Abstract
Background
In the recent decades, the development of novel digital health technologies enables doctors to monitor ECG and vital signs remotely. But the data on applying the noninvasive wearable smartwatch on patients with transcatheter aortic valve replacement (TAVR) are unknown.
Methods and Results
We performed a prospective, observational cohort study to evaluate the feasibility of a novel, virtual, and remote health care strategy for patients with TAVR discharged to home with smart wearable devices. A total of 100 consecutive patients with severe aortic stenosis who underwent elective transfemoral TAVR were enrolled and received the Huawei smartwatch at least 1 day before TAVR. Vital signs, including heart rate, rhythm, oxygen saturation, and activity, were continuously recorded. Single‐lead ECG was recorded twice per day in the week following TAVR discharge and at least 2 days a week for the subsequent month after TAVR discharge. A designated heart team member provided remote health care with the data from the smartwatch when the patient had a need. Thirty‐eight cardiac events were reported in 34 patients after discharge, with most of the events (76.0%) detected and confirmed by the smartwatch. Six patients were advised and readmitted to the hospital for arrhythmia events detected by the smartwatch, of whom 4 patients received pacemaker implantation. The remaining 28 (82.4%) patients received telemedicine monitoring instead of face‐to‐face clinical visits, and 3 of them received new medication treatment under the online guidance of doctors. New‐onset left branch bundle block was found in 48 patients, with transient characteristics, and recovered spontaneously in 30 patients, and new‐onset atrial fibrillation was detected in 4 patients. There were no significant differences in the average weekly heart rates or the ratio of abnormal or low oxygen saturation when compared with the baseline. The average daily steps increased over time significantly (baseline, 870±1353 steps; first week, 1986±2406 steps; second week, 2707±2716 steps; third week, 3059±3036 steps; fourth week, 3678±3485 steps, P<0.001).
Conclusions
Smartwatches can facilitate remote health care for patients discharged to home after undergoing TAVR and enable a novel remote follow‐up strategy. The majority of cardiac clinical events that occurred within 30‐day follow‐up were detected by the smartwatch, mainly because of the record of conduction abnormality.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04454177.
Keywords: arrhythmias, remote health care, smartwatch, transcatheter aortic valve replacement
Subject Categories: Arrhythmias, Aortic Valve Replacement/Transcather Aortic Valve Implantation
Nonstandard Abbreviations and Acronyms
- TAVR
transcatheter aortic valve replacement
Clinical Perspective
What Is New?
Patients who underwent transcatheter aortic valve replacement had shorter lengths of stay and a higher likelihood of being discharge to home.
The noninvasive wearable smartwatch could provide remote health information including continuing 1‐lead ECG, blood oxygen saturation, heart rate, and steps for these patients.
This study was the first to use this promising device to help provide remote health care for patients discharged to home after transcatheter aortic valve replacement; the cardiac clinical events that occurred within 30‐day follow‐up were almost all detected by the smartwatch, mainly because of the record of conduction abnormality.
What Are the Clinical Implications?
Smarter and more intelligent wearable devices enable more patients to benefit from this novel remote health care strategy in follow‐up after cardiac surgery and intervention.
Ambulatory ECG monitoring smartwatch can screen out arrhythmia events in patients discharged to home after transcatheter aortic valve replacement.
Transcatheter aortic valve replacement (TAVR) is a minimally invasive treatment of severe aortic stenosis, which can significantly shorten the in‐hospital time compared with conventional valve surgery and is recommended to all risk profile patients with aortic stenosis in the 2020 American College of Cardiology/American Heart Association updated guideline. 1 , 2 In the past few years, patients undergoing nontransapical approach TAVR had a higher likelihood of home discharge as opposed to skilled nursing facility. 2 However, late postoperative complications, including late conduction disturbances (≥48 hours), remain life‐threatening complications and are impossible to be detected in all patients after home discharge, especially for those who receive next‐day or early discharge. Mobile ECG and vital sign monitoring devices are keys to this issue. However, most prolonged ECG and vital‐sign monitoring devices on the market are wire connected or invasively implanted, which means they are inconvenient and risky for patients’ daily life. Huawei Watch GT 2 Pro ECG edition is a wearable smartwatch that could track health information including continuing 1‐lead ECG, blood oxygen saturation, heart rate, and steps. It can transmit data to most smartphones and upload it to the medical center in time. Therefore, the study was performed for the first time to evaluate the feasibility of clinical events detection and safety of mortality with this promising device to provide remote health care for home discharge patients after the TAVR procedure.
Methods
Study Design and Patient Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. The SMART TAVR (SMART Watch Facilitated Early Discharge in Patients Undergoing Transcatheter Aortic Valve Replacement) registry (NCT04454177) is a prospective cohort study in China. The study protocol was approved by the medical ethics committee of the Second Affiliated Hospital of Zhejiang University and was in accordance with the Declaration of Helsinki. All participants provided informed consent to participating in the study before the procedure.
Participants received a smartwatch paired to their smartphone within 24 hours before the procedure. A smartwatch was employed to record the following parameters: heart rate, steps, pulse oxygen saturation, and single‐lead ECG after triggering. They were instructed how to perform ECGs by placing their finger on the watch’s digital crown using the smartwatch and transmitting smartwatch sensor data via the smartphone application to a web‐based clinical research platform. ECG measurements were required to be at least 15 seconds long to ensure quality and accuracy.
The registry was conducted between July 2020 and March 2021 and a total of 100 consecutive patients undergoing elective transfemoral TAVR were enrolled. The purpose was to telemonitor health for home discharge patients who underwent TAVR through smartwatches during follow‐up. The major exclusion criteria were (1) severe complications of TAVR, such as death, and conversion to surgical aortic valve replacement; (2) life expectancy of <12 months because of non‐heart disease (such as cancer, chronic liver disease, chronic kidney disease, or chronic end‐stage lung disease, etc); (3) severe dementia (cannot sign research informed consent, cannot take care of themselves or cooperate in the study visit); (4) the investigator’s belief that the patient is not suitable to participate in the study or complete the follow‐up prescribed by the protocol from other medical, social and psychological aspects; and (5) current participation in another randomized study. All patients finished a 30‐day follow‐up.
Standard evaluation and diagnostic procedures were performed in preparation for TAVR using standard criteria. Dominant valves implanted in the procedure were Venus A Series (Venus A and Venus A Plus, Venus Medtech, Hangzhou, China). VitaFlow Series (VitaFlow I and VitaFlow II, Microport, Shanghai, China) and SAPIEN 3 valves (Edwards Lifesciences, Irvine, CA) were also applied. Details of TAVR procedures have been previously reported. All TAVR procedures were performed under local anesthesia with sedation, which contributed to early mobilization and early discharge (discharge within 3 days after TAVR). Temporary pacemakers were implanted in all patients during the procedure. ProGlides were used for the puncture site closure to reduce access site‐related complications and facilitate early discharge.
Patients were transferred to the cardiac intensive care unit after the operation and received intensive monitoring. All femoral lines were removed at the end of operation except temporary pacemaker, and radial artery catheterization was removed 4 hours after TAVR. The temporary pacemaker would be removed within 24 hours in the absence of new‐onset or aggravating conduction disturbance. Permanent pacemaker implantation was considered in the case of a high‐grade atrioventricular block or if long intervals occurred.
Data Collection
Data collection included baseline characteristics, procedural data, predischarge outcomes, and follow‐up data. Baseline clinical characteristics, medical comorbidities, and medicines patients were taking were collected the day before the procedure. Periprocedural complications were defined according to Valve Academic Research Consortium‐2 criteria. During hospitalization, 12‐lead ECGs were collected within 24 hours before the procedure, immediately following the procedure, 4 hours following the procedure, and subsequently every day during the hospitalization to determine whether there was a conduction disturbance. Follow‐up data consist of patient‐reported clinical events (include clinical symptoms or new‐onset arrhythmia events), smartwatch sensor data, and examination at 30‐day follow‐up. Patients were required to submit smartwatch biometric data, particularly the 1‐lead ECG data, twice per day in the week following TAVR discharge and at least 2 days a week for the subsequent month after TAVR discharge. If compliance declined, patients would be reminded by a member of the cardiac research team. The final compliance was calculated as the ratio of real recorded times divided by the total planned monitoring times. Furthermore, patients could report their symptoms, such as chest pain, palpitation, and syncope, and record and transmit single‐lead ECGs through the smartphone app simultaneously. Experienced electrophysiologists in the ECG Core‐Lab of this study interpreted ECG on a daily basis to avoid wrong diagnosis and treatment of patients. Patients would be required to seek emergency treatment if necessary. 3 The clinical event committee reviewed and discussed all clinical events regularly to confirm the outcomes in the presented study. The data and safety monitoring board would review data from the ongoing trial and ensure that participants were not exposed to undue risk. Patients were followed up by a face‐to‐face assessment 30 days after the procedure.
Statistical Analysis
All statistical analyses were computed using SPSS 25 (IBM SPSS, Armonk, NY). Kolmogorov‐Smirnov test was used to analyze the distribution of continuous variables. Continuous variables expressed as mean±SD and were compared with Student’s t test or Mann‐Whitney U test based on their distribution. Categorical data were presented as frequency (percentage) and compared with the chi‐square test or Fisher exact test. A 2‐sided P<0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 100 consecutive patients who underwent TAVR between July 16, 2020, and March 16, 2021 received remote health care assisted with Huawei GT 2 Pro ECG edition watches and were included in the study. No patients withdrew from the study and all patients finished 30‐day follow‐up. The average age of the enrolled patients was 73.1±7.6 and 55.0% were men. Overall 71% of the patients had New York Heart Association class III or IV symptoms before the TAVR procedure. Detailed baseline characteristics were provided in Table 1.
Table 1.
Baseline Characteristics
| Number of patients, n=100 | |
|---|---|
| Age, y | 73.1±7.6 |
| Male sex, n (%) | 55 (55.0) |
| Body mass index, kg/m2 | 23.42±3.74 |
| New York Heart Association III/IV, n (%) | 71 (71.0) |
| Society of Thoracic Surgeons score, % | 4.35±3.25 |
| Prior pacemaker, n (%) | 3 (3.0) |
| Hypertension, n (%) | 56 (56.0) |
| Diabetes, n (%) | 21 (21.0) |
| Chronic obstructive pulmonary disease, n (%) | 27 (27.0) |
| Prior percutanous coronary intervention, n (%) | 12 (12.0) |
| Prior electrocardiography | |
| Right branch bundle block, n (%) | 4 (5.0) |
| Left branch bundle block, n (%) | 2 (2.5) |
| First‐degree atrioventricular block, n (%) | 10 (12.5) |
| Atrial fibrillation/atrial flutter, n (%) | 14 (17.5) |
| CHADS‐VASc score | 2.9±1.2 |
| HAS‐BLED score | 1.8±1.0 |
| Anticoagulation, n (%) | 6 (7.5) |
| Echocardiography | |
| Aortic valve area, cm2 | 0.67±0.25 |
| Mean gradient, mm Hg | 55.4±22.2 |
| Maximum velocity, m/s | 4.72±1.11 |
| Left ventricular ejection fraction, % | 59.0±10.5 |
Data are presented as mean±SD or no. (%).
Procedural Characteristics and Clinical Outcome
All patients underwent transfemoral TAVR procedure among whom 91% were implanted with a self‐expanding valve and 9% patients underwent balloon expanding TAVR. Four patients underwent second valve implantation and no patients were transferred to surgical valve replacement. Eighty‐two percent of patients were discharged early, among whom 56% were discharged the next day after the surgery. No death or myocardial infarction occurred during follow‐up. The rate of stroke was 1.0% both in hospital and at 30‐day follow‐up. All patients had New York Heart Association class I or II functions at 30‐day follow‐up. The transvalvular mean gradient was 11.7±5.3 mm Hg and the mean prosthetic valve area was 1.63±0.37 cm2 according to 30‐day echocardiography. Procedural characteristics and clinical outcomes were presented in Table 2 and Table S1.
Table 2.
Clinical Outcome in Hospital and During 30‐Day Follow‐up
| Number of patients, n=100 | |
|---|---|
| In hospital | |
| Mortality, n (%) | 0 (0.0) |
| MI, n (%) | 0 (0.0) |
| Stroke, n (%) | 1 (1.0) |
| Pacemaker implantation, n (%) | 10 (10.0) |
| Next‐day discharge, n (%) | 56 (56.0) |
| Early‐day discharge (≤3 d), n (%) | 82 (82.0) |
| Echocardiography | |
| AVA, cm2 | 1.71±0.48 |
| Mean gradient, mm Hg | 12.4±5.4 |
| Maximum velocity, m/s | 2.41±0.51 |
| LVEF, % | 60.2±9.1 |
| Moderate or severe paravalvular leakage, n (%) | 5 (5.0) |
| 30‐d follow‐up | |
| Mortality, n (%) | 0 (0.0) |
| MI, n (%) | 0 (0.0) |
| Stroke, n (%) | 1 (1.0) |
| New pacemaker implantation, n (%) | 4 (4.0) |
| Rehospitalization, n (%) | 11 (11.0) |
| Cardiac rehospitalization, n (%) | 8 (8.0) |
| Cardiac rehospitalization by watch, n (%) | 6 (6.0) |
| Echocardiography | |
| AVA, cm2 | 1.63±0.37 |
| Mean gradient, mm Hg | 11.7±5.3 |
| Maximum velocity, m/s | 2.32±0.56 |
| LVEF, % | 59.9±9.5 |
| Moderate or severe paravalvular leakage, n (%) | 5 (5.0) |
Data are presented as mean±SD or no. (%). AVA indicates aortic valve area; LVEF, left ventricular ejection fraction; and MI, myocardial infarction.
The Clinical Event, Arrhythmia Findings, and Biometric Parameters Detected by the Smartwatch
The clinical event, arrhythmia findings, and biometric parameters recorded by the watch during 30‐day follow‐up were provided in Table 3 and Figure. The compliance of receiving remote health monitoring reached up to 98.8%. Thirty‐eight cardiac clinical events were reported in 34 patients after discharge. Most of these events (76.0%) were detected and confirmed by the smartwatch, leading to 6 patients readmitted to the hospital. The remaining 28 patients received telemedicine monitoring, and 3 of them received new medication treatment under a doctor’s online guidance. Therapeutic changes occurred in 9 patients, among whom 4 received pacemaker implantation and 5 medical therapy changes (β blocker in 3 patients, potassium treatment in 1, and amiodarone in 1). The therapeutic changes in 9 patients were presented in Table S2.
Table 3.
Clinical Event and Arrhythmias Recorded by Watch During 30‐Day Follow‐up
| Number of patients, n=100 | |
|---|---|
| Clinical event in 30‐d follow‐up | |
| Cardiac clinical event, n | 38 |
| Cardiac clinical event detected by watch, n (%) | 29 (76.0) |
| Cardiac clinical event detected by watch leading to readmission, n (%) | 6 (15.8) |
| Cardiac clinical event detected by watch leading to medication therapy, n (%) | 5 (13.2) |
| Cardiac clinical event detected by watch leading to pacemaker implantation, n (%) | 4 (10.5) |
| Bradyarrhythmias | |
| Patients with bradyarrhythmias, n | 52 |
| Mobitz I, n | 7 |
| Mobitz II, n | 3 |
| Third‐degree atrioventricular block, n | 5 |
| LBBB, n | 48 |
| Baseline LBBB, n | 2 |
| New onset transient LBBB, n | 30 |
| New onset transient LBBB within 1 d after procedure, n (%) | 12 (40.0) |
| New onset persistent LBBB, n | 16 |
| New onset persistent LBBB within 1 d after procedure, n (%) | 13 (81.3) |
| Tachyarrhythmias | |
| Patients with tachyarrhythmias, n | 16 |
| Af/af, n | 16 |
| Baseline Af/af, n | 12 |
| New onset paroxysmal Af/af, n | 3 |
| New onset persistent Af/af, n | 1 |
| Ventricular tachycardia, n | 0 |
| Supraventricular tachycardia, n | 0 |
| Patients with arrhythmias requiring treatment | |
| Pacemaker implantation, n | 4 |
| Change in medical treatment, n | 5 |
Data are presented as mean±SD or no. (%). Af/af indicates atrial fibrillation/atrial flutter; and LBBB, left bundle‐branch block.
Figure 1. Patterns of smartwatch‐facilitated remote health care during follow‐up for home discharge patients undergoing TAVR (top) and clinical event during 30‐day follow‐up with 29 events found by the smartwatch, 6 patients were rehospitalized, 3 patients received new medication remotely, and the remaining 20 patients were in close telemedicine monitoring (Bottom left).
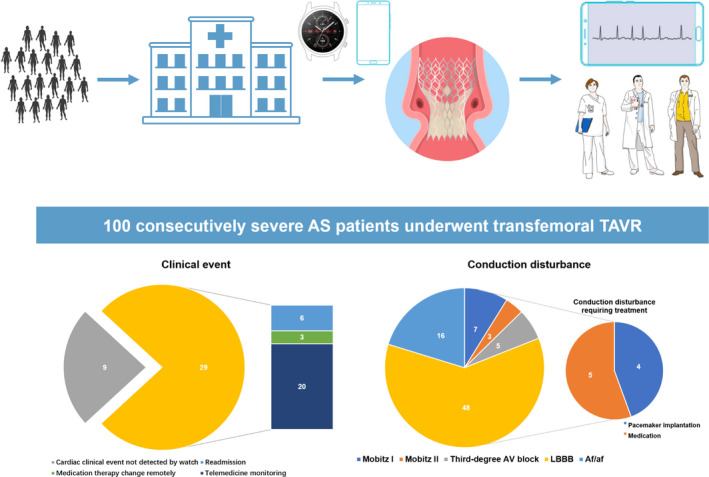
(Bottom right) Conduction disturbance was detected by smartwatch after TAVR, with 48 LBBB patients, 16 atrial fibrillation patients, 7 Mobitz I patients, 3 Mobitz II patients, and 5 third‐degree atrioventricular block patients. Of these patients, 5 patients received medication therapy and 4 patients received pacemaker implantation after discharge with 30‐day follow‐up. Af/af indicates atriabl fibrillation/atrial flutter; AS, aortic stenosis; AV, atrioventricular; LBBB, left bundle‐branch block; and TAVR, transcatheter aortic valve replacement.
The incidence of bradycardia and tachycardiac arrhythmia was diagnosed in 52% and 16% of patients, respectively, from baseline to 30‐day follow‐up. Among 52 patients with bradycardic arrhythmia detected by the smartwatch, left bundle branch‐block (LBBB) was found in 48 patients, 5 patients suffered complete heart block, and 3 developed second‐degree atrioventricular block type 2 (Mobitz II). Six patients developed high‐degree atrioventricular block (including Mobitz II and third‐degree atrioventricular block), of whom 2 patients received pacemaker implantation after discharge. LBBB showed transient characteristics and recovered spontaneously in 30 patients, and persistent LBBB was found in 16 patients. Most of the new‐onset persistent LBBB (81.3%) occurred on the first day after the procedure whereas only 40.0% transient LBBB could be found in this period. As for tachycardiac events, atrial fibrillation or atrial flutter were found in 16 patients, the majority of whom (75.0%) had preoperative atrial fibrillation or flutter (Table 3). No other tachycardiac events such as severe ventricular or supraventricular tachycardia were found by ECG in the hospital or by the smartwatch. In total, permanent pacemakers were implanted in 14 patients, 10 of whom received pacemaker implantation before discharge and 4 received pacemaker implantations during 30‐day follow‐up.
The heart rates, blood oxygen, and steps were recorded by the smartwatch on a real‐time basis every day and were uploaded regularly through smartphone. Each patient had access to his or her own data. The daily steps counted by smartwatch were found to increase over the period from baseline to 30‐day follow‐up (P<0.001). The mean reported steps in 4 weeks were 1986±2406, 2707±2716, 3059±3036, and 3678±3485, respectively, with 870±1353 steps reported at baseline. The average weekly heart rates in the first 4 weeks were 77.6±8.4, 75.9±8.1, 75.1±8.5, and 75.7±8.0, though no trend was found over time. The ratio of abnormality or low oxygen saturation in 4 weeks were 6.5±5.6%, 6.3±6.6%, 6.4±5.8%, and 6.0±5.7% and 11.9±4.6%, 11.6%±4.9%, 12.0%±5.5%, and 11.7%±5.1% respectively. There was also no trend found in oxygen saturation during follow‐up. The biometric parameters recorded by the smartwatch were presented in Table S3 and Figure S1.
Discussion
The main findings of our study are the following: (1) the Huawei smartwatch facilitated postdischarge monitoring can reduce postprocedural medical contact during the follow‐up; (2) the smartwatch‐facilitated early discharge plan is feasible without a significant increase in mortality, clinical outcome, or readmission rates; and (3) post‐TAVR telehealth and monitoring with Huawei smartwatch provide a promising new way to promote remote outpatient management.
Need of Remote Health Care During Follow‐Up
Next‐day or early discharge after TAVR has been validated in multiple studies. 4 , 5 , 6 From 2012 to 2015, patients undergoing TAVR (by a nontransapical approach) had a shorter length of stay and higher likelihood of home discharge as opposed to skilled nursing facility. 2 However, delayed atrioventricular block and other TAVR‐related complications or concomitants require special attention after discharge. 7 Those unsatisfied needs resulted in the rapid development of remote patient management with new technologies and platforms. 8 , 9 Herein, we introduce a novel strategy to provide remote health care for outpatients discharged to home after the TAVR procedure.
To date, ambulatory ECG monitoring devices have been implemented on TAVR patients, which has brought new insight into arrhythmia detection and control. 8 Traditional Holter, external spot single‐lead ECG check (event recorder, smartphone, or smartwatch), mobile cardiovascular telemetry monitoring (lead, patch, or garment based), and implantable cardiac monitor are common ambulatory ECG monitoring devices. 8 Certain monitoring devices may have disadvantages such as signal quality issues, noncontinuous recording, problems of patient acceptance problems, manual trigger, and financial cost. 8 Thus, a new method in the follow‐up with remote health care after TAVR is direly needed.
Clinical Event Detected by Watch Following TAVR
In the study presented in this paper, 29 (76%) cardiac clinical events were detected by the watch, which led to 6 readmission, 5 medication therapy, and 4 pacemaker implantations. For patients enrolled in the SMART TAVR trial, ECG monitoring, blood oxygen saturation, heart rate monitoring, and telemedicine with smartphone app were all able to detect cardiac clinical events. A remote telemonitoring system was introduced by Mathilde C Hermans in 2018. 9 Such a system has not been tested in a real clinical entity yet. Our smartwatch‐facilitated post‐TAVR management plan has been proved to be feasible and safe with zero mortality. With the extra support of vital‐sign monitoring and remote consulting, we managed to avoid the unnecessary clinical visit while distinguishing 4 patients who developed high‐degree atrioventricular block and were in need of pacemaker implantation. It should be noticed that the mean age of our study population was 73.1 years old and all patients showed excellent adherence, which can undoubtedly serve as a reassurance in terms of patient acceptance. During the study period, we promoted our traditional TAVR management plan to a next‐day discharge program. Finally, 82% of patients were discharged early from the hospital and 56% of patients had the next‐day discharge. Of note, frequent premature ventricular contraction of watch ECG was observed in 1 patient with complaint of palpitation. She was referred to a local hospital and confirmed of hypokalemia. The watch ECG recovered after oral potassium chloride. We believe this remote health care system can boost patient confidence and ensure early recognition of a potential pathophysiological change in early home discharge patients, which enables remote assessment and prompts readmission if necessary.
Arrhythmias Following TAVR
As proved by the present study, Huawei Watch GT 2 Pro was able to screen out more than one half of patients with arrhythmias after TAVR. The rate of high atrioventricular block (Mobitz II and third‐degree atrioventricular block) during 30‐day follow‐up was 6%, and the need for pacemaker implantation for high‐degree atrioventricular block was 2%. The results of this study are consistent with previous works. MARE study (Ambulatory Electrocardiographic Monitoring for the Detection of High‐Degree Atrio‐Ventricular Block in Patients With New‐Onset Persistent Left Bundle Branch Block After Transcatheter Aortic Valve Implantation) found 8% of patients after TAVR experienced high atrioventricular block through the invasive implantable cardiac monitor (Reveal XT). 10 Two main studies using mobile cardiovascular telemetry monitoring after TAVR detected 9% of patients with episodes of high atrioventricular block. 11 , 12 Of note, the majority (75%) of bradyarrhythmia patients were asymptomatic, which was in accordance with our finding that only 2 out of 6 high atrioventricular block patients needed the pacemaker. Moreover, the evolution of conduction disturbance was presented in our previous work. 3
On the other hand, we found only 4% of new‐onset atrial fibrillation/atrial flutter (Af/af) during 30‐day follow‐up period. No ventricular or supraventricular tachycardia was recorded in this study. These results match those observed in earlier studies. 10 , 13 , 14 Anticoagulation treatment was initiated in only 1 patient with persistent Af/af, which was in line with suggestions from the 2021 Journal of the American College of Cardiology State‐of‐the‐Art Review. 8 However, the decision of when to transfer a patient to anticoagulation should be individualized given the higher score of both CHA2DS2‐VASc and HAS‐BLED.
Future Perspective
This study, although preliminary, suggests that smartwatch‐facilitated remote health care was feasible in real‐world clinical circumstances. However, further studies are necessary to secure the clinical benefit of such telemonitoring devices. Our preliminary data showed the increased steps gradually after TAVR, to some extent may be related with the recovery of heart function, which could be investigated in the future study.
Study Limitations
This was a single‐center study, and the valves used in this study were mainly first‐generation ones, which may limit generalizability. Secondly, the current version of the Huawei Watch GT 2 Pro ECG edition has to be manually initiated to start recording a single lead ECG. However, with proper predischarge education, we found it could be handled properly by elderly patients with good tolerance. Immediate alarm generation base on real‐time blood oxygen saturation and heart rate monitoring will be upgraded in the next‐generation software. Third, the follow‐up duration of this study was 30 days; longer‐term monitoring of our cohort may provide a better understanding of telemonitoring after TAVR.
Conclusions
Smartwatches can facilitate remote health care for patients discharged to home after undergoing TAVR and enable a novel remote follow‐up strategy. The cardiac clinical events that occurred within 30‐day follow‐up were almost all detected by the smartwatch, mainly because of the record of conduction abnormality.
Sources of Funding
This study was supported by the funding of Zhejiang Province Science and Technology Department Key R&D Program (No. 2021C03097) of China.
Disclosures
None.
Supporting information
Tables S1–S3
Figure S1
Acknowledgments
The authors sincerely thank the Huawei Research Team for the development and optimization of the ECG algorithm, headed by Mr Xiaoxiang He. Team members include Jiabing Yan, Yumei Chen, Lian Wu, Jianhua Guo, Yong Chen, Xingkai Tu, and Xiaoyun Si. We are grateful to Hasan Jilaihawi, from the Department of Cardiology and Cardiothoracic Surgery, NYU Langone Medical Center, New York, NY, for his contribution to the scientific design of the SMART TAVR trial.
Preprint posted on MedRxiv May 23, 2021. doi: https://doi.org/10.1101/2021.05.22.21256870.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023219
For Sources of Funding and Disclosures, see page 7.
References
- 1. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932 [DOI] [PubMed] [Google Scholar]
- 2. Arora S, Strassle PD, Kolte D, Ramm CJ, Falk K, Jack G, Caranasos TG, Cavender MA, Rossi JS, Vavalle JP. Length of stay and discharge disposition after transcatheter versus surgical aortic valve replacement in the United States. Circ Cardiovasc Interv. 2018;11:e006929. doi: 10.1161/CIRCINTERVENTIONS.118.006929 [DOI] [PubMed] [Google Scholar]
- 3. Liu XB, Fan JQ, Guo YC, Chen YW, Li C, Jiang JB, Wang LH, Wang JA. Smart watch measured conduction disturbance after transcatheter aortic valve replacement. J Am Coll Cardiol. 2021;77:1136. doi: 10.1016/S0735-1097(21)02495-5 [DOI] [Google Scholar]
- 4. Kotronias RA, Teitelbaum M, Webb JG, Mylotte D, Barbanti M, Wood DA, Ballantyne B, Osborne A, Solo K, Kwok CS, et al. Early versus standard discharge after transcatheter aortic valve replacement: a systematic review and meta‐analysis. JACC Cardiovasc Interv. 2018;11:1759–1771. doi: 10.1016/j.jcin.2018.04.042 [DOI] [PubMed] [Google Scholar]
- 5. Wood DA, Lauck SB, Cairns JA, Humphries KH, Cook R, Welsh R, Leipsic J, Genereux P, Moss R, Jue J, et al. The Vancouver 3M (multidisciplinary, multimodality, but minimalist) clinical pathway facilitates safe next‐day discharge home at low‐, medium‐, and high‐volume transfemoral transcatheter aortic valve replacement centers: the 3M TAVR study. JACC Cardiovasc Interv. 2019;12:459–469. doi: 10.1016/j.jcin.2018.12.020 [DOI] [PubMed] [Google Scholar]
- 6. Moriyama N, Vento A, Laine M. Safety of next‐day discharge after transfemoral transcatheter aortic valve replacement with a self‐expandable versus balloon‐expandable valve prosthesis. Circ Cardiovasc Interv. 2019;12:e007756. doi: 10.1161/CIRCINTERVENTIONS.118.007756 [DOI] [PubMed] [Google Scholar]
- 7. Mazzella AJ, Hendrickson MJ, Arora S, Sanders M, Li Q, Vavalle JP, Gehi AK. Shifting trends in timing of pacemaker implantation after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2021;14:232–234. doi: 10.1016/j.jcin.2020.09.034 [DOI] [PubMed] [Google Scholar]
- 8. Muntane‐Carol G, Philippon F, Nault I, Faroux L, Alperi A, Mittal S, Rodes‐Cabau J. Ambulatory electrocardiogram monitoring in patients undergoing transcatheter aortic valve replacement: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;77:1344–1356. doi: 10.1016/j.jacc.2020.12.062 [DOI] [PubMed] [Google Scholar]
- 9. Hermans MC, Van Mourik MS, Hermens HJ, Baan J Jr, Vis MM. Remote monitoring of patients undergoing transcatheter aortic valve replacement: a framework for postprocedural telemonitoring. JMIR Cardio. 2018;2:e9. doi: 10.2196/cardio.9075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodes‐Cabau J, Urena M, Nombela‐Franco L, Amat‐Santos I, Kleiman N, Munoz‐Garcia A, Atienza F, Serra V, Deyell MW, Veiga‐Fernandez G, et al. Arrhythmic burden as determined by ambulatory continuous cardiac monitoring in patients with new‐onset persistent left bundle branch block following transcatheter aortic valve replacement: the MARE study. JACC Cardiovasc Interv. 2018;11:1495–1505. doi: 10.1016/j.jcin.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 11. Tian Y, Padmanabhan D, McLeod CJ, Zhang P, Xiao P, Sandhu GS, Greason KL, Gulati R, Nkomo VT, Rihal CS, et al. Utility of 30‐day continuous ambulatory monitoring to identify patients with delayed occurrence of atrioventricular block after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2019;12:e007635. doi: 10.1161/CIRCINTERVENTIONS.118.007635 [DOI] [PubMed] [Google Scholar]
- 12. Ream K, Sandhu A, Valle J, Weber R, Kaizer A, Wiktor DM, Borne RT, Tumolo AZ, Kunkel M, Zipse MM, et al. Ambulatory rhythm monitoring to detect late high‐grade atrioventricular block following transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;73:2538–2547. doi: 10.1016/j.jacc.2019.02.068 [DOI] [PubMed] [Google Scholar]
- 13. Winter JL, Healey JS, Sheth TN, Velianou JL, Schwalm JD, Smith A, Reza S, Natarajan MK. Remote ambulatory cardiac monitoring before and after transcatheter aortic valve replacement. CJC Open. 2020;2:416–419. doi: 10.1016/j.cjco.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skaf M, Makki N, Coressel A, Matre N, O'Neill S, Boudoulas K, Rushing G, Lilly SM. Rhythm disturbances after transcatheter aortic valve replacement: a strategy of surveillance. Cardiovasc Revasc Med. 2020;21:475–478. doi: 10.1016/j.carrev.2019.07.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


