Abstract
Background
Pulmonary arterial end‐diastolic forward flow (EDFF) following repaired tetralogy of Fallot has been thought to represent right ventricular (RV) restrictive physiology, but is not fully understood. This systematic review and meta‐analysis sought to clarify its physiological and clinical correlates, and to define a framework for understanding EDFF and RV restrictive physiology.
Methods and Results
PubMed/MEDLINE, Embase, Scopus, and reference lists of relevant articles were searched for observational studies published before March 2021. Random‐effects meta‐analysis was performed to identify factors associated with EDFF. Forty‐two individual studies published between 1995 and 2021, including a total of 2651 participants (1132 with EDFF; 1519 with no EDFF), met eligibility criteria. The pooled estimated prevalence of EDFF among patients with repaired tetralogy of Fallot was 46.5% (95% CI, 41.6%–51.3%). Among patients with EDFF, the use of a transannular patch was significantly more common, and their stay in the intensive care unit was longer. EDFF was associated with greater RV indexed volumes and mass, as well as smaller E‐wave velocity at the tricuspid valve. Finally, pulmonary regurgitation fraction was greater in patients with EDFF, and moderate to severe pulmonary regurgitation was more common in this population.
Conclusions
EDFF is associated with dilated, hypertrophied RVs and longstanding pulmonary regurgitation. Although several studies have defined RV restrictive physiology as the presence of EDFF, our study found no clear indicators of poor RV compliance in patients with EDFF, suggesting that EDFF may have multiple causes and might not be the precise equivalent of RV restrictive physiology.
Keywords: antegrade diastolic flow, end‐diastolic forward flow, meta‐analysis, restrictive physiology, tetralogy of Fallot
Subject Categories: Meta Analysis, Congenital Heart Disease
Nonstandard Abbreviations and Acronyms
- EDFF
end‐diastolic forward flow
- MD
mean difference
- PR
pulmonary regurgitation
- RA
right atrial
- rToF
repaired tetralogy of Fallot
- RVEDVi
right ventricular end‐diastolic volume indexed
- RVRP
right ventricular restrictive physiology
- ToF
tetralogy of Fallot
Clinical Perspective
What Is New?
In this systematic review and meta‐analysis of 2651 patients with repaired tetralogy of Fallot from 42 individual studies, end‐diastolic forward flow (EDFF) occurred in 46.5%.
EDFF was associated with transannular patch repair, greater right ventricular indexed volumes and mass, smaller E‐wave velocity at the tricuspid valve, increased rates of moderate to severe pulmonary regurgitation, and longer stay in the intensive care unit.
What Are the Clinical Implications?
Although often used as a surrogate marker of right ventricular restrictive physiology, EDFF may have multiple alternative causes and might not be the precise equivalent of right ventricular restrictive physiology.
Our review supports a specific reconciliation of the conflicting EDFF literature, based on the presence of 2 main phenotypes: (1) early‐onset, “primary” EDFF and (2) late‐onset, “secondary” EDFF; the latter has become more prevalent in contemporary practice, with improved perioperative ventricular diastolic function but progressive dilatation resulting from longstanding pulmonary regurgitation.
Future studies should refine the diagnostic criteria for right ventricular restrictive physiology and clarify the potential prognostic relevance of EDFF in various settings.
Tetralogy of Fallot (ToF) is the most common type of cyanotic congenital heart disease. 1 Although great strides have been made in the initial management of this condition, patients with repaired ToF (rToF) carry significant residual hemodynamic burden. 2 Long‐term functional deterioration and adverse outcomes, such as arrhythmias, ventricular dysfunction, and mortality, have been related to longstanding pulmonary regurgitation (PR) and right ventricular (RV) volume overload. 3 , 4 The concept of RV restrictive physiology (RVRP) has been introduced to refer to abnormalities in RV diastolic function, which have been observed both transiently at the time of initial repair 5 and chronically at late follow‐up. 6 Initial reports 5 , 6 , 7 , 8 , 9 , 10 have linked RVRP to the presence of end‐diastolic forward flow (EDFF) into the pulmonary artery (ie, “antegrade diastolic pulmonary flow,” “antegrade diastolic pulmonary artery flow,” and “antegrade diastolic flow”). This phenomenon was thought to result from an RV so “stiff” as to be unfillable late in diastole, as a passive conduit between right atrium (RA) and pulmonary artery during atrial systole. 6
RVRP has been identified on the basis of the presence of EDFF on Doppler echocardiography or cardiac magnetic resonance (CMR), but studies of its physiological and clinical correlates have yielded divergent results. Some authors have suggested that RVRP is beneficial because it decreases PR, RV dilatation, and QRS duration, resulting in improved exercise capacity and lower risk of ventricular arrhythmias. 6 , 7 , 8 Others, in contrast, have found more severe PR, larger RV volumes, and worse exercise capacity in patients with EDFF. 5 , 11 , 12 , 13 , 14 , 15 On the basis of simultaneous catheter pressure monitoring, EDFF can occur whenever RV diastolic pressure equals or exceeds pulmonary artery pressure. 16 An insight emerges that EDFF might not always carry the same implications as true RVRP. The current understanding of the relationship among the various factors leading to EDFF and RVRP remains incomplete. The purpose of this meta‐analysis is to clarify the physiological and clinical correlates of EDFF, and to establish a framework to guide current thinking about EDFF and RVRP.
METHODS
Data used for the analyses in this article will be made available from the corresponding author on reasonable request.
Eligibility Criteria, Databases, and Search Strategy
We followed 2 internationally recognized protocols: Preferred Reporting Items for Systematic Reviews and Meta‐Analyses 17 and Meta‐Analysis of Observational Studies in Epidemiology. 18 Studies were included if (1) the population consisted of patients with ToF, (2) patients had undergone full ToF repair by the time of evaluation, (3) patient characteristics, surgical history, hemodynamic parameters, and/or other measurements were compared between patients with EDFF and those without, and (4) studies were prospective or retrospective observational studies or randomized controlled trials. Exclusion criteria included the following: (1) nonoriginal articles, such as review articles, meta‐analyses, guidelines, consensus statements, conference abstract, editorials, letters, and book reviews, (2) in vitro or in vivo preclinical research, or (3) publications did not include data on EDFF status.
Databases were searched for articles meeting our inclusion criteria and published by March 8, 2021: PubMed/MEDLINE, Embase, Scopus, and reference lists of relevant articles. The detailed search terms that were used for this search are given in Data S1. The following steps were taken: (1) identification of titles of records through databases searching, (2) removal of duplicates, (3) screening and selection of abstracts, (4) assessment for eligibility through full‐text articles, and (5) final inclusion in the study. Studies were selected by 2 independent reviewers (J.V.D.E. and E.D.). Discrepancies were resolved by consensus.
Data Items
All variables that were compared between EDFF and no EDFF groups in least 2 studies were included in the meta‐analysis. These variables included patient characteristics, surgical history, hemodynamic parameters, and other measurements. For studies reporting interquartile ranges, the mean was estimated according to a well‐accepted and commonly used formula. 19 Two reviewers independently extracted the data (J.V.D.E. and E.D.). Discrepancies were resolved by consensus. From each study, we extracted first authors’ name, year of publication, country of origin, study design, years of enrollment, sample size, EDFF prevalence, mean age at initial ToF repair, mean interval between ToF repair and assessment, and mean age at assessment.
Statistical Analysis
Mean differences (MDs) with 95% CI and P values were calculated for continuous variables. For binary variables, odds ratios (ORs) with 95% CI and P values were considered. I², describing the percentage of total variation across studies that is attributable to heterogeneity rather than chance, was calculated to assess the degree of statistical heterogeneity, and its accompanying P value was obtained using the χ2 test of the Cochran Q heterogeneity statistic. 20 The MD and OR were combined across the studies using a random‐effects method (DerSimonian and Laird inverse variance). 21 The choice for random‐effects models was made on the basis of the assumption that the effect sizes in the individual studies represented samples from a mixing distribution. In addition, the results were reanalyzed using fixed‐effects models to explore whether this yielded differences on the summary inferences. Forest plots were used to visualize the individual study and summary effect estimates. These analyses were conducted using the “metacont” and “metabin” functions of the R package “meta” (version 4.19‐0). Funnel plots were produced for visual representation of publication bias, and were analyzed quantitatively by Begg and Mazumdar’s rank correlation method 22 and Egger’s linear regression method, using the “funnel” and “metabias” functions of the R package “meta” (version 4.19‐0). 23 The proportions of patients who had EDFF were pooled into a global estimated prevalence using the same random‐effects method (DerSimonian and Laird inverse variance) as described above, via the “metaprop” function of the R package “meta” (version 4.19‐0).
Subgroup analyses were conducted on the basis of study design (retrospective or prospective), by specifying this grouping variable in the “metacont” and “metabin” functions of the R package “meta” (version 4.19‐0). Furthermore, meta‐regression analyses were performed to determine whether the association of EDFF with the studied variables was modulated by (1) mean year of enrollment, (2) RV end‐diastolic volume indexed (RVEDVi), (3) age at evaluation, or (4) interval from initial repair to evaluation. The regression coefficient describes how the association of EDFF with these variables differs with an increase in each of these variables. These analyses were done using the “metareg” function of the R package “meta” (version 4.19‐0). No attempts were made to correct for multiple testing, given the exploratory nature of this study. All analyses were completed with R Statistical Software (version 4.0.5; Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Selection and Characteristics
A total of 552 citations were identified, of which 83 publications were potentially relevant and retrieved as full text. Forty‐five reports 5 , 6 , 7 , 8 , 11 , 12 , 13 , 14 , 15 , 16 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 of 42 individual studies fulfilled our eligibility criteria (Figure 1). Characteristics of each study and its participants are shown in Table 1. A total of 2651 participants (EDFF: 1132 participants; no EDFF: 1519 participants) were included from studies published between 1995 and 2021. All studies were nonrandomized observational studies, except for one randomized controlled trial. 26 , 36 The pooled mean age of participants was 16.5 years (39 studies, with 2323 participants) at the time of evaluation and 3.37 years (30 studies, with 2175 participants) at initial ToF repair. The interval between initial repair and evaluation was 13.0 years (21 studies, with 1421 participants).
Figure 1. Flow diagram of studies included in data search.
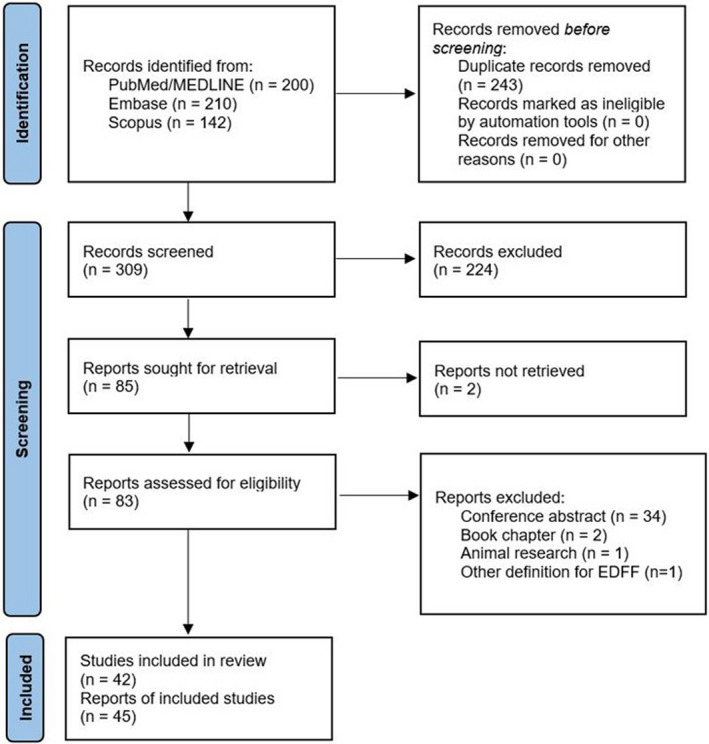
EDFF indicates end‐diastolic forward flow.
Table 1.
Study and Patient Characteristics
| Study | Country of origin | Study design | Years of enrollment | Sample size, N | Imaging tool used to define EDFF | EDFF prevalence, n/total (%) | Mean age at initial ToF repair, y | Mean interval between ToF repair and assessment, y | Mean age at assessment, y |
|---|---|---|---|---|---|---|---|---|---|
| Aburawi 2014 24 | Sweden | Prospective | NR | 20 | CMR | 9/20 (45.0) | NR | NR | 10.2 |
| Ahmad 2012 15 | Canada | Retrospective | 2008–2010 | 112 | Doppler echocardiography | 58/112 (51.8) | 0.9 | NR | 12.9 |
| Apitz 2010 25 | Germany | Prospective | NR | 25 | CMR | 8/25 (32.0) | NR | 7.1 | 17.9 |
| Babu‐Narayan 2012 26 (overlap with Krupickova 2018) | United Kingdom | Prospective | 2002–2005 | 64 | Doppler echocardiography | 27/64 (42.2) | 6.0 | 25.1 | 30.1 |
| Bonello 2013 27 | United Kingdom | Prospective | 2002–2008 | 148 | Doppler echocardiography | 38/148 (25.7) | 4.8 | NR | 32.1 |
| Cardoso 2003 28 | Brazil | Prospective | 2000 | 30 | Doppler echocardiography | 19/30 (63.3) | 3.0 | 3.2 | 8.7 |
| Chaturvedi 1999 29 | United Kingdom | Prospective | NR | 11 | Doppler echocardiography | 4/11 (36.4) | NR | NR | 1.7 |
| Cheng 2019 30 | United States | Retrospective | 1999–2014 | 38 | CMR | 15/38 (39.5) | NR | NR | 13.2 |
| Cheung 2003 31 | Australia | Prospective | 1981–1990 | 45 | Doppler echocardiography | 24/45 (53.3) | 2.1 | 12.5 | 15.0 |
| Choi 2008 32 | Korea | Retrospective | 1997–2000 | 43 | Doppler echocardiography | 15/43 (34.9) | 2.1 | 5.4 | 4.8 |
| Clark 1995 33 (overlap with Gatzoulis 1995) | United Kingdom | Prospective | 1958–1979 | 30 | Doppler echocardiography | 18/30 (60.0) | NR | 21.8 | 27.8 |
| Cullen 1995 5 | United Kingdom | Prospective | 1992–1993 | 35 | Doppler echocardiography | 17/35 (48.6) | NR | NR | 1.9 |
| Eroglu 1999 8 | Turkey | Prospective | 1986–1996 | 44 | Doppler echocardiography | 25/44 (56.8) | 4.0 | NR | 7.7 |
| Gatzoulis 1995 6 (overlap with Clark 1995) | United Kingdom | Prospective | 1958–1979 | 38 | Doppler echocardiography | 20/38 (52.6) | 5.2 | NR | 28.8 |
| Gatzoulis 1998 34 (overlap with Norgard 1996) | United Kingdom | Retrospective | 1985–1994 | 92 | Doppler echocardiography | 36/92 (39.1) | NR | 4.5 | 14.7 |
| Helbing 1996 11 | The Netherlands | Prospective | NR | 19 | Doppler echocardiography | 13/19 (68.4) | 1.5 | 10.0 | 12.0 |
| Kordybach‐Prokopiuk 2018 35 | Poland | Prospective | NR | 83 | Doppler echocardiography | 16/83 (19.3) | 11.9 | 21.6 | 31.5 |
| Krupickova 2018 36 (overlap with Babu‐Narayan 2012) | United Kingdom | Prospective | 2002–2005 | 64 | Doppler echocardiography | 26/64 (40.6) | 6.1 | 25.1 | 31.1 |
| Kutty 2018 37 | United States | Retrospective | 2005–2012 | 399 | Doppler echocardiography | 122/399 (30.6) | 1.1 | 18.5 | 20.5 |
| Latus 2013 38 | Germany | Retrospective | 2007–2011 | 53 | CMR | 15/53 (28.3) | 1.3 | 12.1 | 13.3 |
| Lee 2013 39 | Canada | Retrospective | 2007–2009 | 50 | CMR | 33/50 (66.0) | 1.3 | NR | 13.0 |
| Lu 2010 12 | United States | Prospective | 2008–2009 | 59 | CMR | 40/59 (67.8) | 11.0 | NR | 35.0 |
| Luijnenburg 2013 40 | The Netherlands | Prospective | 2007–2010 | 51 | CMR | 31/51 (60.8) | 2.8 | NR | 21.0 |
| Maskatia 2013 41 | United States | Retrospective | 1997–2011 | 178 | CMR | 77/178 (43.3) | 3.0 | NR | NR |
| Maskatia 2015 42 | United States | Retrospective | NR | 99 | Doppler echocardiography | 43/99 (43.4) | NR | NR | 14.2 |
| Mercer‐Rosa 2018 43 | United States | Prospective | NR | 88 | Doppler echocardiography | 77/88 (87.5) | 0.4 | NR | 12.7 |
| Mori 2017 16 | Japan | Retrospective | 2009–2016 | 62 | Doppler echocardiography | 23/62 (37.1) | 3.1 | NR | 15.7 |
| Munkhammar 1998 44 | United Kingdom | Prospective | 1985–1996 | 47 | Doppler echocardiography | 13/47 (27.7) | 0.7 | NR | 4.4 |
| Munkhammar 2013 45 | Sweden | Prospective | NR | 31 | Doppler echocardiography | 16/31 (51.6) | 1.0 | 9.2 | 10.2 |
| Norgard 1996 46 (overlap with Gatzoulis 1998) | United Kingdom | Retrospective | 1985–1994 | 92 | Doppler echocardiography | 36/92 (39.1) | 11.5 | NR | 14.7 |
| Norgard 1998 7 (early restriction) | United Kingdom | Prospective | 1992–1995 | 34 | Doppler echocardiography | 16/34 (47.1) | 5.9 | 1.8 | NR |
| Norgard 1998 7 (late restriction) | United Kingdom | Prospective | 1992–1995 | 32 | Doppler echocardiography | 10/32 (31.3) | 5.6 | 1.8 | NR |
| Peng 2012 47 | United Kingdom | Prospective | NR | 18 | Doppler echocardiography | 4/18 (22.2) | 1.6 | NR | 1.6 |
| Pijuan‐Domenech 2014 48 | Spain | Prospective | 2009–2012 | 20 | Doppler echocardiography | 16/20 (80.0) | 7.7 | NR | 35.0 |
| Rathore 2006 49 | Australia | Prospective | 2001–2003 | 80 | Doppler echocardiography | 52/80 (65.0) | NR | NR | 7.9 |
| Sachdev 2006 50 | India | Prospective | 2004–2005 | 50 | Doppler echocardiography | 24/50 (48.0) | NR | NR | 5.0 |
| Samyn 2013 13 | United States | Prospective | 2008–2009 | 29 | Doppler echocardiography | 12/29 (41. 4) | 1.4 | 14.0 | 16.3 |
| Sandeep 2019 51 | China | Prospective | 2017–2018 | 50 | Doppler echocardiography | 28/50 (56.0) | NR | NR | 2.2 |
| Sani 2020 52 | Iran | Prospective | 2015–2016 | 30 | CMR | 18/30 (60.0) | NR | 20.2 | 26.5 |
| Shekerdemian 1999 53 | United Kingdom | Prospective | NR | 23 | Doppler echocardiography | 8/23 (34.8) | NR | NR | 2.5 |
| Shin 2016 54 | Korea | Retrospective | 2005–2015 | 116 | Doppler echocardiography | 35/116 (30.2) | 2.3 | 14.2 | NR |
| Sjöberg 2018 55 | Sweden | Prospective | NR | 15 | CMR | 10/15 (66.7) | NR | NR | 29.0 |
| Tominaga 2021 56 | Japan | Retrospective | 2003–2019 | 46 | Doppler echocardiography | 23/46 (50.0) | 3.4 | 31.0 | 37.0 |
| van den Berg 2007 14 | The Netherlands | Prospective | 2002–2004 | 36 | Doppler echocardiography | 24/36 (66. 7) | 0.9 | 15.3 | 16.0 |
| Vukomanovic 2006 57 | Serbia and Montenegro | Prospective | 1995–2004 | 60 | Doppler echocardiography | 18/60 (30.0) | 4.3 | NR | 9.0 |
| Xu 2014 58 | China | Retrospective | 2011–2012 | 80 | Doppler echocardiography | 30/80 (37.5) | 1.2 | NR | 1.2 |
CMR indicates cardiac magnetic resonance; EDFF, end‐diastolic forward flow; NR, not reported; and ToF, tetralogy of Fallot.
Synthesis of Results
Prevalence of EDFF
Overall, the pooled estimated prevalence of EDFF among patients with rToF was 46.5% (95% CI, 41.6%–51.3%; I²=80.9%). The reported prevalence in the 10 studies that used CMR to define EDFF (51.9%; 95% CI, 42.4%–61.1%; I²=70.5%) tended to be marginally higher than that in the 32 studies that defined EDFF based on Doppler echocardiography (45.6%; 95% CI, 40.2%–51.1%; I²=80.7%), although this difference did not reach statistical significance (test for subgroup differences: P=0.263). Subanalyses according to study design revealed that a higher prevalence was reported in prospective studies (49.3%; 95% CI, 42.9%–55.6%; I²=81.2%) than in retrospective studies (40.3%; 95% CI, 35.1%–45.6%; I²=72.9%) (test for subgroup differences: P=0.034). Meta‐regression analysis revealed that the prevalence of EDFF increased with increasing RVEDVi (regression coefficient, 0.017; 95% CI, 0.001–0.034; P=0.049; 24 studies). Other analyses revealed no significant findings.
Meta‐Analysis
The results of the meta‐analysis comparing variables between rToF patients with EDFF and those without are summarized in Table 2. The accompanying forest plots are given in Figures S1 through S14. The use of a transannular patch was significantly more common among patients with EDFF (random‐effects model: OR, 1.98; 95% CI, 1.26–3.11; P=0.005), and intensive care unit length of stay for these patients was longer (random‐effects model: MD, 4.34 days; 95% CI, 1.38–7.29 days; P=0.019) when compared with those having no EDFF.
Table 2.
Meta‐Analysis of EDFF in rToF: Summary of Results
| Variable | Studies, N | Summary measures | Heterogeneity | |||
|---|---|---|---|---|---|---|
| OR/MD | 95% CI | P value | I², % | χ² P value | ||
| Patient characteristics | ||||||
| Age at repair, y | 16 | 0.329 | −0.419 to 1.077 | 0.363 | 95.2 | <0.001 |
| Time of follow‐up since repair, y | 9 | 0.318 | −0.654 to 1.290 | 0.472 | 82.8 | <0.001 |
| Age at study, y | 24 | 0.769 | −0.080 to 1.617 | 0.074 | 90.2 | <0.001 |
| Surgical history | ||||||
| Previous RVPA shunt | 3 | 0.365 | 0.122 to 1.091 | 0.058 | 0 | 0.423 |
| Previous BT shunt | 10 | 0.865 | 0.620 to 1.205 | 0.347 | 0 | 0.960 |
| Aortic cross‐clamp time, min | 7 | 7.786 | −1.053 to 16.624 | 0.075 | 78.7 | <0.001 |
| CPB time, min | 7 | 5.962 | −12.243 to 24.166 | 0.454 | 88.0 | <0.001 |
| Transatrial repair | 4 | 0.474 | 0.100 to 2.233 | 0.223 | 1.9 | 0.383 |
| Transannular patch repair | 21 | 1.983 | 1.264 to 3.112 | 0.005* | 55.9 | 0.001 |
| Outflow patch repair | 4 | 0.323 | 0.095 to 1.099 | 0.061 | 0 | 0.520 |
| ICU length of stay, d | 4 | 4.339 | 1.384 to 7.294 | 0.019* | 75.2 | 0.007 |
| Hemodynamics | ||||||
| RVEDVi, mL/m² | 16 | 14.706 | 4.572 to 24.840 | 0.007* | 91.0 | <0.001 |
| RVESVi, mL/m² | 11 | 16.146 | 1.012 to 31.280 | 0.039* | 94.9 | <0.001 |
| RVSVi, mL/m² | 6 | 9.570 | 0.674 to 18.466 | 0.040* | 98.3 | <0.001 |
| RVMi, g/m² | 7 | 2.873 | 0.139 to 5.606 | 0.042* | 93.9 | <0.001 |
| RVEF, % | 12 | −0.555 | −2.640 to 1.530 | 0.570 | 95.7 | <0.001 |
| RVEDP, mm Hg | 4 | 1.216 | −0.293 to 2.724 | 0.083 | 75.8 | 0.006 |
| RVESP, mm Hg | 5 | 0.824 | −5.563 to 7.210 | 0.738 | 69.9 | 0.010 |
| LVEDVi, mL/m² | 5 | 0.005 | −6.334 to 6.344 | 0.998 | 87.7 | <0.001 |
| LVESVi, mL/m² | 2 | −1.728 | −27.074 to 23.618 | 0.546 | 57.3 | 0.126 |
| LVSVi, mL/m² | 2 | −1.179 | −12.443 to 10.086 | 0.411 | 91.9 | <0.001 |
| LVEF, % | 9 | −0.195 | −1.256 to 0.866 | 0.682 | 74.3 | <0.001 |
| RAAi, cm²/m² | 3 | 1.083 | −0.319 to 2.484 | 0.080 | 92.8 | <0.001 |
| RAVi, mL/m² | 3 | 4.863 | −10.111 to 19.836 | 0.297 | 79.4 | 0.008 |
| E‐wave velocity at the tricuspid valve, cm/s | 11 | −11.586 | −20.850 to −2.321 | 0.019* | 79.3 | <0.001 |
| E‐wave duration at the tricuspid valve, ms | 4 | −7.077 | −33.700 to 19.545 | 0.460 | 85.3 | <0.001 |
| E‐wave deceleration at the tricuspid valve, ms | 8 | −14.507 | −34.448 to 5.434 | 0.129 | 91.5 | <0.001 |
| A‐wave velocity at the tricuspid valve, cm/s | 10 | −1.204 | −5.682 to 3.274 | 0.558 | 76.2 | <0.001 |
| A‐wave duration at the tricuspid valve, ms | 2 | −15.546 | −174.249 to 143.158 | 0.431 | 5.4 | 0.304 |
| E/A at the tricuspid valve | 10 | −0.106 | −0.246 to 0.033 | 0.119 | 59.5 | 0.008 |
| E’ at the tricuspid valve, cm/s | 2 | 0.914 | −12.862 to 14.690 | 0.554 | 73.4 | 0.053 |
| A’ at the tricuspid valve, cm/s | 2 | 0.000 | 0.000 to 0.000 | N/A | 0 | 1.000 |
| E/E’ at the tricuspid valve | 2 | −0.893 | −2.161 to 0.374 | 0.071 | 0 | 0.802 |
| Moderate to severe PR | 3 | 1.268 | 1.090 to 1.476 | 0.021* | 0 | 0.982 |
| PR fraction, % | 8 | 12.662 | 8.912 to 16.411 | <0.001* | 56.3 | 0.025 |
| PR duration, ms | 7 | −46.569 | −100.462 to 7.323 | 0.079 | 95.1 | <0.001 |
| Other | ||||||
| QRS duration, ms | 18 | 4.983 | −4.296 to 14.262 | 0.272 | 89.9 | <0.001 |
| BNP, pg/mL | 3 | 13.264 | −10.052 to 36.581 | 0.134 | 66.8 | 0.049 |
| NT‐proBNP, pg/mL | 3 | 61.125 | −25.398 to 147.647 | 0.093 | 0 | 0.479 |
| Peak VO2, % | 7 | 8.433 | −0.050 to 16.916 | 0.051 | 87.5 | <0.001 |
| Peak VO2, mL/kg per min | 6 | 0.648 | −3.857 to 5.153 | 0.727 | 98.0 | <0.001 |
A' indicates annulus velocity during late atrial filling; BNP, brain natriuretic peptide; BT, Blalock‐Taussig; CPB, cardiopulmonary bypass; E', annulus velocity during early filling; E/A, ratio between early (E) and late atrial (A) ventricular filling velocity; EDFF, end‐diastolic forward flow; ICU, intensive care unit; LVEDVi, left ventricular end‐diastolic volume indexed; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end‐systolic volume indexed; LVSVi, left ventricular stroke volume indexed; MD, mean difference; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OR, odds ratio; PR, pulmonary regurgitation; RAAi, right atrial area indexed; RAVi, right atrial volume indexed; rToF, repaired tetralogy of Fallot; RVEDP, right ventricular end‐diastolic pressure; RVEDVi, right ventricular end‐diastolic volume indexed; RVEF, right ventricular ejection fraction; RVESP, right ventricular end‐systolic pressure; RVESVi, right ventricular end‐systolic volume indexed; RVMi, right ventricular mass indexed; RVPA, right ventricle–pulmonary artery; RVSVi, right ventricular stroke volume indexed; and VO2, oxygen consumption.
P<0.05.
EDFF was found to be associated with dilated RVs, as reflected by a greater RVEDVi (random‐effects model: MD, 14.7 mL/m2; 95% CI, 4.57–24.8 mL/m2; P=0.007), greater RV end‐systolic volume indexed (random‐effects model: MD, 16.1 mL/m²; 95% CI, 1.01–31.3 mL/m2; P=0.039), and greater RV stroke volume indexed (random‐effects model: MD, 9.57 mL/m²; 95% CI, 0.67–18.5 mL/m2; P=0.040). Correspondingly, RV mass indexed was greater in patients with EDFF (random‐effects model: MD, 2.87 g/m²; 95% CI, 0.14–5.61 g/m2; P=0.042).
Furthermore, E‐wave velocity at the tricuspid valve was smaller in patients with EDFF (random‐effects model: MD, −11.6 cm/s; 95% CI, −20.9 to −2.32 cm/s; P=0.019). Last, the PR fraction was greater in patients with EDFF (random‐effects model: MD, 12.7%; 95% CI, 8.91%–16.4%; P<0.001), and moderate to severe PR was more common in this population (random‐effects model: OR, 1.27; 95% CI, 1.09–1.48; P=0.021). No other significant associations with EDFF were found (Table 2).
Funnel plot analysis disclosed asymmetry around the axis for transannular patch repair, RA volume indexed, PR duration, and A‐wave velocity at the tricuspid valve (Figure S15). Consequently, publication bias related to these outcomes cannot be excluded. No publication biases were found in the other short‐term outcomes.
Sensitivity Analysis
The results of the fixed‐effects models were largely comparable to those from random‐effects models, with numerical effect estimates having the same direction and lying close to one another (Figures S1 through S14). However, because of its narrower CIs, the fixed‐effects model additionally suggested a significant association with EDFF for the following variables: younger age at repair (fixed‐effects model: MD, −0.07 years; 95% CI, −0.11 to −0.02 years; P=0.004), older age at study (fixed‐effects model: MD, 0.33 years; 95% CI, 0.04–0.61 years; P=0.024), previous RV–pulmonary artery shunt (fixed‐effects model: OR, 0.35; 95% CI, 0.21–0.60; P<0.001), longer aortic cross‐clamp time (fixed‐effects model: MD, 6.91 minutes; 95% CI, 4.00–9.82 minutes; P<0.001), longer cardiopulmonary bypass time (fixed‐effects model: MD, 8.94 minutes; 95% CI, 4.17–13.71 minutes; P<0.001), outflow patch repair (fixed‐effects model: OR, 0.31; 95% CI, 0.13–0.72; P=0.006), higher RV ejection fraction (fixed‐effects model: MD, 3.91%; 95% CI, 3.65%–4.18%; P<0.001), higher RV end‐diastolic pressure (fixed‐effects model: MD, 0.97 mm Hg; 95% CI, 0.46–1.47 mm Hg; P=0.006), smaller left ventricular (LV) end‐diastolic volume indexed (fixed‐effects model: MD, −4.15 mL/m²; 95% CI, −4.86 to −3.44 mL/m²; P<0.001), smaller LV end‐systolic volume indexed (fixed‐effects model: MD, −2.97 mL/m²; 95% CI, −3.43 to −2.52 mL/m²; P<0.001), smaller LV stroke volume indexed (fixed‐effects model: MD, −1.65 mL/m²; 95% CI, −2.05 to −1.24 mL/m²; P<0.001), greater LV ejection fraction (fixed‐effects model: MD, 0.64%; 95% CI, 0.23%–0.85%; P<0.001), greater RA area indexed (fixed‐effects model: MD, 0.58 cm²/m²; 95% CI, 0.42–0.74 cm²/m²; P=0.028), smaller E‐wave deceleration at the tricuspid valve (fixed‐effects model: MD, −8.62 cm/s; 95% CI, −11.0 to −6.27 cm/s; P<0.001), greater A‐wave velocity at the tricuspid valve (fixed‐effects model: MD, 2.92 cm/s; 95% CI, 0.82–5.03 cm/s; P=0.007), smaller E/A (ratio between early (E) and late atrial (A) ventricular filling velocity) at the tricuspid valve (fixed‐effects model: MD, −0.09; 95% CI, −0.17 to −0.02; P=0.016), longer PR duration (fixed‐effects model: MD, 10.3 ms; 95% CI, 8.68–12.1 ms; P<0.001), shorter QRS duration (fixed‐effects model: MD, −2.90 ms; 95% CI, −4.26 to −1.54 ms; P<0.001), higher brain natriuretic peptide levels (fixed‐effects model: MD, 11.0 pg/mL; 95% CI, 6.53–15.5 pg/mL; P<0.001), and higher NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels (fixed‐effects model: MD, 61.1 pg/mL; 95% CI, 15.2–107 pg/mL; P=0.009). Because these findings were not confirmed by both models, these should be interpreted with caution.
Subgroup Analyses and Meta‐Regression Analyses
In an attempt to explain sources of heterogeneity and to further investigate the underlying mechanisms of EDFF in rToF, subgroup analyses and meta‐regression analyses were performed. The findings of these analyses are presented in Data S1.
DISCUSSION
Summary of Evidence
The current meta‐analysis summarizes the available evidence on associations of EDFF with patient characteristics, hemodynamic findings, and surgical properties in patients with rToF. Our findings, summarized in Figure 2, are as follows: (1) EDFF occurred in 46.5% of all patients, (2) the use of a transannular patch was significantly more common among patients with EDFF, (3) intensive care unit length of stay for these patients was longer, (4) EDFF was associated with greater RV indexed volumes and mass, as well as smaller E‐wave velocity at the tricuspid valve, and (5) PR fraction was greater, and moderate to severe PR was more common with EDFF. Overall, these results suggest that EDFF is associated with dilated, hypertrophied RVs experiencing longstanding PR. However, as no clear indicators of poor RV compliance were found, EDFF may have multiple causes and might not correspond precisely with RVRP.
Figure 2. Summary of the main findings about end‐diastolic forward flow (EDFF) in repaired tetralogy of Fallot (rToF) in the present meta‐analysis.
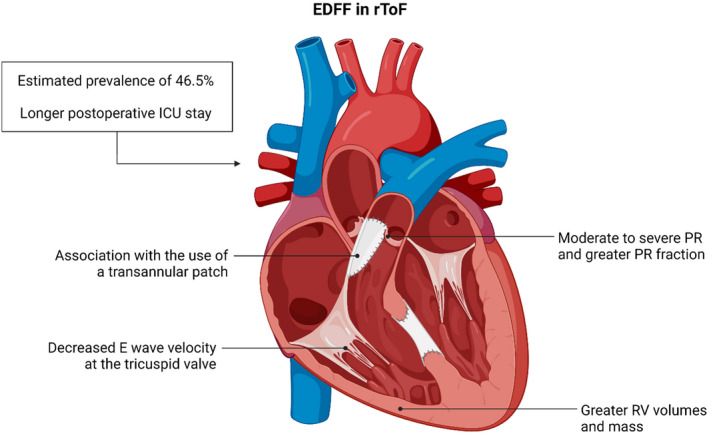
ICU indicates intensive care unit; PR, pulmonary regurgitation; and RV, right ventricular.
EDFF Is Not a Specific Marker of RVRP and May Occur Under Several Other Conditions
Ever since the initial reports on EDFF, 5 , 6 , 7 , 8 , 9 , 10 it has been regarded as a hallmark feature of RVRP. Indeed, studies conducted thereafter, which were included in the present meta‐analysis, defined RVRP solely based on the presence of EDFF. Strictly speaking, however, restrictive physiology implies poor ventricular compliance, or its reciprocal increased myocardial stiffness, which may be either a manifestation of primary cardiomyopathy or secondary to other cardiovascular diseases. 59 The gold standard measure of LV myocardial stiffness is the slope of the end‐diastolic pressure‐volume relationship, 60 but is less practical for the RV, given the trapezoidal nature of the normal RV pressure‐volume relationship. Furthermore, a prerequisite of pressure‐volume analysis is a closed system, meaning that the semilunar valve should be closed such that changes within the ventricle reflect muscle mechanics. As the right heart is a low‐pressure system, RA pressures can at times exceed pulmonary artery pressures, promoting transmission of RA outflow into the pulmonary arteries and thus opening the system. Nonetheless, when this antegrade diastolic pulmonary artery flow occurs, it suggests that the resistance to RV filling is greater than the resistance to pulmonary artery filling; this concept has been the rationale for using EDFF as a surrogate for RVRP. 61
EDFF is a convenient marker that is readily available from conventional Doppler echocardiography or CMR. However, there are several limitations to its value for diagnosis of RVRP, because other factors may modulate EDFF (Table 3). 62 For example, the absence of atrial systole and other conditions that decrease preload may attenuate EDFF. Conversely, increased pulmonary arterial bed capacitance decreases the resistance to pulmonary artery filling and might thereby increase or induce EDFF, even when RV compliance and filling pressures are normal. As shown in our meta‐analysis, the severity of PR and the use of the transannular patch during primary repair of ToF are both significantly associated with EDFF, possibly because of lower pulmonary diastolic pressure. With pressure gradients of only 1 to 2 mm Hg governing EDFF, it is highly susceptible to small changes in preload, pulmonary artery bed capacitance, and PR.
Table 3.
Framework to Think About Factors Influencing EDFF
| Factor | Main findings |
|---|---|
| Atrial contractility | |
| RV volumes |
|
| RV compliance and diastolic function |
|
| Myocardial perfusion |
|
| Ventricular‐ventricular interactions |
|
| Pulmonary regurgitation |
|
| Residual obstruction | |
| Pulmonary arterial bed capacitance and respiration |
|
ACE indicates angiotensin‐converting enzyme; EDFF, end‐diastolic forward flow; LA, left atrial; LV, left ventricular; PR, pulmonary regurgitation; RA, right atrial; RV, right ventricular; RVEDVi, RV end‐diastolic volume indexed; RVOT, RV outflow tract; and ToF, tetralogy of Fallot.
More important, this meta‐analysis found no significant associations of EDFF with typical markers of restrictive filling of the RV, including decreased tricuspid E‐wave deceleration, decreased early diastolic tricuspid annular velocity, increased E/A ratio, increased E/E' (ratio between early ventricular filling velocity (E) and annulus velocity during early filling (E')), or RA enlargement, based on random‐effects models (main analysis) and only limited effects based on fixed‐effects models (sensitivity analysis). This is in accordance with findings by DiLorenzo et al, 63 who found that invasive evaluation of diastolic function with catheter‐based RV end‐diastolic pressure did not correlate with EDFF or any other echocardiographic parameters of diastolic function in patients with ToF. Similarly, Mori et al 16 reported that EDFF was inconsistently associated with RVRP, noting its presence in some patients with low pulmonary diastolic pressure (attributable to severe PR) and normal RA pressure. In fact, our meta‐analysis revealed a lower early (E) inflow velocity through the tricuspid valve in patients with EDFF, in contrast to increased E in the conventional restrictive pattern. This finding could well be a manifestation of the Bernoulli principle, where transtricuspid velocities drop secondary to widening of the tricuspid annulus. However, Sjöberg et al 55 suggested that these decreased velocities might contribute to the lower diastolic kinetic energy observed on 4‐dimensional flow CMR in patients with EDFF. As kinetic energy reflects ventricular performance, it might be a potential early marker of ventricular dysfunction. In summary, clinicians are encouraged to look beyond EDFF to determine if their patients have RV diastolic dysfunction.
A Unifying Theory About the Physiological and Clinical Correlates of EDFF
To reconcile the conflicting results in the literature, the observation of Lee et al, 39 revealing that EDFF most commonly occurs at the ends of the RVEDVi spectrum (at ≤115 and ≥200 mL/m²), is key. Consider that there may be 2 main phenotypes of ToF in which EDFF is observed (Table 4). Representative pressure‐volume curves for each of these phenotypes are presented in Figure 3. The first, which we refer to as early‐onset, “primary” EDFF, matches the original cohorts described by Cullen et al 5 and Gatzoulis et al. 6 This phenotype more closely resembles a “true” RVRP, and occurs in association with small RVs with abnormal diastolic filling. 34 EDFF in these patients has its onset in the period around primary ToF repair. Cardiopulmonary bypass, myocardial edema, ventriculotomy, endomyocardial fibrosis, and the insertion of nonfunctional patches in the ventricular septum and across the right ventricular outflow tract might all be expected to impair RV diastolic performance. 8 Although increased central venous pressure and low cardiac output lead to longer intensive care unit length of stay in these patients, RVRP is eventually beneficial as it prevents further progression of PR, thereby improving exercise tolerance and reducing the risk of adverse outcomes. 6 , 7 , 8 Early‐onset EDFF usually disappears days to months after the primary repair, although it may be maintained into midterm follow‐up in a subset of patients. 5 , 7
Table 4.
Unifying Theory About Physiological and Clinical Correlates of EDFF
| Phenotype 1: early‐onset, “primary” EDFF | Phenotype 2: late‐onset, “secondary” EDFF |
|---|---|
| Physiological correlates | |
| Small RVs with abnormal diastolic filling following directly after primary repair of ToF and probably related to fibrosis, myocardial injury, and other perioperative factors | Dilated RVs at late follow‐up after primary repair of ToF, or may occur as a late stage of phenotype 1 |
| Preventing further progression of PR and limiting the extent of volume overload | Pronounced volume overload attributable to longstanding PR, whereby filling of the RV becomes limited and RV pressure becomes larger than pulmonary artery pressure |
| Usually disappears days to months after the primary repair, but may be maintained into midterm follow‐up in a subset of patients | Usually is maintained during long‐term follow‐up but may disappear after PVR |
| Associated with repair at older age as seen in the initial era of development of ToF repair | Associated with repair at younger age as seen in more contemporary management |
| Corresponds closest to actual RVRP | Only a subset of patients might have actual RVRP |
| Clinical correlates | |
| Longer ICU length of stay attributable to increased central venous pressure and low cardiac output state | Independent predictor of rapid RV enlargement |
| Improved exercise tolerance (higher peak VO2) because of improved oxygenation, as EDFF contributes to forward flow and shortens duration of PR | Related to functional deterioration and worse exercise tolerance |
| Lower risk of arrhythmias and sudden death | Associated with increased risk of adverse outcomes, such as ventricular dysfunction and arrhythmias; persistent EDFF after PVR indicates worse prognosis |
EDFF indicates end‐diastolic forward flow; ICU, intensive care unit; PR, pulmonary regurgitation; PVR, pulmonary valve replacement; RV, right ventricular; RVRP, RV restrictive physiology; ToF, tetralogy of Fallot; and VO2, oxygen consumption.
Figure 3. Representative pressure‐volume curves for the different phenotypes of end‐diastolic forward flow (EDFF).
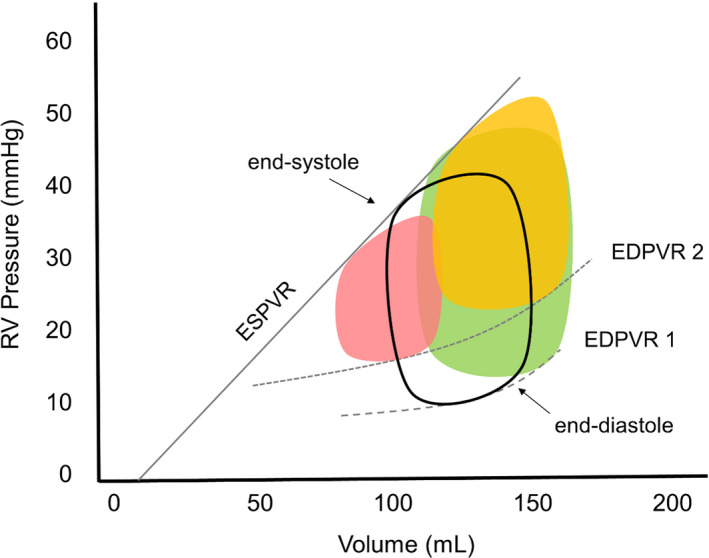
The pressure‐volume curve of the normal right ventricle (RV), which is characterized by its trapezoidal shape, is depicted in the middle (black contours). The early‐onset, “primary” type of EDFF is associated with a small, restrictive RV (red shape on the left) with decreased myocardial compliance (end‐diastolic pressure‐volume relationship [EDPVR] 2 is shifted upward compared with EDPVR 1). In contrast, the late‐onset, “secondary” type of EDFF presents as a dilated RV with a rightward shift of the pressure‐volume relationship, either without (green shape on the right at EDPVR 1) or with marked myocardial stiffening (yellow shape on the right at EDPVR 2). ESPVR indicates end‐systolic pressure‐volume relationship.
The first phenotype was more commonly observed in earlier ToF cohorts, when patients were operated at a later age and perioperative ventricular dysfunction was common. 44 Improvements in surgical techniques and myocardial preservation have led to improved diastolic function in the early and midterm period after repair, but might also have promoted a higher prevalence of a second phenotype. 44 Late‐onset, “secondary” EDFF is a consequence of an overdistended ventricle and rightward shift of the pressure‐volume curve. 16 , 39 The lack of RVRP in early follow‐up allows for continuing RV remodeling and enlargement in the presence of longstanding PR. The severely dilated RV eventually becomes stiff or encounters space constraints attributable to the pericardium and the capacity of the thoracic cavity. In this setting, EDFF occurs without restricted RV filling or decreased RV volume. 12 This dilatation‐related phenotype has been linked to severe PR, 16 fibrosis, 45 accelerated RV enlargement, 54 and increased risk of adverse outcomes. 56 Corroborating these observations, Lee et al 39 demonstrated that EDFF was associated with improved exercise tolerance (peak oxygen consumption) in patients with RVEDVi <170 mL/m², but not in those with RVEDVi ≥170 mL/m².
Perspectives for Future Research and Clinical Practice
EDFF was invariably treated as a binary feature in all studies. However, it is possible that characteristics, such as EDFF duration, mean and peak velocity, velocity time integral, and percentage of contribution to the stroke volume, may have their own implications. Although a few studies have reported such characteristics, 8 , 30 , 34 , 37 , 45 , 48 , 57 it will be a task for future investigations to determine how they correlate with patient characteristics, cardiac morphology and function, and outcomes. Having said that, it is clear that EDFF is an imperfect surrogate for poor RV compliance, so future studies should aim to identify more reliable markers for RVRP. Multiple parameters may be required, including tricuspid inflow characteristics, tricuspid valve annulus, hepatic veins, right atrial size, and collapsibility of the interior vena cava. 64 In addition, more investigations using invasive measurements of filling pressures are warranted to validate findings from noninvasive modalities. Of interest, recent advances have made it possible to measure RV pressure‐volume loops more routinely in clinical and research settings, as described in an outstanding recent review by Brener et al. 65
More research is required to further elucidate how EDFF and different hemodynamics relate to prognosis and anticipated clinical needs. Machine learning techniques could be harnessed to identify phenotypical clusters among patients with EDFF. In addition, the relevance of EDFF for risk stratification for common procedures in rToF, such as placement of implantable cardioverter‐defibrillator and pulmonary valve replacement, should be investigated. 66 , 67 As an example of the latter, Tominaga et al 56 showed that EDFF may disappear after pulmonary valve replacement but signals worse prognosis when it persists. It might be important to interpret this in conjunction with RV size, as patients with smaller RVs (<170 mL/m²) have not consistently shown an effect of persistent EDFF on the risk of arrhythmias. 68 Current surgical practices with more valve‐sparing operations and fewer transannular patches for ToF are likely already influencing the context in which EDFF is observed, so research into the implications of EDFF may differ from the historical baselines established in this analysis. 69
Limitations and Sources of Heterogeneity
Our meta‐analysis was limited to univariate analyses. Residual confounding by year of publication or enrollment, age at initial repair, timing of assessment or pulmonary valve replacement relative to initial repair, as well as anatomical and functional characteristics cannot be excluded. More important, patients from older cohorts underwent initial repair with different techniques and perioperative management compared with contemporary practice. Although subgroup analyses of all investigated factors comparing studies with large RVEDVi versus those with low RVEDVi might have corroborated our framework including the 2 phenotypes, these data were not consistently reported in a sufficient number of studies to perform such analyses. Meta‐regression analyses were conducted instead, but these were likewise limited by modest power. Similarly, subgroup analyses based on the timing of initial repair and subsequent interventions could further enhance our understanding of EDFF and may be the subject of future clinical investigations. Furthermore, it should be considered that our analyses were not corrected for multiple testing given the exploratory nature of our study, such that our estimates might need to be validated in future studies. Finally, the technical limitations of echocardiography and CMR to identify EDFF might have affected our findings. In this regard, 2 of the studies that primarily defined EDFF based on CMR ascertained their results based on Doppler echocardiography. Sani et al 52 found a comparable prevalence of EDFF with both echocardiography (56.7%) and CMR (60.0%; P=0.792). In contrast, Lee et al 39 found that CMR identified a higher prevalence of EDFF (64.4%) compared with Doppler echocardiography (44.4%; P=0.039), with only 58.6% of the CMR cases being confirmed on Doppler echocardiography. Furthermore, they found that Doppler‐based EDFF correlated less well with peak oxygen consumption percentage (r=0.381; P=0.026) than did CMR‐based EDFF (r=0.536; P=0.001). Kutty et al 37 found a modest correlation between both modalities (Fleiss’ κ=0.597). The finding of our subgroup analysis that overall there was only a marginally higher EDFF prevalence with CMR compared with Doppler echocardiography (50.8% versus 45.7%; P=0.332) is reassuring, although future investigations directly comparing both modalities will likely advance our understanding.
CONCLUSIONS
In this meta‐analysis, EDFF occurred in 46.5% of patients with rToF and is associated with the use of a transannular patch, longer intensive care unit length of stay, greater RV indexed volumes and mass, smaller E‐wave velocity at the tricuspid valve, and greater PR. EDFF is not specific of RVRP and has multiple alternative causes. Our review supports a specific reconciliation of the conflicting EDFF literature, based on the presence of 2 main phenotypes: (1) early‐onset, “primary” EDFF and (2) late‐onset, “secondary” EDFF. The latter has become more prevalent in contemporary practice, with improved perioperative ventricular diastolic function but progressive dilatation resulting from longstanding PR. Future studies should refine the diagnostic criteria for RVRP and clarify the potential prognostic relevance of EDFF in various settings.
Sources of Funding
None.
Disclosures
Budts is proctor for Abbott and Occlutech. Kutty is consultant for GE Healthcare. Van den Eynde was supported by the Belgian American Educational Foundation. The remaining authors have no disclosures to report.
Supporting information
Data S1
Figures S1–S15
Acknowledgments
Author Contributions: Van den Eynde: concept/design, data collection, data interpretation, drafting article, critical revision of article, and approval of article; Derdeyn: concept/design, data collection, data interpretation, drafting article, critical revision of article, and approval of article; Schuermans: concept/design, data interpretation, drafting article, critical revision of article, and approval of article; Shivaram: data interpretation, critical revision of article, and approval of article; Budts: data interpretation, critical revision of article, and approval of article; Danford: data interpretation, critical revision of article, and approval of article; Kutty: data interpretation, critical revision of article, and approval of article.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024036
For Sources of Funding and Disclosures, see page 13.
References
- 1. van der Linde D, Konings EEM, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJM, Roos‐Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta‐analysis. J Am Coll Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025 [DOI] [PubMed] [Google Scholar]
- 2. Apitz C, Webb GD, Redington AN. Tetralogy of Fallot. Lancet. 2009;374:1462–1471. doi: 10.1016/S0140-6736(09)60657-7 [DOI] [PubMed] [Google Scholar]
- 3. Carvalho JS, Shinebourne EA, Busst C, Rigby ML, Redington AN. Exercise capacity after complete repair of tetralogy of Fallot: deleterious effects of residual pulmonary regurgitation. Heart. 1992;67:470–473. doi: 10.1136/hrt.67.6.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dennis M, Moore B, Kotchetkova I, Pressley L, Cordina R, Celermajer DS. Adults with repaired tetralogy: low mortality but high morbidity up to middle age. Open Heart. 2017;4:e000564. doi: 10.1136/openhrt-2016-000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cullen S, Shore D, Redington A. Characterization of right ventricular diastolic performance after complete repair of tetralogy of Fallot. Circulation. 1995;91:1782–1789. doi: 10.1161/01.CIR.91.6.1782 [DOI] [PubMed] [Google Scholar]
- 6. Gatzoulis MA, Clark AL, Cullen S, Newman CGH, Redington AN. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot: restrictive physiology predicts superior exercise performance. Circulation. 1995;91:1775–1781. doi: 10.1161/01.CIR.91.6.1775 [DOI] [PubMed] [Google Scholar]
- 7. Norgård G, Gatzoulis MA, Josen M, Cullen S, Redington AN. Does restrictive right ventricular physiology in the early postoperative period predict subsequent right ventricular restriction after repair of tetralogy of Fallot? Heart. 1998;79:481–484. doi: 10.1136/hrt.79.5.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eroglu AG, Sarioglu A, Sarioglu T. Right ventricular diastolic function after repair of tetralogy of Fallot: its relationship to the insertion of a “transannular” patch. Cardiol Young. 1999;9:384–391. doi: 10.1017/S1047951100005187 [DOI] [PubMed] [Google Scholar]
- 9. Gibbs JL, Wilson N, Witsenburg M, Williams GJ, Goldberg SJ. Diastolic forward blood flow in the pulmonary artery detected by doppler echocardiography. J Am Coll Cardiol. 1985;6:1322–1328. doi: 10.1016/S0735-1097(85)80220-5 [DOI] [PubMed] [Google Scholar]
- 10. Redington AN, Penny D, Rigby ML, Hayes A. Antegrade diastolic pulmonary arterial flow as a marker of right ventricular restriction after complete repair of pulmonary atresia with intact septum and critical pulmonary valvar stenosis. Cardiol Young. 1992;2:382–386. doi: 10.1017/S1047951100007988 [DOI] [Google Scholar]
- 11. Helbing WA, Niezen RA, Le Cessie S, van der Geest RJ, Ottenkamp J, de Roos A. Right ventricular diastolic function in children with pulmonary regurgitation after repair of tetralogy of Fallot: volumetric evaluation by magnetic resonance velocity mapping. J Am Coll Cardiol. 1996;28:1827–1835. doi: 10.1016/S0735-1097(96)00387-7 [DOI] [PubMed] [Google Scholar]
- 12. Lu JC, Cotts TB, Agarwal PP, Attili AK, Dorfman AL. Relation of right ventricular dilation, age of repair, and restrictive right ventricular physiology with patient‐reported quality of life in adolescents and adults with repaired tetralogy of fallot. Am J Cardiol. 2010;106:1798–1802. doi: 10.1016/j.amjcard.2010.08.021 [DOI] [PubMed] [Google Scholar]
- 13. Samyn MM, Kwon EN, Gorentz JS, Yan K, Danduran MJ, Cava JR, Simpson PM, Frommelt PC, Tweddell JS. Restrictive versus nonrestrictive physiology following repair of tetralogy of Fallot: is there a difference? J Am Soc Echocardiogr. 2013;26:746–755. doi: 10.1016/j.echo.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 14. Van den Berg J, Wielopolski PA, Meijboom FJ, Witsenburg M, Bogers AJJC, Pattynama PMT, Helbing WA. Diastolic function in repaired tetralogy of fallot at rest and during stress: assessment with MR imaging. Radiology. 2007;243:212–219. doi: 10.1148/radiol.2431060213 [DOI] [PubMed] [Google Scholar]
- 15. Ahmad N, Kantor PF, Grosse‐Wortmann L, Seller N, Jaeggi ET, Friedberg MK, Mertens L. Influence of RV restrictive physiology on LV diastolic function in children after tetralogy of Fallot repair. J Am Soc Echocardiogr. 2012;25:866–873. doi: 10.1016/j.echo.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 16. Mori Y, Murakami T, Inoue N, Kaneko S, Nakashima Y, Koide M. Is the presence of end‐diastolic forward flow specific for restrictive right ventricular physiology in repaired tetralogy of Fallot? Int J Cardiol. 2017;240:187–193. doi: 10.1016/j.ijcard.2017.04.021 [DOI] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. J Am Med Assoc. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 22. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aburawi EH, Munkhammar P, Carlsson M, El‐Sadig M, Pesonen E. Coronary flow dynamics in children after repair of tetralogy of Fallot. Int J Cardiol. 2014;172:122–126. doi: 10.1016/j.ijcard.2013.12.188 [DOI] [PubMed] [Google Scholar]
- 25. Apitz C, Latus H, Binder W, Uebing A, Seeger A, Bretschneider C, Sieverding L, Hofbeck M. Impact of restrictive physiology on intrinsic diastolic right ventricular function and lusitropy in children and adolescents after repair of tetralogy of Fallot. Heart. 2010;96:1837–1841. doi: 10.1136/hrt.2010.203190 [DOI] [PubMed] [Google Scholar]
- 26. Babu‐Narayan SV, Uebing A, Davlouros PA, Kemp M, Davidson S, Dimopoulos K, Bayne S, Pennell DJ, Gibson DG, Flather M, et al. Randomised trial of ramipril in repaired tetralogy of Fallot and pulmonary regurgitation: the APPROPRIATE study (Ace inhibitors for Potential PRevention of the deleterious effects of Pulmonary Regurgitation in Adults with repaired TEtralogy of Fallot). Int J Cardiol. 2012;154:299–305. doi: 10.1016/j.ijcard.2010.09.057 [DOI] [PubMed] [Google Scholar]
- 27. Bonello B, Kempny A, Uebing A, Li W, Kilner PJ, Diller G‐P, Pennell DJ, Shore DF, Ernst S, Gatzoulis MA, et al. Right atrial area and right ventricular outflow tract akinetic length predict sustained tachyarrhythmia in repaired tetralogy of Fallot. Int J Cardiol. 2013;168:3280–3286. doi: 10.1016/j.ijcard.2013.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cardoso SM, Miyague NI. Right ventricular diastolic dysfunction in the postoperative period of tetralogy of Fallot. Arq Bras Cardiol. 2003;80:194–197, 198–201. doi: 10.1590/s0066-782x2003000200008 [DOI] [PubMed] [Google Scholar]
- 29. Chaturvedi RR, Shore DF, Lincoln C, Mumby S, Kemp M, Brierly J, Petros A, Gutteridge JMG, Hooper J, Redington AN. Association with myocardial injury and oxidative stress. Circulation. 1999;100:1540–1547. doi: 10.1155/2020/5732956 [DOI] [PubMed] [Google Scholar]
- 30. Cheng AL, Kaslow AM, Pruetz JD, Lu JC, Wood JC, Detterich JA. Differences in right ventricular physiologic response to chronic volume load in patients with repaired pulmonary atresia intact ventricular septum/critical pulmonary stenosis versus tetralogy of Fallot. Pediatr Cardiol. 2019;40:526–536. doi: 10.1007/s00246-018-2009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheung MMH, Davis AM, Wilkinson JL, Weintraub RG. Evidence for restoration of genetic growth potential. Congenit Heart Dis. 2003;5500:1340–1343. doi: 10.1136/heart.89.11.1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi JY, Kwon HS, Yoo BW, Shin JIl, Sul JH, Park HK, Park YH. Right ventricular restrictive physiology in repaired tetralogy of Fallot is associated with smaller respiratory variability. Int J Cardiol. 2008;125:28–35. doi: 10.1016/j.ijcard.2007.02.020 [DOI] [PubMed] [Google Scholar]
- 33. Clark AL, Gatzoulis MA, Redington AN. Ventilatory responses to exercise in adults after repair of tetralogy of Fallot. Br Heart J. 1995;73:445–449. doi: 10.1136/hrt.73.5.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gatzoulis MA, Norgård G, Redington AN. Biventricular long axis function after repair of tetralogy of Fallot. Pediatr Cardiol. 1998;19:128–132. doi: 10.1007/s002469900260 [DOI] [PubMed] [Google Scholar]
- 35. Kordybach‐Prokopiuk M, Dobrowolski P, Kowalski M, Hoffman P. Differences in regional diastolic function between restrictive and normal right ventricular physiology in adult patients late after tetralogy of Fallot repair. Kardiol Pol. 2018;76:1458–1464. doi: 10.5603/KP.a2018.0145 [DOI] [PubMed] [Google Scholar]
- 36. Krupickova S, Li W, Cheang MH, Rigby ML, Uebing A, Davlouros P, Dimopoulos K, Di Salvo G, Fraisse A, Swan L, et al. Ramipril and left ventricular diastolic function in stable patients with pulmonary regurgitation after repair of tetralogy of Fallot. Int J Cardiol. 2018;272:64–69. doi: 10.1016/j.ijcard.2018.07.132 [DOI] [PubMed] [Google Scholar]
- 37. Kutty S, Valente AM, White MT, Hickey K, Danford DA, Powell AJ, Geva T. Usefulness of pulmonary arterial end‐diastolic forward flow late after tetralogy of Fallot repair to predict a “restrictive” right ventricle. Am J Cardiol. 2018;121:1380–1386. doi: 10.1016/j.amjcard.2018.02.025 [DOI] [PubMed] [Google Scholar]
- 38. Latus H, Gummel K, Rupp S, Valeske K, Akintuerk H, Jux C, Bauer J, Schranz D, Apitz C. Beneficial effects of residual right ventricular outflow tract obstruction on right ventricular volume and function in patients after repair of tetralogy of Fallot. Pediatr Cardiol. 2013;34:424–430. doi: 10.1007/s00246-012-0476-4 [DOI] [PubMed] [Google Scholar]
- 39. Lee W, Yoo SJ, Roche SL, Kantor P, Van Arsdell G, Park EA, Redington A, Grosse‐Wortmann L. Determinants and functional impact of restrictive physiology after repair of tetralogy of Fallot: new insights from magnetic resonance imaging. Int J Cardiol. 2013;167:1347–1353. doi: 10.1016/j.ijcard.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 40. Luijnenburg SE, Peters RE, van der Geest RJ, Moelker A, Roos‐Hesselink JW, de Rijke YB, Mulder BJM, Vliegen HW, Helbing WA. Abnormal right atrial and right ventricular diastolic function relate to impaired clinical condition in patients operated for tetralogy of Fallot. Int J Cardiol. 2013;167:833–839. doi: 10.1016/j.ijcard.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 41. Maskatia SA, Spinner JA, Morris SA, Petit CJ, Krishnamurthy R, Nutting AC. Effect of branch pulmonary artery stenosis on right ventricular volume overload in patients with tetralogy of fallot after initial surgical repair. Am J Cardiol. 2013;111:1355–1360. doi: 10.1016/j.amjcard.2013.01.278 [DOI] [PubMed] [Google Scholar]
- 42. Maskatia SA, Morris SA, Spinner JA, Krishnamurthy R, Altman CA. Echocardiographic parameters of right ventricular diastolic function in repaired tetralogy of Fallot are associated with important findings on magnetic resonance imaging. Congenit Heart Dis. 2015;10:E113–E122. doi: 10.1111/chd.12265 [DOI] [PubMed] [Google Scholar]
- 43. Mercer‐Rosa L, Fogel MA, Paridon SM, Rychik J, Yang W, Goldmuntz E. Revisiting the end‐diastolic forward flow (restrictive physiology) in tetralogy of Fallot: an exercise, echocardiographic, and magnetic resonance study. JACC Cardiovasc Imaging. 2018;11:1547–1548. [DOI] [PubMed] [Google Scholar]
- 44. Munkhammar P, Cullen S, Jögi P, de Leval M, Elliott M, Norgård G. Early age at repair prevents restrictive right ventricular (RV) physiology after surgery for tetralogy of Fallot (TOF): diastolic RV function after TOF repair in infancy. J Am Coll Cardiol. 1998;32:1083–1087. doi: 10.1016/S0735-1097(98)00351-9 [DOI] [PubMed] [Google Scholar]
- 45. Munkhammar P, Carlsson M, Arheden H, Pesonen E. Restrictive right ventricular physiology after tetralogy of Fallot repair is associated with fibrosis of the right ventricular outflow tract visualized on cardiac magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2013;14:978–985. doi: 10.1093/ehjci/jet009 [DOI] [PubMed] [Google Scholar]
- 46. Norgård G, Gatzoulis MA, Moraes F, Lincoln C, Shore DF, Shinebourne EA, Redington AN. Relationship between type of outflow tract repair and postoperative right ventricular diastolic physiology in tetralogy of Fallot. Circulation. 1996;94:3276–3280. doi: 10.1161/01.CIR.94.12.3276 [DOI] [PubMed] [Google Scholar]
- 47. Peng EWK, Spooner R, Young D, Danton MHD. Acute B‐type natriuretic peptide response and early postoperative right ventricular physiology following tetralogy of Fallot’s repair. Interact Cardiovasc Thorac Surg. 2012;15:335–339. doi: 10.1093/icvts/ivs150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pijuan‐Domenech A, Pineda V, Castro MA, Sureda‐Barbosa C, Ribera A, Cruz LM, Ferreira‐Gonzalez I, Dos‐Subirà L, Subirana‐Domènech T, Garcia‐Dorado D, et al. “Pulmonary valve replacement diminishes the presence of restrictive physiology and reduces atrial volumes”: a prospective study in tetralogy of Fallot patients. Int J Cardiol. 2014;177:261–265. doi: 10.1016/j.ijcard.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 49. Rathore KS, Agrawal SK, Kapoor A. Restrictive physiology in tetralogy of Fallot: exercise and arrhythmogenesis. Asian Cardiovasc Thorac Ann. 2006;14:279–283. doi: 10.1177/021849230601400403 [DOI] [PubMed] [Google Scholar]
- 50. Sachdev MS, Bhagyavathy A, Varghese R, Coelho R, Kumar RS. Right ventricular diastolic function after repair of tetralogy of fallot. Pediatr Cardiol. 2006;27:250–255. doi: 10.1007/s00246-005-1186-y [DOI] [PubMed] [Google Scholar]
- 51. Sandeep B, Huang X, Xu F, Su P, Wang T, Sun X. Etiology of right ventricular restrictive physiology early after repair of tetralogy of Fallot in pediatric patients. J Cardiothorac Surg. 2019;14:84. doi: 10.1186/s13019-019-0909-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sani ZA, Samiei N, Khajali Z, Mirrazeghi F, Gholamipoor D, Rouzitalab M, Bayat M, Behjati M, Ghadrdoost B, Rahimi S. Assessment of right ventricular myocardial fibrosis and restrictive physiology in patients with repaired tetralogy of fallot: a comparison between cardiac magnetic resonance and transthoracic echocardiography. Iran Heart J. 2020;21:87–97. [Google Scholar]
- 53. Shekerdemian LS, Bush A, Shore DF, Lincoln C, Redington AN. Cardiorespiratory responses to negative pressure ventilation after tetralogy of fallot repair: a hemodynamic tool for patients with a low‐output state. J Am Coll Cardiol. 1999;33:549–555. doi: 10.1016/S0735-1097(98)00598-1 [DOI] [PubMed] [Google Scholar]
- 54. Shin YR, Jung JW, Kim NK, Choi JY, Kim YJ, Shin HJ, Park YH, Park HK. Factors associated with progression of right ventricular enlargement and dysfunction after repair of tetralogy of Fallot based on serial cardiac magnetic resonance imaging. Eur J Cardiothoracic Surg. 2016;50:464–469. doi: 10.1093/ejcts/ezw049 [DOI] [PubMed] [Google Scholar]
- 55. Sjöberg P, Bidhult S, Bock J, Heiberg E, Arheden H, Gustafsson R, Nozohoor S, Carlsson M. Disturbed left and right ventricular kinetic energy in patients with repaired tetralogy of Fallot: pathophysiological insights using 4D‐flow MRI. Eur Radiol. 2018;28:4066–4076. doi: 10.1007/s00330-018-5385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tominaga Y, Taira M, Kido T, Kanaya T, Araki K, Watanabe T, Sakaniwa R, Toda K, Kuratani T, Ueno T, et al. Persistent end‐diastolic forward flow after pulmonary valve replacement in patients with repaired tetralogy of Fallot. Eur J Cardiothorac Surg. 2021;60:516–523. doi: 10.1093/ejcts/ezab098 [DOI] [PubMed] [Google Scholar]
- 57. Vukomanović V, Stajević M, Jovanović I, Kosutić J, Sehić I, Milovanović V. Echocardiographic analysis of the subtypes of right ventricular restrictive physiology in surgically treated patients with tetralogy of Fallot. Cardiol Young. 2006;16:549–555. doi: 10.1017/S104795110600120X [DOI] [PubMed] [Google Scholar]
- 58. Xu Z, Zhang M, Zhu L, Gong X, Li J. Elevated plasma B‐type natriuretic peptide and C‐reactive protein levels in children with restrictive right ventricular physiology following tetralogy of Fallot repair. Congenit Heart Dis. 2014;9:521–528. doi: 10.1111/chd.12166 [DOI] [PubMed] [Google Scholar]
- 59. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576 [DOI] [PubMed] [Google Scholar]
- 60. Bastos MB, Burkhoff D, Maly J, Daemen J, den Uil CA, Ameloot K, Lenzen M, Mahfoud F, Zijlstra F, Schreuder JJ, et al. Invasive left ventricle pressure–volume analysis: overview and practical clinical implications. Eur Heart J. 2020;41:1286–1297. doi: 10.1093/eurheartj/ehz552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Redington AN, Rigby ML, Hayes A, Penny D. Right ventricular diastolic function in children. Am J Cardiol. 1991;67:329. doi: 10.1016/0002-9149(91)90591-8 [DOI] [PubMed] [Google Scholar]
- 62. Bove T, Vandekerckhove K, Bouchez S, Wouters P, Somers P, Van Nooten G. Role of myocardial hypertrophy on acute and chronic right ventricular performance in relation to chronic volume overload in a porcine model: relevance for the surgical management of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2014;147:1956–1965. doi: 10.1016/j.jtcvs.2013.10.026 [DOI] [PubMed] [Google Scholar]
- 63. DiLorenzo M, Hwang W‐T, Goldmuntz E, Ky B, Mercer‐Rosa L. Diastolic dysfunction in tetralogy of Fallot: comparison of echocardiography with catheterization. Echocardiography. 2018;35:1641–1648. doi: 10.1111/echo.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. DiLorenzo MP, Bhatt SM, Mercer‐Rosa L. How best to assess right ventricular function by echocardiography. Cardiol Young. 2015;25:1473. doi: 10.1017/S1047951115002255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brener MI, Masoumi A, Ng VG, Tello K, Bastos MB, Cornwell WK, Hsu S, Tedford RJ, Lurz P, Rommel K‐P, et al. Invasive right ventricular pressure‐volume analysis: basic principles, clinical applications, and practical recommendations. Circ Heart Fail. 2022;15:e009101. doi: 10.1161/CIRCHEARTFAILURE.121.009101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van den Eynde J, Sá MPBO, Vervoort D, Roever L, Meyns B, Budts W, Gewillig M, Ruhparwar A, Zhigalov K, Weymann A. Pulmonary valve replacement in tetralogy of Fallot: an updated meta‐analysis. Ann Thorac Surg. 2020:S0003‐4975(20)32173‐1. doi: 10.1016/j.athoracsur.2020.11.040 [DOI] [PubMed] [Google Scholar]
- 67. Van den Eynde J, Callahan CP, Lo Rito M, Hussein N, Carvajal H, Guariento A, Ruhparwar A, Weymann A, Budts W, Gewillig M, et al. Tricuspid valve intervention at the time of pulmonary valve replacement in adults with congenital heart disease: a systematic review and meta‐analysis. J Am Heart Assoc. 2021;10:22909. doi: 10.1161/JAHA.121.022909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Van den Eynde J, Derdeyn E, Danford D, Kutty S. Clinical trajectory and the interpretation of end‐diastolic forward flow in tetralogy of Fallot. Eur J Cardiothoracic Surg. 2021;60:1241. doi: 10.1093/EJCTS/EZAB283 [DOI] [PubMed] [Google Scholar]
- 69. Blais S, Marelli A, Vanasse A, Dahdah N, Dancea A, Drolet C, Dallaire F. Comparison of long‐term outcomes of valve‐sparing and transannular patch procedures for correction of tetralogy of Fallot. JAMA Netw Open. 2021;4:e2118141. doi: 10.1001/jamanetworkopen.2021.18141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Figures S1–S15


