Abstract
Background
Chronic vasodilator therapy with long‐acting nitrate is frequently used to treat vasospastic angina. However, the clinical benefits of this approach are controversial. We investigated the prognostic impact of vasodilator therapy in patients with vasospastic angina from the multicenter, prospective VA‐KOREA (Vasospastic Angina in KOREA) registry.
Methods and Results
We analyzed data from 1895 patients with positive intracoronary ergonovine provocation test results. The patients were divided into 4 groups: no vasodilator (n=359), nonnitrate vasodilator (n=1187), conventional nitrate (n=209), and a combination of conventional nitrate and other vasodilators (n=140). The primary end point was a composite of cardiac death, acute coronary syndrome, and new‐onset arrhythmia at 2 years. Secondary end points were the individual components of the primary end point, all‐cause death, and rehospitalization due to recurrent angina.
The groups did not differ in terms of the risk of the primary end point. However, the acute coronary syndrome risk was significantly higher in the conventional nitrate (hazard ratio [HR], 2.49; 95% CI, 1.01–6.14; P=0.047) and combination groups (HR, 3.34; 95% CI, 1.15–9.75, P=0.027) compared with the no‐vasodilator group, as assessed using the inverse probability of treatment weights. Subgroup analyses revealed prominent adverse effects of nitrate in patients with an intermediate positive ergonovine provocation test result and in those with low Japanese Coronary Spasm Association scores.
Conclusions
Long‐acting nitrate‐based chronic vasodilator therapy was associated with an increased 2‐year risk of acute coronary syndrome in patients with vasospastic angina, especially in low‐risk patients.
Keywords: nitrates, outcomes, variant angina pectoris, vasodilator agents
Subject Categories: Cardiovascular Disease, Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- CAG
coronary angiography
- ERGT
ergonovine provocation test
- VSA
vasospastic angina
Clinical Perspective
What Is New?
Chronic long‐acting nitrate‐containing regimen in vasospastic angina was associated with higher risk of acute coronary syndrome in the analysis of the prospective multicenter VA‐KOREA (Vasospastic Angina in Korea) registry.
Association between higher risk of acute coronary syndrome and chronic long‐acting nitrate‐containing regimen was observed only in a low‐risk group with intermediate ergonovine provocation tes or low Japanese Coronary Spasm Association score.
What Are the Clinical Implications?
Caution is required when prescribing chronic long‐acting nitrate for patients with vasospastic angina, especially in low‐risk groups with intermediate ergonovine provocation test or low Japanese Coronary Spasm Association score.
Vasospastic angina (VSA) is distinct from classical atherosclerotic angina pectoris in terms of etiology, treatment, and prognosis. 1 , 2 , 3 VSA is caused by focal or diffuse spasms of an epicardial coronary artery. 4 Established pathogeneses include (1) vascular smooth muscle hyperreactivity, 5 (2) impairment of the autonomic nervous system, 6 and (3) microvascular dysfunction. 7 VSA generally has a favorable long‐term prognosis because coronary artery vasospasm responds well to vasodilator therapy. 8 However, VSA can cause fatal ventricular arrhythmia and sudden cardiac death. 9 , 10 , 11 The mainstays of VSA treatment for the prevention of coronary vasospasm are calcium channel blockers (CCBs), with or without vasodilators. 12 , 13 , 14 However, some recent studies have raised questions regarding the clinical benefits of long‐acting nitrates when used as vasodilators. 15 , 16 In a sample drawn from a Japanese multicenter registry, chronic nitrate therapy did not improve the long‐term prognosis of patients with VSA when combined with CCBs. Furthermore, the application of multiple nitrates increased the risk of adverse cardiac events. 15 Data from a single‐center Korean registry also indicated that chronic nitrate therapy has a harmful effect in patients with VSA. 16 However, nicorandil, which is a nonnitrate vasodilator, had a neutral effect on patients with VSA in the 2 aforementioned studies. As the clinical risks and benefits of chronic vasodilator therapy with long‐acting nitrates have not been fully evaluated in patients with VSA, current guidelines recommend the use of long‐acting nitrates. 13 , 14
In this study, we investigated the prognostic impact of chronic vasodilator therapy in patients with VSA using data from the multicenter, prospective VA‐KOREA (Vasospastic Angina in KOREA) registry.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Protocols
The VA‐KOREA (Vasospastic Angina in Korea) is a prospective, observational, web‐based registry of clinical, angiographic, and prognostic data from patients who underwent intracoronary ergonovine provocation tests (ERGT). The registry includes 2960 patients admitted to 11 major cardiovascular centers between January 2010 and July 2015. All participating institutions were high‐volume centers for coronary angiography (CAG) (>1800 cases/year) and percutaneous coronary interventions (>500 cases/year) and performed intracoronary ERGT using the same protocol. The participating centers and investigators are summarized in Data S1. Patients with suspected VSA underwent CAG with an ERGT according to the discretion of the responsible physician. The exclusion criteria for the VA‐KOREA were as follows: (1) patients with a severe fixed coronary artery stenosis at the baseline CAG (stenosis with a diameter reduction ≥50% at the left main coronary artery or ≥70% at a non‐left main coronary artery according to quantitative coronary analysis) who underwent a percutaneous coronary intervention with or without coronary stenting, (2) those with end‐stage renal disease who were undergoing continuous dialysis, (3) those with a known malignant or inflammatory disease, and (4) those with catheter‐induced spasm at the baseline CAG. The ethics committees at each participating center approved the study protocol (institutional review board approval number: CNUH‐2010‐01‐006), and all procedures followed the principles of the Declaration of Helsinki. All patients provided written informed consent before participation in the registry. The detailed study protocols have been published previously. 17
Among the 2960 patients included in the VA‐KOREA registry, we analyzed data from 1895 with a positive (definite or intermediate) ERGT result. We excluded patients with negative ERGTs (n=1003), those lost to follow‐up (n=58), and those with insufficient data (n=4). The primary outcome was investigated according to vasodilator use, that is, vasodilator (n=1536) or no vasodilator (n=359), at the time of discharge. We also analyzed clinical outcomes according to various vasodilator usage patterns, that is, no vasodilator (n=359), nonnitrate vasodilator (n=1187), conventional nitrate (n=209), or a combination of vasodilators (n=140) including both conventional and nonnitrate vasodilator. Nonnitrate vasodilators included nicorandil, molsidomine, and trimetazidine. Patients in the combination group received a conventional nitrate with at least 1 type of nonnitrate vasodilator.
CAG and Provocation Test for VSA
Vasoactive drugs such as CCBs and nitrates were discontinued for at least 48 hours before CAG. After baseline CAG, an intracoronary ERGT was performed to assess spasm provocation. Incremental doses of 10 (E1), 20 (E2), and 40 (E3) µg were injected into the right coronary artery, and doses of 20 (E1), 40 (E2), 60 (E3) µg were used for the left coronary artery. 17 , 18 If the patient could tolerate ERGTs, they were conducted for both the right and left coronary arteries. Once a spasm had been provoked and the provocation test was complete, intracoronary nitrate (200 µg) was injected. Fixed coronary stenosis with a diameter of ≥2.5 mm and the vascular response to ergonovine were quantitatively analyzed for 6 coronary artery sites (left main, left anterior descending, diagonal branch, left circumflex, obtuse marginal branch, and right coronary artery). The diameter after the intracoronary ergonovine injection was compared with that after the injection of intracoronary nitrate, in the site with the biggest change in diameter.
A definite positive ERGT result was defined as follows: (1) total or subtotal (>90% luminal diameter narrowing) occlusion with ischemic symptoms, with or without electrocardiographic changes; or (2) spontaneous total or subtotal spasm on baseline CAG. An intermediate positive ERGT result was defined as 50% to 90% luminal narrowing with or without ischemic symptoms and/or electrocardiographic changes. Staff at the core laboratory at Seoul St. Mary’s Hospital, Seoul, South Korea analyzed the blinded angiographic data offline via quantitative coronary analysis.
Study Definitions and End Points
Pre‐CAG angina was classified according to the established classification system. Specifically, grade I referred to near‐daily attacks, grade II to ≥4 attacks/month, grade III to ≥1 but <4 attacks/month, and grade IV to <1 attack/month. 17 An ischemic electrocardiographic change was defined as an ST‐segment–elevation or depression >0.1 mV or a negative U‐wave in at least 2 related leads. 17 Fixed coronary artery stenosis was defined as fixed luminal narrowing by <50% at the left main coronary artery, and <70% at the non‐left main coronary artery, as determined by quantitative coronary analysis. Myocardial bridging was defined as systolic narrowing of the coronary artery on CAG, and slow flow referred to a slow passage of contrast in the absence of an obstructive coronary artery lesion. Multivessel spasm was defined as a positive spasm in more than 2 major epicardial coronary arteries. Spasms were classified as focal, diffuse, or mixed. The focal type was defined as a discrete spasm localized in 1 coronary segment, whereas spasms that occurred continuously from the proximal to the distal segments were classified as diffuse. The mixed type referred to multivessel spasms in which at least 1 coronary artery had a focal spasm and the other(s) had a diffuse spasm. The Japanese Coronary Spasm Association (JCSA) risk score enables risk assessment and prognostic stratification of patients with VSA. 18 The JCSA risk score system has 3 risk strata: low (score=0–2), intermediate (3–5), and high (≥6) risk strata.
The primary end point was a composite of cardiac death, acute coronary syndrome (ACS), and symptomatic new‐onset arrhythmia during a 2‐year clinical follow‐up period. The secondary end points were cardiac death, ACS, symptomatic new‐onset arrhythmia, all‐cause mortality, and rehospitalization due to recurrent angina. We also investigated the rate of recurrent angina‐induced changes in medication during the follow‐up. All deaths were considered cardiac deaths unless there was a definite noncardiac cause. ACS was defined as recurrent or continuous chest pain lasting for more than 20 minutes with ischemic electrocardiographic changes or elevation of cardiac biomarkers, including myocardial infarction. For patients presenting with symptoms for the first time, clinically significant symptomatic arrhythmia, such as symptomatic premature beats, sick‐sinus rhythm, atrial or ventricular tachycardia/fibrillation, or atrioventricular block was considered indicative of symptomatic new‐onset arrhythmia. Twelve‐lead electrocardiography was routinely conducted during regular or emergent visits, and 24‐hour Holter monitoring was applied in patients with suspicious symptoms. Patients for whom drugs were added to their existing prescription, who switched to another drug because of recurrent angina, or who stopped all medications were regarded as having medication change. All adverse events were confirmed by consulting the medical records or conducting a telephone interview, and these events were assessed by the Local Events Committee of Seoul St. Mary’s Hospital.
Statistical Analysis
Continuous variables, presented as means±SDs, were compared using an unpaired t‐test, the Mann–Whitney rank‐sum test, or 1‐way analysis of variance. Discrete variables, expressed as counts with percentages, were compared using Pearson’s chi‐square test or Fisher’s exact test. We used Kaplan‐Meier curves and the log‐rank test to compare the groups in terms of the end points.
We conducted 2 different analyses in the current study: (1) no vasodilator versus vasodilator at discharge, and (2) no vasodilator versus nonnitrate vasodilator versus conventional nitrate versus a combination of conventional nitrate and at least 1 type of nonnitrate vasodilator. As differences in baseline characteristics could significantly affect outcomes, sensitivity analyses were performed to adjust for confounding factors. First, a multivariate Cox regression model was used to obtain cutoffs for each of these measures, including covariates with a P value<0.1 in the univariate analysis and a center as a categorical variable. The proportional hazards assumption was evaluated using the log‐minus‐log plot and Schoenfeld residual test. All Cox regression models for the clinical end points satisfied the proportional hazards assumption. Second, we performed propensity score matching to compare the no‐vasodilator and vasodilator groups. A 1:1 matching process without replacements was performed using a greedy algorithm with a caliper width of 0.2; 343 patients in the vasodilator group were matched with 343 controls in the no‐vasodilator group. The standardized mean difference after propensity score matching was within 0.1 for nearly all matched covariates, demonstrating well‐balanced groups (Tables 1 and 2). Third, the inverse of the propensity score was used to compare the 4 groups (no‐vasodilator versus nonnitrate vasodilator versus conventional nitrate versus combination). To assess the inverse probability of treatment weighting, we calculated the absolute standardized mean differences in the covariates used to generate the propensity score. We applied 4 treatment conditions using the multinomial propensity score 19 (Figure S1). All the variables in Tables 1 and 2 were used for propensity score matching and inverse probability of treatment weighting. The Toolkit for Weighting and Analysis of Nonequivalent Groups (https://www.rand.org/statistics/twang/stata‐tutorial.html) was used to calculate the multinomial propensity score.
Table 1.
Baseline Characteristics According to Vasodilator Use
| Unadjusted data (n=1895) | Matched data (n=686) | |||||||
|---|---|---|---|---|---|---|---|---|
|
Vasodilator (n=1536) |
No vasodilator (n=359) |
P value | SMD |
Vasodilator (n=343) |
No vasodilator (n=343) |
P value | SMD | |
| Demographics | ||||||||
| Age, y | 54.9 (11.3) | 55.6 (11.5) | 0.311 | −0.061 | 55.6 (10.5) | 55.7 (11.4) | 0.881 | −0.009 |
| Male sex | 972 (63.3) | 199 (55.4) | 0.006 | 0.161 | 191 (55.7) | 192 (56.0) | 0.939 | −0.006 |
| Medical history | ||||||||
| Ischemic heart disease | 194 (12.6) | 42 (11.7) | 0.631 | 0.028 | 40 (11.7) | 41 (12.0) | 0.906 | −0.009 |
| Stable CAD | 93 (6.1) | 21 (5.8) | 0.883 | 0.013 | 18 (5.2) | 20 (5.8) | 0.739 | −0.026 |
| History of percutaneous coronary intervention | 37 (2.4) | 3 (0.8) | 0.062 | 0.128 | 6 (1.7) | 3 (0.9) | 0.314 | 0.071 |
| Hypertension | 584 (38.0) | 136 (37.9) | 0.961 | 0.002 | 122 (35.6) | 130 (37.9) | 0.526 | −0.048 |
| Diabetes | 150 (9.8) | 28 (7.8) | 0.250 | 0.071 | 23 (6.7) | 28 (8.2) | 0.467 | −0.057 |
| Dyslipidemia | 260 (16.9) | 50 (13.9) | 0.167 | 0.083 | 52 (15.2) | 50 (14.6) | 0.830 | 0.017 |
| Atrial fibrillation or atrial flutter | 12 (0.8) | 6 (1.7) | 0.118 | −0.081 | 3 (0.9) | 3 (0.9) | 1.000 | 0 |
| Cerebrovascular accident | 24 (1.6) | 8 (2.2) | 0.378 | −0.044 | 6 (1.7) | 6 (1.7) | 1.000 | 0 |
| Thyroid disease | 52 (3.4) | 13 (3.6) | 0.825 | −0.011 | 13 (3.8) | 10 (2.9) | 0.525 | 0.050 |
| Hyperthyroidism | 23 (1.5) | 2 (0.6) | 0.160 | 0.088 | 5 (1.5) | 2 (0.6) | 0.254 | 0.088 |
| Hypothyroidism | 17 (1.1) | 9 (2.5) | 0.040 | −0.105 | 6 (1.7) | 6 (1.7) | 1.000 | 0 |
| Chronic airway disease | 21 (1.4) | 5 (1.4) | 0.970 | 0 | 5 (1.5) | 5 (1.5) | 1.000 | 0 |
| Familial history of CAD | 94 (6.1) | 15 (4.2) | 0.155 | 0.086 | 21 (6.1) | 15 (4.4) | 0.304 | 0.076 |
| Current or ex‐smoking | 650 (42.3) | 136 (37.9) | 0.125 | 0.090 | 126 (36.7) | 131 (38.2) | 0.693 | −0.031 |
| Past medication | ||||||||
| Aspirin | 318 (20.7) | 66 (18.4) | 0.325 | 0.058 | 59 (17.2) | 64 (18.7) | 0.619 | −0.039 |
| Thienopyridine | 55 (3.6) | 15 (4.2) | 0.589 | −0.031 | 13 (3.8) | 14 (4.1) | 0.844 | −0.015 |
| Calcium‐channel blocker | 317 (20.6) | 50 (13.9) | 0.004 | 0.178 | 60 (17.5) | 50 (14.6) | 0.298 | 0.079 |
| Angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker | 262 (17.1) | 69 (19.2) | 0.331 | −0.055 | 53 (15.5) | 64 (18.7) | 0.264 | −0.085 |
| Beta blocker | 109 (7.1) | 36 (10.0) | 0.060 | −0.104 | 28 (8.2) | 34 (9.9) | 0.424 | −0.059 |
| Statin | 242 (15.8) | 49 (13.6) | 0.319 | 0.062 | 48 (14.0) | 48 (14.0) | 1.000 | 0 |
| Initial presentation | ||||||||
| Chest pain | 1422 (92.6) | 318 (88.6) | 0.013 | 0.137 | 313 (91.3) | 308 (89.8) | 0.515 | −0.051 |
| Angina class >2 | 1032 (67.2) | 252 (70.2) | 0.272 | −0.065 | 236 (68.8) | 241 (70.3) | 0.678 | −0.033 |
| Dyspnea | 87 (5.7) | 23 (6.4) | 0.588 | −0.029 | 16 (4.7) | 21 (6.1) | 0.398 | −0.062 |
| Syncope | 20 (1.3) | 4 (1.1) | 0.774 | 0.018 | 6 (1.7) | 4 (1.2) | 0.524 | 0.042 |
| Cardiac arrest | 17 (1.1) | 10 (2.8) | 0.016 | −0.123 | 7 (2.0) | 6 (1.7) | 0.779 | 0.022 |
| Ventricular tachycardia or ventricular fibrillation | 6 (0.4) | 6 (1.7) | 0.006 | −0.128 | 4 (1.2) | 2 (0.6) | 0.412 | 0.064 |
| ST‐segment–elevation during chest pain | 90 (5.9) | 15 (4.2) | 0.210 | 0.078 | 25 (7.3) | 15 (4.4) | 0.103 | 0.124 |
| ST‐segment depression during chest pain | 13 (0.8) | 4 (1.1) | 0.628 | −0.030 | 2 (0.6) | 4 (1.2) | 0.412 | −0.064 |
| T‐wave inversion during chest pain | 40 (2.6) | 7 (1.9) | 0.473 | 0.047 | 8 (2.3) | 7 (2.0) | 0.794 | 0.021 |
Values are expressed as mean (SD) or n (%). CAD indicates coronary artery disease; and SMD, standardized mean difference.
Table 2.
Angiographic Characteristics and Medication at Discharge According to Vasodilator Use
| Unadjusted data (n=1895) | Matched data (n=686) | |||||||
|---|---|---|---|---|---|---|---|---|
|
Vasodilator (n=1536) |
No vasodilator (n=359) |
P value | SMD |
Vasodilator (n=343) |
No vasodilator (n=343) |
P value | SMD | |
| Fixed stenosis | 535 (34.8) | 136 (37.9) | 0.276 | −0.064 | 119 (34.7) | 129 (37.6) | 0.427 | −0.060 |
| Left main | 17 (1.1) | 3 (0.8) | 0.651 | 0.031 | 4 (1.2) | 1 (0.3) | 0.178 | 0.104 |
| LAD or diagonal | 368 (24.0) | 100 (27.9) | 0.123 | −0.089 | 91 (26.5) | 96 (28.0) | 0.668 | −0.034 |
| LCX or OM | 157 (10.2) | 37 (10.3) | 0.962 | −0.003 | 29 (8.5) | 36 (10.5) | 0.361 | −0.068 |
| RCA | 272 (17.7) | 61 (17.0) | 0.748 | 0.018 | 55 (16.0) | 58 (16.9) | 0.757 | −0.024 |
| Significant stenosis* | 59 (3.8) | 20 (5.6) | 0.140 | −0.085 | 18 (5.2) | 15 (4.4) | 0.592 | 0.037 |
| LAD or diagonal | 36 (2.3) | 13 (3.6) | 0.170 | −0.077 | 12 (3.5) | 11 (3.2) | 0.832 | 0.017 |
| LCX or OM | 13 (0.8) | 2 (0.6) | 0.578 | 0.024 | 2 (0.6) | 2 (0.6) | 1.000 | 0 |
| RCA | 16 (1.0) | 7 (1.9) | 0.157 | −0.075 | 5 (1.5) | 4 (1.2) | 0.737 | 0.026 |
| Other finding | ||||||||
| Myocardial bridge | 110 (7.2) | 13 (3.6) | 0.014 | 0.160 | 20 (5.8) | 13 (3.8) | 0.212 | 0.094 |
| Slow flow | 86 (5.6) | 12 (3.3) | 0.082 | 0.112 | 7 (2.0) | 12 (3.5) | 0.245 | −0.092 |
| Spasm provocation test | ||||||||
| Spasm positive arteries | ||||||||
| Left main | 17 (1.1) | 1 (0.3) | 0.145 | 0.096 | 4 (1.2) | 1 (0.3) | 0.178 | 0.104 |
| LAD or diagonal | 759 (49.4) | 178 (49.6) | 0.954 | −0.004 | 182 (53.1) | 166 (48.4) | 0.222 | 0.094 |
| LCX or OM | 346 (22.5) | 101 (28.1) | 0.024 | −0.129 | 78 (22.7) | 93 (27.1) | 0.186 | −0.102 |
| RCA | 773 (50.3) | 192 (53.5) | 0.281 | −0.064 | 167 (48.7) | 179 (52.2) | 0.359 | −0.070 |
| Multivessel spasm | 362 (23.6) | 103 (28.7) | 0.042 | −0.116 | 86 (25.1) | 92 (26.8) | 0.601 | −0.039 |
| Spontaneous spasm | 227 (14.8) | 49 (13.6) | 0.585 | 0.034 | 55 (16.0) | 48 (14.0) | 0.454 | 0.056 |
| Diffuse spasm | 951 (61.9) | 234 (65.2) | 0.250 | −0.069 | 207 (60.3) | 219 (63.8) | 0.345 | −0.072 |
| Focal spasm | 596 (38.8) | 134 (37.3) | 0.605 | 0.031 | 140 (40.8) | 127 (37.0) | 0.309 | 0.078 |
| Mixed spasm | 129 (8.4) | 41 (11.4) | 0.071 | −0.101 | 36 (10.5) | 35 (10.2) | 0.900 | 0.010 |
| Spasm on fixed stenosis | 340 (22.1) | 77 (21.4) | 0.777 | 0.017 | 76 (22.2) | 72 (21.0) | 0.710 | 0.029 |
| Chest pain during spasm | 934 (60.8) | 207 (57.7) | 0.273 | 0.063 | 200 (58.3) | 199 (58.0) | 0.938 | 0.006 |
| Result of provocation test | 0.448 | 0.043 | 0.388 | 0.066 | ||||
| Definite positive | 598 (38.9) | 132 (36.8) | 137 (39.9) | 126 (36.7) | ||||
| Intermediate positive | 938 (61.1) | 227 (63.2) | 206 (60.1) | 217 (63.3) | ||||
| ECG change | ||||||||
| ST‐segment–elevation | 128 (8.3) | 29 (8.1) | 0.874 | 0.007 | 30 (8.7) | 28 (8.2) | 0.784 | 0.018 |
| ST‐segment depression | 65 (4.2) | 16 (4.5) | 0.849 | −0.015 | 13 (3.8) | 14 (4.1) | 0.844 | −0.015 |
| T‐wave inversion | 77 (5.0) | 15 (4.2) | 0.508 | 0.038 | 18 (5.2) | 15 (4.4) | 0.592 | 0.037 |
| Medication at discharge | ||||||||
| Calcium‐channel blocker | 1404 (91.4) | 301 (83.8) | <0.001 | 0.232 | 297 (86.6) | 293 (85.4) | 0.660 | 0.035 |
| Angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker | 271 (17.6) | 68 (18.9) | 0.563 | −0.034 | 51 (14.9) | 64 (18.7) | 0.184 | −0.102 |
| Antiplatelet | 715 (46.5) | 166 (46.2) | 0.916 | 0.006 | 153 (44.6) | 156 (45.5) | 0.818 | −0.018 |
| Statin | 755 (49.2) | 161 (44.8) | 0.142 | 0.088 | 150 (43.7) | 155 (45.2) | 0.701 | −0.030 |
| Beta blocker | 100 (6.5) | 21 (5.8) | 0.645 | −0.029 | 21 (6.1) | 19 (5.5) | 0.745 | 0.026 |
| Alpha blocker | 17 (1.1) | 5 (1.4) | 0.649 | −0.027 | 4 (1.2) | 5 (1.5) | 0.737 | −0.026 |
Values are expressed as mean (SD) or n (%). LAD indicates left anterior descending artery; LCX, left circumflex artery; OM, obtuse marginal; RCA, right coronary artery; and SMD, standardized mean difference.
There was no significantly fixed coronary artery stenosis in left main.
Hazard ratios (HRs) and 95% CIs were calculated during the Cox regression analysis. We used a multivariate Cox proportional hazard model to identify independent predictors of primary end points and ACS. All analyses were 2 tailed, and P<0.05 was taken to indicate statistical significance. All analyses were performed using Stata/MP 16.0 (StataCorp LP, College Station, TX, USA).
Results
Characteristics and Clinical Outcomes According to Vasodilator Use
The median follow‐up duration was 756 days (25thpercentile:333 and 75th percentile 1103 days). Patient baseline clinical characteristics, angiographic profiles, and discharge medication are given in Tables 1 and 2, respectively. The vasodilator group had a higher proportion of male patients. Otherwise, there were no significant group differences in age or medical history except for a higher prevalence of hypothyroidism in the no‐vasodilator group (1.1 versus 2.5%, P=0.040). The rate of previous ischemic heart disease (12.6 versus 11.7%, P=0.631) and history of smoking (42.3% versus 37.9%, P=0.125) was not statistically different between the vasodilator and novasodilator groups. Regarding previous medications, CCBs had been prescribed more frequently in the vasodilator group compared with the no‐vasodilator group (20.6 versus 13.9%, P=0.004). At initial presentation, the patients in the no‐vasodilator group were more likely to present with cardiac arrest (1.1 versus 2.8%, P=0.016) and ventricular arrhythmia (0.4 versus 1.7%, P=0.006). The frequency of angina and rate of electrocardiographic changes during chest pain did not differ significantly between the groups. The rate of fixed coronary stenosis and significantly fixed coronary stenosis was comparable between the 2 groups for all epicardial coronary arteries. At the baseline CAG, myocardial bridging was more frequent in the vasodilator group (7.2 versus 3.6%, P=0.014). In the spasm provocation test, multivessel spasm (23.6 versus 28.7%, P=0.042) and spasm of the left circumflex artery or obtuse marginal branch (22.5 versus 28.1%, P=0.024) were more frequently provoked in the no‐vasodilator group. There were no statistically significant group differences in the rate of spontaneous spasm, diffuse spasm, focal spasm, chest pain during spasm, definite positive ERGT result, or electrocardiographic change during provocation. Regarding medications at discharge, CCBs were more frequently prescribed in the vasodilator group (91.4 versus 83.8%, P<0.001). After propensity score matching, the difference between the 2 unmatched groups disappeared. Clinical outcomes at the 2‐year follow‐up are described in Table 3 and Figure 1. The primary end point incidence was not significantly different between the groups (propensity score matched HR: 0.92; 95% CI: 0.48 to 1.77; P=0.806). Secondary end points also occurred at a similar rate between the groups. The rate of medication change during the follow‐up period was not significantly different in both groups (63.1 versus 60.8%). There was no patient who terminated all medications during follow‐up.
Table 3.
Comparison of 2‐Year Clinical Outcomes According to Vasodilator Use
|
Vasodilator (n=1536) |
No vasodilator (n=359) |
Unadjusted | Multivariable‐adjusted | Propensity‐score matched | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Primary end point* | 71 (4.6) | 18 (5.0) | 0.86 (0.51–1.44) | 0.569 | 0.88 (0.51–1.50) | 0.628 | 0.92 (0.48–1.77) | 0.806 |
| Cardiac death | 2 (0.1) | 2 (0.6) | 0.22 (0.03–1.57) | 0.131 | 0.22 (0.03–1.58) | 0.131 | 0.93 (0.13–6.60) | 0.941 |
| Acute coronary syndrome † | 50 (3.3) | 8 (2.2) | 1.36 (0.65–2.87) | 0.417 | 1.30 (0.59–2.84) | 0.512 | 1.38 (0.57–3.38) | 0.478 |
| Non–ST‐segment–elevation myocardial infarction | 4 (0.3) | 0 | ||||||
| Unstable angina | 46 (3.0) | 8 (2.2) | ||||||
| Arrhythmia | 22 (1.4) | 9 (2.5) | 0.53 (0.25–1.15) | 0.110 | 0.62 (0.28–1.38) | 0.242 | 0.51 (0.17–1.51) | 0.224 |
| Atrial fibrillation | 7 (0.5) | 3 (0.8) | ||||||
| Atrioventricular block | 3 (0.2) | 4 (1.1) | ||||||
| Ventricular tachycardia or ventricular fibrillation | 7 (0.5) | 0 | ||||||
| Cardiac arrest | 2 (0.1) | 1 (0.3) | ||||||
| All‐cause death | 7 (0.5) | 6 (1.7) | 0.25 (0.83–0.73) | 0.012 | 0.21 (0.07–0.65) | 0.007 | 0.44 (0.11–1.77) | 0.250 |
| Rehospitalization | 242 (15.8) | 53 (14.8) | 0.99 (0.74–1.33) | 0.942 | 1.08 (0.80–1.46) | 0.634 | 1.00 (0.68–1.48) | 0.994 |
| Medication change ‡ | 969 (63.1) | 203 (60.8) | ||||||
Values are expressed as n (%). HR indicates hazard ratio.
The primary end point was defined as a composite of cardiac death, acute coronary syndrome, or new‐onset arrhythmia.
There was no ST‐segment–elevation myocardial infarction in all groups.
Data for medication change was available in 1535 (99.9%) with vasodilator group and 334 (93.0%) with no vasodilator group. There was no patient who terminated all medications during follow‐up.
Figure 1. Cumulative incidence of primary end point and acute coronary syndrome between vasodilator group and no vasodilator group.
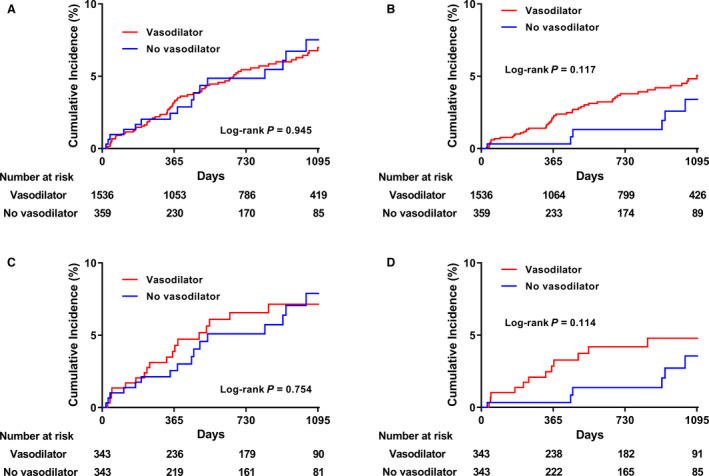
Composite outcome of cardiac death, acute coronary syndrome, and new‐onset arrhythmia before (A) and after (C) propensity score matching; acute coronary syndrome before (B) and after (D) propensity score matching.
Clinical Outcomes According to Various Vasodilator Use
The characteristics and frequencies of clinical outcomes at the 2‐year follow‐up are compared among the 4 groups in Table S1 and Table 4, respectively. In the comparison of baseline characteristics between subgroups, the combination group had higher rate of previous ischemic heart disease (18.6%, P=0.049) with higher rate of stable coronary artery disease (11.4%, P=0.028) compared other groups. The rate of smoking (ex or current) was significantly higher in the conventional nitrate group (52.6%, P=0.005). As described in Table 2, CCB was less frequently prescribed in the no ‐asodilator group compared with all vasodilator subgroups. As shown in Table 4, the crude incidence rates of the primary end point (5.0 versus 3.7 versus 7.2 versus 8.6%, P=0.017) and ACS (2.2 versus 2.1 versus 6.2 versus 8.6%, P<0.001) were significantly higher in the conventional nitrate and combination groups, and the all‐cause mortality rate was lower in the nonnitrate vasodilator group. There were no significant differences in other secondary outcomes according to different vasodilator usage. The risk of the primary end point was similar among the 4 groups; however, the risk of ACS was significantly higher in the conventional nitrate group (HR, 2.49; 95% CI, 1.01 to 6.14; P=0.047) and combination group (HR, 3.34; 95% CI, 1.15 to 9.75, P=0.027) compared with the no‐vasodilator group after inverse probability of treatment weighting adjustment (Table 5 and Figure 2). Furthermore, the risk of all‐cause death was lower in the nonnitrate vasodilator group (HR, 0.11; 95% CI, 0.03 to 0.46, P=0.002). We investigated the detailed characteristics of the patients with death (Table 4). The most deaths were noncardiac death (69.2%), and about 70% of patients were diagnosed as VSA by definite positive ERGT. Information about patients who died during the whole follow‐up period is summarized in Table S2.
Table 4.
Clinical Outcomes at 2 Years According to Various Usage Pattern of Vasodilator
|
Overall (n=1895) |
No vasodilator (n=359) |
Vasodilator | P value* | |||
|---|---|---|---|---|---|---|
|
Nonnitrate vasodilator (n=1187) |
Conventional nitrate (n=209) |
Combination (n=140) |
||||
| Primary end point † | 89 (4.7) | 18 (5.0) | 44 (3.7) | 15 (7.2) | 12 (8.6) | 0.017 |
| Cardiac death | 4 (0.2) | 2 (0.6) | 2 (0.2) | 0 | 0 | 0.410 |
| Acute coronary syndrome ‡ | 58 (3.1) | 8 (2.2) | 25 (2.1) | 13 (6.2) | 12 (8.6) | <0.001 |
| Non–ST‐segment–elevation myocardial infarction | 4 (0.2) | 0 | 1 (0.1) | 1 (0.5) | 2 (1.4) | 0.007 |
| Unstable angina | 54 (2.8) | 8 (2.2) | 24 (2.0) | 12 (5.7) | 10 (7.1) | <0.001 |
| Arrhythmia | 31 (1.6) | 9 (2.5) | 20 (1.7) | 2 (1.0) | 0 | 0.200 |
| Atrial fibrillation | 10 (0.5) | 3 (0.8) | 7 (0.6) | 0 | 0 | 0.460 |
| Atrioventricular block | 7 (0.4) | 4 (1.1) | 2 (0.2) | 1 (0.5) | 0 | 0.063 |
| ventricular tachycardia or ventricular fibrillation | 7 (0.4) | 0 | 6 (0.5) | 1 (0.5) | 0 | 0.473 |
| Cardiac arrest | 3 (0.2) | 1 (0.3) | 2 (0.2) | 0 | 0 | 0.828 |
| All‐cause death | 13 (0.7) | 6 (1.7) | 5 (0.4) | 0 | 2 (1.4) | 0.030 |
| Rehospitalization | 295 (15.6) | 53 (14.8) | 187 (15.8) | 29 (13.9) | 26 (18.6) | 0.654 |
| Medication change § | 1172 (62.7) | 203 (60.8) | 747 (62.9) | 132 (63.2) | 90 (64.7) | 0.844 |
Values are expressed as n (%).
The P values are derived from the chi‐square test for group comparison.
The primary end point was a composite of cardiac death, acute coronary syndrome, and new‐onset arrhythmia.
There was no ST‐segment–elevation myocardial infarction in all groups.
Data for medication change were available in 334 (93.0%) with no vasodilator group, 1187 (100%) with nonnitrate vasodilator group, 209 (100%) with conventional nitrate group, and 139 (99.3%) with combination group.
Table 5.
Comparison of 2‐Year Clinical Outcomes According to Various Usage Pattern of Vasodilator
| Unadjusted | Multivariable‐adjusted | IPTW‐adjusted* | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Primary end point † | ||||||
| No vasodilator (reference) | 1 | 1 | 1 | |||
| Nonnitrate vasodilator | 0.70 (0.40–1.20) | 0.193 | 0.74 (0.42–1.31) | 0.301 | 0.73 (0.39–1.33) | 0.301 |
| Conventional nitrate | 1.29 (0.65–2.56) | 0.468 | 1.25 (0.61–2.56) | 0.539 | 1.25 (0.62–2.54) | 0.537 |
| Combination | 1.59 (0.76–3.29) | 0.217 | 1.48 (0.69–3.18) | 0.320 | 1.44 (0.57–3.62) | 0.436 |
| Cardiac death | ||||||
| No vasodilator (reference) | 1 | 1 | 1 | |||
| Nonnitrate vasodilator | 0.29 (0.04–2.07) | 0.217 | 0.28 (0.04–2.04) | 0.210 | 0.30 (0.01–7.07) | 0.453 |
| Conventional nitrate ‡ | ||||||
| Combination ‡ | ||||||
| Acute coronary syndrome | ||||||
| No vasodilator (reference) | 1 | 1 | 1 | |||
| Nonnitrate vasodilator | 0.88 (0.40–1.95) | 0.754 | 1.05 (0.46–2.38) | 0.912 | 0.92 (0.39–2.13) | 0.841 |
| Conventional nitrate | 2.56 (1.06–6.18) | 0.037 | 2.86 (1.15–7.15) | 0.025 | 2.49 (1.01–6.14) | 0.047 |
| Combination | 3.64 (1.49–8.92) | 0.005 | 4.04 (1.58–10.30) | 0.003 | 3.34 (1.15–9.75) | 0.027 |
| Arrhythmia | ||||||
| No vasodilator (reference) | 1 | 1 | 1 | |||
| Nonnitrate vasodilator | 0.64 (029–1.40) | 0.265 | 0.74 (0.33–1.66) | 0.461 | 0.72 (0.23–2.23) | 0.571 |
| Conventional nitrate | 0.33 (0.71–1.52) | 0.154 | 0.37 (0.08–1.79) | 0.217 | 0.30 (0.06–1.58) | 0.156 |
| Combination ‡ | ||||||
| All‐cause death | ||||||
| No vasodilator (reference) | 1 | 1 | 1 | |||
| Nonnitrate vasodilator | 0.23 (0.07–0.75) | 0.015 | 0.20 (0.06–0.68) | 0.010 | 0.11 (0.03–0.46) | 0.002 |
| Conventional nitrate ‡ | ||||||
| Combination | 0.78 (0.16–3.87) | 0.761 | 0.58 (0.11–3.04) | 0.516 | 2.18 (0.39–12.29) | 0.378 |
| Rehospitalization | ||||||
| No vasodilator (reference) | 1 | 1 | 1 | |||
| Non‐nitrate vasodilator | 0.99 (0.74–1.35) | 0.985 | 1.08 (0.79–1.47) | 0.636 | 1.02 (0.72–1.46) | 0.909 |
| Conventional nitrate | 0.82 (0.52–1.29) | 0.387 | 0.93 (0.59–1.48) | 0.769 | 0.81 (0.51–1.29) | 0.368 |
| Combination | 1.20 (0.75–1.91) | 0.454 | 1.29 (0.80–2.08) | 0.299 | 1.14 (0.63–2.07) | 0.661 |
Values are expressed as n (%). HR indicates hazard ratio; and IPTW, inverse probability of treatment weighting.
IPTW was derived from multinomial propensity score.
The primary end point was defined as a composite of cardiac death, acute coronary syndrome, or new‐onset arrhythmia.
No event.
Figure 2. Cumulative incidence of primary end point and acute coronary syndrome among 4 groups.
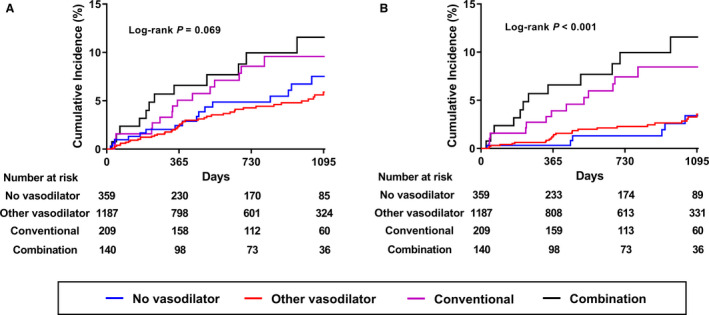
A, Composite outcome of cardiac death, acute coronary syndrome, and new‐onset arrhythmia; (B) acute coronary syndrome.
Subgroup Analyses
We performed subgroup analyses according to the intensity of the positive ERGT results and risk stratification. The higher risk of ACS associated with conventional nitrate usage or combination was observed in an intermediate positive ERGT (multivariable‐adjusted HR, 7.28; 95% CI, 1.48–35.77; P=0.015 for the conventional nitrate group; and HR, 8.16; 95% CI, 1.65–40.30; P=0.010 for the combination group) and those with a low JCSA risk score (multivariable‐adjusted HR, 5.71; 95% CI, 1.15–28.34; P=0.033 for the conventional nitrate group; and 1HR, 0.79; 95% CI, 2.24–51.95; P=0.003 for the combination group). This association was maintained in inverse probability of treatment weighting adjusted analysis. There was no significant difference in the risk of ACS according to vasodilator usage patterns in the subgroup of a definite positive ERGT and an intermediate or high JCSA risk score (Table 6 and Figure 3).
Table 6.
Risks for ACS According to Various Subgroups
| Unadjusted | Multivariable‐adjusted | IPTW adjusted* | |||||
|---|---|---|---|---|---|---|---|
| No. (%) | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Definite positive ERGT † | 730 (38.5) | ||||||
| No vasodilator (reference) | 132 (7.0) | 1 | 1 | 1 | |||
| Nonnitrate vasodilator | 459 (24.2) | 0.51 (0.19–1.37) | 0.505 | 0.41 (0.14–1.22) | 0.109 | 0.33 (0.11–1.01) | 0.052 |
| Conventional nitrate | 81 (4.3) | 1.33 (0.41–4.37) | 0.637 | 0.99 (0.28–3.49) | 0.989 | 1.10 (0.30–3.97) | 0.888 |
| Combination | 58 (3.1) | 2.09 (0.64–6.86) | 0.224 | 1.25 (0.35–4.46) | 0.733 | 1.61 (0.44–5.87) | 0.472 |
| Intermediate positive ERGT † | 1165 (61.5) | ||||||
| No vasodilator (reference) | 227 (12.0) | 1 | 1 | 1 | |||
| Nonnitrate vasodilator | 728 (38.4) | 1.97 (0.45–8.67) | 0.370 | 2.00 (0.45–8.94) | 0.363 | 2.13 (0.47–9.59) | 0.327 |
| Conventional nitrate | 128 (6.8) | 6.15 (1.31–28.95) | 0.022 | 7.28 (1.48–35.77) | 0.015 | 6.89 (1.38–34.45) | 0.019 |
| Combination | 82 (4.3) | 8.30 (1.72–39.96) | 0.008 | 8.16 (1.65–40.30) | 0.010 | 7.52 (1.45–38.86) | 0.016 |
| Intermediate or high JCSA risk score ‡ | 569 (30.0) | ||||||
| No vasodilator (reference) | 122 (6.4) | 1 | 1 | 1 | |||
| Nonnitrate vasodilator | 328 (17.3) | 0.65 (0.24–1.76) | 0.396 | 0.62 (0.22–1.72) | 0.355 | 0.59 (0.21–1.67) | 0.324 |
| Conventional nitrate | 76 (4.0) | 1.36 (0.44–4.22) | 0.594 | 1.11 (0.34–3.63) | 0.858 | 1.17 (0.34–3.65) | 0.857 |
| Combination | 43 (2.3) | 1.17 (0.29–4.67) | 0.827 | 0.91 (0.22–0.3.82) | 0.893 | 1.01 (0.23–4.47) | 0.985 |
| Low JCSA risk score ‡ | 1326 (70.0) | ||||||
| No vasodilator (reference) | 237 (12.5) | 1 | 1 | 1 | |||
| Nonnitrate vasodilator | 859 (45.3) | 1.77 (0.40–7.80) | 0.449 | 1.56 (0.35–6.93) | 0.562 | 1.59 (0.35–7.13) | 0.546 |
| Conventional nitrate | 133 (7.0) | 6.00 (1.24–28.99) | 0.026 | 5.71 (1.15–28.34) | 0.033 | 5.64 (1.13–28.07) | 0.035 |
| Combination | 97 (5.1) | 11.29 (2.43–52.41) | 0.002 | 10.79 (2.24–51.95) | 0.003 | 10.73 (2.23–51.67) | 0.003 |
Values are expressed as n (%). ACS indicates acute coronary syndrome; ERGT, ergonovine provocation test; HR, hazard ratio; IPTW, inverse probability of treatment weighting; and JCSA, Japanese Coronary Spasm Association.
Adjusted by IPTW and variables included in multivariable‐adjusted model.
Adjusted by center identifier, ischemic heart disease, dyslipidemia, atrial fibrillation or flutter, current or ex‐smoking, ST‐T changes during chest pain, and significant coronary stenosis.
Adjusted by center identifier, ischemic heart disease, dyslipidemia, and atrial fibrillation or flutter.
Figure 3. Cumulative incidence of acute coronary syndrome in various subgroups.
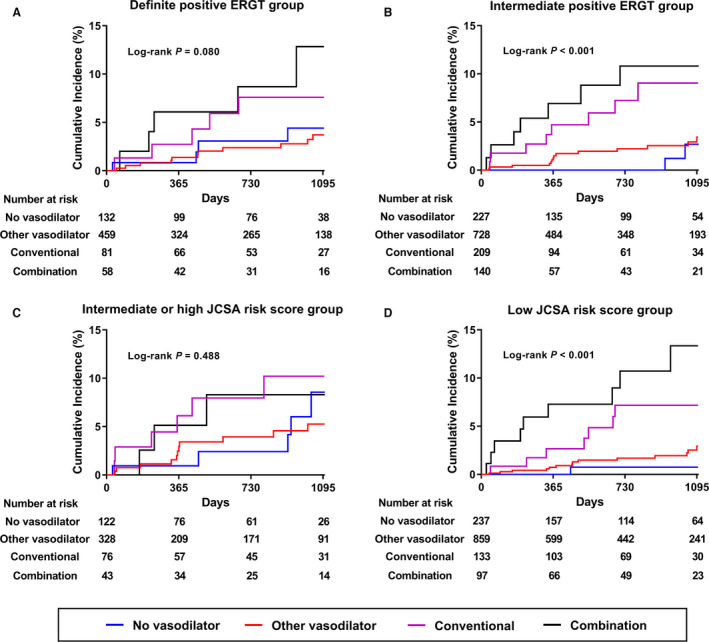
A, Definite positive ERGT group; (B) intermediate positive ERGT group; (C) intermediate or high JCSA risk score group; (D) low JCSA risk score group. ERGT indicates ergonovine provocation test; and JCSA, Japanese Coronary Spasm Association.
Independent Predictors of Primary End Point and ACS
The multivariate Cox proportional hazard model identified independent predictors of the primary and secondary outcomes (Table S3). Atrial fibrillation or flutter at initial electrocardiography and fixed coronary artery stenosis were independent predictors of both the primary end point and ACS. Dyslipidemia predicted the incidence of ACS only during the 2‐year follow‐up and did not predict the primary end point.
Discussion
In the present study, we compared clinical outcomes during a 2‐year period according to the use of various types of vasodilators in patients with ERGT‐confirmed VSA drawn from a nationwide, multicenter, prospective registry. Our main findings were (1) the use of a vasodilator at discharge was not associated with the clinical outcomes of interest, including recurrent angina‐induced rehospitalization and the rate of medication change; and (2) conventional nitrate, or a combination of conventional nitrate with at least one kind of nonnitrate vasodilator, was associated with an increased risk of ACS compared with no vasodilator use at discharge. There were no significant differences in the incidence rates of the study end points between patients who did not use a vasodilator and those who used a nonnitrate vasodilator. However, subgroup analyses according to the intensity of spasm and JCSA risk score revealed that the adverse effects of long‐acting nitrate were prominent in patients with intermediate positive ERGT results and a low JCSA risk score but not in those with a definite positive ERGT result and intermediate‐to‐high JCSA risk score.
Current Evidence for Chronic Vasodilator Therapy in VSA
CCBs administered with or without long‐acting nitrates are mainstays of treatment for VSA 20 that have been found to effectively reduce angina symptoms and provide favorable clinical outcomes. 12 , 13 , 14 Nitrates can dilate veins, arteries, and coronary arteries by relaxing vascular smooth muscle, and their anti‐ischemic effect is mainly due to a decrease in myocardial oxygen demand as a result of systemic vasodilation. 21 However, nitrate has some adverse effects, such as headache, and many patients discontinue nitrate because of such effects. 22 Furthermore, recent studies have raised questions about the clinical benefit of long‐acting nitrate as a vasodilator in patients with VSA. 15 , 16 Retrospective data from 1429 patients with VSA indicated that chronic nitrate therapy did not reduce the rate of cardiac death, nonfatal myocardial infarction, hospitalization due to unstable angina, heart failure, or appropriate implantable cardioverter defibrillator shocks compared with nonnitrate therapy, after propensity‐score matching. 15 This study also showed that the combination of long‐acting nitrate and nicorandil was associated with a 2‐fold increase in the incidence of the composite end point. In another observational study including data from 1154 patients with VSA and positive ERGT results, nitrate use increased the risk of a composite of cardiac death, myocardial infarction, revascularization, and rehospitalization due to recurrent angina. 16 The researchers described putative mechanisms underlying the neutral or deleterious effects of long‐acting nitrates, namely a rebound phenomenon in which angina abruptly increases during nitrate withdrawal and nitrate‐free periods. 23 Increased vasoconstriction during nitrate‐free periods, along with decreased vasodilatory effects of nitric oxide, was proposed as a mechanism. 24
No randomized controlled trials have examined this issue, and few studies have reported on the adverse effects of long‐acting nitrate use in patients with VSA. Therefore, current guidelines still recommend the use of long‐acting nitrates as a second‐line treatment for the treatment of VSA. 12 , 13 , 14 To address this, the current study investigated the prognostic impact of chronic vasodilator therapy, including long‐acting nitrates, in patients with VSA drawn from a multicenter, prospective registry.
Impact of Chronic Vasodilator in Patients With VSA
In the current study, we found no significant differences in clinical outcomes according to vasodilator use at discharge. This result is consistent with the 2 aforementioned observational studies. 15 , 16 We further analyzed the data according to various vasodilator usage patterns.
The analysis revealed adverse effects of conventional nitrate, including in combination with other treatments, compared with nonnitrate vasodilator use. Surprisingly, 77.3% of the patients who used vasodilators were prescribed a nonnitrate vasodilator instead of a conventional nitrate at discharge. Prior studies reported that nicorandil had a neutral effect on clinical outcomes. 15 , 16 In the current study, the nonnitrate vasodilators included nicorandil, molsidomine, and trimetazidine. The mechanisms by which these medications vasodilate are different from those of nitrate. Nicorandil can vasodilate via the cGMP signaling pathway, in which K+ channels open and induce an increase in nitric oxide. 25 Nicorandil is not associated with tolerance or rebound angina. 26 The nonnitrate vasodilator molsidomine also produces nitric oxide by mechanisms other than nitrate. 27 Therefore, we hypothesized that the adverse effects of nitrate in the current study were a result of nitrate tolerance or rebound angina.
Subgroup Analyses
To ascertain whether patients with more severe vasospasms received conventional nitrate alone or in combination with other treatments in the current study, we performed subgroup analyses according to the intensity of spasm provocation and JCSA risk score. Contrary to our hypothesis, adverse effects of nitrate were prominent in patients with intermediate positive ERGT results or a low JCSA risk score but not in high‐risk patients. In a previous report using data from the VA‐KOREA registry, patients with an intermediate positive ERGT result had more favorable clinical outcomes, in terms of lower cardiac mortality, compared with those with a definite positive ERGT result. 17 Patients with a low JCSA risk score also had a lower incidence of major adverse cardiac events compared with those with intermediate or high JCSA risk scores. 18 Although the exact explanation for different clinical outcomes according to risk stratification after vasodilator use is unclear, we think that patients with low risk or intermediate vasospasm have different endothelium‐dependent responsiveness of vascular smooth muscle cell, which is an important pathogenesis of VSA. As far as we know, there have been no basic or clinical studies evaluating this issue, and a large‐scale randomized trial is needed to confirm this.
Study Limitations
This study had several limitations. First, we used observational registry data. Therefore, selection bias was inevitable. However, we performed various sensitivity analyses to adjust for measured or unmeasured confounders for various clinical characteristics. Second, data on the dosage and type of vasodilator were not available in the registry. In a prior study, the type of nitrate was found to affect clinical outcomes. 15 In terms of maintenance of medication during follow‐up, the registry provided only the rate of medication change, which included an addition, switch to different class of medication, or termination of all medications without detailed information about drug type or dosage. Third, there were no data regarding percutaneous coronary interventions during follow‐up. The rate of fixed coronary artery stenosis at baseline CAG was not low (up to 4.1%), and this was an independent predictor of ACS occurrence. However, the revascularization rate according to nitrate usage did not differ from previous findings. 16
Conclusions
Routine prescription of vasodilators did not affect clinical outcomes in patients with ERGT‐confirmed VSA in a nationwide, multicenter, prospective registry. However, long‐acting nitrate‐based chronic vasodilator therapy was associated with an increased 2‐year risk of ACS in patients with VSA, especially in low‐risk patients. However, as this study used a retrospective design, additional large‐scale randomized trials are required for validation.
Sources of Funding
None.
Disclosure
None.
Supporting information
Data S1
Tables S1–S3
Figure S1
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government(MSIT) (No. 2019R1A2C3003547), a grant (BCRI20053) of Chonnam National University Hospital Biomedical Research Institute.
For Sources of Funding and Disclosures, see page 14.
References
- 1. Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283 [DOI] [PubMed] [Google Scholar]
- 2. Keller KB, Lemberg L. Prinzmetal's angina. Am J Crit Care. 2004;13:350–354. doi: 10.4037/ajcc2004.13.4.350 [DOI] [PubMed] [Google Scholar]
- 3. Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN; Coronary Vasomotion Disorders International Study G . International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–2568. doi: 10.1093/eurheartj/ehv351 [DOI] [PubMed] [Google Scholar]
- 4. Kaski JC, Maseri A, Vejar M, Crea F, Hackett D, Halson P. Spontaneous coronary artery spasm in variant angina is caused by a local hyperreactivity to a generalized constrictor stimulus. J Am Coll Cardiol. 1989;14:1456–1463. doi: 10.1016/0735-1097(89)90382-3 [DOI] [PubMed] [Google Scholar]
- 5. Kaski JC, Crea F, Meran D, Rodriguez L, Araujo L, Chierchia S, Davies G, Maseri A. Local coronary supersensitivity to diverse vasoconstrictive stimuli in patients with variant angina. Circulation. 1986;74:1255–1265. doi: 10.1161/01.CIR.74.6.1255 [DOI] [PubMed] [Google Scholar]
- 6. Miyao Y, Kugiyama K, Kawano H, Motoyama T, Ogawa H, Yoshimura M, Sakamoto T, Yasue H. Diffuse intimal thickening of coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 2000;36:432–437. doi: 10.1016/S0735-1097(00)00729-4 [DOI] [PubMed] [Google Scholar]
- 7. Sun H, Mohri M, Shimokawa H, Usui M, Urakami L, Takeshita A. Coronary microvascular spasm causes myocardial ischemia in patients with vasospastic angina. J Am Coll Cardiol. 2002;39:847–851. doi: 10.1016/S0735-1097(02)01690-X [DOI] [PubMed] [Google Scholar]
- 8. Yasue H, Takizawa A, Nagao M, Nishida S, Horie M, Kubota J, Omote S, Takaoka K, Okumura K. Long‐term prognosis for patients with variant angina and influential factors. Circulation. 1988;78:1–9. doi: 10.1161/01.CIR.78.1.1 [DOI] [PubMed] [Google Scholar]
- 9. Matsue Y, Suzuki M, Nishizaki M, Hojo R, Hashimoto Y, Sakurada H. Clinical implications of an implantable cardioverter‐defibrillator in patients with vasospastic angina and lethal ventricular arrhythmia. J Am Coll Cardiol. 2012;60:908–913. doi: 10.1016/j.jacc.2012.03.070 [DOI] [PubMed] [Google Scholar]
- 10. Takagi Y, Yasuda S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, et al. Clinical characteristics and long‐term prognosis of vasospastic angina patients who survived out‐of‐hospital cardiac arrest: multicenter registry study of the Japanese Coronary Spasm Association. Circ Arrhythm Electrophysiol. 2011;4:295–302. doi: 10.1161/CIRCEP.110.959809 [DOI] [PubMed] [Google Scholar]
- 11. Ahn JM, Lee KH, Yoo SY, Cho YR, Suh J, Shin ES, Lee JH, Shin DI, Kim SH, Baek SH, et al. Prognosis of variant angina manifesting as aborted sudden cardiac death. J Am Coll Cardiol. 2016;68:137–145. doi: 10.1016/j.jacc.2016.04.050 [DOI] [PubMed] [Google Scholar]
- 12. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American college of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 13. Group JCSJW . Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2013). Circ J. 2014;78:2779–2801. doi: 10.1253/circj.cj-66-0098 [DOI] [PubMed] [Google Scholar]
- 14. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 esc guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 15. Takahashi J, Nihei T, Takagi Y, Miyata S, Odaka Y, Tsunoda R, Seki A, Sumiyoshi T, Matsui M, Goto T, et al. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: multicentre registry study of the Japanese coronary spasm association. Eur Heart J. 2015;36:228–237. doi: 10.1093/eurheartj/ehu313 [DOI] [PubMed] [Google Scholar]
- 16. Kim CH, Park TK, Cho SW, Oh MS, Lee DH, Seong CS, Gwag HB, Lim AY, Yang JH, Song YB, et al. Impact of different nitrate therapies on long‐term clinical outcomes of patients with vasospastic angina: a propensity score‐matched analysis. Int J Cardiol. 2018;252:1–5. doi: 10.1016/j.ijcard.2017.07.031 [DOI] [PubMed] [Google Scholar]
- 17. Shin DIL, Baek SH, Her SH, Han SH, Ahn Y, Park K‐H, Kim D‐S, Yang T‐H, Choi D‐J, Suh J‐W, et al. The 24‐month prognosis of patients with positive or intermediate results in the intracoronary ergonovine provocation test. JACC Cardiovasc Interv. 2015;8:914–923. doi: 10.1016/j.jcin.2014.12.249 [DOI] [PubMed] [Google Scholar]
- 18. Takagi Y, Takahashi J, Yasuda S, Miyata S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, et al. Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese coronary spasm association. J Am Coll Cardiol. 2013;62:1144–1153. doi: 10.1016/j.jacc.2013.07.018 [DOI] [PubMed] [Google Scholar]
- 19. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–3414. doi: 10.1002/sim.5753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lombardi M, Morales MA, Michelassi C, Moscarelli E, Distante A, L'abbate A. Efficacy of isosorbide‐5‐mononitrate versus nifedipine in preventing spontaneous and ergonovine‐induced myocardial ischaemia. A double‐blind, placebo‐controlled study. Eur Heart J. 1993;14:845–851. doi: 10.1093/eurheartj/14.6.845 [DOI] [PubMed] [Google Scholar]
- 21. Abrams J. Hemodynamic effects of nitroglycerin and long‐acting nitrates. Am Heart J. 1985;110:216–224. doi: 10.1016/0002-8703(85)90490-9 [DOI] [PubMed] [Google Scholar]
- 22. Bagdy G, Riba P, Kecskemeti V, Chase D, Juhasz G. Headache‐type adverse effects of no donors: vasodilation and beyond. Br J Pharmacol. 2010;160:20–35. doi: 10.1111/j.1476-5381.2010.00643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Munzel T, Mollnau H, Hartmann M, Geiger C, Oelze M, Warnholtz A, Yehia AH, Forstermann U, Meinertz T. Effects of a nitrate‐free interval on tolerance, vasoconstrictor sensitivity and vascular superoxide production. J Am Coll Cardiol. 2000;36:628–634. doi: 10.1016/S0735-1097(00)00754-3 [DOI] [PubMed] [Google Scholar]
- 24. Azevedo ER, Schofield AM, Kelly S, Parker JD. Nitroglycerin withdrawal increases endothelium‐dependent vasomotor response to acetylcholine. J Am Coll Cardiol. 2001;37:505–509. doi: 10.1016/S0735-1097(00)01140-2 [DOI] [PubMed] [Google Scholar]
- 25. Tarkin JM, Kaski JC. Vasodilator therapy: nitrates and nicorandil. Cardiovasc Drugs Ther. 2016;30:367–378. doi: 10.1007/s10557-016-6668-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kool MJ, Spek JJ, Struyker Boudier HA, Hoeks AP, Reneman RS, van Herwaarden RH, Van Bortel LM. Acute and subacute effects of nicorandil and isosorbide dinitrate on vessel wall properties of large arteries and hemodynamics in healthy volunteers. Cardiovasc Drugs Ther. 1995;9:331–337. doi: 10.1007/BF00878678 [DOI] [PubMed] [Google Scholar]
- 27. Majid PA, DeFeyter PJ, Van der Wall EE, Wardeh R, Roos JP. Molsidomine in the treatment of patients with angina pectoris. N Engl J Med. 1980;302:1–6. doi: 10.1056/NEJM198001033020101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figure S1


