Abstract
Superfusion of canine tracheal mucosa with 100 μg each of grepafloxacin and ciprofloxacin per ml reduced the electrical transepithelial potential difference in vivo by more than 50%. This effect was dose dependent, specific for new quinolones, and inhibited by Cl channel blockers, indicating that new quinolones attenuate Cl secretion across the airway epithelium.
The amount and physicochemical properties of airway surface fluid are regulated by electrolyte transport across the airway epithelium (10). Epithelial cells absorb Na from and secrete Cl toward the lumen, and the net ion flux across the cells generates a transepithelial electrical potential difference (PD), which concomitantly promotes water movement across the airway mucosa (15, 16). We and others have previously shown that macrolides inhibit Cl secretion by the airway epithelium in vitro, which may lead to a decrease in sputum volume in patients with chronic respiratory tract infections (6, 14). However, the effects of new quinolones remain unknown and, more importantly, the in vitro findings may not necessarily reflect electrolyte transport in vivo because of the lack of innervation and blood supply. Therefore, we studied the effects of grepafloxacin (GPFX) and ciprofloxacin (CPFX) on the tracheal PD in anesthetized dogs and compared them with those of other classes of antimicrobial agents.
Mongrel dogs were anesthetized, and their tracheas were exposed. The cartilage rings of the upper trachea were then incised axially, and the surface of the membranous portion was fully exposed. An exploring bridge constructed of polyethylene tubing was placed on the surface of the posterior membrane. Contact with the tracheal surface was ensured by continuous perfusion through the bridge with warmed (37°C) and gassed (95% O2–5% CO2) Krebs-Henseleit solution. The perfusion reservoir was connected to a calomel electrode via a polyethylene tube filled with 3% agar in saline. The reference bridge, a 21-gauge needle that contained 3% agar in saline, was inserted into the subcutaneous space of the right anterior chest wall. Each bridge was connected by a calomel electrode to a high-impedance voltmeter (CEZ-9100; Nihon Kohden, Tokyo, Japan), and the PD between the tracheal mucosal surface and the subcutaneous space was continuously recorded. Because a preliminary experiment showed that the subcutaneous space is isoelectric with the adventitial surface of the trachea, the PD values measured were assumed to be the transepithelial PDs.
All data were expressed as means ± the standard errors (SE). Statistical analysis was performed by Student's t test, and a P value of less than 0.05 was considered statistically significant.
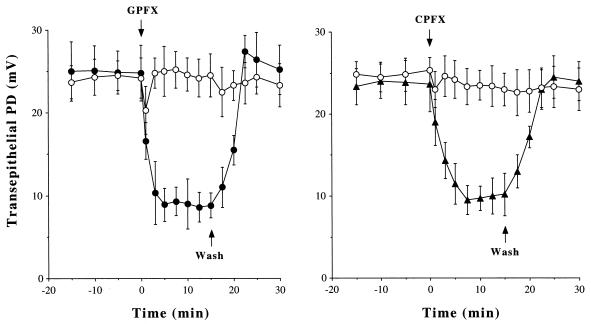
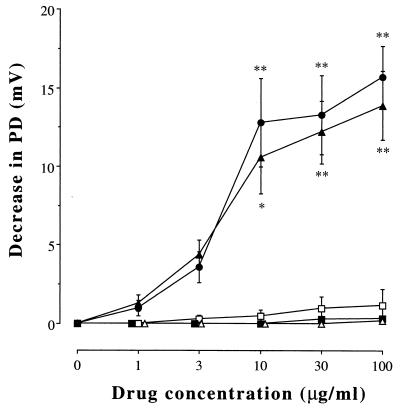
When the PD became stable, the superfusing Krebs-Henseleit solution was changed to a similar solution that contained an antibiotic agent or its vehicle alone. As demonstrated in Fig. 1, perfusion with GPFX (100 μg/ml) rapidly (within 5 min) decreased the PD from 24.8 ± 3.4 to 8.9 ± 2.0 mV (P < 0.01, n = 9) and it remained stable at least for the next 10 min. Perfusion with CPFX produced a similar effect, and the new quinolone-induced decreases in the PD were restored by replacement of the superfusing solution with fresh Krebs-Henseleit solution, indicating that the effects are not due to cytotoxicity. Exposure to GPFX and CPFX (1 to 100 μg/ml) dose dependently decreased the PD, whereas erythromycin, ampicillin, or cefotaxime had no effect (Fig. 2).
FIG. 1.
Time course of the effects of new quinolones on the transepithelial PD in canine tracheal mucosa in vivo. After equilibration, the tracheal mucosa was superfused with GPFX (100 μg/ml; closed circles), CPFX (100 μg/ml; closed triangles), or the vehicle alone (open circles) at time zero (upper arrows). When the PD response became stable, the superfusing solution was replaced with fresh Krebs-Henseleit solution (lower arrows). Values are means ± SE; n = 9 for each point.
FIG. 2.
Dose-dependent effects of GPFX (closed circles), CPFX (closed triangles), erythromycin (open squares), ampicillin (closed squares), and cefotaxime (open triangles) on the transepithelial PD. Increasing concentrations of the drugs were added to the superfusing solution, and the plateau PD value in response to each dose was determined. Values are expressed as decreases in the PD from the baseline value. Data are means ± SE; n = 9 for each point. The symbols * (P < 0.05) and ** (P < 0.01) indicate values that are significantly different from the baseline values.
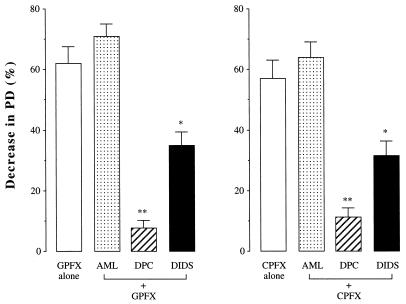
To elucidate whether the new quinolone-induced changes in the PD were associated with Cl secretion and/or Na absorption by the tracheal epithelium, the Na channel blocker amiloride (10−4 M) (1), the blocker of both the cystic fibrosis transmembrane conductance regulator (CFTR) and the outwardly rectifying Cl channel (ORCC) diphenylamine-2-carboxylate (DPC; 10−4 M) (8), or the ORCC blocker 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS; 10−5 M) (7) was added to the superfusing solution and, when the responses of the PD reached a plateau, GPFX or CPFX (100 μg/ml) was applied. As shown in Fig. 3, the GPFX-induced decrease in the PD was not altered by amiloride but was inhibited by DPC and DIDS by 87 ± 7% (P < 0.01) and 44 ± 5% (P < 0.05), respectively (n = 8). The response to CPFX was inhibited by DPC and DIDS in a similar manner.
FIG. 3.
Effects of pharmacologic blocking agents on the transepithelial PD decreases induced by GPFX and CPFX. Tracheal mucosa was treated with amiloride (AML; 10−4 M), DPC (10−4 M), or DIDS (10−5 M), and then GPFX and CPFX (100 μg/ml) were added. Values are expressed as percent decreases in the PD from the baseline value. Data are means ± SE; n = 8 for each column. The symbols * (P < 0.05) and ** (P < 0.01) indicate values that are significantly different from the corresponding values for the new quinolone alone.
Several important conclusions emerged from our study, which evaluated the effects of antibiotics on the tracheal PD in vivo. First, GPFX and CPFX decreased the PD in a dose-dependent fashion, whereas other classes of antibiotics, including macrolides, penicillin, and cephalosporin, had no effect, indicating that the observed inhibition is specific to new quinolones. Second, pretreatment with amiloride to eliminate the component of Na transport across the airway epithelium did not alter the effects of GPFX and CPFX. Thus, the new quinolone-induced decrease in the PD is most likely attributable to inhibition of Cl movement through the epithelial cellular and paracellular paths. However, possible contributions of electrolyte transport processes other than Cl, such as amiloride-insensitive Na, Na-glucose cotransport, and bicarbonate diffusion, cannot be ruled out. Third, the effects of new quinolones were almost completely inhibited when the airway epithelium was pretreated with DPC, which decreases Cl conductance at the apical membrane through inhibition of both CFTR and ORCC. In addition, we examined the effect of DIDS to determine whether ORCC is involved in the new quinolone's action and found that the changes in PD were reduced by more than 40% in the presence of DIDS. These results suggest that new quinolones can attenuate airway epithelial Cl secretion by inhibiting both CFTR and ORCC.
To extrapolate these findings to clinical efficacy, there are some issues to be addressed. Airways usually both secrete Cl and absorb Na, but the proportion of each action varies by region and species (15). Since the cumulative area of the distal airways is much larger than that of the proximal airways, bioelectric properties in small airways may be important. For example, in canines, the bronchial PD is substantially lower than the tracheal PD and Na absorption is predominant in the lower respiratory tract under basal conditions (15). Also, in the human airway epithelium, Cl flux is considered to be symmetric under physiologic conditions (2, 15). However, many inflammatory mediators are known to stimulate Cl secretion across the airway epithelium (3), suggesting that Cl secretion is predominant under pathological conditions. Thus, we speculate that the inhibitory effects of new quinolones on Cl secretion may be beneficial to patients having increased sputum production. Next, there appears to be a discrepancy between the effective quinolone concentrations used in this study and those produced by systemic administration of clinically recommended doses. The maximum mean drug concentration in serum following the ingestion of 400 mg of GPFX by adults has been reported to be 1.8 μg/ml (5), whereas in our experiments, a PD decrease was observed with a concentration of 10 μg/ml. However, the concentrations of new quinolones in serum do not accurately reflect local concentrations, since the GPFX concentration in airway epithelial lining fluids can be 10- to15-fold greater than that in serum (5). Likewise, the peak concentrations of CPFX in serum are in the range of 4 to 6 μg/ml (4) and CPFX penetrates bronchial tissues well, producing tissue/plasma drug ratios of between 1.4 and 4.4 (11). Therefore, our in vivo findings might be relevant to the clinical efficacy of these drugs. Further studies in a clinical setting are needed to determine whether the novel antisecretory action of new quinolones represents inhibition of airway hypersecretion.
The mechanism by which new quinolones inhibit airway epithelial Cl secretion remains uncertain. Several airway epithelial functions are controlled by the autonomic nervous system (9), and we have previously shown that the cholinergic neural pathway plays a role in Cl secretion and, hence, maintenance of the epithelial PD in vivo (12) and that CPFX inhibits the release of acetylcholine from the airway cholinergic nerve terminals (13). Therefore, although it is possible that new quinolones have a direct inhibitory action on Cl channels, the inhibition of cholinergic neurotransmission could also be involved.
Acknowledgments
We thank Yoshimi Sugimura and Masayuki Shino for technical assistance.
This work was supported in part by Scientific Research Grant 04670476 from the Ministry of Education, Science, and Culture, Japan.
REFERENCES
- 1.Al-Bazzaz F J, Zevin R. Ion transport and metabolic effects of amiloride in canine tracheal mucosa. Lung. 1984;162:357–367. doi: 10.1007/BF02715668. [DOI] [PubMed] [Google Scholar]
- 2.Boucher R C. Human airway ion transport: part one. Am J Respir Crit Care Med. 1994;150:271–281. doi: 10.1164/ajrccm.150.1.8025763. [DOI] [PubMed] [Google Scholar]
- 3.Boucher R C. Human airway ion transport: part two. Am J Respir Crit Care Med. 1994;150:581–593. doi: 10.1164/ajrccm.150.2.8049852. [DOI] [PubMed] [Google Scholar]
- 4.Catchpole C, Andrews J M, Woodcock J, Wise R. The comparative pharmacokinetics and tissue penetration of single-dose ciprofloxacin 400 mg iv and 750 mg po. J Antimicrob Chemother. 1994;33:103–110. doi: 10.1093/jac/33.1.103. [DOI] [PubMed] [Google Scholar]
- 5.Cook P J, Andrews J M, Wise R, Honeybourne D, Moudgil H. Concentrations of OPC-17116, a new fluoroquinolone antibacterial, in serum and lung compartments. J Antimicrob Chemother. 1995;35:317–326. doi: 10.1093/jac/35.2.317. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda K, Wu D, Takasaka T. Inhibition of acetylcholine-evoked Cl currents by 14-membered macrolide antibiotics in isolated acinar cells of the guinea pig nasal gland. Am J Respir Cell Mol Biol. 1995;13:449–454. doi: 10.1165/ajrcmb.13.4.7546775. [DOI] [PubMed] [Google Scholar]
- 7.Jovov B, Shlyonsky V G, Berdiev B K, Ismailov I I, Benos D J. Purification and reconstitution of an outwardly rectified Cl− channel from tracheal epithelium. Am J Physiol. 1998;275:C449–C458. doi: 10.1152/ajpcell.1998.275.2.C449. [DOI] [PubMed] [Google Scholar]
- 8.Knowles M R, Olivier K, Noone P, Boucher R C. Pharmacologic modulation of salt and water in the airway epithelium in cystic fibrosis. Am J Respir Crit Care Med. 1995;151:S65–S69. doi: 10.1164/ajrccm/151.3_Pt_2.S65. [DOI] [PubMed] [Google Scholar]
- 9.Marin M G. Pharmacology of airway secretion. Pharmacol Rev. 1986;28:273–289. [PubMed] [Google Scholar]
- 10.Nadel J A, Widdicombe J H, Peatfield A C. Regulation of airway secretion, ion transport, and water movement. In: Fishman A P, editor. Handbook of physiology: the respiratory systems, section 3. Bethesda, Md: American Physiological Society; 1985. pp. 419–445. [Google Scholar]
- 11.Rohwedder R, Bergan T, Caruso E, Thorsteinsson S B, Della Torre H, Scholl H. Penetration of ciprofloxacin and metabolites into human lung, bronchial and pleural tissue after 250 and 500 mg oral ciprofloxacin. Chemotherapy. 1991;37:229–238. doi: 10.1159/000238860. [DOI] [PubMed] [Google Scholar]
- 12.Tamaoki J, Chiyotani A, Tagaya E, Takemura H, Konno K. Cholinergic control of rabbit tracheal transepithelial potential difference in vivo. Eur Respir J. 1996;9:1632–1636. doi: 10.1183/09031936.96.09081632. [DOI] [PubMed] [Google Scholar]
- 13.Tamaoki J, Tagaya E, Chiyotani A, Takemura H, Konno K. Effect of ciprofloxacin on cholinergic neuro-effector transmission in airway smooth muscle. Drugs. 1995;49:298–300. doi: 10.2165/00003495-199500492-00077. [DOI] [PubMed] [Google Scholar]
- 14.Tamaoki J, Takeyama K, Tagaya E, Konno K. Effect of clarithromycin on sputum production and its rheological properties in chronic respiratory tract infection. Antimicrob Agents Chemother. 1995;39:1688–1690. doi: 10.1128/aac.39.8.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welsh M J. Electrolyte transport by airway epithelia. Physiol Rev. 1987;67:1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- 16.Widdicombe J H, Bastacky S J, Wu D X, Lee C Y. Regulation of depth and composition of airway surface liquid. Eur Respir J. 1997;10:2892–2897. doi: 10.1183/09031936.97.10122892. [DOI] [PubMed] [Google Scholar]