Abstract
Background:
Treadmill exercise testing is a crucial diagnostic tool for evaluating congenital and acquired heart disease in the pediatric population. This study aimed to perform a comprehensive evaluation of exercise-induced electrocardiographic (ECG) changes in children. Although there are numerous studies on exercise testing in various cardiac pathologies, studies on exercise-induced ECG changes in normal children with coverage of all ECG parameters of atrial and ventricular depolarization and repolarization are very scant, if any.
Aims and Objectives:
This study aimed to investigate the exercise-induced ECG changes in healthy children and evaluate the effects of gender and four different formulas of heart rate correction of Bazett, Fridericia, Framingham and Hodges on ventricular repolarization parameters pre-and post-exercise.
Materials and Methods:
Between April 2019 and April 2020, all children with normal electrocardiogram, echocardiogram and exercise test, high-quality ECG tracings and consent for participation were enrolled in this prospective study. Twenty electrocardiographic parameters were measured and 25 indices were calculated. P-value < 0.05 was considered significant.
Results:
Seventy-four healthy children were studied. Amplitudes of P, S, and T waves increased significantly after the exercise. All durations, except P wave time to peak and T peak -T end /QT (Tp-e/QT) interval decreased significantly with exercise. Generally, the parameters of ventricular repolarization were not statistically significant between males and females. There were significant differences among the heart-rate corrected values of intervals of QTc, QoTc, JTc, J point to peak T and Tp-e/QTc by various formulas. There was no U wave either at pre-exercise or post-exercise. QT interval was shortened by 24.6 % ± 12.1 % with exercise. The ECG-derived estimated duration of mechanical systole and diastole decreased with exercise. The percentage of decrease in diastole was more than systole (43.79 %± 13.31% versus 33.74% ±15.79 %, respectively, P-value < 0.001).
Conclusion:
Diastolic time decreased more than systolic time with exercise and systolic time to diastolic time increased with exercise. Hodges' and Fridericia's formulas resulted in the longest and shortest QT and QoT, JT, and JTP, respectively. Thus, using a single value as the cut-off for long QT syndrome can lead to under or over-diagnosis. Nomograms incorporating data on age, heart rate, and heart rate correction formula are indispensable for accurate long QT diagnosis. Furthermore, gender differences in ventricular repolarization parameters are not generally present in 5 to 14-year-old healthy children. The lack of U wave in this study may implicate the need for more careful investigation in the presence of U wave in the treadmill exercise testing of healthy children.
Keywords: Electrocardiogram, exercise test, J to peak T interval, J point to end of T wave interval, Onset of Q wave to the end of T wave interval, T peak to T end interval
INTRODUCTION
Treadmill exercise testing (TET) is a crucial diagnostic tool for evaluating congenital and acquired heart disease in the pediatric population.[1,2,3] Nevertheless, although there are numerous studies on exercise testing in various cardiac diseases in children, studies addressing exercise-induced electrocardiographic (ECG) changes in normal children are very old and scant, if any.[4] Furthermore, these studies do not include all ECG parameters of atrial and ventricular depolarization and repolarization.
The aims of this study were four-fold: (1) To evaluate comprehensive ECG changes in the amplitude and duration of P, Q, R, S, T, and U waves, in addition to alterations in the duration of PR, Onset of Q wave to the end of T wave (QT), Q to onset of T wave (QoT), J point to end of T wave (JT), J to peak T (JTP) and peak T to end T (Tp-e) intervals in children before and after exercise testing, (2) To compare four heart rate correction formulas of Bazett, Fridericia, Framingham, and Hodges for QT, QoT, JT, JTP, and Tp-e intervals before and after the exercise test, (3) To compare gender differences of these parameters of ventricular repolarization (QT, QoT, JT, JTP, and Tp-e intervals) before and after exercise, and (4) To compare ECG-derived estimated duration of systole and diastole and systolic-to-diastolic time ratio before and after exercise.
METHODS
Study design and study population
A prospective study was performed. Between April 2019 and April 2020, children who underwent TET and had the following criteria were included in the study: Normal baseline electrocardiogram and echocardiogram, normal reports of exercise testing, acceptable quality of ECG tracing, and consent to participate in the study. Those with a history of congenital or acquired heart disease were excluded from the study. The exercise test was performed to evaluate chest pain with exercise, determination of exercise capacity before participation in competitive sports in children with complaints of exercise-related easy fatigability or palpitation.
Estimation of minimum sample size
Since there was no similar study for sample size estimation, a pilot study was performed on 12 cases and then the sample size was estimated using G * Power software (version 3.1.9.7, Germany).[5] At a power level of 95% and a significance level of 5%, a minimum sample size of 70 was estimated.
Treadmill exercise testing protocol
Following the American Heart Association guidelines regarding clinical stress testing in children, TET (Avicenna Company, Medical Equipment Stress Test System, Version 6.1.0, CE 2195, Iran) was performed, using a modified Bruce protocol, which is shown in Table 1.[6]
Table 1.
Modified Bruce protocol used for graded treadmill exercise testing in this study
| Speed (km/h) | Speed (mile/h) | Duration | Grade (%) | METs | |
|---|---|---|---|---|---|
| Before starting | 0 | 0 | 2 | 0 | 1 |
| Stage 1† | 2.7 | 1.7 | 2 | 0 | 2.3 |
| Stage 2 | 2.7 | 1.7 | 2 | 5 | 3.4 |
| Stage 3 | 2.7 | 1.7 | 2 | 10 | 4.6 |
| Stage 4 | 4 | 2.5 | 2 | 12 | 7 |
| Stage 5 | 5.5 | 3.4 | 2 | 14 | 10.2 |
| Stage 6† | 6.8 | 4.2 | 2 | 16 | 13.6 |
| Stage 7 | 8 | 5 | 2 | 18 | 17.1 |
| Stage 8 | 8.9 | 5.5 | 2 | 20 | 20.5 |
| Stage 9 | 9.7 | 6 | 2 | 22 | 23.9 |
†Preexercise ECG tracing were obtained before start of stage 1 and postexercise ECG tracings were obtained at the end of stage 6 in the erect position. METs: Multiples of resting metabolic equivalent, ECG: Electrocardiographic
Electrocardiographic measurements
All measurements were performed immediately before stage 1 and immediately after stage 6 of the test while the child was in the erect position [Table 1]. Lead II was selected for all measurements. All tracings were recorded and printed at a speed of 100 mm/s. Amplitude was variable between 5 mm (mm)/1 millivolt (mv) and 20 mm/1 mv, adjusted according to the voltages' height. All amplitude calculations were based on millivolts. At the time of measurement, the region of interest of the tracing was magnified using a digital magnifier. Variables shown in Table 2 were manually measured or calculated before and after TET by two experienced pediatric cardiologists [Figures 1 and 2]. The duration of mechanical systole and diastole was estimated from the Wiggers diagram of the cardiac cycle.[7]
Table 2.
Measured and calculated parameters in electrocardiographic tracings at pre-and post-exercise in Lead II of 74 healthy children aged 5 to 12 years
| 20 measured parameters at pre- and post-exercise | |
|---|---|
| Amplitudes of waves | Amplitudes of P wave, Q wave, R wave, S wave, T wave, and U wave |
| Durations or waves | Durations of P wave and P wave time to peak, QRS , T wave and T wave time to peak and U wave |
| Duration of intervals | PR, QT, QoT, JT, JTP, Tp-e [Figure 1] |
| (QT: Onset of Q wave to the end of T wave, QoT: onset of Q to onset of T wave, JT: J point to the end of T wave, JTP: J point to peak of T wave, Tp-e: Peak of T wave to end of T wave, Tp-e/QT: peak of T wave to end of T wave/QT) | |
| ECG-derived estimation of duration of systole=Equivalent to JT interval as shown in the simplified Wiggers diagram in Figure 2 | |
| ECG-derived estimation of duration of diastole=RR interval-JT interval as shown in the simplified Wiggers diagram in Figure 2 | |
|
| |
| 25 Calculated parameters at preexercise and postexercise | |
|
| |
| Heart-rate corrected by four heart-rate correction formulas | QTc by four formulas, QoTc by four formulas, JTc by four formulas, JTPc by four formulas and Tp-e/QTc by four formulas |
| Absolute QT shortening=QT interval at preexercise-QT interval at postexercise | |
| QT shortening fraction=QT interval at preexercise-QT interval at postexercise/QT interval at preexercise×100 | |
| Systolic to diastolic time ratio=duration of systole/duration of diastole | |
| Bazett’s formula=Interval/√RR interval | |
| Fridericia formula=Interval/3√RR interval | |
| Framingham’s formula=Interval+0.154× (1-RR interval) | |
| Hodge’s formula=interval+1.75× (HR-60)] | |
| Percentage of decrease in ECG-derived estimated duration of systole=(preexercise duration of systole-postexercise duration of systole/preexercise duration of systole) ×100 | |
| Percentage of decrease in ECG-derived estimated duration of diastole=(preexercise duration of diastole-postexercise duration of diastole/preexercise duration of diastole) ×100 | |
QoT: Onset of Q to onset of T, JT: J point to end of T wave, QTc: Corrected QT, JTP: J point to peak T interval, Tp-e: T peak-T end interval, QT: Onset of Q wave to the end of T wave, QoTc: Onset of Q to onset of T, ECG: Electrocardiographic, HR: Heart rate, JTc: Corrected JT interval, JTPc: Corrected J point to peak T interval, QRS: QRS complex, PR: PR interval
Figure 1.
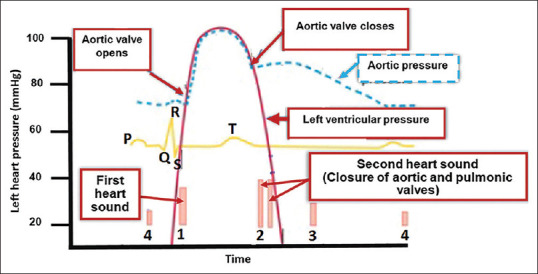
Simplified Wiggers diagram of the cardiac cycle for electrocardiographic-derived estimation of duration of systole and diastole
Figure 2.
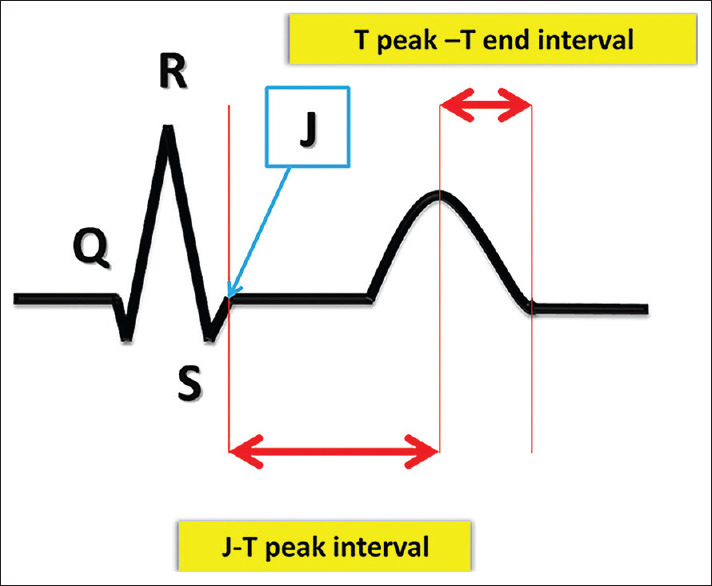
J point to end of T wave interval (from J point to the end of T wave), J to peak T and T peak to T end intervals
Statistical analysis
Categorical variables were expressed as number and percentage and continuous variables as mean ± standard deviation (SD) or median and interquartile range, depending on the variable's distribution. We used the Shapiro–Wilk test to test the normality of the distribution of the variables. A paired t-test was applied to compare the variables with normal distribution and Wilcoxon signed-ranks test was performed for nonnormal distribution variables. An independent-samples t-test was utilized to compare ventricular repolarization parameters in boys and girls. The mean of heart-rate corrected QT, QoT, JT, and JTP by the four formulas of Bazett, Fridericia, Framingham, and Hodges were compared using repeated-measures analysis of variance (ANOVA). IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, N.Y., USA) was used for statistical analysis. P < 0.05 was considered statistically significant.
Ethical considerations
Informed consent was obtained from all participants and their guardians. The study was performed in accordance with the Helsinki Declaration on ethical principles for medical research involving human subjects and was approved by the Institutional Committee for Ethics in Biomedical Research of the medical school of Tehran University of Medical Sciences (ID: IR. TUMS. MEDICINE. REC. 1398. 774).[8]
RESULTS
Seventy-four children aged 5–12 years were enrolled in this study. Basic characteristics and basic data of TET of the study population are shown in Table 3.
Table 3.
Basic characteristics and data of treadmill exercise testing of the study population (74 healthy children)
| Basic Characteristics | Values |
|---|---|
| Age in years (range, mean±SD) | 5-12, 10.3±2.7 |
| Sex, n (%) | |
| Male | 49 (66.2) |
| Female | 25 (33.8) |
| Weight in kg (range, mean±SD) | 18-108 (38.82±16.6) |
| Height in cm (range, mean±SD) | 110-172 (141.46±14.6) |
| Body surface area (m2), mean±SD | 1.29±0.45 |
| BMI (kg/m2), mean±SD | 24.82±5.14 |
| Duration of exercise in min (range, mean±SD) | 11.12-25.31 (18.25±2.28) |
| Estimated METs (range, mean±SD) | 10.20-23.90 (14.62±3.18) |
| Heart rate at the beginning of exercise in beats/min (range, mean±SD) | 70-147 (108±17) |
| Heart rate at the end of exercise in beats/min (range, mean±SD) | 145-212 (181±15) |
| Percentage of achieved target heart rate (range, mean±SD) | 81-120.40 (102.74±0.09) |
| Systolic BP before exercise in mmHg (range, mean±SD) | 75-125 (94.7±12.07) |
| Diastolic BP before exercise in mmHg (range, mean±SD) | 49-90 (64.7±7.63) |
| Systolic BP at the end of exercise in mmHg (range, mean±SD) | 85-140 (110.46±13.72) |
| Diastolic BP at the end of exercise in mmHg (range, mean±SD) | 50-95 (73.43±9.02) |
SD: Standard deviation, BMI: Body mass index, METs: Metabolic equivalents, BP: Blood pressure
Changes in amplitudes of P, Q, R, S, T, and U waves before and after treadmill exercise testing
All amplitudes were significantly increased after exercise except for amplitudes of Q and R waves, which were not increased significantly [Table 4]. Of note, there was no U wave detected either in the preexercise or postexercise ECG tracings. Since U waves are more evident in mid-precordial leads, these leads of ECG tracings were also investigated. Nevertheless, no U waves were detected.
Table 4.
Amplitudes and duration of electrocardiographic waves and intervals before and after exercise
| Electrocardiographic characteristics (mean ± SD) | Preexercise | Postexercise | P |
|---|---|---|---|
| Amplitudes of electrocardiographic waves (mm) | |||
| P wave amplitude | 1.84±0.48 | 2.57±0.76 | <0.001 |
| Q amplitude | 1.74±3.45 | 2.08±2.07 | 0.394 |
| R amplitude | 20.06±6.51 | 20.25±6.97 | 0.651 |
| S amplitude | 2.47±2.55 | 3.03±2.98 | <0.001 |
| T wave amplitude | 2.8±1.30 | 4.06±1.51 | 0.001 |
| Durations of waves and intervals (s) | |||
| PR interval | 0.108±0.018 | 0.089±0.019 | 0.002 |
| P wave duration | 0.075±0.014 | 0.06±0.01 | 0.001 |
| P wave time to peak | 0.04±0.05 | 0.03±0.008 | 0.127 |
| QRS duration | 0.072±0.198 | 0.06±0.01 | 0.006 |
| T wave duration | 0.11±0.02 | 0.09±0.05 | <0.001 |
| T wave time to peak | 0.06±0.01 | 0.04±0.02 | <0.001 |
| QT interval | 0.30±0.4 | 0.23±0.03 | <0.001 |
| QoT interval | 0.19±0.03 | 0.14±0.03 | <0.001 |
| JT interval | 0.23±0.04 | 0.15±0.04 | <0.001 |
| J to peak T interval | 0.17±0.36 | 0.11±0.32 | <0.001 |
| Peak T to end T interval (Tp-e) | 0.06±0.01 | 0.04±0.02 | <0.001 |
| Tp-e/QT interval | 0.20±0.06 | 0.20±0.10 | 0.724 |
| Tp-e/QTc (B) | 0.15±0.04 | 0.12±0.06 | <0.001 |
| Tp-e/QTc (Fridericia’s formula) | 0.17±0.05 | 0.14±0.07 | <0.001 |
| Tp-e/QTc (Framingham’s formula) | 0.16±0.04 | 0.14±0.07 | <0.001 |
| Tp-e/QTc (Hodges’s formula) | 0.16±0.04 | 0.10±0.06 | <0.001 |
| Absolute shortening of QT interval with exercise | 0.226±0.30 | ||
| QT shortening as fraction | 24.6%±12.1% | ||
SD: Standard deviation, QoT: Q to onset of T wave, JT: J point to end of T wave, Tp-e: T peak-T end interval, QTc: Corrected B: Bazett’s formula, QT: Onset of Q wave to the end of T wave, QRS: QRS complex, PR: PR interval
Changes in duration of P wave, P wave time to peak, QRS, T wave, T wave time to peak and PR, Onset of Q wave to the end of T wave, Q to onset of T wave, J point to end of T wave, J to peak T, T to end T and T to end T/Onset of Q wave to the end of T wave intervals
All durations, except P wave time to peak and Tp-e/QT interval, decreased significantly. P wave time to peak decreased at the end of the exercise, but the decrease was not statistically significant [Table 4]. Median (and interquartile range) of Tp-e pre- and post-exercise were 60 (30) and 40 (10) milliseconds, respectively [Figure 3].
Figure 3.
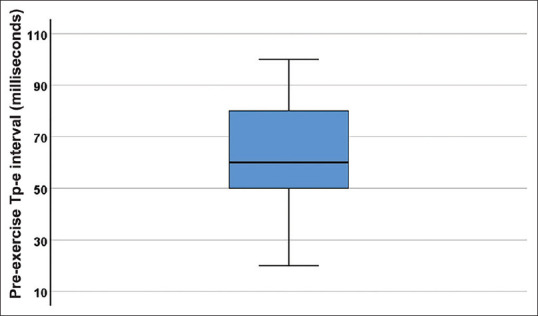
Box-and-whisker plot of Tp-e interval at preexercise in milliseconds, showing the median and lower and upper quartiles
Exercise-induced changes in the duration of heart rate-corrected Onset of Q wave to the end of T wave, Q to onset of T wave, J point to end of T wave, J to peak T intervals by four formulas of Bazett, Fridericia, Framingham, and Hodges
Mean ± SD of values of corrected QT (QTc), QoTc, JTc and JTPc at pre- and post-exercise are shown in Table 5. All intervals significantly decreased with exercise [Table 5].
Table 5.
Heart rate corrected onset of Q wave to the end of T wave, Q to onset of T wave, J point to end of T wave and J point to peak T intervals by four formulas of Bazett, Fridericia, Framingham and Hodges before and after treadmill exercise testing
| Preexercise | 95% CI | Postexercise | 95% CI | P | |
|---|---|---|---|---|---|
| Heart-rate correction of QT interval | |||||
| QT-c-Bazett (mean±SD) | 0.40±0.04 | 0.39-0.41 | 0.38±0.03 | 0.37-0.39 | <0.001 |
| QT-c-Fridericia (mean±SD) | 0.36±0.04 | 0.36-0.37 | 0.32±0.03 | 0.31-0.32 | <0.001 |
| QT-c-Framingham (mean±SD) | 0.37±0.03 | 0.36-0.37 | 0.32±0.02 | 0.32-0.33 | <0.001 |
| QT-c-Hodges (mean±SD) | 0.39±0.04 | 0.38-0.40 | 0.42±0.02 | 0.41-0.43 | <0.001 |
| Heart-rate correction of QoT interval | |||||
| QoT-c-Bazett (mean±SD) | 0.25±0.04 | 0.24-0.26 | 0.22±0.03 | 0.21-0.23 | 0.001 |
| QoT-c-Fridericia (mean±SD) | 0.22±0.04 | 0.21-0.23 | 0.19±0.03 | 0.18-0.19 | <0.001 |
| QoT-c-Framingham (mean±SD) | 0.25±0.03 | 0.24-0.26 | 0.23±0.02 | 0.23-0.24 | <0.001 |
| QoT-c-Hodges (mean±SD) | 0.27±0.04 | 0.26-0.28 | 0.33±0.03 | 0.32-0.34 | <0.001 |
| Heart-rate correction of JT interval | |||||
| JT-c-Bazett (mean±SD) | 0.30±0.04 | 0.29-0.31 | 0.25±0.05 | 0.24-0.26 | <0.001 |
| JT-c-Fridericia (mean±SD) | 0.27±0.04 | 0.26-0.28 | 0.21±0.04 | 0.20-0.22 | <0.001 |
| JT-c-Framingham (mean±SD) | 0.29±0.03 | 0.28-0.30 | 0.25±0.03 | 0.24-0.25 | <0.001 |
| JT-c-Hodges (mean±SD) | 0.31±0.04 | 0.30-0.32 | 0.34±0.03 | 0.34-0.35 | <0.001 |
| Heart-rate correction of J point to peak T interval | |||||
| J-T peak-c-Bazett | 0.22±0.04 | 0.21-0.23 | 0.19±0.05 | 0.17-0.19 | <0.001 |
| J-T peak-c-Fridericia | 0.20±0.04 | 0.19-0.21 | 0.15±0.04 | 0.14-0.16 | <0.001 |
| J-T peak-c-Framingham | 0.23±0.03 | 0.23-0.24 | 0.21±0.03 | 0.20-0.22 | <0.001 |
| J-T peak-c-Hodges | 0.25±0.04 | 0.24-0.26 | 0.32±0.03 | 0.31-0.33 | 0.002 |
C: Corrected, QoT: Q to onset of T wave, JT: J point to end of T wave, JTP: J point to peak T, CI: confidence interval, SD: standard deviation, QT: Onset of Q wave to the end of T wave
Pairwise comparison of effects of four different formulas of heart rate correction of Bazett, Fridericia, Framingham and Hodges on the values of onset of Q wave to the end of T wave, Q to onset of T wave, J point to end of T wave, and J to peak T
Except between preexercise QoTc and postexercise JTc, by Bazett and Framingham formulas, pairwise comparison by multiple measurements of ANOVA revealed significant differences among mean values obtained by different formulas of heart rate correction for these parameters of ventricular repolarization [Table 6].
Table 6.
Pairwise comparison of values of Onset of Q wave to the end of T wave, onset of Q to onset of T, J point to end of T wave, and J point to peak T intervals at preexercise and postexercise by four different formulas of heart rate correction including Bazett, Fridericia, Framingham and Hodges
| Formula | P value (significanceb) |
|---|---|
| QTc: Preexercise | |
| Bazett | |
| Fridericia | <0.001 |
| Framingham | <0.001 |
| Hodges | <0.001 |
| QTc: Postexercise | |
| Bazett | |
| Fridericia | <0.001 |
| Framingham | <0.001 |
| Hodges | <0.001 |
| QoTc: Preexercise | |
| Bazett | |
| Fridericia | <0.001 |
| Framingham | 0.343 |
| Hodges | <0.001 |
| QoTc: Postexercise | |
| Bazett | |
| Fridericia | <0.001 |
| Framingham | 0.002 |
| Hodges | <0.001 |
| JT: Preexercise | |
| Bazett | |
| Fridericia | <0.001 |
| Framingham | <0.001 |
| Hodges | 0.013 |
| JT: Postexercise | |
| Bazett | |
| Fridericia | <0.001 |
| Framingham | <0.001 |
| Hodges | <0.001 |
| JTP interval: Preexercise | |
| Bazett | |
| Fridericia | <0.001 |
| Framingham | <0.001 |
| Hodges | <0.001 |
| JTP interval: Postexercise | |
| Bazett | |
| Fridericia | <0.001 |
| Framingham | <0.001 |
| Hodges | <0.001 |
bAdjustment for multiple comparisons: Bonferroni. QTc: Corrected QT, QoTc: Onset of Q to onset of T, JT: J point to end of T wave, JTP: J point to peak T
Gender differences in ventricular repolarization parameters (onset of Q wave to the end of T wave, Q to onset of T wave, J point to end of T wave, J to peak T, T to end T interval, T to end T/Onset of Q wave to the end of T wave ratio, corrected onset of Q wave to the end of T wave, QoTc, JTc, J to peak Tc and Tp-e/Corrected Onset of Q wave to the end of T wave
Except for the preexercise QT and JT intervals and postexercise JTP corrected by Hodges formula, there was no statistically significant difference between girls and boys for the parameters mentioned above for ventricular repolarization, either in preexercise or postexercise [Table 7]. At preexercise, uncorrected QT was slightly higher in boys (0.31 ± 0.03 in boys versus 0.29 ± 0.03 in girls, P = 0. 022).
Table 7.
Gender differences in ventricular repolarization parameters and their heart rate corrected values at pre- and post-exercise in 74 healthy children
| Parameters | Preexercise | Postexercise | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Male | Female | P | Male | Female | P | |
| Heart rate | 104±16 | 116±16 | 0.005 | 181±15 | 183±15 | 0.668 |
| QT (s) | 0.31±0.03 | 0.29±0.03 | 0.022 | 0.22±0.02 | 0.21±0.02 | 0.140 |
| QTc-Bazett | 0.40±0.04 | 0.40±0.05 | 0.995 | 0.38±0.03 | 0.37±0.02 | 0.172 |
| QTc-Fridericia | 0.37±0.03 | 0.36±0.04 | 0.343 | 0.32±0.03 | 0.31±0.02 | 0.182 |
| QTc-Framingham | 0.37±0.03 | 0.36±0.03 | 0.167 | 0.32±0.02 | 0.32±0.02 | 0.205 |
| QTc-Hodges | 0.38±0.03 | 0.39±0.06 | 0.380 | 0.42±0.02 | 0.42±0.01 | 0.525 |
| QoT | 0.18±0.03 | 0.18±0.03 | 0.456 | 0.13±0.02 | 0.13±0.019 | 0.837 |
| QoTc-Bazett | 0.24±0.04 | 0.25±0.04 | 0.427 | 0.22±0.04 | 0.23±0.02 | 0.597 |
| QoTc-Fridericia | 0.22±0.04 | 0.22±0.04 | 0.794 | 0.19±0.03 | 0.19±0.02 | 0.673 |
| QoTc-Framingham | 0.25±0.03 | 0.25±0.03 | 0.544 | 0.23±0.02 | 0.23±0.01 | 0.677 |
| QoTc-Hodges | 0.26±0.03 | 0.29±0.06 | 0.029 | 0.33±0.03 | 0.33±0.03 | 0.499 |
| JT | 0.24±0.03 | 0.22±0.04 | 0.027 | 0.15±0.04 | 0.15±0.03 | 0.764 |
| JTc-Bazett | 0.31±0.04 | 0.29±0.04 | 0.157 | 0.26±0.06 | 0.26±0.04 | 0.673 |
| JTc-Fridericia | 0.28±0.04 | 0.26±0.04 | 0.068 | 0.22±0.05 | 0.21±0.04 | 0.830 |
| JTc-Framingham | 0.30±0.03 | 0.28±0.03 | 0.128 | 0.25±0.04 | 0.25±0.02 | 0.806 |
| JTc-Hodges | 0.32±0.04 | 0.31±0.02 | 0.873 | 0.36±0.03 (0.034) | 0.36±0.03 (0.026) | 0.794 |
| JTP | 0.17±0.03 | 0.16±0.04 | 0.243 | 0.11±0.03 (0.108±0.034) | 0.11±0.03 (0.105±0.028) | 0.978 |
| JTPc-Bazett | 0.22±0.04 | 0.22±0.04 | 0.725 | 0.18±0.06 | 0.18±0.05 | 0.752 |
| JTPc-Fridericia | 0.20±0.04 | 0.19±0.04 | 0.521 | 0.16±0.05 | 0.15±0.04 | 0.724 |
| JTPc-Framingham | 0.23±0.03 | 0.23±0.03 | 0.924 | 0.21±0.03 | 0.21±0.03 | 0.705 |
| JTPc-Hodges | 0.25±0.04 | 0.26±0.03 | 0.281 | 0.32±0.03 | 0.32±0.03 | 0.949 |
| Tp-e | 0.06±0.01 | 0.05±0.01 | 0.06 | 0.04±0.01 | 0.04±0.04 | 0.65 |
| Tp-e/QT | 0.21±0.06 | 0.19±0.04 | 0.383 | 0.19±0.04 | 0.21±0.16 | 0.521 |
| Tp-e/QTc-Bazett | 0.16±0.05 | 0.14±0.03 | 0.085 | 0.11±0.03 | 0.12±0.10 | 0.535 |
| Tp-e/QTc-Fridericia | 0.17±0.05 | 0.15±0.03 | 0.139 | 0.13±0.03 | 0.15±0.12 | 0.544 |
| Tp-e/QTc-Framingham | 0.17±0.05 | 0.15±0.03 | 0.151 | 0.13±0.03 | 0.14±0.12 | 0.556 |
| Tp-e/QTc-Hodges | 0.16±0.05 | 0.14±0.03 | 0.037 | 0.10±0.02 | 0.11±0.10 | 0.576 |
c: Corrected, J-Tpeak: J point to peak T interval, B: Bazett, JTP: J point to peak T interval, Tp-e: T peak-T end interval, QoT: Onset of Q to onset of T, JT: J point to end of T wave, QTc: Corrected QT, QT: Onset of Q wave to the end of T wave, JTc: Corrected JT interval, JTPc: Corrected J point to peak T interval
Changes in electrocardiographic -derived estimated duration of systole, diastole, and systolic-to-diastolic time ratio before and after treadmill exercise testing
ECG-derived estimated duration of systole and diastole decreased significantly with exercise and the systolic-to-diastolic time ratio increased significantly [Table 8].
Table 8.
Comparison of electrocardiographic-derived estimation of duration of systole and diastole and systolic-to-diastolic time ratio at preexercise and postexercise in 74 healthy children
| Preexercise | Postexercise | P | |
|---|---|---|---|
| Duration of systole (milliseconds) | 230.3±36.86 | 150.9±35.35 | <0.001 |
| Duration of diastole (milliseconds) | 335.8±82.1 | 181.1±32.5 | <0.001 |
| Systolic to diastolic time ratio | 0.72±0.18 | 0.88±0.32 | <0.001 |
| Percentage of decrease in ECG-derived estimation of duration of systole with exercise (mean±SD) | 33.74±15.79 | ||
| Percentage of decrease in ECG-derived estimation of duration of diastole with exercise (mean±SD) | 43.79±13.31 | ||
| Heart rate (beats/min) | 108±17 | 181±15 | <0.001 |
ECG: Electrocardiographic, SD: Standard deviation
DISCUSSION
Exercise-induced electrocardiographic changes in amplitudes and duration
This study showed that amplitudes of all waves increased with exercise, although the increase in Q and R waves were not statistically significant. Lack of U waves either in preexercise or postexercise ECG tracings implicates that U wave does not seem to be a typical feature in 5–12-year-old children. Thus, their detection may warrant further investigation. Except for Tp-e/QT, all the durations were decreased with exercise, although a decrease in P wave time was not statistically significant. These findings show that exercise-induced changes in amplitude and duration of ECG waves and intervals are not the same for atrial and ventricular depolarization and repolarization. The studies on ECG changes with TET in healthy children are old and uncomprehensive and extremely scant if any.[9,10,11]
Simoons and Hugenholtz studied 56 healthy adult males aged 23–62 years undergoing bicycle ergometry. They evaluated one beat from each stage. They reported exercise-induced decreased PR interval and T wave amplitude and increased P wave amplitude associated with no change in QRS duration or vectors. They attributed the increase in P wave amplitude to increased right atrial volume and mildly elevated hematocrit produced by volume loss during the exercise.[12] They did not state that they have chosen one single lead to conduct the study. Comparing complexes from different leads in each case of the study population may confound the comparison between preexercise and postexercise ECGs. The advantage of our study was that we chose lead II for our investigation. Simoons et al. did not explain why the decrease in volume and rise in hematocrit only affected P wave amplitude and not other waves. Furthermore, since this study was done a long time ago, the authors did not include the extensive set of parameters as in our study.
Ogedengbe et al. studied ECG changes in 40 healthy medical students before and after bicycle ergometer exercise testing.[4] They reported an increase in PR, QRS, and QT duration after exercise and they suggested the necessity of further research in this field.
Watanabe et al. showed a correlation between R wave amplitude and spatial QRS vector loop during exercise in 43 individuals undergoing supine bicycle testing, with posterior shift resulting in decreased R wave amplitude and anterior shift causing an increase or no change.[13] This finding implies that body position during exercise may affect R amplitude changes and should probably be considered. Moreover, this denotes the importance of considering the type of exercise testing in studies on exercise-induced ECG changes.
We presume the collective role of body position and Brody effect contributes more to the increase of QRS voltages than a rise in hematocrit since it is less likely for significant hematocrit changes to occur after a short period of exercise.[14,15,16,17]
Body position can affect the distance between the ECG lead and the heart. The amplitude of ECG waves is expected to increase as the distance decreases. Since all four chambers of the heart are not at the same orthogonal location, it is reasonable to observe not precisely the same changes throughout the ECG during exercise.
Exercise-induced electrocardiographic changes in parameters of ventricular repolarization and effect of gender
This is the first report of mean ± SD of JTP pre- and post-exercise in healthy children to the best of our knowledge. JTP represents the early phase of repolarization and is a biomarker for arrhythmias produced by QT shortening medications.[18] The block of hERG potassium channels prolongs JTP interval, while the late sodium current block shortens this interval.[19] Tp-e interval reflects the late phase of repolarization and is valued as a marker for risk stratification in patients with Brugada syndrome.[20,21] Furthermore, it is useful for the prediction of ventricular tachycardia, ventricular fibrillation, and mortality in patients with heart failure and implanted cardioverter-defibrillator and identification of high-risk patients with congenital and acquired long QT syndrome.[20,22,23] In adults, heart rate-corrected Tp-e has been described as a predictor for sudden cardiac arrest (SCA). A heart rate-corrected Tp-e interval beyond 90 msonds has been reported to be associated with a threefold increase in SCA's risk.[24] In a study on 523 healthy adults aged 31.9 ± 8.9 years, Hnatkova et al. reported significantly higher QTc, JTc, and JTPc and lower Tp-e intervals in female individuals.[25]
However, in our study, except for preexercise QT and JT intervals, there was no significant difference between male and female participants in QT, QoT, JTP, Tp-e interval, Tp-e/QT ratio, QTc, QoTc, JTc, JTPc, and Tp-e/QTc. Lower heart rate in boys at preexercise can explain the slightly higher uncorrected QT and JT intervals in this group (heart rate: 104 ± 16 bpm in boys vs. 116 ± 16 bpm in girls, P = 0.005) [Table 7]. Our findings may be explained by the prepubertal age of our study population. We do not expect the effects of sex hormones on ventricular repolarization to be present at this stage.[26,27,28]
We also presented pre- and post-exercise mean ± SD values of Tp-e interval. Tp-e interval is considered as an indicator of transmural dispersion of repolarization. An increased Tp-e interval and Tp-e/QT ratio are accompanied by ventricular arrhythmias and sudden cardiac death.[29,30] However, there is still a dearth of knowledge in the pediatric population in this regard. Demirol et al. studied 110 children with mitral valve prolapse (MVP) and showed increased Tp-e interval, Tp-e/QT, and Tp-e/QTc intervals in children with MVP compared with control groups. They presented their data as median and interquartile ranges for healthy control as 80 and 20 ms, respectively.[31] In our study, the median and IQR were 60 and 30 ms, respectively. Akın et al. reported increased Tp-e interval values, Tp-e/QT, and Tp-e/QTc intervals in 40 children with subclinical hypothyroidism compared with the control group.[32] Their reported mean ± SD of values for Tp-e interval, Tp-e/QT, and Tp-e/QTc intervals in the control group were 63.48 ± 13.03 msonds (ms), 0.19 ± 0.03, and 0.15 ± 0.03, respectively. These values are closely similar to our findings (60 ± 10 ms, 0.20 ± 0.06, and 0.15 ± 0.04, respectively).
Electrocardiographic-derived estimation of duration of systole and diastole with exercise
We introduced a novel concept out of an old Wigger's diagram to estimate the duration of mechanical systole and diastole using ECG. We showed that the duration of systole and diastole decrease by exercise, but in healthy children, the percentage of decrease in duration of diastole (43.79% ±13.31%) exceeds the percentage of decrease in duration of systole (33.74% ±15.79%), P < 0.001.
Effect of the four formula's for heart rate correction on electrocardiographic intervals and the dire need for revisiting the current conventional nomograms
As shown in Table 6, we found significant differences in values of heart-rate-corrected parameters of ventricular repolarization (QT, QoT, JT, and JTP), either at preexercise or postexercise in our study.
However, there was no significant difference between preexercise QoT interval by Bazett's and Framingham's formulas. The reason for this finding is unclear.
Given the difference among the results of QT correction by various formulas, the concept of considering one single value for diagnosing long QT as suggested by Schwartz et al., regardless of the formula used for correction, needs to be revisited. Nomograms considering both heart rate and heart rate correction methods are indispensable for the diagnosis of long QT in children.[33]
Vandenberk et al. studied the impact of different methods of heart rate correction on risk stratification and all-cause mortality in 6609 patients aged more than 18 years. They concluded that Fridericia and Framingham formulas were the best rate correction formulas for predicting mortality.[34] Luo et al. compared four methods of QT correction on 10,303 normal ECGs.[35] They presented nomograms for QT correction based on the heart rate-correction formula and heart rate. They concluded that overall, Hodges' method was the most appropriate method for the correction of QT at any heart rate or any gender.
CONCLUSIONS
To the best of our knowledge, to date, this is the most comprehensive study on exercise-induced ECG changes in healthy children. We presented mean ± SD of amplitude and duration of P, Q, R, S, T, and U, in addition to durations of PR, QT, QoT, JT, JTP, Tp-e, Tp-e/QT, and Tp-e/QTc intervals before and after treadmill exercise test in healthy children aged 5–12 years. This study showed diastolic time decreased more than systolic time with exercise and systolic time to diastolic time increased with exercise. We also calculated heart rate corrected values of ventricular repolarization parameters by four different formulas of Bazett, Fridericia, Framingham, and Hodges. We studied the effect of heart rate correction method and gender on these intervals. This study showed increased amplitude and decreased duration of ECG waves and intervals with TET in 5–12-year-old healthy children. Ventricular repolarization parameters of QTc, QoTc, JTc, JTPc, Jp-e, Tp-e/QT, and Tp-e/QTc did not differ in boys and girls either before or after exercise. In contrast, overall, a significant disparity exists among heart rate corrected values of QT, QoT, JT, and JTP intervals at pre- and post-exercise by different formulas.
Hodges' and Fridericia's formulas of heart rate correction resulted in the longest and shortest ventricular repolarization values, respectively. Therefore, using a single value as the cut-off for long QT syndrome diagnosis may lead to under-diagnosis or over-diagnosis of long QT in children. Therefore, nomograms incorporating age, heart rate, and heart rate correction formula are strongly recommended as a baseline for accurate diagnosis of long QT.
Last but not least, the presence of U waves in ECG tracings of healthy children after the TET may warrant more careful investigation.
Ethics approval and consent to participate
The study was performed in accordance with the Helsinki Declaration on ethical principles for medical research involving human subjects and was approved by the Institutional Committee for Ethics in Biomedical Research of the medical school of Tehran University of Medical Sciences (ID: IR. TUMS. MEDICINE. REC. 1398.774). All participants were included in the study with informed consent of the child and their guardian or guardians.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflict of interest.
Acknowledgment
The authors are grateful to the children and their parents for participation in this study.
REFERENCES
- 1.El Assaad I, Gauvreau K, Rizwan R, Margossian R, Colan S, Chen MH. Value of exercise stress echocardiography in children with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2020;33:888–94.e2. doi: 10.1016/j.echo.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Marcadet DM, Pavy B, Bosser G, Claudot F, Corone S, Douard H, et al. French Society of Cardiology Guidelines on Exercise Tests (part 2): Indications for exercise tests in cardiac diseases. Arch Cardiovasc Dis. 2019;112:56–66. doi: 10.1016/j.acvd.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Massin MM. The role of exercise testing in pediatric cardiology. Arch Cardiovasc Dis. 2014;107:319–27. doi: 10.1016/j.acvd.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Ogedengbe JO, Adelaiye AB, Kolawole OV. Effects of exercise on PR intervals, QRS durations and QTC intervals in male and female students of University of Abuja. J Pak Med Assoc. 2012;62:273–5. [PubMed] [Google Scholar]
- 5.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 6.Hebestreit H, Arets HG, Aurora P, Boas S, Cerny F, Hulzebos EH, et al. Statement on Exercise Testing in Cystic Fibrosis. Respiration. 2015;90:332–51. doi: 10.1159/000439057. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein D. Evaluation of the cardiovascular system and the child with a heart murmur. In: Kliegman RM, St. Geme JW 3rd, Blum NJ, Tasker RC, Shah SS, Wilson KM, et al., editors. Nelson Textbook of Pediatrics. 21st ed. Philadelphia: Elsevier; 2020. p. 2353. [Google Scholar]
- 8.General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14–8. [PubMed] [Google Scholar]
- 9.Deckers JW, Vinke RV, Vos JR, Simoons ML. Changes in the electrocardiographic response to exercise in healthy women. Br Heart J. 1990;64:376–80. doi: 10.1136/hrt.64.6.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilhall M, Riha M, Jern S. Exercise-induced QRS changes in healthy men and women: A multivariate analysis on their relation to background data and exercise performance. Eur Heart J. 1992;13:1316–24. doi: 10.1093/oxfordjournals.eurheartj.a060060. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh RM, Gates GJ, Walsh CA, Schiller MS, Pass RH, Ceresnak SR. The prevalence of arrhythmias, predictors for arrhythmias, and safety of exercise stress testing in children. Pediatr Cardiol. 2015;36:584–90. doi: 10.1007/s00246-014-1053-9. [DOI] [PubMed] [Google Scholar]
- 12.Simoons ML, Hugenholtz PG. Gradual changes of ECG waveform during and after exercise in normal subjects. Circulation. 1975;52:570–7. doi: 10.1161/01.cir.52.4.570. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe K, Bhargava V, Froelicher VF. The relationship between exercise-induced R wave amplitude changes and QRS vector loops. J Electrocardiol. 1981;14:129–38. doi: 10.1016/s0022-0736(81)80047-7. [DOI] [PubMed] [Google Scholar]
- 14.Giraud R, Siegenthaler N, Morel DR, Bendjelid K. The Brody effect to detect hypovolemia in clinical practice. Critical Care. 2012;16(Suppl 1):232. [Google Scholar]
- 15.Amoore JN. The Brody effect and change of volume of the heart. J Electrocardiol. 1985;18:71–5. doi: 10.1016/s0022-0736(85)80037-6. [DOI] [PubMed] [Google Scholar]
- 16.Madias JE. QRS voltage changes in heart failure: A 3-compartment mechanistic model and its implications. Indian Pacing Electrophysiol J. 2010;10:464–73. [PMC free article] [PubMed] [Google Scholar]
- 17.Oreto G, Luzza F, Donato A, Satullo G, Calabrò MP, Consolo A, et al. Electrocardiographic changes associated with haematocrit variations. Eur Heart J. 1992;13:634–7. doi: 10.1093/oxfordjournals.eurheartj.a060227. [DOI] [PubMed] [Google Scholar]
- 18.Qiu B, Wang Y, Li C, Guo H, Xu Y. Utility of the JT peak interval and the JT area in determining the proarrhythmic potential of QT-shortening agents. J Cardiovasc Pharmacol Ther. 2019;24:160–71. doi: 10.1177/1074248418791999. [DOI] [PubMed] [Google Scholar]
- 19.Johannesen L, Vicente J, Mason JW, Erato C, Sanabria C, Waite-Labott K, et al. Late sodium current block for drug-induced long QT syndrome: Results from a prospective clinical trial. Clin Pharmacol Ther. 2016;99:214–23. doi: 10.1002/cpt.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tse G, Gong M, Meng L, Wong CW, Georgopoulos S, Bazoukis G, et al. Meta-analysis of Tpeak-Tend and Tpeak-Tend/QT ratio for risk stratification in congenital long QT syndrome. J Electrocardiol. 2018;51:396–401. doi: 10.1016/j.jelectrocard.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Tse G, Gong M, Wong WT, Georgopoulos S, Letsas KP, Vassiliou VS, et al. The Tpeak-Tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: A systematic review and meta-analysis. Heart Rhythm. 2017;14:1131–7. doi: 10.1016/j.hrthm.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Tse G, Gong M, Meng L, Wong CW, Bazoukis G, Chan MTV, et al. Predictive value of Tpeak-Tend indices for adverse outcomes in acquired QT prolongation: A meta-analysis. Front Physiol. 2018;9:1226. doi: 10.3389/fphys.2018.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue C, Hua W, Cai C, Ding LG, Niu HX, Fan XH, et al. Predictive value of Tpeak-Tend interval for ventricular arrhythmia and mortality in heart failure patients with an implantable cardioverter-defibrillator: A cohort study. Medicine (Baltimore) 2019;98:e18080. doi: 10.1097/MD.0000000000018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua KC, Rusinaru C, Reinier K, Uy-Evanado A, Chugh H, Gunson K, et al. Tpeak-to-Tend interval corrected for heart rate: A more precise measure of increased sudden death risk. Heart Rhythm. 2016;13:2181–5. doi: 10.1016/j.hrthm.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hnatkova K, Vicente J, Johannesen L, Garnett C, Strauss DG, Stockbridge N, et al. Heart Rate Correction of the J-to-Tpeak Interval. Sci Rep. 2019;9:15060. doi: 10.1038/s41598-019-51491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odening KE, Koren G. How do sex hormones modify arrhythmogenesis in long QT syndrome? Sex hormone effects on arrhythmogenic substrate and triggered activity. Heart Rhythm. 2014;11:2107–15. doi: 10.1016/j.hrthm.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sedlak T, Shufelt C, Iribarren C, Merz CN. Sex hormones and the QT interval: A review. J Womens Health (Larchmt) 2012;21:933–41. doi: 10.1089/jwh.2011.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurokawa J, Kodama M, Clancy CE, Furukawa T. Sex hormonal regulation of cardiac ion channels in drug-induced QT syndromes. Pharmacol Ther. 2016;168:23–8. doi: 10.1016/j.pharmthera.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akboğa MK, Gülcihan Balcı K, Yılmaz S, Aydın S, Yayla Ç, Ertem AG, et al. Tp-e interval and Tp-e/QTc ratio as novel surrogate markers for prediction of ventricular arrhythmic events in hypertrophic cardiomyopathy. Anatol J Cardiol. 2017;18:48–53. doi: 10.14744/AnatolJCardiol.2017.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castro-Torres Y, Carmona-Puerta R, Katholi RE. Ventricular repolarization markers for predicting malignant arrhythmias in clinical practice. World J Clin Cases. 2015;3:705–20. doi: 10.12998/wjcc.v3.i8.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demirol M, Karadeniz C, Ozdemir R, Çoban Ş, Katipoğlu N, Yozgat Y, et al. Prolonged Tp-e Interval and Tp-e/QT ratio in children with mitral valve prolapse. Pediatr Cardiol. 2016;37:1169–74. doi: 10.1007/s00246-016-1414-7. [DOI] [PubMed] [Google Scholar]
- 32.Akın A, Unal E, Yıldırım R, Ture M, Balık H, Haspolat YK. Evaluation of QT dispersion and Tp-e interval in children with subclinical hypothyroidism. Pacing Clin Electrophysiol. 2018;41:372–5. doi: 10.1111/pace.13286. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation. 1993;88:782–4. doi: 10.1161/01.cir.88.2.782. [DOI] [PubMed] [Google Scholar]
- 34.Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, Ector J, Willems R. Which QT Correction Formulae to Use for QT Monitoring. J Am Heart Assoc. 2016 Jun 17;5(6):e003264. doi: 10.1161/JAHA.116.003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: The effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37(Suppl):81–90. doi: 10.1016/j.jelectrocard.2004.08.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


