Abstract
Background
The COVID‐19 pandemic resulted in a rapid implementation of telemedicine into clinical practice. This study examined whether early outpatient follow‐up via telemedicine is as effective as in‐person visits for reducing 30‐day readmissions in patients with heart failure.
Methods and Results
Using electronic health records from a large health system, we included patients with heart failure living in North Carolina (N=6918) who were hospitalized between March 16, 2020 and March 14, 2021. All‐cause readmission within 30 days after discharge was examined using weighted logistic regression models. Overall, 7.6% (N=526) of patients received early telemedicine follow‐up, 38.8% (N=2681) received early in‐person follow‐up, and 53.6% (N=3711) did not receive follow‐up within 14 days of discharge. Compared with patients without early follow‐up, those who received early follow‐up were younger, were more likely to be Medicare beneficiaries, had more comorbidities, and were less likely to live in an disadvantaged neighborhood. Relative to in‐person visits, those with telemedicine follow‐up were of similar age, sex, and race but with generally fewer comorbidities. Overall, the 30‐day readmission rate (19.0%) varied among patients who received telemedicine visits (15.0%), in‐person visits (14.0%), or no follow‐up (23.1%). After covariate adjustment, patients who received either telemedicine (odds ratio [OR], 0.55; 95% CI, 0.44–0.72) or in‐person (OR, 0.52; 95% CI, 0.45–0.60) visits were similarly less likely to be readmitted within 30 days compared with patients with no follow‐up.
Conclusions
During the COVID‐19 pandemic, the use of telemedicine visits for early follow‐up increased rapidly. Patients with heart failure who received outpatient follow‐up either via telemedicine or in‐person had better outcomes than those who received no follow‐up.
Keywords: electronic health records, heart failure, hospitalization, telemedicine
Subject Categories: Health Services, Heart Failure, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- ADI
Area Deprivation Index
- DUHS
Duke University Health System
Clinical Perspective
What Is New?
Patients with heart failure who received either an in‐person or telemedicine follow‐up visit after a hospitalization had lower risks of 30‐day readmission compared with those who did not receive a follow‐up visit.
There were no significant differences in patients’ age, sex, race, and/or socioeconomic status between those who were followed up in person and via telemedicine.
What Are the Clinical Implications?
Telemedicine may provide a sustainable, cost‐effective, and patient‐centered approach for helping to reduce rehospitalization in patients with heart failure.
The COVID‐19 pandemic has had a major impact on the delivery of health care in the United States over the past two years. 1 , 2 During the early stages of the pandemic, many US health care systems struggled to address the rapid surge in COVID‐19 cases and were forced to reallocate resources to treat the most critically ill patients. 3 , 4 Many patients with chronic conditions were also reluctant to seek care in person owing to fears of contracting COVID‐19 and/or concerns of inadequate health care resources. 1 , 5 , 6 In response to these challenges, the Centers for Medicare & Medicaid Services and others updated their reimbursement policies to allow health care systems to implement telemedicine visits for patients requiring essential health care services and to help reduce transmission of the virus. 2 , 7 , 8 , 9 As a result, many patients have been using telemedicine services to manage their disease as part of their routine medical care or during transitions of care. 8 , 10 , 11 , 12
Heart failure (HF) is one of the most common chronic conditions in the United States and is the leading cause of 30‐day readmissions in older adults. 13 Prior studies have shown that early outpatient follow‐up with a health care provider reduces 30‐day readmissions and improves quality of life in patients with HF. 14 , 15 , 16 , 17 Current clinical guidelines by the American Heart Association recommend outpatient follow‐up within 7 to 14 days after discharge as a strategy to prevent 30‐day readmissions. 18 , 19 Results from a recent randomized control trial have suggested the feasibility of substituting in‐person visits with telemedicine visits post discharge in patients with HF. 20 Another recent publication demonstrated similar rates of mortality, hospital encounters, and the need for intensive care unit care between patients who received telemedicine visits and those with in‐person visits. 12 To date, however, there is a lack of information on whether telemedicine services can also be as effective as in‐person visits in reducing the risks of readmission in real‐world settings. 17 Furthermore, there is concern that access to telemedicine may be limited among some vulnerable populations—including those of lower socioeconomic status as well as older generations who may face a “digital divide.” 8 , 21 , 22
To address these critical questions, our study examined the use of telemedicine visits in patients with HF at a large academic health system during the COVID‐19 pandemic. We specifically addressed (1) whether the early follow‐up rates differed between the pre‐COVID period versus during the COVID‐19 pandemic; (2) how the COVID‐19 pandemic affected early follow‐up visits overall, in person, and via telemedicine; (3) whether use of telemedicine visits varied by patient demographic and clinical characteristics; and (4) whether telemedicine visits were as effective as in‐person visits in reducing 30‐day readmissions. Results are discussed in the context of whether health care systems should continue incorporating telemedicine into current practice as an effective, long‐term strategy to provide routine outpatient follow‐up and improve outcomes in patients with HF.
Methods
This is a cohort, correlational study that used data from electronic health records (EHRs). Because of the sensitive nature of the data collected for this study, qualified researchers trained in human subject confidentiality protocols may send requests to the corresponding author to access the data that support the findings of this study.
Participants
Data for this study come from patients hospitalized in the Duke University Health System (DUHS) with a discharge diagnosis of HF based on International Classification of Diseases, Ninth Revision (ICD‐9) and Tenth Revision (ICD‐10) codes. On March 10, 2020, North Carolina announced a state of emergency to mitigate the spread of COVID‐19 and on March 16 the DUHS announced additional restrictions within the health system. 23 Thus, we included patients with HF who were 18 years of age or older who were hospitalized from March 16, 2020 through March 14, 2021. We excluded patients who died during their index admission and those who were discharged to another acute hospital, a skilled nursing facility or other types of long‐term care facility, rehabilitation or other intermediate care facility, or hospice. 14 , 16 We also excluded patients who left against medical advice. To ensure that we captured patients’ outpatient follow‐up visits, rehospitalizations, and neighborhood characteristics, we further limited our study participants to those who resided in North Carolina with a valid home address and 9‐digit ZIP code. A pre‐COVID group was selected (from March 17, 2019 to March 15, 2020) based on the same inclusion and exclusion criteria (Figure 1).
Figure 1. Inclusion and exclusion criteria.
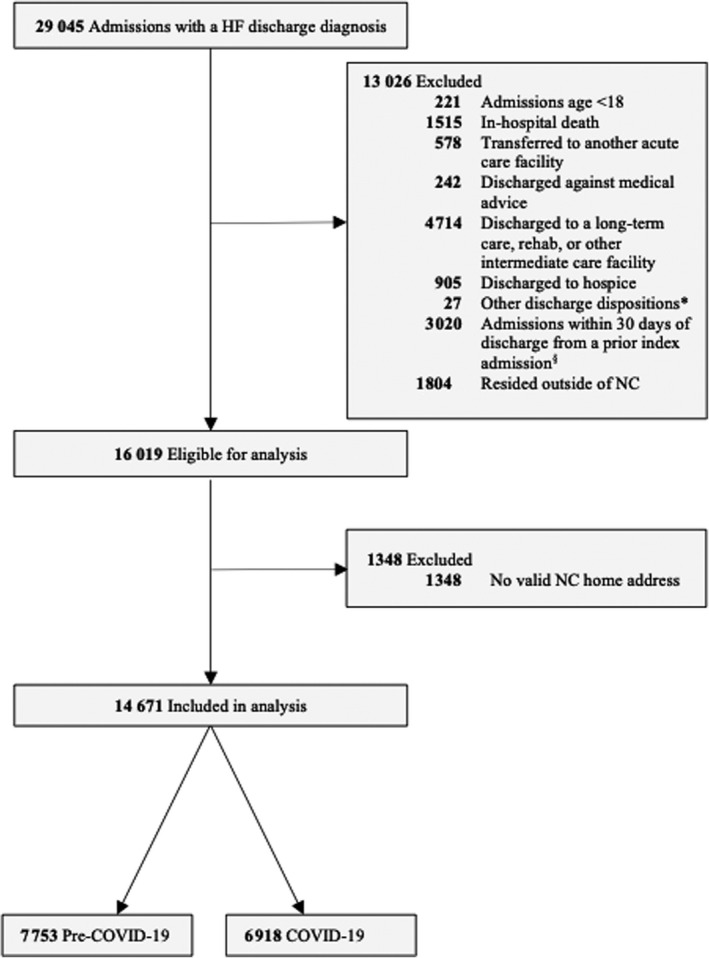
Pre‐COVID 19 period: March 17, 2009 to March 15, 2020. COVID‐19 period: March 16, 2020 to March 14, 2021. *Other discharge dispositions included discharge disposition unknown or discharged to court/law enforcement. §Based on the Centers for Medicare & Medicaid Services 30‐Day Readmission Algorithm, these 3020 hospitalizations were considered readmissions from a prior admission rather than index admissions in this study. HF indicates heart failure; and NC, North Carolina.
Data from patients’ EHRs were extracted using Duke Enterprise Data Unified Content Explorer, a data extraction system that provides access to clinical data stored in an organizational data warehouse. 24 The EHR data were geocoded and linked to census data. The final analytical sample included 6918 patients who were discharged during the COVID‐19 pandemic. The study was approved by the institutional review board at DUHS and no informed consent was required.
Measures
Outpatient Follow‐up Visits
We defined a follow‐up visit within the first 30 days post discharge as an outpatient office visit, clinic visit, or telemedicine visit with a provider from family medicine, internal medicine, geriatrics, or cardiology. From this, we excluded urgent care and emergency department visits as these clinical encounters are unlikely to be planned post discharge follow‐up. 14 , 16 Following current guidelines, early outpatient follow‐up included visits that occurred within the first 14 days of discharge and were categorized into the following categories: (1) in person, (2) telemedicine (eg, audio‐only visits, synchronous audio‐video visits, etc), or (3) no early follow‐up. For patients with multiple outpatient follow‐up visits within 30 days of discharge, we included only the first visit in the analysis. Sensitivity analyses were also conducted for follow‐up visits within the first 7 days after discharge.
Thirty‐Day Readmission
The primary outcome was all‐cause readmission within 30 days of discharge from an index hospitalization. As in prior literature, we identified all‐cause 30‐day readmission (yes/no) based on the number of days after discharge from the index admission to a subsequent inpatient admission using EHRs. 25 We further assessed 30‐day mortality among study participants. Patient mortality was adjudicated by integrating data from the Duke EHR system, the Death Master Files from National Technical Information Services, and the North Carolina death index from the Social Security Administration. 24 A total of 338 patients died within 30 days post discharge (4.9%), and among them, 168 patients had a readmission before death. The 30‐day mortality rate in this study was similar to the rates reported in prior research (2.6%–9.7% in other studies). 25 , 26 , 27 In sensitivity analyses, we used a composite outcome of 30‐day readmission or mortality to account for excess mortality following hospital discharge.
Covariates
We extracted sociodemographic characteristics, diagnoses and procedures, laboratory values, medications, and health care use measures from the patients’ EHRs. Sociodemographic information included age at index admission, sex, race or ethnicity, marital status, and insurance status. Baseline diagnoses and procedures were identified at the index admission using ICD‐9/ICD‐10 codes for acute myocardial infarction, unstable angina, stroke, atrial fibrillation or flutter, mitral or aortic valvular disease, peripheral vascular disease, hypertension, diabetes, hyperlipidemia, chronic obstructive pulmonary disease (COPD), renal disease, dementia, depression, coronary artery bypass graft surgery, percutaneous coronary intervention, implantable cardioverterdefibrillator placement, and permanent pacemaker implantation. Laboratory values during the admission included estimated glomerular filtration rate, serum potassium, and hemoglobin. Baseline medications included beta blockers, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers/angiotensin receptor neprilysin inhibitors, hydralazine, loop diuretics, aldosterone antagonists, statins, and aspirin. Health care use measures included length of stay, admission through emergency department versus other (transfer from other hospitals, admission from clinics, etc), discharge destination (home with versus without home health), any hospitalizations in the past year, and having a primary care provider (PCP) on file.
For patients in the COVID‐19 group, a categorical variable was created to indicate critical periods during the pandemic (early outbreak, March 16–30, 2020; stay at home, March 31–May 4, 2020; reopening, May 5, 2020–March 14, 2021). These periods were defined based on the dates when COVID‐19 related restrictions and executive orders were in place in North Carolina. 28
Patient neighborhood characteristics were measured using the Area Deprivation Index (ADI), a composite measure of neighborhood socioeconomic conditions such as education, income, standard of living, neighborhood quality, and housing quality based on the American Community Survey Five Year Estimates. 29 , 30 Prior research has demonstrated the validity of using ADI in health outcome research. 29 , 31 Details of ADI were documented extensively elsewhere. 29 , 32 We obtained the 2018 ADI from the Neighborhood Atlas and used the 9‐digit ZIP codes from patients’ home address to link the ADI with their EHR data. 29 , 30 As in prior research, we used a dichotomized variable to classify neighborhoods as more disadvantaged if the ADI values ranked in the bottom 15% of the national level. 29 , 31 Preliminary analyses also assessed whether rurality—as defined by the federal rural–urban commuting area codes 33 —played a role in the associations. The results indicated no significant associations and were dropped in the final analyses.
Statistical Analysis
Patient characteristics and outcomes were compared among in‐person, telemedicine, and no early follow‐up groups using Kruskal‐Wallis and Pearson chi‐square tests for continuous and categorical variables, respectively. Patient characteristics, early follow‐up status, and outcomes were also compared between patients who were admitted during the pre‐COVID‐19 period and those in the COVID‐19 period using Mann‐Whitney U and Pearson chi‐square tests for continuous and categorical variables, respectively. Multivariable logistic regression models were used to assess factors associated with early telemedicine versus early in‐person follow‐up. An inverse probability of treatment approach was used to account for potential selection bias related to patients who had more complex health care needs and greater disease severity who were more likely to receive an early outpatient follow‐up after discharge. 34 , 35 We first considered confounders of the treatment outcome relationship and calculated propensity scores (ie. the probability of receiving an early follow‐up) using logistic regression models that included patient age, sex, race or ethnicity, neighborhood characteristics, atrial fibrillation or flutter, mitral or aortic valvular disease, hyperlipidemia, coronary artery bypass graft, percutaneous coronary intervention, estimated glomerular filtration rate, loop diuretics, aldosterone antagonist, principal HF hospitalizations, admission through emergency department, and PCP status (Table S1). We then plotted standardized differences to compare patient characteristics before and after weighting and used a standardized difference of 10 as the marker of balance 12 , 36 (Figure S1). We also used the density curves to show the distributions of the propensity scores in patients who received an early follow‐up and those who did not, before and after weighting. Figure S2 shows adequate overlap between the 2 treatment groups. We used propensity score weighted logistic regression models to evaluate the associations of early outpatient follow‐up and 30‐day readmission. Most variables had no missing data; and variables with missing values (<1%) were imputed to “unknown.” All analyses were performed using Stata 16.0 (StataCorp LP, College Station, TX). P values are reported as 2 sided, with <0.05 used as the threshold for statistical significance.
Results
The number of in‐person, telemedicine, and no early follow‐up by week from March 16, 2020 to March 14, 2021 are presented in Figure 2. The early outpatient follow‐up rates were stable throughout the COVID‐19 pandemic. Compared with the pre‐COVID‐19 period, the proportion of patients receiving early telemedicine follow‐up was significantly higher (0.27% versus 7.60%) during the COVID‐19 period (Table S2). The volume of telemedicine visits during the COVID‐19 period remained high over the course of the early outbreak and stay‐at‐home periods, accounting for nearly 50% of all early outpatient follow‐up visits during that period. However, the number of in‐person follow‐ups began to resume whereas the number of telemedicine follow‐ups declined as North Carlina entered the reopening phase on May 5, 2020. The number of telemedicine follow‐ups remained at a low and stable level throughout the reopening phase.
Figure 2. Early outpatient follow‐up status during the COVID‐19 pandemic.
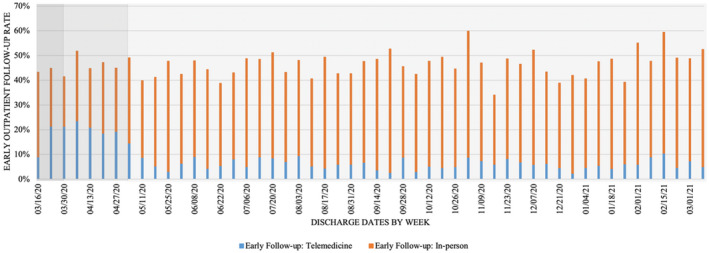
Early outpatient follow‐up was defined as having an outpatient follow up within 14‐days after discharge. The gradient background represents different phases during the COVID‐19 period: Early outbreak period:March 16 to March 31, 2020; Stay‐at‐home period: April 1, 2020 to May 5, 2020; Reopening period: May 6, 2020 to March 14, 2021.
Table 1 displays the patient characteristics and outcomes, stratified by early follow‐up status. The median age (interquartile range) was 67 [19] years, with 45.4% and 51.5% of the patients being female and non‐Hispanic White, respectively. Overall, the early outpatient follow‐up rate was 46.3% during the COVID‐19 period. Among patients who received an early outpatient follow‐up (N=3207), about 1 in 6 had their follow‐up visits via telemedicine (N=526). There was no significant difference in sex and race among patients with in‐person, telemedicine, or no early follow‐up visit. Compared with patients with no early follow‐up, patients with an early follow‐up either in‐person or telemedicine visits were slightly older, were more likely to be Medicare fee‐for‐service (FFS) beneficiaries, and had more comorbidities such as angina, atrial fibrillation or flutter, mitral or aortic valvular disease, hypertension, and hyperlipidemia. Patients who received an early follow‐up were more likely to have a principal index admission for HF, were more likely to be admitted through the emergency department, had slightly longer lengths of stay, and were more likely to have a PCP on file compared with patients with no early follow‐up. In addition, patients who lived in a disadvantaged neighborhood were less likely to receive an early follow‐up. Overall, patient characteristics, follow‐up rate, and readmission rate during the COVID‐19 pandemic were largely the same as those in the pre‐COVID‐19 period (Table S2).
Table 1.
Baseline Characteristics of Study Participants by Outpatient Follow‐up Status During the COVID‐19 Pandemic
|
No follow‐up (N=3711) |
Early follow‐up (N=3207) |
P value Early vs no follow‐up |
|||
|---|---|---|---|---|---|
|
Overall (N=3207) |
In person (N=2681) |
Telemedicine (N=526) |
|||
| Sociodemographic characteristics | |||||
| Age, y, median (IQR) | 67 (19) | 69 (19) | 69 (19) | 70 (19) | <0.001 |
| Female sex | 1685 (45.4) | 1477 (46.1) | 1219 (45.5) | 258 (49.1) | 0.588 |
| Race | 0.051 | ||||
| Non‐Hispanic White | 1912 (51.5) | 1738 (54.2) | 1471 (54.9) | 267 (50.8) | |
| Non‐Hispanic Black | 1510 (40.7) | 1252 (39.0) | 1031 (38.5) | 221 (42.0) | |
| Other || | 289 (7.8) | 217 (6.8) | 179 (6.7) | 38 (7.2) | |
| Currently married † | 1694 (45.7) | 1495 (46.6) | 1282 (47.8) | 213 (40.5) | 0.420 |
| Insurance ‡ | <0.001 | ||||
| Medicare fee‐for‐service | 1504 (40.5) | 1412 (44.0) | 1157 (43.2) | 255 (48.5) | |
| Medicare Advantage | 1112 (30.0) | 967 (30.2) | 805 (30.0) | 162 (30.8) | |
| Medicaid | 366 (9.9) | 238 (7.4) | 190 (7.1) | 48 (9.1) | |
| Other § | 729 (19.6) | 590 (18.4) | 529 (19.7) | 61 (11.6) | |
| Disadvantaged neighborhood | 594 (16.0) | 376 (11.7) | 307 (11.5) | 69 (13.1) | <0.001 |
| Diagnosis and procedures | |||||
| Acute myocardial infarction † | 263 (7.1) | 252 (7.9) | 227 (8.5) | 25 (4.8) | 0.223 |
| Angina | 1944 (52.4) | 1796 (56.0) | 1512 (56.4) | 284 (54.0) | 0.003 |
| Stroke or transient ischemic attack | 114 (3.1) | 105 (3.3) | 92 (3.4) | 13 (2.5) | 0.632 |
| Atrial fibrillation or flutter | 1337 (36.0) | 1524 (47.5) | 1274 (47.5) | 250 (47.5) | <0.001 |
| Mitral or aortic valvular disease ‡ | 739 (19.9) | 766 (23.9) | 675 (25.2) | 91 (17.3) | <0.001 |
| Peripheral vascular disease | 509 (13.7) | 465 (14.5) | 381 (14.2) | 84 (16.0) | 0.350 |
| Hypertension | 3370 (90.8) | 2979 (92.9) | 2484 (92.7) | 495 (94.1) | 0.002 |
| Diabetes | 1916 (51.6) | 1636 (51.0) | 1358 (50.7) | 278 (52.9) | 0.609 |
| Hyperlipidemia | 2215 (59.7) | 2154 (67.2) | 1785 (66.6) | 369 (70.2) | <0.001 |
| Chronic obstructive pulmonary disease ‡ | 948 (25.6) | 832 (25.9) | 661 (24.7) | 171 (32.5) | 0.706 |
| Renal disease | 1893 (51.0) | 1632 (50.9) | 1367 (51.0) | 265 (50.4) | 0.919 |
| Depression | 800 (21.6) | 702 (21.9) | 586 (21.9) | 116 (22.1) | 0.738 |
| Dementia | 309 (8.3) | 222 (6.9) | 176 (6.6) | 46 (8.8) | 0.029 |
| Coronary artery bypass graft surgery* | 88 (2.4) | 112 (3.5) | 103 (3.8) | 9 (1.7) | 0.006 |
| Percutaneous coronary intervention † | 98 (2.6) | 113 (3.5) | 106 (4.0) | 7 (1.3) | 0.033 |
| Permanent pacemaker | 17 (0.5) | 32 (1.0) | 29 (1.1) | 3 (0.6) | 0.008 |
| Implantable cardioverter‐defibrillator | 37 (1.0) | 54 (1.7) | 47 (1.8) | 7 (1.3) | 0.012 |
| Laboratory values | |||||
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | <0.001 | ||||
| ≥60 | 1637 (44.1) | 1378 (43.0) | 1136 (42.4) | 242 (46.0) | |
| 45–60 | 587 (15.8) | 622 (19.4) | 518 (19.3) | 104 (19.8) | |
| 30–45* | 539 (14.5) | 544 (17.0) | 471 (17.6) | 73 (13.9) | |
| <30 | 896 (24.1) | 650 (20.3) | 546 (20.4) | 104 (19.8) | |
| Unknown | 52 (1.4) | 13 (0.4) | 10 (0.4) | 3 (0.6) | |
| Potassium, mEq/L | <0.001 | ||||
| <4.0 | 1632 (44.0) | 1431 (44.6) | 1182 (44.1) | 249 (47.3) | |
| 4.0–4.9 | 1789 (48.2) | 1574 (49.1) | 1329 (49.6) | 245 (46.6) | |
| ≥5.0 | 255 (6.9) | 196 (6.1) | 165 (6.2) | 31 (5.9) | |
| Unknown | 35 (0.9) | 6 (0.2) | 5 (0.2) | 1 (0.2) | |
| Hemoglobin, g/dL | <0.001 | ||||
| <10.0 | 1341 (36.1) | 1028 (32.1) | 867 (32.3) | 161 (30.6) | |
| 10.0–11.9 | 1138 (30.7) | 1013 (31.6) | 847 (31.6) | 166 (31.6) | |
| ≥12.0 | 1194 (32.2) | 1158 (36.1) | 962 (35.9) | 196 (37.3) | |
| Unknown | 38 (1.0) | 8 (0.3) | 5 (0.2) | 3 (0.6) | |
| Medications | |||||
| Beta blocker † | 2951 (79.5) | 2697 (84.1) | 2275 (84.9) | 422 (80.2) | <0.001 |
| Angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker/angiotensin receptor neprilysin inhibitor | 1038 (28.0) | 1028 (32.1) | 876 (32.7) | 152 (28.9) | <0.001 |
| Hydralazine | 929 (25.0) | 711 (22.2) | 605 (22.6) | 106 (20.2) | 0.005 |
| Loop diuretics | 2623 (70.7) | 2545 (79.4) | 2136 (79.7) | 409 (77.8) | <0.001 |
| Aldosterone antagonist | 795 (21.4) | 916 (28.6) | 771 (28.8) | 145 (27.6) | <0.001 |
| Statin | 2519 (67.9) | 2314 (72.2) | 1939 (72.3) | 375 (71.3) | 0.001 |
| Aspirin † | 2464 (66.4) | 2261 (70.5) | 1917 (71.5) | 344 (65.4) | <0.001 |
| Health care use | |||||
| Principal heart failure hospitalizations | 683 (18.4) | 929 (29.0) | 799 (29.8) | 130 (24.7) | <0.001 |
| Admission through emergency department | 2447 (66.0) | 2313 (72.1) | 1905 (71.1) | 408 (77.6) | <0.001 |
| Length of stay, median (IQR) | 4.17 (5.0) | 4.6 (5.2) | 4.58 (5.2) | 4.7 (5.1) | <0.001 |
| Discharge destination ‡ | 0.438 | ||||
| Home without home health | 2626 (70.6) | 2242 (70.0) | 1918 (71.5) | 324 (61.6) | |
| Home with home health | 1085 (29.2) | 965 (30.1) | 763 (28.5) | 202 (38.4) | |
| Hospitalizations in the past year † | 1779 (48.0) | 1604 (50.0) | 1305 (48.7) | 299 (56.8) | 0.085 |
| Has a primary care provider on file | 2992 (80.6) | 2935 (91.5) | 2450 (91.4) | 485 (92.2) | <0.001 |
| Discharge date ‡ | |||||
| Early‐outbreak period | 149 (4.0) | 112 (3.5) | 75 (2.8) | 37 (7.0) | 0.514 |
| Stay‐at‐home period | 265 (7.1) | 362 (11.3) | 128 (4.8) | 106 (20.2) | |
| Reopening period | 3297 (88.8) | 2861 (89.2) | 2478 (92.4) | 383 (72.8) | |
| 30‐d readmission | 856 (23.1) | 456 (14.2) | 377 (14.1) | 79 (15.0) | <0.001 |
| 30‐d readmission or mortality | 921 (24.8) | 471 (14.7) | 386 (14.4) | 85 (16.2) | <0.001 |
P values: comparisons between in person vs telemedicine. IQR indicates interquartile range.
Note, Categorical variables reported as n (%) and continuous variables reported as median (interquartile range).
P<0.05.
P<0.01.
P<0.00.
Self‐pay N=4.
Other includes Hispanic/Latino, Asian, American Indian/Alaskan Native, Native Hawaiian or Other Pacific Islander, 2 or More Races, Not Reported, or Other.
Among patients who received an early follow‐up (N=3207), the factors associated with the modality of follow‐up (in person versus telemedicine) are presented in Tables 1 and 2. Results from bivariate and multivariable analyses showed that those who had other insurance coverage, had mitral or aortic valvular disease, received percutaneous coronary intervention, had estimated glomerular filtration rate between 30 and 45, had prescriptions for aspirin, and had a principal HF hospitalization were less likely to receive an early telemedicine follow‐up. Patients who had COPD, were discharged to home health, and were discharged during the early outbreak and stay‐at‐home periods were more likely to receive a telemedicine follow‐up. No significant differences were found with regard to age, sex, race, or neighborhood characteristics between patients with in‐person or telemedicine follow‐up.
Table 2.
Potential Factors Associated With Early Telemedicine Versus Early In‐Person Follow‐up (N=3207)
| Variables | Unadjusted | P value | Adjusted | P value | ||
|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |||
| Age | 1.01 | (1.00–1.01) | 0.143 | 1.00 | (0.99–1.01) | 0.915 |
| Female sex | 1.15 | (0.95–1.41) | 0.155 | 1.15 | (0.92–1.43) | 0.227 |
| Race (Ref: Non‐Hispanic White) | ||||||
| Non‐Hispanic Black | 1.18 | (0.96–1.45) | 0.116 | 1.13 | (0.87–1.45) | 0.355 |
| Other * | 1.17 | (0.78–1.74) | 0.442 | 1.21 | (0.79–1.84) | 0.376 |
| Currently married | 0.74 | (0.61–0.91) | 0.004 | 0.88 | (0.71–1.11) | 0.278 |
| Insurance (Ref: Medicare fee‐for‐service) | ||||||
| Medicare Advantage | 0.91 | (0.73–1.14) | 0.431 | 0.85 | (0.67–1.08) | 0.192 |
| Medicaid/self‐pay | 1.15 | (0.78–1.69) | 0.489 | 0.99 | (0.62–1.59) | 0.963 |
| Other | 0.52 | (0.39–0.71) | <0.001 | 0.60 | (0.41–0.88) | 0.008 |
| Disadvantaged neighborhood | 0.86 | (0.64–1.15) | 0.310 | 0.96 | (0.69–1.33) | 0.787 |
| Acute myocardial infarction | 0.54 | (0.35–0.82) | 0.004 | 0.76 | (0.46–1.24) | 0.268 |
| Angina | 0.91 | (0.75–1.10) | 0.333 | 1.01 | (0.80–1.28) | 0.909 |
| Stroke or transient ischemic attack | 0.71 | (0.40–1.29) | 0.261 | 0.62 | (0.34–1.13) | 0.120 |
| Atrial fibrillation or flutter | 1.00 | (0.82–1.22) | 0.997 | 1.03 | (0.82–1.30) | 0.775 |
| Mitral or aortic valvular disease | 0.62 | (0.48–0.81) | <0.001 | 0.67 | (0.51–0.89) | 0.005 |
| Peripheral vascular disease | 1.15 | (0.88–1.49) | 0.306 | 1.18 | (0.88–1.58) | 0.259 |
| Hypertension | 1.27 | (0.85–1.90) | 0.251 | 1.00 | (0.64–1.55) | 0.983 |
| Diabetes | 1.09 | (0.90–1.33) | 0.382 | 1.08 | (0.86–1.35) | 0.523 |
| Hyperlipidemia | 1.18 | (0.95–1.46) | 0.126 | 1.36 | (1.04–1.77) | 0.024 |
| Chronic obstructive pulmonary disease | 1.47 | (1.19–1.82) | <0.001 | 1.32 | (1.05–1.66) | 0.016 |
| Renal disease | 0.98 | (0.80–1.19) | 0.809 | 1.15 | (0.86–1.53) | 0.335 |
| Depression | 1.01 | (0.80–1.28) | 0.924 | 0.86 | (0.67–1.11) | 0.262 |
| Dementia | 1.36 | (0.97–1.92) | 0.073 | 1.20 | (0.82–1.74) | 0.348 |
| Coronary artery bypass graft surgery | 0.44 | (0.22–0.87) | 0.018 | 0.61 | (0.29–1.27) | 0.186 |
| Percutaneous coronary intervention | 0.33 | (0.15–0.71) | 0.005 | 0.40 | (0.18–0.92) | 0.030 |
| Permanent pacemaker | 0.52 | (0.16–1.73) | 0.289 | 0.73 | (0.21–2.62) | 0.633 |
| Implantable cardioverter‐defibrillator | 0.76 | (0.34–1.68) | 0.493 | 0.92 | (0.39–2.16) | 0.852 |
| Estimated glomerular filtration rate (Ref: ≥60) | ||||||
| 45–60 | 0.94 | (0.73–1.22) | 0.650 | 0.82 | (0.60–1.12) | 0.220 |
| 30–45 | 0.73 | (0.54–0.98) | 0.036 | 0.60 | (0.41–0.87) | 0.007 |
| <30 | 0.89 | (0.69–1.16) | 0.405 | 0.72 | (0.49–1.05) | 0.085 |
| Unknown | 1.41 | (0.37–5.31) | 0.613 | 1.81 | (0.27–12.2) | 0.543 |
| Potassium (Ref: 4.0–4.9) | ||||||
| <4.0 | 1.14 | (0.94–1.39) | 0.189 | 1.10 | (0.89–1.37) | 0.385 |
| ≥5.0 | 1.02 | (0.68–1.54) | 0.928 | 0.96 | (0.60–1.52) | 0.852 |
| Unknown | 1.08 | (0.13–9.33) | 0.941 | 0.07 | (0.002–2.37) | 0.141 |
| Hemoglobin (Ref: ≥12.0) | ||||||
| <10.0 | 0.91 | (0.72–1.15) | 0.431 | 0.83 | (0.63–1.10) | 0.201 |
| 10.0–11.9 | 0.96 | (0.76–1.21) | 0.741 | 0.93 | (0.72–1.19) | 0.561 |
| Unknown | 2.94 | (0.70–12.4) | 0.141 | 7.23 | (0.61–85.2) | 0.116 |
| Beta blocker | 0.72 | (0.56–0.94) | 0.014 | 0.80 | (0.60–1.07) | 0.130 |
| Angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker/angiotensin receptor neprilysin inhibitor | 0.84 | (0.67–1.04) | 0.108 | 0.91 | (0.72–1.15) | 0.422 |
| Hydralazine | 0.87 | (0.68–1.10) | 0.245 | 0.86 | (0.65–1.13) | 0.270 |
| Loop diuretics | 0.89 | (0.71–1.13) | 0.338 | 1.00 | (0.77–1.30) | 0.980 |
| Aldosterone antagonist | 0.94 | (0.76–1.17) | 0.599 | 0.99 | (0.78–1.26) | 0.959 |
| Statin | 0.95 | (0.76–1.18) | 0.646 | 0.91 | (0.69–1.19) | 0.488 |
| Aspirin | 0.75 | (0.61–0.93) | 0.007 | 0.78 | (0.61–0.99) | 0.042 |
| Principal heart failure hospitalizations | 0.77 | (0.62–0.96) | 0.021 | 0.71 | (0.56–0.90) | 0.005 |
| Admission through emergency department | 1.41 | (1.12–1.77) | 0.003 | 1.05 | (0.80–1.39) | 0.712 |
| Length of stay | 1.00 | (0.99–1.01) | 0.965 | 1.00 | (0.99–1.01) | 0.509 |
| Discharge to home with home health | 1.57 | (1.29–1.90) | <0.001 | 1.29 | (1.04–1.61) | 0.023 |
| Hospitalizations in the past year | 1.39 | (1.14–1.69) | 0.001 | 1.28 | (1.03–1.58) | 0.025 |
| Has a primary care provider on file | 1.12 | (0.77–1.61) | 0.561 | 1.08 | (0.72–1.62) | 0.715 |
| Discharge date (Ref: Reopening) | ||||||
| Early outbreak | 3.19 | (2.11–4.81) | <0.001 | 3.34 | (2.16–5.19) | <0.001 |
| Stay at home | 5.36 | (4.04–7.10) | <0.001 | 5.37 | (3.96–7.29) | <0.001 |
Regression models compare outcomes for patients who received early outpatient follow‐up via telemedicine vs those who received early outpatient follow‐up in person (reference group).
P values are based on logistic regression models for both unadjusted and adjusted ORs. OR indicates odds ratio.
Other includes Hispanic/Latino, Asian, American Indian/Alaskan Native, Native Hawaiian or Other Pacific Islander, 2 or More Races, Not Reported, or Other.
The overall 30‐day readmission rate during the COVID‐19 period was 19.0%. Patients who had an in‐person follow‐up had significantly lower rates of 30‐day readmission than those who had no early follow‐up (14.1% versus 23.1%, odds ratio [OR, unadjusted], 0.53; 95% CI, 0.46–0.61). Similar differences were found when comparing telemedicine follow‐ups to no early follow‐up (15.0% versus 23.1%, OR [unadjusted], 0.58; 95% CI, 0.44–0.76) (Table 3). These differences were robust and remained largely unchanged in the fully adjusted model (in person versus no follow‐up, OR, 0.52; 95% CI, 0.45–0.60; telemedicine versus no follow‐up, OR, 0.55; 95% CI, 0.44–0.72). Predicted probabilities of 30‐day readmission from the fully adjusted models showed that ≈13% of patients who received an in‐person follow‐up and 14% of patients who received a telemedicine follow‐up were readmitted within 30 days after discharge (Figure 3). Conversely, the 30‐day readmission rate was 22% among patients received no early follow‐up. The associations were essentially the same between early outpatient follow‐up and 30‐day readmission or mortality. Findings from sensitivity analyses suggested similar associations between outpatient follow‐ups within 7 days and 30‐day readmission and the composite outcome as presented previously (Table S3).
Table 3.
Unadjusted and Adjusted Odds Ratios for the Association between Early Outpatient Follow‐up Within 14 Days and 30‐Day Readmission and Mortality in Patients With Heart Failure (N=6918)
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | P value | OR | (95% CI) | P value | |
| 30‐d readmission | ||||||
| No early follow‐up | 1.00 | (reference) | 1.00 | (reference) | ||
| In‐person | 0.53 | (0.46–0.61) | <0.001 | 0.52 | (0.45–0.60) | <0.001 |
| Telemedicine | 0.58 | (0.44–0.76) | <0.001 | 0.55 | (0.44–0.72) | <0.001 |
| 30‐d readmission or mortality | ||||||
| No early follow‐up | 1.00 | (reference) | 1.00 | (reference) | ||
| In‐person | 0.49 | (0.43–0.57) | <0.001 | 0.49 | (0.42–0.56) | <0.001 |
| Telemedicine | 0.57 | (0.44–0.74) | <0.001 | 0.53 | (0.42–0.70) | <0.001 |
Adjusted logistic regression models included patient sociodemographic characteristics, diagnoses and procedures, laboratory values, medications, health care use measures, and neighborhood disadvantage. OR indicates odds ratio.
Figure 3. Predicted probability (95% CI) of 30‐day readmission and mortality by early outpatient follow‐up status among patients with heart failure (N=6918).
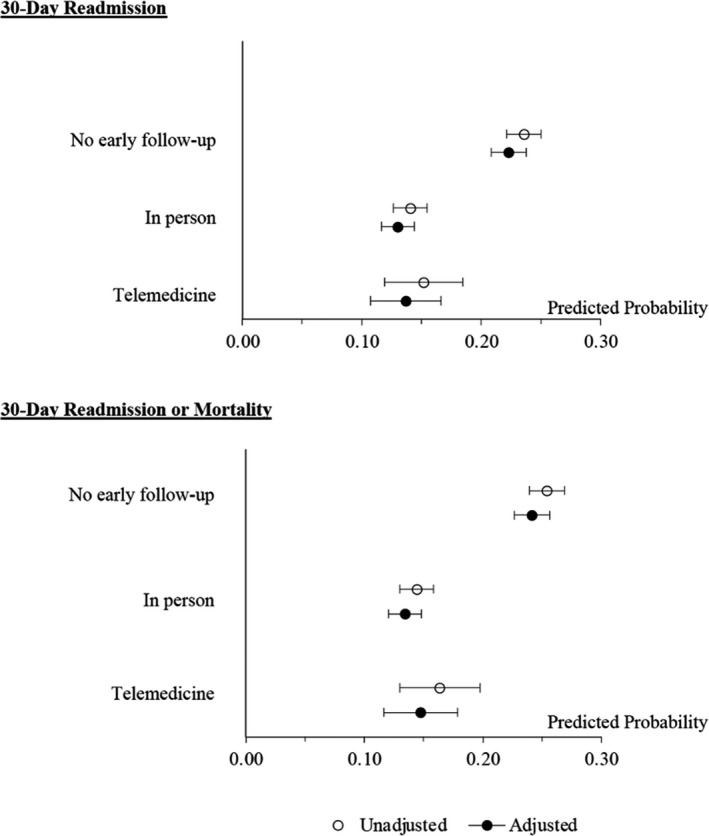
Adjusted models included patient sociodemographic characteristics, diagnoses and procedures, laboratory values, medications, health care use measures, and neighborhood disadvantage.
Discussion
To our knowledge, this is among the first studies to assess the use and outcomes of telemedicine follow‐up visits among patients with HF during the COVID‐19 pandemic. In an analysis of nearly 7000 patients with HF from a large academic medical center, we found that the overall rate of early follow‐up remained largely unchanged during the pandemic—with a rapid increase in the use of telemedicine visits early in the pandemic. We also found no significant differences in patients’ age, sex, race, and/or socioeconomic status between those who received early follow‐up via telemedicine versus in person. Furthermore, patients with HF who received either an in‐person or telemedicine follow‐up visit after a hospitalization had lower risks of 30‐day readmission compared with those who did not receive a follow‐up visit.
In this study, the overall rates of early follow‐up have remained stable throughout the COVID‐19 pandemic. We found that the use of telemedicine visits for early follow‐up increased rapidly in the early stages of the pandemic and subsequently decreased as North Carolina entered its reopening phase. These findings are consistent with prior research showing that the volume of telemedicine visits decreased while in‐person visits started to resume in May 2020. 8 These fluctuations of telemedicine visit volume may be due to a range of factors related to implementation, patient and provider preferences, organizational behavior, and policy adoption at the state and national level. 37 , 38 , 39 For example, it is possible that hospitals were no longer making telemedicine visits a priority and schedulers were less likely to offer telemedicine options to patients. Or patients and/or providers preferred in‐person follow‐up over telemedicine. We also found that more than half of the patients did not receive an outpatient follow‐up within 2 weeks after discharge. Although the DUHS system has implemented a dedicated care transition team at the Heart Center and offers patients with HF access to the Same‐Day HF Clinic, 40 our prior study has found that about 1 in 3 patients experience difficulties accessing their routine medical care. 41 Other research has also indicated that implementing timely outpatient follow‐ups has been a challenge. 14 , 42 Taken together, more research is needed to examine factors associated with the use of telemedicine visits and to assess whether the use of telemedicine can improve patients’ access to care.
Unlike prior research, the results from this study did not find a “digital divide” between patients who used in‐person versus telemedicine follow‐up visits. 8 , 21 , 22 , 43 The reasons are potentially twofold. First, most previous studies assessed the uptake of telemedicine for general outpatient care—rather than during a critical period of transitional care or among a specific patient population (HF) that was recently hospitalized. Second, only around 14% of study participants lived in the most disadvantaged neighborhood and our exploratory analyses suggested that close to 80% of the patients lived in an urban area. Therefore, the nonsignificant finding may be due in part to the small number of patients in this group.
Nevertheless, we found that patients who lived in a disadvantaged neighborhood were less likely to receive any early outpatient follow‐up; and there were no differences in neighborhood characteristics between those who received in‐person versus telemedicine follow‐ups. It might be that with programmatic efforts to promote telemedicine visits, telemedicine can be a viable way to increase access to care among patients from disadvantaged areas. We also found that patients with dementia were less likely to receive an early follow‐up than those without dementia. With prior research demonstrating the high health care use among patients with dementia, 44 additional efforts are needed to further identify and implement practical strategies to improve access to care in this vulnerable population. In the current study, we found a higher proportion of patients with dementia had followed up via telemedicine rather than in person, suggesting the potential for using telemedicine to address the care needs of these high‐risk patients. Our results also showed that patients without a PCP on file and living in a more disadvantaged neighborhood were less likely to receive an outpatient follow‐up. Again, these findings underscore the need for targeted interventions to improve access to care. A key benefit of telemedicine is its flexibility; thus, future research is needed to assess whether the use of telemedicine can help health care practices to expand their connections with patients and to facilitate the establishment of care with a PCP.
We found several factors that were associated with early telemedicine versus in‐person follow‐up. Specifically, patients who had mitral or aortic valvular disease, received percutaneous coronary intervention, had reduced kidney function, were prescribed aspirin, or were hospitalized because of HF were less likely to receive follow‐up via telemedicine than in person. It may be that these patients had more complex conditions that required physical assessments and/or laboratory testing from an outpatient visit. Although there are concerns that some telemedicine visits such as phone calls may have not been sufficient to adequately monitor patients conditions, emerging evidence has suggested the feasibility and validity to perform physical assessments remotely. 45 In addition, the Heart Failure Society of America has issued a statement that promotes the use of telemedicine in a full range of patients with HF. 46 We also found that patients who were discharged to home health were more likely to receive a telemedicine follow‐up. Accordingly, prior research has suggested that the use of telemedicine in patients with HF receiving home services reduces the risks of 30‐day readmission. 10 , 47 It is possible that a combination of telemedicine and home health services contributes to their success in reducing 30‐day readmissions among patients with HF. It might also be that individuals who had home health visits received additional assistance from the home health team to set up their telemedicine appointments. Therefore, more research is needed to develop guidelines or protocols to inform clinicians with regard to who may benefit the most from telemedicine visits post discharge.
We also found that patients with private, commercial, or other health insurance were less likely to receive telemedicine follow‐ups than Medicare fee‐for‐service beneficiaries. It might be that the Centers for Medicare & Medicaid Services provided reimbursement parity for telemedicine visits throughout the COVID‐19 pandemic; whereas for private insurers, the coverage for telemedicine services varied from payer to payer and some of these policies have already expired. 48 Additionally, in our study, most telemedicine follow‐ups occurred during the early phases of the pandemic, and the Centers for Medicare & Medicaid Services were among the first payers that broadened their coverage to reimburse telemedicine visits. 7 Our results also suggested that patients with COPD were more likely to be followed up via telemedicine. Given that many health care systems have implemented policies such as screening for symptoms upon arrival to prevent the transmission of COVID‐19, 49 , 50 patients with COPD may not be able to pass the symptom screening test to be seen in person. Relatedly, patients with COPD may also be reluctant to go to the hospital owing to the fear of contracting the virus and choose telemedicine instead. As a result, it is possible that reimbursement policies for telemedicine visits and hospital‐level visiting polices have had an impact on the use of telemedicine for outpatient follow‐ups.
Results from this study suggest that early telemedicine and in‐person follow‐up are both associated with lower rates of 30‐day readmission among patients with HF. Sensitivity analyses that focused on earlier follow‐ups (within 7 days) yielded similar results. These findings were similar to a prior study that reported no differences in the rates of hospital admissions among patients with HF who received telemedicine visits compared with in‐person visits. 12 Other small‐scale, descriptive studies also showed that 30‐day readmission rates were lower in patients who received a telemedicine follow‐up post discharge than in those who received usual care. 10 , 11 , 47 Together, these findings suggest that telemedicine has the potential to serve as an effective early outpatient follow‐up strategy that can have positive outcomes in patients with HF. In addition, although the readmission rates were essentially the same during the pre‐COVID‐19 and COVID‐19 periods, results from our exploratory analysis suggested that the readmission rates were lower in the earliest phase of the pandemic but quickly returned to the pre‐COVID‐19 level (14.6% in the early‐outbreak period versus 19.2% in the reopening period). These patterns suggest that hospitals may have experienced a lack of beds and/or shortages of staff at the beginning of the pandemic or that patients’ fear of exposure may have played a role.
Currently, despite changes in policy to improve coding for telemedicine visits, North Carolina is one of the few states that does not have a commercial payer telemedicine statute. 51 Given that the COVID‐19 pandemic resulted in a rapid implementation of telemedicine into clinical practice, to what extent health care systems and payers should maintain and/or expand the use of telemedicine warrants further evaluation. We found that early telemedicine follow‐ups can reduce the risks of 30‐day readmission in patients with HF. Still, not all patients with HF can be followed up via telemedicine. To date, there are no guidelines on the clinical consideration of using telemedicine 46 , 52 ; and prior research has shown variability in the acceptance and use of telemedicine across specialties and patient populations. 8 , 21 , 22 , 43 , 53 , 54 , 55 Therefore, future research is needed to inform health care systems and policymakers to establish a sustainable, cost‐effective, equitable, patient‐centered, and evidence‐based adoption plan for telemedicine. In particular, it is critical for health care systems to improve telemedicine access among vulnerable and underserved patient populations. For example, in patients with limited access to technology and/or adequate broadband Internet, the health care team should consider offering a call‐in option to the patient or providing them with information on nearby/free hotspot locations. For patients who are older adults, it may be particularly helpful to offer support services to assist older adults with setting up their smart devices before the telemedicine visit. It should also be noted that the implementation of these strategies often require the health care team to work with other sectors such as community organizations and telecommunication carriers. 56 Therefore, public investment and cross‐sector collaboration are key to the expansion of telemedicine services.
Several limitations of this study should be acknowledged. First, the study was conducted in a single academic medical center, which limits the generalizability of the study findings. Relatedly, we lacked information on outpatient follow‐ups and/or hospitalizations that occurred outside of DUHS. Second, we acknowledge that patients’ physiological indicators such as ejection fraction were not readily available in the current data sources. Nevertheless, prior research has suggested that the outcomes that we examined (ie, 30‐day all‐cause readmission and mortality) are relevant regardless of the severity of HF. 15 Third, the current data sources did not include detailed information on the modality of telemedicine. Therefore, we were unable to compare across different types of telemedicine services. We also lacked data on patients’ satisfaction and comfort level with telemedicine, patients’ and providers’ preference for using telemedicine, and the quality of telemedicine follow‐ups. Relatedly, there may be additional confounding factors contributing to the associations that were not measured in this study. Lastly, we were not able to obtain information on no‐shows and/or refusals. It is possible that some patients with HF were scheduled for a telemedicine or in‐person follow‐up but were unable or chose not to attend.
Conclusions
Patients who received either a telemedicine or in‐person follow‐up within 2 weeks after discharge had a significantly lower rate of 30‐day readmission than those who did not. These findings provide strong evidence for the use of telemedicine post discharge to reduce the risks of 30‐day readmission. Our study also identifies several factors that are associated with the use of telemedicine versus in‐person visits. These findings have important implications for adopting telemedicine into routine medical care to improve outcomes in patients with HF.
Sources of Funding
This research was funded in part by the National Institute on Aging (R21AG061142 for HX, BBG, MED; R03AG064303 for HX, BBG, MED), the National Institute on Minority Health and Health Disparities (U54MD012530 for HX), and the National Heart, Lung, and Blood Institute (K12HL138030 for CD).
Disclosures
None.
Supporting information
Tables S1–S3
Figures S1–S2
Results from this study were presented at the Gerontological Society of America Annual Scientific Meeting, November 10–13, 2021.
For Sources of Funding and Disclosures, see page 13.
References
- 1. Jeffery MM, D’Onofrio G, Paek H, Platts‐Mills TF, Soares WE, Hoppe JA, Genes N, Nath B, Melnick ER. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID‐19 pandemic in the US. JAMA Intern Med. 2020;180:1328–1333. doi: 10.1001/jamainternmed.2020.3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uscher‐Pines L, Sousa J, Jones M, Whaley C, Perrone C, McCullough C, Ober AJ. Telehealth use among safety‐net organizations in California during the COVID‐19 pandemic. JAMA. 2021;325:1106–1107. doi: 10.1001/jama.2021.0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee TH. Creating the new normal: the clinician response to Covid‐19. NEJM Catal Innov Care Deliv. 2020. Available at https://catalyst.nejm.org/doi/full/ 10.1056/CAT.20.0076. Accessed May 24, 2021. [DOI] [Google Scholar]
- 4. Mehrotra A, RayKristin K, Brockmeyer DM, Barnett ML, Bender JA. Rapidly converting to “virtual practices”: outpatient care in the era of Covid‐19. NEJM Catal Innov Care Deliv. 2020. Available at 10.1056/CAT.20.0091. Accessed May 24, 2021. [Google Scholar]
- 5. Tam C‐C, Cheung K‐S, Lam S, Wong A, Yung A, Sze M, Lam Y‐M, Chan C, Tsang T‐C, Tsui M, et al. Impact of coronavirus disease 2019 (COVID‐19) outbreak on ST‐segment–elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13:e006631. doi: 10.1161/CIRCOUTCOMES.120.006631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hartnett KP, Kite‐Powell A, DeVies J, Coletta MA, Boehmer TK, Adjemian J, Gundlapalli AV. Impact of the COVID‐19 pandemic on emergency department visits—United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:699–704. doi: 10.15585/mmwr.mm6923e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Medicare and Medicaid Services . Coronavirus Waivers and Flexibilities. Baltimore: U.S. Centers for Medicare & Medicaid Services; 2020. Available at https://www.cms.gov/about‐cms/emergency‐preparedness‐response‐operations/current‐emergencies/coronavirus‐waivers. Accessed May 20, 2021. [Google Scholar]
- 8. Drake C, Lian T, Cameron B, Medynskaya K, Bosworth HB, Shah K. Understanding telemedicine’s “new normal”: variations in telemedicine use by specialty line and patient demographics. Telemed E‐Health. 2021;28:51–59. doi: 10.1089/tmj.2021.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verma S. Early Impact Of CMS Expansion Of Medicare Telehealth During COVID‐19 | Health Affairs Blog. Health Aff Blog. Available at https://www.healthaffairs.org/do/ 10.1377/hblog20200715.454789/full/. Accessed July 8, 2021 [DOI]
- 10. Khattab M, Jimenez AR, Khattab M, Javed IN, Aston C, Mithilesh S, Kurdgelashvili G, Bray‐Hall S. Early Telephone appointments and home telehealth monitoring may improve 30‐day readmission rates in the COVID‐19 Era. Am J Med Qual. 2021;36:64–65. doi: 10.1097/01.JMQ.0000733456.04111.63 [DOI] [PubMed] [Google Scholar]
- 11. Salzano A, D’Assante R, Stagnaro FM, Valente V, Crisci G, Giardino F, Arcopinto M, Bossone E, Marra AM, Cittadini A. Heart failure management during the COVID ‐19 outbreak in Italy: a telemedicine experience from a heart failure university tertiary referral centre. Eur J Heart Fail. 2020;22:1048–1050. doi: 10.1002/ejhf.1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sammour Y, Spertus JA, Austin BA, Magalski A, Gupta SK, Shatla I, Dean E, Kennedy KF, Jones PG, Nassif ME, et al. Outpatient management of heart failure during the COVID‐19 pandemic after adoption of a telehealth model. JACC Heart Fail. 2021;9:916–924. doi: 10.1016/j.jchf.2021.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 14. Hernandez AF. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716. doi: 10.1001/jama.2010.533 [DOI] [PubMed] [Google Scholar]
- 15. McAlister FA, Youngson E, Kaul P, Ezekowitz JA. Early follow‐up after a heart failure exacerbation: the importance of continuity. Circ Heart Fail. 2016;9:e003194. doi: 10.1161/CIRCHEARTFAILURE.116.003194 [DOI] [PubMed] [Google Scholar]
- 16. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post‐discharge follow‐up characteristics associated with 30‐day readmission after heart failure hospitalization. Med Care. 2016;54:365–372. doi: 10.1097/MLR.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kotb A, Cameron C, Hsieh S, Wells G. Comparative effectiveness of different forms of telemedicine for individuals with heart failure (HF): a systematic review and network meta‐analysis. PLoS One. 2015;10:e0118681. doi: 10.1371/journal.pone.0118681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 19. Albert NM, Barnason S, Deswal A, Hernandez A, Kociol R, Lee E, Paul S, Ryan CJ, White‐Williams C, American Heart Association Complex Cardiovascular Patient and Family Care Committee of the Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research . Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384–409. doi: 10.1161/HHF.0000000000000006 [DOI] [PubMed] [Google Scholar]
- 20. Gorodeski EZ, Moennich LA, Riaz H, Jehi L, Young JB, Tang WHW. Virtual versus in‐person visits and appointment no‐show rates in heart failure care transitions. Circ Heart Fail. 2020;13:e007119. doi: 10.1161/CIRCHEARTFAILURE.120.007119 [DOI] [PubMed] [Google Scholar]
- 21. Lam K, Lu AD, Shi Y, Covinsky KE. Assessing telemedicine unreadiness among older adults in the United States during the COVID‐19 pandemic. JAMA Intern Med. 2020;180:1389–1391. doi: 10.1001/jamainternmed.2020.2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roberts ET, Mehrotra A. Assessment of disparities in digital access among Medicare beneficiaries and implications for telemedicine. JAMA Intern Med. 2020;180:1386–1389. doi: 10.1001/jamainternmed.2020.2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. North Carolina Office of the Governor . Governor Cooper Declares State Of Emergency To Respond To Coronavirus COVID‐19 [press release]. Available at https://governor.nc.gov/news/governor‐cooper‐declares‐state‐emergency‐respond‐coronavirus‐covid‐19#:~:text=Page%20Program‐,Governor%20Cooper%20Declares%20State%20Of%20Emergency%20To%20Respond%20To%20Coronavirus,Issues%20Recommendations%20to%20Slow%20Spread&text=Governor%20Roy%20Cooper%20took%20the,declaring%20a%20state%20of%20emergency
- 24. Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE guided query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform. 2011;44:266–276. doi: 10.1016/j.jbi.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krumholz H, Normand S‐L, Keenan P, Lin Z, Drye EE, Bhat KR, Wang Y, Ross JS, Schuur JD, Stauffer B. Hospital 30‐day heart failure readmission measure methodology. Centers for Medicare & Medicaid Services. 2008.
- 26. Xu H, Farmer HR, Granger BB, Thomas KL, Peterson ED, Dupre ME. Perceived versus actual risks of 30‐day readmission in patients with cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2021;14:e006586. doi: 10.1161/CIRCOUTCOMES.120.006586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pandey A, Patel KV, Liang LI, DeVore AD, Matsouaka R, Bhatt DL, Yancy CW, Hernandez AF, Heidenreich PA, de Lemos JA, et al. Association of hospital performance based on 30‐day risk‐standardized mortality rate with long‐term survival after heart failure hospitalization: an analysis of the get with the guidelines‐heart failure registry. JAMA Cardiol. 2018;3:489–497. doi: 10.1001/jamacardio.2018.0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. North Carolina Department of Health and Human Services . Staying Ahead of the Curve. 2021. Available at https://www.nc.gov/covid‐19/staying‐ahead‐curve. Accessed May 20, 2021.
- 29. Kind AJH, Buckingham WR. Making neighborhood‐disadvantage metrics accessible ‐ the neighborhood Atlas. N Engl J Med. 2018;378:2456–2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. University of Wisconsin School of Medicine and Public Health . 2018 Area Deprivation Index v3.0. Available at https://www.neighborhoodatlas.medicine.wisc.edu/ Accessed May 28, 2021.
- 31. Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M. Neighborhood socioeconomic disadvantage and 30 day rehospitalizations: an analysis of Medicare data. Ann Intern Med. 2014;161:765–774. doi: 10.7326/M13-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. University of Wisconsin School of Medicine and Public Health . Neighborhood Atlas‐2019 Area Deprivation Index version 3.1. Available at https://www.neighborhoodatlas.medicine.wisc.edu/#about‐anchor Accessed April 30, 2021.
- 33. US Department of Agriculture . Rural‐Urban Commuting Area Codes. Available at https://www.ers.usda.gov/data‐products/rural‐urban‐commuting‐area‐codes/ Accessed July 1, 2021.
- 34. Mansournia MA, Altman DG. Inverse probability weighting. BMJ. 2016;352:i189. doi: 10.1136/bmj.i189 [DOI] [PubMed] [Google Scholar]
- 35. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kho J, Gillespie N, Martin‐Khan M. A systematic scoping review of change management practices used for telemedicine service implementations. BMC Health Serv Res. 2020;20:815. doi: 10.1186/s12913-020-05657-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010–2015. Telemed J E‐Health off J Am Telemed Assoc. 2019;25:132–136. doi: 10.1089/tmj.2017.0265 [DOI] [PubMed] [Google Scholar]
- 39. Donelan K, Barreto EA, Sossong S, Michael C, Estrada JJ, Cohen AB, Wozniak J, Schwamm LH. Patient and clinician experiences with telehealth for patient follow‐up care. Am J Manag Care. 2019;25:40–44. [PubMed] [Google Scholar]
- 40. Duke Health. Heart Failure Treated at Same‐Day Access Clinic . Available at https://www.dukehealth.org/blog/heart‐failure‐treated‐same‐day‐access‐clinic. Accessed September 20, 2021.
- 41. Dupre ME, Xu H, Granger BB, Lynch SM, Nelson A, Churchill E, Willis JM, Curtis LH, Peterson ED. Access to routine care and risks for 30‐day readmission in patients with cardiovascular disease. Am Heart J. 2018;196:9–17. doi: 10.1016/j.ahj.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow‐up: an evidence‐based approach for planning after hospital discharge. Ann Fam Med. 2015;13:115–122. doi: 10.1370/afm.1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eberly LA, Khatana SAM, Nathan AS, Snider C, Julien HM, Deleener ME, Adusumalli S. Telemedicine outpatient cardiovascular care during the COVID‐19 pandemic. Circulation. 2020;142:510–512. doi: 10.1161/CIRCULATIONAHA.120.048185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feng Z, Coots LA, Kaganova Y, Wiener JM. Hospital and ED use among Medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff Proj Hope. 2014;33:683–690. [DOI] [PubMed] [Google Scholar]
- 45. Kelly SA, Schesing KB, Thibodeau JT, Ayers CR, Drazner MH. Feasibility of remote video assessment of jugular venous pressure and implications for telehealth. JAMA Cardiol. 2020;5:1194–1195. doi: 10.1001/jamacardio.2020.2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gorodeski EZ, Goyal P, Cox ZL, Thibodeau JT, Reay RE, Rasmusson K, Rogers JG, Starling RC. Virtual visits for care of patients with heart failure in the era of COVID‐19: a statement from the Heart Failure Society of America. J Card Fail. 2020;26:448–456. doi: 10.1016/j.cardfail.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O’Connor M, Asdornwised U, Dempsey ML, Huffenberger A, Jost S, Flynn D, Norris A. Using telehealth to reduce all‐cause 30‐day hospital readmissions among heart failure patients receiving skilled home health services. Appl Clin Inform. 2016;7:238–247. doi: 10.4338/ACI-2015-11-SOA-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Center for Connected Health Policy . COVID‐19 Telehealth Coverage Policies. West Sacramento, CA; 2021. Available at https://www.cchpca.org/resources/covid‐19‐telehealth‐coverage‐policies/. Accessed May 31, 2021.
- 49. Duke Health . COVID‐19 Update. Available at https://www.dukehealth.org/covid‐19‐update. Accessed May 31, 2021.
- 50. Wosik J, Fudim M, Cameron B, Gellad ZF, Cho A, Phinney D, Curtis S, Roman M, Poon EG, Ferranti J, et al. Telehealth transformation: COVID‐19 and the rise of virtual care. J Am Med Inform Assoc JAMIA. 2020;27:957–962. doi: 10.1093/jamia/ocaa067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lacktman NM, Acosta JN, Iacomoni SJ, Levine SJ. 50‐State Survey of Telehealth Commercial Insurance Laws. Foley & Lardner LLP; 2021. Available at https://www.foley.com/‐/media/files/insights/publications/2021/02/21mc30431‐50state‐telemed‐reportmaster‐02082021.pdf. Accessed May 31, 2021.
- 52. Sayer G, Horn EM, Farr MA, Axsom K, Kleet A, Gjerde C, Latif F, Sobol I, Kelley N, Lancet E, et al. Transition of a large tertiary heart failure program in response to the COVID‐19 pandemic. Circ Heart Fail. 2020;13:e007516. doi: 10.1161/CIRCHEARTFAILURE.120.007516 [DOI] [PubMed] [Google Scholar]
- 53. Woo K, Dowding D. Factors affecting the acceptance of telehealth services by heart failure patients: an integrative review. Telemed E‐Health. 2018;24:292–300. doi: 10.1089/tmj.2017.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eberly LA, Kallan MJ, Julien HM, Haynes N, Khatana SAM, Nathan AS, Snider C, Chokshi NP, Eneanya ND, Takvorian SU, et al. Patient characteristics associated with telemedicine access for primary and specialty ambulatory care during the COVID‐19 pandemic. JAMA Netw Open. 2020;3:e2031640. doi: 10.1001/jamanetworkopen.2020.31640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weber E, Miller SJ, Astha V, Janevic T, Benn E. Characteristics of telehealth users in NYC for COVID‐related care during the coronavirus pandemic. J Am Med Inform Assoc. 2020;27:1949–1954. doi: 10.1093/jamia/ocaa216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kronenfeld JP, Penedo FJ. Novel Coronavirus (COVID‐19): telemedicine and remote care delivery in a time of medical crisis, implementation, and challenges. Transl Behav Med. 2021;11:659–663. doi: 10.1093/tbm/ibaa105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S2


