Abstract
Background
Educating cardiologists and health care professionals about cardiovascular genetics and genetic testing is essential to improving diagnosis and management of patients with inherited cardiomyopathies and arrhythmias and those at higher risk for sudden cardiac death. The aim of this study was to understand cardiology and electrophysiology practitioners’ current practices, confidence, and knowledge surrounding genetic testing in cardiology and desired topics for an educational program.
Methods and Results
A one‐time survey was administered through purposive email solicitation to 131 cardiology practitioners in the United States. Of these, 107 self‐identified as nongenetic practitioners. Over three quarters of nongenetic practitioners reported that they refer patients to genetic providers to discuss cardiovascular genetic tests (n=82; 76.6%). More than half of nongenetic practitioners reported that they were not confident about the types of cardiovascular genetic testing available (n=60; 56%) and/or in ordering appropriate cardiovascular genetic tests (n=66; 62%). In addition, 45% (n=22) of nongenetic practitioners did not feel confident making cardiology treatment recommendations based on genetic test results. Among all providers, the most desired topics for an educational program were risk assessment (94%) and management of inherited cardiac conditions based on guidelines (91%).
Conclusions
This study emphasizes the importance of access to genetics services in the cardiology field and the need for addressing the identified deficit in confidence and knowledge about cardiogenetics and genetic testing among nongenetic providers. Additional research is needed, including more practitioners from underserved areas.
Keywords: cardiogenomics, cardiology, continuing medical education, genetic testing
Subject Categories: Genetics
Nonstandard Abbreviations and Acronyms
- SCD
sudden cardiac death
Clinical Perspective
What Is New?
We asked practicing cardiology practitioners about their confidence with regard to the application of cardiogenomics in practice and preferred topics for continuing medical education in cardiogenomics.
What Are the Clinical Implications?
Many cardiology practitioners are not confident about ordering appropriate cardiogenomics tests and making treatment recommendations based on genetic tests and desire additional education in risk assessment incorporating genomics and management of inherited cardiac conditions based on guidelines.
Monogenic diseases of heart rhythm and muscle, such as cardiomyopathies and arrhythmias, affect ≈1 in 500 people, presenting a clinical disease in both children and adults. 1 , 2 Genetic testing yields vary for inherited cardiomyopathies and arrhythmia syndromes, ranging from 20% to 50% in family dilated cardiomyopathy to 75% in long‐QT syndrome. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Genetic testing for inherited cardiac conditions is useful for the clinical management of patients and at‐risk family members. 11 , 12 , 13 Identifying those at increased risk for disease allows for earlier detection and interventions, including lifestyle modifications, drugs to slow disease progression or to prevent thromboembolism, and procedures, drugs, or devices to reduce the risk of sudden cardiac death (SCD).
Despite the benefits of genetic testing, cardiac providers may be unprepared to implement genetic testing in clinical care. 14 , 15 In cardiology and other specialties, nongenetics health professionals have been unable to keep up with the advancements in genomics. 16 , 17 , 18 , 19 , 20 This has been attributed partly to lack of contemporary genetic knowledge and lack of confidence in integrating genetic information and technologies into patient education, management, and referral. 18 , 19 The complexity of current cardiovascular genetic evaluation and testing has led to published guidelines recommending evaluation in specialized centers whenever possible to facilitate access to multidisciplinary teams that include cardiovascular clinicians, geneticists, and genetic counselors. 11 , 21 In 2016, the American Heart Association recommended that every cardiovascular clinician should have the basic skill set to suspect a genetic condition, a basic understanding of genetic testing technology and results interpretation, and the ability to recognize when a genetic diagnosis influences medical and surgical care of patients. 15 Consequently, educating cardiologists and electrophysiology practitioners about cardiovascular genetics and genetic testing is critical to improving patient screening, risk assessment, diagnosis, and clinical care for cardiology patients.
Previous studies have demonstrated that educational programs grounded in evidence‐based principles of adult learning and best‐practice principles of backward design can have a significant impact on health care providers’ performance. 22 , 23 , 24 , 25 , 26 Principles of adult learning include using existing knowledge as the foundation for new knowledge and integration into current practices. Therefore, this study aimed to ascertain cardiology and electrophysiology practitioners’ (1) confidence in cardiomyopathy and arrhythmia genetic testing, (2) current cardiomyopathy and arrhythmia genetic testing ordering practices, (3) knowledge of cardiovascular genetics as it relates to current cardiovascular management guidelines, and (4) desired topics for an educational program for this population of medical professionals.
Methods
A one‐time survey was administered to providers who care directly for children and adults affected with and/or at risk for heritable cardiovascular disease and who evaluate families with a history of SCD. The study was approved by Northwestern University’s Institutional Review Board (STU00210365), and subjects provided informed consent.
Survey Design
Question design was driven by previous qualitative interviews and pilot data obtained from 7 practicing cardiologists. 27 Study data were collected and managed using Research Electronic Data Capture hosted at Northwestern University. 28 , 29
The survey consisted of 5 main sections: (1) current practices around genetic testing for inherited cardiac conditions (focused on inherited cardiomyopathies and arrhythmia syndromes), (2) practitioners’ confidence in their knowledge of cardiovascular genetic testing, (3) practical knowledge based on current practice guidelines for management of patients with ventricular arrhythmias and the prevention of SCD, 12 (4) preferences for continuing medical education topics, and (5) demographics. Assessment of current practices included questions about access to genetics professional(s), frequency of referral for genetic evaluation, performance, and discussion of genetic testing for inherited cardiac conditions, as well as practitioners’ reported barriers and motivations to implementing genetic testing into their practice. Assessment of confidence in knowledge included topics related to understanding of genetics information, risk assessment for inherited cardiac conditions, genetic test ordering, genetic test report interpretation, and management of patients based on genetic test results. Multiple‐choice questions were used to assess demographic information and practice patterns. Knowledge assessment was based on a board‐style vignette and question format. Likert scales were used to assess the respondent’s confidence in each response. The full survey is provided in Data S1.
Participants and Recruitment
Recruitment took place between January 21 and April 5, 2021. To be eligible for inclusion, participants were required to self‐identify as a licensed physician, nurse, physician assistant, or advanced practice nurse (stated as “licensed MD, DO, PA, RN, or APRN”) and provide direct patient care in the United States. Genetic counselors were excluded from this study. In addition, participants were asked to self‐identify as a genetics professional in the field of cardiology (medical geneticist or a physician with genetic expertise) or nongenetics professional in the field of cardiology. This allowed the comparison of current practices, confidence, and practical knowledge between these 2 groups.
Participants were recruited from across the United States via email. Using a purposive approach, key contacts from health care organizations (Northwestern Medicine, Sanford Health, and several pediatric research hospitals), professional organizations (the American Heart Association Strategically Focused Research Networks, the National Society of Genetic Counselors Cardiovascular Genetics Special Interest Group, the Pediatric & Congenital Electrophysiology Society, and local chapters of American College of Cardiology nursing groups), and the advocacy organization Project ADAM were leveraged to disseminate the email. Leaders in all the organizations listed were contacted by email and asked to disseminate the email as widely as possible. Email recipients were also encouraged to forward the email onto other potentially interested and eligible practitioners in a snowball recruitment approach.
Statistical Analysis
Confidence in the respondents’ knowledge of genetic testing for inherited cardiac conditions was evaluated using 13 “I feel confident in…” statements. Participants responded using a 5‐point Likert scale (from strongly disagree to strongly agree). A mean score for each statement was calculated and used to rank these from highest to lowest confidence. An overall confidence score was calculated as the sum of all the 13 confidence responses for each participant. Similarly, an overall knowledge score was calculated for each participant as the number of correct responses to all knowledge questions. Continuous variables were compared using t‐tests. Categorical variables were compared using a χ2 test. A 2‐sided P<0.005 was considered significant to adjust partially for the number of questions asked on the survey. R version 4.0.1 was used for statistical analyses. 30 Data are available on request.
Results
Demographics
A total of 158 individuals agreed to participate; 26 participants were excluded because they did not meet inclusion criteria or did not complete the survey after providing consent, resulting in 131 responses for inclusion in the study. Most survey respondents were physicians (77%; n=101), White race (76.3%; n=100), and non‐Hispanic ethnicity (95.4%; n=125), and had been practicing medicine for 10 to 15 years (17.6%; n=23) or >15 years (42%; n=55). Sex identity was almost equally balanced, with 62 women (47.3%) and 65 men (49.6%). The geographic region and work setting of participants was skewed to university medical centers (81.7%; n=107), primarily located in urban regions (80.9%; n=106). The Table provides demographics for study participants, stratified into those who self‐identified as cardiac genetics providers (hereafter labeled genetics professionals: 17%; n=23) and those who did not (82%; n=108).
Table 1.
Characteristics of the Study Population by Genetics Expertise
| Characteristics |
Genetics professional (N=23) |
Nongenetics professional (N=107) |
Overall (N=131) |
|---|---|---|---|
| Educational background | |||
| Adult cardiologist | 13 (56.5) | 36 (33.6) | 49 (37.4) |
| Pediatric cardiologist | 10 (43.5) | 29 (27.1) | 39 (29.8) |
| Advanced Practice Nurse | 0 (0) | 14 (13.1) | 14 (10.7) |
| Fellow | 0 (0) | 11 (10.3) | 12 (9.2) |
| Other | 0 (0) | 2 (1.9) | 2 (1.5) |
| Physician Assistant | 0 (0) | 3 (2.8) | 3 (2.3) |
| Registered nurse | 0 (0) | 10 (9.3) | 10 (7.6) |
| Resident | 0 (0) | 1 (0.9) | 1 (0.8) |
| Time practicing, y | |||
| Residency | 0 (0) | 3 (2.8) | 3 (2.3) |
| <5 | 5 (21.7) | 24 (22.4) | 30 (22.9) |
| 5–10 | 2 (8.7) | 17 (15.9) | 19 (14.5) |
| 10–15 | 5 (21.7) | 18 (16.8) | 23 (17.6) |
| >15 | 11 (47.8) | 44 (41.1) | 55 (42.0) |
| Geographic region | |||
| Urban | 19 (82.6) | 86 (80.4) | 106 (80.9) |
| Suburban | 4 (17.4) | 19 (17.8) | 23 (17.6) |
| Rural | 0 (0) | 1 (0.9) | 1 (0.8) |
| Work setting | |||
| Private group practice | 2 (8.7) | 2 (1.9) | 4 (3.1) |
| Private solo practice | 0 (0) | 1 (0.9) | 1 (0.8) |
| University medical center | 18 (78.3) | 88 (82.2) | 107 (81.7) |
| Private hospital | 0 (0) | 3 (2.8) | 3 (2.3) |
| Public hospital | 3 (13.0) | 11 (10.3) | 14 (10.7) |
| Sex identity | |||
| Men | 14 (60.9) | 50 (46.7) | 65 (49.6) |
| Women | 9 (39.1) | 53 (49.5) | 62 (47.3) |
| Prefer not to disclose | 0 (0) | 2 (1.9) | 2 (1.5) |
| Hispanic ethnicity | |||
| Hispanic | 0 (0) | 1 (0.9) | 1 (0.8) |
| Non‐Hispanic | 23 (100) | 101 (94.4) | 125 (95.4) |
| Race | |||
| Asian (eg, East/South/Southeast) | 6 (26.1) | 16 (15.0) | 22 (16.8) |
| Mixed | 1 (4.3) | 3 (2.8) | 4 (3.1) |
| White | 15 (65.2) | 84 (78.5) | 100 (76.3) |
| Other | 1 (4.3) | 3 (2.8) | 4 (3.1) |
Data are given as number (percentage). Some categories do not sum to 100% because of missing data.
Current Practices
Figure 1 summarizes the frequency with which practitioners engage patients and colleagues about cardiovascular genetics. Most genetics and nongenetics professionals both referred patients to genetics once a month (43.5% [n=10] of genetics professionals; 45.8% [n=49] of nongenetics professionals). As expected, those who self‐identified as genetics professionals discussed genetic testing more frequently than those who did not identify as genetics professionals (once per week or more for genetic professionals [78.3%; n=18] compared with only once a month [48.6%; n=52] for nongenetics professionals). Self‐identified genetics professionals also reported self‐ordering genetic tests for their patients more often (ie, once a week or more [60.8%; n=14]), compared with nongenetics professionals (ie, once a month or less [84.1%; n=90]). These differences were statistically significant. In addition, 10% (n=11) of nongenetics professionals never discussed genetic testing, 14% (n=15) never referred patients to genetics, and 8% (n=9) never had genetic testing performed for their patients.
Figure 1. Frequency with which genetics and nongenetics professionals discuss, refer, and perform genetic testing for their patients.
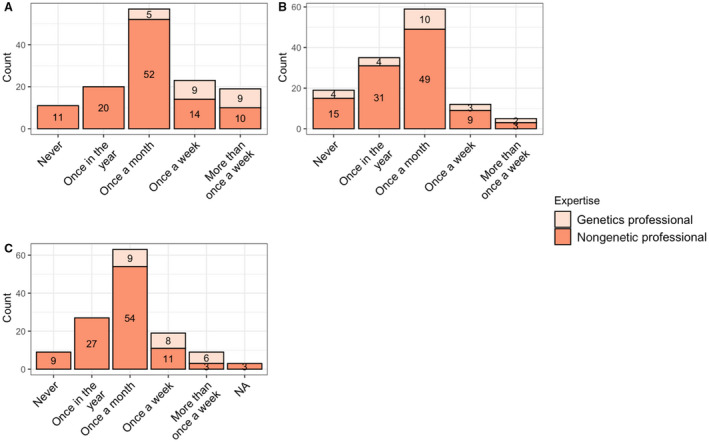
A, Responses to the question, “How often did you discuss genetic testing for inherited cardiac conditions with patients in 2019?” B, Responses to the question, “How often did you refer said patients to genetics?” C, Responses to the question, “How often are genetic tests performed for your patients?” For all 4 panels, responses between genetics and nongenetics professionals were statistically significantly different (P<0.005). NA indicates not applicable.
Most nongenetic professionals preferred to refer their patients to genetics for discussing genetic testing options (76.6%; n=82), implications of positive results (71%; n=76), and implications of uncertain results (86%; n=92), and for discussing implications to family members (78.5%; n=84). In all cases, these were statistically significantly different than the preferences expressed by genetic professionals (Figure 2).
Figure 2. Preferences for discussions about genetic testing options.
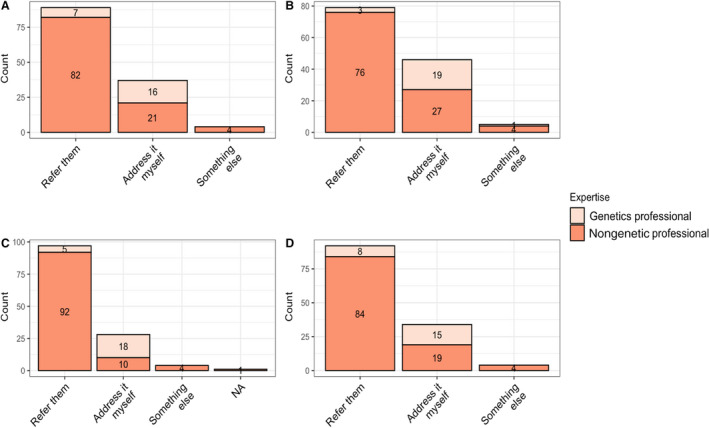
Providers were asked how they handled each of the following clinical scenarios: A, Discussing options for genetic testing. B, Discussing the implications of a positive result with a patient. C, Discussing the implications of variant of uncertain significance with a patient. D, Discussing the implications of genetic testing results for family members. For all 4 panels, responses between genetics and nongenetics professionals were statistically significantly different (P<0.005). NA indicates not applicable.
Most participants reported access to genetic services; 90% (n=119) reported having access to a genetics professional to whom they can refer patients, and 85% (n=112) reported having a genetics professional with whom they can consult about clinical care for issues relating to inherited cardiac conditions. Although we are hesitant to conclude that 85% to 90% of cardiology professionals in the United States have access to a genetics professional to whom they can refer patients or consult about clinical care, these data provide context about the type of providers who responded to the survey.
Barriers and Motivators to Incorporating Genetic Testing of Inherited Cardiac Conditions Into Practice
Thirty‐six participants reported experiencing barriers/frustrations in implementing genetic testing, with the 3 most reported barriers being cost of genetic testing (too costly or not reimbursable) (61.1%; n=22), theoretic risk of increasing insurance discrimination (58.3%; n=21), and requiring more education for implementing genetic testing into their practice (58.3%; n=21). Other reported barriers included insurance coverage (prior authorizations and difficulty of getting letters of medical necessity) and limited availability of genetics providers (half‐time coverage, overbooked, or understaffed). In addition, “literature changes quickly and is often not intuitive” was also specifically reported as a barrier.
Most participants (94.7%; n=124) responded that they would be motivated to incorporate more genetic testing into practice if there was evidence in the medical literature that their patient population was at risk for carrying a pathogenic variant in genes that increase risk for heart disease/SCD. In addition, most (91.5%; n=120) reported that evidence‐based professional society guidelines were a motivator to incorporate more genetic testing into practice. Other reported motivators included genetic testing reference guides and online resources for families.
Confidence in Knowledge About Genetics and Genetic Testing in Cardiology
Figure 3 displays providers’ confidence in their knowledge of cardiovascular genetics and genetic testing. Providers with genetics expertise had a higher overall confidence score than nongenetics professionals (55.6 versus 40.7; P<0.001). With the exception of confidence to “identify the best person to initiate genetic testing,” confidence scores for all questions differed statistically significantly (P<0.005) between genetics and nongenetic professionals. Both genetics and nongenetics professionals felt most confident identifying the best person in the family to initiate genetic testing (96% of genetics professionals [n=22] felt confident, agreed or strongly agreed to feeling confident; 82% of nongenetics professionals [n=88] felt confident, agreed or strongly agreed to feeling confident), followed by knowing when to refer patients to genetics (96% of genetics professionals [n=22] felt confident; 72% of nongenetics professionals [n=77] felt confident) and identifying clinical situations in which genetic testing is indicated (100% of genetics professionals [n=23] felt confident; 65% of nongenetics professionals [n=70] felt confident). However, topics for which health care providers reported the least confidence differed between genetics and nongenetics professionals. Nongenetics professionals are least confident in their knowledge about genetic testing, including interpreting a genetic test result (43% [n=46] did not feel confident, disagreed or strongly disagreed to feeling confident), knowing the types of genetic testing available (56% [n=60] did not feel confident), and identifying the appropriate genetic test to order (62% [n=66] did not feel confident). In addition, 45% (n=48) of nongenetics professionals do not feel confident making treatment recommendations based on genetic test results. In contrast, genetic professionals are mostly confident in most genetic‐related tasks. Genetics professionals reported the least confidence in providing psychosocial support to patients.
Figure 3. Health care providers’ confidence in their knowledge about genetic testing in cardiology.
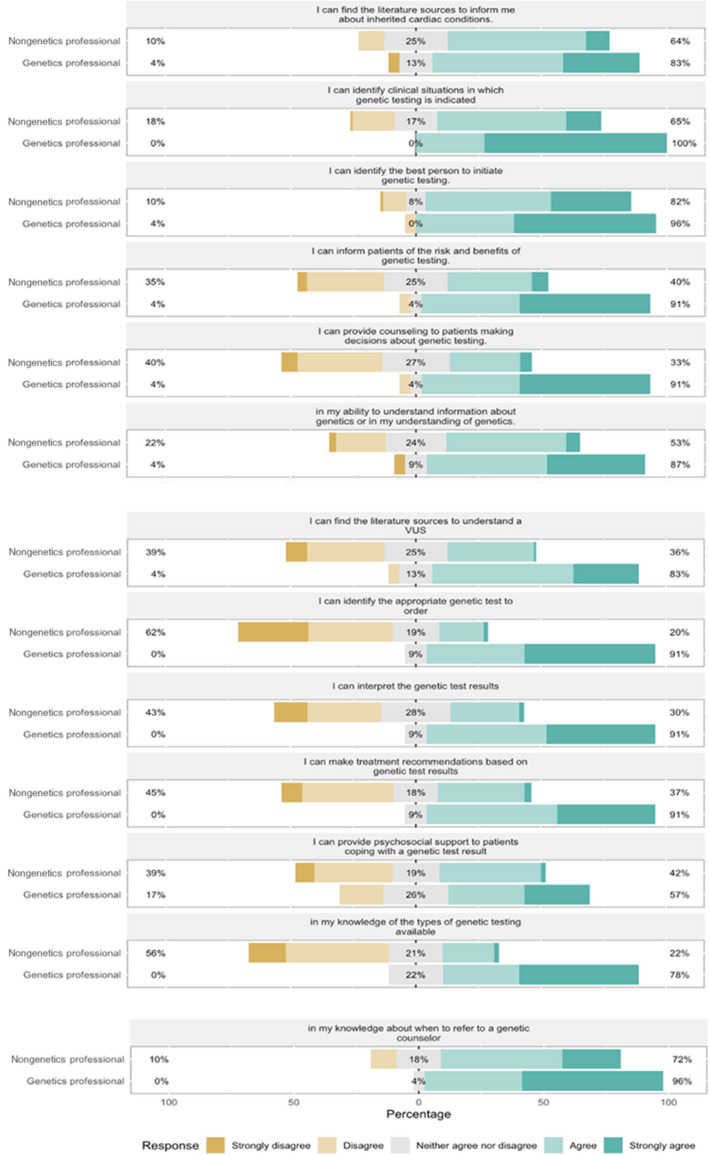
Participants’ responses to 13 “I feel confident in …” statements. Respondents were asked to assess how confident they felt with each task on a Likert scale, from “strongly disagree” to “strongly agree.” Percentages along the left axis represent the percentages of participants who are not confident in the task (participants who “strongly disagreed” or “disagreed”). Percentages centered in the gray boxes represent percentages of participants who neither agree nor disagree to have confidence in the task. Percentages along the right axis represent the percentages of participants who feel confident with the task (participants who “strongly agreed” or “agreed”). With the exception of confidence to “identify the best person to initiate genetic testing,” confidence scores for all questions differed statistically significantly (P<0.005) between genetics and nongenetics professionals. VUS indicates variant of uncertain significance.
Clinical Knowledge of Genetic Testing in Cardiology Based on Practice Guidelines
Providers were evaluated on their practical knowledge of genetic testing in cardiology with questions based on current practice guidelines. 12 Most (93.9%; n=123) correctly identified a clinical situation in which genetic testing for arrhythmia syndromes was warranted. Similarly, 91.6% (n=120) correctly identified the most clearly affected family member as the best person to initiate genetic testing.
Desired Topics for an Educational Program
Among all providers, the most desired topics for a cardiogenetics educational program were how to conduct a risk assessment for inherited cardiac conditions (94%; n=120) and management of inherited cardiac conditions based on guidelines (91%; n=117) (Figure 4). In addition, topics on genetic testing, including both interpreting genetic test results (78%; n=100) and ordering genetic tests (70%; n=89), were also highly desired by most providers. There was no significant difference in desired topics between nongenetic and self‐identified genetic providers, and so pooled data are presented in Figure 4.
Figure 4. Desired topics for an educational program about cardiovascular genetic testing.
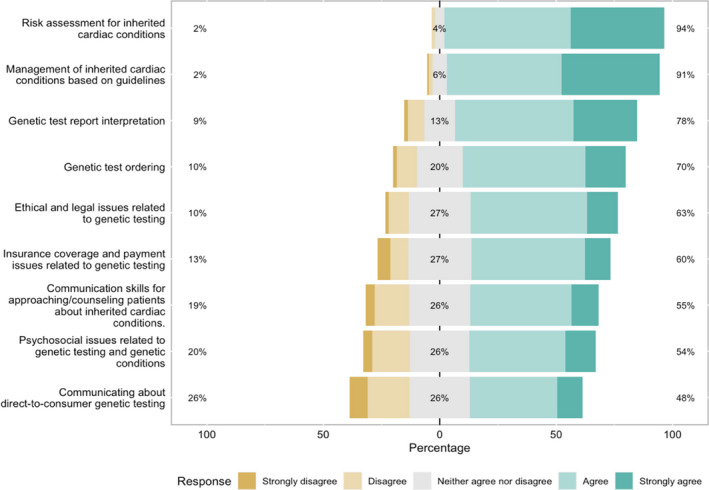
Respondents were asked to indicate whether they would like to see the following topics covered in a continuing medical education program about genetic testing relevant to cardiology using a Likert scale, ranging from strongly disagree to strongly agree. Percentages along the left axis represent the percentages of participants who would not like to have the topic included in the educational program (participants who “strongly disagreed” or “disagreed”). Percentages centered in the gray boxes represent percentages of participants who neither agree nor disagree to have the topic included in the educational program. Percentages along the right axis represent the percentages of participants who would like to have the topic included in the educational program (participants who “strongly agreed” or “agreed”).
Discussion
This study assessed cardiology practitioners’ current practices, confidence, and knowledge surrounding cardiovascular genetics and genetic testing in a US‐based cohort. Our findings reveal 3 overarching themes: (1) nongenetic professionals report deficits in confidence and practical knowledge surrounding genetic testing in cardiology, (2) most providers do not want to be the sole provider of genetic testing for inherited cardiac conditions, and (3) education and the establishment of professional genetic guidelines were identified as possible approaches to increasing the use of genetic testing in the clinical management of patients with inherited cardiac conditions and at‐risk relatives. Our study sample included providers who self‐identified as genetics professionals and those who did not. This allowed the comparison of current practices, confidence, and practical knowledge between these 2 groups. Consistency of the data was a strength of our study. Results showed participants are self‐aware of the deficits in their knowledge of genetic testing for inherited cardiac conditions. Practical knowledge questions on which participants scored poorly were also the skills about which they had the least confidence and were the most desired topics for an educational program.
Our results align with previous studies showing health care providers regard their knowledge and practical cardiovascular genetic skills as insufficient. 18 , 19 , 20 In our study, most nongenetics providers reported high confidence in identifying clinical situations in which genetic testing is warranted and the best person in the family to initiate testing. Practical knowledge was consistent with providers’ confidence as most providers correctly answered the questions covering these 2 topics. Nongenetic providers in this study were less confident about ordering a specific genetic test, interpreting the results, or making treatment recommendations based on results, and thus referred to genetics based on these limitations. Our study did identify some participants who do not routinely refer their patients, and their lack of confidence and practical knowledge about ordering, interpreting, and using genetic testing to guide medical management of cardiology patients is potentially further limiting the use of genetic testing among nongenetic providers.
Participants reported that they do not want to be the sole provider of genetic testing for their patients and preferred to make referrals. Most nongenetic providers in this study reported access to a genetics provider with whom they can consult and to whom they can refer their patients. Notably, most nongenetic providers referred and had genetic testing performed for their patients once a month or less. Despite most reporting access to a genetics provider with whom they can consult and to whom they can refer their patients, most nongenetic providers referred and had genetic testing performed for their patients once a month or less.
A clear need and desire exist to address the knowledge deficit surrounding genetic testing in cardiology among nongenetics professionals. Although a minority of participants reported barriers to implementing genetic testing into their practice, most participants who reported barriers also reported lack of education as a barrier. Moreover, when asked about desired topics for an educational program on cardiovascular genetics, the most desired topics aligned with confidence and knowledge deficits: management of inherited cardiac conditions based on guidelines, risk assessment of inherited cardiac conditions, and topics surrounding genetic testing, including ordering and interpreting genetic test results. Online medical educational modules have proved effective in creating positive change in health care providers’ knowledge and practice. 23 , 31 Educating nongenetics providers on genetics has been shown to improve referral decisions and confidence in genetics and consultation skills in other areas of medicine, like oncology. 16 , 32
Educational programs are a useful approach to begin addressing lack of confidence and knowledge but are not likely sufficient alone. In a previous study, Wilkes and colleagues (2017) assessed the efficacy of a web‐based general genetics curriculum among primary care providers and showed that even though the intervention demonstrated a significant increase in factual learning and retention, there were few differences in clinical behavior. 33 The authors of the study suggested that clinically available resources (eg, evidence‐based information) may promote active engagement. Similarly, many cardiologists stressed the need for published genetic guidelines in cardiology. 18 This aligns with our findings. Most participants reported evidence‐based professional society guidelines as a motivator to incorporating genetic testing into their practice. Although contradictions exist about the impact of professional guidelines on providers’ practice, 34 this study suggests both education and professional guidelines may motivate incorporation of genetics and improvement in clinical care and management of patients with inherited cardiac conditions and those at risk for SCD.
This study was limited by the lack of demographic diversity of its participants. The vast majority of respondents were from urban regions and worked at university medical centers with access to genetics services. Therefore, this sample is not representative of cardiovascular cardiology practitioners in the United States. Health care providers in different geographic regions and work settings who have varied access to genetics services might have different practices, confidence, and knowledge about genetic testing in cardiology. Moreover, most respondents have been practicing for >10 years. It is possible that with a younger cohort, the confidence and knowledge surrounding genetics might be different as medical education continually evolves and there has been an increase in the genetics content incorporated in newer curricula.
Practitioners’ current practices surrounding genetic testing in the cardiology field highlight the importance of access to genetic services. Additional research is required to further explore practitioners’ practices and access to genetics services in different settings like, for example, in rural geographical locations.
Conclusions
As new cardiogenetics knowledge is integrated into health care, it will be essential to ensure that practitioners acquire and maintain the necessary competencies to guide patients with inherited cardiac conditions along the pathways of screening, diagnostics, monitoring, and management. Our study emphasizes the importance of access to genetics services in the cardiology field and the need for addressing the identified deficit in confidence and knowledge surrounding cardiogenetics and genetic testing. The results of this study will be used to guide the development of an online educational program about cardiovascular genetics and genetic testing, with the ultimate goal of reducing morbidity and mortality from these conditions. Ideally, subsequent research in this area will include more practitioners from underserved areas or organizations and hospitals/medical centers that do not have genetic counselors on staff.
Sources of Funding
This work is supported by the American Heart Association Sudden Cardiac Death Strategically Focused Research Network (grants 19SFRN34830054 and 19SFRN34850101). Dr Webster was supported by National Institutes of Health/National Heart, Lung, and Blood Institute K23 HL130554.
Disclosures
EMM has been a consultant for Avidity, Amgen, AstraZeneca, Cytokinetics, Invitae, Janssen, Pfizer, PepGen, Tenaya Therapeutics, Stealth BioTherapeutics; she is also the founder of Ikaika Therapeutics. These activities are unrelated to the content of this manuscript.
Supporting information
Data S1
Acknowledgments
We gratefully acknowledge the contributions of all the medical practitioners who took the time to complete the survey.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023763
For Sources of Funding and Disclosures, see page 9.
References
- 1. Jarcho JA, McKenna W, Pare JAP, Solomon SD, Holcombe RF, Dickie S, Levi T, Donis‐Keller H, Seidman JG, Seidman CE. Mapping a gene for familial hypertrophic cardiomyopathy to chromosome 14q1. N Engl J Med. 1989;321:1372–1378. doi: 10.1056/nejm198911163212005 [DOI] [PubMed] [Google Scholar]
- 2. Geisterfer‐Lowrance AAT, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG. A molecular basis for familial hypertrophic cardiomyopathy: a β cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-I [DOI] [PubMed] [Google Scholar]
- 3. McKenna WJ, Judge DP. Epidemiology of the inherited cardiomyopathies. Nat Rev Cardiol. 2021;18:22–36. doi: 10.1038/s41569-020-0428-2 [DOI] [PubMed] [Google Scholar]
- 4. Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D, Cirino AL, Fox JC, Lakdawala NK, Ware JS, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy. Circulation. 2018;138:1387–1398. doi: 10.1161/CIRCULATIONAHA.117.033200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sen‐Chowdhry S, Morgan RD, Chambers JC, McKenna WJ. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annu Rev Med. 2010;61:233–253. doi: 10.1146/annurev.med.052208.130419 [DOI] [PubMed] [Google Scholar]
- 6. Dunn KE, Ashley EA. Arrhythmogenic right ventricular cardiomyopathy: toward a modern clinical and genomic understanding. Circ Cardiovasc Genet. 2015;8:421–424. doi: 10.1161/CIRCGENETICS.115.001119 [DOI] [PubMed] [Google Scholar]
- 7. Groeneweg JA, Bhonsale A, James CA, te Riele AS, Dooijes D, Tichnell C, Murray B, Wiesfeld ACP, Sawant AC, Kassamali B, et al. Clinical presentation, long‐term follow‐up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet. 2015;8:437–446. doi: 10.1161/CIRCGENETICS.114.001003 [DOI] [PubMed] [Google Scholar]
- 8. Hofman N, Tan HL, Alders M, Kolder I, De Haij S, Mannens MMAM, Lombardi MP, Dit Deprez RHL, van Langen I, Wilde AAM. Yield of molecular and clinical testing for arrhythmia syndromes: report of 15 years’ experience. Circulation. 2013;128:1513–1521. doi: 10.1161/CIRCULATIONAHA.112.000091 [DOI] [PubMed] [Google Scholar]
- 9. Offerhaus JA, Bezzina CR, Wilde AAM. Epidemiology of inherited arrhythmias. Nat Rev Cardiol. 2020;17:205–215. doi: 10.1038/s41569-019-0266-2 [DOI] [PubMed] [Google Scholar]
- 10. Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, et al. Spectrum of mutations in long‐QT syndrome genes: KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.CIR.102.10.1178 [DOI] [PubMed] [Google Scholar]
- 11. Hershberger RE, Givertz MM, Ho CY, Judge DP, Kantor PF, McBride KL, Morales A, Taylor MRG, Vatta M, Ware SM. Genetic evaluation of cardiomyopathy—a Heart Failure Society of America Practice Guideline. J Cardiac Fail. 2018;24:281–302. doi: 10.1016/j.cardfail.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138:e272–e391. doi: 10.1161/CIR.0000000000000549 [DOI] [PubMed] [Google Scholar]
- 13. Musunuru K, Hershberger RE, Day SM, Klinedinst NJ, Landstrom AP, Parikh VN, Prakash S, Semsarian C, Sturm AC. Genetic testing for inherited cardiovascular diseases: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2020;13:e000067. doi: 10.1161/HCG.0000000000000067 [DOI] [PubMed] [Google Scholar]
- 14. Department of Health and Human Services . Genetics Education and Training. Report of the Secretary’s Advisory Committte of Genetics, Health, and Society. Available at: https://www.genome.gov/Pages/Careers/HealthProfessionalEducation/SACGHS‐EducationReport2011.pdf. Accessed February 1, 2022.
- 15. Mital S, Musunuru K, Garg V, Russell MW, Lanfear DE, Gupta RM, Hickey KT, Ackerman MJ, Perez MV, Roden DM, et al. Enhancing literacy in cardiovascular genetics: a scientific statement from the American Heart Association. Circ Cardiovasc Genet. 2016;9:448–467. doi: 10.1161/HCG.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 16. Houwink EJF, Muijtjens AMM, van Teeffelen SR, Henneman L, Rethans JJ, Jacobi F, van der Jagt L, Stirbu I, van Luijk SJ, Stumpel CTRM, et al. Effect of comprehensive oncogenetics training interventions for general practitioners, evaluated at multiple performance levels. PLoS One. 2015;10:e0122648. doi: 10.1371/journal.pone.0122648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Houwink EJF, Muijtjens AMM, van Teeffelen SR, Henneman L, Rethans JJ, van der Jagt LEJ, van Luijk SJ, Dinant GJ, van der Vleuten C, Cornel MC. Effectiveness of oncogenetics training on general practitioners’ consultation skills: a randomized controlled trial. Genet Med. 2014;16:45–52. doi: 10.1038/gim.2013.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Langen IM, Birnie E, Leschot NJ, Bonsel GJ, Wilde AAM. Genetic knowledge and counselling skills of Dutch cardiologists: sufficient for the genomics era? Eur Heart J. 2003;24:560–566. doi: 10.1016/S0195-668X(02)00522-5 [DOI] [PubMed] [Google Scholar]
- 19. Marathe JA, Woodroffe J, Ogden K, Hughes C. General practitioners’ knowledge and use of genetic counselling in managing patients with genetic cardiac disease in non‐specialised settings. J Community Genet. 2015;6:375–382. doi: 10.1007/s12687-015-0229-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Magnusson P, Mörner S. Current knowledge of hypertrophic cardiomyopathy among health care providers in Sweden. Cureus. 2020;12:e12220. doi: 10.7759/cureus.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 22. Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, Grilli R, Harvey E, Oxman A, O’Brien MA. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39:II2–II45. doi: 10.1097/00005650-200108002-00002 [DOI] [PubMed] [Google Scholar]
- 23. Raza A, Coomarasamy A, Khan KS. Best evidence continuous medical education. Arch Gynecol Obstet. 2009;280:683–687. doi: 10.1007/s00404-009-1128-7 [DOI] [PubMed] [Google Scholar]
- 24. Berger AC, Johnson SG, Beachy SH, Olson S. In: Berger AC, Johnson SG, Beachy SH, Olson S, eds. Improving Genetics Education in Graduate and Continuing Health Professional Education: Workshop Summary. The National Academies Press; 2015. Available at: https://www.nap.edu/catalog/18992/improving‐genetics‐education‐in‐graduate‐and‐continuing‐health‐professional‐education. Accessed February 1, 2022. [PubMed] [Google Scholar]
- 25. Dougherty MJ, Wicklund C, Johansen Taber KA. Challenges and opportunities for genomics education: insights from an institute of medicine roundtable activity. J Contin Educ Health Prof. 2016;36:82–85. doi: 10.1097/CEH.0000000000000019 [DOI] [PubMed] [Google Scholar]
- 26. Frenk J, Chen L, Bhutta ZA, Cohen J, Crisp N, Evans T, Fineberg H, Garcia P, Ke Y, Kelley P, et al. Health professionals for a new century: transforming education to strengthen health systems in an interdependent world. Lancet. 2010;376:1923–1958. doi: 10.1016/S0140-6736(10)61854-5 [DOI] [PubMed] [Google Scholar]
- 27. Scherr C, Kalke K, Ramesh S, Fakhari H, Castillo L, Wicklund C, McNally E, Rasmussen‐Torvik L. Integrating clinical genetics in cardiology: current practices and recommendations for education. Genet Med. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020.
- 31. Blazer KR, Christie C, Uman G, Weitzel JN. Impact of web‐based case conferencing on cancer genetics training outcomes for community‐based clinicians. J Cancer Educ. 2012;27:217–225. doi: 10.1007/s13187-012-0313-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carroll JC, Wilson BJ, Allanson J, Grimshaw J, Blaine SM, Meschino WS, Permaul JA, Graham ID. GenetiKit: a randomized controlled trial to enhance delivery of genetics services by family physicians. Fam Pract. 2011;28:615–623. doi: 10.1093/fampra/cmr040 [DOI] [PubMed] [Google Scholar]
- 33. Wilkes MS, Day FC, Fancher TL, McDermott H, Lehman E, Bell RA, Green MJ. Increasing confidence and changing behaviors in primary care providers engaged in genetic counselling. BMC Med Educ. 2017;17:163. doi: 10.1186/s12909-017-0982-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grimshaw J, Thomas R, MacLennan G, Fraser C, Ramsay C, Vale L, Whitty P, Eccles M, Matowe L, Shirran L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8:iii‐iv, 1‐72. doi: 10.3310/hta8060 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


