Abstract
Background
Cardiovascular risk factors are associated with cognitive decline and dementia. Magnetic resonance imaging provides sensitive measurement of brain morphology and vascular brain injury. However, associations of risk factors with brain magnetic resonance imaging findings have largely been studied in White participants. We investigated associations of race, ethnicity, and cardiovascular risk factors with brain morphology and white matter (WM) injury in a diverse population.
Methods and Results
In the Multi‐Ethnic Study of Atherosclerosis, measures were made in 2018 to 2019 of total brain volume, gray matter and WM volume, and WM injury, including WM hyperintensity volume and WM fractional anisotropy. We assessed cross‐sectional associations of race and ethnicity and of cardiovascular risk factors with magnetic resonance imaging measures. Magnetic resonance imaging data were complete in 1036 participants; 25% Black, 15% Chinese‐American, 19% Hispanic, and 41% White. Mean (SD) age was 72 (8) years and 53% were women. Although WM injury was greater in Black than in White participants in a minimally adjusted model, additional adjustment for cardiovascular risk factors and socioeconomic status each attenuated this association, rendering it nonsignificant. Overall, greater average WM hyperintensity volume was associated with older age and current smoking (69% greater vs never smoking); lower fractional anisotropy was additionally associated with higher diastolic blood pressure, use of antihypertensive medication, and diabetes.
Conclusions
We found no statistically significant difference in measures of WM injury by race and ethnicity after adjustment for cardiovascular risk factors and socioeconomic status. In all racial and ethnic groups, older age, current smoking, hypertension, and diabetes were strongly associated with WM injury.
Keywords: brain magnetic resonance imaging, cardiovascular risk factors, race and ethnicity, white matter injury
Subject Categories: Cardiovascular Disease, Epidemiology, Magnetic Resonance Imaging (MRI)
Nonstandard Abbreviations and Acronyms
- FA
fractional anisotropy
- GM
gray matter
- MESA
Multi‐Ethnic Study of Atherosclerosis
- TBV
total brain volume
- WM
white matter
- WMH
white matter hyperintensity
Clinical Perspective
What Is New?
Existing studies of the association of cardiovascular risk factors with brain magnetic resonance imaging measures have included largely White populations.
Our study investigated associations of race, ethnicity and cardiovascular risk factors with measures of white matter injury and brain atrophy in a multiethnic cohort of older individuals.
Our analysis found that although Black participants had more evidence of white matter injury than White participants, these differences were progressively attenuated after adjustment for cardiovascular risk factors and socioeconomic status and became nonsignificant.
What Are the Clinical Implications?
Differences by race in the extent of brain white matter injury were largely explained by the greater burdens of cardiovascular risk factors and socioeconomic disadvantage in Black participants than in White participants.
Cardiovascular health is crucial to brain health, and early treatment of cardiovascular risk factors, including in Black populations, may lessen the burden of white matter injury and brain atrophy later in life.
Alterations in brain structure and function reflect multiple pathologic processes including atrophy and vascular brain injury and are associated with subsequent dementia and Alzheimer’s disease. 1 , 2 , 3 Brain magnetic resonance imaging (MRI) in several large cohort studies has demonstrated associations of traditional cardiovascular risk factors—including age, higher body mass index (BMI), hypertension, smoking, diabetes, and low physical activity level—with structural brain measures such as total brain volume (TBV) and white matter hyperintensity (WMH) volume. 4 , 5 However, studies performed in North America and Europe included predominantly White populations. Available data suggest that compared with White populations, Black populations have a higher burden of cerebral small vessel disease, echoing differences in cardiovascular risk factor distributions and stroke rates, which may arise or be exacerbated by lack of health care access and other forms of structural racism. 6 Comparatively few data are available for Americans of Asian or Hispanic background. 7 , 8 , 9 , 10 , 11 Additionally, some of the available brain MRI data were collected 10 to 25 years ago, and newer MRI protocols provide improved image resolution and quantification.
In the MESA (Multi‐Ethnic Study of Atherosclerosis) Atrial Fibrillation ancillary study, brain MRIs were completed in 2018 and 2019 on 1062 participants from 4 racial and ethnic groups. We provide the brain MRI protocol, describe the participants, and conduct cross‐sectional analyses to characterize associations of race, ethnicity and cardiovascular risk factors with brain morphology and MRI indices of WM injury.
METHODS
The data used in this analysis are available through the MESA Coordinating Center with an approved paper proposal. Instructions for data access may be found at https://www.mesa‐nhlbi.org/.
Study Population
The analysis setting was MESA: a longitudinal study of subclinical atherosclerosis among 6814 men and women 45 to 84 years of age and free of clinically recognized cardiovascular disease at baseline at 6 field centers: Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota. Participants self‐identified as Black, Chinese‐American, Hispanic, or White. Study details have been previously reported. 12 The baseline exam for MESA occurred between 2000 and 2002, and 5 follow‐up exams have been performed, including Exam 6 in 2016 to 2018. At Exam 6, 1942 MESA participants were invited to participate in the Atrial Fibrillation ancillary study involving extended ambulatory cardiac rhythm monitoring 13 followed 1 to 2 years later by brain MRI. Among the 1557 participants who completed cardiac monitoring at Exam 6, 1030 completed the brain MRI in 2018 to 2019, a median of 17 months later. The brain MRI was not completed by 527 participants for the following reasons: metal implant (n=104), claustrophobia (n=63), unable to lie flat or too large for scanner or head coil (n=7), deceased or moved out of the area (n=11), and too ill or declined participation (n=342). A total of 32 participants completed the brain MRI but not the cardiac monitoring, for a total of 1062 participants with brain MRI. Each study site obtained institutional review board approval and all participants provided written informed consent.
Risk Factors
Self‐reported age, sex, race and ethnicity, and maximum attained education level were collected at baseline. All other risk factor data were collected at Exam 6 (2016–2018). Information on smoking status, medication use, and family income was updated; height and weight were measured by study staff. Blood pressure was calculated as the average of the last 2 of 3 seated measurements. Total and high‐density lipoprotein cholesterol, glucose, hemoglobin A1c, and serum creatinine were measured from fasting blood samples; low‐density lipoprotein cholesterol was calculated using the Friedewald equation. Estimated glomerular filtration rate was calculated based on serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation. 14 Diabetes was defined as use of diabetes medication, fasting glucose ≥ 126 mg/dL, or hemoglobin A1c ≥ 6.5%.
At telephone contacts every 9 to 12 months during follow‐up after the baseline visit, participants were asked to identify new hospitalizations and diagnoses. Medical records were obtained and myocardial infarction, heart failure, atrial fibrillation, and stroke during follow‐up were ascertained as previously reported. 12 , 15 , 16 Mean neighborhood socioeconomic status between baseline exam and MESA Exam 5 (2010–2012) was calculated using principal factor analysis of 16 census‐tract‐level variables from American Community Survey 2005 to 2009 and 2007 to 2011 estimates 17 reflecting education, employment, housing, and household income and wealth, as described elsewhere. 18 Missing covariate values were imputed using multiple imputation with chained equations (Stata release 14.2, StataCorp LP, College Station, TX). Covariate data were missing and imputed for fewer than 5% of participants for all covariates.
Brain MRI Acquisition and Processing
Brain MRI scans were acquired on 3‐Tesla (3T) Siemens scanners: Prisma VE11C (University of California Los Angeles, Columbia University, John Hopkins University, Northwestern University, University of Minnesota) and Skyra VD11B (University of California Los Angeles, Wake Forest University). Staff from the University of Pennsylvania Brain MRI Reading Center trained MRI technologists to perform standardized imaging protocols and supervised study quality.
Structural MRI brain sequences included 1 mm isotropic sagittal 3D T1‐weighted, T2‐weighted, and fluid attenuated inversion recovery. Additional imaging acquired for the protocol included axial 2D echo‐planar diffusion‐tensor imaging, axial 3D pseudo‐continuous arterial spin labeling, axial 3D multiecho quantitative susceptibility mapping/susceptibility weighted imaging, and axial 2D resting state and breathhold functional MRI sequences. A detailed brain MRI protocol is included in Data S1.
The participant MRI scans were transferred from the MESA field centers to the Reading Center using a designated, HIPAA‐compliant transfer image and data system (TRIAD, American College of Radiology, Philadelphia, PA). Clinically significant findings identified by the field center or Reading Center radiologist were reported to the participant and with permission, the participant’s physician.
MRI Measures
The present report includes data from the T1, T2, fluid attenuated inversion recovery, and diffusion tensor imaging MRI sequences. Variables of interest derived from the MRI images included TBV, total gray matter (GM) volume, total WM volume, total WMH volume, and WM fractional anisotropy (FA).
An automated pipeline was applied for preprocessing structural MRIs, including inhomogeneity correction 19 and extraction of the intracranial brain tissues and cerebrospinal fluid using multiatlas skull‐stripping. 20 Anatomical regions of interest were identified using a multiatlas label fusion method 21 and were used to segment GM and WM tissues, with the sum of GM and WM defining TBV. Total intracranial volume was defined as the sum of all GM, WM, and cerebrospinal fluid. The volume of WMH, or leukoaraiosis, was measured from inhomogeneity corrected and coregistered fluid attenuated inversion recovery and T1‐weighted images using a deep learning‐based segmentation method. 22 The deep‐learning model was trained using a separate training set with human‐validated segmentation of WMH and was applied to participants to calculate binary WMH masks.
WM FA is a measure of WM integrity calculated from diffusion tensor imaging using automated pipelines. 23 FA is the degree to which water diffusion is limited to a single dimension and is a scalar ranging from 0, indicating equivalent motion in all directions, to 1, indicating motion restricted to a single direction. Here FA is reported as a Z score; low FA is interpreted as indicating poor WM integrity, and characterizes WM injury burden in diseases affecting the WM, including cerebral small vessel disease.
Statistical Analysis
We used linear regression to assess the association of race and ethnicity with TBV, total GM volume, total WM volume, total WMH volume, and WM FA, adjusting for 3 sets of covariates. Model 1 included age, sex, and MESA site; the volumetric measures were also adjusted for total intracranial volume. Model 2 included model 1 covariates plus BMI, smoking status, systolic and diastolic blood pressure, use of hypertension medication, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, diabetes status, and estimated glomerular filtration rate. Model 3 additionally adjusted for educational attainment, income, and neighborhood socioeconomic status. To account for potential clustering by MESA site, all models used robust sandwich estimators. WMH volume is heavily right skewed and was log‐transformed for analysis. Regression estimates for WMH volume are expressed as the percent difference in WMH volume per increment of the exposure, based on the geometric mean ratio.
We used linear regression, including the Model 2 covariates described, to assess the association of established cardiovascular risk factors with brain MRI measures. We used variance inflation factors to investigate the potential for bias due to multicollinearity in this model. We further investigated statistically‐significant associations from this analysis for differences by race and ethnicity and by sex by adding an interaction term for the cardiovascular risk factor of interest and either race and ethnicity or sex, and testing for significance. In sensitivity analyses, we adjusted for or excluded participants with prevalent cardiovascular disease, defined as study‐identified myocardial infarction, heart failure, atrial fibrillation, or stroke before brain MRI. We ran a separate analysis excluding participants with multiple sclerosis (N=3), stroke (N=15), and transient ischemic attack (N=11), and lastly an analysis excluding participants with a self‐reported blow to the head that resulted in loss of consciousness or being “dazed or confused” (N=80). To explore the possibility of nonlinear relationships in the models described, we fit models with restricted cubic splines separately for each continuous cardiovascular risk factor included in the model.
RESULTS
Participant Characteristics
A total of 1062 MESA participants completed brain MRI. Twenty six participants were excluded for focal structural lesions, such as encephalomalacia, tumors, or large infarcts, that could affect image processing, or poor MRI quality control issues, or missing image data, leaving 1036 participants with complete MRI measures in the analysis. Characteristics at Exam 6 of the 1036 participants with brain MRI are described in Table 1. Participants had a mean age of 72 years (SD: 8), 53% were female, and the mean BMI was 28 (SD: 5) kg/m2. Distribution of race and ethnicity was representative of the original MESA cohort: 25% Black, 15% Chinese‐American, 19% Hispanic, and 41% White. Compared with White participants, Black participants had higher BMI, systolic blood pressure, diastolic blood pressure, and use of hypertension medication and diabetes, and lower prevalence of clinical cardiovascular disease; Chinese‐American participants had lower BMI and higher diabetes prevalence; and Hispanic participants had a higher diabetes prevalence (Table 1). Household income and educational attainment also differed in the 4 racial and ethnic groups; larger proportions of White participants had higher levels of income and educational attainment.
Table 1.
Characteristics of MESA Participants With and Without Brain MRI at Exam 6 (2018–2019)
| Characteristic | Exam 6 participants with completed brain MRI | Exam 6 participants without completed brain MRI* | ||||
|---|---|---|---|---|---|---|
| Total | Black | Chinese‐American | Hispanic | White | ||
| N | 1036 | 259 | 155 | 199 | 423 | 2267 |
| Female sex, n (%) | 549 (53) | 154 (59) | 76 (49) | 95 (48) | 224 (53) | 1211 (53) |
| Age, y, mean (SD) | 72 (8) | 73 (8) | 72 (8) | 72 (8) | 73 (8) | 75 (9) |
| Cigarette use | ||||||
| Never, n (%) | 493 (48) | 105 (41) | 108 (70) | 97 (49) | 183 (44) | 1028 (46) |
| Former, n (%) | 482 (47) | 130 (50) | 43 (28) | 92 (46) | 217 (51) | 1106 (49) |
| Current, n (%) | 60 (6) | 24 (9) | 4 (3) | 10 (5) | 22 (5) | 124 (5) |
| Systolic BP, mm Hg, mean (SD) | 127 (21) | 134 (20) | 122 (19) | 125 (18) | 125 (21) | 128 (21) |
| Diastolic BP, mm Hg, mean (SD) | 69 (10) | 72 (10) | 68 (9) | 68 (9) | 68 (10) | 68 (10) |
| Body mass index, kg/m2, mean (SD) | 28 (5) | 30 (5) | 24 (3) | 30 (5) | 28 (5) | 29 (6) |
| High‐density lipoprotein cholesterol, mg/dL, mean (SD) | 60 (18) | 65 (21) | 58 (16) | 53 (14) | 62 (18) | 60 (19) |
| Low‐density lipoprotein cholesterol, mg/dL, mean (SD) | 107 (35) | 108 (37) | 106 (36) | 103 (33) | 109 (35) | 105 (35) |
| Estimated glomerular filtration rate, ml/min/1.73m2, mean (SD) | 77 (19) | 79 (22) | 78 (22) | 79 (19) | 74 (17) | 74 (21) |
| Hypertension medication, n (%) | 602 (58) | 178 (69) | 89 (58) | 116 (58) | 219 (52) | 1430 (64) |
| Diabetes, n (%) | 222 (22) | 67 (26) | 39 (26) | 60 (30) | 56 (13) | 559 (26) |
| Prevalent cardiovascular disease, n (%) | 132 (13) | 29 (11) | 20 (13) | 23 (12) | 60 (14) | 424 (19) |
| Income | ||||||
| <$25,000, n (%) | 230 (23) | 59 (24) | 61 (40) | 68 (35) | 42 (10) | 600 (28) |
| $25,000–$49,999, n (%) | 249 (25) | 69 (28) | 25 (16) | 72 (38) | 83 (21) | 533 (25) |
| $50,000–$99,999, n (%) | 300 (30) | 76 (31) | 37 (24) | 41 (21) | 146 (36) | 571 (26) |
| >$100,000, n (%) | 213 (21) | 39 (16) | 31 (20) | 11 (6) | 132 (33) | 451 (21) |
| Education | ||||||
| <High school, n (%) | 114 (11) | 12 (5) | 31 (20) | 59 (30) | 12 (3) | 310 (14) |
| High school, n (%) | 160 (15) | 45 (17) | 22 (14) | 47 (24) | 46 (11) | 379 (17) |
| Some college, n (%) | 249 (24) | 85 (33) | 20 (13) | 58 (29) | 86 (20) | 539 (24) |
| College degree, n (%) | 271 (26) | 65 (25) | 48 (31) | 23 (12) | 135 (32) | 523 (23) |
| Graduate degree, n (%) | 240 (23) | 51 (20) | 34 (22) | 12 (6) | 143 (34) | 511 (23) |
Prevalent cardiovascular disease=myocardial infarction, stroke, atrial fibrillation, or heart failure
BP indicates blood pressure; MESA, Multi‐Ethnic Study of Atherosclerosis; and MRI, magnetic resonance imaging.
Race and ethnicity distribution of 2265 participants without completed brain MRI:, 26% Black, 12% Chinese‐American, 22% Hispanic, 40% White.
Table 1 also includes characteristics of participants who attended MESA Exam 6 but did not complete the brain MRI. Participants who did not complete brain MRI were older on average and had greater prevalence of hypertension medication, cardiovascular disease, and diabetes. Chinese‐American participants were more likely to be included in our analysis sample and Hispanic participants were slightly less likely to be included. On average, MESA participants not included in this analysis had lower income, and a smaller proportion completed high school or college (Table 1).
Summary statistics for brain MRI measures are presented in Table 2. For all racial and ethnic groups, average TBV was smaller and WMH volume was larger with increasing age (Figure 1).
Table 2.
Summary of MESA Exam 6 (2018–2019) Brain MRI Measures in 1036 Participants
| Brain MRI measure | |
|---|---|
| Total brain volume, mL, mean (SD) | 1092 (114) |
| Total gray matter volume, mL, mean (SD) | 597 (65) |
| Total white matter volume, mL, mean (SD) | 495 (56) |
| Total white matter hyperintensity volume, mL, median (interquartile range) | 2.9 (1.2, 7.5) |
| White matter fractional anisotropy*, mean (SD) | 0.39 (0.03) |
MESA indicates Multi‐Ethnic Study of Atherosclerosis; and MRI, magnetic resonance imaging.
Fractional Anisotropy is a scalar with values between 0 and 1.
Figure 1. Magnetic resonance imaging‐derived total brain volume (A) and white matter hyperintensity volume (B) by age within racial or ethnic groups, with linear fit (line) and 95% CI (shaded).

Association of Race and Ethnicity With Brain MRI Measures
Compared with White participants, Black participants had greater TBV in all models (Table 3). In Model 3, Black participants had on average 11.9 mL larger TBV than White participants (95% CI, 4.8 to 19.1 mL). Black and Hispanic participants had greater WM volume compared with White participants. Black participants also had on average greater WM injury than White participants as measured by higher WMH volume and lower WM FA, but these differences were progressively attenuated after adjustment for cardiovascular risk factors and socioeconomic status and were no longer statistically significant (Table 3, Model 3). No differences in WMH volume or WM FA were detected for Chinese‐American or Hispanic participants compared with White participants.
Table 3.
Associations Between Race, Ethnicity and Brain MRI Measures in MESA from Multivariable Models
| Model 1* | Model 2 † | Model 3 ‡ | ||
|---|---|---|---|---|
| Coef. (95% CI) | Coef. (95% CI) | Coef. (95% CI) | ||
| Total brain volume, mL | White | Ref. | Ref. | Ref. |
| Black | 9.5 (3.0 to 15.9) ‖ | 12.4 (5.5 to 19.4) ‖ | 11.9 (4.8 to 19.1) ‖ | |
| Chinese‐American | −1.6 (−9.5 to 6.3) | −0.8 (−8.9 to 7.3) | −2.5 (−11.4 to 6.3) | |
| Hispanic | 5.9 (−1.4 to 13.1) | 7.8 (0.4 to 15.2) ‖ | 4.7 (−3.2 to 12.6) | |
| Total gray matter volume, mL | White | Ref. | Ref. | Ref. |
| Black | −4.0 (−9.3 to 1.3) | −1.8 (−7.5 to 3.8) | −1.8 (−7.6 to 3.9) | |
| Chinese‐American | −3.9 (−9.7 to 1.8) | −3.1 (−9.1 to 2.8) | −3.8 (−10.3 to 2.7) | |
| Hispanic | 0.8 (−4.7 to 6.2) | 1.6 (−3.9 to 7.2) | 0.0 (−5.9 to 6.0) | |
| Total white matter volume, mL | White | Ref. | Ref. | Ref. |
| Black | 13.5 (9.8 to 17.2) ‖ | 14.3 (10.4 to 18.1) ‖ | 13.8 (9.8 to 17.8) ‖ | |
| Chinese‐American | 2.3 (−2.1 to 6.8) | 2.3 (−2.4 to 7.0) | 1.2 (−3.8 to 6.3) | |
| Hispanic | 5.1 (0.9 to 9.3) ‖ | 6.2 (1.9 to 10.5) ‖ | 4.7 (−0.0 to 9.4) ‖ | |
| Total white matter hyperintensity volume, % difference | White | Ref. | Ref. | Ref. |
| Black | 46.2 (18.5 to 80.4) ‖ | 29.4 (4.0 to 61.0) ‖ | 21.3 (−3.2 to 52.0) | |
| Chinese‐American | 2.1 (−20.9 to 31.9) | −0.1 (−22.9 to 29.4) | −4.8 (−27.2 to 24.4) | |
| Hispanic | 0.7 (−18.7 to 24.6) | 0.1 (−19.6 to 24.7) | −10.4 (−29.2 to 13.5) | |
| White matter fractional anisotropy (SD) § | White | Ref. | Ref. | Ref. |
| Black | −0.19 (−0.34 to −0.05) ‖ | −0.06 (−0.21 to 0.09) | −0.03 (−0.19 to 0.13) | |
| Chinese‐American | 0.01 (−0.17 to 0.20) | 0.04 (−0.15 to 0.23) | 0.05 (−0.14 to 0.25) | |
| Hispanic | 0.06 (−0.10 to 0.22) | 0.12 (−0.04 to 0.28) | 0.14 (−0.03 to 0.31) |
MESA indicates Multi‐Ethnic Study of Atherosclerosis; MRI, magnetic resonance imaging; and WM, white matter.
Model 1 adjusted for age, sex, MESA site, and total intracranial volume (for MRI volumes).
Model 2 adjusted for Model 1 variables and body mass index, smoking status, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, estimated glomerular filtration rate, systolic and diastolic blood pressure, use of antihypertensive medication, and diabetes status.
Model 3 adjusted for Model 2 variables, family income, highest attained education, and neighborhood‐level socioeconomic status.
Fractional anisotropy presented as Z scores; low WM fractional anisotropy is interpreted as indicating poor WM integrity.
Represent those results that reach statistical significance (P≤0.05).
Association of Cardiovascular Risk Factors with Brain MRI Measures
In multivariable models, older age and diabetes were strongly associated with lower TBV, total GM, and total WM volumes, and higher diastolic BP with lower TBV and GM volume (Figure 2, Table S1). Older age and current smoking were associated with greater WMH volume and lower WM FA (Figure 2, Table S2). Higher diastolic blood pressure, use of hypertension medication, and diabetes were all associated with greater WM injury as measured by WM FA. Better kidney function (higher estimated glomerular filtration rate) was associated with higher WM FA, which suggests less WM injury. High‐density lipoprotein and low‐density lipoprotein cholesterol were not associated with brain volumes or measures of WM injury in the multivariable models. We did not find evidence of multicollinearity from variance inflation factors in our main models. In analyses of effect modification by race and ethnicity, compared with White participants, Chinese‐American participants had a slightly weaker association of age with WM FA (interaction P value=0.041). We did not otherwise find evidence of differences by race and ethnicity in the associations of cardiovascular risk factors with the brain MRI measures examined. We found evidence of differences by sex in the associations of age with TBV, age with total GM volume, and age with total WM volume (all interaction P values <= 0.003); advanced age was more strongly associated with low brain volumes in men than women (Table S3). In sensitivity analyses, adjustment for history of clinical cardiovascular disease or exclusion of participants with a history of clinical cardiovascular disease did not materially affect the results presented in Figure 2. Similarly, exclusion of participants with multiple sclerosis, stroke, or transient ischemic attack did not meaningfully influence results, nor did exclusion of participants with a self‐reported history of head injury. Finally, we did not find evidence of meaningful nonlinear relationships of cardiovascular risk factors with brain MRI measures.
Figure 2. Associations between cardiovascular risk factors and brain MRI measures in 1036 MESA participants based on multivariable* models.
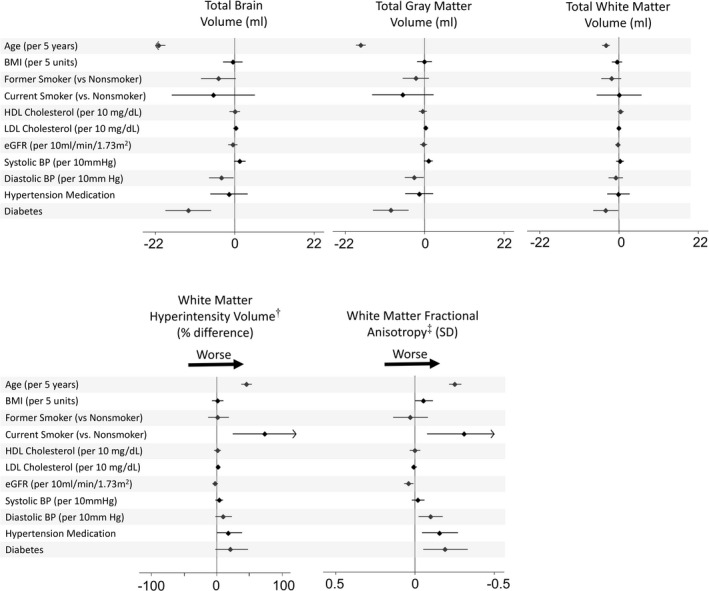
*Regression models include sex, race and ethnicity, MESA site, total intracranial volume (for magnetic resonance imaging volumes), and all cardiovascular risk factors in the leftmost column. †White matter hyperintensity volume presented as percent difference per indicated unit of the exposure based on the geometric mean ratio‡ Fractional anisotropy presented as Z scores; low white matter fractional anisotropy is interpreted as indicating poor white matter integrity. For fractional anisotropy, the x axis has been reversed to aid interpretation. BMI indicates body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MESA, Multi‐Ethnic Study of Atherosclerosis; and MRI, magnetic resonance imaging.
DISCUSSION
In MESA, 3T brain MRI was completed during 2018 to 2019, with complete volumetric and diffusion tensor imaging data available in 1036 participants from 4 racial and ethnic groups. Compared with White participants, Black participants had greater TBV in all models, and Black and Hispanic participants had greater total WM volume. These differences were small, representing about 0.1 SDs of TBV in the analysis sample. The greater burden of WM injury in Black than in White participants in minimally adjusted analyses was progressively attenuated after adjustment for cardiovascular risk factors and socioeconomic status. By contrast, across all 4 racial and ethnic groups, older age and modifiable cardiovascular risk factors were strongly and consistently associated with lower brain volumes and greater WM injury.
Race, Ethnicity, and Brain MRI Measures
Our findings suggest minimal differences in measures of brain atrophy and WM injury by race and ethnicity while supporting established associations between cardiovascular risk factors and brain health. A 2008 study found a higher ratio of brain volume to total intracranial volume among Hispanic participants and Black than in White participants, similar to our findings in Table 3. 7 In our analysis of WM injury measures adjusted only for age, sex, and study site, Black participants had more WM injury than White participants as indicated by higher WMH volume and lower FA. However, adjustment for cardiovascular risk factors and measures of socioeconomic status progressively attenuated these associations, suggesting confounding by the greater burden of cardiovascular risk factors and socioeconomic disadvantage among Black participants. Previous studies have documented greater deep WM hyperintensity volume and more subclinical cerebrovascular disease in participants of African‐American and Afro‐Caribbean descent, respectively, than in White participants. 24 , 25 Our results suggest that some of the previously observed differences may be because of differing burdens of cardiovascular risk factors and socioeconomic disadvantage.
Cardiovascular Risk Factors and Brain MRI Measures
Associations of age and diabetes with brain volumes, as well as associations of age, current smoking, diastolic blood pressure, use of hypertension medication, and diabetes with WM injury have been identified in the Coronary Artery Risk Development in Young Adults study, Atherosclerosis Risk in Communities study, and Framingham Heart Study, among others. 4 , 5 , 26 , 27 , 28 In our analysis, the large magnitude of the association between current smoking status and white matter injury did not differ by race and ethnicity, and this supports the importance of smoking as a modifiable risk factor for WM injury. 29 Similarly, diabetes was associated with 4 of the 5 MRI measures after adjustment for other cardiovascular risk factors, supporting existing evidence of the associations of diabetes with WM injury, cognitive decline, and dementia. In our analysis, the association of older age with smaller brain volumes was stronger in men than in women (Table S3), in agreement with findings from previous studies. 30 , 31 However, in our study, the association of age with brain volumes did not differ by race and ethnicity.
Cognitive function data are available on MESA participants included in this analysis, though they are beyond the scope of this analysis and not assessed here. Additional work is needed to assess the degree to which differences in brain MRI measures in this population are associated with differences in cognitive function.
Characteristics of MESA participants at Exam 6 who were and were not included in this analysis differed in ways that may influence the generalizability of our findings. Compared with participants not included in the analysis, those included were slightly younger and healthier, with more privileged socioeconomic status. Some of these differences may be unavoidable, because brain MRI exclusion criteria (such as metal implants) limited our ability to enroll a representative sample of the MESA population. In addition, older participants and those in poor health may not have wished to undergo the burden of additional testing after Exam 6 participation. These differences may have led to some selection bias, and results should be viewed in light of these considerations.
Our analysis has several limitations that influence our results and their interpretation. MESA participants had a single brain MRI, and the present analysis is cross‐sectional. We cannot demonstrate progression of brain atrophy or WM injury, limiting our ability to make causal inferences. The MESA cohort was free of known cardiovascular disease at enrollment in 2000 to 2002 when the average age was 63 years, and although clinical cardiovascular disease has occurred during follow‐up, MESA participants are not likely fully representative of the larger population of their age and demographic characteristics. Although we have information on blood pressure, diabetes, and other cardiovascular risk factors, we lack detailed data on the severity or control of these risk factors between exams. We do not have detailed data on MRI‐defined infarcts, which may affect values of several measures of interest. However, the prevalence of clinically recognized stroke was low in our MRI cohort, and quality control notes by MRI readers allowed us to identify and exclude participants with large infarcts or clusters of small infarcts that may have affected outcome measures in this analysis. Global measures of brain volume are sensitive but not specific measures, and future studies using these brain MRI data may focus on particular regions of interest. Additionally, our analysis included a large number of tests for significance, increasing the possibility of type I error. We chose not to correct for multiple comparisons because the brain MRI measures are strongly correlated, though many of the associations identified would have remained after Bonferroni multiple‐comparisons adjustment.
CONCLUSIONS
Brain MRI provides a detailed view of structural differences in the brain that may represent atrophy and vascular brain injury, including WM injury, and which may reflect dementia risk. We found that Black and Hispanic participants had slightly greater total brain and WM volumes than White participants. After adjustment for cardiovascular risk factors and socioeconomic status, we did not find consistent evidence of differences in WM injury by race and ethnicity. Rather, the findings from this multiethnic cohort support the importance of modifiable cardiovascular risk factors including smoking, hypertension, and diabetes as risk factors for vascular brain injury in all racial and ethnic groups.
Sources of Funding
This work was supported by the following: National Heart, Lung, and Blood Institute Contracts: 75N92020D00001, HHSN268201500003I, N01HC95159, 75N92020D00005, N01HC95160, 75N92020D00002, N01HC95161, 75N92020D00003, N01HC95162, 75N92020D00006, N01HC95163, 75N92020D00004, N01HC95164, 75N92020D00007, N01HC95165, N01HC95166, N01HC95167, N01HC95168 and N01HC95169, National Heart, Lung, and Blood Institute Grant: R01HL127659, National Center for Advancing Translational Sciences Grants: UL1TR000040, UL1TR001079, and UL1TR001420. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Supporting information
Data S1
Tables S1–S3.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa‐nhlbi.org.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023159.
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, Tanzi RE, Albert MS, Greenberg SM, Guttmann CR. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65:94–100. doi: 10.1001/archneurol.2007.23 [DOI] [PubMed] [Google Scholar]
- 2. Wu A, Sharrett AR, Gottesman RF, Power MC, Mosley TH Jr, Jack CR Jr, Knopman DS, Windham BG, Gross AL, Coresh J. Association of brain magnetic resonance imaging signs with cognitive outcomes in persons with nonimpaired cognition and mild cognitive impairment. JAMA Netw Open. 2019;2:e193359. doi: 10.1001/jamanetworkopen.2019.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brickman AM, Honig LS, Scarmeas N, Tatarina O, Sanders L, Albert MS, Brandt J, Blacker D, Stern Y. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch Neurol. 2008;65:1202–1208. doi: 10.1001/archneur.65.9.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, DeCarli C. Effects of systolic blood pressure on white‐matter integrity in young adults in the Framingham Heart Study: a cross‐sectional study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Launer LJ, Lewis CE, Schreiner PJ, Sidney S, Battapady H, Jacobs DR, Lim KO, D’Esposito M, Zhang Q, Reis J, et al. Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS One. 2015;10:e0122138. doi: 10.1371/journal.pone.0122138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gottesman RF, Fornage M, Knopman DS, Mosley TH. Brain Aging in African‐Americans: The Atherosclerosis Risk in Communities (ARIC) Experience. Curr Alzheimer Res. 2015;12:607–613. doi: 10.2174/1567205012666150701102445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–1061. doi: 10.1001/archneur.65.8.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zahodne LB, Manly JJ, Narkhede A, Griffith EY, DeCarli C, Schupf NS, Mayeux R, Brickman AM. Structural MRI predictors of late‐life cognition differ across african americans, hispanics, and whites. Curr Alzheimer Res. 2015;12:632–639. doi: 10.2174/1567205012666150530203214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mok V, Srikanth V, Xiong Y, Phan TG, Moran C, Chu S, Zhao Q, Chu WWC, Wong A, Hong Z, et al. Race‐ethnicity and cerebral small vessel disease–comparison between Chinese and White populations. Int J Stroke. 2014;9(SA100):36–42. doi: 10.1111/ijs.12270 [DOI] [PubMed] [Google Scholar]
- 10. Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry. 2017;88:669–674. doi: 10.1136/jnnp-2016-315324 [DOI] [PubMed] [Google Scholar]
- 11. Sudre CH, Smith L, Atkinson D, Chaturvedi N, Ourselin S, Barkhof F, Hughes AD, Jager HR, Cardoso MJ. Cardiovascular risk factors and white matter hyperintensities: difference in susceptibility in South Asians compared with Europeans. J Am Heart Assoc. 2018;7:e010533. doi: 10.1161/JAHA.118.010533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 13. Heckbert SR, Austin TR, Jensen PN, Chen LY, Post WS, Floyd JS, Soliman EZ, Kronmal RA, Psaty BM. Differences by race/ethnicity in the prevalence of clinically detected and monitor‐detected atrial fibrillation: MESA. Circ Arrhythm Electrophysiol. 2020;13:e007698. doi: 10.1161/CIRCEP.119.007698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, Zhang Y(L), Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin TR, Wiggins KL, Blackshear C, Yang Y, Benjamin EJ, Curtis LH, Sotoodehnia N, Correa A, Heckbert SR. Atrial fibrillation in an African‐American cohort: The Jackson Heart Study. Clin Cardiol. 2018;41:1049–1054. doi: 10.1002/clc.23020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bureau of the Census US Department of Commerce . American Community Survey 5‐year small area estimates 2005–2009. Washington, DC: Bureau of the Census; 2010. [Google Scholar]
- 18. Moore K, Diez Roux AV, Auchincloss A, Evenson KR, Kaufman J, Mujahid M, Williams K. Home and work neighbourhood environments in relation to body mass index: the Multi‐Ethnic Study of Atherosclerosis (MESA). J Epidemiol Community Health. 2013;67:846–853. doi: 10.1136/jech-2013-202682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi‐atlas skull‐stripping. Acad Radiol. 2013;20:1566–1576. doi: 10.1016/j.acra.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doshi J, Erus G, Ou Y, Resnick SM, Gur RC, Gur RE, Satterthwaite TD, Furth S, Davatzikos C, Alzheimer's NI. MUSE: MUlti‐atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. NeuroImage. 2016;127:186–195. doi: 10.1016/j.neuroimage.2015.11.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doshi J, Erus G, Habes M, DeepMRSeg CD. A convolutional deep neural network for anatomy and abnormality segmentation on MR images. arXiv. preprint posted online July 3, 2019. arXiv:1907.02110.
- 23. Haight T, Nick Bryan R, Erus G, Hsieh M‐K, Davatzikos C, Nasrallah I, D'Esposito M, Jacobs DR, Lewis C, Schreiner P, et al. White matter microstructure, white matter lesions, and hypertension: an examination of early surrogate markers of vascular‐related brain change in midlife. Neuroimage Clin. 2018;18:753–761. doi: 10.1016/j.nicl.2018.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shibata D, Tillin T, Beauchamp N, Heasman J, Hughes AD, Park C, Gedroyc W, Chaturvedi N. African Caribbeans have greater subclinical cerebrovascular disease than Europeans: this is associated with both their elevated resting and ambulatory blood pressure and their hyperglycaemia. J Hypertens. 2013;31:2391–2399. doi: 10.1097/HJH.0b013e328364f5bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nyquist PA, Bilgel MS, Gottesman R, Yanek LR, Moy TF, Becker LC, Cuzzocreo J, Prince J, Yousem DM, Becker DM, et al. Extreme deep white matter hyperintensity volumes are associated with African American race. Cerebrovasc Dis. 2014;37:244–250. doi: 10.1159/000358117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, Shibata DK, Knopman DS, Jack CR, Mosley TH Jr. Blood pressure and white‐matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41:3–8. doi: 10.1161/STROKEAHA.109.566992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, Mosley TH Jr. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76:1879–1885. doi: 10.1212/WNL.0b013e31821d753f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Longstreth WT Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274 [DOI] [PubMed] [Google Scholar]
- 29. Power MC, Deal JA, Sharrett AR, Jack CR Jr, Knopman D, Mosley TH, Gottesman RF. Smoking and white matter hyperintensity progression: the ARIC‐MRI Study. Neurology. 2015;84:841–848. doi: 10.1212/WNL.0000000000001283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS One. 2011;6:e22734. doi: 10.1371/journal.pone.0022734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gur RC, Mozley PD, Resnick SM, Gottlieb GL, Kohn M, Zimmerman R, Herman G, Atlas S, Grossman R, Berretta D. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci U S A. 1991;88:2845–2849. doi: 10.1073/pnas.88.7.2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3.


