Abstract
Background
The aim of this study was to prospectively evaluate the effects of renal artery stenting in consecutive patients with severe atherosclerotic renal artery stenosis and high‐risk clinical presentations as defined in a national protocol developed in 2015.
Methods and Results
Since the protocol was initiated, 102 patients have been referred for revascularization according to the following high‐risk criteria: severe renal artery stenosis (≥70%) with true resistant hypertension, rapidly declining kidney function, or recurrent heart failure/sudden pulmonary edema. At baseline, the mean 24‐hour ambulatory systolic blood pressure was 166.2 mm Hg (95% CI, 162.0–170.4), the defined daily dose of antihypertensive medication was 6.5 (95% CI, 5.8–7.3), and the estimated glomerular filtration rate was 41.1 mL/min per 1.73m2 (95% CI, 36.6–45.6). In 96 patients with available 3‐month follow‐up data, mean 24‐hour ambulatory systolic blood pressure decreased by 19.6 mm Hg (95% CI, 15.4–23.8; P<0.001), the defined daily dose of antihypertensive medication was reduced by 52% (95% CI, 41%–62%; P<0.001), and estimated glomerular filtration rate increased by 7.8 mL/min per 1.73m2 (95% CI, 4.5–11.1; P<0.001). All changes persisted after 24 month follow‐up. Among 17 patients with a history of hospitalization for acute decompensated heart failure, 14 patients had no new episodes after successful revascularization.
Conclusions
In this prospective cohort study, we observed a reduction in blood pressure and antihypertensive medication, an increase in estimated glomerular filtration rate, and a decrease in new hospital admissions attributable to heart failure/sudden pulmonary edema after renal artery stenting.
Registration
URL: https://clinicaltrials.gov. Identifier: NCT02770066.
Keywords: atherosclerotic renal artery stenosis, atherosclerotic renovascular disease, flash pulmonary edema, rapid loss of kidney function, renal revascularization, resistant hypertension
Subject Categories: Hypertension, Atherosclerosis, Vascular Disease, Revascularization
Nonstandard Abbreviations and Acronyms
- ABPM
ambulatory blood pressure monitoring
- CORAL
Cardiovascular Outcomes in Renal Atherosclerotic Lesions
- KDIGO
Kidney Disease: Improving Global Outcomes
- PTRA
percutaneous transluminal renal angioplasty
Clinical Perspective
What Is New?
In this prospective cohort study of well‐defined high‐risk patients with severe atherosclerotic renal artery stenosis established after the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial was published, we observed a reduction in blood pressure, an improvement in kidney function, and a decrease in new hospital admissions attributable to heart failure/sudden pulmonary edema after revascularization.
To increase the validity of our results, we performed 24‐hour ambulatory blood pressure monitoring after nurse‐administered medication to ensure patient adherence and included prestudy results on ambulatory blood pressure monitoring and kidney function to reduce the risk of regression to the mean.
What Are the Clinical Implications?
In carefully selected patients with severe atherosclerotic renal artery stenosis and high‐risk clinical presentations, renal artery stenting may be beneficial, but this should be confirmed in randomized clinical trials.
Our findings are in favor of the current US guideline from the Society for Cardiovascular Angiography and Interventions, which recommends renal artery stenting in patients with hemodynamically significant atherosclerotic renal artery stenosis when there is concomitant cardiac destabilization syndromes, resistant hypertension, or progressive ischemic nephropathy, as compared with the more restrictive European guideline.
Randomized clinical trials have failed to demonstrate benefits of renal artery stenting in addition to medical therapy in patients with atherosclerotic renal artery stenosis, 1 , 2 , 3 but these trials have significant limitations. The main limitation of these trials is that patients with the most severe forms of renovascular disease were excluded or not enrolled. 4 , 5 Accordingly, a recent comparative effectiveness review of renal artery stenosis management strategies commissioned by the Agency for Healthcare Research and Quality found that results from the randomized trials had limited applicability to many patients for whom renal artery stenting is recommended, particularly those who present with pulmonary edema or rapidly declining kidney function. 5 , 6 In the newest US guidelines from the Society for Cardiovascular Angiography and Interventions from 2017, renal artery stenting is considered appropriate for hemodynamically significant atherosclerotic renal artery stenosis (visual stenosis ≥70% or ≥50% with a resting translesional mean gradient ≥10 mm Hg) when there is concomitant cardiac destabilization syndromes (recurrent heart failure, sudden pulmonary edema, or acute coronary syndrome), resistant hypertension, or progressive ischemic nephropathy in patients with bilateral disease or a solitary functioning kidney. 4 , 7 These recommendations are in agreement with the conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference on the Heart, Kidney, and Vasculature held in February 2020. 8 In comparison, the European guideline from 2017 is less specific and more conservative and states that angioplasty may be considered in selected patients with significant atherosclerotic renal artery stenosis and recurrent heart failure or sudden pulmonary edema (class IIb Level of Evidence C) and in rare cases of acute oligo‐anuric kidney failure in patients with bilateral renal artery disease without significant kidney atrophy. 9
After the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) trial was published in 2014, 2 the 3 Danish centers offering percutaneous transluminal renal angioplasty (PTRA) agreed on following a common prospective study protocol limiting PTRA to patients with high‐risk clinical and radiological features of renovascular disease in a proof‐of‐concept study. Thus, the aim of the study was to prospectively evaluate the effects of renal artery stenting on blood pressure (BP), estimated glomerular filtration rate (GFR), and heart failure/pulmonary edema recurrences in a group of well‐defined patients with true resistant hypertension, rapidly declining kidney function, or recurrent heart failure/sudden pulmonary edema and with severe renal artery stenosis assessed by both visual estimation (>70% stenotic) and functional evaluation with Doppler ultrasound and renography.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
The DAN‐PTRA (NCT02770066) study is a prospective, single‐arm cohort study developed in 2015 by the 3 PTRA performing centers in Denmark. Two of the 3 centers adhered to the protocol in January 2015 and November 2016, respectively, and from then on included all patients consecutively referred for PTRA with stent placement according to the national study criteria. Together, the 2 centers covered ≈3.1 million of the 5.7 million inhabitants in Denmark. The study was performed in accordance with the Declaration of Helsinki. The study was reported to the Central Denmark Region Committees on Health Research Ethics and to the Danish Data Protection Agency. All patients provided written informed consent. No funding was provided.
Study Participants
To be eligible for renal artery stenting, patients were required to present with at least 1 of the following high‐risk clinical syndromes: (1) resistant hypertension with average 24‐hour ambulatory systolic BP ≥130 (mostly ≥150) mm Hg despite ≥3 antihypertensive drugs including a diuretic, if tolerated, and each prescribed at optimal doses, 7 (2) rapidly declining kidney function with a reduction in estimated GFR of >5 mL/min per 1.73m2 per year, or (3) hospital admissions with acute decompensated heart failure (≥2 hospitalizations for heart failure or ≥1 hospitalizations for sudden, “flash” pulmonary edema) with no obvious explanations such as nonadherence, left ventricular ejection fraction <40%, or valvular heart disease. In addition, computed tomographic or magnetic resonance angiography should demonstrate a stenosis of ≥70% reduction of the luminal diameter in at least 1 projection. Furthermore, Doppler ultrasound and renography with and without captopril were performed to assess the hemodynamic significance of the stenoses. PTRA was not performed if the length of the affected kidney was <7 cm.
Before referral for renal artery revascularization, each patient was discussed at a multidisciplinary meeting that, depending on the center, could involve the following disciplines: nephrology, cardiology vascular interventional radiology, and vascular surgery. Patients with bilateral disease but only 1 functioning kidney were initially referred for revascularization of the functioning kidney, but if the response was poor, the patient was subsequently evaluated for removal of the nonfunctioning kidney. Before angioplasty, patients not already receiving antiplatelet therapy were started on indefinite treatment with aspirin 75 mg/day. In addition, patients were advised to take lipid‐lowering drugs indefinitely and received counseling regarding smoking cessation, diet, and physical activity. Follow‐up visits after renal artery stenting were planned after 3, 12, 24, 36, 48, and 60 months and included 24‐hour ambulatory blood pressure monitoring (ABPM) after nurse‐administered medication, reassessment of kidney function, and Doppler ultrasound (if performed at the given center). Captopril renography was repeated after 24 months unless the patient had a solitary functioning kidney.
Ambulatory Blood Pressure Monitoring after Nurse‐Administered Medication
To improve the study validity, patient adherence to antihypertensive treatment was ensured by nurse‐administered medication before 24‐hour ABPM at baseline and at all follow‐up visits. According to this procedure, patients brought their medication packages to the clinic and were asked about their medication habits, and the medication was checked and administered from the original packing by a nurse (witnessed drug intake) immediately before the ABPM. ABPM was routinely performed with the oscillometric method (using either Spacelabs 90217, Spacelabs Healthcare, Hawthorne, WA; or Takeda TM‐2430, A&D Company Ltd., Tokyo, Japan) or in patients aged >80 years or with known supraventricular arrhythmia with the auscultatory method using DiaSys Integra II (Novacor, Rueil‐Malmaison, France). Measurement interval was ≤60 minutes both day and night. The defined daily dose of antihypertensive medication was calculated according to the World Health Organization Collaborating Center for Drugs Statistics Methodology Defined Daily Dose system to compare the use of different types of antihypertensive drugs. 10
Kidney Function
Estimated GFR was calculated with use of the Chronic Kidney Disease Epidemiology Collaboration creatinine equation. 11 Available data on kidney function (plasma creatinine and urine albumin‐creatinine ratio) 3, 12, and 24 months before angioplasty were collected retrospectively. If the urine albumin‐creatinine ratio was missing at a given time point but the patient had a negative urine dipstick, the latter was used and set to be equivalent to a urine albumin‐creatinine ratio of 29 mg/g (upper reference limit of a normal urine albumin‐creatinine ratio).
Doppler Ultrasound
Doppler ultrasound was performed by a few experienced operators and mostly included indirect parameters (pulsatility index and resistance index) in the evaluation of hemodynamic significant stenosis/restenosis. 12 , 13 Doppler ultrasound was performed at baseline and at each follow‐up visit.
Renography
Renography was performed with 99mtechnetium‐mercapto‐acetyl‐triglycine or 99mtechnetium‐diethylenetriaminepentaacetic acid. Captopril renography was carried out 60 minutes after 25 mg of captopril had been administered orally. Baseline renography was carried out after the patients had discontinued treatment with angiotensin‐converting enzyme inhibitor (ACEi) and angiotensin‐receptor blocker (ARB) for 5 to 10 days (according to local guidelines). Renograms were classified according to the consensus report on ACEi renography in low or intermediate/high probability for renovascular hypertension. 14 The following was classified as intermediate/high probability for renovascular hypertension: relative function of 1 kidney ≤30% or increased excretion time ≥11 minutes (≥grade 2 renogram) with no change between baseline and captopril renography or an improvement in split kidney function of ≥5% or a decrease in time to peak activity of at least 5 minutes (change ≥1 renogram grades) on the affected side on baseline renography compared with captopril renography. 14 , 15 , 16
Description of Stenoses
All noninvasive imaging and invasive angiographic findings were evaluated and described independently by 2 experienced radiologists, and stenoses were classified as <70%, 70% to 79%, 80% to 89%, ≥90%, or occlusion.
Renal Artery Stenting
PTRA with stent placement was performed via retrograde femoral or brachial approach with the use of different sheaths, guidewires, and balloon‐expandable stents according to local team policy.
Outcomes
The primary outcome measure was changes in 24‐hour ambulatory BP from baseline to 24 months after renal artery stenting in patients with 24‐hour ambulatory average systolic BP ≥150 mm Hg at baseline. Secondary outcome measures included changes in 24‐hour ambulatory BP from baseline to 24 months after renal artery stenting in patients with 24‐hour ambulatory average systolic BP ≥130 mm Hg at baseline, changes in defined daily doses of antihypertensive medication, changes in kidney function, periprocedural events (events ≤30 days after PTRA), and clinical events during follow‐up. Since only 4 patients had 24‐hour ambulatory average systolic BP <130 mm Hg at baseline, we chose to include all patients in the secondary outcome measure analysis of BP changes after renal artery stenting. Clinical events matched clinical end points in the CORAL trial (death from any cause, death from cardiovascular or renal causes, stroke, myocardial infarction, hospitalization for congestive heart failure, progressive kidney insufficiency, and permanent renal‐replacement therapy) and the same definitions were used. 2
Statistical Analysis
Categorical data are expressed as proportions and changes from baseline analyzed using McNemars test. Summary statistics for continuous variables are presented as means with standard deviations for variables with normal distribution or medians with ranges for skewed variables. Changes from baseline were analyzed using multivariate repeated measurements ANOVA. Model validation was performed by comparing observed and expected within‐subject SDs and correlations and by inspecting quantile‐quantile plots of the residuals. Prestudy and baseline systolic and diastolic 24‐hour ambulatory BP measurements were compared with the paired t‐test. Model validation was performed by inspecting Bland‐Altman plots and quantile‐quantile plots of the differences. Results are presented as means or geometric means (after back‐transformation of means of log‐transformed data) with 95% CI, as appropriate. Before log transformation, the defined daily dose of antihypertensive medication was set to 0.1 for patients not taking any antihypertensive medication after revascularization to avoid missing values. The conclusions were the same whether the defined daily dose was 0 or set to either 0.1 or 0.5 before log transformation. If patients needed dialysis before the PTRA procedure, the estimated GFR was set to 10 mL/min per 1.73m2. If patients started permanent renal‐replacement therapy during follow‐up, the estimated GFR was also set to 10 mL/min per 1.73m2, whereas the patients were excluded from further analyses regarding BP and antihypertensive treatment. Univariate and multivariable linear regression models were used to assess the relationship between potential predictors of the changes in 24‐hour ambulatory systolic BP and estimated GFR at 3‐month follow‐up after PTRA. Predictors were listed according to potential clinical significance and findings from previous studies. 12 , 17 , 18 The predictor variables included in the models were chosen by assessing their likely clinical significance, but the number of missing values was also taken into account. Several of the diagnostic predictor variables had missing values, and therefore both basic multivariable regression models including variables with no missing values (age, sex, body mass index, diabetes, 24‐hour ambulatory systolic BP, rapidly declining kidney function, recurrent heart failure/sudden pulmonary edema, and discontinuation of ACEi or ARB because of a ≥30% increase in estimated GFR) and extended multivariable regression models with the addition of variables with missing values (urine albumin‐creatinine ratio, Doppler ultrasound, renography, degree of stenosis, and performance of bilateral renal artery stenting) were considered. P values <0.05 were considered statistically significant. Data were analyzed using Stata version 16.1 (Lakeway Drive, TX).
Results
Study Cohort
From January 2015 to January 2021, a total of 102 patients were referred for PTRA with stent placement according to the common national criteria (Figure 1). Baseline characteristics are shown in Tables 1 and 2. In total, 95 of the 102 patients suffered from resistant hypertension, of whom 36 patients had no additional clinical indication for angioplasty. Only 3 of the 36 patients with resistant hypertension as the sole indication for renal artery stenting had baseline 24‐hour ambulatory systolic BP <150 mm Hg. In 5 patients, angioplasty was not possible because of total occlusion of the renal artery. The remaining 97 patients were successfully revascularized on at least 1 side and followed for a median of 24.4 months (interquartile range, 13.3–36.2) during which 5 patients started permanent renal‐replacement therapy and 10 patients died.
Figure 1. Flowchart for patients referred for angioplasty.

Table 1.
Baseline Characteristics of the Study Participants (N=102)
| Characteristics | |
|---|---|
| Age, y | 69.2 (62.5–76.3) |
| Female sex | 52 (51.0) |
| Body mass index, kg/m2 | 26.2 (22.8–30.1) |
| Ambulatory blood pressure readings, mm Hg | |
| 24‐h systolic ABPM | 166.2±21.6 |
| 24‐h diastolic ABPM | 82.3±12.3 |
| Daytime systolic ABPM | 168.0±21.3 |
| Daytime diastolic ABPM | 84.6±12.8 |
| Nighttime systolic ABPM | 161.9±25.0 |
| Nighttime diastolic ABPM | 77.6±12.7 |
| Duration of antihypertensive treatment, y | 10 (2–20) |
| Number of antihypertensives | 4.0±1.3 |
| Defined daily dose of antihypertensives | 6.3 (4.3–9.0) |
| Estimated GFR,* mL/min per 1.73 m2 | 39.7 (23.5–54.0) |
| Estimated GFR* <60 mL/min per 1.73 m2 | 82 (80.4) |
| Urine albumin‐creatinine ratio, mg/g | 61 (17–396) |
| Single kidney function | |
| Anatomical single kidney | 6 (5.9) |
| Functional single kidney | 17 (16.7) |
| Missing data | 10 (9.8) |
| Medical history and risk factors | |
| Diabetes (all type 2) | 20 (19.6) |
| History of ischemic heart disease | 27 (26.5) |
| History of cerebrovascular disease | 17 (16.7) |
| History of heart failure | 17 (16.7) |
| History of malignancy | 11 (10.8) |
| Current or former smoker | 84 (82.4) |
| Lipid‐lowering drug use | 87 (85.3) |
Data are mean±SD or median (interquartile ranges) for continuous variables and number (%) for categorical variables. ABPM indicates ambulatory blood pressure monitoring; and GFR, glomerular filtration rate.
The estimated GFR was calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration formula.
Table 2.
Indications for Angioplasty and Results of Baseline Investigations (N=102)
| Indications for angioplasty | |
|---|---|
| Resistant hypertension | 95 (93.1) |
| Decline in estimated GFR of ≥5 mL/min per 1.73 m2 per year* | 63 (61.8) |
| Recurrent heart failure/sudden pulmonary edema | 20 (19.6) |
| Subacute angioplasty † performed in | 14 (13.7) |
| Doppler ultrasound | |
| Performed in | 82 (80.4) |
| Size of right kidney, cm | 10.1±1.7 |
| Size of left kidney, cm | 9.9±1.9 |
| No signs of stenosis | 12 (11.8) |
| Signs of unilateral stenosis | 56 (54.9) |
| Signs of bilateral stenosis | 14 (13.7) |
| Missing | 20 (19.6) |
| Resistance index ≥0.8 in successfully treated kidneys (n=112) ‡ | 13 (11.6) |
| Resistance index <0.8 in successfully treated kidneys (n=112) ‡ | 67 (59.8) |
| Resistance index missing in successfully treated kidneys (n=112) ‡ | 32 (28.6) |
| Renography | |
| Low probability of renal artery stenosis | 12 (11.8) |
| Intermediate/high probability of renal artery stenosis | 75 (73.5) |
| Missing | 15 (14.7) |
| Imaging before angioplasty | |
| Computed tomographic angiography | 93 (91.2) |
| Magnetic resonance angiography | 5 (4.9) |
| No imaging before angioplasty | 4 (3.9) |
| Identified renal arteries | 204 |
| Bilateral disease § | 53 (52.0) |
| Angiographic findings | |
| Bilateral disease § | 55 (53.9) |
| Bilateral renal artery stenting | 15 (14.7) |
| Number of renal artery stentings || | 113 |
| Renal artery stenosis <70% ¶ | 3 (2.7) |
| Renal artery stenosis 70%–79% | 22 (19.5) |
| Renal artery stenosis 80%–89% | 32 (28.3) |
| Renal artery stenosis ≥90% | 46 (39.8) |
| Nonassessable | 10 (8.8) |
Data are mean±SD for continuous variables and number (%) for categorical variables.
GFR indicates glomerular filtration rate.
The estimated GFR was calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration formula.
In 14 patients, revascularization was performed subacutely in conjunction with hospitalization for pulmonary edema, severe hypertension, or acute kidney injury refractory to medical therapy.
A total of 97 patients had renal artery stenting performed and in 15 cases on both sides resulting in 112 treated kidneys.
Bilateral disease was defined as stenosis of ≥70% of the diameter of at least 1 artery supplying each kidney.
In 1 patient, 2 renal arteries supplying the same kidney were stented.
Stenting performed in conjunction with stenting of a stenosis of ≥70% of the artery supplying the other kidney.
Stenting and Periprocedural Events
All PTRA procedures in the study included stent placement. Only 3 of the stented arteries were <70% stenotic and these arteries were stented in conjunction with contralateral stenting of a >70% renal artery stenosis (Table 2). In 10 patients referred for renal artery stenting on both sides, revascularization was possible on only 1 side. In 14 patients, revascularization was performed subacutely in conjunction with hospitalization for pulmonary edema, severe hypertension, or acute kidney injury that was refractory to medical therapy. There were 12 procedure‐related complications but no procedure‐related deaths. Four patients experienced either dissection, rupture, or thrombosis of the renal main or branch arteries, which in 1 case resulted in a reduction in estimated GFR from 38 to 18 mL/min per 1.73m2, although, surprisingly, estimated GFR slowly increased and returned to baseline level at 24‐month follow‐up. Two patients had embolism to the kidneys, but this did not affect the kidney function. Two patients who were treated subacutely because of refractory pulmonary edema developed respiratory failure during the procedure, and both patients recovered at the intensive care unit. Four patients developed either femoral or brachial artery pseudoaneurysms located at puncture sites. The observed rate of serious periprocedural events was similar to the event rate observed in the CORAL trial (Table S1). 2
Quality of BP Measurements
A total of 397 BP measurements were available for analysis. Of these measurements, 374 (94%) were performed with a standard cuff‐based oscillometric or auscultatory device and 10 (3%) with a cuffless SOMNOtouch NIBP device (Somnomedics GmbH, Randersacker, Germany), and 96% of these measurements were performed after nurse‐administered medication. The cuff‐less SOMNOtouch NIBP device was used only for 24‐hour ABPM if the patient could not accept use of the standard cuff‐based method. Twelve BP measurements in 8 patients (3%) were made with either automated office BP or home BP measurements because ABPM could not be performed. Automated office BP was recorded with the BpTRU device (BpTRU Medical Devices Ltd., Coquitlam, BC, Canada) set to measure 6 times with an interval of 5 minutes and with the patient resting quietly and alone. The result was the mean of the last 5 measurements. Finally, 1 patient was treated in the intensive care unit with intravenous antihypertensive drugs and hemodialysis before subacute renal artery stenting and baseline BP was calculated as the mean of the 24‐hour invasive BP measurements in the intensive care unit before angioplasty.
Ambulatory Blood Pressure and Antihypertensive Medication
Both systolic and diastolic BP decreased significantly after renal artery stenting (Figure 2 and Table 3). At 3‐month follow‐up, 24‐hour ambulatory systolic BP after witnessed drug intake was reduced by 19.6 mm Hg (95% CI, 15.4–23.8; P<0.001) and 24‐hour ambulatory diastolic BP by 8.4 mm Hg (95% CI, 6.3–10.4; P<0.001) compared with baseline, and the reductions persisted throughout the follow‐up period. Of the 97 successfully treated patients, 60 patients had a meaningful decrease in 24‐hour ambulatory systolic BP of at least 10 mm Hg (13 had a BP reduction between 10 and 19 mm Hg, 18 had a BP reduction between 20 and 29 mm Hg, and 29 had a BP reduction ≥30 mm Hg) from baseline to 3‐month follow‐up. Of the remaining 37 patients, 35 patients had a reduction of <10 mm Hg, and 2 patients had no follow‐up data because one started chronic dialysis and the other was lost to follow‐up. Within a subgroup of 55 patients with available prestudy 24‐hour ambulatory BP measured on average 4.9±3.2 months before baseline, there was no significant difference between prestudy and baseline 24‐hours ambulatory BP (difference in the systolic BP of 1.2 mm Hg (95% CI, −4.5 to 6.8; P=0.68) and in the diastolic BP of 0.6 mm Hg (95% CI, −2.3 to 3.5; P=0.67)) (Figure 2). With regard to the primary outcome measure, 81 patients had ambulatory systolic BP ≥150 mm Hg at baseline, and in this subgroup the 24‐hour ambulatory systolic BP was reduced by 32.2 mm Hg (95% CI, 26.4‐37.9; P<0.001) from baseline to 24‐month follow‐up (Table 3).
Figure 2. 24‐h ambulatory blood pressure (BP) and antihypertensive medication.

A, Mean values for 24‐h ambulatory systolic and diastolic BP; and (B) geometric mean values for the Defined Daily Dose of antihypertensive medication with and without loop diuretics included. In (A) the results of prestudy ambulatory blood pressure monitoring for 55 patients are shown as a dotted circle for mean systolic BP and as a dotted square for mean diastolic BP. Error bars are 95% CIs.
Table 3.
Baseline Values and Changes from Baseline Are Derived From Multivariate Repeated Measurements ANOVA
| Parameter | No. of patients* | Baseline values and changes from baseline (95% CI) * | P value | |
|---|---|---|---|---|
| 24‐h ambulatory BP, full cohort | ||||
| Systolic BP, mm Hg | Mean changes from baseline | |||
| Baseline | 102 | 166.2 | (162.0 to 170.4) | |
| 3 mo | 95 | −19.6 | (−23.8 to −15.4) | <0.001 |
| 12 mo | 85 | −28.2 | (−33.7 to −22.7) | <0.001 |
| 24 mo | 59 | −25.7 | (−30.8 to −20.6) | <0.001 |
| Diastolic BP, mm Hg | Mean changes from baseline | |||
| Baseline | 102 | 82.3 | (79.9 to 84.7) | |
| 3 mo | 95 | −8.4 | (−10.4 to −6.3) | <0.001 |
| 12 mo | 85 | −10.6 | (−13.1 to −8.2) | <0.001 |
| 24 mo | 59 | −9.2 | (−11.7 to −6.8) | <0.001 |
| 24‐h ambulatory BP, subgroup with 24‐h ambulatory systolic BP ≥150 mm Hg at baseline | ||||
| Systolic BP, mm Hg | Mean changes from baseline | |||
| Baseline | 81 | 174.0 | (170.4 to 177.6) | |
| 3 mo | 75 | −22.9 | (−28.2 to −17.7) | <0.001 |
| 12 mo | 66 | −35.8 | (−41.6 to −30.0) | <0.001 |
| 24 mo (primary outcome measure) | 46 | −32.2 | (−37.9 to −26.4) | <0.001 |
| Diastolic BP, mm Hg | Mean changes from baseline | |||
| Baseline | 81 | 84.3 | (81.6 to 86.9) | |
| 3 mo | 75 | −9.5 | (−12.0 to −6.9) | <0.001 |
| 12 mo | 66 | −13.1 | (−15.9 to −10.3) | <0.001 |
| 24 mo (primary outcome measure) | 46 | −11.3 | (−14.0 to −8.6) | <0.001 |
| Antihypertensive medications | ||||
| Defined daily dose of antihypertensives including loop‐diuretics | Geometric mean ratios (ref. baseline) | |||
| Baseline | 102 | 6.5 | (5.8 to 7.3) | |
| 3 mo | 95 | 0.48 | (0.38 to 0.59) | <0.001 |
| 12 mo | 85 | 0.55 | (0.45 to 0.68) | <0.001 |
| 24 mo | 59 | 0.50 | (0.40 to 0.64) | <0.001 |
| Number of antihypertensives | Mean changes from baseline | |||
| Baseline | 102 | 4.0 | (3.7 to 4.2) | |
| 3 mo | 95 | −1.0 | (−1.3 to −0.7) | <0.001 |
| 12 mo | 85 | −0.7 | (−1.0 to −0.4) | <0.001 |
| 24 mo | 59 | −0.9 | (−1.3 to −0.5) | <0.001 |
| Estimated GFR, full cohort | ||||
| Estimated GFR,† mL/min per 1.73 m2 | Mean changes from baseline | |||
| −24 mo | 84 | 19.7 | (15.7 to 23.8) | <0.001 |
| −12 mo | 91 | 12.0 | (8.4 to 15.7) | <0.001 |
| −3 mo | 101 | 3.3 | (0.8 to 5.7) | 0.009 |
| Baseline | 102 | 41.1 | (36.6 to 45.6) | |
| 3 mo | 96 | 7.8 | (4.5 to 11.1) | <0.001 |
| 12 mo | 89 | 5.3 | (2.1 to 8.5) | 0.001 |
| 24 mo | 63 | 7.2 | (3.2 to 11.2) | 0.001 |
| Estimated GFR, subgroup with rapid decline in estimated GFR at baseline | ||||
| Estimated GFR, † mL/min per 1.73 m2 | Mean changes from baseline | |||
| −24 mo | 50 | 30.4 | (25.6 to 35.1) | <0.001 |
| −12 mo | 55 | 19.2 | (14.3 to 24.1) | <0.001 |
| −3 mo | 62 | 6.1 | (2.7 to 9.5) | 0.001 |
| Baseline | 63 | 29.9 | (25.7 to 34.0) | |
| 3 mo | 59 | 12.5 | (7.9 to 17.0) | <0.001 |
| 12 mo | 53 | 8.8 | (4.7 to 13.0) | <0.001 |
| 24 mo | 33 | 12.8 | (7.5 to 18.1) | <0.001 |
| Albuminuria | ||||
| Urine albumin‐creatinine ratio‡ | Geometric mean ratios (ref. baseline) | |||
| −24 mo | 56 | 0.47 | (0.31 to 0.71) | 0.001 |
| −12 mo | 73 | 0.57 | (0.41 to 0.79) | 0.001 |
| −3 mo | 79 | 1.11 | (0.88 to 1.41) | 0.38 |
| Baseline (mg/g) | 95 | 66.9 | (46.5 to 96.3) | |
| 3 mo | 83 | 1.13 | (0.79 to 1.62) | 0.51 |
| 12 mo | 83 | 0.57 | (0.40 to 0.82) | 0.003 |
| 24 mo | 59 | 0.68 | (0.44 to 1.05) | 0.08 |
Data are mean at baseline and mean changes from baseline (95% CI) for ambulatory blood pressure, number of antihypertensives, and estimated GFR and geometric mean at baseline and geometric mean ratios (95% CI) for the defined daily dose of antihypertensives and for the urine albumin‐creatinine ratio. BP indicates blood pressure; and GFR, glomerular filtration rate.
No. of patients* = * If a patient started permanent renal‐replacement therapy during follow‐up, the estimated GFR was set to 10 mL/min per 1.73m2 and the patient was excluded from further analyses regarding BP and antihypertensive treatment.
Baseline values and changes from baseline (95% CI) † = † Using a paired t‐test to calculate the changes in the same patients over time led to only minor changes in the results and did not change the conclusions.
Estimated GFR, ‡ mL/min per 1.73 m2‡ = ‡ The estimated GFR was calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration formula.
Urine albumin‐creatinine ratio§ = § A total of 584 urine albumin‐creatinine ratios were available for the analysis and, of these, 21 (18 before baseline and 3 after baseline) were assigned a value of 29 mg/g because the ratio was not measured but the patient had a negative urine dipstick at the given time point.
At 3‐month follow‐up, the defined daily dose of antihypertensive medication with loop diuretics included was reduced by 52% (95% CI, 41%–62%; P<0.001), and when loop diuretics were excluded from the analysis, the reduction was 48% (95% CI, 36%–58%; P<0.001) compared with baseline and the reductions persisted through follow‐up (Figure 2 and Table 3). The observed reduction in the defined daily dose of antihypertensive medication corresponded to a significant reduction (P<0.001) in the number of antihypertensives used from 4.0 (95% CI, 3.7–4.2) at baseline to 3.0 (95% CI, 2.7–3.2) at 3‐month follow‐up (Table 3). The proportions of patients treated with the different classes of antihypertensives at baseline and last follow‐up visit are shown in Table 4. Of note, the proportion of patients treated with either an ACEi or an ARB increased significantly from 40.6% at baseline to 71.9% at last follow‐up visit, whereas the use of most other drug classes decreased significantly. Importantly, however, only 7 of the 62 patients who were not taking an ACEi/ARB at baseline had never been treated with these drug classes. In the remaining 55 patients, treatment with ACEis/ARBs was discontinued for a median of 5.0 months (interquartile range, 2.1–10.3) before PTRA for the following reasons: increase of ≥30% in P‐creatinine (n=41); hyperkalemia (n=2); suspicion, evaluation, or a diagnosis of renal artery stenosis (n=11); or angioedema (n=1). According to the hospital records, treatment with an ACEi/ARB was given for a median of 24.9 months (interquartile range, 4.1–95.9) before it was discontinued, but this may underestimate the true duration of the treatment because treatment with these drug classes were often started by the patient’s general practitioner before it was registered in the hospital system.
Table 4.
Use of Antihypertensive Medication at Last Visit Compared With Baseline
| Antihypertensive medication | Proportion at baseline, % | Proportion at last visit, % | Difference, % (95% CI) | P value | |
|---|---|---|---|---|---|
| ACEi/ARB | 40.6 | 71.9 | 31.3 | (20.1 to 42.4) | <0.001 |
| Alpha blockers | 36.5 | 12.5 | −24.0 | (−35.7 to −12.2) | <0.001 |
| Alpha and beta blockers | 19.8 | 12.5 | −7.3 | (−14.3 to −0.3) | 0.02 |
| Beta blockers | 61.5 | 41.7 | −19.8 | (−31.5 to −8.1) | 0.001 |
| Calcium channel blockers | 87.5 | 66.7 | −20.8 | (−32.3 to −9.4) | <0.001 |
| Diuretics | 93.8 | 80.2 | −13.5 | (−23.1 to −4.0) | 0.003 |
| Thiazides | 34.4 | 17.7 | −16.7 | (−27.6 to −5.8) | 0.002 |
| Loop diuretics | 64.6 | 54.2 | −10.4 | (−22.1 to 1.2) | 0.06 |
| Potassium‐sparing agents§ | 28.1 | 24.0 | −4.2 | (−15.6 to 7.3) | 0.43 |
| Centrally acting agents† | 14.6 | 1.0 | −13.5 | (−22.0 to −5.1) | <0.001 |
| Direct vasodilators † | 8.3 | 3.1 | −5.2 | (−11.6 to 1.1) | 0.06 |
McNemars test was used to compare proportion at baseline with proportion at last visit. ACEi indicates angiotensin‐converting enzyme inhibitor; and ARB, angiotensin receptor blocker.
Potassium‐sparing agents* = * Spironolactone, eplerenone, or amiloride.
Centrally acting agents† = † Methyldopa or moxonidine.
Direct vasodilators‡= ‡ Hydralazine or minoxidil.
Kidney Function Before Stenting
Data on plasma creatinine 3, 12, and 24 months before renal artery stenting were collected retrospectively and were available for 99% of the patients at −3.0 months (95% CI, −3.2 to −2.9), for 89% at −12.0 months (95% CI, −12.5 to −11.5), and for 82% at −24.5 months (95% CI, −25.2 to −23.8). From −24 months to baseline, estimated GFR decreased by overall 19.7 mL/min per 1.73m2 (95% CI, 15.7–23.8; P<0.001) and in the subgroup of patients with rapidly declining kidney function at baseline by 30.4 mL/min per 1.73m2 (95% CI, 25.6–35.1; P<0.001). The urine albumin‐creatinine ratio before renal artery stenting was available for 77% of the patients at −3.7 months (95% CI, −4.1 to −3.3), for 72% at −11.8 months (95% CI, −12.6 to −10.9), and for 55% at −25.2 months (95% CI, −26.6 to −23.9) and increased significantly by a factor of 2.1 (95% CI, 1.4–3.2, P<0.001) from −24 month to baseline (Figure 3 and Table 3).
Figure 3. Estimated glomerular filtration rate (GFR) and urine albumin‐creatinine ratio.
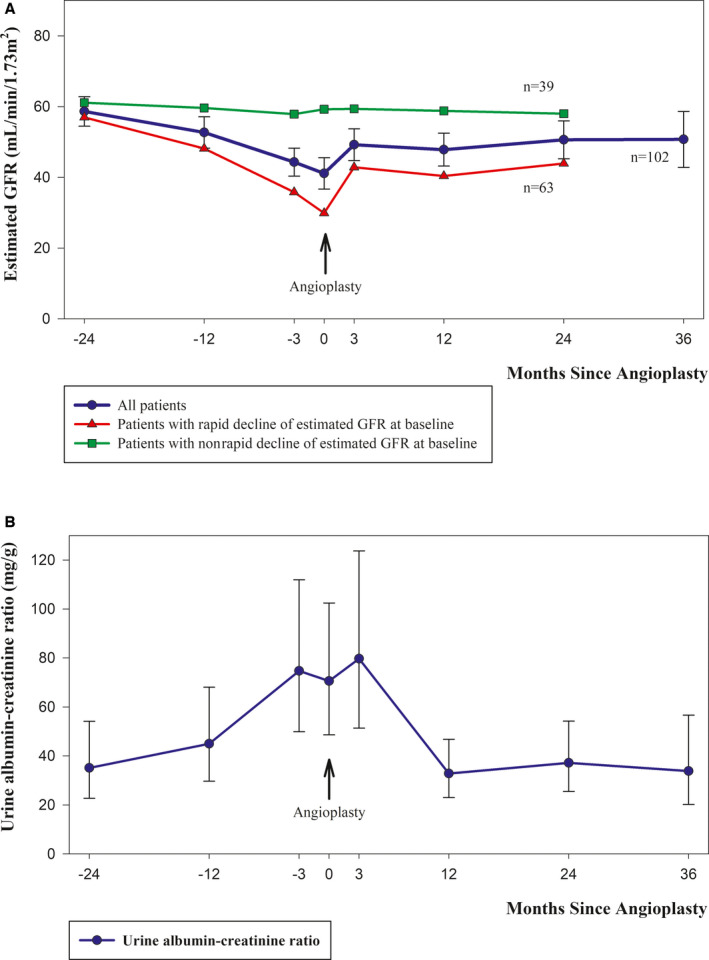
A, Mean values for estimated GFR for all patients and for the subgroups of patients with rapidly declining kidney function and nonrapidly declining kidney function before renal artery stenting. The mean values for estimated GFR for the subgroups are shown until 24 months because of sparse data thereafter. B, Geometric mean values for the urine albumin‐creatinine ratio. Error bars are 95% CIs.
Kidney Function After Stenting
At 3‐month follow‐up after renal artery stenting, the mean estimated GFR had increased significantly by overall 7.8 mL/min per 1.73m2 (95% CI, 4.5–11.1; P<0.001) and in the subgroup of 63 patients with rapidly declining kidney function at baseline by 12.5 mL/min per 1.73m2 (95% CI, 7.9–17.0; P<0.001), and the observed changes persisted throughout the follow‐up period. Four patients required dialysis before renal artery stenting because of acute kidney injury, and 2 patients with successful renal artery stenting regained kidney function, whereas 2 patients with unsuccessful renal artery stenting continued on chronic dialysis. Among the 63 patients with rapidly declining kidney function at baseline, 59 patients had successful revascularization, and among these patients, the estimated GFR at last follow‐up after a median of 23.9 months (interquartile range, 12.2–36.8) was reduced by >5 mL/min per 1.73m2 in 8 patients, of whom 3 had started chronic dialysis; was unchanged (deviated by ≤5 mL/min per 1.73m2 from baseline value) in 24 patients; and had increased by >5 mL/min per 1.73m2 in 27 patients. The urine albumin‐creatinine ratio decreased significantly after renal artery stenting and was reduced by 43% (95% CI, 18%–60%; P=0.003) at 12‐month follow‐up compared with baseline. In 32 patients who were treated for unilateral renal artery stenosis, captopril renography 24 months after revascularization showed that kidney function on the treated side had increased significantly by 13% (95% CI, 7%–20%; P<0.003) compared with captopril renography at baseline.
Recurrent Heart Failure/Sudden Pulmonary Edema
Twenty patients with a history of recurrent heart failure or sudden, “flash” pulmonary edema, were referred to renal artery stenting. In 3 of these patients, renal artery stenting was not possible because of total renal artery occlusion. Of the successfully treated patients, 14 (82%) patients had no new hospital admissions because of congestive heart failure during follow‐up, whereas 3 patients continued to have episodes of heart failure/pulmonary edema and suffered from progressive loss of kidney function, leading to chronic dialysis in 2 patients.
Predictors of Changes in 24‐Hour Ambulatory Systolic BP and Estimated GFR After PTRA
Univariate and multivariable linear regression analyses for changes in systolic BP and estimated GFR after PTRA are presented in Tables S2 and S3. The analyses revealed several interesting findings but should be interpreted with caution since there were missing values in the extended analyses and the 95% CIs were in general rather wide. In the univariate and multivariable models for change in 24‐hour ambulatory systolic BP at 3‐month follow‐up, increasing 24‐hour ambulatory systolic BP at baseline and discontinuation of ACEi/ARB because of an increase of ≥30% in P‐creatinine were associated with a favorable BP response, whereas increasing age was associated with an unfavorable BP response (Table S2). In the similar models for change in estimated GFR at 3‐month follow‐up, female sex, increasing 24‐hour ambulatory systolic BP, rapidly declining kidney function, recurrent heart failure/sudden pulmonary edema, and angiographic stenosis ≥90 were the most important factors associated with a favorable response in estimated GFR after PTRA, whereas a resistance index ≥0.8 with Doppler ultrasound was associated with an unfavorable response in the multivariable model (Table S3).
Clinical Events
Among the 97 patients with successful renal artery stenting, 17 patients had at least 1 clinical event during follow‐up (only the first event in each category was included). The clinical events were stroke (n=3), acute myocardial infarction (n=2), hospitalization for congestive heart failure (n=5), reduction of estimated GFR ≥30% and not on permanent renal‐replacement therapy (n=2), permanent renal‐replacement therapy (n=5), and death (n=10). Causes of death were cardiovascular (n=1), cancer (n=4), infection (n=4), and a perforated peptic ulcer (n=1). The 5 patients requiring permanent renal‐replacement therapy during follow‐up were started on dialysis after 2, 149, 186, 295, and 472 days, respectively. The patient who started chronic dialysis only 2 days after renal artery stenting underwent subacute revascularization because of refractory heart failure and acute kidney injury, but kidney function did not improve. In comparison, the 5 patients with unsuccessful but otherwise uncomplicated angioplasty had a poor prognosis. Four patients died within 6 months from a renal cause (n=1), infection (n=2), and an unknown cause (n=1), and 1 patient with a baseline estimated GFR of 12 mL/min per 1.73m2 started chronic hemodialysis after 37 days. Four of the 5 patients were referred for subacute renal artery stenting because of treatment‐refractory heart failure/pulmonary edema or acute kidney failure.
Reangioplasty and Contralateral Nephrectomy After Primary Renal Artery Stenting
Elective reangioplasty with stent placement was performed successfully in 4 patients with restenosis after a median of 12.5 months (interquartile range, 10.1–24.1). Acute reangioplasty was performed in 1 patient who became anuric on the day after removal of a nonfunctioning kidney because of development of an in‐stent thrombosis in the renal artery to the remaining kidney. Reangioplasty was performed 2 days after the nephrectomy, and the patient needed temporary hemodialysis but regained kidney function, although estimated GFR decreased from ≈45 to 30 mL/min per 1.73m2.
Five patients with a solitary functioning kidney and a poor response to renal artery stenting were subsequently referred to nephrectomy on the contralateral side in an attempt to improve BP control. Three of these nephrectomies were complicated by an incisional hernia, an intra‐abdominal hematoma requiring blood transfusion, and, as already mentioned, an in‐stent thrombosis in the contralateral renal artery, respectively.
Discussion
In this prospective cohort study of patients with severe atherosclerotic renal artery stenosis and well‐defined high‐risk clinical presentations, we observed a reduction in BP and antihypertensive medication, an increase in estimated GFR, and a decrease in new hospital admissions because of heart failure/sudden pulmonary edema after renal artery stenting. Three months after revascularization, mean 24‐hour ambulatory systolic BP after witnessed drug intake was reduced by ≈20 mm Hg, and 60 of 97 successfully treated patients had a meaningful decrease in 24‐hour ambulatory systolic BP of at least 10 mm Hg (including 29 patients with a decrease ≥30 mm Hg), although the mean number of antihypertensive medications was reduced. In the CORAL trial, mean systolic BP declined in the stent group by 16.6 mm Hg and in the medical therapy–only group by 15.6 mm Hg, but in that trial, the number of antihypertensive medications was increased equally in both groups. 2 Patients with severe renovascular hypertension are generally poor responders to antihypertensive treatment. To evaluate the validity and reproducibility of the baseline ambulatory BP measurements, we included prestudy 24‐hour ambulatory BP results available for 55 patients and found no significant difference between prestudy and baseline results. Taken together, renal artery stenting in this selected patient population was associated with improved BP control and tolerance to blockage of the renin‐angiotensin system. A similar effect of renal artery stenting on ABPM in patients with true resistant hypertension has recently been reported in a retrospective single‐center study. 17 Baseline predictors for favorable systolic BP response after successful revascularization were increasing systolic BP and a history of discontinuation of an ACEi/ARB because of a >30% increase in P‐creatinine, whereas increasing age predicted an unfavorable response.
In up to 12% of patients with kidney failure, the attributable cause may be progressive ischemic nephropathy caused by atherosclerotic renal artery stenosis. 4 Our study included data on kidney function from 24 months before renal artery stenting, and our observations suggest that revascularization may change the natural course of ischemic kidney injury in patients with severe renal artery stenosis. Thus, in 85% of successfully treated patients with rapidly declining kidney function at baseline, the estimated GFR was unchanged or had improved at last follow‐up compared with baseline. Furthermore, captopril renography 24 months after renal artery stenting showed a significant improvement of the kidney function on the treated side. Finally, we observed a significant decrease in albuminuria after renal artery stenting. Taken together, our observations suggest that renal artery stenting can stabilize and even increase estimated GFR in patients with rapidly declining kidney function. This conclusion concurs with a recently published retrospective study of the effect of renal artery stenting in patients with rapidly deteriorating kidney function. 19 Baseline predictors for favorable response in kidney function after successful revascularization included severity of clinical presentation at baseline (increasing systolic BP, rapidly declining kidney function, and recurrent heart failure/sudden pulmonary edema) and angiographic stenosis ≥90%. Of interest, a resistance index ≥0.8 was associated with an unfavorable response in estimated GFR after PTRA, which is in agreement with a previous study. 12
Among the patients with a history of recurrent heart failure or sudden pulmonary edema before renal artery stenting, 82% of the successfully treated patients had no new hospital admissions for congestive heart failure in the follow‐up period. The beneficial effect of renal artery stenting in this particular group of high‐risk patients is supported by other nonrandomized trials. 19 , 20 Although our results concur with a number of nonrandomized trials on high‐risk patients with severe atherosclerotic renal artery stenosis, 5 , 6 they are not in accordance with the 3 largest randomized clinical trials. 1 , 2 , 3 Compared with these trials, the patients in our study had more severe renal artery stenoses and more severe clinical presentations. This may also explain why patients in the CORAL trial tolerated treatment with ARB (candesartan), whereas treatment with ACEis/ARBs in this study was discontinued in 55 of 62 patients not taking these drugs at baseline, mostly because of an increase of at least 30% in P‐creatinine (n=41). Although the fraction of patients treated with an ACEi/ARB increased from 41% at baseline to 72% during follow‐up, it is unlikely that this alone should explain the observed reduction in BP as the proportion of patients treated with an ACEi/ARB at 3‐month follow‐up had only increased by 14% (95% CI, 3%–24%; P=0.01) compared with an overall reduction in the defined daily dose of antihypertensive medications of 52%.
Clinical events were defined using the same criteria as in the CORAL study, 2 but patients were followed for a median of 24 months in this study compared with 43 months in the CORAL study. Among the 97 successfully treated patients, there were 10 cardiovascular events in 8 patients (the first event in each of the following categories was included: stroke, acute myocardial infarction, and hospitalization for congestive heart failure), 5 (5%) patients started permanent renal‐replacement therapy, and 10 (10%) patients died from any cause. Among the total number of participants in the CORAL study (n=931), there were 194 cardiovascular events, 24 (3%) patients started permanent renal‐replacement therapy, and 139 (15%) patients died from any cause during follow‐up. Considering the shorter follow‐up in the present study, the clinical event rate seemed higher than observed in the CORAL study, but patients in the present study had higher BP (daytime ambulatory systolic BP 168 mm Hg versus office BP 150 mm Hg in the CORAL study) and lower estimated GFR (40 mL/min per 1.73m2 versus 58 mL/min per 1.73m2 in the CORAL study). In a more comparable retrospective study including patients with high‐risk presentations (n=467), 155 (33%) patients had a cardiovascular event (only 1 event per participant was included), 18% reached end‐stage kidney disease, and 55% died during a median follow‐up of 46 months. 20 Finally, as previously noted, 5 patients in the present study with unsuccessful but otherwise uncomplicated angioplasty had a very poor prognosis, as 4 patients died within 6 months and 1 patient started chronic hemodialysis 37 days after the attempt of revascularization.
Taken together, it seems plausible that some of the discrepancy between nonrandomized and randomized studies may be explained by patient selection, as recently pointed out in a comparative effectiveness review by the Agency for Healthcare Research and Quality. 5
The major limitation of this prospective study is the absence of a control group. However, it may not be possible to include patients with the most severe renovascular syndromes in randomized clinical trials and avoid crossover between groups, since these patients are often relatively refractory to medical therapy and guidelines already recommend renal artery stenting for these patients. 7 To increase the quality and reproducibility of our results, we performed 24‐hour ambulatory BP monitoring after nurse‐administered medication to ensure patient adherence to prescribed medication. Furthermore, we included prestudy results of 24‐hour ambulatory BP monitoring and kidney function to reduce the risk of regression to the mean and to demonstrate a true effect of renal artery stenting on BP control and on kidney function.
If we knew with certainty that alternative therapies would never produce similar results, our data would provide an approach to identify the patients that might benefit from the procedure, despite the severity of their atherosclerotic morbidity/mortality and the unavoidable presence of procedural complications. This would require a randomized comparison of PTRA versus noninvasive management in a population with these high‐risk characteristics. Until such a trial is available, our findings can serve to support current recommendations in the US guideline from the Society for Cardiovascular Angiography and Interventions from 2017 7 and in the KDIGO conference report from 2021. 8
Sources of Funding
None.
Disclosures
Dr Olsen reports grants from Novo Nordic Foundation outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Table S1–S3
Acknowledgments
The authors are indebted to the patients for participating. We also thank Ninna Lundorf, Line Lanstorp, Eva Madsen, Lene Schlamovitz, and Karin Hansen for the skilled performance of nurse‐administered medication before ambulatory blood pressure monitoring, which was of utmost importance for the interpretation and validity of the results in the present study.
For Sources of Funding and Disclosures, see page 14.
References
- 1. Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150:840–841. doi: 10.7326/0003-4819-150-12-200906160-00119 [DOI] [PubMed] [Google Scholar]
- 2. Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, et al. Stenting and medical therapy for atherosclerotic renal‐artery stenosis. N Engl J Med. 2014;370:13–22. doi: 10.1056/NEJMoa1310753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, et al. Revascularization versus medical therapy for renal‐artery stenosis. N Engl J Med. 2009;361:1953–1962. doi: 10.1056/NEJMoa0905368 [DOI] [PubMed] [Google Scholar]
- 4. Prince M, Tafur JD, White CJ. When and how should we revascularize patients with atherosclerotic renal artery stenosis? JACC Cardiovasc Interv. 2019;12:505–517. doi: 10.1016/j.jcin.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 5. Raman G, Adam GP, Halladay CW, Langberg VN, Azodo IA, Balk EM. Comparative effectiveness of management strategies for renal artery stenosis: an updated systematic review. Ann Intern Med. 2016;165:635–649. doi: 10.7326/m16-1053 [DOI] [PubMed] [Google Scholar]
- 6. Balk EM, Raman G, Adam GP, Halladay CW, Langberg VN, Azodo IA, Trikalinos TA. Renal Artery Stenosis Management Strategies: An Updated Comparative Effectiveness Review. Comparative Effectiveness Review No. 179. (Prepared by the Brown Evidence‐based Practice Center under Contract No. 290‐2012‐00012‐I.) AHRQ Publication No. 16‐EHC026‐EF. Rockville, MD: Agency for Healthcare Research and Quality; August 2016. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed March 14, 2022. [PubMed]
- 7. Klein AJ, Jaff MR, Gray BH, Aronow HD, Bersin RM, Diaz‐Sandoval LJ, Dieter RS, Drachman DE, Feldman DN, Gigliotti OS, et al. SCAI appropriate use criteria for peripheral arterial interventions: an update. Catheter Cardiovasc Interv. 2017;90:E90–E110. doi: 10.1002/ccd.27141 [DOI] [PubMed] [Google Scholar]
- 8. Hicks CW, Clark TWI, Cooper CJ, de Bhailis ÁM, De Carlo M, Green D, Małyszko J, Miglinas M, Textor SC, Herzog CA, et al. Artherosclerotic renovascular disease: a KDIGO (Kidney Disease: Improving Global Outcomes) controversies conference. Am J Kidney Dis. 2022;79:289‐301. doi: 10.1053/j.ajkd.2021.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aboyans V, Ricco J‐B, Bartelink M‐L, Björck M, Brodmann M, Cohnert T, Collet J‐P, Czerny M, De Carlo M, Debus S, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO),The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 10. WHO Collaborating Centre for Drug Statistics Methodology . The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults. Available at: https://www.whocc.no/atc_ddd_index/. Accessed March 14, 2022.
- 11. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radermacher J, Chavan A, Bleck J, Vitzthum A, Stoess B, Gebel MJ, Galanski M, Koch KM, Haller H. Use of Doppler ultrasonography to predict the outcome of therapy for renal‐artery stenosis. N Engl J Med. 2001;344:410–417. doi: 10.1056/nejm200102083440603 [DOI] [PubMed] [Google Scholar]
- 13. Zeller T, Bonvini RF, Sixt S. Color‐coded duplex ultrasound for diagnosis of renal artery stenosis and as follow‐up examination after revascularization. Catheter Cardiovasc Interv. 2008;71:995–999. doi: 10.1002/ccd.21525 [DOI] [PubMed] [Google Scholar]
- 14. Taylor A, Nally J, Aurell M, Blaufox D, Dondi M, Dubovsky E, Fine E, Fommei E, Geyskes G, Granerus G, et al. Consensus report on ACE inhibitor renography for detecting renovascular hypertension. Radionuclides in Nephrourology Group. Consensus Group on ACEI Renography. J Nucl Med. 1996;37:1876–1882. [PubMed] [Google Scholar]
- 15. Johansson M, Jensen G, Aurell M, Friberg P, Herlitz H, Klingenstierna H, Volkmann R. Evaluation of duplex ultrasound and captopril renography for detection of renovascular hypertension. Kidney Int. 2000;58:774–782. doi: 10.1046/j.1523-1755.2000.00226.x [DOI] [PubMed] [Google Scholar]
- 16. Stratigis S, Stylianou K, Kyriazis PP, Dermitzaki E‐K, Lygerou D, Syngelaki P, Stratakis S, Koukouraki S, Parthenakis F, Tsetis D, et al. Renal artery stenting for atherosclerotic renal artery stenosis identified in patients with coronary artery disease: does captopril renal scintigraphy predict outcomes? J Clin Hypertens (Greenwich). 2018;20:373–381. doi: 10.1111/jch.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Courand P‐Y, Dinic M, Lorthioir A, Bobrie G, Grataloup C, Denarié N, Soulat G, Mousseaux E, Sapoval M, Azizi M, et al. Resistant hypertension and atherosclerotic renal artery stenosis: effects of angioplasty on ambulatory blood pressure. A retrospective uncontrolled single‐center study. Hypertension. 2019;74:1516–1523. doi: 10.1161/hypertensionaha.119.13393 [DOI] [PubMed] [Google Scholar]
- 18. Mishima E, Suzuki T, Ito S. Selection of patients for angioplasty for treatment of atherosclerotic renovascular disease: predicting responsive patients. Am J Hypertens. 2020;33:391–401. doi: 10.1093/ajh/hpaa016 [DOI] [PubMed] [Google Scholar]
- 19. Vassallo D, Ritchie J, Green D, Chrysochou C, Kalra PA. The effect of revascularization in patients with anatomically significant atherosclerotic renovascular disease presenting with high‐risk clinical features. Nephrol Dial Transplant. 2018;33:497–506. doi: 10.1093/ndt/gfx025 [DOI] [PubMed] [Google Scholar]
- 20. Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA. High‐risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis. 2014;63:186–197. doi: 10.1053/j.ajkd.2013.07.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3


