Abstract
Background
Traumatic experiences have been linked to risk for cardiovascular disease (CVD). Interpersonal violence is a trauma that is prevalent in women. Among midlife women followed up for 2 decades, we examined whether interpersonal violence (childhood abuse, adulthood abuse, or intimate partner violence [IPV]) was related to increased risk of subsequent clinical CVD events.
Methods and Results
A total of 2201 women, aged 42 to 52 years at baseline, underwent up to 16 in‐person visits over 22 years. Measures included questionnaires (including of childhood physical/sexual abuse, adult physical/sexual abuse, and IPV), physical measures, phlebotomy, and reported CVD events (myocardial infarction, stroke, heart failure, and revascularization). Death certificates were collected. Relationships between childhood abuse, adult abuse, and IPV with incident fatal/nonfatal CVD were tested in Cox proportional hazards models. Women with a childhood abuse history had increased risk for incident CVD (versus no abuse; hazard ratio [HR] [95% CI], 1.65 [1.12–2.44]; P=0.01; adjusted for demographics and CVD risk factors); associations were strongest for childhood sexual abuse. Adult abuse was not significantly associated with CVD. Women with IPV had a doubling of risk for incident CVD in demographic‐adjusted models (versus no IPV; IPV: HR [95% CI], 2.06 [1.01–4.23]; P=0.04; no partner: HR [95% CI], 1.79 [0.91–3.53]; P=0.09); systolic blood pressure partially mediated relationships between IPV and CVD.
Conclusions
Childhood abuse, particularly sexual abuse, was associated with increased risk of CVD in women. IPV was associated with risk for CVD, with the higher systolic blood pressure among IPV‐exposed women important in these associations. Interpersonal violence prevention may contribute to CVD risk reduction in women.
Keywords: cardiovascular disease, trauma, violence, women
Subject Categories: Cardiovascular Disease, Epidemiology, Mental Health, Women
Nonstandard Abbreviations and Acronyms
- IPV
intimate partner violence
- SBP
systolic blood pressure
- SWAN
Study of Women’s Health Across the Nation
Clinical Perspective
What Is New?
This study found that a history of childhood abuse and a history of intimate partner violence were each associated with higher incident cardiovascular disease among midlife women, with intimate partner violence–cardiovascular disease associations explained in part by the higher blood pressure of intimate partner violence–exposed women.
What Are the Clinical Implications?
The clinical implications of this work may include inclusion of trauma history assessment as a component of routine health care, close blood pressure surveillance among intimate partner violence–exposed women, and referral to trauma‐focused behavioral health care as needed.
Cardiovascular disease (CVD) is the leading cause of death in women, accounting for approximately a third of all deaths. 1 In addition to traditional CVD risk factors, there is increasing recognition of the importance of psychosocial factors to cardiovascular health. One of these psychosocial factors is psychological trauma, which has been linked to increased risk for CVD after controlling for traditional CVD risk factors. 2
Interpersonal and sexual violence, including assault, sexual abuse, and intimate partner violence (IPV), are traumatic experiences particularly prevalent among women. Estimates indicate that ≈25% of women have experienced childhood maltreatment, 3 , 4 44% have experienced sexual assault, 5 and a quarter of women have experienced IPV in their lifetime. 6 These traumatic experiences are well established as leading risk factors for adverse mental health outcomes. 7 A growing literature also links these experiences to increased risk for poor physical health. For example, women with a history of childhood abuse have been found to have increased risk for CVD. 8 , 9 , 10 Furthermore, women with a history of sexual assault have increased risk of subclinical atherosclerosis 11 and self‐reported history of CVD 12 , 13 ; however, the literature on sexual assault is conflicting 14 and consists largely of cross‐sectional studies. 15 IPV has also been linked to adverse CVD risk factors, such as diabetes and hypertension, 16 , 17 and to clinical CVD in one cross‐sectional study 7 and one case‐control study. 18 However, these findings are not entirely consistent, 19 and longitudinal data on IPV and risk of CVD are lacking. Thus, although prior work suggests a potential increased CVD risk with interpersonal and sexual violence, further research is required.
The SWAN (Study of Women’s Health Across the Nation) is a multisite longitudinal cohort study of 3302 initially midlife women who have been assessed up to 16 times for >20 years. SWAN participants have undergone comprehensive assessments of psychosocial factors, including interpersonal violence during childhood and adulthood, CVD risk factors, and prospective characterization of CVD events and mortality. Among this well‐characterized sample of midlife women, we examined whether a history of interpersonal violence, including physical or sexual abuse in childhood, physical or sexual abuse in adulthood, and IPV, was related to CVD events and CVD mortality. We carefully considered the role of CVD risk factors in these associations, including as potential pathways linking interpersonal violence to CVD. This analysis seeks to shed light on relationships between interpersonal violence and women’s risk for clinical CVD as they age.
Methods
SWAN provides access to public use data sets that include data from SWAN screening, the baseline visit, and follow‐up visits (https://agingresearchbiobank.nia.nih.gov/). To preserve participant confidentiality, some, but not all, of the data used for this article are contained in the public use data sets. A link to the public use data sets is also located on the SWAN web site: http://www.swanstudy.org/swan‐research/data‐access/. Investigators who require assistance accessing the public use data set may contact the SWAN Coordinating Center at the following e‐mail address: swanaccess@edc.pitt.edu.
SWAN is a prospective cohort study of women conducted at 7 sites in the United States (Boston, MA; Chicago, IL; southeast Michigan; Los Angeles, CA; Newark, NJ; Pittsburgh, PA; and Oakland, CA). 20 SWAN was designed to investigate the natural history of the menopause transition and its implications for women’s health as they age. Each site recruited non‐Hispanic White women and one racial or ethnic minority group (Black race, Chinese race, Japanese race, or Hispanic ethnicity). Women were recruited from lists of names or household addresses. Select sites supplemented primary sampling frames to obtain adequate numbers of racial or ethnic minority women. Baseline eligibility criteria included being aged 42 to 52 years, having a uterus and at least one ovary, not being pregnant or lactating, not using oral contraceptives/hormone therapy, and having at least one menstrual cycle in the prior 3 months. Annual clinic assessments began in 1996 to 1997. Protocols were approved by the institutional review boards at each site. Each participant provided written informed consent at each visit.
A total of 3302 women were enrolled in the SWAN cohort at baseline. Trauma assessments began at visit 12 (child and adult abuse at visit 12; IPV at visits 12, 13, and 15). Of the 3302 women, 2320 attended at least one of the visits in which trauma assessments occurred and completed at least one of the trauma assessments. Of these 2320 women, 6 women were excluded from all models because of missing data on the time of the CVD event, 47 women were excluded because of reporting CVD at baseline, and 66 women were excluded because of missing covariate data. Thus, 2201 women were included in at least one of the trauma models (n=2004 in child abuse models, n=1911 in adult abuse models, and n=2090 in IPV models).
Interpersonal Trauma
At visit 12, women were asked about their experience of physical and sexual abuse during childhood and adulthood via 4 questions: “As a child, were you ever beaten, physically attacked, or physically abused? As a child, were you ever sexually attacked, raped, or sexually abused? As an adult, have you ever been beaten, physically attacked, or physically abused? As an adult, have you ever been sexually attacked, raped, or sexually abused?” These child abuse questions have been found to have strong correlations with the validated Child Trauma Questionnaire 21 (physical abuse: r=0.51, P<0.0001; sexual abuse: r=0.73, P<0.0001). At visits 12, 13, and 15, women were also asked about IPV via 2 questions: “Does your partner physically abuse or hurt you when he/she is upset?” and “Does your partner verbally or emotionally abuse you when he/she is upset? By verbal or emotional abuse, we mean insult you, yell at you, curse you, or humiliate you?” Endorsing either of these IPV questions at any of the 3 visits was considered positive for IPV. Women were classified as being partnered and having IPV, being partnered and not having IPV, or having no partner at the time of IPV assessment. In addition, the cumulative interpersonal trauma a woman experienced was calculated as the sum of the number of traumas assessed herein that were endorsed (childhood physical abuse, childhood sexual abuse, adult physical abuse, adult sexual abuse, physical IPV, and emotional IPV; range, 0–6).
CVD Events/CVD Mortality
At each SWAN visit, participants reported the occurrence of CVD events (myocardial infarction, cerebrovascular accident/stroke, heart failure, and revascularization procedures [percutaneous coronary intervention or coronary artery bypass grafting]). More extensive information about CVD events was obtained at SWAN visits 12, 13, and 15. At visit 15, adjudication of events began. Women were asked to provide consent to obtain medical record information related to events, and attempts were made to obtain medical records for each CVD event. When medical records were obtained, the SWAN Coordinating Center assembled information for each event (eg, admission history, physical examination, discharge summary, laboratory data, diagnostic test results, and operative/procedure reports). Two cardiologist reviewers blinded to trauma status reviewed this information and returned their determination to the SWAN Coordinating Center as to the status of the event (yes, no, or indeterminate). If there was agreement between the 2 members, the case was considered complete. If there was not agreement, a third cardiologist resolved the difference. For participants with multiple events, the first event was used.
The women in this analysis reported 146 CVD events (myocardial infarction: n=31; cerebrovascular accident/stroke: n=56; revascularization: n=22; heart failure: n=21; and multiple events simultaneously: n=16). Of these events, 58 were confirmed through adjudication. In addition to nonfatal events, fatal CVD events were identified via systematic review of death certificates that began at SWAN visit 15, and coded as a fatal CVD event if CVD was listed as an underlying cause of death on the death certificate, yielding 5 CVD‐related deaths. The primary outcome was the first nonfatal CVD event (myocardial infarction, stroke, heart failure, or revascularization) or fatal CVD event, which included 151 events (146 nonfatal events and 5 fatal CVD events). Analyses of childhood abuse were restricted to women with childhood abuse data and included 129 CVD events (over a median of ≈19 follow‐up years). For analyses of adult abuse and IPV, only events occurring after the trauma assessment were considered (52 CVD events for adult abuse models and 57 CVD events for IPV models over a median of ≈6 follow‐up years). The multiple trauma models included 56 CVD events (over a median of ≈5.5 follow‐up years). In secondary models of adult exposures, all events over the follow‐up period were considered.
Covariates
Race or ethnicity and education (high school or less, some college/vocational, or college or higher) were reported at baseline via standardized questionnaires. Age and smoking (current versus past/never) were derived from questionnaires and clinical interviews at each visit. Depressive symptoms were assessed at each visit via the Center for Epidemiologic Studies Depression scale. 22 At each visit, medication use was ascertained by self‐report and confirmed via visual inspection of pill bottles at the study visit. The therapeutic class and subclass for each medication were coded according to the Iowa Drug Information System. 23 Use of cardiovascular medications (blood pressure lowering, lipid lowering, and antidiabetic) was classified using these classifications. At each visit, height and weight were measured and body mass index was calculated (kg/m2). Systolic blood pressure (SBP) and diastolic blood pressure were averaged from 2 seated measurements; the blood pressure value with the statistically strongest associations with outcomes was considered in models (ie, SBP).
Phlebotomy was performed at each visit following overnight fast. When women were regularly cycling (eg, during the premenopause), blood sampling was timed to the menstrual cycle (early follicular phase, days 2–5 of the menstrual cycle). When women were no longer regularly cycling (eg, during the perimenopause or postmenopause), a random fasting blood sample was taken. EDTA‐treated plasma was separated, frozen at −20 °C, and sent on dry ice to the Medical Research Laboratories (Highland Heights, KY). From baseline through the seventh follow‐up visit, total cholesterol and triglycerides concentrations were determined by enzymatic methods (Hitachi 747 analyzer; Boehringer Mannheim Diagnostics, Indianapolis, IN). High‐density lipoprotein cholesterol was quantitated following precipitation of low‐density lipoprotein cholesterol with heparin and manganese chloride by the modified Lipid Research Clinics procedure. Cholesterol in the supernate was measured by an automated cholesterol oxidase assay on a Hitachi 747–200 clinical analyzer using RAICHEM reagents. 24 , 25 For subsequent visits, total cholesterol, ADVIA assay methods were used for total cholesterol, triglycerides, and high‐density lipoprotein cholesterol. 26 , 27 Calibration was based on 340 samples selected to be representative of the SWAN cohort. Low‐density lipoprotein cholesterol was calculated by the Friedewald equation, and values of estimated low‐density lipoprotein cholesterol and triglycerides were set to missing when the triglycerides >400 mg/dL. 28 Glucose was measured in serum by automated enzymatic assay on a Hitachi 747–200 chemistry analyzer using the hexokinase reaction and Roche Diagnostic reagents (baseline–SWAN visit 7) or by the ADVIA Chemistry Glucose Hexokinase 3 Concentrated Reagents 26 (subsequent visits). Insulin was measured in serum in duplicate by competitive binding radioimmunoassay using reagent from Diagnostic Products Corporation (baseline–SWAN visit 7) or using the ADVIA Centaur Insulin assay as a 2‐site sandwich immunoassay 29 , 30 (subsequent visits). Calibration equations for glucose and insulin were developed on the basis of random samples of 565 and 400 samples, respectively. Homeostatic model assessment insulin resistance was calculated (glucose [mmol/L]*insulin [mIU/mL]/22.5). 31
Statistical Analysis
All study variables were examined for distributions, cell sizes, and outliers. Triglycerides and homeostatic model assessment were natural log transformed for analyses. Differences between included and excluded women were determined via t‐tests and χ2 tests. We considered the associations between interpersonal violence types in logistic regression models. We next separately considered childhood abuse, adult abuse, or IPV in relation to combined fatal and nonfatal CVD events in several Cox proportional hazards models. For child abuse models, covariates were derived from baseline. For adult abuse and IPV models, covariates were derived from the visit coincident with the trauma assessment (typically visit 12). Covariates were determined on the basis of their association with the outcome at P<0.10 and included age, site, education, and race or ethnicity for minimally adjusted models, and additionally CVD risk factors (body mass index, SBP, lipids, smoking, homeostatic model assessment, medication for blood pressure, lipids, and diabetes) in demographic and CVD risk factor–adjusted models. In secondary models, we explored the role of CVD risk factors, and particularly blood pressure given its links to IPV in prior work, 17 as mediators of IPV‐CVD associations in Weibull accelerated failure time models and product of coefficients methods. 32 CVD risk factors were derived from the visit coincident with the exposure assessment. For child abuse models, CVD events from baseline through visit 15 were included. For models of adult exposures, CVD events included were those occurring after the adult exposure assessment; in secondary models, all CVD events from baseline through visit 15 were considered (conducted via logistic regression because of uncertainly around the timing of trauma relative to CVD). Exposures were considered in separate models; thus, women experiencing >1 type of violence were represented in >1 of the models. To address the issue of women experiencing multiple types of violence, we considered the number of interpersonal violence types a woman experienced (categorized as 0, 1, or ≥2) in relation to incident CVD in Cox proportional hazards models. For these models, covariates were derived from the adult trauma/IPV assessment visit; CVD events for these models were those occurring after the IPV assessment. In secondary models, similar to adult models, all CVD events from baseline through visit 15 were considered in logistic regression models. Additional sensitivity analyses were conducted with further adjustment for depressive symptoms and in which the outcome was restricted to adjudicated CVD events (for childhood abuse models only attributable to limits in the number of events). All models were evaluated for and met proportional hazards assumptions. Analyses were performed with SAS v9.4 (SAS, Cary, NC).
Results
At baseline, women were on average 46 years old, overweight, and nonsmoking (Table 1). Women with an interpersonal trauma history varied on few characteristics relative to those without this history, with the exception of education, homeostatic model assessment, and use of diabetes medications. A quarter of the women endorsed a history of childhood abuse, 23% reported a history of adult abuse, and over a quarter of women reported IPV, principally emotional IPV (Table 2). Almost a quarter of women reported ≥2 traumas. A history of childhood abuse was associated with an almost 4‐fold odds of adult abuse (odds ratio [OR] [95% CI], 3.78 [3.02–4.73]; P<0.0001) and increased odds of IPV (OR [95% CI], 1.87 [1.45–2.41]; P<0.0001, partnered women). Similarly, a history of adult abuse was associated with increased odds of IPV (OR [95% CI], 1.84 [1.40–2.42]; P<0.0001, partnered women).
Table 1.
Baseline Sample Characteristics by Interpersonal Trauma in SWAN (n=2201)
| Characteristic |
No interpersonal trauma (n=1107) |
Any interpersonal trauma (n=1094) |
|---|---|---|
| Age, mean (SD), y | 45.96 (2.70) | 45.83 (2.64) |
| Race or ethnicity, N (%) | ||
| White | 539 (48.69) | 546 (49.91) |
| Black | 263 (23.67) | 289 (26.42) |
| Chinese | 106 (9.58) | 100 (9.14) |
| Japanese | 130 (11.74) | 108 (9.87) |
| Hispanic/Latina | 69 (6.23) | 51 (4.66) |
| Education, N (%)* | ||
| High school or less | 262 (23.67) | 202 (18.46) |
| Some college/vocational | 316 (28.55) | 388 (35.47) |
| College or higher | 529 (47.78) | 504 (46.07) |
| BMI, mean (SD), kg/m2 | 27.46 (6.82) | 27.94 (7.43) |
| Smoking, N (%) | 136 (12.33) | 161 (14.84) |
| SBP, mean (SD), mm Hg | 116.31 (16.48) | 115.91 (16.06) |
| DBP, mean (SD), mm Hg | 74.77 (10.34) | 74.67 (10.14) |
| LDL‐C, mean (SD), mg/dL | 113.75 (29.30) | 115.81 (30.99) |
| HDL‐C, mean (SD), mg/dL | 57.22 (13.64) | 56.19 (14.11) |
| Triglycerides, median (IQR), mg/dL | 87 (64–122) | 91 (67–128) |
| HOMA, median (IQR)* | 1.68 (1.23–2.66) | 1.84 (1.28–2.94) |
| Medications, N (%) | ||
| Blood pressure | 124 (11.20) | 125 (11.43) |
| Lipids | 10 (0.90) | 6 (0.55) |
| Diabetes* | 14 (1.26) | 27 (2.47) |
Interpersonal trauma included childhood abuse, adult abuse, and intimate partner violence. BMI indicates body mass index; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; HOMA, homeostatic model assessment; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; and SWAN, Study of Women’s Health Across the Nation.
P<0.05 for interpersonal trauma vs no interpersonal trauma.
Table 2.
Prevalence of Child hood Abuse, Adult Abuse, and IPV in SWAN (n=2201)
| Variable | No. (%) of women |
|---|---|
| Childhood abuse | |
| Sexual abuse | 296 (14.77) |
| Physical abuse | 361 (17.94) |
| Any childhood abuse | 505 (25.17) |
| Adult abuse | |
| Sexual abuse | 224 (11.09) |
| Physical abuse | 360 (17.80) |
| Any adult abuse | 454 (22.51) |
| IPV | |
| Emotional IPV | 578 (26.32) |
| Physical IPV | 75 (3.42) |
| Any IPV | 581 (26.46) |
| No partner | 748 (34.06) |
| No. of interpersonal traumas | |
| 0 | 1107 (50.29) |
| 1 | 581 (26.40) |
| ≥2 | 513 (23.31) |
Abuse types are not mutually exclusive. IPV indicates intimate partner violence; and SWAN, Study of Women’s Health Across the Nation.
We first considered associations between abuse and CVD events. Women who had a history of childhood abuse had a greater risk of incident CVD relative to women without this history; these associations persisted when adjusting for demographic and CVD risk factors (Table 3 and Figure [A]). When child abuse types were considered separately, findings were more pronounced for childhood sexual abuse (childhood sexual abuse: hazard ratio [HR] [95% CI], 1.92 [1.25–2.97], P=0.003; childhood physical abuse: HR [95% CI], 1.52 [0.98–2.37], P=0.06; models adjusted for demographics and CVD risk factors). Adult abuse was not significantly related to incident CVD (Table 3 and Figure [B]), with findings similar when considering adult physical and sexual abuse separately (data not shown).
Table 3.
Association Between Childhood or Adult Abuse and Incident CVD in SWAN
| Variable | CVD | |
|---|---|---|
| Model 1 | Model 2 | |
| Childhood abuse | ||
| Yes | 1.71 (1.19–2.45)* | 1.65 (1.12–2.44) † |
| No | Referent | Referent |
| Adult abuse | ||
| Yes | 0.94 (0.50–1.78) | 0.95 (0.48–1.89) |
| No | Referent | Referent |
Data are given as hazard ratio (95% CI). Model 1: site, age, race, and education; model 2: model 1 plus body mass index, systolic blood pressure, lipids (high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and triglycerides), homeostatic model assessment, smoking, and medication use (for lipid lowering, diabetes, and blood pressure). CVD indicates cardiovascular disease; and SWAN, Study of Women’s Health Across the Nation.
P<0.01.
P<0.05.
Figure . Incidence of cardiovascular disease (CVD) over time among (A) women with and without a history of childhood abuse, (B) women with and without a history of adult abuse, (C) women with and without intimate partner violence in The Study of Women’s Health Across the Nation.
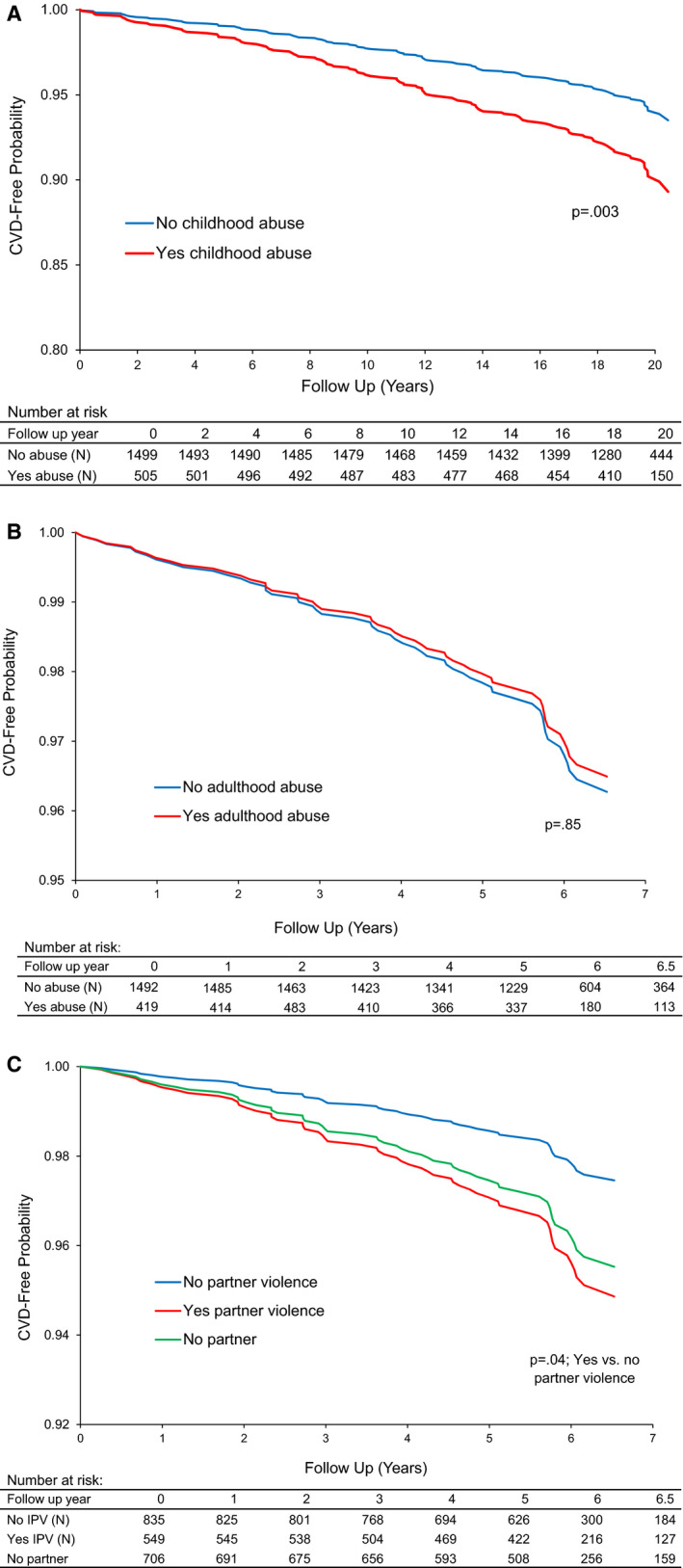
All models adjusted for site, age, race, and education.
We next considered IPV in relation to incident CVD. Women who had experienced IPV had an approximate doubling of incident CVD risk, after adjusting for site, age, race, and education (Table 4 and Figure [C]). These associations were attenuated with the addition of SBP to the models. In secondary models, we considered SBP as a mediator; SBP emerged as a significant mediator of the relationship between IPV and CVD (indirect effect=0.031 [SE=0.018], P=0.04, adjusted for site, age, race, and education). Notably, women with IPV had significantly higher SBP than partnered women without IPV (relative to partnered without IPV: IPV: B (SE)=1.89 (0.86), P=0.03; unpartnered: B (SE)=1.32 (0.82), P=0.11, adjusted for site, age, race, and education), with an adjusted mean SBP of 122.16 mm Hg versus 120.27 mm Hg for partnered women with IPV and without IPV, respectively.
Table 4.
Associations Between IPV and Incident CVD in SWAN
| CVD | |||
|---|---|---|---|
| Variable | Model 1 | Model 2 | Model 3 |
| IPV* | |||
| Yes | 2.06 (1.01–4.23) † | 1.91 (0.91–4.01) ‡ | 1.84 (0.84–4.01) |
| No partner | 1.79 (0.91–3.53) ‡ | 1.51 (0.73–3.09) | 0.99 (0.45–2.18) |
| Partnered, no IPV | Referent | Referent | Referent |
Data are given as hazard ratio (95% CI). Model 1: site, age, race, and education; model 2: model 1 plus systolic blood pressure; model 3: model 2 plus body mass index, lipids (high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and triglycerides), homeostatic model assessment, smoking, and medication use (for lipid lowering, diabetes, and blood pressure). CVD indicates cardiovascular disease; IPV, intimate partner violence; and SWAN, Study of Women’s Health Across the Nation.
Physical or emotional IPV.
P<0.05.
P<0.10.
We next considered the number of interpersonal trauma exposures women had in relation to incident CVD. In demographic and CVD risk factor–adjusted models, women who experienced ≥2 interpersonal traumas had a >2‐fold risk of incident CVD relative to women with no exposures (Table 5).
Table 5.
Association of Number of Interpersonal Traumas and Incident CVD in SWAN
| Variable | CVD | |
|---|---|---|
| Model 1 | Model 2 | |
| No. of interpersonal traumas | ||
| 0 | Referent | Referent |
| 1 | 1.70 (0.89–3.23) | 1.90 (0.91–3.95)* |
| ≥2 | 1.71 (0.90–3.27) | 2.15 (1.02–4.51) † |
Data are given as hazard ratio (95% CI). Model 1: site, age, race, and education; model 2: model 1 plus body mass index, systolic blood pressure, lipids (high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and triglycerides), homeostatic model assessment, smoking, and medication use (for lipid lowering, diabetes, and blood pressure). CVD indicates cardiovascular disease; and SWAN, Study of Women’s Health Across the Nation.
P<0.10.
P<0.05.
We conducted several additional analyses. First, we restricted childhood abuse–CVD models to adjudicated CVD events. These associations were not significant (childhood abuse: HR [95% CI], 1.36 [0.70–2.66], P=0.36, relative to no childhood abuse, adjusted for demographics and CVD risk factors); however, findings should be interpreted in light of the few CVD events in these models (42 events). We next considered additional adjustment for depressive symptoms in primary child abuse–CVD models; associations between child abuse and CVD persisted (HR [95% CI], 1.60 [1.08–2.37], P=0.02, adjusted for demographics, CVD risk factors, and depressive symptoms). Third, to address potential “double counting” of physical IPV and adult physical abuse, in models testing the number of types of abuse in relation to CVD, we considered adult physical abuse and physical IPV as one type of abuse; models were unchanged (data not shown).
Furthermore, because of uncertainty about the timing of the exposure relative to the CVD event, we also considered logistic regression models relating adult interpersonal trauma to all CVD events from baseline through the entire follow‐up period. Conclusions were unchanged for adult abuse (adult abuse versus no adult abuse in relation to CVD: OR [95% CI], 1.18 [0.74–1.87], P=0.48, adjusted for demographics and CVD risk factors). For IPV, associations between IPV and CVD were statistically significant when adjusting for both demographic factors and CVD risk factors, including SBP (IPV: OR [95% CI], 1.91 [1.12–3.28], P=0.02; unpartnered: OR [95% CI], 1.23 [0.73–2.07], P=0.43; relative to partnered without IPV, adjusted for demographics and CVD risk factors). Moreover, the number of interpersonal trauma exposures was related to incident CVD in a dose‐response manner in models including events across the follow‐up period, similar to that of primary models (≥2 traumas: OR [95% CI], 2.05 [1.23–3.42], P=0.006; 1 trauma: OR [95% CI], 1.88 [1.14–3.10], P=0.01; relative to no traumas, models adjusted for demographics, and CVD risk factors).
Discussion
In this study of midlife and older women, we found that interpersonal violence was associated with increased risk for incident CVD. Specifically, we found that women with a history of child abuse had a 65% increased risk of incident CVD relative to women without this history after accounting for traditional CVD risk factors. Childhood sexual abuse was a particularly potent risk factor and associated with a doubling of risk of incident CVD. Furthermore, women who had experienced IPV had an increased risk of incident CVD relative to partnered women without this history, and SBP emerged as a mediator of relationships between IPV and CVD. Study findings point to the importance of understanding a woman’s interpersonal trauma history in assessing her risk for CVD and of targeting modifiable CVD risk factors in trauma‐exposed women.
A quarter of women endorsed a history of childhood abuse, and 22% of the women endorsed a history of adult abuse. Associations between abuse and CVD were most pronounced for childhood abuse. This finding is broadly consistent with prior literature showing particularly consistent associations for childhood abuse and CVD. 9 , 10 , 14 In interpreting the differences between findings for childhood and adult abuse, a methodological consideration is that our childhood abuse models used CVD events over a median follow‐up of 19 years, whereas adult abuse models examined events from a median follow‐up of 6 years. However, findings for adult abuse were similar when models used all events across the follow‐up period. It is also notable that only adult physical and sexual abuse, and not emotional abuse, was assessed herein, which may be important in understanding the divergence of findings between adult trauma and IPV. Furthermore, study findings pointed to the particular importance of childhood sexual abuse to women’s cardiovascular health. Other work has underscored the importance of childhood sexual abuse. Childhood sexual abuse is the form of childhood abuse particularly prevalent among women 10 and a potent trauma that can place individuals on a trajectory of social, behavioral, and biological risk over one’s life. 33
The present study is the first to show an increased risk for incident CVD with IPV. IPV was prevalent in the sample, with over a quarter of the women endorsing a history of IPV. This study advanced the prior work on IPV and CVD risk, which, although suggesting an association between IPV and CVD, relied largely on cross‐sectional designs, case‐control studies, or studies of CVD risk factors; herein, we consider associations between IPV and incident CVD in a prospective cohort study. It is notable the majority of IPV experienced was emotional IPV (only 3% of the women endorsed physical IPV), and thus findings were largely driven by emotional IPV, underscoring the importance of emotional IPV for women’s health. Other work has indicated the importance of emotional IPV, with or without physical IPV, for women’s physical and mental health. 17 , 34 Furthermore, we found that the relationships between IPV and incident CVD were mediated in part by SBP. Some prior work has found IPV, notably severe emotional IPV, associated with increased risk of self‐reported hypertension 7 , 17 ; we now show links between IPV and measured blood pressure. The present findings point to blood pressure as a key mechanistic pathway linking IPV and CVD and a potential target of intervention.
Many women experience multiple traumatic experiences in their life. In fact, although approximately half of the women experienced at least one trauma, almost a quarter of women studied experienced ≥2 trauma types. We examined potential compounding effects of having multiple traumatic exposures in relation to CVD; results indicated that women with ≥2 interpersonal traumas had an over doubling of risk of incident CVD in demographic and CVD risk factor–adjusted models. Notably, childhood abuse and its sequelae can place individuals at increased risk for later life trauma. 33 These findings underscore the adverse impact of multiple interpersonal traumas throughout life.
Several mechanisms may underlie these associations. For childhood abuse, standard CVD risk factors did not account for these associations. We further considered the role of depressive symptoms in child abuse–CVD associations, and these symptoms did not account for these associations. In the case of IPV, SBP played an important role in associations between IPV and CVD. Notably, although not entirely consistent, 7 prior work has pointed to the links of IPV (particularly emotional IPV) and other forms of interpersonal violence to hypertension risk in women. 17 , 35 Future work should also consider the role of additional factors that may link interpersonal trauma to CVD risk, including other psychological factors, such as posttraumatic stress symptoms; social and behavioral factors, such as impaired social mobility, disrupted interpersonal relationships, and adverse health behaviors over the lifecourse 36 , 37 , 38 ; and other potential physiological processes, such as inflammatory mechanisms, altered autonomic nervous system function, impaired hypothalamic pituitary adrenal axis function, or epigenetic changes. 10 , 39
Several limitations deserve consideration. First, the number of CVD events was limited, particularly for models that examined adult traumas. CVD events studied herein reflected early CVD events, as participants were somewhat younger than the ages when events typically accumulate in women. The adjudication rate was low, in part because of the limitations in obtaining medical records and the fact that event adjudication largely began at visit 15. Limitations in assessment of trauma exposures reflect their implementation in a large epidemiologic study and included study questions being brief and limited in scope and detail. Nonvalidated questions were used, yet it is notable that child abuse questions correlated highly with a well‐validated child abuse measure. 21 CVD events were collected prospectively, yet some of the trauma assessments, particularly childhood abuse assessments, were retrospective in nature. Furthermore, our examination of the role of CVD risk factors in child abuse–CVD risk associations was limited by their evaluation only during adulthood. Child and adult abuse assessments were performed only once over the follow‐up period; thus, women who did not attend this visit or experienced their adult abuse after the study assessment would have not been captured. Future work should be conducted with more extensive multidimensional, validated assessments.
Despite these limitations, this study had several strengths. It was conducted in a well‐characterized large sample of midlife women followed up over midlife and early old age. CVD events were captured prospectively. Multiple types of interpersonal trauma were assessed, including childhood abuse, adult abuse, and IPV, which is particularly understudied in relation to women’s cardiovascular health. Women underwent comprehensive, direct measurements of CVD risk factors, advancing prior work that relied on self‐reported CVD risk factor information.
In conclusion, in a well‐characterized sample of midlife women followed up for over 2 decades, we found that women who had experienced a history of childhood abuse, particularly childhood sexual abuse, or IPV were at increased risk for CVD later as they age. In the case of childhood abuse, these associations were not accounted for by CVD risk factors. For IPV, SBP played an important role in relationships between IPV and CVD, suggesting the importance of blood pressure management among IPV‐exposed women. Notably, midlife is an important time for women’s cardiovascular health; it is a time of accelerating CVD risk and an important window for prevention, occurring directly before the onset of most clinical CVD events for women. These findings underscore the importance of assessing interpersonal trauma history in a comprehensive CVD risk assessment and referral to trauma‐focused behavioral health care as needed. These steps have the potential to enhance mental health and reduce CVD risk among women.
Sources of Funding
The SWAN (Study of Women's Health Across the Nation) has grant support from the National Institutes of Health (NIH), US Department of Health and Human Services, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Disclosures
Dr Thurston is a consultant/advisory board member for Astellas Pharma, Bayer, Vira Health, and Happify Health. All other authors have no conflicts.
Acknowledgments
We thank the study staff at each site and all the women who participated in SWAN (Study of Women's Health Across the Nation).
Clinical Centers: University of Michigan, Ann Arbor: Carrie Karvonen‐Gutierrez, principal investigator (PI) 2021 to present; Siobán Harlow, PI 2011 to 2021; MaryFran Sowers, PI 1994 to 2011; Massachusetts General Hospital, Boston, MA: Sherri‐Ann Burnett‐Bowie, PI 2020 to present; Joel Finkelstein, PI 1999 to 2020; Robert Neer, PI 1994 to 1999; Rush University, Rush University Medical Center, Chicago, IL: Imke Janssen, PI 2020 to present; Howard Kravitz, PI 2009 to 2020; Lynda Powell, PI 1994 to 2009; University of California, Davis/Kaiser: Elaine Waetjen and Monique Hedderson, PIs 2020 to present; Ellen Gold, PI 1994 to 2020; University of California, Los Angeles: Arun Karlamangla, PI 2020 to present; Gail Greendale, PI 1994 to 2020; Albert Einstein College of Medicine, Bronx, NY: Carol Derby, PI 2011 to present; Rachel Wildman, PI 2010 to 2011; Nanette Santoro, PI 2004 to 2010; University of Medicine and Dentistry–New Jersey Medical School, Newark: Gerson Weiss, PI 1994 to 2004; and the University of Pittsburgh, Pittsburgh, PA: Rebecca Thurston, PI 2020 to present; Karen Matthews, PI 1994 to 2020.
National Institutes of Health Program Office: National Institute on Aging, Bethesda, MD: Rosaly Correa‐de‐Araujo 2020 to present; Chhanda Dutta 2016 to present; Winifred Rossi 2012 to 2016; Sherry Sherman 1994 to 2012; Marcia Ory 1994 to 2001; National Institute of Nursing Research, Bethesda, MD: program officers.
Central Laboratory: University of Michigan, Ann Arbor: Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA: Maria Mori Brooks, PI 2012 to present; Kim Sutton‐Tyrrell, PI 2001 to 2012; New England Research Institutes, Watertown, MA: Sonja McKinlay, PI 1995 to 2001.
Steering Committee: Susan Johnson, current chair; Chris Gallagher, former chair.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2. Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew‐Blais J, Chen Q, Cerdá M, Rexrode KM, Rich‐Edwards JW, Spiegelman D, et al. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132:251–259. doi: 10.1161/circulationaha.114.014492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wildeman C, Emanuel N, Leventhal JM, Putnam‐Hornstein E, Waldfogel J, Lee H. The prevalence of confirmed maltreatment among US children, 2004 to 2011. JAMA Pediatr. 2014;168:706–713. doi: 10.1001/jamapediatrics.2014.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim H, Wildeman C, Jonson‐Reid M, Drake B. Lifetime prevalence of investigating child maltreatment among US children. Am J Public Health. 2017;107:274–280. doi: 10.2105/AJPH.2016.303545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith SG, Zhang X, Basile KC, Merrick MT, Wang J, Kresnow M, Chen J. The National Intimate Partner and Sexual Violence Survey (NISVS): 2015 Data Brief – Updated Release. National Center for Injury Prevention and Control; 2018. [Google Scholar]
- 6. Black MC, Basile KC, Breiding MJ, Smith SG, Walters ML, Merrick MT, Chen J, Stevens MR. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 7. Breiding MJ, Black MC, Ryan GW, Chronic disease and health risk behaviors associated with intimate partner violence‐18 U.S. states/territories, 2005. Ann Epidemiol. 2008;18:538–544. [DOI] [PubMed] [Google Scholar]
- 8. Basu A, McLaughlin KA, Misra S, Koenen KC. Childhood maltreatment and health impact: the examples of cardiovascular disease and type 2 diabetes mellitus in adults. Clin Psychol (New York). 2017;24:125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jakubowski KP, Cundiff JM, Matthews KA. Cumulative childhood adversity and adult cardiometabolic disease: a meta‐analysis. Health Psychol. 2018;37:701–715. doi: 10.1037/hea0000637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suglia SF, Koenen KC, Boynton‐Jarrett R, Chan PS, Clark CJ, Danese A, Faith MS, Goldstein BI, Hayman LL, Isasi CR, et al; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Functional Genomics and Translational Biology; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research . Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation. 2018;137:e15–e28. doi: 10.1161/CIR.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thurston RC, Jakubowski K, Chang Y, Koenen K, Maki PM, Mitchell EB. Sexual assault and carotid plaque among midlife women. J Am Heart Assoc. 2021;10:e017629. doi: 10.1161/JAHA.120.017629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santaularia J, Johnson M, Hart L, Haskett L, Welsh E, Faseru B. Relationships between sexual violence and chronic disease: a cross‐sectional study. BMC Public Health. 2014;14:1286. doi: 10.1186/1471-2458-14-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frayne SM, Skinner KM, Sullivan LM, Tripp TJ, Hankin CS, Kressin NR, Miller DR. Medical profile of women veterans administration outpatients who report a history of sexual assault occurring while in the military. J Womens Health Gend Based Med. 1999;8:835–845. doi: 10.1089/152460999319156 [DOI] [PubMed] [Google Scholar]
- 14. Jakubowski KP, Murray V, Stokes N, Thurston RC. Sexual violence and cardiovascular risk: a systematic review and meta‐analysis. Maturitas. 2021;153:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibson CJ, Maguen S, Xia F, Barnes DE, Peltz CB, Yaffe K. Military sexual trauma in older women veterans: prevalence and comorbidities. J Gen Intern Med. 2020;35:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mason SM, Wright RJ, Hibert EN, Spiegelman D, Jun HJ, Hu FB, Rich‐Edwards JW. Intimate partner violence and incidence of type 2 diabetes in women. Diabetes Care. 2013;36:1159–1165. doi: 10.2337/dc12-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mason SM, Wright RJ, Hibert EN, Spiegelman D, Forman JP, Rich‐Edwards JW. Intimate partner violence and incidence of hypertension in women. Ann Epidemiol. 2012;22:562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chandan JS, Thomas T, Bradbury‐Jones C, Taylor J, Bandyopadhyay S, Nirantharakumar K. Risk of cardiometabolic disease and all‐cause mortality in female survivors of domestic abuse. J Am Heart Assoc. 2020;9:e014580. doi: 10.1161/JAHA.119.014580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gass JD, Stein DJ, Williams DR, Seedat S. Intimate partner violence, health behaviours, and chronic physical illness among South African women. S Afr Med J. 2010;100:582–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, et al. SWAN: a multicenter, multiethnic, community‐based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, Lobo AR, eds. Menopause: Biology and Pathology. Academic Press; 2000:175–188. [Google Scholar]
- 21. Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress. 2001;14:843–857. [DOI] [PubMed] [Google Scholar]
- 22. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 23. Iowa Drug Information Service . IDIS Drug Vocabulary and Thesaurus Description. Coralville, IA: College of Pharmacy, University of Iowa; 2012. [Google Scholar]
- 24. Steiner PM, Freidel J, Bremner WF, Stein EA. Standardization of micro‐methods for plasma cholesterol, triglyceride and HDL‐cholesterol with the lipid research clinics’ methodology. J Clin Chem Clin Biochem. 1981;19:850. [Google Scholar]
- 25. Warnick GR, Albers JJ. A comprehensive evaluation of the heparin‐manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. doi: 10.1016/S0022-2275(20)41577-9 [DOI] [PubMed] [Google Scholar]
- 26. Siemans Healthcare Solutions Diagnostics . Advia 1800 Chemistry System. Tarrytown, NY: Siemens Healthcare Diagnostics Inc; 2020. [Google Scholar]
- 27. Baruch L, Agarwal S, Gupta B, Haynos A, Johnson S, Kelly‐Johnson K, Eng C. Is directly measured low‐density lipoprotein clinically equivalent to calculated low‐density lipoprotein? J Clin Lipidol. 2010;4:259–264. doi: 10.1016/j.jacl.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 28. Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499 [DOI] [PubMed] [Google Scholar]
- 29. Siemans Medical Solutions Diagnostics . Advia Centaur XP Reference Manual. Tarrytown, NY: Siemens Healthcare Diagnostics Inc; 2007. [Google Scholar]
- 30. El Kenz H, Bergmann P. Evaluation of immunochemiluminometric assays for the measurement of insulin and C‐peptide using the ADVIA centaur. Clin Lab. 2004;50:171–174. [PubMed] [Google Scholar]
- 31. Matthews D, Hosker J, Rudenski A, Naylor B, Teacher D, Turner R. Homeostasis model assessment: insulin resistance and β cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 32. VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology. 2011;22:582–585. doi: 10.1097/EDE.0b013e31821db37e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ullman SE, Najdowski CJ, Filipas HH. Child sexual abuse, post‐traumatic stress disorder, and substance use: predictors of revictimization in adult sexual assault survivors. J Child Sex Abus. 2009;18:367–385. doi: 10.1080/10538710903035263 [DOI] [PubMed] [Google Scholar]
- 34. Fisher BS, Regan SL. The extent and frequency of abuse in the lives of older women and their relationship with health outcomes. Gerontologist. 2006;46:200–209. doi: 10.1093/geront/46.2.200 [DOI] [PubMed] [Google Scholar]
- 35. Thurston RC, Chang Y, Matthews KA, von Känel R, Koenen K. Association of sexual harassment and sexual assault with midlife women’s mental and physical health. JAMA Intern Med. 2019;179:48–53. doi: 10.1001/jamainternmed.2018.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zielinski DS. Child maltreatment and adult socioeconomic well‐being. Child Abuse Negl. 2009;33:666–678. doi: 10.1016/j.chiabu.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 37. Kendall‐Tackett K. The health effects of childhood abuse: four pathways by which abuse can influence health. Child Abuse Negl. 2002;26:715–729. doi: 10.1016/S0145-2134(02)00343-5 [DOI] [PubMed] [Google Scholar]
- 38. Sperry DM, Widom CS. Child abuse and neglect, social support, and psychopathology in adulthood: a prospective investigation. Child Abuse Negl. 2013;37:415–425. doi: 10.1016/j.chiabu.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hao G, Youssef NA, Davis CL, Su S. The role of DNA methylation in the association between childhood adversity and cardiometabolic disease. Int J Cardiol. 2018;255:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]


