Abstract
Tissue engineering strategies, notably biomaterials, can be modularly designed and tuned to match specific patient needs. Although many challenges within tissue engineering remain, the incorporation of diagnostic strategies to create theranostic (combined therapy and diagnostic) biomaterials presents a unique platform to provide dual monitoring and treatment capabilities and advance the field toward personalized technologies. In this review, we summarize recent developments in this young field of regenerative theranostics and discuss the clinical potential and outlook of these systems from a tissue engineering perspective. As the need for precision and personalized medicines continues to increase to address diseases in all tissues in a patient-specific manner, we envision that such theranostic platforms can serve these needs.
Keywords: Tissue engineering, Theranostics, Biomaterials, Nanoparticles, Scaffolds, Cell-based devices
Introduction
In 2017, the National Academy of Engineering identified the need to engineer better medicines as one of the fourteen grand engineering challenges [1]. This call for the development of precision and personalized medicines to diagnose and tailor therapies toward individual patient biology has been echoed in recent years as the next frontier of medicine [2,3]. Tissue engineering strategies can be used to address patient needs through the rational design of biomaterials that promote tissue-specific functions in the body [4]. In particular, these strategies can drive in situ tissue engineering by leveraging the body’s innate cellular populations to drive this regeneration without ex vivo cell manipulation [5]. Tissue engineering still faces many challenges [6-9], notably the difficulty to monitor treatment processes. Personalized medicine technologies capable of monitoring and tracking treatment efficacy and disease conditions are vital to ensure their success. By integrating diagnostic modalities with a therapeutic biomaterial, theranostic biomaterials present an opportunity to integrate precision medicine with tissue engineering [10]. Precisely, these platforms can noninvasively monitor engineered tissues of interest in real time while providing therapeutic cues to promote regeneration and repair. In this review, we examine recent efforts to design theranostic systems for tissue engineering applications, categorized by their material form factor as particles, scaffolds, and cellular or biomolecular platforms. In the following sections, we review the current state of these regenerative and diagnostic platforms.
Nanoparticle and microparticle platforms
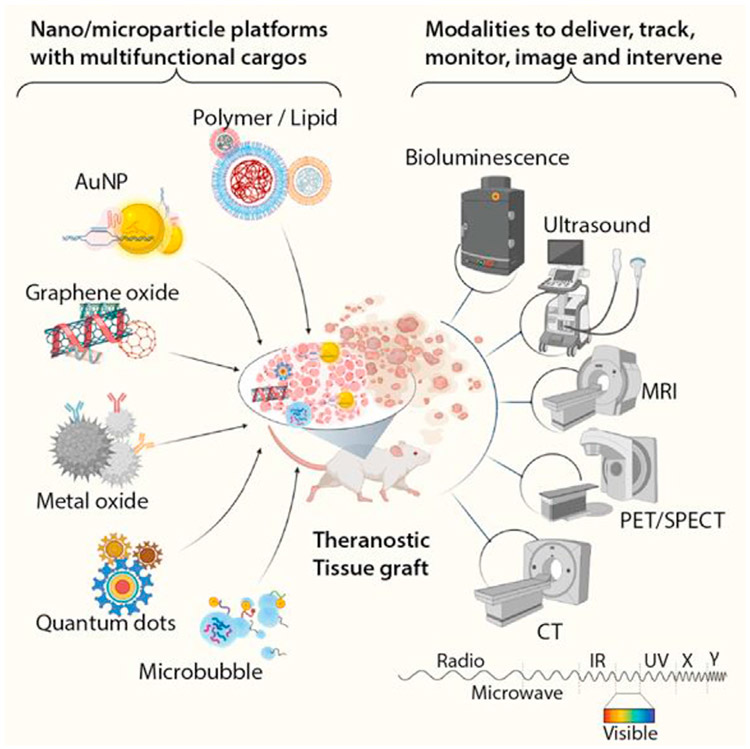
Nanoparticle (NP) systems are among the earliest theranostic systems, dating back to the early 2000s as imaging and delivery systems for photodynamic therapies [11,12]. Since then, significant advances have been made in designing NPs for regenerative medicine [13]. These systems hold immense promise in serving as platforms that advance regenerative medicine through treatment and real-time monitoring. Here, we highlight exciting advances in the design and implementation of NP systems to serve dual functions as therapies and diagnostics in tissue engineering (Figure 1).
Figure 1. Theranostic nanoparticles and microparticles.
Nanoparticles and microparticles can be synthesized using a wide range of suitable biomaterials ranging from metals and graphenes to polymers and lipids. These particles can be functionalized with targeting entities and loaded with therapeutic cargos for systemic or localized delivery. The particles themselves can also serve as contrast agents for various techniques such as ultrasound, bioluminescence, MRI, Positron emission tomography (PET)/Single-photon emission computerized tomopgrahy (SPECT), and CT to achieve the theranostic objectives. AuNP, gold nanoparticle; CT, computed tomography; MRI, magnetic resonance imaging.
Superparamagnetic iron-based metal oxide NPs (SPIONs) have broad implications in various regenerative engineering strategies by delivering cargos to tissues and cells while enhancing magnetic resonance imaging (MRI) signaling contrast to monitor drug therapies, cell migration, and cell viability at sites of interest. This platform served as a promising theranostic when functionalized with micro-ribonucleic acid (miRNA) and small interfering RNA (siRNA) to protect pancreatic islets and increase insulin levels while improving MRI diagnosis of cell viability in vivo [14]. Additional work has shown the ability of SPIONs to monitor immune cells and induce cholesterol efflux via high-density lipoprotein coatings to reduce atherosclerosis plaques [15]. More recently, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-CRISPR associated (Cas)9 gene editing systems were coated on SPIONs and delivered with guide RNA and donor RNA into cells in vitro, demonstrating a promising alternative strategy to traditional transfection approaches [16]. In a similar approach, Zhang et al. [17] developed SPIONs coated with nanoscale graphene oxide to label dental pulp stem cells and organize cell sheets and growth factors into various patterns using magnetic fields for improved bone formation in vivo. The magnetic NP-based system used in these studies can have extended use in MRI diagnostic systems for other intracellular delivery applications. In another set of studies, the intrinsic magnetic properties of NPs were leveraged to manipulate cell fate and monitor migration behavior. Singh et al. [18] developed a novel microrobot platform derived from human hair, termed Hairbot, that was coated with SPIONs for potential bone healing applications. Under magnetic stimulation, the Hairbots induced osteogenesis of mesenchymal stem cells and enhanced ultrasound imaging via the empty medullary cavity within the hair. In addition, Hairbots could be loaded with doxorubicin and exhibited sustained release and subsequent cell death, highlighting their therapeutic potential in other clinical applications [18]. Interestingly, Sejesh et al. [19] were able to demonstrate that iron-doped nano hydroxyapatite magnetic NPs could emulate SPIONs and provide adequate T2-weighted MRI contrast that allowed for evaluation of cell migration and matrix deposition overtime in rat calvarial bone defect models. Although these studies highlight the most recent efforts in regenerative theranostics using iron-based metal oxide NPs, readers are referred to another comprehensive review that highlights their role in clinical practice [20].
In addition to SPION-based systems, other NP platforms have also demonstrated theranostic potential in a variety of tissue engineering applications. Gold NPs (AuNPs) have shown promise for such systems as the tunability of their surface chemistry facilitates specialized loading of cargo while the particles themselves can function as contrast agents for computed tomography (CT) or near-infrared (NIR) imaging. These systems have demonstrated recent success in gene delivery for musculoskeletal repair [21] and in delivery of therapeutics to combat acute liver failure [22]. Lee et al. [21] developed one such system described as CRISPR-Gold, using an AuNP core conjugated with donor DNA to load guide RNA–Cas9 complexes. This complex was encapsulated in a cationic polymer, promoting cellular uptake and endosomal escape of CRISPR-Gold. This system demonstrated efficient delivery with minimal off-target editing, decreased muscle fibrosis, and promoted the full functional expression of dystrophin in mouse models of Duchenne muscular dystrophy [20]. Other inorganic particle systems capable of NIR visualization have also shown efficacy in targeting and delivering therapeutics to tissues of interest [22-25]. One study showcases the ability of multifunctional quantum dot–based systems to track and monitor cardiovascular disease. In this system, quantum dots were encapsulated alongside an antithrombotic agent within a peptide NP shell based on simian virus 40. By including peptide sequences to target components of atherosclerotic plaques, NIR fluorescence of the quantum dots allowed them to visualize the location and maturity of plaques while also facilitating localized delivery of therapeutic molecules [23].
Polymer NPs present a class of highly modular synthetic platforms that can be engineered for regenerative medicine and diagnostics. The tunability of polymer chemistry alongside the potential for postsynthesis modification allows for a myriad of approaches to target, treat, and visualize tissues and cells of interest [26-32]. One interesting trend observed in polymer NPs is their use as a photoacoustic contrast agent. In one study, ketalized maltodextrin NPs served as an ultrasound contrast agent in acidic inflammatory conditions through hydrolytic degradation and production of carbon dioxide bubbles. This system also demonstrated a pH-responsive delivery of anti-inflammatory therapeutics to minimize hepatic damage [27]. Other NP systems have similarly harnessed the potential of photoacoustic imaging for antithrombotic therapies and rheumatoid arthritis, allowing for real-time monitoring of disease progression throughout the time course of treatment [27,28,30,31]. Chen et al. [28] demonstrated this very nicely with their polymer nanoprobe system, conjugating an antirheumatic antibody to target and treat arthritic joints (Figure 2a). Disease progression was monitored over the course of months using NIR-II photoacoustic tomography, with results consistent with traditional clinical micro-CT approaches [28].
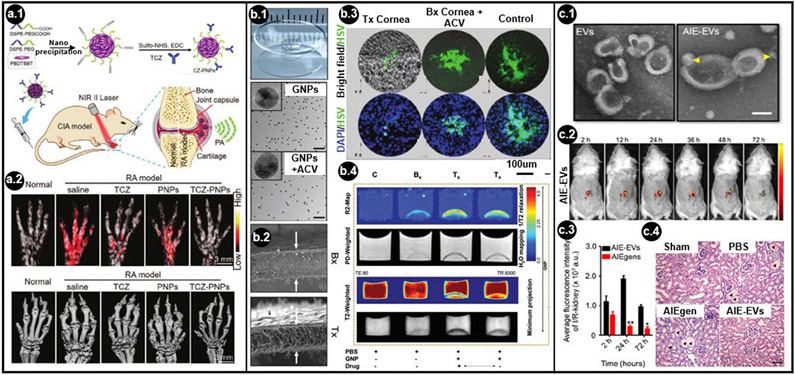
Figure 2. Applications of theranostic nanoparticles, scaffolds, and cell-derived platforms.
(a.1) Schematic representation of a polymer nanoparticle (PNP) conjugated with an antirheumatic targeted drug, tocilizumab (TCZ), for near-infrared (NIR) II photoacoustic (PA) imaging and treatment of rheumatoid arthritis (RA). (a.2) Maximum amplitude projection PA (top) and micro-CT (bottom) images of RA animal forepaws at day 57 with and without TCZ PNP treatment (adapted with permission from Go et al. [27]. Copyright (2021) John Wiley and Sons.). (b.1) Representative images of cornea collagen implants, gold nanoparticles (GNPs), and GNPs conjugated with acyclovir (ACV). (b.2) Cross-sectional backscattered electron images of unmodified implants (Bx) and implants with ACV-conjugated GNPs (Tx) (white arrows). (b.3). Immunostained herpes simplex virus type 1 treated with Tx implants, Bx + ACV, or Bx. (b.4) Scans of Bx and Tx implants with MRI in transverse relaxivity (R2), proton density–weighted, and transverse relaxation (T2)–weighted modes with and without ACV (bottom arrow) (adapted with permission from Moroni et al. [39]. Copyright (2021) John Wiley and Sons.). (c.1) Representative transmission electron images of extracellular vesicles (EVs) without aggregation-induced emission luminogens (AIEgens) and with AIEgens (AIE-EVs) (yellow arrowheads) to treat renal ischemia-reperfusion injury (I/IR-kidney). (c.2) In vivo imaging of AIE-EVs in a mouse model of acute kidney injury over 72 h. (c.3) Quantification of AIEgens and AIE-EVs from the I/IR-kidney. (c.4) Micrograph for hematoxylin and eosin staining of kidney sections on day 5 after treatment with AIEgens or AIE-EVs. Black arrowheads indicate tubular protein cast and the necrotic area (adapted with permission from Saxena et al. [59]. Copyright (2021) American Chemical Society.). CT, computed tomography; MRI, magnetic resonance imaging.
Microbubbles are a unique class of particles that also harness photoacoustic properties and have recently demonstrated theranostic potential for tissue engineering [33]. These colloidal systems composed of amphiphilic molecules can carry essential nutrients or therapeutic molecules to regions of interest, while their gaseous core also serves as a contrast enhancer for ultrasound-based imaging and therapies [34,35]. For example, in one study, microbubbles with fibrin-targeting peptide sequences allowed for the monitoring of surgical adhesion sites and enabled disruption of fibrin clots without the need for a secondary surgery [36].
Scaffolds and hydrogels
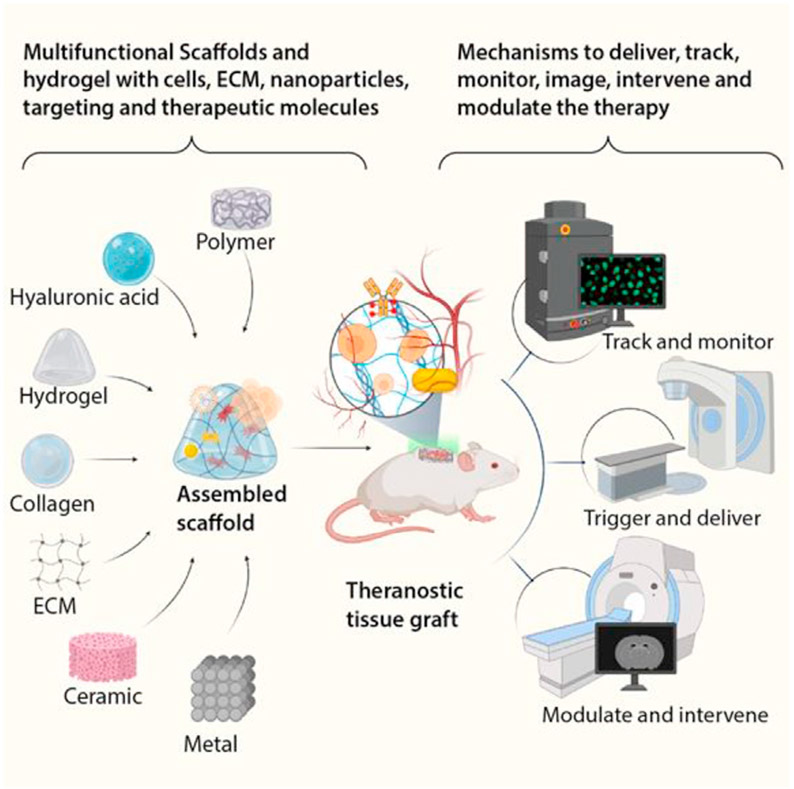
Scaffolds have been a key strategy for tissue engineering ever since the field was first coined around the 1990s [37]. This broad class of materials provides structural support and biochemical signals to cells to promote native tissue growth and regeneration [38,39]. However, there are limited ways to noninvasively evaluate the success of these scaffolds in vivo within the context of their degradation and interaction with native tissue. We will now explore recent approaches to incorporate diagnostic modalities into scaffolds, allowing for real-time monitoring and imaging to assess the functionality of these constructs and their surrounding tissue (Figure 3).
Figure 3. Scaffolds and hydrogel platforms.
Scaffolds and hydrogels can be made from a multitude of suitable biomaterials including natural and synthetic polymers, components of the extracellular matrix, ceramics, and metals. The scaffolds by themselves and in combination with engineered cells, nanomaterials/micromaterials, and therapeutic molecules can be used as theranostic tissue-engineered grafts. They, in turn, can track and monitor for any anomalies associated with grafts, intervene by releasing therapeutic molecules, and perform the therapeutic course correction. These real-time monitoring approaches guide the decision-making process of whether to continue or discontinue the grafted tissue.
Collagen and other natural polymers, such as gelatin and hyaluronic acid (HA), are extensively used as scaffolds owing to their biocompatibility and ability to closely recapitulate native tissue structures and biophysical cues. Recent work has shown that incorporating diagnostic monitoring with these materials does not impede their functionality and enables noninvasive tracking of degradation and incorporation into native tissues [40,41]. The incorporation of SPIONs as an MRI contrast agent in these natural polymer scaffolds has been a recent trend [41-43]. Composite gelatin and SPION scaffolds demonstrate promise and show synergistic effects that achieve greater new bone growth than control gelatin scaffolds [41]. Furthermore, drug release from AuNPs and degradation of collagen scaffolds can be monitored in real time through the inclusion of SPIONs, highlighting the potential for such scaffolds to function as corneal implants (Figure 2b). Incorporation of these modalities affects neither the optical clarity of these scaffolds nor cellular function, while successfully being able to monitor drug release for up to 60 days via MRI transverse relaxivity mapping [42]. In an alternative approach, Janke et al. [40] used a novel hemin–l-lysine complex as an MRI contrast agent to label type I collagen–based scaffolds of various morphologies. These scaffolds showed excellent biocompatibility in vivo and enhanced MRI contrast over twelve weeks compared with their unlabeled counterparts [40]. In addition, other imaging modalities have been incorporated into natural polymer scaffolds. Tunable HA and gelatin scaffolds modified with fluorophores for multispectral NIR imaging can monitor neural proliferation and track scaffold degradation while recapitulating native brain tissue composition [44]. Another study also expanded the abilities of injectable HA systems for cardiac applications by loading and retaining the CT contrast agent Iohexol to improve guided injection and prolong detection of infarcted tissue (Avendano et al. Abstract 14819, AHA Scientific Sessions, Philadelphia, November 2019).
Beyond natural polymers, ceramic and synthetic scaffolds can also incorporate imaging agents to allow for monitoring of tissue regeneration. Although ceramic-based systems are often used in scenarios involving mineralized tissue regeneration, they provide limited contrast between the material and mineralized tissue, which prevents accurate engraftment monitoring. One study aimed to address these limitations through the use of calcium phosphate cement labeled with fluorine isotope–loaded poly(lactic-co-glycolic acid) (PLGA) NPs and AuNPs to provide MRI and CT contrast, respectively, without affecting bone formation [45]. Of particular interest for therapeutic strategies, the incorporation of SPIONs can synergistically improve osteogenesis [17,18,41,45,46]. Hao et al. [46] found that nanofibrous composite scaffolds of poly(lactic acid), hydroxyapatite, and SPIONs demonstrated the highest osteogenic enhancement with magnetic stimulation compared with varying gelatin and polyurethane composite scaffolds with different microstructures. Although this study did not address the composite scaffolds as diagnostic systems, it provides a valuable pipeline for the development of future osteogenic scaffolds containing SPION contrast agents for theranostic applications. Mastrogiacomo et al. [45] developed SPIONs coated with AuNPs in a silica matrix functionalized with calcium phosphate cements and Bone Morphogenetic Protein-2 to improve imaging and repair of mineralized dentin tissue. The composite scaffold improved MRI and CT imaging ex vivo in human molars as well as improved dentin repair and scaffold evaluation up to 7 weeks in vivo in a goat dentin defect model, highlighting the potential of such composite scaffolds for dental pulp capping [45].
Using synthetic and natural scaffolds as a foundation, microelectronic systems have been incorporated to synergistically monitor electrical signals and improve tissue function in bioelectric organs. Feiner et al. [47,48] used electrospinning to create two generations of flexible cardiac patch scaffolds with electrical signal monitoring. The first-generation system used polycaprolactone nanofiber–coated microelectronics [47], with the group subsequently switching to a biodegradable albumin nanofiber system with deposited gold electrodes in the second generation [48]. These scaffolds promoted alignment and striation of cardiomyocytes without impacting viability, with folded configurations of the scaffolds enabling sensing and interactions between multiple layers of cells. In both scaffolds, electrodes provided high-fidelity monitoring of extracellular electrical signals indicative of spontaneous cardiac tissue beating. Furthermore, the presence of electroactive polypyrrole on these electrodes enabled the release of negatively charged drugs upon stimulation without interfering with cardiomyocyte function, enabling remote treatment of cardiac dysfunction based on measured cardiac performance.
Cell and biomolecular platforms
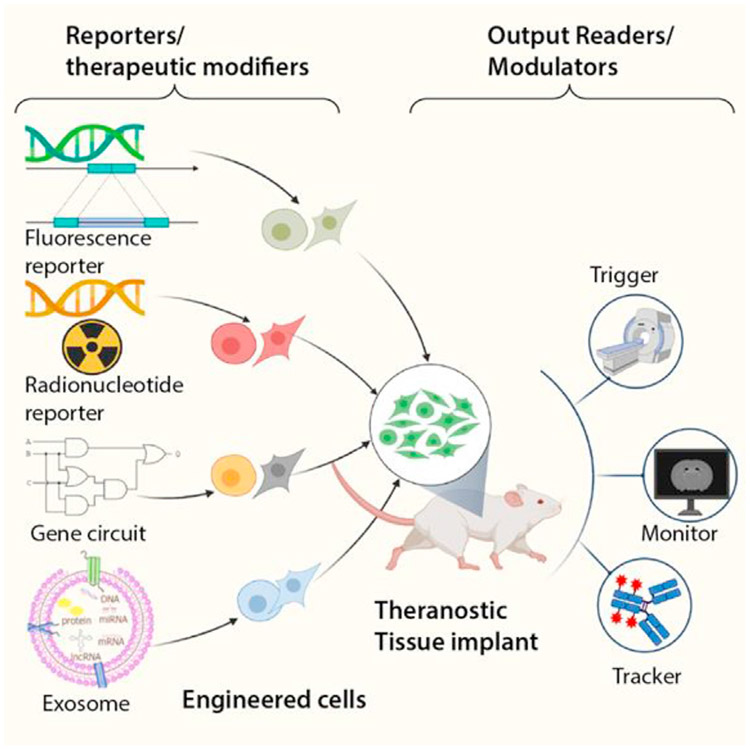
Cells are complex in nature and create a plethora of endogenous biomolecules that maintain their function and survival and allow communication between other cells. As such, cells, biomolecules, and extracellular vesicles (EVs) are powerful paradigms to help recover function of damaged tissues in situ without relying on host-driven regeneration [49,50]. Significant advances have been made in cell and biomolecular platforms from a therapeutic standpoint [51], but it remains very difficult to monitor their functionality and precisely track these systems in vivo for extended periods of time. Intrinsic and extrinsic imaging agents have recently improved the therapeutic efficacy and diagnostic capabilities of transplanted cells and their derived factors by modifying their core architecture. Here, we review recent innovations that show how cellular and biomolecular tools have been leveraged to track and evaluate cell therapies (Figure 4).
Figure 4. Cell and biomolecular platforms.
Cells and biomolecules can be engineered to provide diagnostic output alongside their innate biological capacity. This can be achieved by through genetic engineering in fluorescent or radionucleotide reporters, building synthetic gene circuits, or using exosomes loaded with intervening nucleotide sequences or proteins. These platforms allow for synchronous diagnostics and autonomous regulation of corrective therapeutic actions.
Cellular therapies are uniquely positioned as theranostics to be genetically modified for the production and expression of imaging markers without compromising normal cellular function. These systems have demonstrated the capacity to monitor cell engraftment and differentiation upon transplantation [52-54]. In one such study, lentiviral transduction drove the expression of a radionucleotide sodium iodide symporter gene in human-induced pluripotent stem cells without affecting the differentiation process to hepatocyte-like cells. This allowed for live-cell tracking via Single-photon emission computerized tomopgrahy (SPECT)/CT after injection without any impact on cell survival in vivo [53]. Similarly, Morikawa et al. [52] established a dual green fluorescence protein/luciferase transgenic rodent reporter system that allowed for visualization of the location and fate of labeled stem cells isolated and transplanted from these reporters. Cells have also been labeled through more traditional approaches, using microelectrodes to monitor neural stem cell differentiation [55], fluorescent small molecules to identify pluripotent stem cells [56], and micron-sized SPIONs to visualize and monitor bioprinted cell tissues [57].
Beyond tracking cellular incorporation and differentiation, theranostics have also demonstrated the ability to report biochemical and mechanical signals. These signals from the microenvironment play key roles in regulating tissue function, and recent advances have allowed for the identification and, in some cases, treatment of diseased tissue. One such approach has used gene circuits to create cell-based biosensors [58,59]. Of note, Smole et al. [58] designed an autonomous anti-inflammatory gene circuit, in which inflammatory signaling was used to drive fluorescent reporter production alongside anti-inflammatory proteins to minimize dysregulated and chronic inflammation. Treatment with this gene circuit system in a murine model of colitis led to retention of healthy tissue morphology through reduction of disease activity and minimization of inflammatory damage [58]. Biomolecular sensors have also demonstrated microenvironment detection capabilities, notably in the evaluation of mechanical strain. Shiwarski et al. [60] developed a fluorescently labeled fibronectin nanomechanical biosensor to map strain on 3D surfaces, including single cells, cell monolayers, and developing ovarioles in Drosophila. Notably, this system was able to monitor cardiomyocyte beat frequency and regional dilation, highlighting its potential to detect impaired force generation of dilated cardiomyopathy and correspondingly guide regenerative therapies [60].
Exosomes and EVs have recently been harnessed as theranostics owing to their role in paracrine signaling. These cell-derived platforms carry biomolecular cargo that can be leveraged for regenerative purposes and labeled to determine their fate and therapeutic efficacy. Stem cell–derived exosomes and EVs have demonstrated such therapeutic potential, promoting angiogenesis and osteogenesis [61-63], as well as recovery from acute liver and kidney injuries [64,65]. Cao et al. [64] used electrostatic interactions to label the negatively charged lipid membranes of stem cell EVs with a novel positively charged fluorophore and tracked these EVs through traditional fluorescence. These fluorophores demonstrated superior retention and tracking of EVs compared with commonly used EV dyes, with no influence on the native EV structure and function and no cytotoxic effects in vivo. EVs could be tracked for 12 days and demonstrated anti-inflammatory and proliferative effects in models of acute liver disease [64]. In a followup study, these EVs also demonstrated renoprotective capabilities by preserving mitochondrial function while providing strong spatiotemporal fluorescence resolution (Figure 2c) [65]. These studies highlight the potential of cell-derived and biomolecular systems to not only improve tissue regeneration but also serve as diagnostic modalities to monitor their distribution and toxicity.
Future perspective/conclusion
Engineered platforms with diagnostic and regenerative capabilities have advanced the ability to monitor the repair of tissues of interest as well as biomaterial platforms and their payloads, but many classical challenges of tissue engineering remain. As described in this short review, the past several years have seen innovative development of novel theranostics such as NPs, scaffolds, and cell-based platforms. These studies have largely focused on the fabrication and characterization of these platforms, with some exploration into their in vivo functionality. However, further work remains to ensure that these systems are translatable. Theranostic platforms must be thoroughly investigated in preclinical models with longitudinal studies to assess toxicity, engraftment, and regenerative capacity to ensure adequate safety profiles for potential clinical use. These platforms must also demonstrate extended functionality and retention, especially when treating chronic diseases.
The grand challenge of engineering better medicines remains unsolved. Additional technological development and integration are needed to implement personalized biomaterial theranostics at increasing levels of structural and functional complexity for tissue engineering applications. Despite being a relatively young field, regenerative theranostics boasts a significant amount of work showing the promising potential of these systems in various applications, including bone defects, liver disease, cardiac dysfunction, and pancreatic islet regeneration. In the long term, we envision that regenerative theranostics holds immense potential as personalized medicine platforms, enabling patient-specific treatment plans based on diagnostic output and improving patient outcomes.
Acknowledgements
This work was supported in part by awards from the Diabetes Research Connections (Early Career Award A135548 to BNK), the National Science Foundation (Graduate Research Fellowship 1650113 to JXZ), UCSF School of Dentistry (Resource Allocation Program to DLC and TAD), the National GEM Consortium (full fellowship to CAP), and the National Institutes of Health (HL137209 and GM008155 to TAD). The authors would like to thank Gauree S. Chendke, Priya Mohindra, and Tannia M. Rodriguez for reviewing the manuscript.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.NAE: Grand challenges - 14 grand challenges for engineering. Natl Acad Eng 2019. [Google Scholar]
- 2.Collins FS, Varmus H: A new initiative on precision medicine. N Engl J Med 2015, 372:793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho D, Quake SR, McCabe ERB, Chng WJ, Chow EK, Ding X, Gelb BD, Ginsburg GS, Hassenstab J, Ho CM, et al. : Enabling technologies for personalized and precision medicine. Trends Biotechnol 2020, 38:497–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguado BA, Grim JC, Rosales AM, Watson-Capps JJ, Anseth KS: Engineering precision biomaterials for personalized medicine. Sci Transl Med 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaharwar AK, Singh I, Khademhosseini A: Engineered biomaterials for in situ tissue regeneration. Nat Rev Mater 2020, 5:686–705. [Google Scholar]
- 6.Grounds MD: Obstacles and challenges for tissue engineering and regenerative medicine: Australian nuances. Clin Exp Pharmacol Physiol 2018, 45:390–400. [DOI] [PubMed] [Google Scholar]
- 7.Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, Pillay M, Motaung KSCM: Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int 2018, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kharbikar BN, Chendke GS, Desai TA: Modulating the foreign body response of implants for diabetes treatment. Adv Drug Deliv Rev 2021. 10.1016/j.addr.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sneddon JB, Tang Q, Stock P, Bluestone JA, Roy S, Desai T, Hebrok M: Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell 2018. 10.1016/j.stem.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong JPK, Stevens MM: Emerging technologies for tissue engineering: from gene editing to personalized medicine. Tissue Eng Part A 2019, 25:688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy JR, Jaffer FA, Weissleder R: A macrophage-targeted theranostic nanoparticle for biomedical applications. Small 2006, 2:983–987. [DOI] [PubMed] [Google Scholar]
- 12.Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, Dougherty TJ, Prasad PN: Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: a novel drug-carrier system for photodynamic therapy. J Am Chem Soc 2003, 125:7860–7865. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R: Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2021, 20:101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pomposelli T, Wang P, Takeuchi K, Miyake K, Ariyoshi Y, Watanabe H, Chen X, Shimizu A, Robertson N, Yamada K, et al. : Protection of pancreatic islets using theranostic silencing nanoparticles in a baboon model of islet transplantation. Diabetes 2020, 69:2414–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandwana V, Ryoo SR, Kanthala S, McMahon KM, Rink JS, Li Y, Venkatraman SS, Thaxton CS, Dravid VP: High-density lipoprotein-like magnetic nanostructures (HDL-MNS): theranostic agents for cardiovascular disease. Chem Mater 2017, 29:2276–2282. [Google Scholar]
- 16.Rohiwal SS, Dvorakova N, Klima J, Vaskovicova M, Senigl F, Slouf M, Pavlova E, Stepanek P, Babuka D, Benes H, et al. : Polyethylenimine based magnetic nanoparticles mediated non-viral CRISPR/Cas9 system for genome editing. Sci Rep 2020, 10:4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. •. Zhang W, Yang G, Wang X, Jiang L, Jiang F, Li G, Zhang Z, Jiang X: Magnetically controlled growth-factor-immobilized multilayer cell sheets for complex tissue regeneration. Adv Mater 2017, 29. Iron-doped hydroxyapatite magnetic nanoparticles functionalized alginate-gelatin scaffolds and improved non-invasive MRI and bone tissue regeneration
- 18.Singh AV, Dad Ansari MH, Dayan CB, Giltinan J, Wang S, Yu Y, Kishore V, Laux P, Luch A, Sitti M: Multifunctional magnetic hairbot for untethered osteogenesis, ultrasound contrast imaging and drug delivery. Biomaterials 2019, 219. [DOI] [PubMed] [Google Scholar]
- 19.Sajesh KM, Ashokan A, Gowd GS, Sivanarayanan TB, Unni AKK, Nair SV, Koyakutty M: Magnetic 3D scaffold: a theranostic tool for tissue regeneration and non-invasive imaging in vivo. Nanomed Nanotechnol Biol Med 2019, 18:179–188. [DOI] [PubMed] [Google Scholar]
- 20. •. Dadfar SM, Roemhild K, Drude NI, von Stillfried S, Knuchel R, Kiessling F, Lammers T: Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev 2019, 138:302–325. Functionalized gold nanoparticles facilitated non-viral delivery of CRISPR-Cas9 systems to restore muscle strength in Duchenne muscular dystrophy.
- 21.Lee K, Conboy M, Park HM, Jiang F, Kim HJ, Dewitt MA, Mackley VA, Chang K, Rao A, Skinner C, et al. : Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat Biomed Eng 2017, 1:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Li Y, Li W, Xiao C, Liu D, Dong C, Zhang M, Makila E, Kemell M, Salonen J, et al. : Multifunctional nanohybrid based on porous silicon nanoparticles, gold nanoparticles, and acetalated dextran for liver regeneration and acute liver failure theranostics. Adv Mater 2018, 30, e1703393. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Li W, Zhang X, Qi M, Zhang Z, Zhang XE, Cui Z: In vivo targeting and imaging of atherosclerosis using multifunctional virus-like particles of simian virus 40. Nano Lett 2016, 16:6164–6171. [DOI] [PubMed] [Google Scholar]
- 24.Parodi A, Evangelopoulos M, Arrighetti N, Cevenini A, Livingston M, Khaled SZ, Brown BS, Yazdi IK, Paradiso F, Campa-Carranza JN, et al. : Endosomal escape of polymercoated silica nanoparticles in endothelial cells. Small 2020, 16. [DOI] [PubMed] [Google Scholar]
- 25.Khaled SZ, Cevenini A, Yazdi IK, Parodi A, Evangelopoulos M, Corbo C, Scaria S, Hu Y, Haddix SG, Corradetti B, et al. : One-pot synthesis of pH-responsive hybrid nanogel particles for the intracellular delivery of small interfering RNA. Biomaterials 2016, 87:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan C, Lin G, Huang Y, Wang Z, Zeng F, Wu S: A dopamine-precursor-based nanoprodrug for in-situ drug release and treatment of acute liver failure by inhibiting NLRP3 inflammasome and facilitating liver regeneration. Biomaterials 2021, 268:120573. [DOI] [PubMed] [Google Scholar]
- 27. ••. Go Y, Lee H, Jeong L, Sun S, Hong E, Jung E, Ko C, Noh J, Park S, Lee M, et al. : Acid-triggered echogenic nanoparticles for contrast-enhanced ultrasound imaging and therapy of acute liver failure. Biomaterials 2018, 186:22–30. This study demonstrated the use of acid-mediated hydrolysis to generate carbon dioxide bubbles from polymer nanoparticles, allowing for ultrasound imaging alongside pH responsive delivery of an anti-inflammatory.
- 28. •. Chen J, Qi J, Chen C, Chen J, Liu L, Gao R, Zhang T, Song L, Ding D, Zhang P, et al. : Tocilizumab-conjugated polymer nanoparticles for NIR-II photoacoustic-imaging-guided therapy of rheumatoid arthritis. Adv Mater 2020, 32, e2003399. Polymer nanoparticles conjugated with monoclonal antibody, tozilizumab, targeted and suppressed rheumatoid arthritis in mouse animal models and provided high signal-to-noise ratios of non-invasive photoacoustic imaging of the diseased tissue.
- 29.Evans MA, Huang PJ, Iwamoto Y, Ibsen KN, Chan EM, Hitomi Y, Ford PC, Mitragotri S: Macrophage-mediated delivery of light activated nitric oxide prodrugs with spatial, temporal and concentration control. Chem Sci 2018, 9:3729–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juenet M, Aid-Launais R, Li B, Berger A, Aerts J, Ollivier V, Nicoletti A, Letourneur D, Chauvierre C: Thrombolytic therapy based on fucoidan-functionalized polymer nanoparticles targeting P-selectin. Biomaterials 2018, 156:204–216. [DOI] [PubMed] [Google Scholar]
- 31.Jung E, Kang C, Lee J, Yoo D, Hwang DW, Kim D, Park SC, Lim SK, Song C, Lee D: Molecularly engineered theranostic nanoparticles for thrombosed vessels: H2O2-activatable contrast-enhanced photoacoustic imaging and antithrombotic therapy. ACS Nano 2018, 12:392–401. [DOI] [PubMed] [Google Scholar]
- 32.Clegg JR, Irani AS, Ander EW, Ludolph CM, Venkataraman AK, Zhong JX, Peppas NA: Synthetic networks with tunable responsiveness, biodegradation, and molecular recognition for precision medicine applications. Sci Adv 2019, 5, eaax7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulsipher KW, Hammer DA, Lee D, Sehgal CM: Engineering theranostic microbubbles using microfluidics for ultrasound imaging and therapy: a review. Ultrasound Med Biol 2018, 44: 2441–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osborn J, Aliabouzar M, Zhou X, Rao R, Zhang LG, Sarkar K: Enhanced osteogenic differentiation of human mesenchymal stem cells using microbubbles and low intensity pulsed ultrasound on 3D printed scaffolds. Adv Biosyst 2019, 3, e1800257. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Liu Q, Hu J, Asenso J, Wise MJ, Wu X, Ma C, Chen X, Yang J, Tang D: Structure and evolution of glycogen branching enzyme N-termini from bacteria. Front Microbiol 2018, 9:3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. •. Gormley CA, Keenan BJ, Buczek-Thomas JA, Pessoa A, Xu J, Monti F, Tabeling P, Holt RG, Nagy JO, Wong JY: Fibrin-targeted polymerized shell microbubbles as potential theranostic agents for surgical adhesions. Langmuir 2019, 35:10061–10067. The development of fibrin-targeting microbubbles allows for the monitoring and in situ disruption of fibrin clots without secondary surgery.
- 37.Langer R, Vacanti JP: Tissue engineering. Science 1993, 260:920–926. [DOI] [PubMed] [Google Scholar]
- 38.Hussey GS, Dziki JL, Badylak SF: Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater 2018, 3:159–173. [Google Scholar]
- 39.Moroni L, Burdick JA, Highley C, Lee SJ, Morimoto Y, Takeuchi S, Yoo JJ: Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater 2018, 3:21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. ••. Janke HP, Guvener N, Dou W, Tiemessen DM, YantiSetiasti A, Cremers JGO, Borm PJA, Feitz WFJ, Heerschap A, Kiessling F, et al. : Labeling of collagen type I templates with a naturally derived contrast agent for noninvasive MR imaging in soft tissue engineering. Adv Heal Mater 2018, 7, e1800605. Naturally-derived hemin-l-lysine complexes conjugated to collagen type I scaffolds enhanced non-invasive MRI, tissue remodeling, and vascularization after subcutaneous implantation.
- 41.Hu S, Zhou Y, Zhao Y, Xu Y, Zhang F, Gu N, Ma J, Reynolds MA, Xia Y, Xu HHK: Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J Tissue Eng Regen Med 2018, 12:e2085–e2098. [DOI] [PubMed] [Google Scholar]
- 42. ••. Patra HK, Azharuddin M, Islam MM, Papapavlou G, Deb S, Osterrieth J, Zhu GH, Romu T, Dhara AK, Jafari MJ, et al. : Rational nanotoolbox with theranostic potential for medicated pro-regenerative corneal implants. Adv Funct Mater 2019, 29:1903760. Incorporation of therapeutic-loaded gold nanoparticles and iron oxide nanoparticles into collagen scaffolds allowed for real-time monitoring of drug elution and scaffold degradation.
- 43.Zhou S, Yang R, Zou Q, Zhang K, Yin T, Zhao W, Shapter JG, Gao G, Fu Q: Fabrication of tissue-engineered bionic urethra using cell sheet technology and labeling by ultrasmall superparamagnetic iron oxide for full-thickness urethral reconstruction. Theranostics 2017, 7:2509–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park GK, Kim SH, Kim K, Das P, Kim BG, Kashiwagi S, Choi HS, Hwang NS: Dual-channel fluorescence imaging of hydrogel degradation and tissue regeneration in the brain. Theranostics 2019, 9:4255–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mastrogiacomo S, Guvener N, Dou W, Alghamdi HS, Camargo WA, Cremers JGO, Borm PJA, Heerschap A, Oosterwijk E, Jansen JA, et al. : A theranostic dental pulp capping agent with improved MRI and CT contrast and biological properties. Acta Biomater 2017, 62:340–351. [DOI] [PubMed] [Google Scholar]
- 46.Hao S, Shen Y, Wu H, Meng J, Xie L, Wen T, Gu N, Liu J, Zhang Y, Xu H: Modulatory effects of the composition and structure on the osteogenic enhancement for superparamagnetic scaffolds. Eng Sci 2018. 10.30919/es8d782. [DOI] [Google Scholar]
- 47. ••. Feiner R, Engel L, Fleischer S, Malki M, Gal I, Shapira A, Shacham-Diamand Y, Dvir T: Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat Mater 2016, 15:679–685. This study showcased the use of flexible polymer scaffolds integrated with microelectronics and electroactive polymers to provide therapeutic advantages for cardic function.
- 48. •. Feiner R, Fleischer S, Shapira A, Kalish O, Dvir T: Multifunctional degradable electronic scaffolds for cardiac tissue engineering. J Control Release 2018, 281:189–195. Electrospun albumin scaffolds with deposited gold electrodes provide cellular support and monitoring of cardiomyocytes while also allowing for externally-initiated release of therapeutics.
- 49.Soekmadji C, Li B, Huang Y, Wang H, An T, Liu C, Pan W, Chen J, Cheung L, Falcon-Perez JM, et al. : The future of Extracellular Vesicles as Theranostics–an ISEV meeting report. J Extracell Vesicles 2020, 9. Taylor and Francis Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray P, De A: Reporter gene imaging in therapy and diagnosis. Theranostics 2012, 2:333–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shafiee A, Atala A: Tissue engineering: toward a new era of medicine. Annu Rev Med 2017, 68:29–40. [DOI] [PubMed] [Google Scholar]
- 52.Morikawa K, Nakamura K, Suyama Y, Yamamoto K, Fukuoka K, Yagi S, Shirayoshi Y, Ohbayashi T, Hisatome I: Novel dual-reporter transgenic rodents enable cell tracking in animal models of stem cell transplantation. Biochem Biophys Rep 2019, 18:100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. •. Ashmore-Harris C, Blackford SJ, Grimsdell B, Kurtys E, Glatz MC, Rashid TS, Fruhwirth GO: Reporter gene-engineering of human induced pluripotent stem cells during differentiation renders in vivo traceable hepatocyte-like cells accessible. Stem Cell Res 2019, 41:101599. Lentiviral gene transfer was used during differentiation of iPSC-derived hepatocyte-like cells, making them traceable in vivo using SPECT/CT.
- 54.Lopez-Yrigoyen M, Fidanza A, Cassetta L, Axton RA, Taylor AH, Meseguer-Ripolles J, Tsakiridis A, Wilson V, Hay DC, Pollard JW, et al. : A human iPSC line capable of differentiating into functional macrophages expressing ZsGreen: a tool for the study and in vivo tracking of therapeutic cells. Philos Trans R Soc Lond B Biol Sci 2018, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seidel D, Obendorf J, Englich B, Jahnke HG, Semkova V, Haupt S, Girard M, Peschanski M, Brüstle O, Robitzki AA: Impedimetric real-time monitoring of neural pluripotent stem cell differentiation process on microelectrode arrays. Biosens Bioelectron 2016, 86:277–286. [DOI] [PubMed] [Google Scholar]
- 56.Park MR, Nam D, Lee H, Chang YT, Zaehres H, Kim JB: Direct monitoring of live human pluripotent stem cells by a highly selective pluripotency sensor. Bioorg Med Chem Lett 2020, 30:127347. [DOI] [PubMed] [Google Scholar]
- 57.Kérourédan O, Ribot E, Fricain JC, Devillard R, Miraux S: Magnetic resonance imaging for tracking cellular patterns obtained by laser-assisted bioprinting. Sci Rep 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. ••. Smole A, Lainscek D, Bezeljak U, Horvat S, Jerala R: A synthetic mammalian therapeutic gene circuit for sensing and suppressing inflammation. Mol Ther 2017, 25:102–119. In this study, a modular genetic circuit was built in mammalian cells and encoded to autonomously activate anti-inflammatory pathways to suppress inflammatory pathology.
- 59.Saxena P, Bojar D, Zulewski H, Fussenegger M: Generation of glucose-sensitive insulin-secreting beta-like cells from human embryonic stem cells by incorporating a synthetic lineage-control network. J Biotechnol 2017, 259:39–45. [DOI] [PubMed] [Google Scholar]
- 60.Shiwarski DJ, Tashman JW, Tsamis A, Bliley JM, Blundon MA, Aranda-Michel E, Jallerat Q, Szymanski JM, McCartney BM, Feinberg AW: Fibronectin-based nanomechanical biosensors to map 3D surface strains in live cells and tissue. Nat Commun 2020, 11:5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hao D, Swindell HS, Ramasubramanian L, Liu R, Lam KS, Farmer DL, Wang A: Extracellular matrix mimicking nanofibrous scaffolds modified with mesenchymal stem cell-derived extracellular vesicles for improved vascularization. Front Bioeng Biotechnol 2020, 8:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, Millard RW, Xiao DS, Ashraf M, Xu M: Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8:45200–45212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diomede F, Gugliandolo A, Cardelli P, Merciaro I, Ettorre V, Traini T, Bedini R, Scionti D, Bramanti A, Nanci A, et al. : Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther 2018, 9:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao H, Yue Z, Gao H, Chen C, Cui K, Zhang K, Cheng Y, Shao G, Kong D, Li Z, et al. : In vivo real-time imaging of extracellular vesicles in liver regeneration via aggregation-induced emission luminogens. ACS Nano 2019, 13:3522–3533. [DOI] [PubMed] [Google Scholar]
- 65. ••. Cao H, Cheng Y, Gao H, Zhuang J, Zhang W, Bian Q, Wang F, Du Y, Li Z, Kong D, et al. : In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia-reperfusion injury. ACS Nano 2020, 14:4014–4026. Aggregation Induced Emission was used for real-time tracking the fate of mesenchymal stem cell EVs in renal tissue repair.