Cardiogenic shock (CS) affects between 40 000 and 50 000 individuals in the United States per year and is the leading cause of in‐hospital mortality following acute myocardial infarction (AMI). 1 , 2 , 3 Even with advances in acute cardiovascular care, including primary percutaneous coronary intervention, 30‐day mortality for patients with AMI‐CS remains ≈40%. 1 Despite the hemodynamic advantage of mechanical circulatory support devices in AMI‐CS, there are limited randomized data supporting their use. 4 Over the past decade, there has been an increase in the use of veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) in the management of refractory cardiogenic shock, because it offers not only a high cardiac output with biventricular support but also respiratory support. 5 However, peripheral VA‐ECMO is limited by the significant increase in afterload because of retrograde aortic flow, which may be deleterious in CS, especially from AMI. 5 Multiple techniques have been proposed to offload the left ventricle (LV) in patients on VA‐ECMO, including a pigtail catheter in the LV or pulmonary artery, atrial septostomy, and concomitant mechanical circulatory support such as the intra‐aortic balloon pump (IABP) or a percutaneous left ventricular assist device. The IABP has remained the most commonly used modality of LV decompression studied in literature because of its ubiquitous availability, ease of insertion, relatively small arteriotomy, theoretical benefit of diastolic augmentation and therefore coronary perfusion, and lastly, the ease of maintenance in the cardiac intensive care unit. 6
In this issue of the Journal of the American Heart Association (JAHA), Nishi et al 7 use the nationwide Japanese Registry of All Cardiac and Vascular Disease and describe a large retrospective cohort of 3815 patients with AMI‐CS who underwent primary percutaneous coronary intervention and compared the outcomes of VA‐ECMO+IABP (n=2964) to VA‐ECMO alone (n=851). Though patients in the VA‐ECMO+IABP group were younger, they were more likely to have higher rates of comorbidities including hypertension, dyslipidemia, diabetes, and atrial fibrillation. IABP was more likely to be used in hospitals with teaching status, more hospital beds, and availability of cardiac surgery. The authors concluded that patients managed with VA‐ECMO+IABP demonstrated significantly lower in‐hospital (adjusted odds ratio [OR], 0.47 [95% CI, 0.38–0.59]), 7‐day (adjusted OR, 0.41 [95% CI, 0.33–0.51]), and 30‐day mortality (adjusted OR, 0.30 [95% CI, 0.25–0.37]) in comparison to those managed with VA‐ECMO alone. These findings align with the results from a meta‐analysis that noted the benefit of IABP combined with VA‐ECMO in patients with AMI‐CS. 6 We would like to commend the authors for their work on addressing an important question using the largest cohort to date. However, there are certain points that merit discussion (Figure).
Figure 1. Current state of the evidence for LV unloading in VA‐ECMO for AMI‐CS.
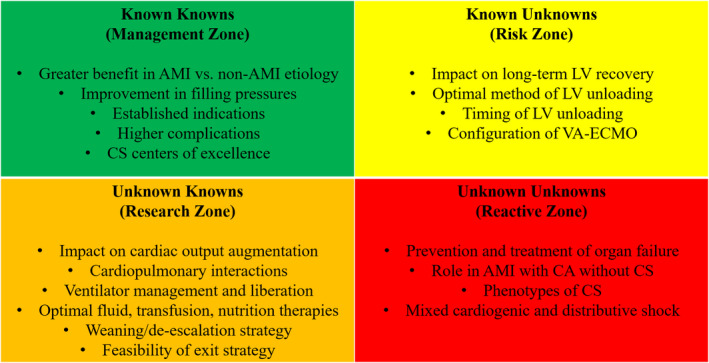
AMI indicates acute myocardial infarction; CA, cardiac arrest; CS, cardiogenic shock; LV, left ventricular; and VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation.
First, as the authors mention in the Limitations section, the information pertaining to the detailed timing of CS, IABP placement and ECMO placement were not available. Early use of VA‐ECMO in patients with AMI‐CS has been shown to be associated with a significant survival benefit. 8 Delaying the initiation of mechanical circulatory support may be deleterious, because prolonged microcirculatory dysfunction leads to irreversible end‐organ injury, rendering any subsequent hemodynamic intervention futile. 9 The results of the ongoing ECMO‐CS (Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock), 10 a multicenter trial of patients with AMI‐CS randomized to early ECMO versus standard of care, are eagerly awaited to understand the timing and role of VA‐ECMO in CS better. Furthermore, among the patients requiring VA‐ECMO, early unloading of the LV appears to be associated with increased success of weaning and reduced short‐term mortality. In a meta‐analysis by Al‐Fares et al, 8 early (<12 hours) VA‐ECMO+IABP (7 studies) was associated with a reduced short‐term mortality (risk ratio [RR], 0.80 [95% CI, 0.68–0.94]). For late (>12 hours and beyond) unloading with IABP, only 1 study met the inclusion criteria in this meta‐analysis. Therefore, further studies are needed to understand the timing of unloading the LV with IABP in patients on VA‐ECMO with AMI‐CS. Similarly, in a multicenter study by Schrage et al, 11 early left ventricular unloading with Impella was defined as <2 hours, which was associated with a decrease in mortality among patients with CS as compared with late left ventricular unloading. Left ventricular distension is an increasingly appreciated nuance of VA‐ECMO support. Therefore, it is of utmost importance to recognize the triggers of left ventricular unloading in patients on VA‐ECMO including (1) an elevated pulmonary capillary wedge pressure, (2) an aortic valve that remains closed throughout the cycle, (3) persistent pulmonary edema on chest x‐ray film, (4) a distended hypocontractile LV, and (5) refractory ventricular arrhythmias. 12 Nishi et al 7 illustrate that in‐hospital mortality was higher in patients who were started on IABP with subsequent VA‐ECMO than the others, including those who started on VA‐ECMO with subsequent IABP and those who had VA‐ECMO+IABP on the same day. Early IABP placement followed by escalation to VA‐ECMO may demonstrate a rapidly worsening phenotype portending worse outcomes, whereas a patient started on VA‐ECMO with subsequent IABP implantation for the purpose of left ventricular unloading may denote delayed myocardial recovery and is reported to have a better prognosis. 13
Second, this study by Nishi et al 7 was different from the previous study from the Japanese inpatient database by Aso et al, 14 in that they included patients with cardiac arrest (CA) at the time of admission, which were missing in the latter. Around 17% of the patients had cardiac arrest at the time of admission. There were no significant differences in the percentage of patients with CA between the VA‐ECMO+IABP versus VA‐ECMO alone groups. It is well established that the combination of CS and CA is associated with higher rates of in‐hospital mortality, and acute noncardiac organ failure in both ST‐segment–elevation 15 and non–ST‐segment–elevation 16 AMI admissions as compared with CS or CA alone. In patients with CA, VA‐ECMO–facilitated cardiopulmonary resuscitation can be used to restore circulation, prevent end‐organ damage, and facilitate percutaneous coronary intervention. 17 Unlike in CS, where the mechanical circulatory support devices serve to support the circulatory function, VA‐ECMO in CA is used to maintain cerebral and other vital organ perfusion despite the lack of return of spontaneous circulation. One of the limitations of the present study was that it could not be determined if the VA‐ECMO device was placed as a part of the facilitated resuscitation protocol or to support circulatory function. It is important to determine that, because the phenotype of combined AMI‐CS+CA is different from CS alone. Encouragingly, the authors demonstrated improved survival with combined VA‐ECMO+IABP in both patients with and without CA.
Lastly, it is important to mention about the unique end points in cardiac critical care especially in patients with CS. The authors mention that the risk of mechanical ventilation use was lower in patients in the VA‐ECMO+IABP group (RR, 0.49 [95% CI, 0.45–0.53]). This is a significant pertinent point. A previous study 18 has shown that use of IABP with VA‐ECMO was independently associated with a lower frequency of hydrostatic pulmonary edema and more days off the mechanical ventilation. An additional unique and important end point includes acute noncardiac organ failure. Acute organ failure in AMI‐CS is presumed to be secondary to systemic inflammation and microcirculatory abnormalities in addition to primary pump failure. 19 Presence of multiorgan failure in AMI‐CS is independently associated with higher in‐hospital mortality and greater resource use. 20 Given that this data set did not have information related to the noncardiac organ failure, the authors could not measure this important end point. Further studies in acute cardiovascular care, and specifically CS, should consider these unique end points pertinent to critical care cardiology, in addition to traditional metrics used in cardiovascular medicine.
Although limited by the caveats mentioned above, this study by Nishi et al supports the use of IABP for left ventricular unloading in AMI‐CS being treated with VA‐ECMO. However, these results cannot be extrapolated to other causes of CS. 6 The results from the observational studies point to a need for a randomized trial evaluating the outcomes of VA‐ECMO+IABP in patients with AMI‐CS to better define the management practice in this acutely ill patient population.
Disclosures
None.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 3.
See Article by Nishi et al.
REFERENCES
- 1. Samsky MD, Morrow DA, Proudfoot AG, Hochman JS, Thiele H, Rao SV. Cardiogenic shock after acute myocardial infarction: a review. JAMA. 2021;326:1840–1850. doi: 10.1001/jama.2021.18323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vallabhajosyula S, Bhopalwala HM, Sundaragiri PR, Dewaswala N, Cheungpasitporn W, Doshi R, Prasad A, Sandhu GS, Jaffe AS, Bell MR, et al. Cardiogenic shock complicating non‐ST‐segment elevation myocardial infarction: an 18‐year study. Am Heart J. 2022;244:54–65. doi: 10.1016/j.ahj.2021.11.002 [DOI] [PubMed] [Google Scholar]
- 3. Vallabhajosyula S, Dewaswala N, Sundaragiri PR, Bhopalwala HM, Cheungpasitporn W, Doshi R, Miller PE, Bell MR, Singh M. Cardiogenic shock complicating ST‐segment elevation myocardial infarction: an 18‐year analysis of temporal trends, epidemiology, management and outcomes. Shock. 2021. doi: 10.1097/SHK.0000000000001895 [DOI] [PubMed] [Google Scholar]
- 4. Thiele H, Zeymer U, Neumann F‐J, Ferenc M, Olbrich H‐G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al.; IABP‐SHOCK II Trial Investigators . Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 5. Meani P, Gelsomino S, Natour E, Johnson DM, Rocca H‐P, Pappalardo F, Bidar E, Makhoul M, Raffa G, Heuts S, et al. Modalities and effects of left ventricle unloading on extracorporeal life support: a review of the current literature. Eur J Heart Fail. 2017;19(suppl 2):84–91. doi: 10.1002/ejhf.850 [DOI] [PubMed] [Google Scholar]
- 6. Vallabhajosyula S, O’Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Eleid MF, Dunlay SM, Gersh BJ, Rihal CS, et al. Concomitant intra‐aortic balloon pump use in cardiogenic shock requiring veno‐arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv. 2018;11:e006930. doi: 10.1161/CIRCINTERVENTIONS.118.006930 [DOI] [PubMed] [Google Scholar]
- 7. Nishi T, Ishii M, Tsujita K, Okamoto H, Satoshi K, Nakai M, Yoko S, Iwanaga Y, Matoba S, Kobayashi Y, et al. Outcomes of VA‐ECMO plus IABP versus VA‐ECMO alone for treatment of acute myocardial infarction complicated by cardiogenic shock: insight from the JROAD‐DPC registry. J Am Heart Assoc. 2022;11:e023713. doi: 10.1161/JAHA.121.023713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al‐Fares AA, Randhawa VK, Englesakis M, McDonald MA, Nagpal AD, Estep JD, Soltesz EG, Fan E. Optimal strategy and timing of left ventricular venting during veno‐arterial extracorporeal life support for adults in cardiogenic shock: a systematic review and meta‐analysis. Circ Heart Fail. 2019;12:e006486. doi: 10.1161/CIRCHEARTFAILURE.119.006486 [DOI] [PubMed] [Google Scholar]
- 9. Wong ASK, Sin SWC. Short‐term mechanical circulatory support (intra‐aortic balloon pump, Impella, extracorporeal membrane oxygenation, TandemHeart): a review. Ann Transl Med. 2020;8:829. doi: 10.21037/atm-20-2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ostadal P, Rokyta R, Kruger A, Vondrakova D, Janotka M, Smíd O, Smalcova J, Hromadka M, Linhart A, Bělohlávek J. Extra corporeal membrane oxygenation in the therapy of cardiogenic shock (ECMO‐CS): rationale and design of the multicenter randomized trial. Eur J Heart Fail. 2017;19(suppl 2):124–127. doi: 10.1002/ejhf.857 [DOI] [PubMed] [Google Scholar]
- 11. Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, Colson P, Cudemus Deseda G, Dabboura S, Eckner D, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020;142:2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cevasco M, Takayama H, Ando M, Garan AR, Naka Y, Takeda K. Left ventricular distension and venting strategies for patients on venoarterial extracorporeal membrane oxygenation. J Thorac Dis. 2019;11:1676–1683. doi: 10.21037/jtd.2019.03.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vallabhajosyula S, Verghese D. Mechanical circulatory support in post‐cardiac arrest: one two many? Resuscitation. 2021;167:390–392. doi: 10.1016/j.resuscitation.2021.08.019 [DOI] [PubMed] [Google Scholar]
- 14. Aso S, Matsui H, Fushimi K, Yasunaga H. The effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: an analysis using a nationwide inpatient database. Crit Care Med. 2016;44:1974–1979. doi: 10.1097/CCM.0000000000001828 [DOI] [PubMed] [Google Scholar]
- 15. Vallabhajosyula S, Dunlay SM, Prasad A, Sangaralingham LR, Kashani K, Shah ND, Jentzer JC. Cardiogenic shock and cardiac arrest complicating ST‐segment elevation myocardial infarction in the United States, 2000–2017. Resuscitation. 2020;155:55–64. doi: 10.1016/j.resuscitation.2020.07.022 [DOI] [PubMed] [Google Scholar]
- 16. Vallabhajosyula S, Jentzer JC, Prasad A, Sangaralingham LR, Kashani K, Shah ND, Dunlay SM. Epidemiology of cardiogenic shock and cardiac arrest complicating non‐ST‐segment elevation myocardial infarction: 18‐year US study. ESC Heart Fail. 2021;8:2259–2269. doi: 10.1002/ehf2.13321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yannopoulos D, Bartos JA, Aufderheide TP, Callaway CW, Deo R, Garcia S, Halperin HR, Kern KB, Kudenchuk PJ, Neumar RW, et al.; American Heart Association Emergency Cardiovascular Care Committee . The evolving role of the cardiac catheterization laboratory in the management of patients with out‐of‐hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;139:e530–e552. doi: 10.1161/CIR.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 18. Bréchot N, Demondion P, Santi F, Lebreton G, Pham T, Dalakidis A, Gambotti L, Luyt C‐E, Schmidt M, Hekimian G, et al. Intra‐aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial‐extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care. 2018;7:62–69. doi: 10.1177/2048872617711169 [DOI] [PubMed] [Google Scholar]
- 19. den Uil CA, Lagrand WK, van der Ent M, Jewbali LS, Cheng JM, Spronk PE, Simoons ML. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2010;31:3032–3039. doi: 10.1093/eurheartj/ehq324 [DOI] [PubMed] [Google Scholar]
- 20. Vallabhajosyula S, Dunlay SM, Prasad A, Kashani K, Sakhuja A, Gersh BJ, Jaffe AS, Holmes DR Jr, Barsness GW. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2019;73:1781–1791. doi: 10.1016/j.jacc.2019.01.053 [DOI] [PubMed] [Google Scholar]


