Abstract
Necrotizing enterocolitis (NEC) remains a prominent surgical emergency among infant population, associated with a significant mortality, as well as various subsequent morbidities. Congenital heart disease (CHD) has an increased associated incidence with NEC in infant population. Recent research has provided insight into the pathophysiology of NEC in patients with CHD and how this differs from those without CHD. The deviation from normal circulatory physiology has a suggested association in the pathophysiology of NEC in CHD, which may have implications for the risk factors of NEC in infants with CHD, the effect on outcomes of NEC, and whether alternative approaches to management may need to be considered in comparison to classical NEC. This review aims to highlight studies that provide insight and awareness into the relationship between NEC and CHD, in order that clinicians may direct themselves more clearly toward optimal management for infants in this category.
Keywords: Bowel surgery, interventions, outcomes, pediatrics
INTRODUCTION
Necrotizing enterocolitis (NEC) is a commonly encountered gastrointestinal emergency and a leading cause of morbidity and mortality in the neonatal population.[1,2] The typical characteristics of NEC include breaching of the gut mucosal barrier by pathogenic enteric bacteria, which results in intestinal inflammation, hypoxia, ischemia, and necrosis.[3,4] In the last few decades, although mortality rates in premature infants have decreased significantly due to advancements in the management of respiratory distress syndrome and other aspects of neonatal care, the incidence of NEC has generally remained the same as a result of greater ability to distinguish NEC from similarly presenting conditions balanced against the increased risk of NEC in infants born at younger gestational ages.[5,6] This disease entity typically afflicts 5%–7% of preterm infants, particularly those infants who are of very low birth weight (VLBW <1500 g).[7] However, in full-term neonates, NEC has an association with certain congenital anomalies such as congenital heart disease (CHD), with an incidence ranging between 1.6% and 6% in full-term infants with NEC and CHD.[4,8,9,10,11,12] Prevalence and mortality of NEC in CHD have varied significantly among different studies.[9,13,14,15,16,17,18,19] Infants with complex CHD have a notably higher risk of developing NEC.[11,20] Numerous studies have been performed aimed at identifying unique risk factors contributing to NEC in patients with CHD as well as the underlying pathophysiology so that provision of care may be anticipated and optimized.
PATHOPHYSIOLOGY
The pathophysiology of NEC has not been completely elucidated. Proposed theories include a multifactorial disease process, resulting in intraluminal bacteria disrupting and invading intestinal epithelial cells.[21,22] Subsequently, adherence of leukocytes and platelets to the endothelium prevents microvascular blood flow in the small intestine, resulting in tissue injury.[23]
Several studies suggest alternative pathophysiology in infants who develop NEC with coexisting CHD. It has been theorized that CHD infants have low diastolic pressures and consequently lower bowel perfusion pressures, in addition to low systemic oxygenated blood.[2,18,24] Ultimately, the bowel is hypoperfused and ischemic.[18,24] Different types of CHDs can contribute to NEC development. Patent ductus arteriosus and significant left-to-right shunting are thought to lead to pulmonary hyperperfusion and systemic hypoperfusion – resulting in superior mesenteric diastolic blood flow being restricted.[25,26] Cyanotic CHD can predispose the infant to a generalized state of hypoxia, which may facilitate development of NEC.[27] Similarly, ductal-dependent (DD) CHD (e.g., coarctation of the aorta and atrioventricular canal defect) can lower diastolic gut perfusion pressures and restrict oxygenated blood flow in the systemic circulation, directly leading to gastrointestinal circulatory insufficiency and ischemia.[28] Infants with atrioventricular canal experience pulmonary over circulation and accompanying atrioventricular valve regurgitation, leading to reduced systemic output.[29] A summary of the proposed pathophysiology is summarized in Figure 1.
Figure 1.

Pathophysiology of necrotizing enterocolitis in patients with congenital heart disease
However, during interventions involving arch reconstruction, the use of deep hypothermia aims to protect the bowels from ischemia.[30] The infant may still be predisposed to NEC postoperatively due to reperfusion injury and the increase of proinflammatory cytokines.[30] It was observed that in infants who developed NEC, the occurrence of tissue damage in the colon was more frequent in infants with CHD as compared to those without CHD. This corroborates the theory of alternative pathophysiology of NEC in patients with CHD as the colon is at a comparatively increased risk of hypoxic or ischemic injury.[2] It has been observed that infants who developed NEC with CHD had significantly lower APGAR scores at 1 and 5 min after birth and required a higher level of respiratory support after delivery, further supporting the role of ischemia as a major contributor of NEC in infants with CHD.[31]
RISK FACTORS OF NECROTIZING ENTEROCOLITIS IN INFANTS WITH CONGENITAL HEART DISEASE
A retrospective case–control study found that neonates with hypoplastic left heart syndrome (HLHS) are at the highest risk of developing NEC when compared with other CHDs.[9] Children with truncus arteriosus and aortopulmonary window also carry a significantly increased risk.[9] DD lesions have been shown to have increased rates of developing NEC as compared to non-DD lesions.[19,28] However, in premature or VLBW infants, the presence of atrioventricular canal defect is associated with the highest risk of NEC development.[29]
Certain studies identify prostaglandin (PGE) use as another possible risk factor for NEC in infants with CHD, attributable to side effects such as apnea and hypotensive episodes, particularly at infusion rates greater than 0.05 μg/kg/min.[9] However, in a recent study, no association was observed between use of PGE and the risk of developing NEC in infants with CHD.[15] In another study, the risk of NEC in infants with DD lesions on PGE therapy was observed to be 0.3%,[28] thus suggesting that PGE usage is a nonmajor risk factor. Further studies on larger cohorts will be required to decisively conclude the magnitude of PGE as a risk factor compared to others discussed.
Previously, feeding practices in infants with CHD have been analyzed with no clear significance of the risk between enteral feeding or parenteral nutrition and the subsequent development of NEC.[17,19] However, recent studies shed further light upon the risk of enteral feeding and its role in the development of NEC. No significant difference was found in either enteral or parenteral feeds, therefore supporting the use of the enteral route in CHD.[28,32] One observational study found that initiating enteral feeding in the preoperative period leads to low risk (0.9%) of NEC in infants.[33] Further studies will be required, with larger cohorts, to conclude the relationship of enteral feeding as a risk factor for NEC in infants with CHD. However, consideration can be given to the benefits of initiating early enteral feeding postoperatively in infants, including reduced length of stay, decreased time till full enteral nutrition, and reduced time till first stool.[34]
Studies have shown that NEC may be more common in the postoperative period,[17,33] following cardiac surgery. With regard to specific procedures, NEC rates were higher in infants who had received a systemic to pulmonary shunt procedure compared to other procedures.[12] In addition, one study showed a higher incidence of NEC associated with red blood cell transfusion.[35] The risk factors described so far relate to infants with CHD who develop NEC. Other risk factors such as prematurity or gestational age are generally considered significant and important risk factors for NEC in neonates born without CHD.[36] These risk factors have also been shown in studies including neonates with CHD, indicating that prematurity or gestational age has a significant association in the development of NEC in this group.[9,37] A summary of possible risk factors discussed is shown in Figure 2.
Figure 2.
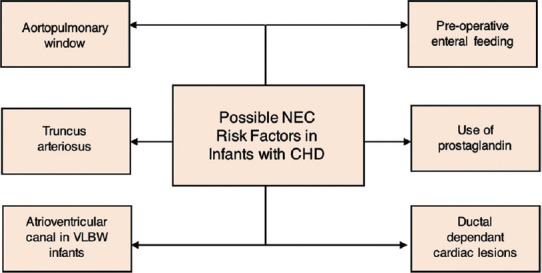
Risk factors for necrotizing enterocolitis in patients with congenital heart disease
DIAGNOSTIC APPROACH
Clinically, NEC can present in many different ways among infants, which can make it more difficult for clinicians to diagnose the condition at the earliest and least severe stage of pathogenesis. NEC may present anywhere on the clinical spectrum, ranging from slow and insidious to rapid and progressive.[38,39] The diagnosis of NEC is based on variable clinicoradiologic signs and extent of involvement. Staging criteria are utilized to assign disease severity and determine treatment. Bell's classification has traditionally been the standard for severity assessment in NEC [Table 1].
Table 1.
Modified Bell’s staging criteria for necrotizing enterocolitis
| Stage | Classification | Systemic signs | Intestinal signs | Radiologic signs |
|---|---|---|---|---|
| IA | Suspected NEC | Bradycardia, lethargy | Mild abdominal distention, vomiting, occult fecal blood | Normal mild ileus |
| IB | Suspected NEC | Same as above | Macroscopic rectal bleeding | Same as above |
| IIA | Proven NEC-mildly ill | Same as above | Same as above, + absent bowel sounds, ± tenderness | Intestinal dilation, ileus, pneumatosis intestinalis |
| IIB | Proven NEC-moderately ill | Same as above, + mild metabolic acidosis and/or thrombocytopenia | Same as above+absent bowel sounds, definite tenderness±abdominal cellulitis or mass | Same as IIA, + portal venous gas, ± ascites |
| IIIA | Advanced NEC - severely ill, bowel intact | Same as IIB, + hypotension, disseminated intravascular coagulation | Same as above, + signs of generalized peritonitis, marked tenderness, and distention of abdomen | Same as IIB, + definite ascites |
| IIIB | Advanced NEC - severely ill, bowel perforated | Same as IIIA | Same as IIIA | Same as IIB, + pneumoperitoneum |
NEC: Necrotizing enterocolitis
Most commonly, both small and large bowels are affected; the next most frequent location of disease involvement is the small bowel alone. In infants with congenital heart disease and NEC, the colon is most commonly affected.[36] Interestingly, a retrospective study showed seven patients with CHD (0.9%) developed NEC, of which all had nontypical radiologic findings, resulting in delayed diagnosis with five patients having developed bowel perforation.[40] This suggests that clinicians may need to have a higher suspicion of NEC occurrence in infants with CHD.
TREATMENT
Necrotizing enterocolitis management
Management of NEC in infants with CHD, where the underlying pathophysiology itself consists of distinct features, may need to be approached with an alternative perspective. Current management for NEC in infants with CHD is generally derived from guidance on classic NEC, with little unique consideration given to patients with CHD.[2,41] Management of NEC involves supportive care, empirical antibiotic therapy, parenteral nutrition, and bowel rest with gastric decompression initiated as soon as NEC is suspected.[42] In a retrospective study, it was observed that no patient among the entire cohort (n = 251) developed NEC preoperatively when the protocol of enteral feeding in neonates with DD lesions included continuous trophic feeds if nil per os since birth or trophic transpyloric feeds if the neonate was not stable.[8] This same study additionally suggested a provisional antibiotic guideline to address the variation of regimens found in infants with CHD.[8] Briefly, they suggest ampicillin, gentamicin ± metronidazole for suspected NEC; ampicillin if hemodynamically stable or vancomycin and piperacillin-tazobactam if unstable with confirmed NEC; and vancomycin and piperacillin-tazobactam in advanced NEC.[8] Surgical therapy is indicated according to clinical signs or investigations suggesting bowel perforation, such as pneumoperitoneum on an abdominal radiograph, or in the case of failure of medical therapy.[42]
Timing of surgical intervention
Current literature suggests that severity, of NEC in infants with CHD, is generally less when compared to classical NEC.[17] Infants with CHD have a reduced likelihood of developing clinically important morbidities such as perforation of the bowel, resulting in a need for stomas or development of short bowel syndrome, or sepsis, in comparison to classical NEC.[43] A recent study also showed that a smaller percentage of infants with CHD who developed NEC underwent surgical intervention as compared to infants without CHD, suggesting consistency with findings of reduced severity of NEC in infants with CHD in the literature.[2]
However, after excluding suspected NEC cases (Stage I), Cheng et al.[27] found that earlier surgery in proven NEC cases without perforation, i.e., Stages II and IIIA, resulted in higher survival than those managed medically (n = ¾, 75% vs. n = 4/9, 44%).[27] In patients with CHD and NEC, where surgery was clearly indicated, surgical intervention was successful in saving 33% of these patients (n = 3/6 vs. n = 0/2).[27] This suggests that surgical management did not present a greater risk of mortality and resulted in a higher survival in comparison to medical treatment, although not statistically significant.[27] Therefore, suggesting the consideration of possible surgery earlier before it is clinically indicated with evidence of bowel perforation.
A more recent study found that macroscopic intestinal necrosis was present with greater frequency intraoperatively in infants with CHD and NEC in comparison to infants without CHD.[44] This should prompt the earlier consideration of surgical intervention for NEC in patients with CHD. It is difficult to evaluate current literature for queries such as whether surgical intervention should occur earlier, delayed after medical management fails, or reserved until clinical signs of perforation are apparent. Further studies are required into this aspect of management for NEC in infants with CHD to provide clearer and consistent conclusions.
Cardiac surgical interventions
In neonates with DD lesions, important components of the management during an acute situation comprise initiation of a PGE infusion at a rate of 5–10 ng/kg/min and consideration for definitive repair through either surgical therapy or transcatheter therapy as diastolic pressures remain low in this form of CHD.[45,46] Having discussed the possible nature of PGE infusion as a risk factor for NEC, along with other potentially detrimental side effects, the infusion rates are set to the minimally effective dose.[9,45]
Stenting of the ductus arteriosus may be considered early in DD systemic lesions followed with bidirectional cavopulmonary connection for definitive repair.[46] However, in cases involving transposition of great arteries, consideration can be made for a balloon atrial septostomy.[45,46] For single ventricle heart defects such as HLHS, one retrospective study compared the risk of gastrointestinal complications such as NEC between first-stage palliation procedures such as the Norwood modified Blalock-Taussig shunt, Norwood right ventricle to pulmonary artery conduit (Sano repair), and a hybrid procedure, finding that the gastrointestinal complications were minimal in infants who underwent a Sano repair compared to the other procedures.[47] However, the incidence of NEC was not significantly distinguishable between the procedures.[47] Further research will be required to elucidate the association of NEC following cardiac surgical interventions.
Initial surgical intervention – Necrotizing enterocolitis surgery or cardiac surgery?
In a retrospective study spanning from 2008 to 2011, records of neonates who developed severe NEC postoperatively after surgical correction of a CHD were reviewed.[41] In three patients, managing NEC before surgical correction of the CHD significantly relapsed in the postoperative period.[40]
A case–control study looked at NEC in four CHD infants who were undergoing surgical cardiac procedures.[48] Although this study was comprised of a very small cohort, the authors reported that NEC may have occurred due to mesenteric ischemia which correlated with a perfusion state that was lower in the perioperative period.[48] It is therefore logical that the initial surgical intervention should tackle the CHD to possibly prevent or reverse NEC occurrence, given that there are no contraindications.[27]
There does not appear to be extensive research available in this niche area, and thus, more studies in this cohort of patients are required to ascertain and clarify the risk–benefit profile.
OUTCOMES
Mortality
Surprisingly, Pickard et al.[43] showed that patients with NEC who also suffered from a CHD had a significant survival advantage.[43] However, there was notable heterogeneity between the study groups (NEC with CHD vs. NEC without CHD), which may have influenced the study outcome. First, Pickard et al.[43] included neonates with suspected NEC at an unbalanced ratio of 29% in neonates with CHD versus 21% in neonates without CHD.[43] Moreover, there were fewer patients with advanced Grade III NEC in CHD group (22%) than in patients without CHD (44%), resulting in better outcomes for patients with NEC and CHD.[43]
However, a recent retrospective study by Kessler et al.[49] that only included patients with confirmed NEC (Bell Stage ≥II) and a comparable rate of severe disease (Bell Stage III) in both groups, found that patients with CHD and confirmed NEC had higher rates of overall mortality.[49] This is in keeping with other studies which found that neonates with both NEC and CHD had worse outcomes in terms of mortality than patients with a single disease.[29,50,51,52]
When stratified by type of CHD, Lau et al.[19] found that although patients with DD lesions and complex patients with RACHS-1 >2 were more likely to develop NEC after cardiac surgery, mortality is similar regardless of DD.[19] Cheng et al.[27] found that cyanotic patients had higher mortality than the acyanotic group (n = 5/13, 71% vs. n = 12/17, 39% respectively).[27] Table 2 summarizes the studies to date comparing mortality rates in NEC patients with and without CHD.
Table 2.
Summary of mortality rates in necrotizing enterocolitis patients with and without congenital heart disease
| Author | Type of study | Time period of study | Notable inclusion/exclusion criteria | Demographics | Mortality | Main findings | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Gestational age, mean±SD, weak/median (IQR) | Birth weight, mean±SD, kg/median (IQR) | Classical NEC (%) | CHD-NEC (%) | P | |||||||
|
|
|
||||||||||
| Classical NEC | CHD-NEC | Classical NEC | CHD-NEC | ||||||||
| Pickard et al. 2009[43] | Retrospective cohort study | May 1999 to August 2007 | Included neonates with suspected NEC (Bell Stage I) | 27.3±2.56 | 35.8±4.60 | 1.44±0.69 | 1.49±0.87 | 18/126 (14) | 6/76 (8) | NS | Infants with CHD had a significant decreased risk of perforating (OR: 0.42 [95% CI: 0.22-0.81]), needing a bowel operation (OR: 0.30 [CI: 0.15-0.58]), developing a stricture (OR: 0.06 [CI: 0.01-0.50]), needing a stoma (OR: 0.46 [CI: 0.23-0.93]), becoming septic (OR: 0.41 [CI: 0.18-0.96]), and developing SBS |
| Cozzi et al. 2013[52] | Retrospective cohort study | January 2000 to December 2011 | Only included neonates who were treated surgically | 28±4 | 34±5 | 1.178±0.580 | 2106±0.252 | 43/147 (29) | 13/18 (72) | 0.001 | No difference in the location of NEC between non-CHD and CHD patients, with the predominant location being the small intestine in both No difference in the location of NEC between preterm non-CHD patients and full-term CHD patients with the small intestine again being the primary site |
| Short et al. 2014[50] | Retrospective cohort study | 1990-2012 | Only included full-term neonates | 39 (38-40) | 3.2±0.59 | 3/30 (10) | 4/9 (44) | 0.04 | Univariate predictors of mortality included congenital heart disease and placement of an UA catheter Multivariate analysis revealed late onset of NEC to be an independent predictor of mortality (OR 90.8, 95% CI 2.6-3121) |
||
| Fisher et al. 2015[29] | Prospective cohort study | January 2006 to December 2011 | Only included patients with a birth weight <1500 g | 26.4±2.4 | 28.8±3.0 | 0.889±0.266 | 1.017±0.302 | 6496/23,201 (28) | 139/253 (55) | <0.0001 | Mortality for neonates with CHD and no NEC was 34%, versus 55% for those with CHD and NEC (P<0.0001) Both groups of CHD patients had higher mortality than infants with NEC without CHD (28%, P<0.0001) Although NEC mortality overall decreases with higher birth weight, mortality for NEC and CHD together does not |
| Velazco et al. 2017[51] | Prospective cohort study | 2009-2015 | Only included birth weight >2500 g | 36 (37-39) | Medical NEC: 3.035 (2.754-3.453) Surgical NEC: 3.010 (2.739-3.405) |
94/1336 (7) | 85/293 (29) | <0.0001 | Of 1629 neonates with NEC, 45% had major congenital anomalies, most commonly gastrointestinal defects (20%), congenital heart defects (18%), and chromosomal anomalies (7%) CHD (P<0.0001) was an independent predictor of mortality and increased length of stay (P<0.0001) |
||
| Kessler et al. 2018[49] | Retrospective cohort study | December 2004 to May 2017 | Excluded patients with isolated patent ductus arteriosus and suspected NEC | 32.6 (95% CI: 31.9-33.3) | 37.1 (95% CI: 34.5-37.2) | 1.700 (95% CI: 1.633-1.938) | 2.483 (95% CI: 2.086-2.634) | 7/91 (8) | 19/38 (50) | <0.001 | Patients with CHD were more mature than those without CHD (P<0.01) The presence of CHD did not influence the frequencies of severe disease (overall 21% Bell Stage III), nor surgical interventions (overall 30%), the occurrence of intestinal complications (overall 13%), nor the duration of hospitalization (overall 38 days in survivors) NEC-related mortality was increased with the presence of CHD, when compared to neonates without CHD CHD and advanced NEC Stage III were independent predictors of NEC-associated fatalities with multivariable OR (95% CI) of 7.0, 1.3-39.5 for CHD, and of 3.4, 1.6-7.5 for Stage III disease |
| Bubberman et al. 2019[2] | Retrospective cohort study | 2004-2014 | PT-NEC versus CHD-NEC | 28.3 (range: 25-35.6) | 38.6 (range: 31.7-40.7) | 1135 (range: 615-2280) | 2895 (range: 1545-3700) | 8/36 (22) | 2/18 (11) | 0.47 | Postnatal age at onset was significantly lower in CHD-NEC patients (4 [2-24] vs. 11 [4-41] days, P<0.001) Lowest pH levels were lower (7.21 [7.01-7.47] vs. 7.27 [6.68-7.39], P=0.02), and highest CRP levels were higher (112.5 mg/L [5.0-425.0] vs. 66.0 [5.2-189.0], P=0.05) in PT-NEC versus CHD-NEC The colon was significantly more often involved in CHD-NEC versus PT-NEC (86% vs. 33%, P=0.03) |
CHD: Congenital heart disease, OR: Odds ratio, CI: Confidence interval, SBS: Sick building syndrome, SD: Standard deviation, IQR: Interquartile range, NS: Nonsignificant, NEC: Necrotizing enterocolitis, UA: Umbilical artery, PT NEC: Preterm NEC, CRP: C-reactive protein
Complications
A recent meta-analysis of 58 studies, including 4260 patients, found that gastrointestinal sequelae in neonates surviving surgery for NEC are a frequent problem, which should not be underestimated when assessing disease outcome.[9] Strictures (24%), interstitial fluid (13%), recurrence of NEC (8%), and adhesion ileus (6%) were the most commonly reported complications.[53] After controlling for birth weight and gestational age, Pickard et al.[43] found that neonates with CHD-NEC had decreased risk of perforation, requiring an operation, strictures, need for a stoma, sepsis, and short bowel syndrome compared with neonates without CHD.[43]
Kessler et al.[49] found that surviving CHD-NEC neonates do not have more gastrointestinal complications than patients without CHD (overall 13%).[49] Similarly, Bubberman et al.[2] found that the complication rates were comparable between both groups.[2] When stratified by type of CHD, Cheng et al.[27] found that gut perforation was more common in acyanotic CHD neonates compared to those with cyanotic CHD (n = 6/13, 46% vs. n = 5/17, 29%).[27] McElhinney et al.[9] reported that the mean hospital stay was significantly longer in CHD patients that developed NEC than those who did not develop NEC (36 ± 22 days vs. 19 ± 14 days).9
CONCLUSION
In infants with CHD, ischemia and hypoxic damage are the major risk factors within the pathophysiology of NEC. Furthermore, the major risk factors for NEC in the context of CHD may be distinct with specific forms of CHD, such as DD lesions and atrioventricular canal in VLBW infants, carrying a significantly higher risk of NEC. Literature suggests the logical approach that the initial surgical interventions address the cardiac defect to tackle the driving pathophysiology of NEC. PGE use and enteral feeding studies do not provide any conclusions collectively, and further research will be required using larger cohorts.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: Clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6:6–23. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bubberman JM, van Zoonen A, Bruggink JL, van der Heide M, Berger RM, Bos AF, et al. Necrotizing enterocolitis associated with congenital heart disease: A different entity. J Pediatr Surg. 2019;54:1755–60. doi: 10.1016/j.jpedsurg.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: Recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–83. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 5.Thyoka M, Eaton S, Hall NJ, Drake D, Kiely E, Curry J, et al. Advanced necrotizing enterocolitis part 2: Recurrence of necrotizing enterocolitis. Eur J Pediatr Surg. 2012;22:13–6. doi: 10.1055/s-0032-1306264. [DOI] [PubMed] [Google Scholar]
- 6.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23:278–85. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 7.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuchardt EL, Kaufman J, Lucas B, Tiernan K, Lujan SO, Barrett C. Suspected necrotising enterocolitis after surgery for CHD: An opportunity to improve practice and outcomes. Cardiol Young. 2018;28:639–46. doi: 10.1017/S1047951117002815. [DOI] [PubMed] [Google Scholar]
- 9.McElhinney DB, Hedrick HL, Bush DM, Pereira GR, Stafford PW, Gaynor JW, et al. Necrotizing enterocolitis in neonates with congenital heart disease: Risk factors and outcomes. Pediatrics. 2000;106:1080–7. doi: 10.1542/peds.106.5.1080. [DOI] [PubMed] [Google Scholar]
- 10.Ostlie DJ, Spilde TL, St Peter SD, Sexton N, Miller KA, Sharp RJ, et al. Necrotizing enterocolitis in full-term infants. J Pediatr Surg. 2003;38:1039–42. doi: 10.1016/s0022-3468(03)00187-8. [DOI] [PubMed] [Google Scholar]
- 11.Giannone PJ, Luce WA, Nankervis CA, Hoffman TM, Wold LE. Necrotizing enterocolitis in neonates with congenital heart disease. Life Sci. 2008;82:341–7. doi: 10.1016/j.lfs.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee D, Zhang Y, Chang DC, Vricella LA, Brenner JI, Abdullah F. Outcomes analysis of necrotizing enterocolitis within 11 958 neonates undergoing cardiac surgical procedures. Arch Surg. 2010;145:389–92. doi: 10.1001/archsurg.2010.39. [DOI] [PubMed] [Google Scholar]
- 13.Jeffries HE, Wells WJ, Starnes VA, Wetzel RC, Moromisato DY. Gastrointestinal morbidity after Norwood palliation for hypoplastic left heart syndrome. Ann Thorac Surg. 2006;81:982–7. doi: 10.1016/j.athoracsur.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Davies RR, Carver SW, Schmidt R, Keskeny H, Hoch J, Pizarro C. Gastrointestinal complications after stage I Norwood versus hybrid procedures. Ann Thorac Surg. 2013;95:189–95. doi: 10.1016/j.athoracsur.2012.05.130. [DOI] [PubMed] [Google Scholar]
- 15.Carlo WF, Kimball TR, Michelfelder EC, Border WL. Persistent diastolic flow reversal in abdominal aortic Doppler-flow profiles is associated with an increased risk of necrotizing enterocolitis in term infants with congenital heart disease. Pediatrics. 2007;119:330–5. doi: 10.1542/peds.2006-2640. [DOI] [PubMed] [Google Scholar]
- 16.Scahill CJ, Graham EM, Atz AM, Bradley SM, Kavarana MN, Zyblewski SC. Preoperative Feeding Neonates With Cardiac Disease. World J Pediatr Congenit Heart Surg. 2017;8:62–8. doi: 10.1177/2150135116668833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iannucci GJ, Oster ME, Mahle WT. Necrotising enterocolitis in infants with congenital heart disease: The role of enteral feeds. Cardiol Young. 2013;23:553–9. doi: 10.1017/S1047951112001370. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan G, Anne SR, Aggarwal S. Outcomes of congenital heart disease in late preterm infants: Double jeopardy. Acta Paediatr. 2011;100:1104–7. doi: 10.1111/j.1651-2227.2011.02245.x. [DOI] [PubMed] [Google Scholar]
- 19.Lau PE, Cruz SM, Ocampo EC, Nuthakki S, Style CC, Lee TC, et al. Necrotizing enterocolitis in patients with congenital heart disease: A single center experience. J Pediatr Surg. 2018;53:914–7. doi: 10.1016/j.jpedsurg.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Spinner JA, Morris SA, Nandi D, Costarino AT, Marino BS, Rossano JW, et al. Necrotizing enterocolitis and associated mortality in neonates with congenital heart disease: A multi-institutional study. Pediatr Crit Care Med. 2020;21:228–34. doi: 10.1097/PCC.0000000000002133. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Sherman MP, Prince LS, Bader D, Weitkamp JH, Slaughter JC, et al. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech. 2012;5:522–32. doi: 10.1242/dmm.009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molteni M, Gemma S, Rossetti C. The role of Toll-like receptor 4 in infectious and noninfectious inflammation. Mediators Inflamm. 2016;2016:6978936. doi: 10.1155/2016/6978936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alganabi M, Lee C, Bindi E, Li B, Pierro A. Recent advances in understanding necrotizing enterocolitis. F1000Res. 2019;8:107. doi: 10.12688/f1000research.17228.1. F1000 Faculty Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stapleton GE, Eble BK, Dickerson HA, Andropoulos DB, Chang AC. Mesenteric oxygen desaturation in an infant with congenital heart disease and necrotizing enterocolitis. Tex Heart Inst J. 2007;34:442–4. [PMC free article] [PubMed] [Google Scholar]
- 25.Hundscheid T, Onland W, van Overmeire B, Dijk P, van Kaam AH, Dijkman KP, et al. Early treatment versus expectative management of patent ductus arteriosus in preterm infants: A multicentre, randomised, non-inferiority trial in Europe (BeNeDuctus trial) BMC Pediatr. 2018;18:262. doi: 10.1186/s12887-018-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung YF, Ho MH, Cheng VY. Mesenteric blood flow response to feeding after systemic-to-pulmonary arterial shunt palliation. Ann Thorac Surg. 2003;75:947–51. doi: 10.1016/s0003-4975(02)04627-1. [DOI] [PubMed] [Google Scholar]
- 27.Cheng W, Leung MP, Tam PK. Surgical intervention in necrotizing enterocolitis in neonates with symptomatic congenital heart disease. Pediatr Surg Int. 1999;15:492–5. doi: 10.1007/s003830050647. [DOI] [PubMed] [Google Scholar]
- 28.Becker KC, Hornik CP, Cotten CM, Clark RH, Hill KD, Smith PB, et al. Necrotizing enterocolitis in infants with ductal-dependent congenital heart disease. Am J Perinatol. 2015;32:633–8. doi: 10.1055/s-0034-1390349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher JG, Bairdain S, Sparks EA, Khan FA, Archer JM, Kenny M, et al. Serious congenital heart disease and necrotizing enterocolitis in very low birth weight neonates. J Am Coll Surg. 2015;220:1018–26.e14. doi: 10.1016/j.jamcollsurg.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Raees MA, Morgan CD, Pinto VL, Westrick AC, Shannon CN, Christian KG, et al. Neonatal aortic arch reconstruction with direct splanchnic perfusion avoids deep hypothermia. Ann Thorac Surg. 2017;104:2054–63. doi: 10.1016/j.athoracsur.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 31.van der Heide M, Mebius MJ, Bos AF, Roofthooft MT, Berger RM, Hulscher JB, et al. Hypoxic/ischemic hits predispose to necrotizing enterocolitis in (near) term infants with congenital heart disease: A case control study. BMC Pediatr. 2020;20:553. doi: 10.1186/s12887-020-02446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day TG, Dionisio D, Zannino D, Brizard C, Cheung MM. Enteral feeding in duct-dependent congenital heart disease. J Neonatal Perinatal Med. 2019;12:9–12. doi: 10.3233/NPM-1861. [DOI] [PubMed] [Google Scholar]
- 33.Nordenström K, Lannering K, Mellander M, Elfvin A. Low risk of necrotising enterocolitis in enterally fed neonates with critical heart disease: An observational study. Arch Dis Child Fetal Neonatal Ed. 2020;105:609–14. doi: 10.1136/archdischild-2019-318537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greer D, Karunaratne YG, Karpelowsky J, Adams S. Early enteral feeding after pediatric abdominal surgery: A systematic review of the literature. J Pediatr Surg. 2020;55:1180–7. doi: 10.1016/j.jpedsurg.2019.08.055. [DOI] [PubMed] [Google Scholar]
- 35.Baxi AC, Josephson CD, Iannucci GJ, Mahle WT. Necrotizing enterocolitis in infants with congenital heart disease: The role of red blood cell transfusions. Pediatr Cardiol. 2014;35:1024–9. doi: 10.1007/s00246-014-0891-9. [DOI] [PubMed] [Google Scholar]
- 36.Partridge E, Rintoul N. Congenital heart disease (CHD) and necrotizing enterocolitis (NEC) Prog Pediatr Cardiol. 2019;54:101146. [Google Scholar]
- 37.Steurer MA, Baer RJ, Keller RL, Oltman S, Chambers CD, Norton ME, et al. Gestational age and outcomes in critical congenital heart disease. Pediatrics. 2017;140:e20170999. doi: 10.1542/peds.2017-0999. [DOI] [PubMed] [Google Scholar]
- 38.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: Is it time to abandon Bell's criteria. J Perinatol. 2007;27:661–71. doi: 10.1038/sj.jp.7211782. [DOI] [PubMed] [Google Scholar]
- 40.Kargl S, Maier R, Gitter R, Pumberger W. Necrotizing enterocolitis after open cardiac surgery for congenital heart defects—A serious threat. Klin Padiatr. 2013;225:24–8. doi: 10.1055/s-0032-1331724. [DOI] [PubMed] [Google Scholar]
- 41.Siano E, Lauriti G, Ceccanti S, Zani A. Cardiogenic necrotizing enterocolitis: A clinically distinct entity from classical necrotizing enterocolitis. Eur J Pediatr Surg. 2019;29:14–22. doi: 10.1055/s-0038-1668144. [DOI] [PubMed] [Google Scholar]
- 42.Knell J, Han SM, Jaksic T, Modi BP. Current status of necrotizing enterocolitis. Curr Probl Surg. 2019;56:11–38. doi: 10.1067/j.cpsurg.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Pickard SS, Feinstein JA, Popat RA, Huang L, Dutta S. Short-and long-term outcomes of necrotizing enterocolitis in infants with congenital heart disease. Pediatrics. 2009;123:e901–6. doi: 10.1542/peds.2008-3216. [DOI] [PubMed] [Google Scholar]
- 44.Diez S, Tielesch L, Weiss C, Halbfass J, Müller H, Besendörfer M. Clinical Characteristics of necrotizing enterocolitis in preterm patients with and without persistent ductus arteriosus and in patients with congenital heart disease. Front Pediatr. 2020;8:257. doi: 10.3389/fped.2020.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalil M, Jux C, Rueblinger L, Behrje J, Esmaeili A, Schranz D. Acute therapy of newborns with critical congenital heart disease. Transl Pediatr. 2019;8:114–26. doi: 10.21037/tp.2019.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kutty S, Zahn EM. Interventional therapy for neonates with critical congenital heart disease. Catheter Cardiovasc Interv. 2008;72:663–74. doi: 10.1002/ccd.21705. [DOI] [PubMed] [Google Scholar]
- 47.Weiss SL, Gossett JG, Kaushal S, Wang D, Backer CL, Wald EL. Comparison of gastrointestinal morbidity after Norwood and hybrid palliation for complex heart defects. Pediatr Cardiol. 2011;32:391–8. doi: 10.1007/s00246-010-9864-9. [DOI] [PubMed] [Google Scholar]
- 48.Fatica C, Gordon S, Mossad E, McHugh M, Mee R. A cluster of necrotizing enterocolitis in term infants undergoing open heart surgery. Am J Infect Control. 2000;28:130–2. [PubMed] [Google Scholar]
- 49.Kessler U, Hau EM, Kordasz M, Haefeli S, Tsai C, Klimek P, et al. Congenital heart disease increases mortality in neonates with necrotizing enterocolitis. Front Pediatr. 2018;6:312. doi: 10.3389/fped.2018.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Short SS, Papillon S, Berel D, Ford HR, Frykman PK, Kawaguchi A. Late onset of necrotizing enterocolitis in the full-term infant is associated with increased mortality: Results from a two-center analysis. J Pediatr Surg. 2014;49:950–3. doi: 10.1016/j.jpedsurg.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velazco CS, Fullerton BS, Hong CR, Morrow KA, Edwards EM, Soll RF, et al. Morbidity and mortality among “big” babies who develop necrotizing enterocolitis: A prospective multicenter cohort analysis. J Pediatr Surg. 2017;53(1):108–12. doi: 10.1016/j.jpedsurg.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 52.Cozzi C, Aldrink J, Nicol K, Nicholson L, Cua C. Intestinal location of necrotizing enterocolitis among infants with congenital heart disease. J Perinatol. 2013;33:783–5. doi: 10.1038/jp.2013.49. [DOI] [PubMed] [Google Scholar]
- 53.Hau EM, Meyer SC, Berger S, Goutaki M, Kordasz M, Kessler U. Gastrointestinal sequelae after surgery for necrotising enterocolitis: A systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2019;104:F265–73. doi: 10.1136/archdischild-2017-314435. [DOI] [PubMed] [Google Scholar]


