Abstract
Background
Amid stagnating declines in national cardiovascular disease (CVD) mortality, documenting trends in county‐level hypertension‐related CVD death rates can help activate local efforts prioritizing hypertension prevention, detection, and control.
Methods and Results
Using death certificate data from the National Vital Statistics System, Bayesian spatiotemporal models were used to estimate county‐level hypertension‐related CVD death rates and corresponding trends during 2000 to 2010 and 2010 to 2019 for adults aged ≥35 years overall and by age group, race or ethnicity, and sex. Among adults aged 35 to 64 years, county‐level hypertension‐related CVD death rates increased from a median of 23.2 per 100 000 in 2000 to 43.4 per 100 000 in 2019. Among adults aged ≥65 years, county‐level hypertension‐related CVD death rates increased from a median of 362.1 per 100 000 in 2000 to 430.1 per 100 000 in 2019. Increases were larger and more prevalent among adults aged 35 to 64 years than those aged ≥65 years. More than 75% of counties experienced increasing hypertension‐related CVD death rates among patients aged 35 to 64 years during 2000 to 2010 and 2010 to 2019 (76.2% [95% credible interval, 74.7–78.4] and 86.2% [95% credible interval, 84.6–87.6], respectively), compared with 48.2% (95% credible interval, 47.0–49.7) during 2000 to 2010 and 66.1% (95% credible interval, 64.9–67.1) for patients aged ≥65 years. The highest rates for both age groups were among men and Black populations. All racial and ethnic categories in both age groups experienced widespread county‐level increases.
Conclusions
Large, widespread county‐level increases in hypertension‐related CVD mortality sound an alarm for intensified clinical and public health actions to improve hypertension prevention, detection, and control and prevent subsequent CVD deaths in counties across the nation.
Keywords: cardiovascular disease, epidemiology, hypertension, mortality
Subject Categories: Cardiovascular Disease, Epidemiology, High Blood Pressure, Hypertension, Race and Ethnicity
Nonstandard Abbreviations and Acronyms
- AI/AN
American Indian/Alaska Native
- CrI
credible interval
Clinical Perspective
What Is New?
Against a background of stagnating national declines in cardiovascular disease mortality, hypertension‐related deaths from cardiovascular disease increased in counties across the nation since 2000.
During 2010 to 2019, 86.2% and 66.1% of counties in the United States had rising hypertension‐related cardiovascular disease death rates among adults aged 35 to 64 and ≥65 years, respectively.
All racial or ethnic and sex categories experienced widespread county‐level increases; the highest death rates for both age groups were among men and Black populations.
What Are the Clinical Implications?
Widespread county‐level increases in hypertension‐related cardiovascular disease mortality sound an alarm and call for intensified national and local efforts to improve hypertension prevention, detection, and control, especially among working‐aged adults and in locations where the burden is high and growing.
In the United States, nearly half of adults (47.3% or 116.0 million) live with hypertension, 1 with the prevalence increasing since 2013. 2 This burden costs ≈$48.6 billion annually, including spending on health care services and antihypertensive medications, and on productivity loss from premature death. 3
Hypertension is the leading risk factor for cardiovascular disease (CVD) and premature death. 4 Hypertension‐related deaths have increased since 2000, representing >500 000 deaths in 2019. 5 From 2000 to 2013, hypertension‐related mortality attributable to all causes increased 23.1% among adults. 6 Likewise, hypertension‐related mortality caused by CVD increased during the first 2 decades of this century among race and ethnicity, age, and sex. 7 , 8 After decades of declines, stagnating or increasing national death rates have also been observed in conditions where uncontrolled hypertension heightens the risk, including stroke, coronary heart disease, and heart failure. 9 , 10 , 11 , 12 , 13
Nevertheless, national and state‐level data mask geographic disparities in CVD mortality. 14 , 15 , 16 , 17 , 18 By relying on data that lack geographic granularity, efforts to address the burden of hypertension may not reach appropriate populations in specific locations. Additionally, national surveillance systems do not capture county‐level hypertension prevalence and incidence data, leaving county‐level hypertension‐related death data as a critical data source to address the nation’s hypertension burden and the concerning recent trends in CVD mortality. 9 , 10 , 11 , 12 , 13 , 19 Local hypertension‐related CVD death rates and trends, and corresponding differences by demographic group, can provide key guiding evidence for clinicians, public health professionals, and local decision‐makers in locating and tailoring hypertension prevention and control initiatives to the needs of specific communities.
Examining these trends assumes increasing urgency amid the COVID‐19 pandemic. Hypertension and conditions resulting from uncontrolled hypertension have been associated with increased risk of SARS‐CoV‐2 infection and subsequent severe COVID‐19 illness and death. 20 , 21 Consequently, understanding prepandemic trends in hypertension‐related mortality and differences by demographic groups are pivotal to help equitably respond to, alleviate, and build resilience to COVID‐19 and future health threats.
Therefore, we analyzed county‐level hypertension‐related CVD death rates and trends among adults aged ≥35 years from 2000 to 2019.
Methods
Hypertension‐Related CVD Mortality Data
We obtained annual hypertension‐related CVD death counts by county of residence, age group, race or ethnicity, and sex during 2000 to 2019 from the National Vital Statistics System (NVSS) of the National Center for Health Statistics (NCHS). The study population included US residents aged ≥35 years. This age group represented 99.4% of all CVD deaths in 2019. 5 Data were stratified into 2 age groups (35–64 years and ≥65 years) and 5 racial and ethnic categories: non‐Hispanic American Indian/Alaska Native (AI/AN), non‐Hispanic Asian/Pacific Islander, non‐Hispanic Black, Hispanic, and non‐Hispanic White. Hypertension‐related CVD deaths were defined as deaths with any mention of hypertension or hypertension‐related disease (including essential hypertension, hypertension‐related heart and/or renal disease, and secondary hypertension) (International Statistical Classification of Diseases, Tenth Revision [ICD‐10]: I10–I15), where the underlying cause of death was any disease of the circulatory system (ICD‐10: I00–I99). We used NCHS bridged‐race estimates for annual county‐level populations. 22
Although NVSS mortality data included 3142 counties and county equivalents, given changes in county definitions during the study period, deaths and populations for some counties were merged to create a final set of 3136 counties.
Estimating County‐Level Hypertension‐Related CVD Death Rates
We estimated county‐level hypertension‐related CVD death rates by age groups, racial or ethnic categories, and sex using a statistical model that produces precise, reliable rates, even in the presence of small case counts and populations. 23 , 24 Specifically, we used a Bayesian multivariate space‐time conditional autoregressive model to estimate county‐level hypertension‐related CVD death rates and 95% credible intervals (CrIs). This model’s statistical details have been previously published and used extensively for heart disease and stroke mortality. 14 , 15 , 23 , 25 , 26 , 27 , 28 Briefly, this model is based on the Besag‐York‐Mollié conditional autoregressive model for spatially referenced count data. 29 These models iterative estimate parameters within a Markov chain Monte Carlo (MCMC) algorithm and incorporate correlation across space, time, and demographic groups. Hypertension‐related CVD death rates were estimated as the medians of the posterior distributions defined by the MCMC iterations. Death rates were age‐standardized to the 2010 US population using 10‐year age groups.
Estimating Trends in Death Rates
To account for potential nonlinearity in trends, we calculated total percent change across 2 intervals (2000–2010 and 2010–2019). In addition to 2010 being the midpoint of this time period, national death rates for many CVD subtypes have stagnated or increased since ≈2010. 9 , 13 , 30 , 31 For each demographic group within each county and among each interval, we used separate log‐linear regression models to estimate total percent change (ie, the trend) in hypertension‐related CVD death rates. Models were run within each MCMC iteration and included rates for all years within each interval. The resulting posterior distributions of coefficients were then transformed to produce posterior distributions of total percent change over each internal. We then estimated the county’s total percent change as the median of each posterior distribution.
To summarize the distributions of estimated death rates and percent change among counties, we calculated the medians and the 25th and 75th percentiles. We also used the posterior distributions of total percent change to calculate the percentage of counties and 95% CrI with increasing hypertension‐related CVD death rates for each interval and demographic group. By using the posterior distributions of hypertension‐related CVD death rates and percent change (rather than the point estimates), all measures account for uncertainty in the underlying estimates.
Inclusion Criteria
Inclusion criteria ensured that we only reported reliable rates in sufficiently large populations, and each demographic group used a common set of counties for the entire study period. For a given demographic group within a given county to be included in this analysis, we required that the estimated rates were reliable (ie, the 95% CrI width was less than the point estimate) and that the group‐specific population was ≥500 for all years in the study period. 23 This requirement is effectively a Bayesian analogue of the suppression criteria used in reporting US cancer statistics. 32
Given these inclusion criteria, counties included in our analysis represented almost all of the population and deaths for each demographic group (Table 1). The one exception was AI/AN populations, for whom our analysis included 295 counties representing 65.9% of the population and 76.3% of deaths for those aged 35 to 64 years, and 22 counties representing 33.2% of the population and 41.4% of deaths for those aged ≥65 years.
Table 1.
Distributions of County‐Level Age‐Standardized Hypertension‐Related CVD Death Rates and Total Percent Change in Hypertension‐Related CVD Death Rates by Year, Age Group, Racial or Ethnic Group, and Sex—United States, 2000–2019
| Counties, n* | Population included, % | Deaths included, % | Median county‐level rates, per 100 000 (IQR) | Median county‐level total percent change, % (IQR) † | Counties with increasing death rates, % (95% CrI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2010 | 2019 | 2000–2010 | 2010–2019 | 2000–2010 | 2010–2019 | |||||
| Age 35 to 64 y | |||||||||||
| Overall | 2896 | 99.3 | 99.5 | 23.2 (17.6–32.5) | 30.7 (21.9–43.3) | 43.4 (31.5–61.5) | 28.1 (4.8–57.4) | 43.1 (17.6–74.5) | 76.2 (74.7–78.4) | 86.2 (84.6–87.6) | |
| Women | 2547 | 97.5 | 98.2 | 15.7 (11.4–23.0) | 18.9 (13.0–28.2) | 25.9 (18.1–38.6) | 16.9 (−5.2 to 45.1) | 36.3 (11.2–68.5) | 66.5 (64–68.5) | 81.2 (79.2–83.1) | |
| Men | 2737 | 98.8 | 99.4 | 31.6 (24.1–44.0) | 43.6 (31.0–60.9) | 61.3 (45.3–87.0) | 32.8 (8.5–64.1) | 44.4 (18.4–76.1) | 78.5 (77.2–80.5) | 86.7 (84.6–88.1) | |
| AI/AN, non‐Hispanic | 295 | 65.9 | 76.3 | 23.8 (20.5–27.3) | 38.4 (33.4–47.6) | 58.3 (47.5–75.3) | 66.0 (40.1–93.6) | 50.7 (28.6–77.4) | 95.9 (92.9–99) | 92.2 (85.8–95.6) | |
| Asian/Pacific Islander, non‐Hispanic | 421 | 94.2 | 95.7 | 17.4 (15.4–20.4) | 16.3 (13.8–19.5) | 18.0 (14.8–22.1) | −13.6 (−22.7 to−1.3) | 8.2 (−4.4 to 24.6) | 28.7 (20.2–37.8) | 61.8 (56.8–68.2) | |
| Black, non‐Hispanic | 1212 | 97.7 | 98.5 | 88.6 (69.8–107.9) | 81 (63.4–102.7) | 92.2 (69.4–119.6) | −7.0 (−22.8 to 9.9) | 13.6 (−1.3 to 35.5) | 39.4 (36.9–41.8) | 68.9 (64.6–72.1) | |
| Hispanic | 832 | 96.0 | 97.5 | 21.6 (18.3–25.0) | 22.6 (18.4–28.3) | 27.2 (21.7–35.3) | 5.2 (−8.8 to 19.8) | 23.0 (6.5–43.6) | 57.7 (51.1–62.6) | 78 (73.1–82.3) | |
| White, non‐Hispanic | 2686 | 98.1 | 98.4 | 20.1 (16.1–26.4) | 28.1 (20.5–38.2) | 40.5 (29.4–57.9) | 36.1 (9.5–72.2) | 47.1 (19.1–79.8) | 79.6 (78–81.8) | 86.2 (84.4–87.3) | |
| Age ≥65 y | |||||||||||
| Overall | 2952 | 99.8 | 99.8 | 362.1 (281.2–459.9) | 371 (292.4–470.6) | 430.1 (339.1–548.7) | −1.2 (−17.2 to 19.4) | 16.2 (−6.8 to 43.3) | 48.2 (47.0–49.7) | 66.1 (64.9–67.1) | |
| Women | 2741 | 99.4 | 99.1 | 362.3 (283.0–459.1) | 357.6 (279.2–451.6) | 389.6 (305.7–493.6) | −5.7 (−21.1 to 15.5) | 7.7 (−13.6 to 34.1) | 43.1 (41.8–44.8) | 58 (56.2–59.3) | |
| Men | 2615 | 98.9 | 99.0 | 356.1 (277.0–452.6) | 384.5 (300–485.3) | 485.4 (377.3–608.1) | 3.3 (−13.1 to 26.2) | 25.0 (1.9–54.2) | 54.5 (53–56.2) | 75.0 (73.7–76.2) | |
| AI/AN, non‐Hispanic | 44 | 33.2 | 41.4 | 311.5 (254.6–358.9) | 430.5 (343.4–538.5) | 460.1 (366–645.5) | 29.2 (16.6–72.1) | 18.2 (−7.7 to 36.7) | 84.1 (72.7–90.9) | 68.2 (59.1–79.5) | |
| Asian/Pacific Islander, non‐Hispanic | 154 | 87.5 | 92.3 | 314.1 (261.7–411.6) | 288.4 (240.3–384.9) | 272.0 (213.1–357.5) | −10.7 (−22.0 to 0.6) | −4.4 (−14.9 to 8.0) | 27.9 (21.4–39.6) | 42.2 (36.4–48.7) | |
| Black, non‐Hispanic | 719 | 93.3 | 94.3 | 694.9 (567.2–871.9) | 601.9 (474.6–752.9) | 568.9 (452.0–739.6) | −18.1 (−29.1 to −2.3) | −4.4 (−18.3 to 15.4) | 25.2 (22.8–27.5) | 44.4 (41.6–47.3) | |
| Hispanic | 287 | 89.9 | 93.9 | 291.6 (246.1–375.6) | 318.1 (247.3–414.3) | 338.0 (249.5–423.8) | −5.2 (−15.6 to 8.0) | 3.6 (−9.2 to 16.0) | 41.1 (36.2–45.3) | 55.1 (50.2–59.9) | |
| White, non‐Hispanic | 2888 | 99.7 | 99.7 | 345.8 (268.5–432.6) | 359.8 (280.9–452.4) | 429.4 (331.8–543.0) | 0.8 (−16.4 to 21.9) | 18.2 (−5.4 to 47.1) | 50.6 (49.3–52.3) | 68.0 (66.9–69.0) | |
AI/AN indicates American Indian/Alaska Native; CrI, credible interval; CVD, cardiovascular disease; and IQR, interquartile range.
Numbers of counties are based on inclusion criteria, which result in a common set of counties for each demographic group across all years. See Methods for details.
Percent change was calculated using log‐linear regression on all years of data within the given interval.
Analyses were conducted using R version 4.0.3 (The R Foundation). Models were run using user‐developed code, which is available on request. The first author had full access to all of the data in the study and takes responsibility for its integrity and the data analysis. The Centers for Disease Control and Prevention (CDC) reviewed this study for human subjects protection and determined it to be nonresearch. Institutional Review Board approval and participant consent are not required.
Results
National Hypertension‐Related CVD Death Rates and Trends
A total of 4 270 415 hypertension‐related CVD deaths were reported during 2000 to 2019 among US adults aged ≥35 years, representing 8.8% of all deaths and 25.4% of CVD deaths during this period. The national hypertension‐related CVD death rate in 2019 among adults aged 35 to 64 years was 44.6 per 100 000 (95% CI, 44.3–45.0), with increases during both 2000 to 2010 and 2010 to 2019 (Table 2, Figure 1). In 2019, among adults aged 35 to 64 years, men had higher death rates than women (63.8 per 100 000 [95% CI, 63.2–64.4] and 26.4 per 100 000 [95% CI, 26–26.8], respectively). Both men and women experienced increasing hypertension‐related CVD death rates during 2000 to 2010 and 2010 to 2019, with men having stronger increases. By race or ethnicity, Black populations aged 35 to 64 years had the highest rates (96.3 per 100 000 [95% CI, 94.8–97.8] in 2019). The largest magnitude of increases among both intervals occurred in AI/AN and White populations.
Table 2.
National Age‐Standardized Hypertension‐Related CVD Death Rates and Total Percent Change in Hypertension‐Related CVD Death Rates by Year, Age Group, Racial or Ethnic Group, and Sex—United States, 2000 to 2019
| National rates, per 100 000 (95% CI) | National total percent change, % (95% CI) | ||||
|---|---|---|---|---|---|
| 2000 | 2010 | 2019 | 2000–2010 | 2010–2019 | |
| Age 35 to 64 y | |||||
| Overall | 32.3 (31.9–32.6) | 36.5 (36.1–36.8) | 44.6 (44.3–45.0) | 15.0 (11.3–18.9) | 23.2 (21.8–24.6) |
| Women | 21.9 (21.4–22.3) | 22.4 (22.0–22.7) | 26.4 (26.0–26.8) | 3.3 (0.1–6.6) | 19.8 (17.0–22.6) |
| Men | 43.3 (42.6–43.9) | 51.3 (50.7–51.9) | 63.8 (63.2–64.4) | 21.1 (16.6–25.9) | 24.7 (23.0–26.5) |
| AI/AN, non‐Hispanic | 23.8 (19.9–27.6) | 40.2 (36.1–44.3) | 60.9 (56.0–65.7) | 66.7 (37.6–101.9) | 54 (38.7–71.0) |
| Asian/Pacific Islander, non‐Hispanic | 21.1 (19.5–22.7) | 18.7 (17.6–19.8) | 21.6 (20.6–22.6) | −14.7 (−21.8 to −6.8) | 14.3 (7.2–22.0) |
| Black, non‐Hispanic | 102.1 (100.0–104.2) | 91.3 (89.7–92.9) | 96.3 (94.8–97.8) | −10.4 (−14 to −6.6) | 7.5 (4.6–10.5) |
| Hispanic | 27.9 (26.6–29.2) | 27.8 (26.9–28.7) | 31.0 (30.2–31.9) | −0.5 (−6.1 to 5.4) | 15.2 (10.0–20.6) |
| White, non‐Hispanic | 23.7 (23.3–24.1) | 30 (29.7–30.4) | 39.6 (39.2–40.0) | 30.5 (26–35.3) | 31.6 (29.3–33.8) |
| Age ≥65 y | |||||
| Overall | 413.6 (411.4–415.7) | 396.5 (394.5–398.4) | 439.6 (437.8–441.4) | −6.3 (−12.1 to −0.1) | 11.1 (5.3–17.2) |
| Women | 407.1 (404.4–409.8) | 375.4 (373–377.9) | 394.0 (391.7–396.2) | −10.0 (−16.1 to −3.5) | 5.0 (−0.8 to 11.2) |
| Men | 408.0 (404.4–411.6) | 416.7 (413.5–420) | 494.1 (491–497.1) | 0.3 (−5.3 to 6.1) | 19 (13.3–24.9) |
| AI/AN, non‐Hispanic | 292.6 (261.6–323.7) | 359.7 (329.6–389.8) | 380.0 (356.9–403.2) | 17.3 (−4.2 to 43.5) | 9.2 (3.5–15.2) |
| Asian/Pacific Islander, non‐Hispanic | 406.9 (391.3–422.4) | 359.5 (348.8–370.1) | 323.1 (315.8–330.3) | −13.5 (−17.9 to −8.9) | −5.4 (−14.9 to 5.2) |
| Black, non‐Hispanic | 773.4 (762.8–784) | 648 (639.1–656.9) | 619.6 (612.2–626.9) | −17.6 (−23.2 to −11.7) | −3.9 (−9.2 to 1.8) |
| Hispanic | 377.4 (367.2–387.6) | 382.2 (374.3–390.1) | 386.1 (380.1–392.2) | −5.6 (−13.2 to 2.6) | 3.5 (−2.8 to 10.2) |
| White, non‐Hispanic | 380.8 (378.6–383) | 372.3 (370.3–374.4) | 429.5 (427.4–431.5) | −4.1 (−10.1 to 2.2) | 15.0 (8.8–21.5) |
AI/AN indicates American Indian/Alaska Native; and CVD, cardiovascular disease.
Figure 1. National hypertension‐related cardiovascular disease (CVD) death rates by age group, race or ethnicity, and sex—United States, 2000 to 2019.
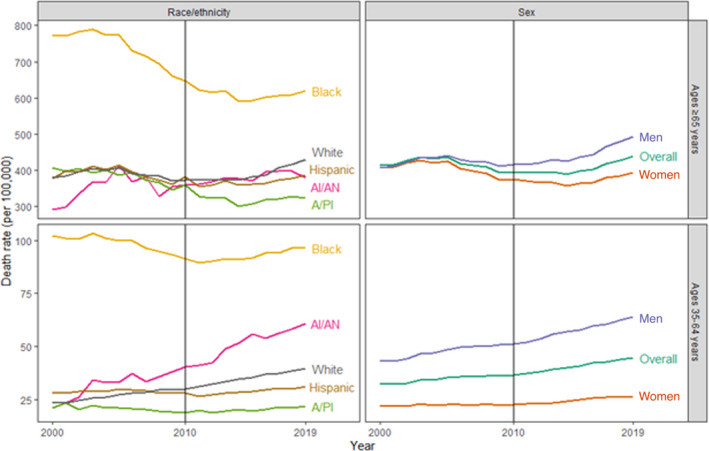
Note that the scales of the y‐axes differ to ease visualization. A/PI indicates Asian/Pacific Islander; and AI/AN, American Indian/Alaska Native
Among adults aged ≥65 years, the national hypertension‐related CVD death rate in 2019 was 439.6 per 100 000 (95% CI, 437.8–441.4), with decreases during 2000 to 2010 and increases during 2010 to 2019 (Table 2, Figure 1). As with adults aged 35 to 64 years, men had higher rates than women, and Black populations had the highest rates by race or ethnicity in 2019. During 2010 to 2019, AI/AN and White populations experienced increases, whereas death rates plateaued among the other racial and ethnic categories.
County‐Level Hypertension‐Related CVD Death Rates Among Patients Aged 35 to 64 Years
Among adults aged 35 to 64 years, the highest hypertension‐related CVD death rates were in the southern United States, especially in the Mississippi Delta region (Figure 2). County‐level hypertension‐related CVD death rates increased from a median county‐level death rate of 23.2 per 100 000 in 2000 to 43.4 per 100 000 in 2019 (Table 1). Over three quarters of counties experienced increases during 2000 to 2010 and 2010 to 2019 (76.2% [95% CrI, 74.7–78.4] and 86.2% [95% CrI, 84.6–87.6], respectively) (Table 1, Figure 2).
Figure 2. Hypertension‐related cardiovascular disease (CVD) death rates, 2019, and trends, 2010 to 2019, overall and by race or ethnicity, in patients aged 34 to 64 years—United States.
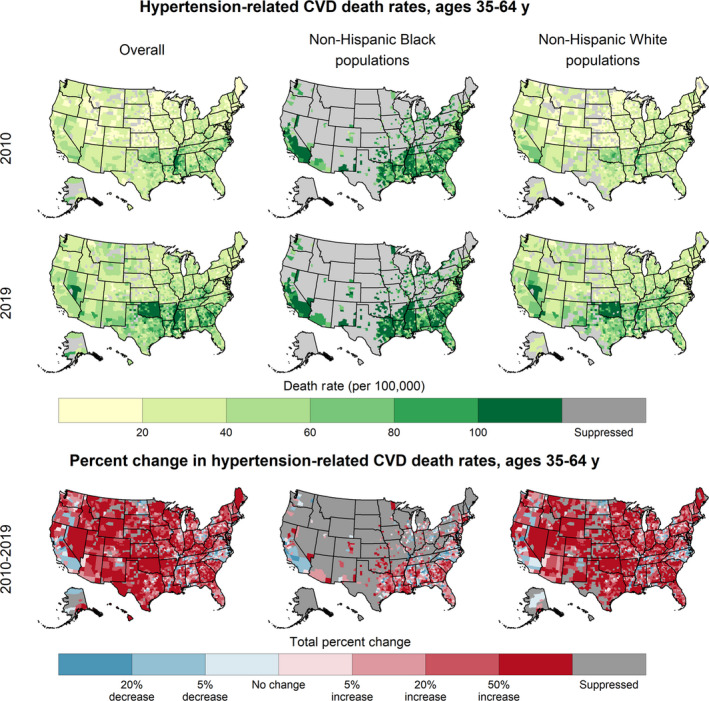
By sex, the distributions of county‐level hypertension‐related CVD death rates among men and women were almost completely distinct, with the 75th percentile of death rates among women in 2019 (38.6 per 100 000) being comparable to the 25th percentile of death rates among men (45.3 per 100 000). Both sexes experienced widespread increases during both intervals (Table 1, Figure S1).
By race or ethnicity, the highest death rates for all years were among Black populations. In 2019, the median county‐level death rate among Black adults aged 35 to 64 years was more than double that of their White counterparts (92.2 per 100 000 and 40.5 per 100 000, respectively) (Table 1, Figure 2). The highest death rates for both Black and White populations were concentrated in the southern United States (Figure 2). Although all racial and ethnic categories experienced widespread county‐level increases, especially during 2010 to 2019 (Figure 2, Figure S2), the magnitudes of the increases varied. White and AI/AN populations aged 35 to 64 years experienced median increases of ≈50% during 2010 to 2019 (47.1% [interquartile range, 19.1%–79.8%] and 50.7% [interquartile range, 28.6%–77.4%], respectively). Among Black populations, on average, death rates decreased during 2000 to 2010 (median percent change, −7.0%) and increased during 2010 to 2019 (median county‐level percent change, 13.6%).
County‐Level Hypertension‐Related CVD Death Rates and Trends Among Adults Aged ≥65 Years
Among adults aged ≥65 years, the highest hypertension‐related CVD death rates were concentrated in the southern United States (Figure 3). County‐level hypertension‐related CVD death rates increased from a median of 371.0 per 100 000 in 2010 to 430.1 per 100 000 in 2019 (Table 1). Approximately half of the counties experienced increasing death rates during 2000 to 2010 (48.2%; 95% CI, 47.0%–49.7%) and two‐thirds during 2010 to 2019 (66.1%; 95% CI, 64.9%–67.1%).
Figure 3. Hypertension‐related cardiovascular disease (CVD) death rates, 2019, and trends, 2010 to 2019, overall and by race or ethnicity, in patients aged ≥65 years—United States.
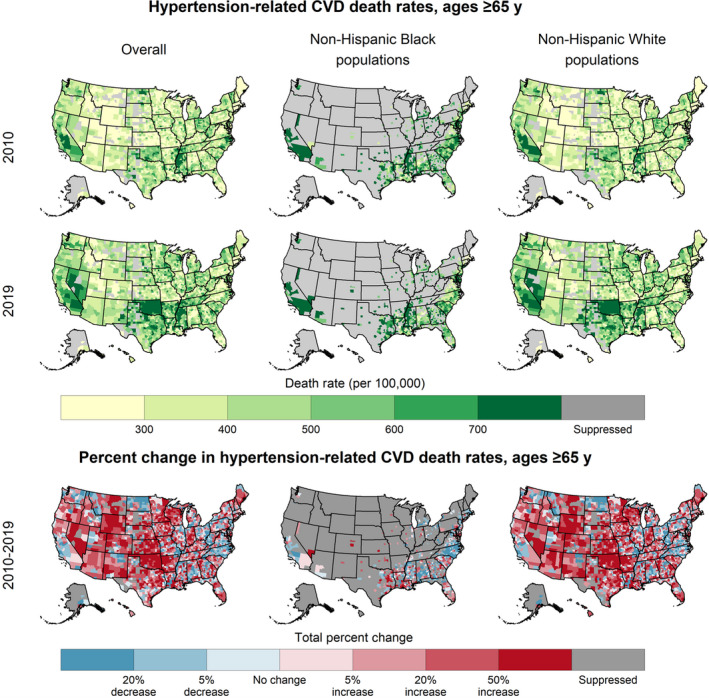
By sex, the distributions of county‐level hypertension‐related CVD death rates among men and women were similar in 2000 but diverged over time; by 2019 the median death rate among women was 389.6 per 100 000 and among men was 485.4 per 100 000. Both sexes experienced widespread increases during both intervals (Table 1, Figure S3). During 2010 to 2019, relative increases in death rates were larger among men than among women (median county‐level percent change: 25.0% and 7.7%, respectively).
By race and ethnicity, the highest death rates for all years were among Black populations. The median county‐level death rate among Black adults aged ≥65 years in 2019 was ≈30% higher than that of their White counterparts (568.9 per 100 000 and 429.4 per 100 000, respectively) (Table 1, Figure 3). Although all racial and ethnic categories experienced widespread increases, especially during 2010 to 2019, the magnitudes of the increases varied markedly among counties (Figure 3, Figure S4). White and AI/AN populations aged ≥65 years experienced median increases of 18.2% during 2010 to 2019. Among Black populations, death rates in many counties decreased during both 2000 to 2010 and 2010 to 2019 (median percent change: −18.1% and −4.4%, respectively).
All county‐level hypertension‐related CVD death rates and trends generated by this study are available at https://chronicdata.cdc.gov/Heart‐Disease‐Stroke‐Prevention/Rates‐and‐Trends‐in‐Hypertension‐related‐Cardiovas/uc9k‐vc2j.
Discussion
Uncontrolled hypertension increases the risk of stroke, coronary heart disease, atherosclerotic CVD, and heart failure, and of subsequent all‐cause and CVD mortality. 33 , 34 Using national vital statistics data, our study documented alarming trends in county‐level hypertension‐related CVD mortality in counties across the United States among all age, race and ethnicity, and sex categories. In the past decade, hypertension‐related CVD death rates among adults aged 35 to 64 years increased in >85% of counties; rates among adults aged ≥65 years increased in approximately two thirds of counties. From 2010 to 2019, the median county‐level percent increase in hypertension‐related CVD death rates among adults aged 35 to 64 years was 43.1%, almost 3 times that of adults aged ≥65 years. These findings accentuate the need for clinicians, public health professionals, and policy makers in counties across the country to prioritize the prevention, detection, and control of hypertension with multisector, coordinated efforts at national, state, and local levels, and with an emphasis on working‐aged adults.
The observed local changes in hypertension‐related CVD death rates occur within the context of concurrent national trends in hypertension prevalence, treatment, and control. Hypertension prevalence among US adults remained relatively constant during 1999 to 2018. 35 Likewise, by 2018, hypertension awareness, treatment, and control had improved compared with their 2019 levels among age, race and ethnicity, and sex, although those gains have recently stalled. 35 , 36 , 37 Despite these improvements, hypertension prevalence and subsequent lack of control remain high. Approximately half (47.3%) of US adults were living with hypertension in 2015 to 2018, and 79.4% of those had uncontrolled hypertension. 1 Of those with uncontrolled hypertension, 70.6% are aged <65 years. 1
These national improvements in hypertension measures are consistent with our observed national declines in hypertension‐related CVD mortality among adults aged ≥65 years during 2000 to 2010, but appear to oppose the observed national trends for adults aged 35 to 64 years and widespread county‐level increases among age, race and ethnicity, and sex. First, this apparent inconsistency may stem from the latent effect of uncontrolled hypertension of mortality. Although uncontrolled hypertension is a key risk factor for CVD death, survival is still measured in decades. 33 Second, this apparent inconsistency between improved national hypertension measures and worsening national and county‐level hypertension‐related CVD mortality may stem from comparing national hypertension measures with county‐level death rates. Although county‐level estimates of trends in hypertension prevalence, treatment, and control are not available, national data mask variation at the county level. 14 , 16 , 26 , 27 , 28 Therefore, the high national prevalence of hypertension and lack of hypertension control combined with the recent stalling of improvements in these measures suggest that there may be many counties with even higher prevalence measures and potential increases in these measures. Our results may therefore serve as indicators of worsening hypertension prevalence, treatment, and control among counties, age groups, race and ethnicity, and sex, especially among younger adults. 1 Supplemental analyses of national CVD mortality lend further support to this statement. Total CVD mortality among all US adults declined during 2000 to 2010. 9 , 35 This decline was driven by decreasing CVD death rates that did not list hypertension on the death certificate and overwhelmed increases in the hypertension‐related CVD death rate (Figure S5). Since 2010, CVD mortality has plateaued, while CVD death rates without hypertension listed on the death certificate continued to decline. Additionally, supplemental analyses also found that during the study period the proportion of CVD deaths with hypertension listed on the death certificate almost doubled from 21.4% to 37.8% for patients aged 35 to 64 years and from 17.7% to 31.3% for patients aged ≥65 years (Figure S6). Within this national context of hypertension and in the absence of national surveillance systems to directly measure county‐level trends in hypertension prevalence, our findings reveal vital insights into the local epidemiology of hypertension and illuminate populations that need attention for hypertension prevention and control. Mimicking the geographic patterns of other CVD death rates, 14 , 16 , 17 , 38 hypertension‐related CVD death rates were highest in the southern United States and among men and Black adults. Increases in hypertension‐related CVD mortality were not confined to counties with the highest rates. Among sex and racial and ethnic categories, the largest and most pervasive increases were among adults aged 35 to 64 years, especially among AI/AN and White populations.
The large increases in hypertension‐related cardiovascular disease mortality among working‐aged adults in the majority of counties across the country is particularly troubling given that these deaths are largely preventable. 11 Younger adults are less likely than older adults to be aware of their hypertension status and to subsequently receive treatment for hypertension, partially as a result of not being engaged with the health care system as adults. 1 , 36 , 37 , 39 However, clinical engagement during the pediatric and adolescent years is high. 40 Supporting the transition from the pediatric to the adult medical home could sustain preventive messages, identify hypertension earlier, and mitigate long‐term consequences. Within the context of limited clinical engagement of working‐aged adults, extending multisector hypertension‐related prevention and treatment opportunities to community and workplace settings may provide opportunities for reaching this age group. Proven clinical interventions that have successfully improved hypertension management have been demonstrated among diverse communities and settings. 41 , 42 These interventions should be adopted and adapted by those counties and among those populations with increasing hypertension‐related CVD death rates. For example, the well‐recognized Barbershop Initiative demonstrated effective blood pressure (BP) reductions among Black men and could serve as a model for reaching populations outside of clinical settings. 42 , 43 , 44 , 45
Improving hypertension control is a key clinical component to addressing these troubling county‐level trends, particularly among working‐aged adults. Given the widespread nature of the county‐level increases in hypertension‐related CVD mortality, national calls to improve hypertension control may inform actions in communities with the highest burden of hypertension in southern US states and those with increasing burden of hypertension‐related CVD mortality in counties across the country. To galvanize national action, the Surgeon General’s Call to Action to Control Hypertension (Call to Action) identified evidence‐based interventions that can be implemented, adapted, and expanded in diverse settings across the United States. 19 Building on previous clinical practice guidelines, 46 the Call to Action seeks to improve hypertension control by making it a national priority, promoting environments that support hypertension prevention, detection, and control, and optimizing patient care. Likewise, Healthy People 2030, the national objectives aiming to improve health over the next decade, included improved hypertension control in its prioritized list of leading health indicators. 47 Additionally, Million Hearts, a national initiative co‐led by the Centers for Disease Control and Prevention and the Centers for Medicare & Medicaid Services, has drawn on best practices from the Million Hearts Hypertension Control Champions and other high‐performing health systems to develop numerous resources to guide clinical systems seeking to improve hypertension control, with an emphasis on Black populations and working‐aged adults. 48 , 49 , 50 , 51 Other national organizations, including the American Heart Association and the American College of Cardiology, have developed recognition programs and resources that can enhance hypertension control in counties across the United States. 52 , 53
Optimizing clinical care is key to reducing hypertension and increasing hypertension control among communities and thereby reducing subsequent hypertension‐related CVD mortality. Three promising directions for optimizing clinical care to local populations with increasing hypertension‐related death rates are fixed‐dose combination medications, improved medication adherence, and improved telehealth opportunities. Fixed‐dose combinations are evidence‐based but underutilized, comprising only 12% of all national BP medication fills in 2017, with considerably lower values in southern states where hypertension‐related CVD death rates are high. 54 Increasing adherence to these medication regimens is critical. Despite the 706.5 million BP medication prescriptions filled in the United States in 2017, only half of treated patients followed physicians’ instructions, with lower adherence among Black adults and in the South. 54 , 55 , 56 Finally, improved telehealth capabilities could optimize clinical care, especially in counties with limited access to care. Telehealth visits have increased during the COVID‐19 pandemic. 57 Making these telehealth policy changes permanent might continue to support increased access after the pandemic. Within telehealth visits, self‐measured BP monitoring, an evidence‐based approach, represents a key opportunity for clinicians to help their patients achieve and maintain optimal BP levels. 58 , 59 zCritically, given the stark differences in hypertension‐related CVD mortality by race and ethnicity and place, actions to prevent hypertension and improve subsequent clinical outcomes must be equitably implemented to avoid exacerbating existing disparities and to ultimately eliminate all disparities. Disparities in hypertension prevention, detection, and control are aided by unequal access to and engagement with healthy environments and the medical system, which, in turn, are rooted in more upstream social determinants of health, including poverty, stress, and racial and ethnic discrimination. 60 , 61 , 62 , 63 , 64 Attention to place‐specific social determinants of health when addressing the troubling trends in hypertension‐related CVD mortality will enable all communities to experience equitable improvement in cardiovascular health.
This analysis is subject to several limitations. First, the use of “any disease of the circulatory system” as the underlying cause of death is likely an overestimate of hypertension’s influence on CVD, as hypertension is not a risk factor for all of these ICD‐10 codes. However, this definition provided a temporally stable baseline for comparison and acknowledges the widespread influence of the condition on the most common CVD causes of death. Second, death certificates may be subject to misreporting and ascertainment bias. The accuracy of hypertension coding on death certificates and potential temporal changes in misclassification have not been investigated. However, the increasing prevalence of hypertension and decreasing prevalence of hypertension control, especially in younger adults, 36 , 37 and the sustained increases over time and place together suggest that misreporting alone is unlikely to explain the observed increases. Additionally, this limitation is minimized through our use of hypertension as a multiple cause of death, rather than an underlying cause of death. 65 Third, we suppressed counties because of limited precision or small populations, which might differentially impact counties with low rates. This limitation was pronounced for AI/AN populations; their data are reported for completeness. For other demographic groups, our analysis included most of the population and most reported deaths (Table 1). The potential suppression of low rates is balanced by our model’s ability to generate estimates that are more precise than other methods, allowing the inclusion of more counties than other small‐area estimation methods. 23 , 24 Additionally, sensitivity analysis performed using unsuppressed data yielded results that were not meaningfully different than the results using the suppressed data. Finally, categorization of race and Hispanic origin is a known concern with death certificate data. Misclassification of AI/AN deaths is common, resulting in lower rates but similar trends compared with data corrected for AI/AN race. 66 , 67 Likewise, the government‐defined non‐Hispanic Asian/Pacific Islander and Hispanic categories are heterogeneous in terms of origin, culture, and CVD risk. Therefore, our results might mask differences within racial or ethnic categories. 68 , 69 , 70
Hypertension‐related deaths from all causes and from CVD increased during the past 2 decades. Our documentation of large, widespread county‐level increases in hypertension‐related CVD mortality, especially among working‐aged adults, sounds an alarm calling for intensified, coordinated multisector efforts at national, state, and local levels for improving hypertension prevention, detection, and control, and preventing subsequent CVD deaths in communities across the country. These efforts will require specific and sustained action by public health and health care professionals, along with the public and private sectors, especially in locations where the burden is high and growing.
Sources of Funding
None.
Disclosures
None.
Supporting information
Figures S1–S6
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024785
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Centers for Disease Control and Prevention . Hypertension cascade: hypertension prevalence, treatment and control estimates among us adults aged 18 years and older applying the criteria from the American college of cardiology and American Heart Association’s 2017 hypertension guideline—NHANES 2015–2018. 2019. Available at: https://millionhearts.hhs.gov/data‐reports/hypertension‐prevalence.html. Accessed May 18, 2021.
- 2. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United states, 2017–2018. NCHS Data Brief. 2020;364:1–7. [PubMed] [Google Scholar]
- 3. Kirkland EB, Heincelman M, Bishu KG, Schumann SO, Schreiner A, Axon RN, Mauldin PD, Moran WP. Trends in healthcare expenditures among us adults with hypertension: National estimates, 2003–2014. J Am Heart Assoc. 2018;7:e008731. doi: 10.1161/JAHA.118.008731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, Abbafati C, Abbasi N, Abbastabar H, Abd‐Allah F, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1923–1994. doi: 10.1016/s0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Center for Health Statistics . Underlying cause of death 1999–2019 on CDC wonder online database website. Available at: http://wonder.cdc.gov/ucd‐icd10.html. Accessed August 13, 2021.
- 6. Kung HC, Xu J. Hypertension‐related mortality in the United States, 2000–2013. NCHS Data Brief. 2015:193:1–8. [PubMed] [Google Scholar]
- 7. Nambiar L, Lewinter MM, Vanburen PC, Dauerman HL. Decade‐long temporal trends in U.S. hypertension‐related cardiovascular mortality. J Am Coll Cardiol. 2020;75:2644–2646. doi: 10.1016/j.jacc.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 8. Forrester SJ, Dolmatova EV, Griendling KK. An acceleration in hypertension‐related mortality for middle‐aged and older Americans, 1999–2016: an observational study. PLoS One. 2020;15:e0225207. doi: 10.1371/journal.pone.0225207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sidney S, Quesenberry CP Jr, Jaffe MG, Sorel M, Nguyen‐Huynh MN, Kushi LH, Go AS, Rana JS. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1:594–599. doi: 10.1001/jamacardio.2016.1326 [DOI] [PubMed] [Google Scholar]
- 10. Yang Q, Tong X, Schieb L, Vaughan A, Gillespie C, Wiltz JL, King SC, Odom E, Merritt R, Hong Y, et al. Vital signs: recent trends in stroke death rates—United States, 2000–2015. MMWR. 2017;66:933–939. doi: 10.15585/mmwr.mm6635e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ritchey MD, Wall HK, George MG, Wright JS. Us trends in premature heart disease mortality over the past 50 years: where do we go from here? Trends Cardiovasc Med. 2020;30:364–374. doi: 10.1016/j.tcm.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ritchey MD, Loustalot F, Bowman BA, Hong Y. Trends in mortality rates by subtypes of heart disease in the United States, 2000–2010. JAMA. 2014;312:2037–2039. doi: 10.1001/jama.2014.11344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sidney S, Quesenberry CP, Jaffe MG, Sorel M, Go AS, Rana JS. Heterogeneity in national U.S. mortality trends within heart disease subgroups, 2000–2015. BMC Cardiovasc Disord. 2017;17. doi: 10.1186/s12872-017-0630-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall EW, Vaughan AS, Ritchey MD, Schieb L, Casper M. Stagnating national declines in stroke mortality mask widespread county‐level increases, 2010–2016. Stroke. 2019;50:3355–3359. doi: 10.1161/STROKEAHA.119.026695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vaughan AS, Ritchey MD, Hannan J, Kramer MR, Casper M. Widespread recent increases in county‐level heart disease mortality across age groups. Ann Epidemiol. 2017;27:796–800. doi: 10.1016/j.annepidem.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaughan AS, Schieb L, Casper M. Historic and recent trends in county‐level coronary heart disease death rates by race, gender, and age group, United States, 1979‐2017. PLoS One. 2020;15:1979–2017. doi: 10.1371/journal.pone.0235839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vaughan AS, Woodruff RC, Shay CM, Loustalot F, Casper M. Progress toward achieving national targets for reducing coronary heart disease and stroke mortality: a county‐level perspective. J Am Heart Assoc. 2021;10:e019562. doi: 10.1161/JAHA.120.019562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woodruff RC, Casper M, Loustalot F, Vaughan AS. Unequal local progress towards healthy people 2020 objectives for stroke and coronary heart disease mortality. Stroke. 2021;52:e229–e232. doi: 10.1161/STROKEAHA.121.034100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. U.S. Department of Health and Human Services . The surgeon general’s call to action to control hypertension. 2020. Available at: https://www.hhs.gov/surgeongeneral/reports‐and‐publications/index.html. Accessed July 25, 2021.
- 20. Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID‐19. Am J Hypertens. 2020;33:373–374. doi: 10.1093/ajh/hpaa057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bae S, Kim SR, Kim MN, Shim WJ, Park SM. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID‐19 according to age: a systematic review and meta‐analysis. Heart. 2021;107:373–380. doi: 10.1136/heartjnl-2020-317901 [DOI] [PubMed] [Google Scholar]
- 22. National Center for Health Statistics US census populations with bridged race categories. 2017. Available at: https://www.cdc.gov/nchs/nvss/bridged_race.htm. Accessed August 13, 2021.
- 23. Quick H, Casper M, Waller LA. A multivariate space‐time model for analysing county level heart disease death rates by race and sex. Journal of the Royal Statistical Society Applied Statistics Series C. 2017;67:291–304. doi: 10.1111/rssc.12215 [DOI] [Google Scholar]
- 24. Vaughan AS, Kramer MR, Waller LA, Schieb LJ, Greer S, Casper M. Comparing methods of measuring geographic patterns in temporal trends: an application to county‐level heart disease mortality in the United States, 1973 to 2010. Ann Epidemiol. 2015;25:329–335. doi: 10.1016/j.annepidem.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casper M, Kramer MR, Quick H, Schieb LJ, Vaughan AS, Greer S. Changes in the geographic patterns of heart disease mortality in the United States: 1973 to 2010. Circulation. 2016;133:1171–1180. doi: 10.1161/CIRCULATIONAHA.115.018663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaughan AS, Quick H, Pathak EB, Kramer MR, Casper M. Disparities in temporal and geographic patterns of declining heart disease mortality by race and sex in the United States, 1973‐2010. J Am Heart Assoc. 2015;4:e002567. doi: 10.1161/JAHA.115.002567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vaughan AS, Quick H, Schieb L, Kramer MR, Taylor HA, Casper M. Changing rate orders of race‐gender heart disease death rates: an exploration of county‐level race‐gender disparities. SSM Popul Health. 2019;7:100334. doi: 10.1016/j.ssmph.2018.100334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaughan AS, Schieb L, Quick H, Kramer MR, Casper M. Before the here and now: what we can learn from variation in spatiotemporal patterns of changing heart disease mortality by age group, time period, and birth cohort. Soc Sci Med. 2018;217:97–105. doi: 10.1016/j.socscimed.2018.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Besag J, York J, Mollié A. Bayesian image restoration, with two applications in spatial statistics. Ann Inst Stat Math. 1991;43:1–20. doi: 10.1007/BF00116466 [DOI] [Google Scholar]
- 30. Shah NS, Molsberry R, Rana JS, Sidney S, Capewell S, O'Flaherty M, Carnethon M, Lloyd‐Jones DM, Khan SS. Heterogeneous trends in burden of heart disease mortality by subtypes in the United States, 1999–2018: observational analysis of vital statistics. BMJ. 2020;370:m2688. doi: 10.1136/bmj.m2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma J, Ward EM, Siegel RL, Jemal A. Temporal trends in mortality in the United States. JAMA. 2015;314:1969–2013. doi: 10.1001/jama.2015.12319 [DOI] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention . United States cancer statistics (USCS): suppression of rates and counts. Available at: https://www.cdc.gov/cancer/uscs/technical_notes/stat_methods/suppression.htm. Accessed June 17, 2021.
- 33. Zhou D, Xi B, Zhao M, Wang L, Veeranki SP. Uncontrolled hypertension increases risk of all‐cause and cardiovascular disease mortality in US adults: the NHANES III linked mortality study. Sci Rep. 2018;8. doi: 10.1038/s41598-018-27377-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rana JS, Liu JY, Moffet HH, Karter AJ, Nasir K, Solomon MD, Jaffe MG, Ambrosy AP, Go AS, Sidney S. Risk of atherosclerotic cardiovascular disease by cardiovascular health metric categories in approximately 1 million patients. Eur J Prev Cardiol. 2021;28:e29–e32. doi: 10.1177/2047487320905025 [DOI] [PubMed] [Google Scholar]
- 35. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Moran AE. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension. 2017;70:736–742. doi: 10.1161/HYPERTENSIONAHA.117.09801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among us adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vaughan AS, George MG, Jackson SL, Schieb L, Casper M. Changing spatiotemporal trends in county‐level heart failure death rates in the United States, 1999 to 2018. J Am Heart Assoc. 2021;10:e018125. doi: 10.1161/JAHA.120.018125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson HM, Thorpe CT, Bartels CM, Schumacher JR, Palta M, Pandhi N, Sheehy AM, Smith MA. Undiagnosed hypertension among young adults with regular primary care use. J Hypertens. 2014;32:65–74. doi: 10.1097/HJH.0000000000000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White PH, Cooley WC, Boudreau ADA, Cyr M, Davis BE, Dreyfus DE, Forlenza E, Friedland A, Greenlee C, Mann M, et al. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2018;142. doi: 10.1542/peds.2018-2587 [DOI] [PubMed] [Google Scholar]
- 41. Fontil V, Gupta R, Moise N, Chen E, Guzman D, McCulloch CE, Bibbins‐Domingo K. Adapting and evaluating a health system intervention from kaiser permanente to improve hypertension management and control in a large network of safety‐net clinics. Circ Cardiovasc Qual Outcomes. 2018;11. doi: 10.1161/circoutcomes.117.004386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Egan BM, Sutherland SE, Rakotz M, Yang J, Hanlin RB, Davis RA, Wozniak G. Improving hypertension control in primary care with the measure accurately, act rapidly, and partner with patients protocol. Hypertension. 2018;72:1320–1327. doi: 10.1161/HYPERTENSIONAHA.118.11558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Victor RG, Blyler CA, Li N, Lynch K, Moy NB, Rashid M, Chang LC, Handler J, Brettler J, Rader F, et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10–19. doi: 10.1161/CIRCULATIONAHA.118.038165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx‐Drew D, et al. A cluster‐randomized trial of blood‐pressure reduction in black barbershops. N Engl J Med. 2018;378:1291–1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sulaica EM, Wollen JT, Kotter J, Macaulay TE. A review of hypertension management in black male patients. Mayo Clin Proc. 2020;95:1955–1963. doi: 10.1016/j.mayocp.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 46. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, Depalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical pr. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 47. US Department of Health and Human Services . Healthy people 2030: leading health indicators. 2020.
- 48. Centers for Disease Control and Prevention . Hypertension management program (HMP) toolkit. 2021.
- 49. Ritchey MD, Hannan J, Wall HK, George MG, Sperling LS. Notes from the field: characteristics of million hearts hypertension control champions, 2012–2019. MMWR Morb Mortal Wkly Rep. 2020;69:196–197. doi: 10.15585/mmwr.mm6907a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Centers for Disease Control and Prevention . Available at: https://millionhearts.hhs.gov/tools‐protocols/action‐guides/htn‐change‐package/index.html. Accessed September 1, 2021.
- 51. Wright JS, Wall HK, Ritchey MD. Million Hearts 2022: small steps are needed for cardiovascular disease prevention. JAMA. 2018;320:1857–1858. doi: 10.1001/jama.2018.13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Go AS, Bauman MA, Coleman King SM, Fonarow GC, Lawrence W, Williams KA, Sanchez E. An effective approach to high blood pressure control. Hypertension. 2014;63:878–885. doi: 10.1161/HYP.0000000000000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. American Heart Association & American Medical Association . 2020. Target:BP. Available at: https://targetbp.org/. Accessed August 1, 2021.
- 54. Yang PK, Ritchey MD, Tsipas S, Loustalot F, Wozniak GD. State and regional variation in prescription‐ and payment‐related promoters of adherence to blood pressure medication. Prev Chronic Dis. 2020;17:E112. doi: 10.5888/pcd17.190440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986 [DOI] [PubMed] [Google Scholar]
- 56. Ritchey M, Chang A, Powers C, Loustalot F, Schieb L, Ketcham M, Durthaler J, Hong Y. Vital signs: disparities in antihypertensive medication nonadherence among Medicare part D beneficiaries—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:967–976. doi: 10.15585/mmwr.mm6536e1 [DOI] [PubMed] [Google Scholar]
- 57. Demeke HB, Merali S, Marks S, Pao LZ, Romero L, Sandhu P, Clark H, Clara A, McDow KB, Tindall E, et al. Trends in use of telehealth among health centers during the COVID‐19 pandemic—United States, June 26–November 6, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:240–244. doi: 10.15585/mmwr.mm7007a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Duan Y, Xie Z, Dong F, Wu Z, Lin Z, Sun N, Xu J. Effectiveness of home blood pressure telemonitoring: a systematic review and meta‐analysis of randomised controlled studies. J Hum Hypertens. 2017;31:427–437. doi: 10.1038/jhh.2016.99 [DOI] [PubMed] [Google Scholar]
- 59. Meador M, Hannan J, Roy D, Whelihan K, Sasu N, Hodge H, Lewis JH. Accelerating use of self‐measured blood pressure monitoring (SMBP) through clinical‐community care models. J Community Health. 2021;46:127–138. doi: 10.1007/s10900-020-00858-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu MY, Li N, Li WA, Khan H. Association between psychosocial stress and hypertension: a systematic review and meta‐analysis. Neurol Res. 2017;39:573–580. doi: 10.1080/01616412.2017.1317904 [DOI] [PubMed] [Google Scholar]
- 61. Cuevas AG, Williams DR, Albert MA. Psychosocial factors and hypertension: a review of the literature. Cardiol Clin. 2017;35:223–230. doi: 10.1016/j.ccl.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz‐Flores S, Davey‐Smith G, Dennison‐Himmelfarb CR, Lauer MS, Lockwood DW, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 63. Churchwell K, Elkind MS, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 64. Harper S, Lynch J, Smith GD. Social determinants and the decline of cardiovascular diseases: understanding the links. Annu Rev Public Health. 2011;32:39–69. doi: 10.1146/annurev-publhealth-031210-101234 [DOI] [PubMed] [Google Scholar]
- 65. Lu TH, Anderson RN, Kawachi I. Effect of coding rules on reporting of hypertension mortality. Epidemiology. 2013;24:168–169. doi: 10.1097/EDE.0b013e3182788a04 [DOI] [PubMed] [Google Scholar]
- 66. Arias E, Heron M, Hakes J. The validity of race and Hispanic‐origin reporting on death certificates in the United States: an update. Vital Health Stat. 2016;2:1–21. [PubMed] [Google Scholar]
- 67. Rhoades DA. Racial misclassification and disparities in cardiovascular disease among American Indians and Alaska natives. Circulation. 2005;111:1250–1256. doi: 10.1161/01.CIR.0000157735.25005.3F [DOI] [PubMed] [Google Scholar]
- 68. Narayan KM, Aviles‐Santa L, Oza‐Frank R, Pandey M, Curb JD, McNeely M, Araneta MR, Palaniappan L, Rajpathak S, Barrett‐Connor E, et al. Report of a national heart, lung, and blood institute workshop: heterogeneity in cardiometabolic risk in Asian Americans in the U.S. Opportunities for research. J Am Coll Cardiol. 2010;55:966–973. doi: 10.1016/j.jacc.2009.07.075 [DOI] [PubMed] [Google Scholar]
- 69. Shah NS, Kandula NR. Addressing Asian American misrepresentation and underrepresentation in research. Ethn Dis. 2020;30:513–516. doi: 10.18865/ed.30.3.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rodriguez F, Chung S, Blum MR, Coulet A, Basu S, Palaniappan LP. Atherosclerotic cardiovascular disease risk prediction in disaggregated Asian and Hispanic subgroups using electronic health records. J Am Heart Assoc. 2019;8. doi: 10.1161/jaha.118.011874 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S6


