Abstract
Essential genes encode the processes that are necessary for life. Until recently, commonly applied binary classifications left no space between essential and non-essential genes. In this review, we frame bacterial gene essentiality in the context of genetic networks. We explore how the quantitative properties of gene essentiality are influenced by the nature of the encoded process, environmental conditions and genetic background, including a strain's distinct evolutionary history. The covered topics have important consequences for antibacterials, which inhibit essential processes. We argue that the quantitative properties of essentiality can thus be used to prioritize antibacterial cellular targets and desired spectrum of activity in specific infection settings. We summarize our points with a case study on the core essential genome of the cystic fibrosis pathobiome and highlight avenues for targeted antibacterial development.
Keywords: essential gene, antibiotic discovery, genetic interaction, conditional essentiality, transposon mutagenesis, CRISPRi, cystic fibrosis
This review explores the spectrum of gene essentiality in the context of the environment, genetic networks and evolution, with the goal of highlighting its applicability to antimicrobial research targeting specific pathogens and infection settings.
Introduction
Bacteria have adapted to survive in virtually every niche on the planet. The resulting diversity has driven an equally large diversity of genetic material enabling growth. Over recent decades, the advances in DNA sequencing technology have given researchers an unparalleled view into the molecular biology pieces that make up the vast puzzle of life on Earth. We know that microbial genomes can house thousands of genes, but which are truly required for growth and survival, that is, which are essential? Answering this question has been an active research area as essential genes encode fundamental and conserved process of bacterial life. A foundational study by Eugene Koonin found that bacteria belonging to two different ancient lineages, Mycoplasma genitalium and Haemophilus influenzae, shared 240 homologous genes, suggesting that this gene set may represent a minimal gene set for microbial viability and thus contains many essential genes (Mushegian and Koonin 1996). Later, the Koonin group compared the rate of evolution between experimentally confirmed essential genes and non-essential genes in three species of bacteria and concluded that essential genes tend to be more evolutionarily conserved than their non-essential counterparts (Jordan et al. 2002). Therefore, essential genes are not only important physiologically, but their coding products are enticing targets for broad range antibiotic discovery endeavours.
An early example of gene essentiality is from the auxotrophy studies of Beadle and Tatum. X-ray generated mutants of Neurospora crassa and N. sitophila exhibited virtually no growth in medium each lacking either pyridoxine, thiamine and p-aminobenzoic acid (Beadle and Tatum 1941). We now know that gene essentiality is complex and depends on many environmental and genetic factors. Indeed, many of Beadle and Tatum's mutants displayed titrable growth with intermediate nutrient concentrations and were also able to achieve wild-type growth with adequate nutrient supplementation (Beadle and Tatum 1941; Tatum and Beadle 1942; Horowitz et al. 1945). A simple binary classification of genes as essential or non-essential oversimplifies biology. Our understanding of gene essentiality has evolved considerably by acknowledging that the contribution of each gene to microbial growth and survival is on a continuous spectrum. To describe this scenario, we survey the methodology used to propel this field of study forward. Then, we lay out the key points that led us to expand the concept of gene essentiality to that of essentiality gradients. Finally, we summarize how the intricacies of gene essentiality can have broad implications for antibiotic discovery and development. As an example, we present a case study on the core and accessory essential genomes of the cystic fibrosis pathobiome and how it can be used to guide the development of infection setting- and pathogen-specific therapeutics.
Genetic tools for identifying essential genomes and characterizing essential genes
In efforts to answer the question about which genes are essential in a given microorganism, several targeted and untargeted methods have been employed. For targeted approaches, genes are systematically deleted or interrupted. For untargeted methodologies, genes are randomly inactivated by a genetic element, such as a transposon. Indeed, it was Craig Venter who set the foundations of essential genome projects by first determining the essential gene set in Mycoplasma genitalium by transposon mutagenesis (Hutchison et al. 1999). Now, over twenty years since that seminal work, hundreds of large-scale essential gene and genome (the so-called ‘essentialome’) projects have been performed with these methodologies in multiple species and conditions. We will survey examples of such endeavours with a focus on the methods employed. An important distinction that we explore for each technology is whether they allow the creation and recovery of essential gene conditional mutants or only generate a catalog of putative essential genes. Below, we briefly survey the most common of these technologies, highlighting similarities, advantages and shortcomings (Table 1).
Table 1.
Summary of common tools used to identify essential genomes.
| Method | Recover Essential Gene Mutants? | Advantages | Disadvantages | Notable Sources |
|---|---|---|---|---|
| Transposon Mutagenesis | ||||
| Subtype: Gene/promoter Disruption | No | Rapid generation of mutant libraries; amenable to many species | Not all genes may be disrupted; potential insertion biases; difficult to isolate mutants from pool; polar effects in operons; statistical analysis differences may alter essential gene calls | Akerley et al. 2002; Gerdes et al. 2003; Chaudhuri et al. 2009; Langridge et al. 2009; Christen et al. 2011; Griffin et al. 2011; Goodall et al. 2018 |
| Subtype: Promoter Replacement | Yes | Rapid generation of mutant libraries; tunable levels of gene expression; amenable to many species | Not all genes may be disrupted; may have insertion biases; difficult to isolate mutants from pool; polar effects in operons; statistical analysis differences may alter essential gene calls; disrupts natural gene regulation | Judson and Mekalanos 2000; Jacobs et al. 2003; Christen et al. 2011; Wang et al. 2011; Lee et al. 2015; Gislason et al. 2017; Hogan et al. 2018; Santiago et al. 2018 |
| Protein Degradation Tags | Yes | Ordered library; tunable levels of gene depletion | Tag may affect protein folding/localization; time-consuming to create | Kim et al. 2013; Cameron et al. 2014; Johnson et al. 2019 |
| Gene Deletion | No (in haploid organisms); Yes (heterozygotes in diploid organisms) | Ordered library; no chance of residual gene expression; ‘gold-standard’ for identifying absolutely essential genes | Time-consuming to create; initial screening medium defines which (essential) mutants cannot be recovered for study | Kobayashi et al. 2003; Baba et al. 2006; Berardinis et al. 2008; Xu et al. 2011; Muir et al. 2020 |
| CRISPRi | ||||
| Subtype: Individual sgRNA Synthesis | Yes | Ordered library; tunable levels of repression; flexibility in target site; amenable to many species | Polar effects in operons; time-consuming to create; potential toxicity of dCas9; uncommon PAM sites can limit target sites | Peters et al. 2016; Singh et al. 2016; Liu et al. 2017; de Wet et al. 2020; Shields et al 2020; Silvis et al. 2021 |
| Subtype: Pooled sgRNA Synthesis | Yes | Rapid mutant generation; tunable levels of repression; flexibility in target site; amenable to many species | Polar effects in operons; potential toxicity of dCas9; difficult to isolate mutants from pool; uncommon PAM sites can limit target sites | Cui et al. 2018; Rousset et al. 2018; Wang et al. 2018; Lee et al. 2019; Peters et al. 2019; Donati et al. 2020; Hawkins et al. 2020; Liu et al. 2020; Mathis et al. 2021 |
| asRNA | ||||
| Subtype: Shotgun Cloning | Yes | Rapid generation of mutant libraries; tunable levels of gene depletion | Polar effects in operons; potential for toxicity in E. coli propagation host; may not achieve complete silencing | Ji et al. 2001; Forsyth et al. 2002; Knuth et al. 2004; Wang and Kuramitsu 2005; Meng et al. 2012; Rusmini et al. 2014 |
| Subtype: Custom asRNA Design | Yes | Ordered library; tunable levels of gene depletion | Polar effects in operons; potential for toxicity in E. coli propagation host; may not achieve complete silencing | Goh et al. 2009; Nakashima and Tamura 2009; Goh et al. 2015; |
Transposon mutagenesis
Mycoplasma genitalium and M. pneumoniae have small genomes of 580 and 816 kb, respectively (Fraser et al. 1995; Himmelreich et al. 1996) and thus are logical experimental platforms to develop genome-wide studies. In particular, the Venter group chose these bacteria to apply transposon mutagenesis and identify the first essential genome in laboratory conditions (Hutchison et al. 1999). The experimental approach centered on the generation of thousands of transposon insertion mutants by introduction (i.e. electroporation) of a plasmid-borne Tn4001 harbouring an antibiotic resistance gene into M. genitalium cells. Then, they collected thousands of transposon mutants on antibiotic selective medium. The transposon-genome junctions were identified by an inverse PCR protocol entailing restriction enzyme digestion of isolated genomic DNA, followed by ligation then PCR amplification and sequencing of the resulting amplicons. The absence of transposon insertions within the 5’-most 80% of a gene suggested that gene was essential for growth. In total, ∼2200 unique insertion sites were identified, allowing them to approximate the number of essential genes in M. genitalium as 265–350 of the 482 protein-coding genes.
The identification of the minimal Mycoplasma genome was an important foundational study in the field, and highlights how much experimental designs can influence which genes are regarded as essential in laboratory conditions. Mathematical modelling predicts that competitive outgrowth of a mutant population featuring different growth rates can affect the outcome of essentiality screens (Grenov and Gerdes 2008). Therefore, after a transposition event, the transposon mutants must be collected and grown as pure clonal populations to avoid competitive outgrowth effects. Indeed, the Venter group refined the essential gene set of M. genitalium to 382 after passing cell suspensions through a filter to remove cell clumps before plating to select for antibiotic-resistant transposon mutants (Glass et al. 2006). In this way, single cells were deposited on the plates, thus ensuring pure colonies and removing competitive outgrowth effects.
Like the differences in outgrowth of transposon mutant populations, the density of transposon insertions in a genome can greatly influence which genes are identified as essential. An analysis of more than 20 000 transposon mutants by the Mekalanos group (Cameron, Urbach and Mekalanos 2008) used a neutral base-pair model (every base pair in the genome has the same chance to contain an insertion) to estimate the number of genes not being hit by a transposon by chance. They found that the probability of mis-annotating a gene as essential decreased as gene length increased, as longer genes are more likely to be disrupted by a transposon insertion. Currently, next generation sequencing (NGS) techniques allow the creation of high-density transposon mutant libraries comprising hundreds of thousands of unique mutants, thus increasing insertion density and the accuracy of gene essentiality annotations. With NGS, identification of transposon junctions and quantification of mutant abundance can be performed directly from genomic DNA extracted from a mutant population in toto, facilitating the identification of insertion sites without the need to isolate individual mutants. Several methods of transposon sequencing by NGS now exist such as TnSeq (Opijnen, Bodi and Camilli 2009), TnSeq-circle (Gallagher, Shendure and Manoil 2011), TraDIS (Langridge et al. 2009; Barquist, Boinett and Cain 2013), HITS (Gawronski et al. 2009) and INSeq (Goodman, Wu and Gordon 2011) (collectively summarized here as TnSeq). In essence, these methods are very similar in that they rely on PCR to amplify transposon-genome junctions, and only differ in technical details (e.g. methods of shearing DNA and ligating adapters).
An improvement on TnSeq methods is the introduction of unique, random DNA barcodes to the transposon elements. Once the unique DNA barcodes are delivered with the transposon element to a genomic region, an initial TnSeq analysis is necessary to link insertion sites with DNA barcodes (random barcode, TnSeq, RB-TnSeq). In subsequent experiments using the same mutant library, quantitative tracking of the transposon mutant population in different conditions can be achieved by simple PCR amplification of the DNA barcodes followed by NGS (barcode sequencing, BarSeq) (Wetmore et al. 2015; Price et al. 2018; Simpkins et al. 2019). For experiments with multiple conditions, BarSeq offers increased throughput by substantially reducing the DNA library preparation times.
Another aspect that influences essential gene identification is the use of thresholds for the number of insertions allowed and the growth phenotypes. Choice of thresholding for interpreting transposon insertion density data varies across the literature with combinations of comparison to Poisson distributions (as in (Hutchison et al. 1999)), gamma distributions, Gaussian mixture models, sliding windows, average insertion site densities corrected for gene length and Monte Carlo pseudo-data sets, among others (Hutchison et al. 1999; Langridge et al. 2009; Griffin et al. 2011; Zhang et al. 2012; Zomer et al. 2012; DeJesus and Ioerger 2013; Sarmiento, Mrázek and Whitman 2013; Solaimanpour, Sarmiento and Mrázek 2015; Freed, Bumann and Silander 2016; Gislason et al. 2017; Miravet-Verde et al. 2020). For a thorough review, the Waldor group provides expert advice on the design and analysis of such high-density transposon mutant libraries (Chao et al. 2016). There are also databases, the Transposon Registry (https://transposon.lstmed.ac.uk/) and TnCentral (Ross et al. 2021), to catalogue and organize nomenclature for the thousands of natural transposon variants (Tansirichaiya, Rahman and Roberts 2019), and tools to customize transposons for non-model bacteria (Liu et al. 2018).
In principle, transposon-based approaches can only identify essential genes indirectly through the lack of their corresponding mutants. Mutants with disrupted essential genes, or their essential promoters, are not viable (Fig. 1A). However, transposons can be modified to include an outward-facing constitutive promoter allowing expression of genes downstream of the insertion. Thus transposon mutants with insertions in the promoter region, 5’ end of a gene, or non-essential gene upstream of an essential gene in an operon can be recovered, albeit with altered (non-native) essential gene expression (Fig. 1B) (Hutchison et al. 1999; Jacobs et al. 2003; Wang et al. 2011; Lee et al. 2015; Santiago et al. 2018; Coe et al. 2019; Shaw et al. 2020). Variable levels of expression can be obtained through a pool of transposons harbouring constitutive promoters with different strengths (Wang et al. 2011). Titratable control of expression can be achieved with inducible promoters, allowing the creation of conditional growth mutants that depend on the inducer for growth (Fig. 1C) (Chow and Berg 1988; Takiff et al. 1992; Rappleye and Roth 1997; Judson and Mekalanos 2000; Hu et al. 2007; Bloodworth, Gislason and Cardona 2013; Le Breton et al. 2015; Gislason et al. 2017; Hogan et al. 2018; Yasir et al. 2020). Essential gene mutants can then be isolated from a library of transposon mutants by screening for growth only in the presence of the inducer (Judson and Mekalanos 2000; Bloodworth, Gislason and Cardona 2013) or by selectively killing non-essential gene mutants (Hogan et al. 2018).
Figure 1.

Outcomes of transposon mutagenesis with engineered transposons in the context of essential genes. (A) Regardless of operon arrangement, disruption of either an essential promoter or essential gene results in non-viable cells. Disruption of non-essential promoters or genes results in viable cells. (B) Modified transposons with outward-facing promoters yield mutants with variable growth defects. Only promoters with equal strength to the native promoter yield mutants with equal growth to the wild-type. However, if the transposon insertion places the promoter on the opposite strand, the mutant would not be viable. (C) An outward-facing inducible promoter allows recovery of conditional growth mutants with titratable growth and essential gene expression.
In summary, transposon mutagenesis has become a common method for essential gene identification. While the popularity of transposon mutagenesis has come with diversification in protocols and tools (Zomer et al. 2012; Barquist, Boinett and Cain 2013; Pritchard et al. 2014; DeJesus et al. 2015; Solaimanpour, Sarmiento and Mrázek 2015; Chao et al. 2016; Zhao et al. 2017; Liu et al. 2018; Poulsen et al. 2019; Shields and Jensen 2019; Page et al. 2020); comparative studies of essential genomes should consider the biases produced by this diversification.
Gene deletion collections
The availability of the first microbial genome sequences (Fleischmann et al. 1995; Fraser et al. 1995; Goffeau et al. 1996; Blattner et al. 1997) enabled precise mutations to interrogate gene function. Several multinational teams quickly moved on the ability to accurately manipulate genomes and focused on targeted methods for gene-by-gene inactivation, based on homologous recombination. The technical requirements of these endeavours were challenging, but they ultimately resulted in the creation of the first well-defined libraries in yeast (Winzeler et al. 1999; Giaever et al. 2002) and bacteria (Kobayashi et al. 2003; Baba et al. 2006). In particular, the handful of ordered bacterial mutant libraries that now exist have contributed greatly to the understanding of genotype-phenotype relationships (Kobayashi et al. 2003; Baba et al. 2006; Berardinis et al. 2008; Xu et al. 2011; Porwollik et al. 2014; Koo et al. 2017). The utility of gene-by-gene deletion libraries is also demonstrated by their continuing creation in other bacteria of interest (Muir et al. 2020). Additionally, the E. coli Keio collection (Baba et al. 2006) has over 3100 citations on PubMed in the 15 years since its creation and continues to yield new insights (Klobucar et al. 2020; Tong et al. 2020; Casanova-Hampton et al. 2021; Wang et al. 2021). However, it should be noted that during the construction of gene deletion mutant collections, hundreds of mutants for each bacterial species could not be isolated, leading to the compilation of lists of essential genes.
Creating comprehensive gene deletion mutant libraries is technically and logistically challenging. An allelic exchange strategy is employed to either insert a selection marker in place of each gene or delete the gene entirely. Briefly, primers must be designed to amplify DNA fragments with homology to the flanking target region. Then, the homologous fragments need to be combined with a DNA fragment encoding a selection marker. Finally, the construct is introduced into the desired bacterial strain and the resulting mutant, in which the selection marker has replaced the target gene, is selected and isolated. The specifics of the method are organism-dependent in terms of the length of the homologous region and factors increasing recombination efficiency (e.g. λ Red), but all result in replacement of a gene of interest by a selectable marker (Wach et al. 1994; Datsenko and Wanner 2000; Vries and Wackernagel 2002; Metzgar et al. 2004; Xu et al. 2011; Koo et al. 2017). Some studies include removal of the selection marker by a specific recombination system such as Cre-Lox or FLP-FRT, creating (nearly) scarless gene deletions (Datsenko and Wanner 2000; Baba et al. 2006). Although the use of naturally competent organisms, such as Bacillus subtilis (Kobayashi et al. 2003) and Acinetobacter baylyi (Berardinis et al. 2008), and single recombination approaches (Giaever et al. 2002; Baba et al. 2006; Kim et al. 2010) have halved the effort required to delete genes, making single gene deletion libraries remains difficult, as evidenced by the paucity of such libraries when compared to transposon libraries.
While targeted gene deletion collections remain a ‘gold standard’ for establishing gene-to-function links at the genome level and to identify essential genes, they do present limitations. For example, genes could be misannotated as non-essential if suppressor mutations quickly arise after deleting essential genes. Such is the case of the Keio collection, where several genes were misannotated as non-essential due to a high frequency of suppressor mutations (Riber et al. 2006; Durand et al. 2016). Solutions are to re-sequence the genomes of mutants of interest or to independently recreate them using inducible genetic tools, such as CRISPR-interference (see below). Another limitation is that mutants of small non-protein coding RNA (ncRNA) genes are missing from deletion collections simply because many ncRNA genes were not identified at the time of library construction. Annotating ncRNA genes remains an active research area as, for example, a deep sequencing analysis recently identified over 10000 putative ncRNAs in E. coli alone (Raad et al. 2021). In summary, phenotypic profiling of existing deletion collections can interrogate the function of some but not all genetic elements of the bacterial cell. Of particular interest for this review, the identification of essential genes as part of deletion libraries does not render essential gene mutants for further characterization of gene function.
Antisense RNA
Non-coding RNAs are a broad family of important regulators in both eukaryotes and prokaryotes. Among the members are antisense RNAs (asRNA), that generally cause sequence-specific inhibition of gene expression by interfering with mRNA transcription and translation, or by signaling mRNA degradation (Sesto et al. 2013; Saberi et al. 2016). The first use of asRNA to probe essential genes in bacteria was an untargeted approach (Ji et al. 2001). The Rosenberg group ligated randomly sheared Staphylococcus aureus genomic DNA fragments into a replicative plasmid featuring a promoter under anhydrotetracycline-inducible control. The random ligation allowed gene fragments to be transcribed in the reverse direction, thus generating an asRNA. Reintroduction of the plasmid constructs into S. aureus and screening for anhydrotetracycline-induced growth defects allowed identification and isolation of conditional growth mutants or knockdowns; hence, asRNA methods can be used to both identify and characterize essential genes. Since then, small- (Nakashima and Tamura 2009; Goh et al. 2015) and large-scale asRNA approaches have used inducible control (Forsyth et al. 2002; Knuth et al. 2004; Wang and Kuramitsu 2005; Meng et al. 2012; Rusmini et al. 2014).
Drawbacks of asRNA knockdown include the possibility of off-target effects and incomplete silencing of gene expression, resulting in a high proportion of false negatives when screening for growth defects. In fact, most reports of essential genes determined by asRNA knockdown identify substantially fewer essential genes than deletion and transposon methods (Ji et al. 2001; Knuth et al. 2004; Meng et al. 2012; Rusmini et al. 2014). Hence, there have been no recent essential genome projects using asRNA.
Protein degradation tags
Instead of interfering with transcription or translation of essential genes, fully formed essential proteins can be targeted for destruction by exploiting natural protein degradation pathways. There are many proteases in bacteria (Mahmoud and Chien 2018), and some have been manipulated for targeted degradation (Herman et al. 1998; Sekar et al. 2016; Butzin and Mather 2018); however, the Lon and ClpXP proteases have been the focus of attention with respect to essential proteins. The general approach is to insert a DNA sequence encoding a degradation tag (degron) at the 3’ end of the gene of interest within the genome and introduce a plasmid-borne protease or adapter (which binds the degron and shuttles the targeted protein to the protease) under inducible control, yielding mutants with titratable essential protein abundance and conditional growth (Davis, Baker and Sauer 2011; Kim et al. 2011, 2013; Johnson et al. 2019). As each mutant must be custom-made, this is a targeted approach to study essential gene function. The most commonly used system relies on the tmRNA-encoded ssrA peptide, used by the adapter protein SspB to shuttle truncated proteins to the ClpXP and ClpAP proteases (Janssen and Hayes 2012). The Schnappinger group demonstrated that the ssrA degron and SspB adapter can be functionally transferred to mycobacteria and used to achieve dose-dependent essential gene knockdown and cell death, both in vitro (Kim et al. 2011) and in murine models of acute and chronic infection (Kim et al. 2013). On a larger scale, custom-made libraries of essential protein depletion mutants can be used to investigate chemical genetic interactions and physiological consequences of essential gene depletion (Cameron and Collins 2014; Johnson et al. 2019).
However, there have been no reports using degrons for de novo essential genome identification, in bacteria, likely due to the laborious nature required to tag every protein-coding gene. Furthermore, the expression of essential RNAs (e.g. tRNAs) cannot be modulated by degron tagging. Even the libraries that do exist in E. coli (Cameron and Collins 2014) and Mycobacterium tuberculosis (Johnson et al. 2019) do not represent more than 80% of a complete essential genome. It has been suggested that the degron tag may interfere with protein localization or function, limiting the ability to examine every essential gene (Johnson et al. 2019).
CRISPR-interference
Another RNA-mediated silencing method that has gained traction with researchers recently is CRISPR-interference, or CRISPRi. The bacterial CRISPR/Cas system provides acquired sequence-specific immunity against phages and foreign DNA (Garneau et al. 2010; Jinek et al. 2012). The Cas9 component of this system is an RNA-guided endonuclease; however, a ‘dead’ Cas9, dCas9, from Streptococcus pyogenes has been modified to retain sequence-specific binding of DNA, but without nuclease activity (Bikard et al. 2013; Qi et al. 2013). Synthetic guide RNAs (sgRNAs) can be introduced to direct dCas9 to bind any DNA sequence of interest downstream of a protospacer-adjacent motif (PAM), a small, but critical, sequence motif. Targeting the promoter region sterically inhibits binding of the RNA polymerase complex and transcription initiation, while targeting the gene body inhibits elongation (Bikard et al. 2013; Qi et al. 2013), both causing a polar effect in operons. In this way, gene expression can be repressed up to 3 500-fold (Reis et al. 2019). When the exact location of the promoter is unknown, as is the case for most genes in non-model organisms, the 5’ end of the gene near the ATG start codon can be targeted (Peters et al. 2016; Singh et al. 2016; Liu et al. 2017; Lee et al. 2019).
Since its inception, there has been rapid adoption of CRISPRi methods to study many different aspects of diverse microbes. Typical approaches to CRISPRi use a constitutively expressed sgRNA and place the dcas9 gene under control of an inducible promoter, both either on a plasmid or in the chromosome (Larson et al. 2013). Dynamic levels of targeted gene knockdown can be achieved by titrating levels of inducer, changing sgRNA binding location or by introducing mismatches in the sgRNA (Qi et al. 2013; Hawkins et al. 2020; Mathis, Otto and Reynolds 2021). There are several distinct features of CRISPRi that address shortcomings of other methods: (i) sgRNA constructs can be made with relative ease; (ii) multiple genes can be simultaneously repressed (multiplexing); (iii) no genomic modifications are made to the target gene. In order to probe large numbers of genes, many sgRNA-expressing plasmids must be constructed. This can be done quickly in 96-well format using inverse PCR and blunt-end ligation, allowing hundreds of custom mutants to be constructed in a matter of days (Larson et al. 2013). Furthermore, with multiplexing, several sgRNA cassettes can be introduced into the same plasmid, allowing investigations into high order knockdown mutants (Ni et al. 2019; Reis et al. 2019).
The broad applicability of CRISPRi tools has also enabled discovery in diverse microbes (Schultenkämper, Brito and Wendisch 2020). As expression of the Cas proteins is not tolerated in some species, a recent review (Todor et al. 2021) on the use of different Cas orthologues for CRISPRi in bacteria highlights the need to avoid toxicity effects. In any case, high levels of dCas9 expression may induce morphological and growth defects (Rock et al. 2017; Cho et al. 2018; Depardieu and Bikard 2019; Hogan et al. 2019). The lethal effect may be mitigated by titrating dCas9 expression or using other less toxic Cas orthologues. The Fortune group found that the S. pyogenes dCas9 was both toxic and yielded only moderate repression in M. tuberculosis (Rock et al. 2017). Profiling 10 Cas9 orthologues identified the S. thermophilus dCas9 as being both well-tolerated and providing > 150-fold gene knockdown (Rock et al. 2017). The diversity of CRISPRI/Cas systems can provide a wealth of potential alternatives. However, cloning nuclease-inactive mutants directly into the host of interest may not always yield expression levels required for effective gene silencing due to codon usage and differences in GC content. Several codon-optimized dcas9 genes have therefore been developed (Farzadfard, Perli and Lu 2013; Maeder et al. 2013; Choudhary et al. 2015; Tong et al. 2015; Rock et al. 2017; Peng et al. 2018; Hogan et al. 2019) and mobilized on broad-range replicating or integrative plasmids (Depardieu and Bikard 2019; Hogan et al. 2019; Peters et al. 2019).
The fact that gene expression can be turned on and off with CRISPRi has been exploited for the isolation and characterization of essential gene mutants. Reports on essential genes using CRISPRi can be separated on whether they use targeted or untargeted approaches, dictating how the sgRNAs are designed and cloned individually vs. in pools. Targeted CRISPRi validated previously identified essential genomes and focused on essential genetic networks (Peters et al. 2016; Liu et al. 2017; de Wet et al. 2020; Shields et al. 2020; Silvis et al. 2021). Untargeted methods use pools of sgRNAs in parallel to target all PAM sites or known genetic elements, enabling genome-wide screens and mutant tracking by sequencing of the gRNA genes, which are unique for each mutant (Cui et al. 2018; Rousset et al. 2018, 2021; Wang et al. 2018; Lee et al. 2019; Peters et al. 2019; Liu et al. 2020; Donati et al. 2021; Mathis, Otto and Reynolds 2021). The logic behind untargeted CRISPRi is similar to transposon mutagenesis: CRISPRi mutants in essential genes are recovered in lower abundance, resulting in lower gRNA sequencing read density (termed CRISPRi-Seq). Interestingly, it has been reported that CRISPRi screens are more efficient than transposon mutagenesis in identifying essential genes, requiring at least ten-fold fewer mutants (Rousset et al. 2018, 2021; Wang et al. 2018; Calvo-Villamañán et al. 2020). A recent study from the Bikard group only required on average 3.4 sgRNAs per gene to accurately identify the essential genome of E. coli K-12; thus substantially lowering the number of total mutants screened to ∼11600 (Rousset et al. 2021). To aid in genome-wide screens, new computational tools can automate sgRNA design and selection (Mohr et al. 2016; Spoto et al. 2020) to reduce chances of confounding off-target effects (Cui et al. 2018); however, mismatches are sometimes desired as they can predictably titrate gene repression (Hawkins et al. 2020; Jost et al. 2020; Mathis, Otto and Reynolds 2021). Furthermore, there was a method recently developed to turn cells with hyperactive CRISPR/Cas9 systems into ‘factories’ to produce large numbers of CRISPR RNA gene cassettes that can be cloned into a desired target organism (CRISPR adaptation-mediated library manufacturing; CALM), substantially increasing gene coverage over other sgRNA design methods (Jiang, Oikonomou and Tavazoie 2020). However, these tools cannot produce sgRNAs for every gene, as some genes lack appropriate PAM sites, limiting genome-wide approaches (Shields et al. 2020). The requirement of a PAM site is a drawback for all CRISPR/Cas approaches, but can be partially compensated for by using dCas9 enzymes that recognize different (Rock et al. 2017) or synthetically reduced PAM sites (Hu et al. 2018; Nishimasu et al. 2018; Kulcsár et al. 2020; Walton et al. 2020).
Comparing targeted and untargeted approaches
A comparison between the essential genomes determined by transposon insertion methods and gene deletion approaches reveals differences in the number and nature of the identified essential genes. A study by the Soberón-Gonzalez group (Martínez-Carranza et al. 2018) compared five datasets (targeted deletion (Baba et al. 2006), large deletions (Kato and Hashimoto 2007), two transposon studies (Gerdes et al. 2003; Goodall et al. 2018), and the Profiling of E. coli Chromosome (PEC) database (Yamazaki, Niki and Kato 2008)), and found large variations in the number of essential genes determined for each, ranging from 303 (Baba et al. 2006) to 620 (Gerdes et al. 2003), and only 164 were common to all studies. Another study (Goodall et al. 2018) thoroughly re-examined the essential genome of E. coli K-12 by saturating transposon mutagenesis. Despite defining a geometric distribution to correct for assumptions in other models, they concluded that purely automated analysis of transposon sequencing data overlooks important details such as small essential coding regions within genes and insertion orientation-dependent effects. One of the complicating factors was the occurrence of essential protein domains, resulting in a void of insertions in an otherwise disrupted gene. Therefore, manual curation of transposon insertion datasets remains necessary (Goodall et al. 2018). Furthermore, growing mutant populations in pools, as exemplified by transposon mutant collections, can result in out-competition of certain mutants. In this context, gene fitness can be defined as the relative abundance of a mutant during competitive growth. However, the concept of gene fitness can have broader meanings, such as the ratio between the growth rate of a mutant vs. the wild-type strain when cultured competitively, or the differences in relative abundance of a clone before and after a condition is applied (Wiser and Lenski 2015). Together, untargeted approaches, such as transposon mutagenesis have been powerful to collect gene fitness data in different conditions, while targeted approaches, such as deletion methods have been applied to the identification of minimal gene sets in standard growth conditions (Gerdes et al. 2006).
Determinants of essentiality gradients
The nuances of growth conditions, experimental techniques, and genetic backgrounds are critical to defining the nature of gene essentiality. Considering an operational definition of essential genes as those indispensable for growth in rich medium, the percentage of essential genes varies from 80% to 7% in intracellular parasitic and free-living bacteria, respectively. Overall, the number of essential genes decreases with genome size (Rancati et al. 2018). However, it is clear that gene essentiality is a quantitative trait. Many studies have pondered how to classify genes with moderate or strong fitness contributions. In transposon mutant libraries, these genes are likely to tolerate some transposon insertions, could display slow colony growth if deleted, or could have a slow growth rate in liquid medium if gene expression is knocked down by CRISPRi. Because of their growth phenotype, genes that fall under this ‘grey area’ have been called ‘quasi-essential’ (Hutchison et al. 1999; Glass et al. 2006; Breuer et al. 2019) or ‘essential for fitness’ (Gerdes et al. 2006). Quantifying fitness of ‘intermediate’ essential genes was proposed to lead to fuller characterization of the essentiality spectrum (Gerdes et al. 2006). Then, fitness values can be used to bin genes by essentiality categories and we argue that defining bins is much more representative of actuality than a simple binary gene essentiality threshold. Indeed, there is a gradient of growth phenotypes between mutants in classically defined non-essential genes (for which no fitness defect is noted) and mutants in classically defined essential genes (mutation abolishes growth). As we will describe below, gene essentiality can be modified by the environment and the genetic context and captured in continuous quantities. In addition, the depletion levels of an essential gene product, dominated by a combination of gene expression and product turnover, can create gradients of growth phenotypes even within the same gene. We call these diversities of growth phenotypes ‘essentiality gradients’. Below, we will explain that essentiality gradients can be captured by quantifying gene fitness in different environmental conditions, genetic contexts, cellular copies of an essential product, and during the selection of compensatory mutations that render essential genes dispensable.
Environment-gene interactions
The contribution of a gene and its product to overall fitness depends on the context, both with respect to environmental conditions and genetic background. Then, genes which are essential in some, but not all conditions are called conditionally essential genes (Fig. 2A). A classic example of conditional essentiality due to environmental constraints is auxotrophy, where organisms are deficient in various nutrient synthesis genes. While said auxotrophic mutants would grow in rich medium containing essential nutrients, they would not grow in a minimal medium that requires their de novo synthesis. For example, screening the Keio collection in E. coli revealed that many amino acid and cofactor biosynthetic pathways (e.g. pyridoxal phosphate, histidine, and leucine) are conditionally essential for growth in glycerol-supplemented minimal medium, in good agreement with metabolic modelling predictions (Joyce et al. 2006). Early metabolic modeling in S. cerevisiae suggested that up to 68% of protein-coding genes could contribute to fitness in at least one condition (Papp, Pal and Hurst 2004), compared to 18.7% being essential for growth on rich medium (Giaever et al. 2002).
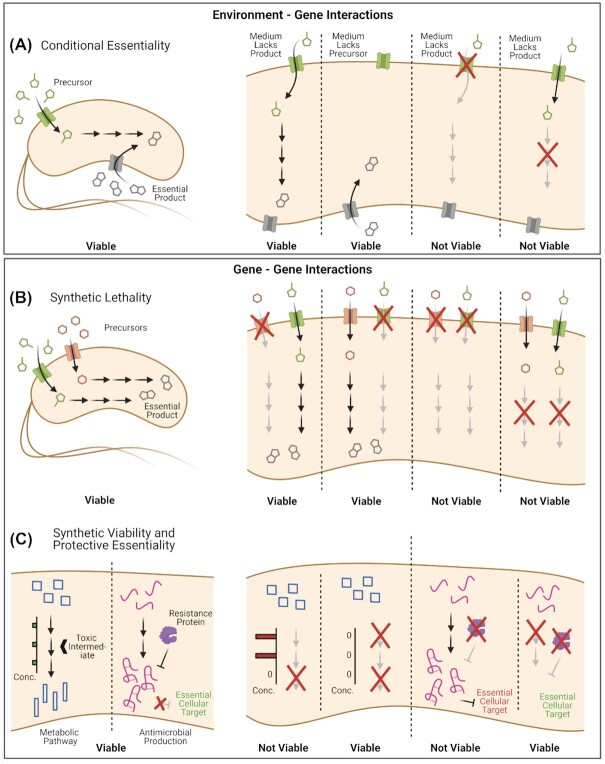
Figure 2.
Diagram of environment-gene and gene-gene interactions creating gene essentiality gradients. (A) Prototypical environment-gene interactions leading to conditional essentiality. On the left, a rich medium contains both an essential product (amino acid, co-factor, etc.) and the precursors for de novo synthesis that are taken up by specific transporters/channels. On the right, if the medium lacks the essential product (either naturally or synthetically), the cell relies on de novo synthesis for viability. Cells may also directly take up the essential product from the medium if a transporter/channel exists. Growth is not possible if the medium contains only precursors and the cells lack the appropriate transporters/channels or biosynthetic pathways. (B) Prototypical gene-gene interactions leading to synthetic lethality. On the left, an essential product can be synthesized by two independent and redundant pathways, relying on precursors present in the medium. On the right, the cells remain viable if either are deleted or inactivated; however, if both of the transporters/channels are deleted, or if both the biosynthetic pathways are inactivated, growth is not possible. The transporters/channels (and likewise genes in the biosynthetic pathways) are said to exist in synthetic lethal pairs. (C) Some gene–gene interactions leading to synthetic viability and protective essentiality. A cell may contain non-essential pathways with toxic intermediates that are rapidly turned over to ensure a low concentration. If there is a genetic or chemical block in the pathway, toxic intermediates accumulate leading to a growth defect/stasis. Viability can be restored by an upstream block in the pathway preventing the initial accumulation of toxic intermediates. A cell may also encode biosynthetic gene clusters for antimicrobial production that exhibit autoinhibition lest a resistance gene is present to protect the cell. The resistance gene may be inactivated only if first the antimicrobial synthesis gene(s) are inactivated.
Large experimental screens using gene deletion collections across hundreds of conditions have confirmed at least minor fitness contributions for 97% of S. cerevisiae genes (Hillenmeyer et al. 2008), and less than 50% of E. coli genes (Nichols et al. 2011). By applying a more stringent threshold, Nichols et al. identified 197 conditionally essential genes, each displaying no growth in at least one of 324 conditions, in addition to the approximately 296 essential genes for growth on rich medium (Baba et al. 2006; Yamamoto et al. 2009). Recently, the Brown group found 342 genes, which their deletion mutants showed a growth defect phenotype on at least one of 30 different carbon sources (Tong et al. 2020). Thus, it is clear that with new, larger studies, conditional essentiality will be observed for an increasing number of genes.
Transposon mutagenesis studies also provide multiple examples of conditional essentiality. For example, the Baughn group (Minato et al. 2019) used transposon mutagenesis to compare the essential genome of M. tuberculosis H37Rv in minimal and rich media. They identified up to 601 essential genes in rich, non-defined medium (MtbYM), while an additional 130 genes were conditionally essential when grown on minimal medium. As expected, the majority of these conditionally essential genes encoded biosynthetic functions, such as vitamin/cofactor, amino acid, and nucleotide biosynthesis (Minato et al. 2019). Indeed, entire pathways became essential, such as that for pantothenate (vitamin B5) biosynthesis. The group also identified 98 genes that were conditionally essential in MtbYM rich medium, as mutants were able to grow in minimal medium but not in rich medium. Penicillin-binding protein genes ponA1 and ponA2, and the major transpeptidase-encoding gene ltdB were among them. The conditional essentiality of these genes and the fact that the growth rate is slower in minimal medium suggest that defective peptidoglycan synthesis is tolerated during slow growth but not during fast growth in M. tuberculosis. Additionally, with their experimental data, the group was able to suggest important refinements to the metabolic model for M. tuberculosis (Ma et al. 2015). Other studies comparing essentiality between in vivo and in silico data have also suggested ways to improve metabolic models and vice versa (Molina-Henares et al. 2010; Orth and Palsson 2012; Yang et al. 2014; Burger et al. 2017; Vitkin et al. 2018; Blazier and Papin 2019; diCenzo, Mengoni and Fondi 2019; Mazharul Islam et al. 2020).
Defining the conditions in which a gene is essential can shed light on the characterization of genes of unknown function. To functionally link genes to metabolic pathways, the Deutschbauer and Arkin groups (Price et al. 2018) created randomly barcoded transposon mutant libraries in 32 different species and strains from 23 genera comprising six diverse bacterial taxa and grew them in hundreds of different conditions (carbon source, nitrogen source, and stressors). The authors linked genes to functions by identifying (i) highly specific phenotypes observed under one or a few conditions, and (ii) cofitness patterns, where more than one gene would display the same fitness across conditions. Together, they identified functional associations for 25276 genes and provided functional annotations for 11779 genes that were not previously associated with a specific function (Price et al. 2018). The results of this extensive study are available through their Fitness Browser for further analysis (http://fit.genomics.lbl.gov).
Upregulation of a gene in response to an environmental cue usually implies a function for the gene in the condition. On the contrary, the expression of many genes in a variety of conditions are rarely correlated with contribution to fitness (Deutschbauer et al. 2011; Turner et al. 2014; Murray et al. 2015; Jensen, Zhu and van Opijnen 2017; Helmann, Deutschbauer and Lindow 2019). This is succinctly demonstrated by comparing transposon mutant fitness to transcriptomic analyses. For example, in the Pseudomonas syringae-Phaseolus vulgaris bean plant infection model, mutants in amino acid biosynthetic pathways had severe fitness defects, but the same genes were actually repressed compared to in vitro growth (Helmann, Deutschbauer and Lindow 2019). Additionally, expression-fitness discord was recently demonstrated in the random and uncoordinated transcriptomic response to antibiotics (Jensen, Zhu and van Opijnen 2017; Zhu et al. 2020b). The number of encoded bacterial transcription factor families and their relative proportion to other genes increases with genome size to maintain adaptive control over more complex regulatory networks (Konstantinidis and Tiedje 2004; Sanchez et al. 2020). However, the fact that several gene products are expressed in a given condition does not necessarily mean that they will perform a unified set of functions. Under standard laboratory conditions, expression of 24% of the genes in Shewanella oneidensis are actually detrimental to fitness, and 21% of genes are either constitutively expressed or growth rate-dependent (coregulated with genes functioning in transcription or translation), creating noise when trying to correlate expression with contribution to fitness (Price et al. 2013). This phenomenon is thought to be widespread among bacteria (Price et al. 2013), as demonstrated when exposed to antibiotics (Jensen, Zhu and van Opijnen 2017; Zhu et al. 2020b) or when growing in mammalian (Turner et al. 2014) and plant (Helmann, Deutschbauer and Lindow 2019) infection models. Thus, the lack of direct correlation between a given gene's expression and importance for fitness has important implications and suggests care be taken when broadly interpreting transcriptomic data.
A special case of environment-gene interaction: gene essentiality in infection settings
Although in vitro growth phenotypes can be used to assign specific functions for uncharacterized genes, these conditions may not reflect real-world scenarios. For example, the nutritional environment that bacteria encounter during infection are difficult to reproduce in a test tube. Many conditions may be different or inexistent such as nutrient content and external stress, respectively (Umland et al. 2012; Grazziotin, Vidal and Venancio 2015; Liu et al. 2020; Miller and Scott 2020; Zhu et al. 2020a; Bjarnsholt et al. 2021). Several studies have shown poor overlap of essential gene sets identified in rich medium and either synthetic medium designed to mimic infection conditions or medium supplemented with ex vivo components. Studying which genes are essential in more realistic conditions is therefore very informative. One such condition is the lung environment of patients with cystic fibrosis (CF), which is a dense mucus matrix of lipids, mucin, and extracellular DNA easily colonized by several microbial species simultaneously (Gibson, Burns and Ramsey 2003; Filkins and O'Toole 2015). The desire to simulate the CF lung environment led the Whiteley group to create a synthetic CF sputum medium (SCFM) (Palmer, Aye and Whiteley 2007). This group later created SCFM2 by adding biological molecules and polymers (DNA, mucin, N-acetylglucosamine, and dioleoyl phosphatidylcholine) and compared the essential genome of Pseudomonas aeruginosa in CF sputum, SCFM2, and defined minimal medium. As many amino acids and carbon sources are present in both SCFM2 and CF sputum, few genes specifically related to their synthetic pathways were essential. On the contrary, cofactors such as riboflavin, pyridoxal phosphate, and biotin were not abundant in the media; thus, many cofactor biosynthetic genes were conditionally essential (Turner et al. 2015). By showing an overlapping set of essential genes in both SCFM2 and CF sputum, these authors demonstrated that SCFM2 can mimic CF sputum conditions for microbiological studies.
Although technically easy to use, media that mimic infection conditions may not fully recapitulate them. Defining conditionally essential genes in an in vivo infection model can then identify genes that are required for in vivo persistence, immune evasion, and infection progression. M. tuberculosis was the subject of early intensive transposon mutagenesis schemes to dissect genes essential for in vivo replication and survival (Cox et al. 1999; Sassetti and Rubin 2003; Rengarajan, Bloom and Rubin 2005; Joshi et al. 2006; Pandey and Sassetti 2008; Dhar and McKinney 2010; Dutta et al. 2010; Griffin et al. 2011). These multiple efforts are not surprising as M. tuberculosis is the causative agent of tuberculosis and responsible for millions of infections each year (WHO 2021). Through various infection models, including macrophages, mice, and non-human primates, the virulence gene arsenal and intracellular survival determinants were revealed. In addition to secretion systems (e.g. the type VII ESX-1 system) (Sassetti and Rubin 2003; Rengarajan, Bloom and Rubin 2005) and cell wall factors required for cell entry and immune system modulation (e.g. lipoarabinomannan and phthiocerol dimycocerosate) (Cox et al. 1999; Dutta et al. 2010), it became clear that M. tuberculosis extensively modulates its energy metabolism upon intracellular infection (Rengarajan, Bloom and Rubin 2005; Joshi et al. 2006). Many genes related to fatty acid β-oxidation, gluconeogenesis, and saccharide transport were found to be conditionally essential. Moreover, it was later discovered that catabolism of host cholesterol was essential for intracellular persistence (Pandey and Sassetti 2008), thus necessitating β-oxidation and gluconeogenesis. Furthermore, by analysing the mutant population at timepoints during murine infection, the temporal importance of genes was exposed. Genes required for energy metabolism and cofactor biosynthesis (e.g. bioF and sugA) were required during the initial week of infection, which is characterized by rapid bacterial growth (Sassetti and Rubin 2003). At subsequent timepoints, the effect of the immune system was more evident as genes related to oxidative and DNA damage (e.g. kefB and xthA) became essential (Sassetti and Rubin 2003). Overall, only by using appropriate infection models, key insights into the conditionally essential genes for M. tuberculosis infection were revealed.
While the use of CRISPRi in the identification of conditionally essential genes has been so far limited (Qu et al. 2019; Liu et al. 2020; Mathis, Otto and Reynolds 2021), CRISPRi and CRISPRi-Seq hold promise for the identification of essential genes in infection conditions. Recently, the first genome-wide CRISPRi investigation of genes required for infection was published (Liu et al. 2020), using an S. pneumoniae murine model of pneumonia and bacteremia, and influenza virus superinfection. Nearly every transcriptional unit, including those harbouring essential genes, was targeted by an sgRNA, which was sequenced following infection to determine mutant abundance and identity. Among the conditionally essential genes were purA, encoding adenylosuccinate synthetase which is important for purine biosynthesis, and multiple capsule biosynthetic genes, all known to be critical for S. pneumoniae infection (Liu et al. 2020). They also demonstrated that ply, encoding the major pneumococcal toxin pneumolysin, was in fact not required for infection progression, agreeing with a report instead suggesting a role in bacterial shedding and transmission (Zafar et al. 2017).
An important consideration is that the lower complexity of CRISPRi libraries compared to transposon mutant collections can contribute to minimize the so called ‘bottleneck effects’, which can cause mutants of high-complexity libraries to be lost due to fitness-independent processes (Abel et al. 2015). Compared to transposon mutant libraries, CRISPRi libraries often require fewer mutants per gene to achieve the same predictive power (Rousset et al. 2018). Thus, in vivo CRISPRi studies should suffer less from bottlenecks, due to lower required inoculum size.
Gene-gene interactions
Similar to the interactions of environmental conditions with genetic backgrounds, there are also epistatic interactions between genes (or lack thereof) that can change an observed phenotype. The term epistasis refers to an event where expression of a gene affects the expression of a second gene. Epistatic interactions are also known as synthetic genetic interactions, whereby the effect of combinations of mutations is statistically greater than the expected result if observed independently (Fisher 1919; Ruiz, Kahne and Silhavy 2006). In the context of growth, synthetic interactions can either be negative and result in lower fitness—or death—than expected (synthetic lethal/sick or aggravating epistasis) or can be positive and result in greater fitness (synthetic viability or alleviating epistasis). Interestingly, antibiotic drug interactions phenocopy genetic interactions wherein one drug can either antagonize or synergize the activity of another, (Brochado et al. 2018; Tyers and Wright 2019). Synthetic lethality primarily occurs when pairs of functionally overlapping non-essential genes that control an essential function are perturbed. On the contrary, synthetic viability involves the opposite scenario, that is, when pairs of non-functionally overlapping essential (He et al. 2010) or non-essential genes (Baryshnikova et al. 2010) are disrupted viability and fitness increases. In this section, we focus our review on synthetic genetic interactions; select topics in chemical-genetic interactions will be briefly examined later (see section Outlook). The fundamentals of chemical-genetic interactions have also been reviewed more thoroughly elsewhere (Lopez et al. 2008; Beltrao, Ryan and Krogan 2012; Cacace, Kritikos and Typas 2017; Klobucar and Brown 2018).
Gene deletion and knockdown collections can serve as a template for genetic interaction studies (Davierwala et al. 2005; Butland et al. 2008; Yong et al. 2013). A large number of gene deletions can be made in each mutant background, creating a series called a synthetic genetic array, either by individual deletion (Tong et al. 2001; Butland et al. 2008; Typas et al. 2008) or transposon mutagenesis strategies (Meeske et al. 2015; Wivagg et al. 2016). Many methods also exist for quantifying the subsequent interactions (Baryshnikova et al. 2010; Collins, Roguev and Krogan 2010; Babu et al. 2014; Côté et al. 2016; Simpkins et al. 2019). Foreseeably, high throughput methods for array creation are, for now, limited to model organisms. The number of crosses increases with the square of genome size: over 17 million and 27 million mutants are required to probe the entire (non-essential) gene sets of E. coli and S. cerevisiae, respectively. In S. cerevisiae, the interactions of nearly every gene in the genome (including many essential genes) have been examined in double mutants (Costanzo et al. 2016), and substantial strides have been made in the direction of probing collections of triple mutants (Braberg et al. 2014; Kuzmin et al. 2018, 2020). Currently, the largest screen in E. coli represents only ∼3.5% of the total interactions among non-essential genes (Babu et al. 2014).
Classic examples of synthetic lethality include two parallel pathways for essential nutrient biosynthesis. When one of the pathways is inactivated, the other can compensate in performing the essential function, avoiding lethality. However, inactivation of both pathways results in cell death, resulting in a synthetic phenotype as the double mutant has a more severe defect than would be expected from combination of the single mutations alone (Fig. 2B) (Costanzo et al. 2011).
Often, nutrient transporters and biosynthetic pathways are functionally redundant and found in synthetic lethal pairs. To characterize the interactions of nutrient acquisition and biosynthesis genes, the Brown group (Côté et al. 2016) examined the interaction of all non-essential genes in E. coli with 82 nutrient stress-associated genes, resulting in over 315000 interaction pairs. Pantothenate is a common component of rich medium and E. coli encodes the PanF transporter import it; however, E. coli can also synthesize pantothenate de novo. Consequently, panF and pantothenate biosynthetic genes are not individually essential in rich media (Baba et al. 2006). However, deletion of any of the pantothenate biosynthetic genes formed a synthetic lethal interaction in a ΔpanF background (Côté et al. 2016). Interactions can also yield information about transport systems, as there were no transporter interactions featuring gene deletion mutants for isoleucine and valine synthesis, as no single transporter is solely responsible for their uptake (Guardiola et al. 1974). In other cases, synthetic lethality can report on functionally redundant enzymes, such as metL and thrA, both of which encode bifunctional aspartokinase/homoserine dehydrogenases (Zakin et al. 1983) and form a synthetic lethal pair (Côté et al. 2016).
More recently, the considerable power of synthetic lethality screening was used to assess interactions of outer membrane permeability determinants in E. coli (Klobucar et al. 2020). The permeability barrier of the Gram-negative outer membrane is an important determinant of antibiotic resistance, and is an attractive antibiotic target in its own right (Silver 2016; Lehman and Grabowicz 2019). To that end, the Brown group generated a large double deletion screen of 39 membrane-associated genes with every non-essential protein-coding and small RNA gene in E. coli (Klobucar et al. 2020). The resulting double mutants were screened in the presence of sub-inhibitory concentrations of vancomycin or rifampicin, both of which are excluded by the Gram-negative cell envelope, to identify mutants with defects in membrane integrity (Klobucar et al. 2020). Among the many interactions were several synthetic sick interactions between core LPS biosynthesis genes and the uncharacterized gene, yhdP, in the presence of vancomycin. LPS, enterobacterial common antigen (ECA) and peptidoglycan biosynthesis all share a common undecaprenol phosphate (Und-P) intermediate. Constant Und-P recycling is essential for cell viability, with disruption of any one pathway leading to intermediate buildup and decreased flux through the other two pathways (Jorgenson and Young 2016; Jorgenson et al. 2016). Und-P intermediate accumulation then causes competition for Und-P with essential peptidoglycan synthesis, resulting in morphological defects (Jorgenson et al. 2016), a weakened cell wall, and vancomycin sensitivity (Klobucar et al. 2020). The Silhavy group found that vancomycin susceptibility of a ΔyhdP mutant could be spontaneously suppressed by inactivating genes in the ECA pathway, and that deleting yhdP reduced levels of a cyclic form of ECA that was required for outer membrane integrity (Mitchell, Srikumar and Silhavy 2018). Vancomycin susceptibility of double mutants in yhdP and core LPS synthesis genes could be reversed by overexpressing murA, encoding the first committed step in peptidoglycan synthesis, suggesting that a block in the ECA pathway decreases flux of Und-P towards cell wall synthesis (Mitchell, Srikumar and Silhavy 2018; Klobucar et al. 2020). Going one step further, the Jorgenson group created a genetic screen in E. coli for synthetic interactions with Und-P recycling, finding wide-spread interactions with cell wall biogenesis, division, DNA replication and signal transduction (Jorgenson and Bryant 2021). However, YhdP has recently been suggested to be an anterograde phospholipid transporter (Ruiz, Davis and Kumar 2021) and may have indirect roles in regulating ECA synthesis (Mitchell, Srikumar and Silhavy 2018). Together, these studies show the power of selective screening conditions and the wealth of information held in synthetic interactions.
Synthetic viable interactions occur in the opposite direction as synthetic lethality, with the combined mutant being more fit than expected from accumulation of each single mutation. In general, synthetic viability is observed less frequently than synthetic lethality. However comprehensive studies such as those using CRISPRi (Jaffe et al. 2019; Jiang, Oikonomou and Tavazoie 2020) are able to detect them. The Tavazoie group (Jiang, Oikonomou and Tavazoie 2020) recently identified many cases of synthetic viability in S. aureus that alter susceptibility to gentamicin, a clinical antibiotic. Their success may perhaps be due to the innovative experimental approach and method of identifying interactions. Using a novel CALM method (see section CRISPR-interference) targeting every gene in the genome, they first identified single genes that when knocked down resulted in reduced gentamicin susceptibility. Focusing their efforts on the quinol oxidase subunit qoxA mutant background, they added a second layer of knockdowns. The proton-motive force promotes gentamicin uptake (Taber et al. 1987; Girgis, Hottes and Tavazoie 2009); therefore, knockdown in many genes upstream of,and in, the electron transport chain (e.g. hemABD in heme synthesis, menABC in quinone synthesis, and mvaDS in mevalonate synthesis) showed positive interactions with a knockdown in qoxA expression. Indeed, individually, knockdown of these genes reduced gentamicin susceptibility. Importantly, they also uncovered a strong interaction between qoxA and the cyd operon (encoding the second terminal cytochrome oxidases) that could not be identified by screening single knockdown mutants alone, showing how potentiating genetic interactions can be used to elucidate new cause and effect scenarios (Jiang, Oikonomou and Tavazoie 2020).
Together, genetic interaction studies highlight that essential genes are part of a complex network and that these network connections may not be intuitive, especially when uncharacterized genes are involved (Côté et al. 2016). In fact, during the construction of the smallest minimal genome to date, synthetic lethal pairs were noted as the biggest design challenge, with the lethal phenotype depending on the order of gene deletion (Hutchison et al. 2016). To counter this, efforts are being made to predict genetic and chemical-genetic interactions (Simpkins et al. 2018), some that include using machine learning (Madhukar, Elemento and Pandey 2015).
A special case of gene-gene interaction: toxic intermediates and protective essential genes
An often-overlooked essential function is to prevent the accumulation of toxic intermediates and waste products. A subset of genes, called protective essential genes, form a series of checks and balances in pathways to mitigate the negative effects of blockages in normal cellular functions (Fig. 2C). Bacillus subtilis, for example, may contain at least 13 protective essential genes out of 257 identified essential genes (Commichau, Pietack and Stulke 2013; Michna et al. 2014; Koo et al. 2017). The encoded functions are diverse, including catabolic pathways (e.g. pncB and hlpB), prophage repressors (e.g. sknR and xre), antitoxins (e.g. yezG and wapI), anti-sigma proteins (e.g. yxlC and yhdL), protection from reactive oxygen species and maintaining redox homeostasis (e.g. trxB) and resistance to endogenous antimicrobial production (e.g. sunI). Interestingly, viable B. subtilis mutants can be constructed of some protective essential genes to counter the toxicity of a single process. For example, the HlpB nuclease is thought to derive its essentiality from degrading toxic intermediates created by the AddAB recombinase; consequently, hlpB can be deleted in a ΔaddAB background (Pediaditakis, Kaufenstein and Graumann 2012). Similarly, SunI provides resistance to the glycocin antibiotic sublancin, produced by SunA; sunI can only be deleted when sunA is also absent (Dubois et al. 2009). It can be said that the interactions of protective essential genes are more extreme than synthetic viability. With synthetic viability, both single mutants typically display a mild growth defect; however, in the case of protective essentiality, viable cells can only be recovered when the gene responsible for a buildup of toxic intermediates is first inactivated. Protective essentiality has also been characterized in other bacteria across a range of processes. In Gram-negatives, proper assembly and maintenance of the outer membrane is critical for viability (Silhavy, Kahne and Walker 2010). Remarkably, the essential LolABCDE major lipoprotein intermembrane trafficking components have been recently characterized as protective in E. coli by preventing the toxic accumulation of Lpp and OsmB at the inner membrane; lolB could therefore be deleted in a Δlpp ΔosmB background (Grabowicz and Silhavy 2017). Similarly, under denitrifying conditions with nitrate as a terminal electron acceptor, double deletion of the norB and norZ nitric oxide reductase isoforms in the biotechnologically important species Cupriavidus necator was lethal, owing to nitric oxide toxicity (Cramm, Siddiqui and Friedrich 1997). However, an additional deletion of the upstream gene in the pathway, nirS, encoding nitrite reductase, restored viability, leaving nitrite as the final pathway product.
Protective essential genes display inconsistent patterns of essentiality across species and strains, reflecting differing evolutionary histories and interactions in genetic circuitry. Case in point, sunI and sunA are encoded by the SPβ prophage, which is generally limited to strains of subspecies B. subtilis subtilis (Brito et al. 2017). Foreseeably, the presence of protective essential prophage repressors also varies depending on individual strain histories and phage infection (Canchaya et al. 2003; Atsumi and Little 2006; Glinkowska et al. 2010; Rousset et al. 2021). Recently, in E. coli it was found that horizontally acquired genetic elements associated with bacterial immunity could induce the essentiality of endogenous genes needed to control activity of the acquired genes (Rousset et al. 2018). There are also large differences in the conservation of essentiality in genes involved with cellular redox homeostasis (e.g. superoxide dismutases, and the thioredoxin and glutathione systems). In addition to reducing cytoplasmic protein disulfide bonds necessary for their function, these systems are also important for detoxification of reactive oxygen species from physiological processes such as aerobic metabolism (Carmel-Harel and Storz 2000; Staerck et al. 2017). For example, thioredoxin (trxA) and/or thioredoxin reductase (trxB) are individually essential in many species, such as B. subtilis (Kobayashi et al. 2003; Koo et al. 2017), Acinetobacter baylyi (Berardinis et al. 2008), Pseudomonas aeruginosa (Jacobs et al. 2003; Liberati et al. 2006), Staphylococcus aureus (Chaudhuri et al. 2009; Santiago et al. 2015). Neither are individually essential in E. coli (Baba et al. 2006; Yamazaki, Niki and Kato 2008), due to functional overlap with other antioxidant similar systems. However, synthetic lethal interactions among antioxidant-encoding genes demonstrates the overall essentiality of the processes (Carmel-Harel and Storz 2000; Staerck et al. 2017). Indeed, E. coli depends on either a functional thioredoxin or glutathione system for viability and robust growth but cannot tolerate severe defects in both pathways simultaneously (Russel and Holmgren 1988; Prinz et al. 1997).
Vulnerability to essential gene depletion
Natural levels of expression and regulation vary between genes to coordinate metabolic and structural processes. Different critical thresholds of essential product abundance exist for each essential function, below which cell viability is compromised. These thresholds are defined by combinations of gene expression, protein activity and degradation, and metabolic and regulatory buffering, among other factors (Hawkins et al. 2020; Poyatos 2020; Donati et al. 2021). Studying a cell's response to catastrophic blockage in essential functions—say by CRISPRi or protein degradation—yields important insight into the nature of gene essentiality. Over successive generations, the levels of essential gene products are depleted in the daughter cells, eventually crossing a critical functional threshold and halting cell growth (Herring and Blattner 2004; Gallagher, Bailey and Manoil 2020; Hart and Silhavy 2020; Hawkins et al. 2020). Many methods have been used to study the effects of essential gene depletion, and this idea has been variably called ‘stringency’ (Goh et al. 2009), ‘vulnerability’ (Wei et al. 2011; Bosch et al. 2021; McNeil et al. 2021), ‘depletion sensitivity’ (Gallagher, Bailey and Manoil 2020) and the expression-fitness relationship (Hawkins et al. 2020). First measured using asRNA and synthetic antisense peptide nucleic acids, the relationship between growth inhibition and mRNA transcript abundance could be quantified and compared between genes (Goh et al. 2009), a metric called MTL50 (the minimum transcript level for a 50% reduction in growth rate). It seems logical that genes with higher MTL50 values (i.e. higher stringency), such as acpP and ftsZ, are also highly connected in genetic networks (Butland et al. 2005) suggesting that vulnerability is affected by more than just initial expression levels (Goh et al. 2009, 2015; Hawkins et al. 2020). However, mRNA levels do not correlate 1:1 with protein levels, as they are affected by post-transcriptional and post-translational regulation (Li et al. 2014). In support of the findings by Goh et. al, depleting essential proteins in Mycobacterium smegmatis with a degradation tag also showed no correlation of depletion with growth defect (Wei et al. 2011). While moderate depletion of RpoB (RNA polymerase β subunit) and InhA (enoyl-ACP reductase) caused growth arrest and cell death, respectively, depleting DHFR (dihydrofolate reductase) and Alr (alanine racemase) by 97% caused only minor growth defects (Wei et al. 2011). These findings illustrate that different essential functions have different threshold levels of essential products below of which a differential growth phenotype can be observed.
Large-scale studies also show that essentiality gradients are created due to essential gene depletion. Transposons with outward-facing promoters of variable strength can permit study of mutant phenotypes when essential genes are depleted (Wang et al. 2011; Hogan et al. 2018; Santiago et al. 2018). Recently, the Manoil group (Gallagher, Bailey and Manoil 2020) made clever use of a coupled transformation-transposon mutagenesis method (TFNseq) in A. baylyi to rank processes based on the timing of mutant depletion from a pool. Unlike most other screens of transposon mutant libraries, the mutant population generated by TFNseq was assessed by sequencing at many early timepoints immediately after mutagenesis, giving finer temporal resolution as essential processes were inactivated from essential protein depletion. Overall, disruptions to ATP synthesis, dNTP synthesis, and ribosome production more rapidly arrested growth. Conversely, cells could better tolerate disruptions in cell division and outer membrane protein synthesis. However, they also noted heterogeneity in the vulnerability profiles within functional classes, such as DNA replication (Gallagher, Bailey and Manoil 2020). The Manoil group argued that inactivating processes rapidly inhibiting growth may be attractive antibiotic targets. It may be relevant then, to measure cell death as cells can remain viable for several hours even when absolutely essential genes are knocked out (Bailey et al. 2019). Foreseeably, there would be a much different pattern by mutagenizing persister cells from stationary phase (Brauner et al. 2016; Caro, Place and Mekalanos 2019) or using a genetic background with higher persister formation (Wu et al. 2015; Harms, Maisonneuve and Gerdes 2016). It would be interesting to test the mettle of these findings by comparing trends in vulnerability with sensitivity to chemical inhibition.
Alternatively, CRISPRi can be used to deplete essential genes then track mutant abundance using NGS. Recently, the Gross group (Hawkins et al. 2020) tiled the essential genome of E. coli and B. subtilis with a panel of 10 fully complementary and 90 single mismatched sgRNAs per gene for a wide range of repression. Expectedly, there was a large diversity of expression-fitness responses (vulnerability profiles); however, the responses within functional classes were generally similar, as noted before in S. cerevisiae (Keren et al. 2016) and A. baylyi (Gallagher, Bailey and Manoil 2020). The vulnerability profiles of essential homologues between E. coli and B. subtilis were also similar, demonstrating the importance and conservation of central processes even in bacteria with ∼2 billion years of evolutionary separation (Hawkins et al. 2020). Specifically, there were shared trends in genes involved in cofactor biosynthesis, translation, and peptidoglycan synthesis. With some exceptions, strong knockdowns in co-factor synthesis genes resulted in only minor fitness defects, moderate defects for translation-associated genes, whereas modest knockdown in peptidoglycan synthesis-associated genes caused severe fitness defects (Hawkins et al. 2020). Variations in vulnerability, both between classes and species, may be caused by differences in initial levels of proteins and metabolites versus the critical threshold for viability, and the capacity of metabolic (Poyatos 2020; Donati et al. 2021) and regulatory buffering (Hawkins et al. 2020). Indeed, it can take over a dozen generations for the true depleted phenotype to develop (Mathis, Otto and Reynolds 2021).
Examining bacterial species side-by-side can reveal unique and conserved buffering mechanisms that effectively changes the efficiency of the depletion. For example, B. subtilis was highly susceptible to knockdown in genes involved in the synthesis and incorporation of peptidoglycan precursors (Hawkins et al. 2020). By contrast, E. coli displayed only a minor defect over several generations when the same pathways were knocked down. In E. coli, the minor defect for knockdowns in asd and glmS (encoding aspartate-semialdehyde dehydrogenase and D-fructose-6-phosphate amidotransferase, respectively, both involved in peptidoglycan precursor synthesis), were due to low levels of transcriptional repression and were attributed to regulatory mechanisms buffering the cell to changes in these important processes (Rodionov et al. 2003; Barreteau et al. 2008). Species-specific differences may also explain why other groups (Gallagher, Bailey and Manoil 2020) found peptidoglycan synthesis defects to have a less pronounced fitness defect in A. baylyi. The differences may also be due to the methods employed; CRISPRi maintains genetically wild-type loci, whereas transpositions are static interruption events that over-ride the regulatory buffering mechanisms at each locus. Overall, the buffering capacity and tolerance of genetic networks to essential gene depletion contribute to the quantitative nature of gene essentiality. Highly vulnerable essential genes are more functionally important and thus incur higher fitness costs and are more likely to result in cell death when depleted, at least in M. tuberculosis (McNeil et al. 2021). Conversely, genes with moderate or low vulnerability are more likely result in cell stasis when depleted (McNeil et al. 2021). Together, characterization of growth phenotypes during different levels of essential gene depletion highlights that essentiality gradients are observed not only with changes of environment and genetic context, but also by changes in the abundance of an essential product. We expect that further studies on essential gene depletion will further unravel the genetic determinants of gene essentiality gradients in more species, especially as they relate to differences in bacterial physiology and lifestyle.
Compensatory mutations
Above we presented recent evidence of how environmental conditions, genetic backgrounds, and the nature of the encoded function strongly affect gene essentiality status, thereby creating essentiality gradients. Despite essential genes being conserved across bacterial lineages, compensatory mutations can arise that cause essential genes to be dispensable, adding another path through which essentiality gradients can be observed. Bolstered by the creation of tools such as the Keio gene deletion collection (Baba et al. 2006) and ASKA library (Kitagawa et al. 2005), some early medium-scale reports in bacteria described a phenomenon whereby the overexpression of non-homologous genes rescued growth when essential genes are depleted or deleted, called compensation or multicopy suppression (Campbell et al. 2007; Patrick et al. 2007; Campbell and Brown 2008; Handford et al. 2009; Bergmiller, Ackermann and Silander 2012). Despite being non-homologous, compensating gene pairs often share functional relationships. For example, in E. coli the lack of the 4-hydroxy-tetrahydrodipicolinate synthase gene dapA can be compensated by the promiscuous activity of the N-acetylneuraminate lyase, encoded by nanA (Bergmiller, Ackermann and Silander 2012). Specifically, it is known that a single amino acid substitution allows NanA to take over the essential 4-hydroxy-tetrahydrodipicolinate synthase activity of DapA (Joerger, Mayer and Fersht 2003), and likewise for how a single substitution in FtsA can bypass the requirement of another essential, but poorly conserved, division protein ZipA (Geissler, Elraheb and Margolin 2003). More recently, the Maurelli group (Ranjit, Liechti and Maurelli 2020) showed that exogenous expression together of MreB and RodZ from Chlamydia trachomatis (which lacks ftsZ) can circumvent the essentiality of ftsZ in E. coli, as they localize to the midcell and likely aid in directing peptidoglycan synthesis in the absence of FtsZ. Hence, in each of these scenarios the essential functions are still being performed, but by different cellular components. To date, the largest study of compensation in bacteria was performed in 2007 and was limited to 104 conditionally essential genes (Patrick et al. 2007). We therefore expect that further studies of this area will yield important findings on the intersection of bacterial essential gene conservation and compensation.
The ability of a gene to compensate for another's loss is a measure of biochemical function but is more strongly affected by their coordinating binding partners. In this context, genes that encode for proteins that interact with multiple partners may be less likely to be replaced. For example, high connectivity in genetic networks is a stronger barrier to horizontal gene transfer than a gene's function (Cohen, Gophna and Pupko 2011). Studying a specific example, the Kacar group assessed the ability for evolutionarily distant homologues of the essential protein elongation factor (EF)-Tu (encoded by the ancient paralogs tufA and tufB) to compensate for the native gene in E. coli (Kacar et al. 2017). EF-Tu delivers aminoacyl-tRNAs to the ribosome and uses GTP hydrolysis for tRNA release. By replacing the endogenous tufA with homologues from across the bacterial phylogenetic tree (ranging in divergence from 0.7 to 3.6 billion years), it was found that the longer the evolutionary distance of the foreign tufA gene with E. coli, the more likely the viability depended on the endogenous tufB. Fitness also correlated with EF-Tu protein levels and the rate of protein synthesis. Overall, the resulting strain's relative fitness depended on the presence of the evolutionary distance of the tufA homologue. It was then speculated that the distantly related EF-Tu homologues have lower capacity to coordinate activity within the highly connected environment of the ribosome and that lineage-specific essential interactions may have arisen (Kacar et al. 2017). More broadly, these findings demonstrate that critical thresholds exist in essential protein networks that must be met for cell viability, and that these thresholds move over evolutionary time, either allowing or preventing other cellular components from participating in genetic interactions. In terms of quantitative essentiality, high-connectivity essential genes, such as those encoding ribosomal components, are very important for fitness. Indeed, many ribosomal genes were found to be highly vulnerable in M. tuberculosis (Bosch et al. 2021).
Essentiality gradients shed light on essential gene evolution and drug target prioritization
As we stated at the beginning of this review, foundational studies suggested the functional importance of essential genes results in stringent purifying selection and high conservation (Jordan et al. 2002). However this early study along with more recent studies (Gong et al. 2008; Dotsch et al. 2010; Ish-Am, Kristensen and Ruppin 2015) recognized that this pattern could not be uniformly applied across all bacterial genes and species. An essential gene may be conserved in a different organism, but its essentiality may not be conserved. In other cases, some essential genes may be completely lost from the genomes of other bacteria. To experimentally address which essential genes were more likely to be replaced, at least in laboratory conditions, conditional growth mutants were constructed in E. coli by placing an arabinose-inducible promoter upstream of 26 essential genes within the genome (Bergmiller, Ackermann and Silander 2012). These strains were transformed with the overexpression ASKA library and screened for loss of the conditional growth phenotype. With this procedure, the authors isolated a gene set that was able to compensate the conditional lethal phenotype. The majority of the compensating genes where non-homologous to the under-regulated essential genes. Further, the group demonstrated that those dispensable essential genes were more likely to be non-essential or absent in other bacteria. Interestingly, essential gene vulnerability, defined as a quantifiable trait that links the effect on fitness with the magnitude of gene depletion, sheds light on why some essential genes may be more conserved than others. A recent report from the Rock group studied vulnerability in M. tuberculosis with CRISPRi by systematically titrating gene expression with sgRNAs of varying targeting sequence length (Bosch et al. 2021). They found that essential genes displayed a spectrum of vulnerability and that highly vulnerable M. tuberculosis genes were more likely to have a homologue in other bacterial species and more likely to be essential than invulnerable genes. For example, of the most highly vulnerable genes in M. tuberculosis, 70% have an essential homologue in E. coli, while only 29% of invulnerable genes have an essential homologue in E. coli. Additionally, these trends in conservation extended to whole pathways, as between E. coli, B, subtilis and M. tuberculosis, genes involved in translation were highly vulnerable, while genes involved in cofactor biosynthesis were generally less vulnerable (Hawkins et al. 2020; Bosch et al. 2021). Importantly, quantification of vulnerability allowed the Rock group to suggest ranks for essential genes as drug targets in M. tuberculosis. United, these findings highlight the importance of characterizing gene essentiality across different levels of essential product depletion. Furthermore, genomic quantification of essentiality gradients can shed light on essential genes that may represent more attractive antibacterial targets.
Outlooks
Prioritizing and validating targets for antibiotic discovery
Rising rates of antibiotic resistance around the world continue to threaten public health. Essential gene products and processes are important in this regard as they are targeted by antibiotics. We propose that essentiality gradients may be used to prioritize targets and unveil new avenues for discovery, although it would be unwise to use essentiality as the only criterion for a tractable target (Projan 2008). Preferred antibiotic targets would be encoded by indispensable essential genes within highly connected networks. Targeting these gene products would maximize the damage to essential cellular functions and minimize the likelihood of resistance by genetic rewiring. Computational tools can be recruited to support this effort to both catalogue and model across taxa the ways by which essentiality can be bypassed (Barve, Rodrigues and Wagner 2012; Guzmán et al. 2015, 2018).
Ideally, cells would also be highly vulnerable to inhibition of the target to immediately arrest growth or cause cell death. There is a broad range in the rate of killing for bactericidal antibiotics (Baquero and Levin 2021), with the rapid cell death associated with physical membrane disruption by detergents and biocides being an aspirational target for new antibiotics. However, the factors leading to vulnerability are still largely unknown in bacteria and these areas require further work, such as genome-wide vulnerability profiling (Gallagher, Bailey and Manoil 2020; Hawkins et al. 2020; Bosch et al. 2021; McNeil et al. 2021). As proof of principle of the link between vulnerability and target prioritization, the Rock group's genome-wide vulnerability profiling in M. tuberculosis identified that the targets of the first-line anti-tuberculosis drugs isoniazid (inhA) and rifampicin (rpoB) are highly vulnerable (Bosch et al. 2021), supporting a previous report (Wei et al. 2011). However, they also point to many other highly vulnerable potential drug targets, such as the aminoacyl-tRNA synthetases, which are under active investigation (Bouz and Zitko 2021) with one compound (GSK656) entering clinical trials (Li et al. 2017). Additionally, the Rock group also used vulnerability to explain the differences in susceptibility of two strains of M. tuberculosis to DNA gyrase and cytochrome bc1 inhibitors. (Bosch et al. 2021). Separately, the Cook group examined their genome-wide CRISPRi vulnerability screen in M. tuberculosis in the context of targeted pathway and cell fate (bacteriostatic vs. bactericidal) (McNeil et al. 2021). They found that knockdown of genes related to transcription, translation, cell wall synthesis and division were generally bactericidal, while knockdown of genes related to oxidative phosphorylation and amino acid synthesis were generally bacteriostatic. Such studies could form guiding frameworks with implications for antibiotic development and target selection. Alternatively, if target inhibition does not render complete growth arrest, the gene may exist in a synthetic lethal pair which could be exploited by combination therapy, as recently proposed for cancer therapy (Huang et al. 2020; Neggers et al. 2020; Akimov and Aittokallio 2021; Thng, Toh and Chow 2021), a theory called collateral vulnerability (Wang et al. 2019). For example, the recently approved antitubercular bedaquiline depletes the ATP pool from M. tuberculosis, thereby sensitizing cells to inhibition of glycolysis (Mackenzie et al. 2020) and the essential ATP-intensive process of glutamine synthesis (Wang et al. 2019). Furthermore, genetic and physiological factors must be integrated with the biochemistry and pharmacology of the target, such as druggability and Km (Murima, McKinney and Pethe 2014), which is beyond our current scope.
The intrinsic link between essential genes and antibiotics can also be used to identify targets and mechanisms of action for uncharacterized compounds. This is a critical step in antibiotic discovery and development, but also represents a bottleneck in the pipeline (Farha and Brown 2016). Chemical-genetic approaches for target over- and under-expression change the cellular response to antibiotics, and not always with opposing effects (Pathania et al. 2009; Xu et al. 2010; Bloodworth, Gislason and Cardona 2013; Cameron and Collins 2014; Palmer and Kishony 2014; Peters et al. 2016, 2019; Gingras et al. 2018, 2019; Hogan et al. 2018; Johnson et al. 2019; Jiang, Oikonomou and Tavazoie 2020). While many studies have used gene deletion or simple transposon mutant libraries exposed to antimicrobials to profile resistance mechanisms or susceptibility determinants (Liu et al. 2010; Gallagher, Shendure and Manoil 2011; Nichols et al. 2011; Murray et al. 2015; Rajagopal et al. 2016; Piotrowski et al. 2017; Geisinger et al. 2020; Klobucar et al. 2020; Weber et al. 2020), they generally lack the fundamental ability to examine interactions with essential targets. Importantly, only methods that permit targeted manipulation of essential genes can bridge this gap (e.g. CRISPRi, protein degradation tags, asRNA, and transposons bearing an outward-facing promoter). Furthermore, the coupling of NGS to target-based assays greatly increases the throughput, and while there are no studies on the three-way interactions between libraries of essential gene mutants, infection-relevant conditions (in vitro medium or in vivo models), and antibiotics, we expect this will come shortly. In fact, this is the pinnacle of what the field is trying to achieve: rapid profiling to characterize new antibiotics in settings immediately applicable to clinical/pre-clinical trials (Bjarnsholt et al. 2021). Until that time, there have been some recent uses of libraries of essential gene mutants to identify the target and mechanism of action of known (de Wet et al. 2020; Yasir et al. 2020) and uncharacterized antimicrobials (Peters et al. 2016; Hogan et al. 2018; Johnson et al. 2019; Nunvar et al. 2019). After standardizing the response of a CRISPRi library in B. subtilis to 35 diverse inhibitory compounds, the Gross, Huang, and Qi groups identified that knockdown of uppS, encoding the undecaprenyl pyrophosphate synthase, was highly susceptible to MAC-0170636, which was validated with biochemical assays (Peters et al. 2016). Our group used an enriched library of essential gene mutants, created by mutagenesis with a transposon bearing an outward-facing inducible promoter, to identify and validate the essential cell division protein FtsZ as the target of the broad-spectrum inhibitor C109 (Hogan et al. 2018). More recently, a large collaborative group lead by the Hung lab (Johnson et al. 2019) profiled nearly 50000 diverse compounds against a library of degron-tagged essential protein mutants in M. tuberculosis. The group identified over 40 new inhibitory compounds, of which some have novel scaffolds, targeting DNA gyrase, mycolic acid biosynthesis, folate metabolism, and the essential efflux pump EfpA, a new antimicrobial target.
Core and accessory essential genomes guide broad- and narrow-spectrum antibiotic discovery
The comparison of multiple genomes enables the identification of core and accessory genomes, and in a similar fashion we can define core and accessory essential genomes. The conservation of bacterial core essential genes and bacterial essential genes overall (Jordan et al. 2002; Ish-Am, Kristensen and Ruppin 2015), presents these genes are favourable targets for broad-spectrum antibiotics, such as the β-lactams, fluoroquinolones and aminoglycosides. Highly conserved pathways are attractive for broad-spectrum inhibitor design and discovery, as evidenced by the recent interest in darobactin (targeting BamA) (Imai et al. 2019), complestatin and corbomycin (interfering with autolysins and peptidoglycan remodeling) (Culp et al. 2020), the arylomycin G0775 (targeting signal peptidase I) (Schimana et al. 2002; Smith et al. 2018), and new β-lactam/β-lactamase inhibitor combinations (targeting peptidoglycan synthesis) (Durand-Reville et al. 2021; Lomovskaya et al. 2021). Remarkably, we have come to a point where claiming to have identified a core or accessory essential genome has become ambiguous. Originally, the core and accessory essential genomes referred to the universal prokaryote/eukaryote and individual species/genera gene sets, respectively (Juhas, Eberl and Church 2012); however, the vast amount of data has forced a change in that the core essential genome usually refers to species, and that the accessory essential genome refers to strain-specific differences (Lin and Zhang 2011; Le Breton et al. 2015; Gislason et al. 2017; Narayanan et al. 2017; Martínez-Carranza et al. 2018; Coe et al. 2019; Lewin et al. 2019; Poulsen et al. 2019). Pangenomes can be very large (Freschi et al. 2019; Mangas et al. 2019), but the Hung group was able to define a reliable core essential genome for P. aeruginosa by comparing only four strains; however, they also noted that the methods for mutant generation and analysis substantially affected core essential genome calls (Poulsen et al. 2019). This was recently remedied by the Bikard group (Rousset et al. 2021) via a unified large CRISPRi screen in 18 diverse strains of E. coli representing common phylogroups and lifestyles. Similarly, they found diminishing returns in the number of core essential genes as the number of strains increased. There were also striking strain-specific differences: 266 core essential genes were required for growth in LB, versus 506 pan-essential genes were required for growth in LB in at least one strain (Rousset et al. 2021).
The investigation of narrow-spectrum antibiotics is driven by the prospect of reduced negative effects on the healthy microbiome (Melander, Zurawski and Melander 2017). In theory, activity can be finely tuned to exploit clade-specific conditional essentiality. For example, the Xu group (Stone et al. 2015) took advantage of differences in meso-diaminopimelate synthesis in oral commensal streptococci versus pathogenic Porphyromonas gingivalis, to design and validate an inhibitor of the essential P. gingivalis meso-diaminopimelate dehydrogenase. Importantly, as this enzyme is lacking in oral commensal streptococci, the inhibitor had a 7-fold lower MIC against P. gingivalis than Streptococcus sanguinis (Stone et al. 2015). The activity of this inhibitor is driven by the essentiality of L-lysine biosynthesis from L-aspartate in P. gingivalis only.
Gene essentiality in antibiotic mechanisms is critical, as mutations leading to resistance may arise in non-essential genes. Case in point, although the pacidamycins are potent MraY inhibitors and are selectively transported into P. aeruginosa by an oligopeptide transporter, the transporter is non-essential; thus, there is a high frequency of pacidamycin resistance (Mistry et al. 2013). Very recently, the Lewis group reported that hygromycin A, a protein synthesis inhibitor identified almost 70 years ago (Pittenger et al. 1953), was a selective inhibitor of Borrelia burgdorferi, the causative agent of Lyme disease, and related spirochaetes such as Treponema pallidum, the causative agent of syphilis (Leimer et al. 2021). The group found that hygromycin A likely mimicked the substrate of the essential BmpDEFG nucleoside transporter, thus gaining entry into the cell. B. burdorferi is a purine auxotroph and depends on the BmpDEFG transporter as the only mechanism of purine uptake (Cuellar et al. 2020). This selective uptake mechanism explains the very narrow spectrum of activity and consequently mild effect on the gut microbiome in a murine model (Leimer et al. 2021).
As discussed above, conditionally essential gene sets for survival in nutrient-poor or infection-relevant settings are larger than those required for growth on laboratory medium due to altered biosynthetic and stress-management demands. Recently, several studies have exploited the changes in microbial physiology and metabolism that occur in infection settings to identify potent antimicrobials (Carfrae et al. 2019; Stanway et al. 2019; Curran et al. 2020; Weber et al. 2020). For example, human plasma has low levels of biotin, both necessitating de novo synthesis by invading pathogens lacking high affinity transporters and making the biosynthetic pathway an attractive drug target (Carfrae et al. 2019). The Brown group exploited this dependence and identified that the biotin synthesis inhibitor MAC13772 inhibits growth of important pathogens such as A. baumannii and M. tuberculosis, in biotin-deficient medium and human serum but not rich laboratory medium (Zlitni, Ferruccio and Brown 2013; Carfrae et al. 2019). Similarly, a screen of 11862 compounds with increased activity in serum-supplemented nutrient-poor medium identified the RNA polymerase inhibitor rifabutin as having 200-fold reduced MIC against an extensively drug-resistant strain of A. baumannii. In nutrient-limited conditions only, non-specific uptake by the outer membrane protein FhuE permitted entry of rifabutin, but not the closely related rifampicin (Luna et al. 2020). A much larger screen of 170000 compounds highlights three of the underlying mechanisms of differential activity in the presence of serum (Weber et al. 2020). First, the Brown group observed an interaction with a dipyridyl compound that chelated iron and prevented uptake of this essential micronutrient (Weber et al. 2020). This form of interaction is similar in theory to antimetabolites that inhibit the biosynthesis of conditionally essential nutrients (van der Westhuyzen et al. 2012; Zlitni, Ferruccio and Brown 2013; Gehrke et al. 2017). The second mechanism accounts for the enzymatic properties of the serum, as an otherwise low potency compound was cleaved by biotinidase to liberate 6-aminoindole, a much more potent putative tryptophan antimetabolite (Weber et al. 2020) Of note, this type of interactions would be completely missed by screening in any synthetic laboratory medium. The third mechanism relies on more cryptic changes to physiology in different growth conditions. By analyzing the growth of transposon mutants exposed to ruthenium red, the Brown group found that many mutants in DNA repair and replication genes had severe growth defects (Weber et al. 2020), and they hypothesized that growth in serum exacerbates the sensitivity.
Case study: The core and accessory essential genomes of the cystic fibrosis pathobiome and avenues for targeted antibiotics
An interesting case study on target prioritization that encompasses conditional and species-specific considerations can be seen in CF lung infections. A genetic condition caused by loss-of-function mutations in the CF transmembrane conductance regulator, CF is characterized by mucus accumulation in the lungs, among other pathologies, which is prone to recurrent and difficult to treat polymicrobial infection (Gibson, Burns and Ramsey 2003; Filkins and O'Toole 2015). Common CF lung pathogens include S. aureus, P. aeruginosa, A. fumigatus, Achromobacter spp., members of the B. cepacia complex, Haemophilus influenzae, Stenotrophomonas maltophilia, and atypical mycobacteria (Surette 2014; Zemanick et al. 2017; Cuthbertson et al. 2020). Notably, antibiotherapy is prolonged, intense, and often fails to fully clear the pathogen(s) from the lungs (Hewer and Smyth 2017; Garcia et al. 2018; Claude, Rochat and Hafen 2019; Regan and Bhatt 2019). The composition of the mucus has been determined to permit formulation in a lab setting mostly from chemically defined components, even down to proper viscosity and the presence of lipids and extracellular DNA (Palmer, Aye and Whiteley 2007; Turner et al. 2015). With some exceptions, these synthetic media closely mimic authentic sputum from the CF lung, showing that certain metabolic pathways are conditionally essential, e.g. biotin, pantothenate, riboflavin, pyrimidines, pyridoxal phosphate (Turner et al. 2015). Given their relevance to infection settings, there has been recent interest in exploring these biosynthetic pathways as antibiotic targets (van der Westhuyzen et al. 2012; Gehrke et al. 2017; Serer et al. 2019; Barra et al. 2020).
In addition to these avenues, focusing on the pathogens themselves reveals some important trends. Due to the possibility of severe polymicrobial infection, developing an antibiotic that targets core essential processes in CF pathogens may be of interest. Alternatively, antibiotics targeting the accessory essential genome may yield targeted approaches to limit the effects on beneficial organisms. While several studies profile the essential genome of CF pathogens, none have compared them to determine the core and accessory essential genomes. With some important limitations, we compared essential genomes from several strains each of P. aeruginosa, B. cenocepacia, and S. aureus (Box 1) to highlight the potential for new broad- and narrow-spectrum antibiotics to target CF pathogens. While the standard essential gene studies using rich medium are still needed, conditionally essential studies in infection models or infection-relevant media are more urgently needed. The Whiteley group recently went a step further to show that in a murine abscess model, Aggregatibacter actinomycetemcomitans, an opportunistic pathogen associated with periodontitis and abscesses, had substantially different essential gene sets in pairwise abscess infection with 25 other oral and non-oral microbes (Lewin et al. 2019). Despite there being only 59 genes required for fitness in all conditions (the ‘core’), up to 33% of the entire genome was important for fitness in at least one coinfection. Additionally, their large dataset enabled them to define sets of community-dependent essential genes (Ibberson et al. 2017), that were in general smaller if the coinfecting microbe had a larger metabolic capacity, highlighting the potential for cross-feeding complementation (Ramsey, Rumbaugh and Whiteley 2011; Stacy et al. 2016; Lewin et al. 2019; Fritts, McCully and McKinlay 2021). Similar to our analysis here (for P. aeruginosa and B. cenocepacia), they also determined that the core essential genome contained several genes for aerobic respiration, suggesting possible therapeutic routes for severe A. actinomycetemcomitans infections.
Box 1. The core and accessory essential protein-coding genomes of CF lung pathogens
We collected data from published experimental genome-wide searches for essential genes in the CF pathogens P. aeruginosa (Poulsen et al. 2019), B. cenocepacia (Wong et al. 2016; Gislason et al. 2017; Higgins et al. 2017), and S. aureus (Bae et al. 2004; Chaudhuri et al. 2009; Fey et al. 2013; Christiansen et al. 2014; Valentino et al. 2014; Coe et al. 2019). Essential orthologues were identified by BLASTP (Supplemental File 1) and functionally annotated with eggNOG-mapper (Supplemental File 2) (Pearson 2013; Huerta-Cepas et al. 2017, 2019). Only one genome-wide study was available for H. influenzae, so it was not considered here. Also, there is insufficient data to exclusively use CF isolates probed in CF-relevant conditions.
Based on 20 strains (3 of B. cenocepacia, 9 of P. aeruginosa, and 8 of S. aureus), we identified 81 core essential protein-coding genes from 16 COG categories (Fig. 3A) (Tatusov et al. 2000, 2003). We did not include genes encoding only RNA products as proteins are more tractable drug targets. Nearly as many (80) are common only between P. aeruginosaand B. cenocepacia, while many fewer are shared only between S. aureus and P. aeruginosa (23) and B. cenocepacia(7), reflecting their vastly different structure and physiology. Expectedly, translation is by far the most abundant functional category of core essential genes, composed primarily of ribosomal proteins and aminoacyl-tRNA synthetases. This class is followed by DNA repair and replication, then by cell wall and membrane biogenesis. Components of translation, DNA replication, and cell wall synthesis have indeed proven many times to be valid targets of broad-spectrum antibiotics. However, from a compound and target diversity perspective, there are some functional classes worth highlighting. Nucleotide metabolism (COG category F), coenzyme metabolism (COG category H), and trafficking and secretion (COG category U) are each represented by at least 5 core essential genes but are under-exploited antibiotic targets. Furthermore, many of these genes products function in the same pathway: thyA, nrdA, nrdB, pyrG and pyrH are used in the forked pathway of dTTP and dCTP synthesis (Biocyc.org; (Karp et al. 2019)); yidC, secY, secA, ffh and ftsY function together in co-translational protein secretion (Loeffelholz et al. 2015; Sachelaru et al. 2017).
There is an abundance of lipid metabolism genes (COG category I) that are uniquely essential in S. aureus, such as mvk, mvaD, mvaK2 for mevalonate-based isoprene synthesis. These genes, together with the essential teichoic acid synthesis genes tagH and tagG, could represent aims for targeted antibiotic development (Ferrand et al. 2011; Lee et al. 2010; Skaff et al. 2015; Wang et al. 2013).
Expectedly, P. aeruginosaand B. cenocepacia share many essential genes prototypical of Gram-negatives, such as those for LPS synthesis and transport (lpxAB, lpxD, kdsA, kdsB, and lptCAD, and lptG) and outer membrane biogenesis (lolC, lolD and bamA). Several of these have been investigated recently as attractive antibiotic targets (Alexander et al. 2018; Hart et al. 2019; Imai et al. 2019; Krause et al. 2019; Moura et al. 2020). Additionally, shared glucose-nonfermenting lifestyles are reflected in the essentiality of many genes in energy production (COG category C), such as components of the F1F0 ATPase (atpBHAD) and electron-transfer proteins (sucAB, fpr, etfA), which are non-essential in fermentative bacteria such as E. coli (Goodall et al. 2018). B. cenocepacia displays further sensitivity in this regard as many NADH and succinate dehydrogenase genes (nuoABCEFGILMN and sdhCDAB) are uniquely essential for growth, representing tempting antibiotic targets that may rapidly halt growth (Gallagher, Bailey and Manoil 2020). This should be approached carefully though, as ATP depletion is a proposed mechanism of persister cell formation (Conlon et al. 2016; Shan et al. 2017), which is known to antagonize clearance during infection (Fisher Gollan and Helaine 2017). As noted before (Gislason et al. 2017), the genes required for constitutive 4-amino-4-deoxy-L-arabinose (Ara4N) synthesis and attachment to LPS (arnT and arnBC) are also uniquely essential in B. cenocepacia. Ara4N decreases the net negative charge on LPS, reducing affinity for cationic antibiotics, the primary cause of the ineffectiveness of these antibiotics against many Burkholderia species (Loutet and Valvano 2011). P. aeruginosa is also capable of this modification; however, it is typically inducible under stressful conditions and is non-essential (Lam et al. 2011). Theoretically, it may be possible to chemically inhibit the Ara4N modification, thereby preventing B. cenocepacia growth while also causing severe polymyxin and cationic peptide susceptibility in P. aeruginosa. Alternatively, the accessory essential genome of P. aeruginosa appears to be rich with genes involved in coenzyme metabolism such as ubiA, ubiD, ubiE, ubiG, and ubiH (ubiquinone synthesis), folA, folB and folK (folate synthesis), and ribA, ribD and ribE (riboflavin synthesis).
We note some places for improvement not just for application to CF but to all settings: (i) only using strains isolated from a specific infection/condition; (ii) identifying essential genes in both rich laboratory medium and the relevant environmental medium and (iii) performing the screen in one study with the same method for each strain and condition (Coe et al. 2019; Lewin et al. 2019; Martínez-Carranza et al. 2018; Poulsen et al. 2019; Rousset et al. 2021). Out of necessity, we had to rely on essential genes identified in rich laboratory medium, which certainly reduces the number of core and accessory essential genes. We expect that future studies adding to the conditionally essential knowledgebase will identify many more core and accessory conditionally essential genes, opening up new opportunities for designer, infection-specific antibiotics.
This analysis also identifies areas needing more study: although Stenotrophomonas maltophilia and Achromobacter spp. are present in approximately 12% and 4% of lung infections in CF patients (Zemanick et al. 2017), respectively, their essential genomes remain experimentally undetermined. Colonization by S. maltophilia and Achromobacter spp. are associated with increased frequency of pulmonary exacerbation, hospitalization and death (Berdah et al. 2018; Edwards et al. 2017; Somayaji et al. 2017; Waters et al. 2011, 2013). Individual study of these organisms will yield a fuller picture of the CF pathogen core and accessory essential genomes and may highlight additional routes for antibiotic development.
Figure 3.
Core and accessory essential protein-coding genes of CF lung pathogens. (A) Venn diagram showing the comparison between the three species investigated here. (B) Descriptions of the COG categories identified in the essential gene sets here. (C) COG category breakdown of the protein-coding essential genes in A (same colour scheme).
Concluding remarks
While genomes encode complex regulatory and metabolic pathways to support survival in various conditions, only a fraction of the coding capacity is essential for survival. The development of new methods, especially those coupled to NGS, are very powerful and are now within reach for most laboratory groups. Historically, the bulk of our knowledge on the identity of essential genes comes from transposon mutagenesis studies, but innovative new methods are poised to shift that balance. We expect that the broad application of CRISPRi tools to generate libraries of mutants in essential genes will narrow the gap between essential gene identification and characterization.
Our methods are the scientific lenses we use to understand the microbial world, and through them it is clear that gradients of essentiality more aptly explain the observed behaviour of essential genes than a simple binary classification. Growth in different conditions, including those reflective of natural settings, reveals that many more genes contribute to fitness than previously thought. An additional layer of complexity is added by the strain background and nature of encoded processes, resulting in differences in genetic network connectivity. New high-throughput techniques can deeply probe the matrix of environmental conditions and (essential) genes, systematically elucidating the foundations of microbial life. Present day genetic strain backgrounds are a product of evolution, but the genetic repertoire is still capable of change and flexibility. Moreover, compensation and bypasses of essentiality can dramatically change the conservation of essential genes. While for now much of our knowledge of essential gene dispensability centres around model organisms, we believe that new methods will bridge the gap for non-model organisms.
With the crisis of antibiotic resistance becoming more urgent every day, new angles of attack are desperately needed. We argue that the concept of quantitative essentiality gradients is well-suited to guide antibiotic target prioritization, in terms of vulnerability and dispensability. Furthermore, a target may be precisely selected for specific infection settings based on theories of conditional essentiality and on knowledge of core and accessory essential genomes of specific pathogens.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Dr Ann Karen Brassinga (University of Manitoba) for critical review of the manuscript and helpful discussions.
Figs 1 and 2 were created with the aid of BioRender (biorender.com)
Contributor Information
Andrew M Hogan, Department of Microbiology, University of Manitoba, 45 Chancellor's Circle, Winnipeg, Manitoba R3T 2N2, Canada.
Silvia T Cardona, Department of Microbiology, University of Manitoba, 45 Chancellor's Circle, Winnipeg, Manitoba R3T 2N2, Canada; Department of Medical Microbiology and Infectious Diseases, Max Rady College of Medicine, University of Manitoba, Room 543 - 745 Bannatyne Avenue, Winnipeg, Manitoba, R3E 0J9, Canada.
Funding
Our investigations have been supported by a Vanier Canada Graduate Scholarship to AMH and grants from Cystic Fibrosis Canada (609522), the Cystic Fibrosis Foundation (CARDON1810), and the Canadian Institutes of Health Research (CIHR project Grant 169121) to STC.
Conflict of interest statement. The authors have no conflicts of interest to declare.
REFERENCES
- Abel S, Abel zur Wiesch P, Davis BMet al. Analysis of bottlenecks in experimental models of infection. PLoS Pathog. 2015;11:e1004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimov Y, Aittokallio T. Re-defining synthetic lethality by phenotypic profiling for precision oncology. Cell Chemical Biology. 2021;28:246–56. [DOI] [PubMed] [Google Scholar]
- Alexander MK, Miu A, Oh Aet al. Disrupting Gram-negative bacterial outer membrane biosynthesis through inhibition of the lipopolysaccharide transporter msbA. Antimicrob Agents Chemother. 2018;62:e01142–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi S, Little JW. Role of the lytic repressor in prophage induction of phage λ as analyzed by a module-replacement approach. Proc Natl Acad Sci. 2006;103:4558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa Met al. Construction of Escherichiacoli K-12 in-frame, single-gene knockout mutants: the keio collection. Mol Syst Biol. 2006;2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu M, Arnold R, Bundalovic-Torma Cet al. Quantitative genome-wide genetic interaction screens reveal global epistatic relationships of protein complexes in Escherichiacoli. PLos Genet. 2014;10:e1004120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae T, Banger AK, Wallace Aet al. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci. 2004;101:12312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J, Cass J, Gasper Jet al. Essential gene deletions producing gigantic bacteria. PLos Genet. 2019;15:e1008195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquero F, Levin BR. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat Rev Microbiol. 2021;19:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquist L, Boinett CJ, Cain AK. Approaches to querying bacterial genomes with transposon-insertion sequencing. RNA Biology. 2013;10:1161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra ALC, Dantas LdeOC, Morão LGet al. Essential metabolic routes as a way to ESKAPE from antibiotic resistance. Public Health Front. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreteau H, Kovač A, Boniface Aet al. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:168–207. [DOI] [PubMed] [Google Scholar]
- Barve A, Rodrigues JFM, Wagner A. Superessential reactions in metabolic networks. Proc Natl Acad Sci. 2012;109:E1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryshnikova A, Costanzo M, Kim Yet al. Quantitative analysis of fitness and genetic interactions in yeast on a genome scale. Nat Methods. 2010;7:1017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle GW, Tatum EL. Genetic control of biochemical reactions in neurospora. Proc Natl Acad Sci. 1941;27:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrao P, Ryan C, Krogan NJ. Comparative interaction networks: Bridging genotype to phenotype. In: Soyer OS (ed). Evolutionary Systems Biology. New York, NY: Springer, 2012, 139–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardinis V, Vallenet D, Castelli Vet al. A complete collection of single-gene deletion mutants of Acinetobacterbaylyi ADP1. Mol Syst Biol. 2008;4:174–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdah L, Taytard J, Leyronnas Set al. Stenotrophomonas maltophilia: a marker of lung disease severity. Pediatr Pulmonol. 2018;53:426–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmiller T, Ackermann M, Silander OK. Patterns of evolutionary conservation of essential genes correlate with their compensability. PLos Genet. 2012;8:e1002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikard D, Jiang W, Samai Pet al. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013;41:7429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T, Whiteley M, Rumbaugh KPet al. The importance of understanding the infectious microenvironment. Lancet Infect Dis. 2021. 10.1016/S1473-3099(21)00122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner FR, Plunkett G, Bloch CAet al. The complete genome sequence of Escherichiacoli K-12. Science. 1997;277:1453–62. [DOI] [PubMed] [Google Scholar]
- Blazier AS, Papin JA. Reconciling high-throughput gene essentiality data with metabolic network reconstructions. PLoS Comput Biol. 2019;15:e1006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodworth RA, Gislason AS, Cardona ST. Burkholderia cenocepacia conditional growth mutant library created by random promoter replacement of essential genes. MicrobiologyOpen. 2013;2:243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B, DeJesus MA, Poulton NCet al. Genome-wide gene expression tuning reveals diverse vulnerabilities of M. tuberculosis. Cell. 2021;184:4579–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouz G, Zitko J. Inhibitors of aminoacyl-tRNA synthetases as antimycobacterial compounds: an up-to-date review. Bioorg Chem. 2021;110:104806. [DOI] [PubMed] [Google Scholar]
- Braberg H, Alexander R, Shales Met al. Quantitative analysis of triple mutant genetic interactions. Nat Protoc. 2014;9:1867–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner A, Fridman O, Gefen Oet al. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol. 2016;14:320–30. [DOI] [PubMed] [Google Scholar]
- Breuer M, Earnest TM, Merryman Cet al. Essential metabolism for a minimal cell. ELife. 2019;8:e36842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito PH, Chevreux B, Serra CRet al. Genetic competence drives genome diversity in Bacillussubtilis. Genome Biol Evol. 2017;10:108–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochado AR, Telzerow A, Bobonis Jet al. Species-specific activity of antibacterial drug combinations. Nature. 2018;559:259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger BT, Imam S, Scarborough MJet al. Combining genome-scale experimental and computational methods to identify essential genes in Rhodobactersphaeroides. MSystems. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butland G, Babu M, Díaz-Mejía JJet al. eSGA: E. coli synthetic genetic array analysis. Nat Methods. 2008;5:789–95. [DOI] [PubMed] [Google Scholar]
- Butland G, Peregrin-Alvarez JM, Li Jet al. Interaction network containing conserved and essential protein complexes in Escherichiacoli. Nature. 2005;433:531–7. [DOI] [PubMed] [Google Scholar]
- Butzin NC, Mather WH. Crosstalk between diverse synthetic protein degradation tags in Escherichiacoli. ACS Synth Biol. 2018;7:54–62. [DOI] [PubMed] [Google Scholar]
- Cacace E, Kritikos G, Typas A. Chemical genetics in drug discovery. Curr Opin Syst Biol. 2017;4:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Villamañán A, Ng JW, Planel Ret al. On-target activity predictions enable improved CRISPR–dCas9 screens in bacteria. Nucleic Acids Res. 2020;48:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DE, Collins JJ. Tunable protein degradation in bacteria. Nat Biotechnol. 2014;32:1276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DE, Urbach JM, Mekalanos JJ. A defined transposon mutant library and its use in identifying motility genes in Vibriocholerae. Proc Natl Acad Sci. 2008;105:8736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TL, Brown ED. Genetic interaction screens with ordered overexpression and deletion clone sets implicate the Escherichiacoli GTPase YjeQ in late ribosome biogenesis. J Bacteriol. 2008;190:2537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TL, Ederer CS, Allali-Hassani Aet al. Isolation of the rstA gene as a multicopy suppressor of YjeE, an essential ATPase of unknown function in Escherichiacoli. J Bacteriol. 2007;189:3318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canchaya C, Proux C, Fournous Get al. Prophage genomics. Microbiol Mol Biol Rev. 2003;67:238–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfrae LA, MacNair CR, Brown CMet al. Mimicking the human environment in mice reveals that inhibiting biotin biosynthesis is effective against antibiotic-resistant pathogens. Nat Microbiol. 2019;1–9. [DOI] [PubMed] [Google Scholar]
- Carmel-Harel O, Storz G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu Rev Microbiol. 2000;54:439–61. [DOI] [PubMed] [Google Scholar]
- Caro F, Place NM, Mekalanos JJ. Analysis of lipoprotein transport depletion in Vibriocholerae using CRISPRi. Proc Natl Acad Sci. 2019;116:17013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova-Hampton K, Carey A, Kassam Set al. A genome-wide screen reveals the involvement of enterobactin-mediated iron acquisition in Escherichiacoli survival during copper stress. Metallomics. 2021;13:mfab052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MC, Abel S, Davis BMet al. The design and analysis of transposon insertion sequencing experiments. Nat Rev Microbiol. 2016;14:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri RR, Allen AG, Owen PJet al. Comprehensive identification of essential staphylococcusaureus genes using transposon-mediated differential hybridisation (TMDH). BMC Genomics. 2009;10:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Choe D, Lee Eet al. High-level dCas9 expression induces abnormal cell morphology in Escherichiacoli. ACS Synth Biol. 2018;7. [DOI] [PubMed] [Google Scholar]
- Choudhary E, Thakur P, Pareek Met al. Gene silencing by CRISPR interference in mycobacteria. Nat Commun. 2015;6:ncomms7267. [DOI] [PubMed] [Google Scholar]
- Chow WY, Berg DE. Tn5tac1, a derivative of transposon Tn5 that generates conditional mutations. Proc Natl Acad Sci. 1988;85:6468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen MT, Kaas RS, Chaudhuri RRet al. Genome-wide high-throughput screening to investigate essential genes involved in methicillin-resistant Staphylococcusaureus sequence type 398 survival. PLoS One. 2014;9:e89018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude F, Rochat I, Hafen GM. No benefit of longer eradication therapy of Pseudomonasaeruginosa primoinfections in pediatric cystic fibrosis. BMC Res Notes. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe KA, Lee W, Stone MCet al. Multi-strain tn-seq reveals common daptomycin resistance determinants in Staphylococcusaureus. PLoS Pathog. 2019;15:e1007862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen O, Gophna U, Pupko T. The complexity hypothesis revisited: connectivity rather than function constitutes a barrier to horizontal gene transfer. Mol Biol Evol. 2011;28:1481–9. [DOI] [PubMed] [Google Scholar]
- Collins SR, Roguev A, Krogan NJ. Quantitative genetic interaction mapping using the E-MAP approach. Methods Enzymol. 2010;470:205–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commichau FM, Pietack N, Stulke J. Essential genes in Bacillussubtilis: a re-evaluation after ten years. Mol Biosyst. 2013;9:1068–75. [DOI] [PubMed] [Google Scholar]
- Conlon BP, Rowe SE, Gandt ABet al. Persister formation in Staphylococcusaureus is associated with ATP depletion. Nature Microbiology. 2016;1:16051. [DOI] [PubMed] [Google Scholar]
- Costanzo M, Baryshnikova A, Myers CLet al. Charting the genetic interaction map of a cell. Curr Opin Biotechnol. 2011;22:66–74. [DOI] [PubMed] [Google Scholar]
- Costanzo M, VanderSluis B, Koch ENet al. A global genetic interaction network maps a wiring diagram of cellular function. Science. 2016;353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté J-P, French S, Gehrke SSet al. The genome-wide interaction network of nutrient stress genes in Escherichiacoli. MBio. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JS, Chen B, McNeil Met al. Complex lipid determines tissue-specific replication of Mycobacteriumtuberculosis in mice. Nature. 1999;402:79–83. [DOI] [PubMed] [Google Scholar]
- Cramm R, Siddiqui RA, Friedrich B. Two isofunctional nitric oxide reductases in Alcaligeneseutrophus H16. J Bacteriol. 1997;179:6769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar J, Åstrand M, Elovaara Het al. Structural and biomolecular analyses of Borreliaburgdorferi BmpD reveal a substrate-binding protein of an ABC-type nucleoside transporter family. Infect Immun. 2020;88:e00962–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Vigouroux A, Rousset Fet al. A CRISPRi screen in E. coli reveals sequence-specific toxicity of dCas9. Nat Commun. 2018;9:1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp EJ, Waglechner N, Wang Wet al. Evolution-guided discovery of antibiotics that inhibit peptidoglycan remodelling. Nature. 2020;1–6. [DOI] [PubMed] [Google Scholar]
- Curran DM, Grote A, Nursimulu Net al. Modeling the metabolic interplay between a parasitic worm and its bacterial endosymbiont allows the identification of novel drug targets. ELife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson L, Walker AW, Oliver AEet al. Lung function and microbiota diversity in cystic fibrosis. Microbiome. 2020;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichiacoli K-12 using PCR products. Proc Natl Acad Sci. 2000;97:6640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davierwala AP, Haynes J, Li Zet al. The synthetic genetic interaction spectrum of essential genes. Nat Genet. 2005;37:1147–52. [DOI] [PubMed] [Google Scholar]
- Davis JH, Baker TA, Sauer RT. Small-molecule control of protein degradation using split adaptors. ACS Chem Biol. 2011;6:1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus MA, Ambadipudi C, Baker Ret al. TRANSIT - A software tool for himar1 tnseq analysis. PLoS Comput Biol. 2015;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus MA, Ioerger TR. A hidden markov model for identifying essential and growth-defect regions in bacterial genomes from transposon insertion sequencing data. BMC Bioinf. 2013;14:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depardieu F, Bikard D. Gene silencing with CRISPRi in bacteria and optimization of dCas9 expression levels. Methods. 2019. [DOI] [PubMed] [Google Scholar]
- Deutschbauer A, Price MN, Wetmore KMet al. Evidence-Based annotation of gene function in shewanella oneidensis MR-1 using genome-wide fitness profiling across 121 conditions. PLos Genet. 2011;7:e1002385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar N, McKinney JD. Mycobacterium tuberculosis persistence mutants identified by screening in isoniazid-treated mice. Proc Natl Acad Sci. 2010;107:12275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- diCenzo GC, Mengoni A, Fondi M. Tn-Core: a toolbox for integrating Tn-seq gene essentiality data and constraint-based metabolic modeling. ACS Synthetic Biology. 2019;8:158–69. [DOI] [PubMed] [Google Scholar]
- Donati S, Kuntz M, Pahl Vet al. Multi-omics analysis of CRISPRi-knockdowns identifies mechanisms that buffer decreases of enzymes in E. coli metabolism. Cell Syst. 2021;12:56–67. [DOI] [PubMed] [Google Scholar]
- Dotsch A, Klawonn F, Jarek Met al. Evolutionary conservation of essential and highly expressed genes in Pseudomonasaeruginosa. BMC Genomics. 2010;11:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J-YF, Kouwen TRHM, Schurich AKCet al. Immunity to the bacteriocin sublancin 168 is determined by the SunI (YolF) protein of Bacillussubtilis. Antimicrob Agents Chemother. 2009;53:651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand A, Sinha AK, Dard-Dascot Cet al. Mutations affecting potassium import restore the viability of the Escherichiacoli DNA polymerase III holD mutant. PLos Genet. 2016;12:e1006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand-Reville TF, Miller AA, O'Donnell JPet al. Rational design of a new antibiotic class for drug-resistant infections. Nature. 2021;597:698–702. [DOI] [PubMed] [Google Scholar]
- Dutta NK, Mehra S, Didier PJet al. Genetic requirements for the survival of tubercle bacilli in primates. J Infect Dis. 2010;201:1743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BD, Greysson-Wong J, Somayaji Ret al. Prevalence and outcomes of Achromobacter species infections in adults with cystic fibrosis: a north American cohort study. J Clin Microbiol. 2017;55:2074–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farha MA, Brown ED. Strategies for target identification of antimicrobial natural products. Nat Prod Rep. 2016;33:668–80. [DOI] [PubMed] [Google Scholar]
- Farzadfard F, Perli SD, Lu TK. Tunable and multifunctional eukaryotic transcription factors based on CRISPR/Cas. ACS Synthetic Biology. 2013;2:604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrand S, Tao J, Shen Xet al. Screening for mevalonate biosynthetic pathway inhibitors using sensitized bacterial strains. J Biomol Screening. 2011;16:637–46. [DOI] [PubMed] [Google Scholar]
- Fey PD, Endres JL, Yajjala VKet al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcusaureus genes. MBio. 2013;4:e00537–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins LM, O'Toole GA. Cystic fibrosis lung infections: polymicrobial, complex, hard to treat. PLoS Pathog. 2015;11:e1005258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persister cells. Nat Rev Microbiol. 2017;15:453–64. [DOI] [PubMed] [Google Scholar]
- Fisher RA. XV.—The correlation between relatives on the supposition of Mendelian inheritance. Transactions of the Royal Society of Edinburgh. 1919;52:399–433. [Google Scholar]
- Fleischmann RD, Adams MD, White Oet al. Whole-genome random sequencing and assembly of Haemophilusinfluenzae rd. Science. 1995;269:496–512. [DOI] [PubMed] [Google Scholar]
- Forsyth RA, Haselbeck RJ, Ohlsen KLet al. A genome-wide strategy for the identification of essential genes in Staphylococcusaureus. Mol Microbiol. 2002;43:1387–400. [DOI] [PubMed] [Google Scholar]
- Fraser CM, Gocayne JD, White Oet al. The minimal gene complement of Mycoplasmagenitalium. Science. 1995;270:397–404. [DOI] [PubMed] [Google Scholar]
- Freed NE, Bumann D, Silander OK. Combining shigella Tn-seq data with gold-standard E. coli gene deletion data suggests rare transitions between essential and non-essential gene functionality. BMC Microbiol. 2016;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi L, Vincent AT, Jeukens Jet al. The Pseudomonasaeruginosa pan-genome provides new insights on its population structure, horizontal gene transfer, and pathogenicity. Genome Biology and Evolution. 2019;11:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritts RK, McCully AL, McKinlay JB. Extracellular metabolism sets the table for microbial cross-feeding. Microbiol. Mol Biol Rev. 2021;85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher LA, Bailey J, Manoil C. Ranking essential bacterial processes by speed of mutant death. Proc Natl Acad Sci. 2020;117:18010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher LA, Shendure J, Manoil C. Genome-scale identification of resistance functions in Pseudomonasaeruginosa using Tn-seq. MBio. 2011;2:e00315–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia BA, Carden JL, Goodwin DLet al. Implementation of a successful eradication protocol for Burkholderiacepacia complex in cystic fibrosis patients. BMC Pulmonary Medicine. 2018;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau JE, Dupuis M-È, Villion Met al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–71. [DOI] [PubMed] [Google Scholar]
- Gawronski JD, Wong SMS, Giannoukos Get al. Tracking insertion mutants within libraries by deep sequencing and a genome-wide screen for Haemophilus genes required in the lung. Proc Natl Acad Sci. 2009;106:16422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke SS, Kumar G, Yokubynas NAet al. Exploiting the sensitivity of nutrient transporter deletion strains in discovery of natural product antimetabolites. ACS Infectious Diseases. 2017;3:955–65. [DOI] [PubMed] [Google Scholar]
- Geisinger E, Mortman NJ, Dai Yet al. Antibiotic susceptibility signatures identify potential antimicrobial targets in the Acinetobacterbaumannii cell envelope. Nat Commun. 2020;11:4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler B, Elraheb D, Margolin W. A gain-of-function mutation in ftsA bypasses the requirement for the essential cell division gene zipA in Escherichiacoli. Proc Natl Acad Sci. 2003;100:4197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes S, Edwards R, Kubal Met al. Essential genes on metabolic maps. Curr Opin Biotechnol. 2006;17:448–56. [DOI] [PubMed] [Google Scholar]
- Gerdes SY, Scholle MD, Campbell JWet al. Experimental determination and system level analysis of essential genes in Escherichiacoli MG1655. J Bacteriol. 2003;185:5673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni Let al. Functional profiling of the Saccharomycescerevisiae genome. Nature. 2002;418:387–91. [DOI] [PubMed] [Google Scholar]
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–51. [DOI] [PubMed] [Google Scholar]
- Gingras H, Dridi B, Leprohon Pet al. Coupling next-generation sequencing to dominant positive screens for finding antibiotic cellular targets and resistance mechanisms in Escherichiacoli. Microbial Genomics. 2018;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras H, Patron K, Bhattacharya Aet al. Gain- and loss-of-function screens coupled to next-generation sequencing for antibiotic mode of action and resistance studies in Streptococcuspneumoniae. Antimicrob Agents Chemother. 2019;63:e02381–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis HS, Hottes AK, Tavazoie S. Genetic architecture of intrinsic antibiotic susceptibility. PLoS One. 2009;4:e5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gislason AS, Turner K, Domaratzki Met al. Comparative analysis of the burkholderiacenocepacia K56-2 essential genome reveals cell envelope functions that are uniquely required for survival in species of the genus Burkholderia. Microb. Genomics. 2017;3:e000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JI, Assad-Garcia N, Alperovich Net al. Essential genes of a minimal bacterium. Proc Natl Acad Sci. 2006;103:425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinkowska M, Łoś JM, Szambowska Aet al. Influence of the escherichiacoli oxyR gene function on λ prophage maintenance. Arch Microbiol. 2010;192:673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau A, Barrell BG, Bussey Het al. Life with 6000 genes. Science. 1996;274:546, 563–7. [DOI] [PubMed] [Google Scholar]
- Goh S, Boberek JM, Nakashima Net al. Concurrent growth rate and transcript analyses reveal essential gene stringency in Escherichiacoli. PLoS One. 2009;4:e60601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S, Hohmeier A, Stone TCet al. Silencing of essential genes within a highly coordinated operon in Escherichiacoli. Appl Environ Microbiol. 2015;81:5650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Fan S, Bilderbeck Aet al. Comparative analysis of essential genes and nonessential genes in Escherichiacoli K12. Mol Genet Genomics. 2008;279:87–94. [DOI] [PubMed] [Google Scholar]
- Goodall ECA, Robinson A, Johnston IGet al. The essential genome of Escherichiacoli K-12. MBio. 2018;9:e02096–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, Wu M, Gordon JI. Identifying microbial fitness determinants by insertion sequencing (INSeq) using genome-wide transposon mutant libraries. Nat Protoc. 2011;6:1969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowicz M, Silhavy TJ. Redefining the essential trafficking pathway for outer membrane lipoproteins. Proc Natl Acad Sci. 2017;114:4769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazziotin AL, Vidal NM, Venancio TM. Uncovering major genomic features of essential genes in bacteria and a methanogenic archaea. FEBS J. 2015;282:3395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenov AI, Gerdes SY. Modeling competitive outgrowth of mutant populations: why do essentiality screens yield divergent results?. In: AL Osterman et al. (ed). Microbial Gene Essentiality: Protocols and Bioinformatics. Totowa, NJ: Humana Press, 2008;361–7. [DOI] [PubMed] [Google Scholar]
- Griffin JE, Gawronski JD, Dejesus MAet al. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 2011;7:e1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J, Felice MD, Klopotowski Tet al. Multiplicity of isoleucine, leucine, and valine transport systems in Escherichiacoli K-12. J Bacteriol. 1974;117:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán GI, Olson CA, Hefner Yet al. Reframing gene essentiality in terms of adaptive flexibility. BMC Syst Biol. 2018;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán GI, Utrilla J, Nurk Set al. Model-driven discovery of underground metabolic functions in Escherichiacoli. Proc Natl Acad Sci. 2015;112:929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford JI, Ize B, Buchanan Get al. Conserved network of proteins essential for bacterial viability. J Bacteriol. 2009;191:4732–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms A, Maisonneuve E, Gerdes K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science. 2016;354:aaf4268. [DOI] [PubMed] [Google Scholar]
- Hart EM, Mitchell AM, Konovalova Aet al. A small-molecule inhibitor of BamA impervious to efflux and the outer membrane permeability barrier. Proc Natl Acad Sci. 2019;201912345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EM, Silhavy TJ. Functions of the BamBCDE lipoproteins revealed by bypass mutations in bamA. J Bacteriol. 2020;202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JS, Silvis MR, Koo B-Met al. Mismatch-CRISPRi reveals the co-varying expression-fitness relationships of essential genes in Escherichiacoli and Bacillussubtilis. Cell Syst. 2020;11:523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Qian W, Wang Zet al. Prevalent positive epistasis in E. coli and S. cerevisiae metabolic networks. Nat Genet. 2010;42:272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann TC, Deutschbauer AM, Lindow SE. Genome-wide identification of pseudomonas syringae genes required for fitness during colonization of the leaf surface and apoplast. Proc Natl Acad Sci. 2019;116:18900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman C, Thévenet D, Bouloc Pet al. Degradation of carboxy-terminal-tagged cytoplasmic proteins by the Escherichiacoli protease HflB (FtsH). Genes Dev. 1998;12:1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring CD, Blattner FR. Conditional lethal amber mutations in essential Escherichiacoli genes. J Bacteriol. 2004;186:2673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewer SCL, Smyth AR. Antibiotic strategies for eradicating Pseudomonasaeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev. 2017;CD004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S, Sanchez-Contreras M, Gualdi Set al. The essential genome of Burkholderiacenocepacia H111. J Bacteriol. 2017;199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenmeyer ME, Fung E, Wildenhain Jet al. The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science. 2008;320:362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelreich R, Hilbert H, Plagens Het al. Complete sequence analysis of the genome of the bacterium Mycoplasmapneumoniae. Nucleic Acids Res. 1996;24:4420–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan AM, Rahman ASMZ, Lightly TJet al. A broad-host-range CRISPRi toolkit for silencing gene expression in Burkholderia. ACS Synthetic Biology. 2019;8:2372–84. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Scoffone VC, Makarov Vet al. Competitive fitness of essential gene knockdowns reveals a broad-spectrum antibacterial inhibitor of the cell division protein ftsZ. Antimicrob Agents Chemother. 2018;62:e01231–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz NH, Bonner D, Mitchell HKet al. Genic control of biochemical reactions in neurospora. Am Nat. 1945;79:304–17. [Google Scholar]
- Hu JH, Miller SM, Geurts MHet al. Evolved cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018;556:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Sillaots S, Lemieux Set al. Essential gene identification and drug target prioritization in Aspergillusfumigatus. PLoS Pathog. 2007;3:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Garraway LA, Ashworth Aet al. Synthetic lethality as an engine for cancer drug target discovery. Nat Rev Drug Discovery. 2020;19:23–38. [DOI] [PubMed] [Google Scholar]
- Huerta-Cepas J, Forslund K, Coelho LPet al. Fast genome-wide functional annotation through orthology assignment by eggNOG-Mapper. Mol Biol Evol. 2017;34:2115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Cepas J, Szklarczyk D, Heller Det al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019;47:D309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CA, Chuang R-Y, Noskov VNet al. Design and synthesis of a minimal bacterial genome. Science. 2016;351:aad6253. [DOI] [PubMed] [Google Scholar]
- Hutchison CA, Peterson SN, Gill SRet al. Global transposon mutagenesis and a minimal Mycoplasma genome. Science. 1999;286:2165–9. [DOI] [PubMed] [Google Scholar]
- Ibberson CB, Stacy A, Fleming Det al. Co-infecting microorganisms dramatically alter pathogen gene essentiality during polymicrobial infection. Nature Microbiology. 2017;2:17079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Meyer KJ, Iinishi Aet al. A new antibiotic selectively kills Gram-negative pathogens. Nature. 2019;576:459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Am O, Kristensen DM, Ruppin E. Evolutionary conservation of bacterial essential metabolic genes across all bacterial culture media. PLoS One. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MA, Alwood A, Thaipisuttikul Iet al. Comprehensive transposon mutant library of Pseudomonasaeruginosa. Proc Natl Acad Sci. 2003;100:14339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M, Dziulko A, Smith JDet al. Improved discovery of genetic interactions using CRISPRiSeq across multiple environments. Genome Res. 2019;29:668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BD, Hayes CS. The tmRNA ribosome rescue system. Adv Protein Chem Struct Biol. 2012;86:151–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PA, Zhu Z, van Opijnen T. Antibiotics disrupt coordination between transcriptional and phenotypic stress responses in pathogenic bacteria. Cell Rep. 2017;20:1705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Zhang B, Van SFet al. Identification of critical staphylococcal genes using conditional phenotypes generated by antisense RNA. Science. 2001;293:2266–9. [DOI] [PubMed] [Google Scholar]
- Jiang W, Oikonomou P, Tavazoie S. Comprehensive genome-wide perturbations via CRISPR adaptation reveal complex genetics of antibiotic sensitivity. Cell. 2020;180:1002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara Iet al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger AC, Mayer S, Fersht AR. Mimicking natural evolution in vitro: an N-acetylneuraminate lyase mutant with an increased dihydrodipicolinate synthase activity. Proc Natl Acad Sci. 2003;100:5694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, LaVerriere E, Office Eet al. Large-scale chemical–genetics yields new M. tuberculosis inhibitor classes. Nature. 2019;571:72–8. [DOI] [PubMed] [Google Scholar]
- Jordan IK, Rogozin IB, Wolf YIet al. Essential genes are more evolutionarily conserved than are nonessential genes in bacteria. Genome Res. 2002;12:962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson MA, Bryant JC. A genetic screen to identify factors affected by undecaprenyl phosphate recycling uncovers novel connections to morphogenesis in Escherichiacoli. Mol Microbiol. 2021;115:191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson MA, Kannan S, Laubacher MEet al. Dead-end intermediates in the enterobacterial common antigen pathway induce morphological defects in Escherichiacoli by competing for undecaprenyl phosphate. Mol Microbiol. 2016;100:mmi.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson MA, Young KD. Interrupting biosynthesis of o-antigen or the lipopolysaccharide core produces morphological defects in Escherichiacoli by sequestering undecaprenyl phosphate. J Bacteriol. 2016;198:3070–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SM, Pandey AK, Capite Net al. Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc Natl Acad Sci. 2006;103:11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M, Santos DA, Saunders RAet al. Titrating gene expression using libraries of systematically attenuated CRISPR guide RNAs. Nat Biotechnol. 2020;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce AR, Reed JL, White Aet al. Experimental and computational assessment of conditionally essential genes in Escherichiacoli. J Bacteriol. 2006;188:8259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson N, Mekalanos JJ. TnAraOut, a transposon-based approach to identify and characterize essential bacterial genes. Nat Biotechnol. 2000;18:740–5. [DOI] [PubMed] [Google Scholar]
- Juhas M, Eberl L, Church GM. Essential genes as antimicrobial targets and cornerstones of synthetic biology. Trends Biotechnol. 2012;30:601–7. [DOI] [PubMed] [Google Scholar]
- Kacar B, Garmendia E, Tuncbag Net al. Functional constraints on replacing an essential gene with its ancient and modern homologs. MBio. 2017;8:e01276–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp PD, Billington R, Caspi Ret al. The biocyc collection of microbial genomes and metabolic pathways. Briefings Bioinf. 2019;20:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J, Hashimoto M. Construction of consecutive deletions of the Escherichiacoli chromosome. Mol Syst Biol. 2007;3:msb4100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren L, Hausser J, Lotan-Pompan Met al. Massively parallel interrogation of the effects of gene expression levels on fitness. Cell. 2016;166:1282–94. [DOI] [PubMed] [Google Scholar]
- Kim D-U, Hayles J, Kim Det al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomycespombe. Nat Biotechnol. 2010;28:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-H, Wei J-R, Wallach JBet al. Protein inactivation in mycobacteria by controlled proteolysis and its application to deplete the beta subunit of RNA polymerase. Nucleic Acids Res. 2011;39:2210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, O'Brien KM, Sharma Ret al. A genetic strategy to identify targets for the development of drugs that prevent bacterial persistence. Proc Natl Acad Sci. 2013;110:19095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Ara T, Arifuzzaman Met al. Complete set of ORF clones of Escherichiacoli ASKA library (a complete set of e. coli K-12 ORF archive): unique resources for biological research. DNA Res Int J Rapid Publ Rep Genes Genomes. 2005;12:291–9. [DOI] [PubMed] [Google Scholar]
- Klobucar K, Brown ED. Use of genetic and chemical synthetic lethality as probes of complexity in bacterial cell systems. FEMS Microbiol Rev. 2018;42:femsre/fux054. [DOI] [PubMed] [Google Scholar]
- Klobucar K, French S, Côté J-Pet al. Genetic and chemical-genetic interactions map biogenesis and permeability determinants of the outer membrane of Escherichiacoli. MBio. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuth K, Niesalla H, Hueck CJet al. Large-scale identification of essential Salmonella genes by trapping lethal insertions. Mol Microbiol. 2004;51:1729–44. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ehrlich SD, Albertini Aet al. Essential Bacillussubtilis genes. Proc Natl Acad Sci. 2003;100:4678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidis KT, Tiedje JM. Trends between gene content and genome size in prokaryotic species with larger genomes. Proc Natl Acad Sci. 2004;101:3160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B-M, Kritikos G, Farelli JDet al. Construction and analysis of two genome-scale deletion libraries for Bacillussubtilis. Cell Syst. 2017;4:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KM, Haglund CM, Hebner Cet al. Potent LpxC inhibitors with in vitro activity against multidrug-resistant Pseudomonasaeruginosa. Antimicrob Agents Chemother. 2019;63:e00977–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcsár PI, Tálas A, Tóth Eet al. Blackjack mutations improve the on-target activities of increased fidelity variants of spcas9 with 5′G-extended sgRNAs. Nat Commun. 2020;11:1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin E, VanderSluis B, Ba ANNet al. Exploring whole-genome duplicate gene retention with complex genetic interaction analysis. Science. 2020;368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin E, VanderSluis B, Wang Wet al. Systematic analysis of complex genetic interactions. Science. 2018;360:eaao1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JS, Taylor VL, Islam STet al. Genetic and functional diversity of pseudomonasaeruginosa lipopolysaccharide. Front Microbiol. 2011;2, 10.3389/fmicb.2011.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge GC, Phan MD, Turner DJet al. Simultaneous assay of every salmonellatyphi gene using one million transposon mutants. Genome Res. 2009;19:2308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MH, Gilbert LA, Wang Xet al. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc. 2013;8:2180–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Breton Y, Belew AT, Valdes KMet al. Essential genes in the core genome of the human pathogen streptococcuspyogenes. Sci Rep. 2015;5:9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Ostrov N, Wong BGet al. Functional genomics of the rapidly replicating bacterium Vibrionatriegens by CRISPRi. Nature Microbiol. 2019;4. [DOI] [PubMed] [Google Scholar]
- Lee K, Campbell J, Swoboda JGet al. Development of improved inhibitors of wall teichoic acid biosynthesis with potent activity against staphylococcusaureus. Bioorg Med Chem Lett. 2010;20:1767–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Gallagher LA, Thongdee Met al. General and condition-specific essential functions of Pseudomonasaeruginosa. Proc Natl Acad Sci. 2015;112:5189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman KM, Grabowicz M. Countering Gram-negative antibiotic resistance: recent progress in disrupting the outer membrane with novel therapeutics. Antibiotics. 2019;8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimer N, Wu X, Imai Yet al. A selective antibiotic for lyme disease. Cell. 2021;184:5405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Stacy A, Michie KLet al. Large-scale identification of pathogen essential genes during coinfection with sympatric and allopatric microbes. Proc Natl Acad Sci. 2019;116:19685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G-W, Burkhardt D, Gross Cet al. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell. 2014;157:624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hernandez V, Rock FLet al. Discovery of a potent and specific M. tuberculosis leucyl-tRNA synthetase inhibitor: (S)-3-(aminomethyl)-4-chloro-7-(2-hydroxyethoxy)benzo[c][1,2]oxaborol-1(3H)-ol (GSK656). J Med Chem. 2017;60:8011–26. [DOI] [PubMed] [Google Scholar]
- Liberati NT, Urbach JM, Miyata Set al. An ordered, nonredundant library of Pseudomonasaeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci. 2006;103:2833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhang RR. Putative essential and core-essential genes in Mycoplasma genomes. Sci Rep. 2011;1:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Tran L, Becket Eet al. Antibiotic sensitivity profiles determined with an Escherichiacoli gene knockout collection: generating an antibiotic bar code. Antimicrob Agents Chemother. 2010;54:1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Price MN, Waters RJet al. Magic pools: parallel assessment of transposon delivery vectors in bacteria. MSystems. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gallay C, Kjos Met al. High-throughput CRISPRi phenotyping identifies new essential genes in Streptococcuspneumoniae. Mol Syst Biol. 2017;13:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kimmey JM, Matarazzo Let al. Exploration of bacterial bottlenecks and Streptococcuspneumoniae pathogenesis by CRISPRi-Seq. Cell Host Microbe. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffelholz O, Jiang Q, Ariosa Aet al. Ribosome–SRP–FtsY cotranslational targeting complex in the closed state. Proc Natl Acad Sci. 2015;112:3943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya O, Rubio-Aparicio D, Nelson Ket al. In vitro activity of the ultra-broad-spectrum beta-lactamase inhibitor QPX7728 in combination with multiple beta-lactam antibiotics against Pseudomonasaeruginosa. Antimicrob Agents Chemother. 2021;65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A, Parsons AB, Nislow Cet al. Chemical-genetic approaches for exploring the mode of action of natural products. Prog Drug Res. 2008;66:239–71. [DOI] [PubMed] [Google Scholar]
- Loutet SA, Valvano MA. Extreme antimicrobial peptide and polymyxin b resistance in the genus Burkholderia. Front Microbiol. 2011;2. DOI: 10.3389/fmicb.2011.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Trebosc V, Lee Bet al. A nutrient-limited screen unmasks rifabutin hyperactivity for extensively drug-resistant Acinetobacterbaumannii. Nature Microbiology. 2020;5:1134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Minch KJ, Rustad TRet al. Integrated modeling of gene regulatory and metabolic networks in Mycobacteriumtuberculosis. PLoS Comput Biol. 2015;11:e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie JS, Lamprecht DA, Asmal Ret al. Bedaquiline reprograms central metabolism to reveal glycolytic vulnerability in Mycobacteriumtuberculosis. Nat Commun. 2020;11:6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhukar NS, Elemento O, Pandey G. Prediction of genetic interactions using machine learning and network properties. Front Bioengineer Biotechnol. 2015;3:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder ML, Linder SJ, Cascio VMet al. CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013;10:977–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud SA, Chien P. Regulated proteolysis in bacteria. Annu Rev Biochem. 2018;87:677–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangas EL, Rubio A, Álvarez-Marín Ret al. Pangenome of acinetobacterbaumannii uncovers two groups of genomes, one of them with genes involved in CRISPR/Cas defence systems associated with the absence of plasmids and exclusive genes for biofilm formation. Microb Genomics. 2019;5:e000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Carranza E, Barajas H, Alcaraz L-Det al. Variability of bacterial essential genes among closely related bacteria: the case of Escherichiacoli. Front Microbiol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis AD, Otto RM, Reynolds KA. A simplified strategy for titrating gene expression reveals new relationships between genotype, environment, and bacterial growth. Nucleic Acids Res. 2021;49:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazharul Islam M, Thomas VC, Van Beek Met al. An integrated computational and experimental study to investigate Staphylococcusaureus metabolism. Syst Biol Appl. 2020;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil MB, Keighley LM, Cook JRet al. CRISPR interference identifies vulnerable cellular pathways with bactericidal phenotypes in Mycobacteriumtuberculosis. Mol Microbiol. 2021. [DOI] [PubMed] [Google Scholar]
- Meeske AJ, Sham L-T, Kimsey Het al. MurJ and a novel lipid II flippase are required for cell wall biogenesis in Bacillussubtilis. Proc Natl Acad Sci. 2015;112:6437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander RJ, Zurawski DV, Melander C. Narrow-spectrum antibacterial agents. MedChemComm. 2017;9:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Kanzaki G, Meas Det al. A genome-wide inducible phenotypic screen identifies antisense RNA constructs silencing Escherichiacoli essential genes. FEMS Microbiol Lett. 2012;329:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzgar D, Bacher JM, Pezo Vet al. Acinetobacter sp. ADP1: an ideal model organism for genetic analysis and genome engineering. Nucleic Acids Res. 2004;32:5780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna RH, Commichau FM, Tödter Det al. SubtiWiki-a database for the model organism Bacillussubtilis that links pathway, interaction and expression information. Nucleic Acids Res. 2014;42:D692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DP, Scott DA. Inherently and conditionally essential protein catabolism genes of Porphyromonasgingivalis. Trends Microbiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato Y, Gohl DM, Thiede JMet al. Genome-wide assessment of Mycobacteriumtuberculosis conditionally essential metabolic pathways. MSystems. 2019;4:e00070–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miravet-Verde S, Burgos R, Delgado Jet al. FASTQINS and ANUBIS: two bioinformatic tools to explore facts and artifacts in transposon sequencing and essentiality studies. Nucleic Acids Res. 2020;48:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry A, Warren MS, Cusick JKet al. High-level pacidamycin resistance in Pseudomonasaeruginosa is mediated by an opp oligopeptide permease encoded by the opp-fabI operon. Antimicrob Agents Chemother. 2013;57:5565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AM, Srikumar T, Silhavy TJ. Cyclic enterobacterial common antigen maintains the outer membrane permeability barrier of Escherichiacoli in a manner controlled by yhdP. MBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr SE, Hu Y, Ewen-Campen Bet al. CRISPR guide RNA design for research applications. FEBS J. 2016;283:3232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Henares MA, de la Torre J, García-Salamanca Aet al. Identification of conditionally essential genes for growth of Pseudomonasputida KT2440 on minimal medium through the screening of a genome-wide mutant library. Environ Microbiol. 2010;12:1468–85. [DOI] [PubMed] [Google Scholar]
- Moura ECCM, Baeta T, Romanelli Aet al. Thanatin impairs lipopolysaccharide transport complex assembly by targeting LptC–LptA interaction and decreasing LptA stability. Front Microbiol. 2020;11. 10.3389/fmicb.2020.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A, Gurung I, Cehovin Aet al. Construction of a complete set of Neisseriameningitidis mutants and its use for the phenotypic profiling of this human pathogen. Nat Commun. 2020;11:5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murima P, McKinney JD, Pethe K. Targeting bacterial central metabolism for drug development. Chem Biol. 2014;21:1423–32. [DOI] [PubMed] [Google Scholar]
- Murray JL, Kwon T, Marcotte EMet al. Intrinsic antimicrobial resistance determinants in the superbug Pseudomonasaeruginosa. MBio. 2015;6:e01603–01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushegian AR, Koonin EV. A minimal gene set for cellular life derived by comparison of complete bacterial genomes. Proc Natl Acad Sci. 1996;93:10268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima N, Tamura T. Conditional gene silencing of multiple genes with antisense RNAs and generation of a mutator strain of Escherichiacoli. Nucleic Acids Res. 2009;37:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan AM, Ramsey MM, Stacy Aet al. Defining genetic fitness determinants and creating genomic resources for an oral pathogen. Appl Environ Microbiol. 2017;83:AEM.00797–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers JE, Paolella BR, Asfaw Aet al. Synthetic lethal interaction between the ESCRT paralog enzymes VPS4A and VPS4B in cancers harboring loss of chromosome 18q or 16q. Cell Rep. 2020;33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Zhang G, Qin Let al. Simultaneously down-regulation of multiplex branch pathways using CRISPRi and fermentation optimization for enhancing β-amyrin production in Saccharomycescerevisiae. Synthetic and Systems Biotechnology. 2019;4:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RJ, Sen S, Choo YJet al. Phenotypic landscape of a bacterial cell. Cell. 2011;144:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimasu H, Shi X, Ishiguro Set al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science. 2018;361:1259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunvar J, Hogan AM, Buroni Set al. The effect of 2-thiocyanatopyridine derivative 11026103 on Burkholderiacenocepacia: resistance mechanisms and systemic impact. Antibiotics. 2019;8:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opijnen T, Bodi KL, Camilli A. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods. 2009;6:767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JD, Palsson B. Gap-filling analysis of the iJO1366 Escherichiacoli metabolic network reconstruction for discovery of metabolic functions. BMC Syst Biol. 2012;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Bastkowski S, Yasir Met al. AlbaTraDIS: Comparative analysis of large datasets from parallel transposon mutagenesis experiments. PLoS Comput Biol. 2020;16:e1007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AC, Kishony R. Opposing effects of target overexpression reveal drug mechanisms. Nat Commun. 2014;5:4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer KL, Aye LM, Whiteley M. Nutritional cues control Pseudomonasaeruginosa multi-cellular behavior in cystic fibrosis sputum. J Bacteriol. 2007;189:8079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci. 2008;105:4376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp B, Pal C, Hurst LD. Metabolic network analysis of the causes and evolution of enzyme dispensability in yeast. Nature. 2004;429:661–4. [DOI] [PubMed] [Google Scholar]
- Pathania R, Zlitni S, Barker Cet al. Chemical genomics in Escherichiacoli identifies an inhibitor of bacterial lipoprotein targeting. Nat Chem Biol. 2009;5:849–56. [DOI] [PubMed] [Google Scholar]
- Patrick WM, Quandt EM, Swartzlander DBet al. Multicopy suppression underpins metabolic evolvability. Mol Biol Evol. 2007;24:2716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WR. An introduction to sequence similarity (“homology”) searching. Curr Protoc Bioinforma. 2013;3:ebi0301s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediaditakis M, Kaufenstein M, Graumann PL. Bacillus subtilis hlpB encodes a conserved stand-alone HNH nuclease-like protein that is essential for viability unless the hlpB deletion is accompanied by the deletion of genes encoding the AddAB DNA repair complex. J Bacteriol. 2012;194:6184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R, Wang Y, Feng Wet al. CRISPR/dCas9-mediated transcriptional improvement of the biosynthetic gene cluster for the epothilone production in Myxococcusxanthus. Microb Cell Fact. 2018;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Colavin A, Shi Het al. A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell. 2016;165:1493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Koo B-M, Patino Ret al. Enabling genetic analysis of diverse bacteria with Mobile-CRISPRi. Nature Microbiology. 2019;4:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski JS, Li SC, Deshpande Ret al. Functional annotation of chemical libraries across diverse biological processes. Nat Chem Biol. 2017;13:982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger RC, Wolfe RN, Hoehn MMet al. Hygromycin. I. Preliminary studies on the production and biologic activity of a new antibiotic. Antibiot Chemother Northfield Ill. 1953;3:1268–78. [PubMed] [Google Scholar]
- Porwollik S, Santiviago CA, Cheng Pet al. Defined single-gene and multi-gene deletion mutant collections in Salmonellaenterica sv Typhimurium. PLoS One. 2014;9:e99820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen BE, Yang R, Clatworthy AEet al. Defining the core essential genome of Pseudomonasaeruginosa. Proc Natl Acad Sci. 2019;116:10072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyatos JF. Genetic buffering and potentiation in metabolism. PLoS Comput Biol. 2020;16:e1008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MN, Deutschbauer AM, Skerker JMet al. Indirect and suboptimal control of gene expression is widespread in bacteria. Mol Syst Biol. 2013;9:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MN, Wetmore KM, Waters RJet al. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature. 2018;557:503–9. [DOI] [PubMed] [Google Scholar]
- Prinz WA, Åslund F, Holmgren Aet al. The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichiacoli cytoplasm. J Biol Chem. 1997;272:15661–7. [DOI] [PubMed] [Google Scholar]
- Pritchard JR, Chao MC, Abel Set al. ARTIST: high-resolution genome-wide assessment of fitness using transposon-insertion sequencing. PLoS Genet. 2014;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projan SJ. Whither antibacterial drug discovery?. Drug Discovery Today. 2008;13:279–80. [DOI] [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LAet al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Prasad NK, Yu MAet al. Modulating pathogenesis with mobile-CRISPRi. J Bacteriol. 2019;201:e00304–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad N, Luidalepp H, Fasnacht Met al. Transcriptome-wide analysis of stationary phase small ncRNAs in e. coli. Int J Mol Sci. 2021;22:1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal M, Martin MJ, Santiago Met al. Multidrug intrinsic resistance factors in Staphylococcusaureus identified by profiling fitness within high-diversity transposon libraries. MBio. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey MM, Rumbaugh KP, Whiteley M. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog. 2011;7:e1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancati G, Moffat J, Typas Aet al. Emerging and evolving concepts in gene essentiality. Nat Rev Genet. 2018;19:34–49. [DOI] [PubMed] [Google Scholar]
- Ranjit DK, Liechti GW, Maurelli AT. Chlamydial MreB directs cell division and peptidoglycan synthesis in Escherichiacoli in the absence of FtsZ activity. MBio. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, Roth JR. A Tn10 derivative (T-POP) for isolation of insertions with conditional (tetracycline-dependent) phenotypes. J Bacteriol. 1997;179:5827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan KH, Bhatt J. Eradication therapy for Burkholderiacepacia complex in people with cystic fibrosis. Cochrane Database Syst Rev. 2019;4:CD009876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis AC, Halper SM, Vezeau GEet al. Simultaneous repression of multiple bacterial genes using nonrepetitive extra-long sgRNA arrays. Nat Biotechnol. 2019;37. [DOI] [PubMed] [Google Scholar]
- Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacteriumtuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci. 2005;102:8327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riber L, Olsson JA, Jensen RBet al. Hda-mediated inactivation of the DnaA protein and dnaA gene autoregulation act in concert to ensure homeostatic maintenance of the Escherichiacoli chromosome. Genes Dev. 2006;20:2121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JM, Hopkins FF, Chavez Aet al. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nature Microbiology. 2017;2:16274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DA, Vitreschak AG, Mironov AAet al. Regulation of lysine biosynthesis and transport genes in bacteria: yet another RNA riboswitch?. Nucleic Acids Res. 2003;31:6748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K, Varani AM, Snesrud Eet al. TnCentral: a prokaryotic transposable element database and web portal for transposon analysis. MBio. 2021;12:e02060–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F, Cabezas-Caballero J, Piastra-Facon Fet al. The impact of genetic diversity on gene essentiality within the Escherichiacoli species. Nature Microbiology. 2021;6:301–12. [DOI] [PubMed] [Google Scholar]
- Rousset F, Cui L, Siouve Eet al. Genome-wide CRISPR-dCas9 screens in e. coli identify essential genes and phage host factors. PLos Genet. 2018;14:e1007749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N, Davis RM, Kumar S. YhdP, TamB, and YdbH are redundant but essential for growth and lipid homeostasis of the Gram-negative outer membrane. MBio. 2021;12:e0271421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N, Kahne D, Silhavy TJ. Advances in understanding bacterial outer-membrane biogenesis. Nat Rev Microbiol. 2006;4:57–66. [DOI] [PubMed] [Google Scholar]
- Rusmini R, Vecchietti D, Macchi Ret al. A shotgun antisense approach to the identification of novel essential genes in Pseudomonasaeruginosa. BMC Microbiol. 2014;14. DOI: 10.1186/1471-2180-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M, Holmgren A. Construction and characterization of glutaredoxin-negative mutants of Escherichiacoli. Proc Natl Acad Sci. 1988;85:990–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi F, Kamali M, Najafi Aet al. Natural antisense RNAs as mRNA regulatory elements in bacteria: a review on function and applications. Cell Mol Biol Lett. 2016;21. 10.1186/s11658-016-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachelaru I, Winter L, Knyazev DGet al. YidC and SecYEG form a heterotetrameric protein translocation channel. Sci Rep. 2017;7:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez I, Hernandez-Guerrero R, Mendez-Monroy PEet al. Evaluation of the abundance of DNA-binding transcription factors in prokaryotes. Genes. 2020;11:E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago M, Lee W, Fayad AAet al. Genome-wide mutant profiling predicts the mechanism of a lipid II binding antibiotic. Nat Chem Biol. 2018;14:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago M, Matano LM, Moussa SHet al. A new platform for ultra-high density Staphylococcusaureus transposon libraries. BMC Genomics. 2015;16:s12864–015-1361-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento F, Mrázek J, Whitman WB. Genome-scale analysis of gene function in the hydrogenotrophic methanogenic archaeon Methanococcusmaripaludis. Proc Natl Acad Sci. 2013;110:4726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci. 2003;100:12989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimana J, Gebhardt K, Höltzel Aet al. Arylomycins a and b, new biaryl-bridged lipopeptide antibiotics produced by Streptomyces sp. Tü 6075. I. Taxonomy, fermentation, isolation and biological activities. J Antibiot (Tokyo). 2002;55:565–70. [DOI] [PubMed] [Google Scholar]
- Schultenkämper K, Brito LF, Wendisch VF. Impact of CRISPR interference on strain development in biotechnology. Biotechnol Appl Biochem. 2020;67:bab.1901. [DOI] [PubMed] [Google Scholar]
- Sekar K, Gentile AM, Bostick JWet al. N-terminal-based targeted, inducible protein degradation in Escherichiacoli. PLoS One. 2016;11:e0149746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serer MI, Carrica MC, Trappe Jet al. A high-throughput screening for inhibitors of riboflavin synthase identifies novel antimicrobial compounds to treat brucellosis. FEBS J. 2019;286:2522–35. [DOI] [PubMed] [Google Scholar]
- Sesto N, Wurtzel O, Archambaud Cet al. The excludon: a new concept in bacterial antisense RNA-mediated gene regulation. Nat Rev Microbiol. 2013;11:75–82. [DOI] [PubMed] [Google Scholar]
- Shan Y, Brown Gandt A, Rowe SEet al. ATP-dependent persister formation in Escherichiacoli. MBio. 2017;8:e02267–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D, Miravet-Verde S, Piñero-Lambea Cet al. LoxTnSeq: random transposon insertions combined with cre/lox recombination and counterselection to generate large random genome reductions. Microb Biotechnol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RC, Jensen PA. The bare necessities: uncovering essential and condition-critical genes with transposon sequencing. Molecular Oral Microbiology. 2019;34:39–50. [DOI] [PubMed] [Google Scholar]
- Shields RC, Walker AR, Maricic Net al. Repurposing the Streptococcusmutans CRISPR-Cas9 system to understand essential gene function. PLoS Pathog. 2020;16:e1008344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver LL. A gestalt approach to Gram-negative entry. Bioorg Med Chem. 2016;24:6379–89. [DOI] [PubMed] [Google Scholar]
- Silvis MR, Rajendram M, Shi Het al. Morphological and transcriptional responses to CRISPRi knockdown of essential genes in Escherichiacoli. MBio. 2021;12:e02561–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins SW, Deshpande R, Nelson Jet al. Using BEAN-counter to quantify genetic interactions from multiplexed barcode sequencing experiments. Nat Protoc. 2019;14:415–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins SW, Nelson J, Deshpande Ret al. Predicting bioprocess targets of chemical compounds through integration of chemical-genetic and genetic interactions. PLoS Comput Biol. 2018;14:e1006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Carette X, Potluri LPet al. Investigating essential gene function in Mycobacteriumtuberculosis using an efficient CRISPR interference system. Nucleic Acids Res. 2016;44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaff DA, McWhorter WJ, Geisbrecht BVet al. Inhibition of bacterial mevalonate diphosphate decarboxylase by eriochrome compounds. Arch Biochem Biophys. 2015;566:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PA, Koehler MFT, Girgis HSet al. Optimized arylomycins are a new class of Gram-negative antibiotics. Nature. 2018;561:189. [DOI] [PubMed] [Google Scholar]
- Solaimanpour S, Sarmiento F, Mrázek J. Tn-seq explorer: a tool for analysis of high-throughput sequencing data of transposon mutant libraries. PLoS One. 2015;10:e0126070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somayaji R, Stanojevic S, Tullis DEet al. Clinical outcomes associated with achromobacter species infection in patients with cystic fibrosis. Annals of the American Thoracic Society. 2017;14:1412–8. [DOI] [PubMed] [Google Scholar]
- Spoto M, Guan C, Fleming Eet al. A universal, Genome-wide guidefinder for CRISPR/Cas9 targeting in microbial genomes. MSphere. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy A, Fleming D, Lamont RJet al. A commensal bacterium promotes virulence of an opportunistic pathogen via cross-respiration. MBio. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerck C, Gastebois A, Vandeputte Pet al. Microbial antioxidant defense enzymes. Microb Pathog. 2017;110:56–65. [DOI] [PubMed] [Google Scholar]
- Stanway RR, Bushell E, Chiappino-Pepe Aet al. Genome-scale identification of essential metabolic processes for targeting the plasmodium liver stage. Cell. 2019;179:1112–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone VN, Parikh HI, El-rami Fet al. Identification of small-molecule inhibitors against meso-2, 6-diaminopimelate dehydrogenase from porphyromonasgingivalis. PLoS One. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surette MG. The cystic fibrosis lung microbiome. Ann Am Thoracic Soc. 2014;11:Suppl 1:S61–5. [DOI] [PubMed] [Google Scholar]
- Taber HW, Mueller JP, Miller PFet al. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987;51:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiff HE, Baker T, Copeland Tet al. Locating essential Escherichiacoli genes by using mini-Tn10 transposons: the pdxJ operon. J Bacteriol. 1992;174:1544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansirichaiya S, Rahman MdA, Roberts AP. The transposon registry. Mobile DNA. 2019;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatum EL, Beadle GW. Genetic control of biochemical reactions in neurospora: an “aminobenzoicless” mutant. Proc Natl Acad Sci. 1942;28:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusov RL, Fedorova ND, Jackson JDet al. The COG database: an updated version includes eukaryotes. BMC Bioinf. 2003;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusov RL, Galperin MY, Natale DAet al. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thng DKH, Toh TB, Chow EK-H. Capitalizing on synthetic lethality of MYC to treat cancer in the digital age. Trends Pharmacol Sci. 2021. [DOI] [PubMed] [Google Scholar]
- Todor H, Silvis MR, Osadnik Het al. Bacterial CRISPR screens for gene function. Curr Opin Microbiol. 2021;59:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AHY, Evangelista M, Parsons ABet al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science. 2001;294:2364–8. [DOI] [PubMed] [Google Scholar]
- Tong M, French S, Zahed SSEet al. Gene dispensability in Escherichiacoli grown in thirty different carbon environments. MBio. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Charusanti P, Zhang Let al. CRISPR-Cas9 based engineering of actinomycetal genomes. ACS Synthetic Biology. 2015;4:1020–9. [DOI] [PubMed] [Google Scholar]
- Turner KH, Everett J, Trivedi Uet al. Requirements for Pseudomonasaeruginosa acute burn and chronic surgical wound infection. PLos Genet. 2014;10:e1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KH, Wessel AK, Palmer GCet al. Essential genome of Pseudomonasaeruginosa in cystic fibrosis sputum. Proc Natl Acad Sci. 2015;112:4110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers M, Wright GD. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat Rev Microbiol. 2019;17:141. [DOI] [PubMed] [Google Scholar]
- Typas A, Nichols RJ, Siegele DAet al. High-throughput, quantitative analyses of genetic interactions in e. coli. Nat Methods. 2008;5:781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umland TC, Schultz LW, MacDonald Uet al. In vivo-validated essential genes identified in Acinetobacterbaumannii by using human ascites overlap poorly with essential genes detected on laboratory media. MBio. 2012;3. DOI: 10.1128/mBio.00113-12. Print 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino MD, Foulston L, Sadaka Aet al. Genes contributing to Staphylococcusaureus fitness in abscess- and infection-related ecologies. MBio. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkin E, Solomon O, Sultan Set al. Genome-wide analysis of fitness data and its application to improve metabolic models. BMC Bioinf. 2018;19:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vries J, Wackernagel W. Integration of foreign DNA during natural transformation of Acinetobacter sp. by homology-facilitated illegitimate recombination. Proc Natl Acad Sci. 2002;99:2094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pöhlmann Ret al. New heterologous modules for classical or PCR-based gene disruptions in Saccharomycescerevisiae. Yeast. 1994;10:1793–808. [DOI] [PubMed] [Google Scholar]
- Walton RT, Christie KA, Whittaker MNet al. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science. 2020;368:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Kuramitsu HK. Inducible antisense RNA expression in the characterization of gene functions in Streptococcusmutans. Infect Immun. 2005;73:3568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Lau CY, Ma Fet al. Genome-wide screen identifies curli amyloid fibril as a bacterial component promoting host neurodegeneration. Proc Natl Acad Sci. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Claveau D, Vaillancourt JPet al. High-frequency transposition for determining antibacterial mode of action. Nat Chem Biol. 2011;7:720–9. [DOI] [PubMed] [Google Scholar]
- Wang H, Gill CJ, Lee SHet al. Discovery of wall teichoic acid inhibitors as potential anti-MRSA beta-lactam combination agents. Chem Biol. 2013;20:272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Guan C, Guo Jet al. Pooled CRISPR interference screening enables genome-scale functional genomics study in bacteria with superior performance. Nat Commun. 2018;9:2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Soni V, Marriner Get al. Mode-of-action profiling reveals glutamine synthetase as a collateral metabolic vulnerability of M. tuberculosis to bedaquiline. Proc Natl Acad Sci. 2019;116:19646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters V, Atenafu EG, Lu Aet al. Chronic Stenotrophomonasmaltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J Cyst Fibros. 2013;12:482–6. [DOI] [PubMed] [Google Scholar]
- Waters V, Yau Y, Prasad Set al. Stenotrophomonas maltophilia in cystic fibrosis. Am J Respir Crit Care Med. 2011;183:635–40. [DOI] [PubMed] [Google Scholar]
- Weber BS, De Jong AM, Guo ABYet al. Genetic and chemical screening in human blood serum reveals unique antibacterial targets and compounds against Klebsiellapneumoniae. Cell Rep. 2020;32:107927. [DOI] [PubMed] [Google Scholar]
- Wei JR, Krishnamoorthy V, Murphy Ket al. Depletion of antibiotic targets has widely varying effects on growth. Proc Natl Acad Sci. 2011;108:4176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhuyzen van der R, Hammons JC, Meier JLet al. The antibiotic CJ-15,801 is an antimetabolite that hijacks and then inhibits CoA biosynthesis. Chem Biol. 2012;19:559–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wet de TJ, Winkler KR, Mhlanga Met al. Arrayed CRISPRi and quantitative imaging describe the morphotypic landscape of essential mycobacterial genes. ELife. 2020;9:e60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore KM, Price MN, Waters RJet al. Rapid quantification of mutant fitness in diverse bacteria by sequencing randomly bar-coded transposons. MBio. 2015;6:e00306–00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Tuberculosis (TB). 2021. [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff Aet al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–6. [DOI] [PubMed] [Google Scholar]
- Wiser MJ, Lenski RE. A comparison of methods to measure fitness in Eschrichiacoli. PLoS One. 2015;10:e0126210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wivagg CN, Wellington S, Gomez JEet al. Loss of a class a penicillin-binding protein alters β-lactam susceptibilities in Mycobacteriumtuberculosis. ACS Infectious Diseases. 2016;2:104–10. [DOI] [PubMed] [Google Scholar]
- Wong YC, Ghany MAE, Naeem Ret al. Candidate essential genes in Burkholderiacenocepacia J2315 identified by genome-wide TraDIS. Frontiers in Microbiology. 2016;7:1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, He L, Cui Pet al. Ranking of persister genes in the same Escherichiacoli genetic background demonstrates varying importance of individual persister genes in tolerance to different antibiotics. Frontiers in Microbiology. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HH, Trawick JD, Haselbeck RJet al. Staphylococcus aureus targetarray: comprehensive differential essential gene expression as a mechanistic tool to profile antibacterials. Antimicrob Agents Chemother. 2010;54:3659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Ge X, Chen Let al. Genome-wide essential gene identification in Streptococcussanguinis. Sci Rep. 2011;1:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Nakahigashi K, Nakamichi Tet al. Update on the keio collection of Escherichiacoli single-gene deletion mutants. Mol Syst Biol. 2009;5:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Niki H, Kato J. Profiling of Escherichiacoli chromosome database. Methods Mol Biol Clifton NJ. 2008;416:385–9. [DOI] [PubMed] [Google Scholar]
- Yang H, Krumholz EW, Brutinel EDet al. Genome-scale metabolic network validation of Shewanellaoneidensis using transposon insertion frequency analysis. PLoS Comput Biol. 2014;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasir M, Turner AK, Bastkowski Set al. TraDIS-Xpress: a high-resolution whole-genome assay identifies novel mechanisms of triclosan action and resistance. Genome Res. 2020;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong HT, Yamamoto N, Takeuchi Ret al. Development of a system for discovery of genetic interactions for essential genes in Escherichiacoli K-12. Genes Genet Syst. 2013;88:233–40. [DOI] [PubMed] [Google Scholar]
- Zafar MA, Wang Y, Hamaguchi Set al. Host-to-host transmission of Streptococcuspneumoniae is driven by its inflammatory toxin, pneumolysin. Cell Host & Microbe. 2017;21:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin MM, Duchange N, Ferrara Pet al. Nucleotide sequence of the metL gene of Escherichiacoli. Its product, the bifunctional aspartokinase ii-homoserine dehydrogenase II, and the bifunctional product of the thrA gene, aspartokinase I-homoserine dehydrogenase i, derive from a common ancestor. J Biol Chem. 1983;258:3028–31. [PubMed] [Google Scholar]
- Zemanick ET, Wagner BD, Robertson CEet al. Airway microbiota across age and disease spectrum in cystic fibrosis. Eur Respir J. 2017;50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Ioerger TR, Huttenhower Cet al. Global assessment of genomic regions required for growth in Mycobacteriumtuberculosis. PLoS Pathog. 2012;8:e1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Anderson MT, Wu WTet al. TnseqDiff: identification of conditionally essential genes in transposon sequencing studies. BMC Bioinf. 2017;18:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Green SP, Ge Xet al. Genome-wide identification of Streptococcussanguinis fitness genes in human serum and discovery of potential selective drug targets. Mol Microbiol. 2020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Surujon D, Ortiz-Marquez JCet al. Entropy of a bacterial stress response is a generalizable predictor for fitness and antibiotic sensitivity. Nat Commun. 2020b;11:4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlitni S, Ferruccio LF, Brown ED. Metabolic suppression identifies new antibacterial inhibitors under nutrient limitation. Nat Chem Biol. 2013;9:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A, Burghout P, Bootsma HJet al. ESSENTIALS: software for rapid analysis of high throughput transposon insertion sequencing data. PLoS One. 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.