Abstract
Background
Abdominal aortic aneurysm (AAA) screening programs have been active in the United States since 2005, but are not the only way AAAs are detected. AAA management and outcomes have not been investigated broadly in the context of “implicit AAA screening,” whereby radiologic examinations not intended for focused screening can identify AAAs.
Methods and Results
We examined the association between imaging‐based AAA screening, both explicit and implicit, and various outcomes for ≈1.6 million veterans in the Veterans Affairs health care system from 2005 to 2015. Screened‐positive, screened‐negative, and unscreened veterans were identified in the overall cohort and within a subgroup of veterans aged 65 years in 2005. The yearly composite screening rate increased over 10 years, from 11.7% to 18.3%, whereas the screened‐positive rate decreased from 7.3% to 4.9%. Only 12.9% of screening examinations were explicit AAA screening ultrasounds. The subgroup’s composite screening rate was 74% within its 10‐year eligibility window, with implicit screening accounting for 91.8% of examinations. In the 2005 subgroup, all‐cause mortality and Charlson comorbidity scores were higher for veterans who underwent screening compared with those unscreened (31.2% versus 23.1% and 0.47 versus 0.25, respectively; P<0.001). AAA rupture rates were similar between those unscreened and screened‐negative individuals.
Conclusions
Accounting for both explicit and implicit screening, AAA screening in the Veterans Affairs population has moderate reach. Efforts to expand explicit AAA screening are not likely to impact either all‐cause mortality or AAA rupture on the population scale as significantly as a careful accounting for and use of implicit screening data.
Keywords: abdominal aortic aneurysm, imaging, radiology, screening
Subject Categories: Imaging, Ultrasound, Magnetic Resonance Imaging (MRI), Computerized Tomography (CT), Aneurysm
Nonstandard Abbreviations and Acronyms
- USPSTF
US Preventative Services Task Force
- VA
Veterans Affairs
Clinical Perspective
What Is New?
This analysis of abdominal aortic aneurysm (AAA) screening in an integrated national health care system introduces the concept of “implicit screening,” whereby radiologic examinations not intended for focused screening can identify the target pathology and are used to satisfy screening recommendations.
AAA screening has a much larger reach than previously recognized when implicit screening practices are considered.
What Are the Clinical Implications?
Implicit screening is an often‐overlooked adjunct to formal screening programs, and in the case of AAA accounts for most screening activity.
Efforts to expand formal AAA screening programs are not likely to impact either all‐cause mortality or AAA rupture on the population scale as significantly as a careful accounting for and use of implicit screening data.
Abdominal aortic aneurysm (AAA) disease, typically considered an abdominal aortic diameter >3 cm, has a pooled global prevalence of 4.8% and is commonly seen in men aged ≥65 years. 1 Most small AAAs (<4 cm) are asymptomatic and pose little danger, whereas larger AAAs have a significant risk of fatal rupture. 2 At a diameter of 5.5 cm, asymptomatic aneurysms are considered for surgical or endovascular repair to prevent rupture. Given the significant risk associated with larger AAAs, screening programs have been implemented in the United States and other countries to detect aneurysms earlier and at smaller diameters, so that surveillance and intervention can reduce the incidence of rupture.
The US Preventative Services Task Force (USPSTF) recommended in 2005 that men aged 65 to 75 years with any history of smoking undergo a 1‐time AAA screening via ultrasonography. 3 This recommendation was based largely on the outcomes of 4 population‐based randomized controlled trials of sonographic AAA screening performed in the United Kingdom and Australia, 4 , 5 , 6 , 7 which at that time collectively demonstrated a reduction in AAA‐specific mortality, which favored screening (odds ratio [OR], 0.57; 95% CI, 0.45–0.74). An extrapolation of the trials’ findings to a hypothetical cohort of 100 000 US men aged 65 to 74 years showed that 89% of AAA‐attributable death could be prevented by screening only those with smoking history. 3
Few studies have assessed screening outside of a highly controlled trial‐type environment. Several studies have looked at institutional, local, and regional implementation of the USPSTF recommendation for AAA screening, 8 , 9 , 10 , 11 , 12 , 13 focusing on the appropriateness of screening referrals with regard to age, sex, and smoking history criteria, rather than on screening benefit. Other studies have investigated AAA screening on a broader geographic scale, but in a more narrow sense 14 , 15 (eg, considering only a single examination [G‐code G0389, AAA] for screening). More important, nearly all of these works considered only examinations explicitly intended to screen for AAA.
Little attention has been paid to the significance of “implicit AAA screening,” defined as screening via any imaging study that evaluates abdominal aortic caliber, including radiology examinations performed for a range of abdominal indications (eg, a computed tomography [CT] scan of the abdomen and pelvis performed to evaluate diffuse abdominal pain). Implicit AAA screening reflects the common post hoc screening practices of providers in a wide diversity of clinical settings, and is an often overlooked but key adjunct to explicit screening examinations targeting fulfillment of the USPSTF screening recommendation. Possibly related to the lack of detailed study of this de facto AAA screening implementation, there has been minimal modification of the USPSTF AAA screening recommendation since 2005, with no change affecting the most at‐risk population, men aged 65 to 75 years who have ever smoked. 16 In contrast, other imaging‐based screening programs have undergone more significant periodic and recent updating, such as with age in colorectal cancer screening, 17 , 18 screening mammography, 19 , 20 and lung cancer screening CT, 21 or pulmonary nodule size in lung cancer screening CT. 22
To better understand the impact of implicit AAA screening, we conducted a retrospective observational analysis of the association between AAA screening, via explicit and implicit mechanisms, and various outcomes, including mortality, in a large cohort of male veterans who received care in the US Veterans Affairs (VA) health care system over a period of 10 years.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
Veterans receiving care within the VA health care system between February 1, 2005, and December 31, 2015, were included in this retrospective analysis if they met the USPSTF criteria for AAA screening: men, aged 65 to 75 years, and positive history of smoking. From the veterans meeting these criteria over the 10‐year period, yearly cohorts were constructed after the exclusion of veterans with a preexisting or unexplained diagnosis of AAA without imaging, recent AAA screening (explicit or implicit screening examination within 3 years), or history of AAA repair. Veterans were also excluded if they were enrolled in Medicare Advantage (Medicare part C) for ≥1 month during the study period, as complete health care records of imaging and outcomes were not available. Exclusion criteria were not mutually exclusive. The final pool of included veterans was temporally stratified into a rolling yearly cohort, changing in size depending on the year. Although a veteran could be within multiple yearly cohorts if not screened during his first year of eligibility, each veteran was counted only once in longitudinal analysis. The San Francisco VA Medical Center Research and Development Committee approved the study and waived the requirement for informed consent because the study used deidentified data.
Implicit and Explicit Screening
Implicit and explicit AAA screening were assessed for all veterans on a yearly basis. Veterans were categorized as (1) having undergone AAA screening and “screened positive”; (2) having undergone AAA screening and “screened negative”; or (3) “not screened.” To determine the outcome of a screening examination, the veteran’s medical record was queried for the addition of International Classification of Diseases, Ninth Revision (ICD‐9)/International Classification of Diseases, Tenth Revision (ICD‐10), codes for AAA within the 12‐month period following the examination. Veterans not undergoing screening in a given year were carried into the cohort for the following year if they were still within the screening age range.
Medical Record Abstraction and Vital Status
Whether or not a veteran underwent screening was ascertained by searching the VA Corporate Data Warehouse and Centers for Medicare and Medicaid Services database (for those enrolled in Medicare parts A and B) for Common Procedural Terminology (CPT) codes for qualifying radiology examinations for the previous 3 years (for prior screening) and within the current year of screening eligibility. The first qualifying examination performed within the year of eligibility was counted, either explicit or implicit, and whether or not a follow‐up explicit screening examination was performed later that year was also recorded. The full list of CPT codes considered is provided in Data S1. More important, only 2 of the 29 CPT codes represent ultrasound examinations explicitly tailored to AAA screening. The remaining 27 CPT codes represent ultrasound, CT, and magnetic resonance imaging examinations performed for a variety of acute and routine indications, such as assessing for acute diverticulitis or restaging of malignancy. Although no information was available on the intended focus of any particular examination, detection of AAA is but one of hundreds of common indications for imaging, and thus most imaging examinations performed were expected to represent implicit AAA screenings.
The principal outcomes for analysis were AAA repair (open surgical or endovascular), AAA rupture, and all‐cause mortality during the study period. Dates of death were determined from the VA Master Veteran Index. Patient characteristics, including sex, age, race, ethnicity, and marital status, were obtained from VA patient care files. Comorbidities were determined by searching ICD‐9/ICD‐10 codes for inpatient hospitalizations or any outpatient encounters during the eligibility period and quantified using the Charlson comorbidity index. 23 , 24
Subgroup Analysis
Because follow‐up data are more limited for veterans entering the screening‐eligible cohort in the later years of data collection, a separate outcomes analysis was made for a subgroup of veterans entering the screening‐eligible cohort at age 65 years in 2005, thus allowing for 10‐years’ follow‐up that coincided with the veterans’ period of screening eligibility.
Statistical Analysis
Summary statistics were calculated, including means, medians, and SDs for continuous variables, and frequencies and percentages for categorical variables. We compared patient characteristics for subgroups, evaluating differences using χ2 test statistics. Statistical significance was set a priori with 2‐sided tests at P<0.05. All analyses were performed using SAS Enterprise Guide statistical software version 7.1.
Results
Overall Cohort
A total of 3 072 384 veterans met AAA screening eligibility criteria during the study period. Enrollment in Medicare part C resulted in exclusion of 1 075 658 veterans. Prior screening (either explicit or implicit) within the preceding 3 years resulted in the exclusion of 377 167 veterans, whereas prior diagnosis of AAA or prior AAA repair resulted in exclusion of 66 635 and 9675 veterans, respectively. As exclusion criteria were not mutually exclusive, the total number of excluded veterans numbered 1 492 727. The remaining 1 579 657 veterans were stratified into a rolling yearly cohort that ranged in size from 425 154 to 602 765, depending on the year. Figure 1 presents a flowchart of the cohort generation. The average age of screening‐eligible veterans over the period of data collection was 67.22±2.88 years. A total of 75.2% of eligible veterans were characterized as “White” race, 8.9% were characterized as “Black” race, 2.0% were characterized as “Asian, Pacific Islander, or American Indian” race, and 13.8% of eligible veterans either declined to identify their race or it was otherwise unknown. Additional demographic data for the full cohort over the entire study period are shown in Table 1.
Figure 1. Inclusion and exclusions resulting in yearly rolling cohorts.
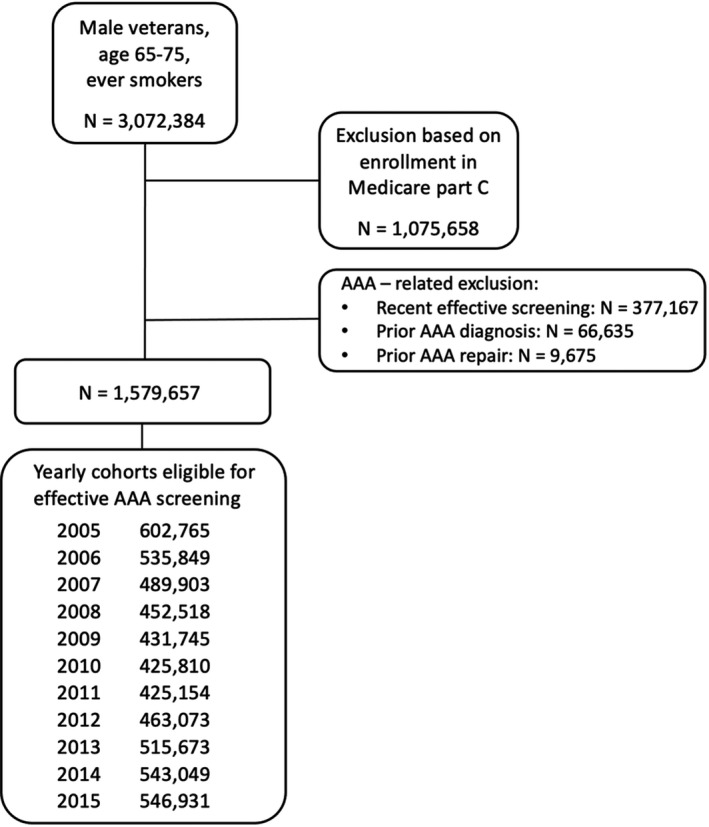
Approximately 3 million veterans met criteria for abdominal aortic aneurysm (AAA) screening, and approximately half were excluded based on AAA‐specific or other patient factors, resulting in yearly cohorts of ≈500 000 veterans.
Table 1.
Demographics of Overall Cohort
| Variable |
Total (N=1 579 657) |
Screened and AAA positive (N=56 899) | Screened and AAA negative (N=777 392) |
Not screened (N=745 366) |
P value |
|---|---|---|---|---|---|
| Eligible age, y | |||||
| Mean±SD | 67.22±2.88 | 67.04±2.50 | 66.69±2.29 | 67.79±3.32 | <0.001 |
| Race | |||||
| White | 1 188 199 (75.2) | 44 022 (77.4) | 584 404 (75.2) | 559 773 (75.1) | <0.001 |
| Black | 141 008 (8.9) | 3434 (6.0) | 76 641 (9.9) | 60 933 (8.2) | |
| Asian/PI/AM IND | 32 179 (2.0) | 885 (1.6) | 14 917 (1.9) | 16 377 (2.2) | |
| Declined to answer | 41 190 (2.6) | 1470 (2.6) | 21 319 (2.7) | 18 401 (2.5) | |
| Unknown/missing | 177 081 (11.2) | 7088 (12.5) | 80 111 (10.3) | 89 882 (12.1) | |
| Ethnicity | |||||
| Not Hispanic or Latino | 1 368 755 (86.6) | 49 406 (86.8) | 679 503 (87.4) | 639 846 (85.8) | <0.001 |
| Hispanic or Latino | 50 506 (3.2) | 953 (1.7) | 24 121 (3.1) | 25 432 (3.4) | |
| Declined to answer | 26 999 (1.7) | 1047 (1.8) | 13 874 (1.8) | 12 078 (1.6) | |
| Unknown/missing | 133 397 (8.4) | 5493 (9.7) | 59 894 (7.7) | 68 010 (9.1) | |
| Marriage | |||||
| Married | 976 694 (61.8) | 36 579 (64.3) | 476 587 (61.3) | 463 528 (62.2) | <0.001 |
| Others | 587 574 (37.2) | 19 807 (34.8) | 294 614 (37.9) | 273 153 (36.6) | |
| Unknown/missing | 15 389 (1.0) | 513 (0.9) | 6191 (0.8) | 8685 (1.2) | |
| Charlson comorbidity score | |||||
| Mean±SD | 0.48±1.10 | 0.58±1.15 | 0.63±1.28 | 0.32±0.84 | <0.001 |
| Time of eligibility, y | |||||
| Mean±SD | 5.00±2.82 | 6.04±2.69 | 5.81±2.72 | 4.07±2.64 | <0.001 |
Data are given as number (percentage), unless otherwise indicated. AAA indicates abdominal aortic aneurysm; AM IND, American Indian or Alaska Native; and PI, Native Hawaiian or other Pacific Islander.
Figure 2 shows yearly eligibility and exclusion data, after exclusion of veterans enrolled in Medicare part C, used to achieve the final yearly cohorts, and the yearly prevalence and outcomes of screening. After an initial exclusion of ≈138 000 veterans in 2005, the number of excluded veterans increased nearly every year, from ≈9400 in 2006 to 47 000 in 2013, remaining essentially stable thereafter. The number of veterans remaining eligible for AAA screening ranged from 425 154 to 602 765, and the fraction of veterans undergoing screening ranged from 11.7% in 2005 to 18.3% in 2015, increasing nearly every year. In total, 834 291 veterans underwent screening during the follow‐up period, 726 838 (87.1%) via implicit screening examinations and 107 453 (12.9%) via explicit ultrasound AAA screening examinations. The frequency of screening events by CPT code is presented in Data S1. Between 3931 and 4981 AAAs were detected at screening each year, constituting a “screen‐positive” rate of 4.9% to 7.3%. For veterans who underwent screening examinations, 73 459 (8.8%) underwent an explicit AAA ultrasound later in the same year as their primary screening examination.
Figure 2. Cohort size, screened percentage, and screened positive by year.
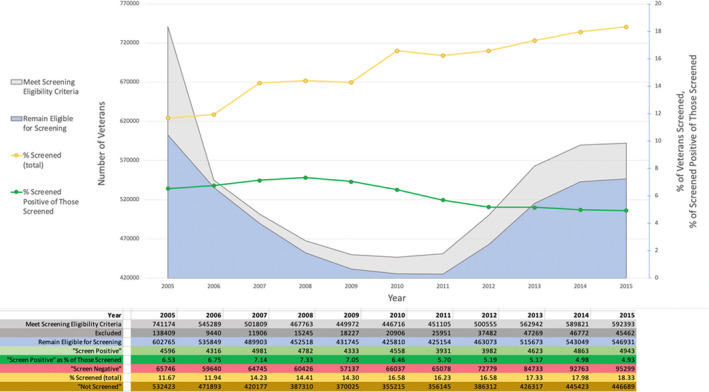
Details of how many veterans met abdominal aortic aneurysm screening criteria, how many veterans met exclusion criteria, and how many veterans were effectively screened with what outcome. Yearly effective screening rate increases steadily, whereas positive effective screening tends to decrease.
AAA‐Related Outcomes and Mortality
Outcome data of AAA repair, AAA rupture, and all‐cause mortality within the period of data collection are shown in Table 2 for the entire cohort over 10 years. Approximately 16% of veterans who screened positive for AAA underwent open (4.3%) or endovascular (11.8%) aneurysm repair. The rate of open or endovascular aneurysm repair in veterans who screened positive for AAA was 64 times that for veterans who screened negative and 40 times greater than that for veterans who never underwent AAA screening. AAA rupture occurred in 2.9% of veterans who screened positive for AAA, at a rate 31 times greater than veterans who screened negative and 27 times greater than veterans who did not undergo AAA screening. Death rate during the data collection period was highest for veterans screening positive for AAA, lower for those screening negative, and lowest for veterans going unscreened (27.8% versus 23.6% versus 17.7%, respectively; P<0.001). Among 834 291 screened versus 745 366 unscreened veterans, AAA screening was associated with a 57% greater odds of death (OR, 1.57; 95% CI, 1.55–1.58) after adjustment for age, race, ethnicity, years of eligibility, and Charlson comorbidity score.
Table 2.
Outcomes Data for Overall Cohort
| Variable |
Total (N=1 579 657) |
Total screened (positive and negative) (N=834 291) |
Screened and AAA positive (N=56 899) |
Screened and AAA negative (N=777 392) |
Not screened (N=745 366) |
P value |
|---|---|---|---|---|---|---|
| Open repair | 3437 (0.2) | 2804 (0.3) | 2437 (4.3) | 367 (0.0) | 633 (0.1) | <0.001 |
| Endovascular repair | 10 676 (0.7) | 8299 (1.0) | 6701 (11.8) | 1598 (0.2) | 2377 (0.3) | <0.001 |
| AAA rupture | 3186 (0.2) | 2391 (0.3) | 1663 (2.9) | 728 (0.1) | 795 (0.1) | <0.001 |
| Death | 331 305 (21.0) | 199 621 (23.9) | 15 808 (27.8) | 183 813 (23.6) | 131 684 (17.7) | <0.001 |
Data are given as number (percentage). AAA indicates abdominal aortic aneurysm.
2005 Subgroup Analyses
Approximately three quarters (73.7%) of the 59 965 veterans with unknown AAA status who entered eligibility for screening in 2005 at age 65 years underwent screening during their 10‐year eligibility window. In total, 44 215 veterans underwent screening during the follow‐up period, 40 611 (91.8%) via implicit screening examinations and 3604 (8.2%) via explicit ultrasound AAA screening examinations. Demographic and outcomes data for this subgroup are shown in Tables 3 and 4, respectively. Among the 15 750 veterans who did not undergo AAA screening in their eligibility period, 98.1% had at least one clinical visit within or coordinated by the VA health care system during the data collection period. The rate of open or endovascular AAA repair was slightly greater in this subgroup followed up for their entire eligibility window, with 18.6% of those screening positive for AAA undergoing intervention within the 10‐year period, compared with 16.1% of those screening positive in the entire 10‐year cohort. The rate of aneurysm repair in veterans within the 2005 subgroup who screened positive was 47 times that for veterans who screened negative and 266 times greater than that for veterans who never underwent AAA screening. AAA rupture occurred in 3.5% of veterans (114 of 3297) who screened positive, at a rate 26 times greater than veterans who screened negative (55 of 40 918) and 42 times greater than veterans who did not undergo AAA screening (13 of 15 750). Death rate during the data collection period was statistically significantly lower for veterans who did not undergo AAA screening when compared with veterans who screened positive or negative (23.1% versus 33.5% versus 31%, respectively; P<0.001). In 44 215 screened versus 15 750 unscreened veterans, AAA screening was associated with a 46% greater odds of mortality (OR, 1.46; 95% CI, 1.39–1.53) after adjustment for age, race, ethnicity, and Charlson comorbidity score. Although statistically significant, the difference in death rate for those screening positive or negative for AAA was small (33.5% versus 31%, respectively; P=0.001).
Table 3.
Demographics of 2005 Cohort
| Variable |
Total (N=59 965) |
Screened and AAA positive (N=3297) |
Screened and AAA negative (N=40 918) |
Not screened (N=15 750) |
P value |
|---|---|---|---|---|---|
| Eligible age, y | |||||
| Mean±SD | 65.50±0.29 | 65.49±0.29 | 65.49±0.29 | 65.51±0.29 | <0.001 |
| Race | |||||
| White | 44 389 (74.0) | 2514 (76.3) | 30 313 (74.1) | 11 562 (73.4) | <0.001 |
| Black | 4571 (7.6) | 178 (5.4) | 3386 (8.3) | 1007 (6.4) | |
| Asian/PI/AM IND | 1173 (2.0) | 42 (1.3) | 780 (1.9) | 351 (2.2) | |
| Declined to answer | 1604 (2.7) | 83 (2.5) | 1166 (2.8) | 355 (2.3) | |
| Unknown/missing | 8228 (13.7) | 480 (14.6) | 5273 (12.9) | 2475 (15.7) | |
| Ethnicity | |||||
| Not Hispanic or Latino | 50 800 (84.7) | 2815 (85.4) | 34 970 (85.5) | 13 015 (82.6) | <0.001 |
| Hispanic or Latino | 1515 (2.5) | 42 (1.3) | 1047 (2.6) | 426 (2.7) | |
| Declined to answer | 1188 (2.0) | 61 (1.9) | 857 (2.1) | 270 (1.7) | |
| Unknown/missing | 6462 (10.8) | 379 (11.5) | 4044 (9.9) | 2039 (12.9) | |
| Marriage | |||||
| Married | 37 424 (62.4) | 2147 (65.1) | 25 461 (62.2) | 9816 (62.3) | <0.001 |
| Others | 21 941 (36.6) | 1127 (34.2) | 15 110 (36.9) | 5704 (36.2) | |
| Unknown/missing | 600 (1.0) | 23 (0.7) | 347 (0.8) | 230 (1.5) | |
| Charlson comorbidity score | |||||
| Mean±SD | 0.41±1.02 | 0.42±0.88 | 0.47±1.08 | 0.25±0.86 | <0.001 |
Data are given as number (percentage), unless otherwise indicated. AAA indicates abdominal aortic aneurysm; AM IND, American Indian or Alaska Native; and PI, Native Hawaiian or other Pacific Islander.
Table 4.
Outcomes Data for 2005 Cohort
| Variable |
Total (N=59 965) |
Total screened (positive and negative) (N=44 215) |
Screened and AAA positive (N=3297) |
Screened and AAA negative (N=40 918) |
Not screened (N=15 750) |
P value |
|---|---|---|---|---|---|---|
| Open repair | 202 (0.3) | 199 (0.5) | 174 (5.3) | 25 (0.1) | 3 (0.0) | <0.001 |
| Endovascular repair | 583 (1.0) | 575 (1.3) | 439 (13.3) | 136 (0.3) | 8 (0.1) | <0.001 |
| AAA rupture | 182 (0.3) | 169 (0.4) | 114 (3.5) | 55 (0.1) | 13 (0.1) | <0.001 |
| Death | 17 433 (29.1) | 13 796 (31.2) | 1104 (33.5) | 12 692 (31.0) | 3637 (23.1) | <0.001 |
Data are given as number (percentage). AAA indicates abdominal aortic aneurysm.
Discussion
We report the trends and outcomes of AAA screening in the national VA health care system over the 10‐year period since the USPSTF screening recommendation was made in 2005. Our retrospective observational study specifically sought to characterize screening reach and impact when accounting for the commonly used but rarely acknowledged “implicit screening” that occurs when an imaging examination not targeted to abdominal aortic evaluation can fulfill USPSTF screening recommendations. We studied the impact of screening in a population at risk for the disease process, strongly targeted by the screening eligibility criteria, and available for a large‐scale investigation with long‐term follow‐up. We assessed AAA screening by querying the VA Corporate Data Warehouse and Centers for Medicare and Medicaid Services records for eligible veterans with coding indicating that they had undergone a radiologic examination that evaluates the abdominal aorta.
AAA screening programs were widely implemented 17 years ago based on data from 4 population‐based, randomized, controlled trials performed in the United Kingdom and Australia. With the exception of the original trials, no population‐scale investigations of screening implementation or impact have occurred since, and there has been a paucity of data comprehensively describing screening efforts in the United States. Smaller studies have examined the implementation of screening, but these have typically been limited to assessing the adherence to screening eligibility criteria, factors influencing screening, or near‐term outcomes of limited screening programs in tightly managed patient populations. 9 , 10 , 11 , 12 , 13 , 15 More important, prior works have almost universally neglected the substantial contribution of implicit screening practices toward fulfilling the USPSTF AAA screening recommendations.
Our data demonstrate that AAA screening is accomplished with moderate success: 73.7% of veterans followed up for their full 10‐year eligibility period underwent screening. This is similar to screening rates for eligible adults in the United States of 68.8% for colorectal cancer in 2018 25 and 78.3% for screening mammography in 2018, 26 but is higher than the 2.0% reported for low‐dose CT lung cancer screening in 2016. 27
The yearly rate of AAA screening for all eligible veterans increased from 11.7% to 18.3% over the 10‐year study period, and explicit screening constituted a minority (12.9%) of screening examinations performed. For screened veterans, the rate of AAA diagnosis ranged from 7.3% to 4.9%, declining every year since 2008. The total yearly screening rates estimated herein are substantially higher than the 1% estimated in the study by Olchanski et al of Medicare beneficiaries from 2005 to 2009, 14 as their data only considered explicit screening. The yearly explicit screening rate from our data is 1.5% to 2.4%, which compares well with the study by Olchanski et al. The predominance of implicit screening examinations in our data compares well to that observed in the smaller study by Ruff et al 12 , where implicit AAA screening outweighed explicit screening by 4.5× or 8.6× in a regional health care system, depending on the year. That study also found that 31% of patients undergoing an explicit ultrasound screening examination had already undergone implicit screening via CT, magnetic resonance imaging, or another type of ultrasound examination. A total of 8.8% of subjects who underwent (overwhelmingly implicit) screening in our study underwent explicit ultrasound screening later in the same year, amounting to 73 459 redundant and likely unnecessary examinations.
The rates of AAA repair and rupture are significantly higher in veterans who screened positive for AAA compared with those who screened negative or never underwent explicit or implicit screening. However, screening is not associated with better overall outcomes. For the subgroup of veterans who entered eligibility for AAA screening in 2005 at the age of 65 years but went unscreened, all‐cause mortality was 8.1% lower than that for veterans who were screened for AAA within their 10‐year eligibility. This finding seems to go against the traditional thinking that increased screening efforts are linked to better health and decreased mortality, and appears to contradict the results of the 4 large AAA screening trials, where no significant difference in all‐cause mortality occurred between the screened subjects and unscreened controls. More important, our results reflect the selection bias inherent to implicit screening practices, and highlight that implicit screening practices must be considered differently than explicit screening programs, the benefits of which were established by large randomized controlled trials. Patients undergoing implicit screening via radiology examinations are being imaged to evaluate a known or suspected pathology. This in itself is associated with greater odds of mortality in the follow‐up period, regardless of demographic factors or comorbidities reflected in the Charlson index. Interestingly, all‐cause mortality was only slightly higher in the 2005 subgroup for veterans who screened positive for AAA compared with those who screened negative (33.5% versus 31%; P=0.001), likely reflecting the fact that AAA‐specific mortality is low compared with other comorbidities common in the older veteran population, ≈1.5 per 100 000 in the United States in 2019. 28
Although our observational study cannot assess the effectiveness or benefits of explicit AAA screening, which have been previously established in 4 large trials, our data suggest that efforts to increase the percentage of eligible veterans undergoing explicit AAA screening may not be warranted. Rather, implementing practice patterns that definitively exploit available implicit screening data could more significantly extend the reach of screening efforts. In our data, all‐cause mortality in the unscreened group is less than in those screened, which reflects that imaging decisions are informed by many factors and in most cases imaging is performed for reasons other than AAA screening. The significantly lower rate of AAA rupture in the unscreened veterans compared with the screened veterans (0.1% versus 0.38% overall, with 3.5% rupture rate in screen‐positive veterans) in the 2005 subgroup suggests that factors influencing implicit screening might also stratify patients for AAA rupture risk.
Our study has several limitations. Although the nationwide VA population is well suited to study AAA screening given the epidemiology of the disease, extrapolation to other populations may not be appropriate. In addition to possible differences in patient demographics and comorbidities, VA health care system practices may differ from those of private and other public health care systems. Our analysis is retrospective and relies on medical coding, which is susceptible to inaccuracy and delays in entry that can distort or obscure the temporal relationship between clinical events. Given the large cohort size and associated medical coding data included in this work, it is challenging to fully mitigate these hazards. An additional limitation of our coding‐based investigation is that we could not fully assess certain imaging and clinical factors. For example, we could not determine the diameter of a particular AAA, but only detect that an AAA diagnosis was made. We assume that a reasonable threshold aortic diameter (eg, 3 cm) was observed. Similarly, follow‐up care after AAA diagnosis and cause of death could not be reliably assessed. Such limitations are the unavoidable cost of population‐scale assessments of screening programs and other public health measures.
We have attempted to comprehensively account for additional medical imaging studies and diagnoses occurring outside the VA health care system by incorporating Medicare data where we are confident in our ability to capture health care events and outcomes. Veterans who received some portion of their care outside the VA system under Medicare part C were excluded, as we could not guarantee that this portion of their clinical picture was represented in the available medical records. Reassuringly, the vast majority (98.1%) of unscreened veterans in the 2005 subgroup had coding events for at least one clinical care visit either at or coordinated through the VA system, indicating that the absence of screening does not reflect any significant incompleteness of data capture using our method.
Conclusions
Our data suggest that although AAA screening in the VA population is only moderately successful in terms of reach, it is substantially greater than other analyses have suggested because of consideration of implicit screening. Efforts to expand explicit AAA screening are not likely to impact either all‐cause mortality or AAA rupture on the population scale as significantly as a careful accounting for and use of implicit screening data.
Sources of Funding
None.
Disclosures
None.
Supporting information
Data S1
For Sources of Funding and Disclosures, see page 9.
References
- 1. Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population–a meta‐analysis. PLoS One. 2013;8:e81260. doi: 10.1371/journal.pone.0081260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e2. doi: 10.1016/j.jvs.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 3. Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best‐evidence systematic review for the U.S. preventive services task force. Ann Intern Med. 2005;142:203–211. doi: 10.7326/0003-4819-142-3-200502010-00012 [DOI] [PubMed] [Google Scholar]
- 4. Lindholt JS, Juul S, Fasting H, Henneberg EW. Hospital costs and benefits of screening for abdominal aortic aneurysms: results from a randomised population screening trial. Eur J Vasc Endovasc Surg. 2002;23:55–60. doi: 10.1053/ejvs.2001.1534 [DOI] [PubMed] [Google Scholar]
- 5. Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RAP, Thompson SG, Walker NM, Multicentre Aneurysm Screening Study Group . The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/S0140-6736(02)11522-4 [DOI] [PubMed] [Google Scholar]
- 6. Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RA. Fifteen‐year follow‐up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696–701. doi: 10.1002/bjs.5780 [DOI] [PubMed] [Google Scholar]
- 7. Norman PE, Jamrozik K, Lawrence‐Brown MM, Le MT, Spencer CA, Tuohy RJ, Parsons RW, Dickinson JA. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329:1259. doi: 10.1136/bmj.38272.478438.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Federman DG, Carbone VG, Kravetz JD, Kirsner RS, Bravata DM. Are screening guidelines for abdominal aortic aneurysms being implemented within a large VA primary health care system? Postgrad Med. 2009;121:132–135. doi: 10.3810/pgm.2009.01.1962 [DOI] [PubMed] [Google Scholar]
- 9. Chun KC, Dolan KJ, Smothers HC, Irwin ZT, Anderson RC, Gonzalves AL, Lee ES. The 10‐year outcomes of a regional abdominal aortic aneurysm screening program. J Vasc Surg. 2019;70:1123–1129. doi: 10.1016/j.jvs.2019.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chun KC, Teng KY, Van Spyk EN, Carson JG, Lee ES. Outcomes of an abdominal aortic aneurysm screening program. J Vasc Surg. 2013;57:376–381. doi: 10.1016/j.jvs.2012.08.038 [DOI] [PubMed] [Google Scholar]
- 11. Lee ES, Pickett E, Hedayati N, Dawson DL, Pevec WC. Implementation of an aortic screening program in clinical practice: implications for the Screen for Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act. J Vasc Surg. 2009;49:1107–1111. doi: 10.1016/j.jvs.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 12. Ruff AL, Teng K, Hu B, Rothberg MB. Screening for abdominal aortic aneurysms in outpatient primary care clinics. Am J Med. 2015;128:283–288. doi: 10.1016/j.amjmed.2014.10.036 [DOI] [PubMed] [Google Scholar]
- 13. Eaton J, Reed D, Angstman KB, Thomas K, North F, Stroebel R, Tulledge‐Scheitel SM, Chaudhry R. Effect of visit length and a clinical decision support tool on abdominal aortic aneurysm screening rates in a primary care practice: visit length and abdominal aortic aneurysm screening. J Eval Clin Pract. 2012;18:593–598. doi: 10.1111/j.1365-2753.2010.01625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olchanski N, Winn A, Cohen JT, Neumann PJ. Abdominal aortic aneurysm screening: how many life years lost from underuse of the Medicare screening benefit? J Gen Intern Med. 2014;29:1155–1161. doi: 10.1007/s11606-014-2831-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hye RJ, Smith AE, Wong GH, Vansomphone SS, Scott RD, Kanter MH. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535–1543. doi: 10.1016/j.jvs.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 16. O’Donnell TFX, Schermerhorn ML. Abdominal aortic aneurysm screening guidelines: United States preventative services task force and society for vascular surgery. J Vasc Surg. 2020;71:1457–1458. doi: 10.1016/j.jvs.2020.01.054 [DOI] [PubMed] [Google Scholar]
- 17. Draft recommendation: colorectal cancer: screening | United States Preventive Services Taskforce . https://www.uspreventiveservicestaskforce.org/uspstf/draft‐recommendation/colorectal‐cancer‐screening3. Accessed January 30, 2021.
- 18. Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih Y‐C, et al. Colorectal cancer screening for average‐risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–281. doi: 10.3322/caac.21457 [DOI] [PubMed] [Google Scholar]
- 19. History of ACS recommendations for the early detection of cancer in people without symptoms . https://www.cancer.org/health‐care‐professionals/american‐cancer‐society‐prevention‐early‐detection‐guidelines/overview/chronological‐history‐of‐acs‐recommendations.html. Accessed January 30, 2021.
- 20. Oeffinger KC, Fontham ETH, Etzioni R, Herzig A, Michaelson JS, Shih Y‐C, Walter LC, Church TR, Flowers CR, LaMonte SJ, et al. Guideline update from the American Cancer Society. JAMA. 2015;314:1599. doi: 10.1001/jama.2015.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jonas DE, Reuland DS, Reddy SM, Nagle M, Clark SD, Weber RP, Enyioha C, Malo TL, Brenner AT, Armstrong C, et al. Screening for lung cancer with low‐dose computed tomography: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325:971. doi: 10.1001/jama.2021.0377 [DOI] [PubMed] [Google Scholar]
- 22. Lung rads . https://www.acr.org/Clinical‐Resources/Reporting‐and‐Data‐Systems/Lung‐Rads. Accessed January 30, 2021.
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 24. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel J‐M, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 25. Use of colorectal cancer screening tests (2018 Behavioral Risk Factor Surveillance System) | CDC . 2020; https://www.cdc.gov/cancer/colorectal/statistics/use‐screening‐tests‐BRFSS.htm. Accessed January 30, 2021.
- 26. Centers for Disease Control and Prevention (CDC) . Behavioral Risk Factor Surveillance System Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 27. Pham D, Bhandari S, Pinkston C, Oechsli M, Kloecker G. Lung cancer screening registry reveals low‐dose CT screening remains heavily underutilized. Clin Lung Cancer. 2020;21:e206–e211. doi: 10.1016/j.cllc.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention, National Center for Health Statistics . Underlying cause of death 1999‐2019 on CDC WONDER Online Database, released in 2020. Data are from the Multiple Cause of Death Files, 1999‐2019, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed January 30, 2021. http://wonder.cdc.gov/ucd‐icd10.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


