minimal SpO2, %) | 72.4±10.4 |
| Mean SpO2, %) | 91.2±3.9 |
Data are mean±SD or percentages. ACE indicates angiotensin‐converting enzyme; AHI, apnea‐hypopnea index; DBP, diastolic blood pressure; PR, pulse rate; SBP, systolic blood pressure; and SpO2, oxyhemoglobin saturation.
Figure 1. Distribution of the hours of continuous positive airway pressure (CPAP) use per night in each individual during study.
Model: systolic blood pressure=MM; c240; age 65 years; male sex; body mass index b25; habits: drink, current smoking; diabetes; cardiovascular disease; antHT; MM*c240. ID indicates identification.
Table 2 shows the association between good CPAP adherence and home BP. Good CPAP adherence was negatively associated with morning home SBP (β, −0.670; P=0.004), and this association remained after adjustment for covariates (β, −0.663; P=0.004). When we also stratified the individuals according to demographics (<65 years old versus ≥65 years old; women or men; <25 versus ≥25 kg/m2 in BMI; and the absence versus presence of smoking, drinking, diabetes, CVD, and the use of an antihypertensive drug), adjusted multiple linear regression analysis showed that good CPAP adherence was negatively associated with morning home SBP in individuals ≥65 years old, men, and those with BMI ≥25 kg/m2, diabetes, CVD, or the use of an antihypertensive drug. In the subgroup analysis, this association was larger in participants with BMIs ≥25 kg/m2.
Table 2.
Association Between Good Continuous Positive Airway Pressure Adherence (≥4 h per Night) and Morning Systolic Blood Pressure
| Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | No. of days | β (95% CI) | P value | P int value | β (95% CI) | P value | P int value | |
| All patients | 104 | 206.6±122.7 | −0.670 (−1.124 to −0.215) | 0.004 | NA | −0.663 (−1.118 to −0.208) | 0.004 | NA |
| Age | ||||||||
| <65 y | 61 | 162.1±114.8 | −0.345 (−0.969 to 0.280) | 0.280 | 0.143 | −0.342 (−0.967 to 0.283) | 0.283 | 0.143 |
| ≥65 y | 43 | 269.7±105.5 | −1.027 (−1.690 to −0.364) | 0.002 | −1.023 (−1.686 to −0.360) | 0.003 | ||
| Sex | ||||||||
| Women | 18 | 214.0±116.2 | −0.539 (−1.753 to 0.676) | 0.385 | 0.826 | −0.549 (−1.764 to 0.667) | 0.376 | 0.827 |
| Men | 86 | 205.1±124.6 | −0.686 (−1.177 to −0.196) | 0.006 | −0.683 (−1.174 to −0.193) | 0.006 | ||
| BMI | ||||||||
| <25 kg/m2 | 24 | 261.0±123.1 | 0.077 (−0.720 to 0.875) | 0.849 | 0.044 | 0.077 (−0.721 to 0.874) | 0.851 | 0.046 |
| ≥25 kg/m2 | 80 | 190.3±118.5 | −0.962 (−1.513 to −0.410) | <0.001 | −0.955 (−1.507 to 0.404) | <0.001 | ||
| Drinking | ||||||||
| Yes | 32 | 221.8±110.2 | −0.097 (−0.975 to 0.781) | 0.828 | 0.102 | −0.093 (−0.971 to 0.785) | 0.836 | 0.102 |
| No | 72 | 199.9±128.0 | −0.921 (−1.448 to −0.394) | <0.001 | −0.911 (−1.437 to −0.384) | <0.001 | ||
| Smoking | ||||||||
| Yes | 15 | 97.5±86.2 | −1.806 (−3.408 to −0.203) | 0.027 | 0.129 | −1.793 (−3.400 to −0.186) | 0.029 | 0.125 |
| No | 89 | 225.0±118.6 | −0.558 (−1.033 to −0.084) | 0.021 | −0.552 (−1.027 to −0.078) | 0.023 | ||
| Diabetes | ||||||||
| Yes | 29 | 172.4±135.1 | −1.402 (−2.373 to −0.431) | 0.005 | 0.102 | −1.394 (−2.366 to −0.423) | 0.005 | 0.104 |
| No | 75 | 219.8±115.8 | −0.473 (−0.989 to 0.042) | 0.072 | −0.467 (−0.982 to 0.048) | 0.076 | ||
| Prevalent cardiovascular disease | ||||||||
| Yes | 17 | 248.1±110.6 | −1.346 (−2.458 to −0.234) | 0.018 | 0.144 | −1.353 (−2.465 to −0.241) | 0.017 | 0.143 |
| No | 87 | 198.5±123.9 | −0.500 (−0.997 to −0.004) | 0.048 | −0.493 (−0.989 to 0.004) | 0.052 | ||
| Use of an antihypertensive drug | ||||||||
| Yes | 85 | 208.5±124.3 | −0.862 (−1.385 to −0.339) | 0.001 | 0.144 | −0.853 (−1.377 to −0.330) | 0.001 | 0.150 |
| No | 19 | 198.1±118.3 | −0.075 (−0.996 to 0.845) | 0.873 | −0.064 (−0.985 to 0.856) | 0.891 | ||
BMI indicates body mass index; int, interaction; and NA, not applicable.
Similar findings were observed in the association between good CPAP adherence and morning home DBP (Table 3). On the other hand, there was no significant association between good CPAP adherence and evening home SBP or DBP (Tables S1 and S2). Of 105 participants, 21 (20%) changed their antihypertensive medication during follow‐up (15.2% increased the amount of similar antihypertensive drugs or added another antihypertensive drug; 14.3% decreased the amount of similar antihypertensive drugs or decreased the number of antihypertensive drugs). After additional adjustment for the change in antihypertensive medication, the association between high CPAP adherence and morning home SBP remained (β, −0.664; P=0.004). When we further added uncontrolled office SBP at baseline (presence or absence of ≥140 mm Hg in office SBP) as a covariate, the result did not change from those of the previous model (β, −0.680 [95% CI, −1.136 to −0.224]; P=0.003). After adding the residual apnea‐hypopnea index (presence or absence of a score ≥5 as noted by CPAP devices) to the covariates, the association between good CPAP adherence and morning home SBP remained (β, −0.693 [95% CI, −1.149 to −0.238]; P=0.003). After dividing the respective nights within each patient into tertiles dependent on the total hours of daily CPAP use, we analyzed the association between the tertiles and morning home SBP. Compared with the nights with the lowest of the time of CPAP use, the nights with the second (β, 0.09 [95% CI, −0.21 to 0.39]) and third tertiles (β, 0.03 [95% CI, −0.26 to 0.33]) showed no significantly greater association with morning home SBP. In the sensitivity analysis, good CPAP adherence defined as >3 hours per night was negatively associated with morning home SBP (β, −1.146 [95% CI, −1.700 to −0.592]; P<0.001), and this association remained after adjustment for covariates (β, −1.137 [95% CI, −1.691 to −0.583]; P<0.001).
Table 3.
Association Between Good Continuous Positive Airway Pressure Adherence (≥4 h per Night) and Morning Diastolic Blood Pressure
| Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | No. of days | β (95% CI) | P value | P int value | β (95% CI) | P value | P int value | |
| All patients | 104 | 206.6±122.7 | −0.835 (−1.129 to −0.541) | <0.001 | NA | −0.829 (−1.123 to −0.535) | <0.001 | NA |
| Age | ||||||||
| <65 y | 61 | 162.1±114.8 | −0.472 (−0.899 to −0.044) | 0.031 | 0.012 | −0.468 (−0.895 to −0.040) | 0.032 | 0.011 |
| ≥65 y | 43 | 269.7±105.5 | −1.230 (−1.636 to −0.824) | <0.001 | −1.231 (−1.637 to −0.825) | <0.001 | ||
| Sex | ||||||||
| Women | 18 | 214.0±116.2 | −0.650 (−1.451 to 0.152) | 0.112 | 0.607 | −0.631 (−1.433 to 0.172) | 0.123 | 0.581 |
| Men | 86 | 205.1±124.6 | −0.864 (−1.180 to −0.549) | <0.001 | −0.862 (−1.177 to −0.546) | <0.001 | ||
| BMI | ||||||||
| <25 kg/m2 | 24 | 261.0±123.1 | −0.544 (−1.031 to −0.057) | 0.028 | 0.221 | −0.542 (−1.028 to −0.055) | 0.029 | 0.227 |
| ≥25 kg/m2 | 80 | 190.3±118.5 | −0.951 (−1.314 to −0.588) | <0.001 | −0.945 (−1.308 to −0.582) | <0.001 | ||
| Drinking | ||||||||
| Yes | 32 | 221.8±110.2 | −0.912 (−1.478 to −0.346) | 0.002 | 0.758 | −0.913 (−1.479 to −0.347) | 0.002 | 0.715 |
| No | 72 | 199.9±128.0 | −0.803 (−1.144 to −0.462) | <0.001 | −0.791 (−1.132 to −0.451) | <0.001 | ||
| Smoking | ||||||||
| Yes | 15 | 97.5±86.2 | −1.052 (−2.142 to 0.037) | 0.058 | 0.680 | −0.982 (−2.074 to 0.111) | 0.078 | 0.665 |
| No | 89 | 225.0±118.6 | −0.813 (−1.119 to −0.508) | <0.001 | −0.809 (−1.114 to −0.503) | <0.001 | ||
| Diabetes | ||||||||
| Yes | 29 | 172.4±135.1 | −0.721 (−1.375 to −0.066) | 0.031 | 0.707 | −0.706 (−1.360 to −0.051) | 0.035 | 0.671 |
| No | 75 | 219.8±115.8 | −0.864 (−1.193 to −0.535) | <0.001 | −0.861 (−1.189 to −0.532) | <0.001 | ||
| Prevalent cardiovascular disease | ||||||||
| Yes | 17 | 248.1±110.6 | −0.922 (−1.614 to −0.230) | 0.009 | 0.772 | −0.926 (−1.618 to −0.234) | 0.009 | 0.751 |
| No | 87 | 198.5±123.9 | −0.814 (−1.139 to −0.490) | <0.001 | −0.805 (−1.130 to −0.481) | <0.001 | ||
| Use of an antihypertensive drug | ||||||||
| Yes | 85 | 208.5±124.3 | −0.896 (−1.230 to −0.563) | <0.001 | 0.474 | −0.890 (−1.223 to −0.557) | <0.001 | 0.477 |
| No | 19 | 198.1±118.3 | −0.647 (−1.282 to −0.012) | 0.046 | −0.639 (−1.274 to −0.003) | 0.049 | ||
BMI indicates body mass index; int, interaction; and NA, not applicable.
Figure 2 shows the average morning and evening home SBP measurements for each month in individuals with or without high adherence to CPAP. Average morning home SBP in December, January, February, and March in the individuals with good adherence to CPAP was significantly lower than that in those without good adherence, respectively. The largest difference in monthly morning SBP between the group with good adherence to CPAP and those without was 3.7 mm Hg in January. In the group with good adherence to CPAP, the highest and lowest morning home SBP were 129.5 mm Hg in November and 124.8 mm Hg in July, respectively, whereas the highest and lowest morning home SBP were 132.5 mm Hg in January and 124.7 mm Hg in June in those without good adherence to CPAP. There was a significant interaction between adherence to CPAP and morning home SBP throughout the 1‐year study period (P<0.001), but not between adherence to CPAP and evening home SBP. Although there was a significant difference in evening home SBP in June between those with and without good adherence to CPAP, there was no interaction between those with and without good adherence to CPAP. For seasonal variation of morning and evening home DBP, the trend was almost identical to that for morning and evening home SBP (Figure S1).
Figure 2. Monthly means of (A) home morning and (B) home evening systolic blood pressure (SBP).
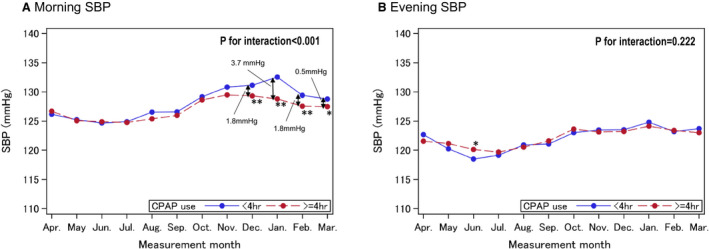
Significant at *P<0.05, **P<0.01 vs poor adherence to continuous positive airway pressure (CPAP) therapy (<4 hours per night) after adjustment by age, sex, body mass index, drinking, smoking, diabetes, prevalent cardiovascular disease, and use of an antihypertensive drug.
Discussion
In this study, we observed several important findings. First, when we investigated the association between night‐to‐night good adherence to CPAP therapy (defined as >4 hours per night of CPAP use) and morning home BP in patients with OSA over a 1‐year observation period, night‐to‐night good adherence to CPAP therapy was attributed to a reduction in home SBP on the next morning, but not to a reduction in evening home BP. In the subgroup analysis, this association was larger in older participants, men, and participants with obesity, smoking, prevalent diabetes and CVD, or the use of antihypertensive drugs. Second, the favorable effect of good CPAP adherence on morning home SBP was more pronounced in winter than in the 3 other seasons. Our findings confirm that the amelioration of hypertension previously associated with good adherence to CPAP therapy extends to a reduction in home BP, which could lead to the prevention of CVD events.
To the best of our knowledge, this is the first study to examine the association between night‐to‐night adherence to CPAP therapy and changes in BP on the next day as assessed by home BP measurement. International guidelines have recommended that hypertension be managed using out‐of‐office BP measurement (ie, ambulatory BP monitoring and HBPM), 3 , 4 , 5 because both ambulatory and HBPM exhibit stronger prognostic power compared with office BP. Several meta‐analyses showing a beneficial effect of CPAP therapy on BP reduction have been reported. 7 , 21 , 22 However, in those studies, the evaluation of BP was performed by office BP and/or ambulatory BP monitoring. Evidence of an association between morning home BP and the progression of target organ damage or cardiovascular events has been accumulating. 9 , 10 , 23 The results of our study contribute the clinically relevant information that CPAP therapy is associated with morning home BP, which in turn would help to prevent cardiovascular events. A previous study examined the association between the severity of sleep disordered breathing (SDB) assessed by pacemakers with implanted SDB monitoring and incident atrial fibrillation. 24 That result showed that, on a per‐patient basis, the nights in the highest quartile of respiratory disturbance index were associated with a risk of incident atrial fibrillation compared with the nights with the lowest oxygen desaturation index. The present and previous study confirms that nightly severity of SDB may be a more accurate daily risk factor for CVD than SDB severity per se.
In the subgroup analysis, the reduction of morning home BP by good CPAP adherence was remarkable in the individuals with BMI ≥25 kg/m2. Moreover, good CPAP adherence was negatively associated with morning home SBP in individuals with other cardiovascular risk factors (ie, old age, male sex, and diabetes). The population with these cardiovascular risk factors is known to have increased sympathetic nervous activity. 25 , 26 , 27 In addition to a mechanism by which sympathetic activation causes a BP response in patients with OSA 28 and the linkage between sympathetic nervous activity after awaking and morning BP, 29 , 30 the presence of cardiovascular risk may deteriorate morning BP, which in turn would result in a greater response to CPAP therapy. Although several studies have investigated whether CPAP therapy is beneficial for secondary prevention in patients with CVD, 20 , 31 , 32 those studies did not examine whether CPAP therapy is beneficial even in patients with poor adherence to CPAP therapy. In addition, those previous studies assessed CPAP adherence using the internal clock built into the CPAP device, as we did in the present study. Although this method has been widely used in clinical practice, not only the evaluation of night‐to‐night CPAP adherence but also home BP measurements on the next day might augment the benefit of long‐term CPAP therapy, which would contribute to a prevention of CVD events.
In the meta‐analysis comparing the association of CPAP treatment versus untreated controls with changes in office SBP and DBP, the reductions in office SBP and DBP were 2.5 mm Hg and 2.0 mm Hg, respectively. 7 Compared with those results and others, the effect of CPAP adherence on home BP in this study was small. This discrepancy in outcomes may be explained by differences in study settings between our study and previous studies. These previous studies compared BP changes between groups with and without CPAP treatment, whereas our study enrolled patients who had already been introduced to CPAP treatment to determine the daily effect of CPAP adherence on home BP. In addition, the follow‐up duration was longer in our study than in previous studies. One can speculate that in populations in which CPAP has been used for a long period of time, the daily effect of CPAP adherence on BP may be small.
Several studies have demonstrated a significant seasonal variation in home BP, with values higher in winter than summer. 13 , 14 , 15 One previous study on the seasonal variation in home BP among 64 536 Japanese subjects showed that the maximum winter–summer difference of home morning SBP was 6.2 mm Hg in men and 7.3 mm Hg in women. 13 In our present study, the maximum winter–summer difference of home morning SBP among individuals without good adherence to CPAP therapy was 7.8 mm Hg (132.5 mm Hg versus 124.7 mm Hg; see Figure 2), which was marginally similar to the seasonal home SBP difference in the previous study. In contrast, the individuals with good adherence to CPAP therapy showed less seasonal home BP variation. We previously reported that morning home BP is higher in winter, and this increase may lead to a higher incidence of cardiovascular events in winter compared with the other 3 seasons. 15 In addition, a previous study about the association between seasonal variation in home BP and cardiovascular events demonstrated that a small increase in morning home BP (0–4.8 mm Hg in SBP, 0–2.4 mm Hg in DBP) from summer to winter was associated with better cardiovascular outcome compared with a larger increase between the 2 seasons (≥9.1 mm Hg in SBP, ≥4.5 mm Hg in DBP) in 2787 treated patients with hypertension. 33 The results of the present study also suggest that good adherence to CPAP therapy would reduce the incidence of cardiovascular events throughout the year in patients with OSA . The reason the effect of CPAP adherence on morning SBP differs according to season may be partly explained by the results of a previous study. That study reported that the apnea‐hypopnea index was recorded more frequently in the winter season than in other seasons in patients with a suspected sleep disorder. 34 The authors speculated that the seasonal change in the severity of SDB is caused by winter‐related fat redistribution, use of medication, fluid displacement to the neck, and/or air pollution. Another possible explanation for seasonal change in the severity of SDB may be seasonal changes in serum 25‐hydroxyvitamin D levels, 35 which are lower in the winter season. 36 Low 25‐hydroxyvitamin D is associated with reduced musculoskeletal function. 37 Because impaired upper airway muscle tone has an important role in OSA, low 25‐hydroxyvitamin D may increase the risk of worse OSA. 35 Thus, the pathogenesis of increased risk for OSA in the winter season may be complex.
Strengths and Limitations
The strengths of the present study include its use of the same, validated home BP device, with BP data automatically transmitted to a server, in all participants. This is the first study about the association between internight variability of adherence to CPAP and the home BP level on the following morning.
There are several limitations in this study. First, the number of participants was small. In particular, the small number of subjects limits the statistical power and the feasibility of subgroup analysis. However, the number of home BP readings was over 20 000. Second, risk factors related to BP were assessed at baseline, and changes in these risk factors over time were not taken into account, except for the change in antihypertensive drugs. Third, detailed information about other residual confounding risk factors, which may have influenced home BP, was not available. Fourth, these results may not be generalizable to other settings, particularly for patients with uncontrolled or resistant hypertension.
Perspectives
Good night‐to‐night adherence to CPAP therapy was associated with a reduction in morning home BP in patients with OSA. In addition, the individuals with good CPAP adherence exhibited a reduction in seasonal variation of morning home BP compared with those without good adherence. Further studies will be needed to determine whether the results of this study would be relevant to the prevention of CVD events.
Sources of Funding
This study was funded by Teijin Incorporated.
Disclosures
Dr Kario receives funding from Teijin Incorporated. The remaining authors have no disclosures to report.
Supporting information
Acknowledgments
The authors thank the staff (C. Iwashita and Y. Okawara) and participants of this study.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024865
For Sources of Funding and Disclosures, see page 8.
References
- 1. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 2. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Resp Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080 [DOI] [PubMed] [Google Scholar]
- 3. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 4. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 5. Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–1481. doi: 10.1038/s41440-019-0284-9 [DOI] [PubMed] [Google Scholar]
- 6. Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, Velkeniers B. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta‐analysis of placebo‐controlled randomized trials. Arch Intern Med. 2007;167:757–764. doi: 10.1001/archinte.167.8.757 [DOI] [PubMed] [Google Scholar]
- 7. Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta‐analysis. JAMA. 2015;314:2280–2293. doi: 10.1001/jama.2015.16303 [DOI] [PubMed] [Google Scholar]
- 8. Posadas T, Campos‐Rodriguez F, Sapiña‐Beltrán E, Oscullo G, Torres G, Martinez‐Garcia MA. Obstructive sleep apnea and arterial hypertension: implications of treatment adherence. Curr Hypertens Rep. 2020;22:12. doi: 10.1007/s11906-020-1015-y [DOI] [PubMed] [Google Scholar]
- 9. Hoshide S, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, Matsui Y, Nakamura A, Fukutomi M, Eguchi K, et al. Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the Japanese general practice population: the Japan morning surge‐home blood pressure study. Hypertension. 2016;68:54–61. doi: 10.1161/HYPERTENSIONAHA.116.07201 [DOI] [PubMed] [Google Scholar]
- 10. Kario K, Iwashita M, Okuda Y, Sugiyama M, Saito I, Kushiro T, Teramukai S, Shimada K. Morning home blood pressure and cardiovascular events in Japanese hypertensive patients. Hypertension. 2018;72:854–861. doi: 10.1161/HYPERTENSIONAHA.118.11388 [DOI] [PubMed] [Google Scholar]
- 11. Han SH, Kim HJ, Lee SA. The effect of high evening blood pressure on obstructive sleep apnea‐related morning blood pressure elevation: does sex modify this interaction effect? Sleep Breath. 2019;23:1255–1263. doi: 10.1007/s11325-019-01869-5 [DOI] [PubMed] [Google Scholar]
- 12. Kario K. Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertens Res. 2009;32:428–432. doi: 10.1038/hr.2009.56 [DOI] [PubMed] [Google Scholar]
- 13. Iwahori T, Miura K, Obayashi K, Ohkubo T, Nakajima H, Shiga T, Ueshima H. Seasonal variation in home blood pressure: findings from nationwide web‐based monitoring in Japan. BMJ Open. 2018;8:e017351. doi: 10.1136/bmjopen-2017-017351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanazawa T, Asayama K, Watabe D, Hosaka M, Satoh M, Yasui D, Obara T, Inoue R, Metoki H, Kikuya M, et al. Seasonal variation in self‐measured home blood pressure among patients on antihypertensive medications: HOMED‐BP study. Hypertens Res. 2017;40:284–290. doi: 10.1038/hr.2016.133 [DOI] [PubMed] [Google Scholar]
- 15. Narita K, Hoshide S, Fujiwara T, Kanegae H, Kario K. Seasonal variation of home blood pressure and its association with target organ damage: the J‐HOP study (Japan Morning Surge‐Home Blood Pressure). Am J Hypertens. 2020;33:620–628. doi: 10.1093/ajh/hpaa027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]
- 18. Barbé F, Durán‐Cantolla J, Sánchez‐de‐la‐Torre M, Martínez‐Alonso M, Carmona C, Barceló A, Chiner E, Masa JF, Gonzalez M, Marín JM, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–2168. doi: 10.1001/jama.2012.4366 [DOI] [PubMed] [Google Scholar]
- 19. Takahashi H, Yoshika M, Yokoi T. Validation of two automatic devices: Omron HEM‐7252G‐HP and Omron HEM‐7251G for self‐measurement of blood pressure according to the European society of hypertension international protocol revision 2010. Blood Press Monit. 2015;20:286–290. doi: 10.1097/MBP.0000000000000127 [DOI] [PubMed] [Google Scholar]
- 20. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 21. Pengo MF, Soranna D, Giontella A, Perger E, Mattaliano P, Schwarz EI, Lombardi C, Bilo G, Zambon A, Steier J, et al. Obstructive sleep apnoea treatment and blood pressure: which phenotypes predict a response? A systematic review and meta‐analysis. Eur Respir J. 2020;55:1901945. doi: 10.1183/13993003.01945-2019 [DOI] [PubMed] [Google Scholar]
- 22. Green M, Ken‐Dror G, Fluck D, Sada C, Sharma P, Fry CH, Han TS. Meta‐analysis of changes in the levels of catecholamines and blood pressure with continuous positive airway pressure therapy in obstructive sleep apnea. J Clin Hypertens. 2021;23:12–20. doi: 10.1111/jch.14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoshide S, Kario K, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, Matsui Y, Nakamura A, Fukutomi M, et al. Association of morning and evening blood pressure at home with asymptomatic organ damage in the J‐HOP study. Am J Hypertens. 2014;27:939–947. doi: 10.1093/ajh/hpt290 [DOI] [PubMed] [Google Scholar]
- 24. Linz D, Brooks AG, Elliott AD, Nalliah CJ, Hendriks JML, Middeldorp ME, Gallagher C, Mahajan R, Kalman JM, McEvoy RD, et al. Variability of sleep apnea severity and risk of atrial fibrillation: the VARIOSA‐AF study. JACC. Clin Electrophysiol. 2019;5:692–701. doi: 10.1016/j.jacep.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 25. Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol. 1992;263:H798–H803. doi: 10.1152/ajpheart.1992.263.3.H798 [DOI] [PubMed] [Google Scholar]
- 26. Frontoni S, Bracaglia D, Gigli F. Relationship between autonomic dysfunction, insulin resistance and hypertension, in diabetes. Nutr, Metab, Cardiovasc Dis: NMCD. 2005;15:441–449. doi: 10.1016/j.numecd.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 27. Narkiewicz K, van de Borne PJ, Hausberg M, Cooley RL, Winniford MD, Davison DE, Somers VK. Cigarette smoking increases sympathetic outflow in humans. Circulation. 1998;98:528–534. doi: 10.1161/01.CIR.98.6.528 [DOI] [PubMed] [Google Scholar]
- 28. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Investigat. 1995;96:1897–1904. doi: 10.1172/JCI118235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metabo. 1985;60:1210–1215. doi: 10.1210/jcem-60-6-1210 [DOI] [PubMed] [Google Scholar]
- 30. Johnson AW, Hissen SL, Macefield VG, Brown R, Taylor CE. Magnitude of morning surge in blood pressure is associated with sympathetic but not cardiac baroreflex sensitivity. Front Neurosci. 2016;10:412. doi: 10.3389/fnins.2016.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC [DOI] [PubMed] [Google Scholar]
- 32. Sánchez‐de‐la‐Torre M, Sánchez‐de‐la‐Torre A, Bertran S, Abad J, Duran‐Cantolla J, Cabriada V, Mediano O, Masdeu MJ, Alonso ML, Masa JF, et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8:359–367. doi: 10.1016/S2213-2600(19)30271-1 [DOI] [PubMed] [Google Scholar]
- 33. Hanazawa T, Asayama K, Watabe D, Tanabe A, Satoh M, Inoue R, Hara A, Obara T, Kikuya M, Nomura K, et al. Association between amplitude of seasonal variation in self‐measured home blood pressure and cardiovascular outcomes: HOMED‐BP (Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure) study. J Am Heart Associ. 2018;7:e008509. doi: 10.1161/JAHA.117.008509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cassol CM, Martinez D, da Silva F, Fischer MK, Lenz M, Bós ÂJG. Is sleep apnea a winter disease?: meteorologic and sleep laboratory evidence collected over 1 decade. Chest. 2012;142:1499–1507. doi: 10.1378/chest.11-0493 [DOI] [PubMed] [Google Scholar]
- 35. Goswami U, Ensrud KE, Paudel ML, Redline S, Schernhammer ES, Shikany JM, Stone KL, Kunisaki KM. Vitamin d concentrations and obstructive sleep apnea in a multicenter cohort of older males. Ann Am Thorac Soc. 2016;13:712–718. doi: 10.1513/AnnalsATS.201507-440OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Schoor NM, Knol DL, Deeg DJ, Peters FP, Heijboer AC, Lips P. Longitudinal changes and seasonal variations in serum 25‐hydroxyvitamin d levels in different age groups: results of the longitudinal aging study Amsterdam. Osteoporos Int. 2014;25:1483–1491. doi: 10.1007/s00198-014-2651-3 [DOI] [PubMed] [Google Scholar]
- 37. Janssen HC, Samson MM, Verhaar HJ. Vitamin d deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611–615. doi: 10.1093/ajcn/75.4.611 [DOI] [PubMed] [Google Scholar]
- 38. Rechtschaffen AKA. Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Los angeles, CA: Ucla brain information service/brain research institute; 1968. [Google Scholar]
- 39. Suzuki S, Yoshihisa A, Sato YU, Watanabe S, Yokokawa T, Sato T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, et al. Association between sleep‐disordered breathing and arterial stiffness in heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail. 2018;5:284–291. doi: 10.1002/ehf2.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pinna GD, Robbi E, La Rovere MT, Taurino AE, Bruschi C, Guazzotti G, Maestri R. Differential impact of body position on the severity of disordered breathing in heart failure patients with obstructive vs. central sleep apnoea. Eur J Heart Fail. 2015;17:1302–1309. doi: 10.1002/ejhf.410 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


