Abstract
Background
The association between blood pressure control and clinical outcomes is unclear among patients with heart failure with preserved ejection fraction. Both too high and too low of systolic blood pressure (SBP) have been reported to be related to poor clinical prognosis. This study aimed to assess the association between time in SBP target range and adverse clinical events among patients with heart failure with preserved ejection fraction.
Methods and Results
This study was a secondary analysis of the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial, a randomized clinical trial that compared the efficacy and safety of spironolactone in patients with heart failure with preserved ejection fraction. Time in target range (TTR) was calculated using linear interpolation, with the target range of SBP defined as 110 to 130 mm Hg. The association between TTR with adverse outcomes was estimated using multivariable Cox regression to adjust for multiple confounders. Participants with greater TTR were younger, more likely to be White, had less comorbidities, and lower body mass index. After adjusting for multiple covariates including mean SBP, 1‐SD increment (38.3%) of TTR was significantly associated with a decreased risk of primary composite end point (hazard ratio [HR], 0.81 [0.73–0.90]), as well as a lower risk of all‐cause mortality (HR, 0.81 [0.73–0.90]), cardiovascular death (HR, 0.78 [0.68–0.90]), and heart failure hospitalization (HR, 0.85 [0.74–0.97]). Results were similar when participants were categorized by TTR groups. Subgroup analyses showed that the associations were more significant in young people than in the old (P interaction=0.028).
Conclusions
In patients with heart failure with preserved ejection fraction, greater time in SBP target range was statistically associated with a decreased risk of cardiovascular outcomes and mortality events beyond blood pressure level, especially among younger patients.
Keywords: blood pressure, heart failure, time in target range
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- HFpEF
heart failure with preserved ejection fraction
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist
- TTR
time in target range
Clinical Perspective
What Is New?
In patients with heart failure with preserved ejection fraction (HFpEF), our study found that greater time in systolic blood pressure target range was associated with decreased risk of cardiovascular outcomes, mortality events and hospitalization for heart failure, especially for those with younger age.
Time in target range could be a value that characterize the extent of blood pressure control among patients with HFpEF.
What Are the Clinical Implications?
The results of this study highlight the importance of taking time in target range as a modifiable risk factor to reduce the risk of cardiovascular and mortality events in patients with HFpEF.
Time in systolic blood pressure target range may provide an assessment of the efficacy of therapy and guide adjustments in medications and lifestyle to achieve better blood pressure control.
Time in systolic blood pressure target range may provide effective risk stratification across patients with HFpEF; identifying patients at higher risk through time in target range, reinforcing clinical management and providing sufficient therapeutic intervention may make it possible to alter mortality outcome in HFpEF.
Heart failure with preserved ejection fraction (HFpEF) has grown to be the predominant form of heart failure (HF) and still presents a diagnostic and therapeutic challenge for clinicians. 1 Little medical intervention has been proved to alter mortality outcome in HFpEF, and therefore treatment is mainly focused on management of comorbidities. 1 Hypertension is one of the most common comorbidities in patients with HFpEF 2 and most medications recommended by guideline to improve prognosis in this population have an effect on reducing blood pressure concomitantly. 3 Management of systolic blood pressure (SBP) remains an important part of therapy and has reduced adverse clinical events in the general population, but there still lacks a dedicated trial using blood pressure targets in patients with HFpEF to determine the relationship between blood pressure control and clinical outcomes. Thus, whether blood pressure control is associated with clinical benefit among patients with HFpEF is still unclear.
Despite limited evidence to confirm the effect of blood pressure lowering in HFpEF, an SBP goal of <130 mm Hg has been recommended by clinical guideline. 3 Previous studies have observed that there existed a J‐shaped relationship between SBP and adverse clinical outcomes, 4 , 5 , 6 , 7 indicating that both too high and too low of SBP may lead to poor prognosis in patients with HFpEF. It is conjectured that controlling blood pressure in a specific target range might help to achieve the maximum benefit. Time in target range (TTR) of SBP is a value that estimates the time with SBP within target range and characterize the extent of blood pressure control. 8 , 9 In this study, we aimed to evaluate the association between TTR of SBP and risk of clinical outcomes among patients with HFpEF, using data from the TOPCAT trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist; URL: https://www.clinicaltrials.gov; Unique identifier: NCT00094302). We hypothesized that greater TTR would be associated with a decreased risk of adverse clinical outcomes.
Methods
The data and materials from the TOPCAT study have been made available on request, located on the National Institutes of Health website (https://biolincc.nhlbi.nih.gov/studies/topcat/). A request for assessing the data of TOPCAT Trial with a protocol for the intended post hoc analysis has been submitted, and the data were provided by National Heart, Lung, and Blood Institute after a Research Materials Distribution Agreement was signed.
Study Population
This study was a secondary cohort analysis of the TOPCAT trial and deidentified data were obtained from the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Coordinating Center. The detailed design and protocol of the TOPCAT trial have been published and described previously. 10 , 11 Briefly, TOPCAT was a multi‐center, international, randomized, double‐masked clinical trial that evaluated the efficacy and safety of spironolactone in 3445 patients with HFpEF. To meet the inclusion criteria for TOPCAT, patients diagnosed as HF with left ventricular ejection fraction ≥45% were aged >50 years and required to have a controlled SBP (<140 mm Hg or 140–160 mm Hg with at least 3 anti‐hypertensive medications). Patients with a life expectancy of <3 years, severe chronic kidney disease (estimated glomerular filtration rate <30 mL/min per 1.73 m2 body surface area or serum creatinine level ≥2.5 mg/dL) or known chronic hepatic disease (alanine aminotransferase or aspartate aminotransferase levels >3 times of the upper limit) were excluded. All patients enrolled in the TOPCAT trial provided written informed consent.
The cohort for this analysis included all the participants with ≥3 blood pressure measurements and free of primary composite outcome event within the first 4 months after enrollment. Additionally, participants with missing covariate data were excluded. The flowchart is shown in Figure S1. In total, 3194 participants from the TOPCAT trial were included in the final analysis.
Blood Pressure Measurements and Definition of Time in Target Range
Participants recruited in the TOPCAT study underwent detailed evaluation at baseline. Blood pressure was measured by trained physicians or nurses in the seated posture at least 3 times after 5‐minutes rest, and the average of 3 blood pressure measurements was calculated as the blood pressure for that particular visit. Blood pressure was measured at baseline, 4 weeks, 8 weeks, 4 months, and every 4 months during the rest of the first year and every 6 months thereafter for up to 6 years. We extracted data from the first 4 months and excluded participants with <3 available blood pressure measurements. Since the therapeutic target range for blood pressure is varied across different population, a specific time in target range should be calculated based on the recommended blood pressure target among different population group. In our analysis, the target range for SBP was defined as 110 to 130 mm Hg, because a target of SBP <130 mm Hg has been recommended for patients with HFpEF by professional society guidelines 3 and the same SBP target range has been used in previous study. 8 Time in target range was estimated using linear interpolation, 12 which assumed a linear relationship existed between 2 consecutive blood pressure values and determined the proportion of time for which the blood pressure was within the target range (Figure 1A).
Figure 1. Diagram of time in systolic blood pressure (SBP) target range and stacked bar graphs of mean SBP by time in target range groups.
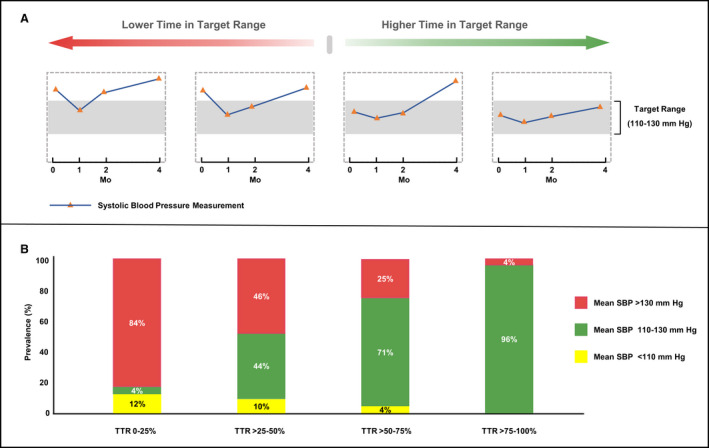
This figure depicts example of low to high time in SBP target range (A). Participants with higher time in SBP target range tend to have a greater proportion for which the mean SBP was within the target range (B). SBP indicates systolic blood pressure; and TTR, time in target range.
Clinical Outcomes
The primary outcome for this analysis was consistent with the primary end point of the TOPCAT trial, 11 as a composite of cardiovascular death, aborted cardiac arrest, or hospitalization for HF. The secondary outcomes of interest included all‐cause mortality, cardiovascular death, and hospitalization for HF. Cardiovascular death included mortality from sudden death, pump failure, myocardial infarction, stroke, pulmonary embolism, and cardiovascular procedure‐related events. All outcome events were adjudicated by a clinical end point committee at Brigham and Women’s Hospital according to pre‐specified criteria as previously described. 10 Time origin (landmark) for follow‐up was defined as 4 months after randomization. Time‐to‐event was measured as the time from 4 months to the date of first event occurrence.
Statistical Analysis
Patients were stratified into 4 groups according to TTR level (0%–25%, >25%–50%, >50%–75%, >75%–100%), and baseline characteristics at the time of randomization were compared across these 4 groups as mean (SD) for continuous variables and number (percentage) for categorical variables. To detect the statistical significance across groups, χ2 test, 1‐way ANOVA, or Kruskal‒Wallis test were used as appropriate.
The analyses of primary end point associated with TTR were performed as primary analyses and the other analyses designated as confirmatory. Kaplan‒Meier survival analysis was performed to compare the survival estimates of primary and secondary outcomes across TTR groups. Multivariable Cox proportional hazards regression models were conducted to evaluate the associations of TTR with clinical outcomes. The following potential confounders were adjusted: age, sex, race, smoking status, left ventricular ejection fraction, estimated glomerular filtration rate, body mass index (BMI), medical history (previous HF hospitalization, diabetes, and hypertension), medicine uses (angiotensin‐converting enzyme inhibitors, angiotensin receptor blocker, beta‐blocker, calcium‐channel blockers, diuretic agents) and mean corresponding SBP. The results were presented using hazard ratio (HR) and 95% CI. Restricted cubic spline models with 5 knots at the 5th, 25th, 50th, 75th, and 95th percentiles were performed to assess the non‐linearity between TTR and primary end point. Given the design of the TOPCAT trial, we further examined the association between TTR with primary and secondary outcomes stratified by trial arms (spironolactone versus placebo). Using visual inspection and testing of the Schoenfeld residuals as a function of time didn’t suggest deviation from proportionality for any model.
To examine whether the association of primary outcome with TTR differed by subgroups, we performed pre‐specified subgroup analysis by sex, age, race, treatment arm, BMI, New York Heart Association class, chronic kidney disease, and diabetes. To test the robustness of the association between TTR and risk of the primary composite outcome, several sensitivity analyses were performed. First, sensitivity analysis was performed limited to participants enrolled in North or South America. 13 Second, we performed a sensitivity analysis limited to participants with hypertension using a SBP target range of 120 to 130 mm Hg according to the global hypertension practice guidelines by International Society of Hypertension. 14 Third, we performed an analysis using blood pressure values from 4 weeks to 4 months because prior studies have found that SBP was significantly decreased soon after the usage of spironolactone but reached flat after 4 weeks. 6 Fourth, to examine the effects of TTR over a longer time span, we calculate TTR using data from baseline to 12 months.
Analyses were performed using Stata (version 14.0, StataCorp LP, College Station, TX) and R statistical software (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria), with a 2‐sided P value <0.05 considered statistically significant.
Results
Baseline Characteristics
The current study included 3194 patients with HFpEF from the TOPCAT study. The mean age of the study population was 68±10 years, 1636 (51%) were women, 2877 (90%) were White, and 2920 (91%) had a comorbidity of hypertension. The baseline characteristics of participants were shown in Table 1. Participants with greater TTR were younger, more likely to be White, had a lower BMI and New York Heart Association class than those with TTR of 0% to 25%. Participants with history of previous HF hospitalization, diabetes, and hypertension and usage of anti‐hypertensive agents comprised a large proportion in those with TTR of 0% to 25% than of the group with TTR of >75% to 100%. The relationship between TTR groups and mean SBP is shown in Figure 1B, which demonstrated that participants with greater TTR had a higher proportion of mean SBP within target range (110–130 mm Hg).
Table 1.
Baseline Characteristics of Participants According to SBP TTR
| Characteristics | TTR groups | ||||
|---|---|---|---|---|---|
|
0%–25% (n=1055) |
>25%–50% (n=463) |
>50%–75% (n=526) |
>75%–100% (n=1150) |
P value | |
| TTR, % | 5.7 (8.6) | 38.0 (7.3) | 61.9 (7.2) | 94.5 (8.5) | <0.001 |
| Age, y | 68.0±9.5 | 69.5±9.5 | 69.6±9.9 | 67.8±9.3 | <0.001 |
| Women | 568 (53.8) | 227 (48.9) | 260 (49.3) | 582 (50.6) | 0.187 |
| White race* | 922 (87.4) | 402 (86.6) | 461 (87.5) | 1094 (95.1) | <0.001 |
| Current smoker | 111 (10.5) | 47 (10.2) | 41 (7.8) | 133 (11.6) | 0.001 |
| BMI, kg/m2 | 32.6±7.2 | 32.65±7.6 | 32.23±7.2 | 30.8±6.0 | <0.001 |
| NYHA class III and IV | 345 (32.7) | 164 (35.3) | 167 (31.7) | 338 (29.4) | 0.058 |
| LVEF, % | 57.6±7.3 | 57.5±7.7 | 57.4±7.5 | 56.4±7.4 | <0.001 |
| Previous HFH | 792 (75.1) | 345 (74.4) | 354 (67.2) | 834 (72.5) | 0.008 |
| Diabetes | 372 (35.3) | 179 (38.6) | 175 (33.2) | 274 (23.8) | <0.001 |
| Hypertension | 1001 (94.9) | 416 (89.8) | 481 (91.4) | 1022 (88.9) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 68.4±20.4 | 65.7±20.0 | 67.4±19.9 | 68.7±18.9 | 0.032 |
| ACEI/ARB | 916 (86.8) | 385 (83.0) | 432 (82.1) | 966 (84.0) | 0.054 |
| Beta‐blocker | 843 (79.9) | 360 (77.6) | 403 (76.6) | 879 (76.4) | 0.223 |
| CCB | 431 (40.9) | 172 (37.1) | 200 (38.0) | 398 (34.6) | 0.026 |
| Diuretic agents | 905 (85.8) | 400 (86.2) | 446 (84.8) | 858 (74.6) | <0.001 |
| Baseline SBP, mm Hg | 135.2±15.1 | 127.4±14.2 | 128.0±12.4 | 125.4±10.0 | <0.001 |
| Mean SBP, mm Hg | 136.1±15.1 | 126.7±9.9 | 125.1±7.6 | 122.6±4.8 | <0.001 |
Values are presented as mean±SD or number (%). ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium‐channel blockers; eGFR, estimated glomerular filtration rate; HFH, heart failure hospitalization; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure; and TTR, time in target range.
In this study, participants are White race or Black race. White race is compared with Black race.
Associations Between TTR and Clinical Outcomes
During a mean (SD) follow‐up of 3.0 (1.7) years, 537 (16.8%) primary end point events occurred. In adjusted analyses that accounted for multiple confounders, 1‐SD increment (38.3%) of TTR was associated with a decreased risk of primary composite outcome (HR, 0.80 [0.72–0.88]; P<0.001), even after adjusting for mean SBP (Table 2). The spline regression analysis confirmed that TTR was inversely associated with the risk of the primary outcome (Figure 2). In secondary analyses, 440 (13.8%) all‐cause mortality events, 277 (8.7%) cardiovascular death events, and 342 (10.7%) HF hospitalization events occurred separately. After adjusting for multiple confounders, greater TTR was significantly associated with lower risk of all‐cause mortality (HR, 0.79 [0.71–0.88]; P<0.001), cardiovascular death (HR, 0.77 [0.67–0.88]; P<0.001), and HF hospitalization (HR, 0.83 [0.73–0.94]; P=0.003) (Table 2). Results were similar when participants were categorized into 4 groups: the lowest risk of primary composite outcome was observed in the greatest (>75%–100%) TTR group (Table 3, Figure S2). When examining across treatment arms, similar association was found in spironolactone arm and placebo arm, separately. (Table S1).
Table 2.
Association Between Time in SBP Target Range with Clinical Outcomes
| n/N (%) | Unadjusted model | Fully adjusted model | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Primary end point | 537/3194 (16.8) | 0.77 (0.71–0.84) | <0.001 | 0.80 (0.72–0.88) | <0.001 |
| All‐cause mortality | 440/3194 (13.8) | 0.82 (0.75–0.90) | <0.001 | 0.79 (0.71–0.88) | <0.001 |
| Cardiovascular death | 277/3194 (8.7) | 0.79 (0.71–0.89) | <0.001 | 0.77 (0.67–0.88) | <0.001 |
| HF hospitalization | 342/3194 (10.7) | 0.77 (0.69–0.85) | <0.001 | 0.83 (0.73–0.94) | 0.003 |
HRs (95% CIs) express the difference in primary end point, all‐cause mortality, cardiovascular death, and HF hospitalization associated with 1‐SD (38.3%) increment in time in target range in 3194 patients. Fully adjusted model: age, sex, race, current smoker, left ventricular ejection fraction, estimated glomerular filtration rate, body mass index, previous HF hospitalization, diabetes, hypertension, anti‐hypertensive medication uses (inhibitors of the renin‐angiotensin, beta‐blocker, calcium‐channel blocker, diuretics), and mean corresponding systolic blood pressure. HF indicates heart failure; HR, hazard ratio; and SBP, systolic blood pressure.
Figure 2. Restricted cubic spline plots for primary end point by time in systolic blood pressure target range.
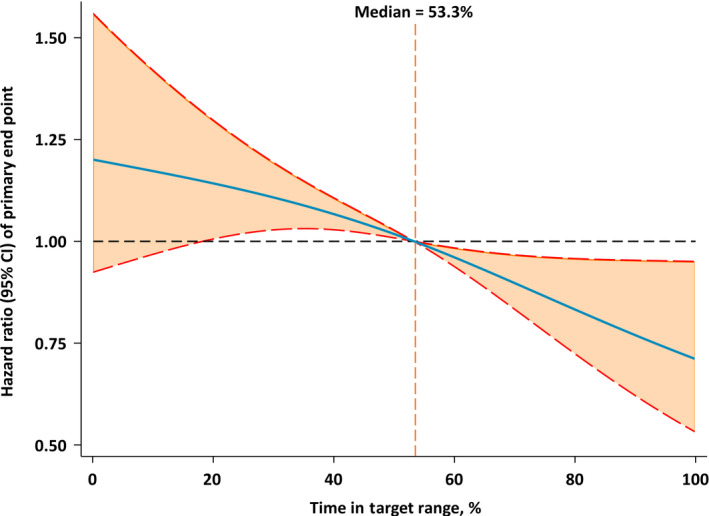
The figure showed the adjusted hazard ratios of primary end point by time in systolic blood pressure target range. Each hazard ratio was compared with a median time in target range of 53.3%. The blue line represents the hazard ratio of time in systolic blood pressure target range across the whole range. The red lines represent the 95% CI.
Table 3.
Association Between Time in SBP Target Range With Clinical Outcomes Across TTR Groups
| TTR groups | |||||
|---|---|---|---|---|---|
| 0%–25% | >25%–50% | >50%–75% | >75%–100% | P for trend | |
| Primary end point | |||||
| No. (%) | 206/1055 (19.5) | 111/463 (24.0) | 99/526 (18.8) | 121/1150 (10.5) | |
| Crude model | 1.00 (Ref) | 1.30 (1.04–1.64) | 0.94 (0.74–1.20) | 0.52 (0.42–0.65) | <0.001 |
| Fully adjusted model | 1.00 (Ref) | 1.13 (0.89–1.43) | 0.85 (0.66–1.09) | 0.59 (0.46–0.75) | <0.001 |
| All‐cause mortality | |||||
| No. (%) | 159/1055 (15.1) | 88/463 (19.0) | 81/526 (15.4) | 112/1150 (9.7) | |
| Crude model | 1.00 (Ref) | 1.34 (1.04–1.74) | 1.01 (0.77–1.32) | 0.64 (0.51–0.82) | <0.001 |
| Fully adjusted model | 1.00 (Ref) | 1.12 (0.86–1.46) | 0.86 (0.65–1.13) | 0.63 (0.48–0.81) | <0.001 |
| Cardiovascular death | |||||
| No. (%) | 106/1055 (10.0) | 55/463 (11.9) | 51/526 (9.7) | 65/1150 (5.7) | |
| Crude model | 1.00 (Ref) | 1.26 (0.91–1.75) | 0.95 (0.68–1.33) | 0.56 (0.41–0.76) | <0.001 |
| Fully adjusted model | 1.00 (Ref) | 1.10 (0.78–1.53) | 0.88 (0.62–1.24) | 0.55 (0.39–0.77) | <0.001 |
| HF hospitalization | |||||
| No. (%) | 131/1055 (12.4) | 71/463 (15.3) | 66/526 (12.5) | 74/1150 (6.4) | |
| Crude model | 1.00 (Ref) | 1.30 (0.98–1.74) | 0.99 (0.73–1.32) | 0.50 (0.38–0.67) | <0.001 |
| Fully adjusted model | 1.00 (Ref) | 1.10 (0.82–1.49) | 0.87 (0.64–1.19) | 0.64 (0.47–0.88) | <0.001 |
Hazard ratios and 95% CIs were calculated with the use of the Cox proportional hazards regression model. Fully adjusted model was adjusted for age, sex, race, current smoker, left ventricular ejection fraction, estimated glomerular filtration rate, body mass index, previous HF hospitalization, diabetes, hypertension, anti‐hypertensive medication uses (inhibitors of the renin‐angiotensin, beta‐blocker, calcium‐channel blocker, diuretics), and mean corresponding SBP. HF indicates heart failure; Ref, reference; SBP, systolic blood pressure; and TTR, time in target range.
Sensitivity Analyses
In adjusted analyses across key subgroups of interest, a consistent pattern of association between greater TTR and lower risk of the primary outcome was observed among male patients and female patients, among spironolactone and placebo arms and across different BMI class (BMI <30 kg/m2 versus BMI ≥30 kg/m2) (Figure 3). When stratified by age, race, New York Heart Association class, and diabetes, the association was stronger in White participants, younger participants (aged ≤75 years), patients with lower class of New York Heart Association, and patients without diabetes (Figure 3). Specifically, the interaction between TTR with age was statistically significant (P for interaction <0.05). The hazards of greater TTR on primary end point were prominent in the young than in the old. In sensitivity analyses, greater TTR was associated with a decreased risk of primary end point with restriction of the study cohort to participants enrolled in North or South America (HR, 0.89 [0.80–0.99]; P=0.026) (Table S2), to participants with hypertension (HR, 0.82 [0.74 to 0.91]; P<0.001) (Table S3) and when using blood pressure data from week 4 to month 4 (HR, 0.82 [0.74–0.90]; P<0.001; Table S4). Besides, time in target range over a longer time span (months, 0 to 12) also significantly associated with a reduced risk of primary end point (HR, 0.81 [0.71–0.92]; P=0.001) (Table S5).
Figure 3. Pre‐specified subgroups analyses of the association between time in systolic blood pressure target range and primary composite end point.
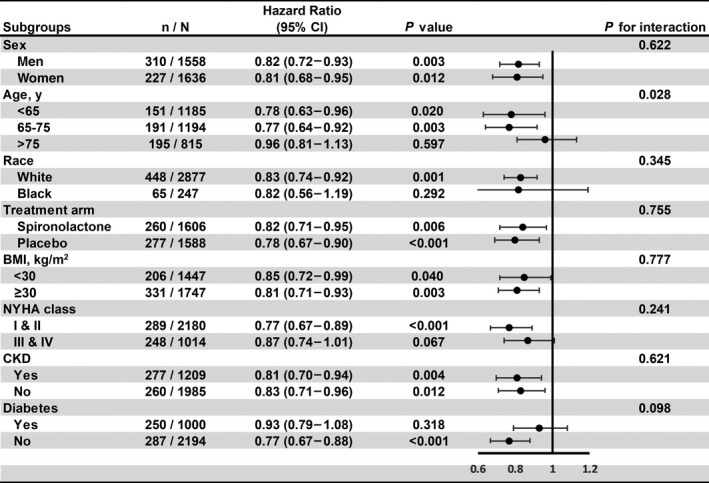
In this forest plot, circles represent the value of hazard ratio, and bars represent the 95% CI. BMI indicates body mass index; CKD, chronic kidney disease; NYHA, New York Heart Association; and SBP, systolic blood pressure.
Discussion
In this analysis of patients with HFpEF from the TOPCAT trial, greater time in SBP target range was associated with a decreased risk of primary composite end point, mortality events, and hospitalization for HF. Additionally, subgroup analyses showed that there was a significant interaction between age and TTR, indicating that the associations were more significant in young people than in the old (P interaction=0.028). To the best of our knowledge, it is the first large cohort study to assess the relationship of time in SBP target range with risk of adverse clinical outcomes among patients with HFpEF.
Several prior studies have examined the association between blood pressure level and clinical outcomes in patients with HFpEF. A secondary analysis of TOPCAT didn’t observe a significant relationship between baseline SBP quartiles and adverse outcomes, but a J‐shaped relationship was observed when SBP was analyzed continuously. 6 The OPTIMIZE‐HF (Medicare‐linked Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) registry found that a discharge SBP <120 mm Hg predicted a higher risk of mortality events. 4 The PARAGON‐HF (Prospective Comparison of ARNI With ARB Global Outcomes in HF With Preserved Ejection Fraction) trial demonstrated that the relationship between baseline SBP and cardiovascular outcomes was noted to be J‐shaped, with baseline SBP of 120–129 mm Hg identifying the lowest risk of cardiovascular outcome. 7 Besides, a J‐shaped relationship was also observed between long‐term SBP and clinical outcomes with nadir risk occurring at 120 to 130 mm Hg. 5 , 7 , 15 The J‐curve relationship between SBP and adverse clinical outcomes in HFpEF indicated that the risk of adverse events may increase at both too high or too low levels of blood pressure. The potential mechanism of J‐curve relationship between SBP and adverse outcome has not been totally understood, but high SBP could lead to left ventricular hypertrophy, diastolic dysfunction, and vascular stiffening, while low SBP reflects low stroke volume and poor tissue perfusion. 16
Time in target range was estimated using linear interpolation to account for time with an SBP within target range using blood pressure values during a specific exposure period. Time in target range was found to account for the extent of blood pressure control and predicted major adverse cardiovascular outcomes beyond mean blood pressure in previous analysis of Veterans Affairs study and the SPRINT (Systolic Blood Pressure Intervention Trial). 8 , 9 However, either the Veterans Affairs study or the SPRINT included little patients with HF. The prognostic significance of TTR in patients with HFpEF is still unclear. In this study, we used TTR to analyze the risk of cardiovascular outcomes and mortality events.
After adjusting for multiple potential confounders, 1‐SD (38.3%) increase of TTR was associated with 20%, 21%, 23%, and 17% lower risk of primary end point, all‐cause mortality, cardiovascular death, and hospitalization for HF, respectively. When participants were categorized into 4 groups by TTR value, the lowest risk of adverse clinical outcomes was observed in the highest TTR group (>75%–100%), while no significant difference was found in the middle groups (>25%–50% and >50%–75%) compared with the lowest TTR group (0%–25%). This may be because of the relatively short follow‐up time (mean [SD]=3.0 [1.7]) and the trend of TTR associated with clinical outcomes was observed among these 4 groups. The interaction between age and TTR was significantly different (P for interaction=0.028), indicating that the associations between TTR and adverse clinical outcomes were more pronounced in younger patients, and might not apply to patients of advanced age (>75 years). This result is in line with the guidelines, which aims for a conservative blood pressure target and avoids treated SBP <130 mm Hg in elderly patients aged >75 years. 17 Previous secondary analysis of TOPCAT has observed that spironolactone reduced SBP by 4.4±0.6 mm Hg compared with placebo. 6 We analyzed the association between TTR with clinical outcomes in spironolactone and placebo arm, respectively, and the association remained consistent across these 2 treatment arms.
The findings of our study are of major clinical importance for blood pressure control of patients with HFpEF. First, patients with greater TTR are associated with a decreased risk of cardiovascular events and mortality outcomes, which indicates that TTR may provide effective risk stratification across patients with HFpEF. Identifying patients at higher risk through TTR, reinforcing clinical management and providing sufficient therapeutic intervention may make it possible to alter mortality outcome in HFpEF. Second, TTR incorporates both the average blood pressure value over time and the degree of blood pressure variation. It provides a method to evaluate the consistency of blood pressure control as well as an estimate of future cardiovascular outcomes. Third, the monitoring of blood pressure status is considered a cornerstone of blood pressure management. It’s important to point out that most participants with HFpEF in this study have a poor control of blood pressure, with <40% of participants achieving a time in SBP target range of >75%. Previous studies have found that TTR may be a feasible surrogate outcome to estimate the effect of blood pressure control. 18 , 19 Time in target range may also be useful for clinicians to adjust anti‐hypertensive therapies since TTR can provide a long‐term view of individual condition for blood pressure management. Thus, time in target range may provide an assessment of the efficacy of anti‐hypertensive therapy and guide adjustments in medications and lifestyle to achieve better blood pressure control. Fourth, patients with HFpEF have a heavy medication burden that >50% of patients were prescribed with >10 medications. 20 Time in target range may be useful to evaluate the treatment compliance and assist to start with the most achievable goal for each patient. Besides, using an optimal blood pressure target range for blood pressure control may contribute to achieving maximal therapeutic benefit without overtreatment. Thus, it helps to lighten the medication burden, ease the pain, and improve therapeutic compliance of patients.
There exists some limitations in our study. First, the study population of the TOPCAT trial were required to control SBP well, which would be expected to bias the result toward null because well‐controlled SBP would limit changes in blood pressure and lead TTR to converge. However, in this study we still observed an association between greater TTR with decreased risk of cardiovascular outcome, highlighting the usefulness of TTR among HFpEF in the real world. Second, because of the observational nature of this study, specific inclusion and exclusion criteria for the trial made it possible for selection bias or unknown confounding and may not be generalizable to other diverse HFpEF cohorts. Third, about 91% of the participants in this study had a comorbidity of hypertension, over the prevalence of hypertension in other HFpEF clinical trials (42.9% to 86.6%). 3 Since a strict blood pressure control (120‒130 mm Hg) was recommended for patients with HF and hypertension, we performed a sensitivity analysis limited to participants with hypertension, and consistent results were found in this population. Last but not least, the purpose of the TOPCAT study was not to evaluate the effect of blood pressure in patients with HFpEF. Therefore, it’s warranted to examine if treating to a specified TTR would ensure maximal benefit while also reducing the risk of overtreatment with antihypertensive agents in clinical trials among patients with HFpEF.
In conclusion, time in target range of SBP is inversely associated with cardiovascular outcomes and mortality events among patients with HFpEF, especially in younger patients. Further randomized clinical trials are warranted to certain the best cut‐off of time in SBP target range to integrate the optimal therapeutic strategy and pharmacoeconomic for patients with HFpEF.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (81600206 to Z.X.D.; 81870195 to L.X.X.), and Natural Science Foundation of Guangdong Province (2016A030310140 to Z.X.D.; 2016A020220007 and 2019A1515011582 to L.X.X.). The supporting organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
None.
Supporting information
Tables S1–S5
Figures S1–S2
Acknowledgments
We thank the staff and participants of the TOPCAT study for their contributions.
R. Huang, Y. Lin, and M. Liu contributed equally.
Contributor Information
Xiaodong Zhuang, Email: zhuangxd3@mail.sysu.edu.cn.
Xinxue Liao, Email: liaoxinx@mail.sysu.edu.cn.
References
- 1. Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–573. doi: 10.1038/s41569-020-0363-2 [DOI] [PubMed] [Google Scholar]
- 2. Khan MS, Samman Tahhan A, Vaduganathan M, Greene SJ, Alrohaibani A, Anker SD, Vardeny O, Fonarow GC, Butler J. Trends in prevalence of comorbidities in heart failure clinical trials. Eur J Heart Fail. 2020;22:1032–1042. doi: 10.1002/ejhf.1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 4. Tsimploulis A, Lam PH, Arundel C, Singh SN, Morgan CJ, Faselis C, Deedwania P, Butler J, Aronow WS, Yancy CW, et al. Systolic blood pressure and outcomes in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2018;3:288–297. doi: 10.1001/jamacardio.2017.5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee SE, Lee H‐Y, Cho H‐J, Choe W‐S, Kim H, Choi J‐O, Jeon E‐S, Kim M‐S, Hwang K‐K, Chae SC, et al. Reverse J‐curve relationship between on‐treatment blood pressure and mortality in patients with heart failure. JACC Heart Fail. 2017;5:810–819. doi: 10.1016/j.jchf.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 6. Selvaraj S, Claggett B, Shah SJ, Anand I, Rouleau JL, Desai AS, Lewis EF, Pitt B, Sweitzer NK, Pfeffer MA, et al. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: an analysis of the TOPCAT trial. Eur J Heart Fail. 2018;20:483–490. doi: 10.1002/ejhf.1060 [DOI] [PubMed] [Google Scholar]
- 7. Selvaraj S, Claggett BL, Böhm M, Anker SD, Vaduganathan M, Zannad F, Pieske B, Lam CSP, Anand IS, Shi VC, et al. Systolic blood pressure in heart failure with preserved ejection fraction treated with sacubitril/valsartan. J Am Coll Cardiol. 2020;75:1644–1656. doi: 10.1016/j.jacc.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fatani N, Dixon DL, Van Tassell BW, Fanikos J, Buckley LF. Systolic blood pressure time in target range and cardiovascular outcomes in patients with hypertension. J Am Coll Cardiol. 2021;77:1290–1299. doi: 10.1016/j.jacc.2021.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doumas M, Tsioufis C, Fletcher R, Amdur R, Faselis C, Papademetriou V. Time in therapeutic range, as a determinant of all‐cause mortality in patients with hypertension. J Am Heart Assoc. 2017;6. doi: 10.1161/JAHA.117.007131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972.e10. doi: 10.1016/j.ahj.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 11. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 12. Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: comparative evaluation of measures of time‐in‐therapeutic range. J Thromb Thrombolysis. 2003;15:213–216. doi: 10.1023/B:THRO.0000011377.78585.63 [DOI] [PubMed] [Google Scholar]
- 13. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255 [DOI] [PubMed] [Google Scholar]
- 14. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 15. Huang P, Yu Y, Wei F, Zhu W, Xue R, Dong Y, Liu C. Association of long‐term SBP with clinical outcomes and quality of life in heart failure with preserved ejection fraction: an analysis of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. J Hypertens. 2021;39:1378–1385. doi: 10.1097/HJH.0000000000002807 [DOI] [PubMed] [Google Scholar]
- 16. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83 [DOI] [PubMed] [Google Scholar]
- 17. Kjeldsen SE, Stenehjem A, Os I, Van de Borne P, Burnier M, Narkiewicz K, Redon J, Agabiti Rosei E, Mancia G. Treatment of high blood pressure in elderly and octogenarians: European Society of Hypertension statement on blood pressure targets. Blood Press. 2016;25:333–336. doi: 10.1080/08037051.2016.1236329 [DOI] [PubMed] [Google Scholar]
- 18. Dixon DL, Parod ED, Sisson EM, Van Tassell BW, Nadpara PA, Dow A. Impact of a pharmacist‐physician collaborative care model on time‐in‐therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404–409. doi: 10.1002/jac5.1115 [DOI] [Google Scholar]
- 19. Dixon DL, Sisson EM, Parod ED, Van Tassell BW, Nadpara PA, Carl D, W. Dow A. Pharmacist‐physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88–95. doi: 10.1111/jch.13150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y, Zhu W, He X, Xue R, Liang W, Wei F, Wu Z, Zhou Y, Wu D, He J, et al. Influence of polypharmacy on patients with heart failure with preserved ejection fraction: a retrospective analysis on adverse outcomes in the TOPCAT trial. Br J Gen Pract. 2021;71:e62–e70. doi: 10.3399/bjgp21X714245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S2


