Abstract
Purpose of Review
The management of advanced hepatocellular (HCC) has drastically changed in the past few years with approval of several first line and second line systemic therapies. In this review we present an overview of the recent progress in the treatment of advanced HCC and discuss future perspectives.
Recent Findings
The phase 3 clinical trial IMBRAVE150 has recently shown the combination of an immune checkpoint inhibitor, atezolizumab, with an anti-angiogenic agent, bevacizumab, to be superior to sorafenib monotherapy for treatment-naive advanced HCC. Moreover, patients now have multiple options available in second-line therapy including targeted therapies like sorafenib, lenvatinib, regorafenib, cabozantinib, ramucirumab and immunotherapies like atezolizumab, and nivolumab either alone or combined with ipilimumab.
Summary
There has been tremendous recent progress in the management of advanced HCC. Combination therapy with atezolizumab-bevacizumab has recently become the standard first line of therapy for patients with advanced HCC. Additionally, immunotherapy agents are poised to play a significant role in the management of HCC either alone or in combination with molecular targeted therapies.
Keywords: HCC, Liver cancer, Immunotherapy, trials, targeted therapy
Background
Liver cancer is one of the leading causes of cancer incidence and mortality around the world, causing more than 800,000 deaths annually1. Hepatocellular carcinoma (HCC) is the most common form of liver cancer and accounts for more than 90% of primary cancer in the liver. Early detection of HCC can facilitate access to several curative and non-curative therapies including surgical and locoregional options. However, until recently, patients with advanced HCC had very limited systemic therapy options. There are several reasons for this including the apparent chemoresistance of HCC to conventional chemotherapy and low tolerance for such therapy due to underlying hepatic dysfunction. In this background, the approval of Sorafenib, the first systemic agent that was shown to improve overall survival (OS) in advanced HCC, was heralded as a major breakthrough in 20072. Unfortunately, it has taken more than 10 years since the initial approval of sorafenib for new therapies to emerge.
Since 2016, we have now entered a new era in HCC treatment with the approval of several targeted therapies and immunotherapies for the management of advanced HCC. In this review, we highlight the recent changes in the landscape of systemic therapy for HCC and discuss future perspectives. Before we evaluate the various clinical trials for advanced HCC, we need to understand the definitions of staging criteria used to enroll patients in clinical trials and also the endpoints used to assess efficacy and safety of the therapies in clinical trials. In table 1 we have presented a broad overview of these definitions.
Table 1.
Commonly used Terms in the Enrollment and Assessment of Clinical Trials for Hepatocellular Carcinoma (HCC)
| TERM | ACRONYM | DESCRIPTION |
|---|---|---|
| Clinical Trial Endpoints for Efficacy and Safety | ||
| Overall Survival | OS | Time between randomization in trial allocation until death, of any cause. |
| Progression Free Survival | PFS | Time from randomization until objective tumor progression or death, whichever occurs first. |
| Time to Progression | TTP | Time between trial allocation and radiological tumor progression. |
| Objective Response Rates | ORR | Percentage of patients who achieve an objective tumor response |
| Complete Response | CR | No detectable evidence of tumor |
| Quality of life | QOL | effect of the disease on an individual’s physical, psychological and social functioning and well-being |
| Common Terminology Criteria for Adverse Events | CTCAE | Criteria for the standardized classification of adverse events and lab abnormalities with cancer therapy. Grade refers to the severity of the AE. Grade of severity ranges from 1 to 5. In general, grade 1- asymptomatic or mild adverse events; 2- mild symptoms; 2- moderate symptoms; 3- severe symptoms; 4- life threatening consequences; 5- death due to adverse events |
| Staging HCC and Liver Disease for Clinical Trials | ||
| Barcelona Clinic Liver Cancer system | BCLC | Determines cancer stage and patient prognosis based on tumor burden, severity of liver disease, and the patient’s performance status. Stage 0- Very early stage; Stage A- Early; Stage B- Intermediate; Stage C- Advanced; Stage D- Terminal disease. |
| Child Pugh Class | CP class | Scale to assess severity of liver disease. Uses- severity of ascites and hepatic encephalopathy and blood tests (bilirubin, albumin, and prothrombin time). A CP score of 5–6 is class A (well-compensated); 7– 9 is class B (moderate severity); 10–15 is class C (decompensated disease). |
| Performance status | PS | The Eastern Cooperative Oncology Group (ECOG) criteria measures patient’s functional status- ability to perform self-care, activities of daily living and physical ability. |
| Response Evaluation Criteria in Solid Tumors | RECIST | Criteria to measure radiological response based on size (RECIST 1.1). Complete response (CR)- absence of all target lesions; partial response (PR) and progressive disease (PD) >30 % decrease and >20 % increase, respectively, in the sum of the longest diameters of the target lesions; and stable disease (SD), neither PR nor PD. Modified RECIST (mRECIST) criteria also includes evaluation of tumor viability or enhancement. |
The table defines major clinical trials endpoints and staging systems used in the enrollment for clinical trials evaluating systemic therapies for HCC.
Timeline of HCC Systemic Therapy
Multiple trials prior to 2007 had shown that conventional chemotherapies and hormonal therapies do not improve survival of patients with advanced HCC and are associated with significant adverse events3–5. The sorafenib era began in 2007 when two pivotal phase 3 multicenter randomized trials of sorafenib, a multikinase inhibitor, showed prolonged time to progression (TTP) and overall survival (OS) in patients with advanced HCC2,6. Based on these results it became the standard of care for management of advanced HCC around the world, despite having only modest benefits. Also, it raised expectations that newer and better therapies would soon be approved for HCC. However, more than ten clinical trials of molecular targeted therapies including brivanib and sunitinib, which were indeed successful in treating other cancers, failed to perform better than sorafenib for HCC7–10.
The long stretch of failed trials was broken in 2017, when the phase 3 RESORCE trial showed that regorafenib provided survival benefit to patients with advanced HCC who had failed sorafenib therapy11. This led to FDA approval of regorafenib as the first second-line agent for HCC in 2017. Meanwhile, the phase 1/2 trial CHECKMATE 040 of the immunotherapeutic, nivolumab, showed a promising overall response rate (ORR) of 14% and surprisingly, 91% of responders had responses lasting longer than 6 months and 55% had responses lasting longer than 12 months12. These promising early data led to accelerated FDA approval for nivolumab in late 2017 for HCC that had failed sorafenib therapy. Similarly, promising high overall response rates, durable response in a subset of patients and relatively favorable safety profile further led to the approval of pembrolizumab in 2018 and the combination of ipilimumab and nivolumab in 2020 as second-line therapies, thus opening the door to the era of immunotherapy for HCC. Eventually, lenvatinib became the first approved first-line therapy for advanced HCC since sorafenib, and this was based on data from the phase 3 REFLECT trial showing that lenvatinib was noninferior to sorafenib for advanced HCC13. In 2019, two other molecular targeted therapies, cabozantinib and ramucirumab, received approvals as second-line therapies based on trials showing modest improvement in overall survival for patients who had failed sorafenib.
Lastly, the most recent drug approval for advanced HCC is the combination of PDL1 inhibitor atezolizumab and VEGF inhibitor bevacizumab, which showed significant improvement in overall survival when compared to sorafenib and as hence been approved as first-line therapy14. The results from the IMBRAVE-150 are a breakthrough since this is the first trial to show improvement in survival beyond sorafenib, the first trial of successful combination therapy for HCC and also the first trial to show the benefit of immunotherapy as first-line agents. Thus, we currently have three therapies approved as first-line and six agents in the second line, figure 1 summarizes the timeline of drug approvals for HCC and table 1 describes the pivotal phase 3 trials which led to the approval of these agents. Most trials in both first- and second-line therapy included patients with preserved liver function or Child-Pugh (CP) class A disease and a performance status score of 0 or 1 on the Eastern Cooperative Oncology Group (ECOG) scale (scores range from 0 to 5, with higher numbers reflecting greater disability).
Figure 1. Timeline of approval of systemic therapy for HCC.
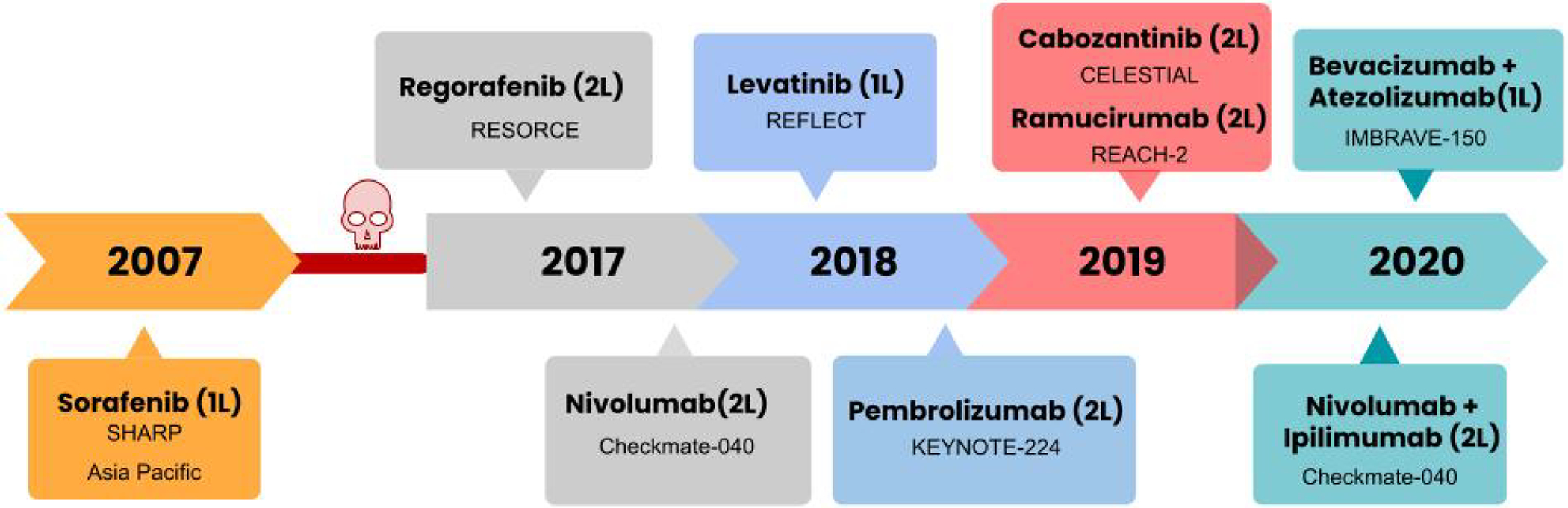
This figure depicts the chronological order in which systemic therapies have been approved in the management of HCC and the name of the clinical trial which led to the approval of the respective drugs.
1L- first line; 2L- second line.
We will now discuss the various systemic therapies currently approved for advanced HCC in the first line and second-line setting.
First-line systemic therapies for advanced HCC
Atezolizumab in combination with bevacizumab for advanced HCC
The combination of atezolizumab and bevacizumab has now replaced sorafenib as the first line treatment for advanced HCC. Atezolizumab is an immune checkpoint inhibitor which binds to PDL1 and bevacizumab is a humanized recombinant monoclonal antibody against circulating vascular endothelial growth factor (VEGF-A). The rationale for this combination comes from earlier studies in solid tumors, such as renal cell carcinoma, which showed that VEGF has a strong immunomodulatory effect in the tumor microenvironment, thus suggesting that anti-VEGF therapy could potentially augment the response to checkpoint inhibitors by reprogramming the tumor microenvironment from an immunosuppressive to an immune permissive profile15,16.
The early randomized phase 1b study of atezolizumab plus bevacizumab in patients with treatment-naive HCC showed an acceptable side-effect profile and promising antitumor activity, with an objective response rate of 36% and a median progression-free survival of 7 months17. This was followed by the phase 3 IMbrave 150 trial which evaluated the combination of atezolizumab (atezo) plus bevacizumab (bev) compared to sorafenib in the setting of first-line therapy of HCC, in patients with CP class A disease, ECOG status of 0–1, with no prior systemic therapy for HCC14. Patients had locally advanced or metastatic and/or unresectable HCC. Due to known elevated risk of GI bleeding with the use of bevacizumab, all patients underwent an upper endoscopy within 6 months of study enrollment to assess for varices or other high-risk lesions. Patients with autoimmune hepatitis, coinfection with hepatitis B or C, untreated esophageal or gastric varices were excluded from the trial. The study showed improvement in overall survival (OS) and progression free survival (PFS) with combination of atezolizumab-bevacizumab (atezo-bev) compared to sorafenib (6-month OS 85% vs. 72%, HR 0.58 [95% CI 0.42–0.79], p=0.0006; and PFS 6.8 vs. 4.3 months, HR 0.59 [0.47–0.76], p<0.001). The objective response rate (ORR) and complete response rate (CR) in HCC tumors, assessed by modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria, was significantly higher with atezo-bev compared to sorafenib (ORR- 33.2% vs 13.3%, CR- 10.2% vs 1.9%; p<0.001).
Grade 3 or 4 adverse events occurred in a similar percentage of patients in each group (57% vs 55%), and the rate of upper GI bleeding was not significantly different between atezo-bev and sorafenib groups either (7% vs 4.5%). Adverse events leading to dose modification or interruption occurred in 49.5% of patients who were receiving atezo–bev and in 60.9% who were receiving sorafenib. Further details of side effects of systemic therapy in HCC will be discussed later in the article. Patient-reported rates for quality of life (QOL) deterioration was significantly lower in the atezo-bev group compared to sorafenib group (median time to deterioration, 11.2 mo vs. 3.6 mo, respectively; HR 0.63; 95% CI, 0.46 to 0.85). Thus, IMbrave 150 is the first positive phase 3 randomized clinical trial that showed a superior survival benefit over sorafenib. Based on these data, the combination of atezolizumab plus bevacizumab was approved by FDA in May of 2020 as the first-line treatment of advanced HCC. Recently, investigators provided an update on the IMBRAVE150 trial with extended median follow up, of 15.6 months18. They were able to confirm longer OS (atezo-bev 19.2 mo vs sor 13.4 mo; HR, 0.66 [95% CI, 0.52, 0.85]; P=0.0009), higher ORR of 29.8%, and higher rates of complete response, 7.7%, than previously reported18. Thus, atezo-bev therapy leads to clinically meaningful survival benefit, confirming it to be the first-line standard-of-care systemic therapy for advanced HCC
Sorafenib
Sorafenib is a tyrosine kinase inhibitor (TKI) with action against many protein kinases including VEGF receptor, PDGF receptor and RAF kinases. It has been the standard first-line systemic therapy for patients with CP class A with Barcelona Clinic Liver Cancer (BCLC)-C or earlier stage tumors progressing upon or unsuitable for loco-regional therapies, since 2007. Sorafenib showed a modest but significant survival benefit compared with placebo in the SHARP trial (10.7 versus 7.9 months)2 as well as the Asia Pacific trial (6.5 versus 4.2 months)6. But the objective response rate (ORR) with sorafenib is low, in the 2–5% range. The safety profile of sorafenib is reasonable, with grade 3–4 adverse events like diarrhea, hypertension and dermatologic complications in 8–11% of patients. Multiple real-world studies have subsequently confirmed the clinical benefits of sorafenib and have explored factors which are predictive of response. For example, chronic hepatitis C-related HCC appears to respond more favorably with sorafenib than other etiologies like hepatitis B19–21. Also, several studies have suggested that incidence of adverse events like skin rash or diarrhea are predictive of response22–24. Expression of proteins like phospho ERK or presence of FGF3/4 fusions have also been proposed to serve as predictive biomarkers for response to sorafenib25,26. However, none of these factors have been approved as standard biomarkers which can be used in clinical practice. Thus, there is yet no clear way to identify the subset of patients who might benefit more favorably from sorafenib.
Given the longstanding use of sorafenib for HCC, several aspects of its clinical use have been studied in real-world settings. For instance, despite the phase 3 clinical trials only enrolling CP class A disease, the benefit of sorafenib in CP class B has been explored further. A prospective, multinational registry study of 3203 patients showed that sorafenib appears equally effective and relatively safe in CP class B (n=666) as in CP class A disease (n=1968)27. However, a meta-analysis of 30 studies (12 Asian) with 8678 patients showed that CP B liver function was associated with worse OS than CP class A status with sorafenib, but the safety and tolerability were similar28. Despite these contradictory results, sorafenib use is generally restricted to CP class A disease, with selected patients with CP class B receiving it on a case-by-case basis. Also, the role of sorafenib as adjuvant therapy has been extensively evaluated. Addition of sorafenib to doxorubicin29 or gemcitabine-based chemotherapy30 or to chemoembolization31,32 has not shown any clinical benefit. Given the recent approval of atezo-bev as first line therapy, sorafenib use will likely be moved to the second line and probably reserved for patients who fail atezo-bev but have preserved liver function and functional status.
Lenvatinib
Lenvatinib is a TKI with action against multiple kinases including VEGF and FGF. The phase 3 randomized trial (REFLECT) that compared lenvatinib with sorafenib in patients with treatment-naive HCC led to its FDA approval in 2018 for first-line treatment of unresectable HCC13. Enrolled patients had CP class A and ECOG status 0–1 with BCLC B or C (with vascular invasion and/or extrahepatic disease) who were ineligible for locoregional therapy or showed disease progression after treatment according to mRECIST. In this trial, lenvatinib was found to be non-inferior to sorafenib with respect to the median overall survival (13.6 months vs. 12.3 months), moreover, it did improve all the secondary endpoints including ORR (24% vs. 9%), time to progression (8.9 mo vs. 3.7 mo), and PFS (7.4 mo vs. 3.7 mo)13. Moreover, lenvatinib showed clinically significant improvement in QOL scores. Multiple real-world studies have subsequently confirmed the improved survival and relatively higher ORR noted in the REFLECT trial33–35. A recent real-world comparative analysis showed that lenvatinib was more efficacious than sorafenib in patients who had failed transarterial therapies, suggesting there may be room to expand indications for lenvatinib36. Going forward, lenvatinib like sorafenib is more likely to be used as a second-line agent. However, results of combination of lenvatinib with immune checkpoint inhibitors are eagerly anticipated to lead their approval, potentially as first-line therapy.
Second-line Systemic Therapies for Advanced HCC
Nivolumab
Nivolumab is a monoclonal antibody that binds and neutralizes programmed cell death 1 (PD-1), thus removing breaks on the immune system and reinstating T-cell mediated anti-tumor immunity. CheckMate 040 was the first phase 1/2 trial that evaluated the safety and tolerability of nivolumab, in patients with advanced HCC who were not eligible for resection and/or locoregional therapies and whose HCC had progressed on sorafenib or who refused or were intolerant of the drug12. The study included 48 patients in the dose-escalation cohort and 214 in the expansion cohort. Eligible patients had CP scores of 7 or less (CP class A or B7) for the dose-escalation phase and 6 or less (CP class A) for the dose-expansion phase, and an ECOG status of 0–1. Patients with HBV infection had to be receiving effective antiviral therapy but antiviral therapy was not required for patients with HCV infection. The objective response rate was 20% in the dose-expansion phase and 15% in the dose-escalation phase. Moreover, the investigators observed durable responses with median duration of response >9.9 months. The safety profile of nivolumab looked encouraging with most adverse events being transient. Only 5% of the patients had severe enough immune-mediated hepatitis to require steroids. Health-related QOL profile was in favor of Nivolumab over sorafenib. These promising early results led to accelerated approval of Nivolumab in 2017 for advanced HCC after the failure of sorafenib. The trial included a cohort of patients with CP score B7 or B8 patients, in contrast to the majority of TKI trials in patients with HCC which enrolled only patients with CP class A. The ORR was reduced to about 12% in these patients, but the durability appeared similar to the other cohorts in the study. The types and frequency of adverse events (AEs) were not different overall in the CP class B cohort, except for a slight increase in hepatic related adverse events. Based on these results, the most recent National Comprehensive Cancer Network (NCCN) guideline recommends the use of Nivolumab in both CP class A and B737.
Checkmate 040 included a small subset of sorafenib-naive patients and a subsequent analysis of these sorafenib-naïve patients demonstrated a promising ORR of 23%38. These results raised the possibility that nivolumab can potentially be offered as a first-line option for advanced HCC. This was evaluated in phase III CheckMate 459 trial which compared first-line nivolumab directly with sorafenib for advanced, previously untreated HCC39. Although nivolumab did have a higher ORR (15 versus 7 percent), it did not improve PFS (3.7 vs. 3.8 months) or OS (16.4 vs. 14.7 months) compared to sorafenib. The side effect profile of nivolumab did look better than sorafenib with fewer grade 3/4 adverse events (22% versus 49%), and fewer instances of drug discontinuation therapy due to side effects (4% vs. 8%). Despite these negative results, there appears to be a role for nivolumab in the first-line, instead of sorafenib, for a subset of patients who have contraindications for TKI or are in need for rapid decrease in tumor size due to symptomatic tumor burden. However, with the approval of atezo-bev in the first line, the justification for using nivolumab as first-line appears weaker. At present, the NCCN guidelines recommend nivolumab as the first-line option if patients are ineligible for TKIs or other antiangiogenic agents37.
Combination of nivolumab and ipilimumab
The combination of nivolumab and ipilimumab integrates two checkpoint inhibitors with different mechanisms of action. Nivolumab, as mentioned above, is an anti PD-1 antibody and ipilimumab is an anti-CTLA4 antibody. This combination has been shown to be effective in other cancers like melanoma and lung cancer40,41. The phase 1/2 CheckMate 040 clinical trial evaluated the efficacy of this combination in HCC. Eligible patients with CP class A, ECOG status of 0–1, and BCLC B or C who had disease progression after or intolerance to sorafenib were randomly assigned to three different dosing regimens. Patients with or without HBV or HCV infection were included. Patients who had active coinfection with HBV and HCV, or HBV and hepatitis D virus, were not eligible. The combination therapy showed much higher response rates than nivolumab monotherapy with an ORR of 31%, and a subset of patients showed strong durable responses. Recent updates were reported from this trial and some patients were noted to achieve very significantly durable responses, even up to 4 years, and the median duration of response was as high as 22.2 months in one of the arms42. As expected, immune mediated adverse events were more common than with nivolumab monotherapy, with 57% of these patients requiring steroids. Moreover, around 10% of the patients with viral hepatitis B or C experienced viral breakthroughs. Based on promising ORR and durable responses this combination has been approved as a second-line for sorafenib-experienced patients. But what role this combination therapy will play in patients who do not tolerate or fail the combination of atezolizumab and bevacizumab is not yet clear.
Pembrolizumab
Pembrolizumab is an anti PD-1 antibody, similar in action to nivolumab. The Keynote-224 phase 2 trial evaluated the safety and tolerability of pembrolizumab as a monotherapy option in advanced HCC43. Patients who had previously been treated with sorafenib and were either intolerant to this treatment or showed disease progression according to RECIST v.1.1 were included in this cohort. Eligible patients were not amenable to or refractory to locoregional therapy and had CP class A and ECOG status of 0–1. Patients who had received prior immunotherapy, including anti PD-1, anti PD-L1, or previous systemic therapy for HCC other than sorafenib were excluded. The ORR was 18% (95% CI 11–26) which was quite similar to the data with nivolumab as a single agent. Serious adverse events occurred in 15% of patients, with hepatitis being the most common. These data resulted in accelerated FDA approval of pembrolizumab in 2018 for sorafenib-experienced patients. Following this phase 2 study, a randomized, double-blind, phase 3 trial, Keynote-240 was designed with two primary endpoints including OS and PFS44. The median OS was longer with pembrolizumab compared to placebo (13.9 months vs. 10.6 months) (HR, 0.781 [0.611–0.998]; p=0.023). However, the pre-specified statistical significance (p=0.0178) was not met and this was considered a negative trial. Median PFS for pembrolizumab was 3.0 months versus 2.8 months for placebo at final analysis (HR, 0.718; 95% CI, 0.570 to 0.904; p=0.002). An extended follow up confirmed that these improvements in OS and PFS were maintained over time, along with higher ORR (18.3% vs. 4.4%)45. Immune-mediated AEs occurred in 18.3% in the pembrolizumab group and 8.2% in the placebo group. Only 8.2% in the pembrolizumab group and one (0.7%) in the placebo group received steroids for possible immune-mediated AEs. No cases of hepatitis B or C viral flares were identified. Overall, pembrolizumab was well tolerated in these patients. Several questions regarding the role of pembrolizumab remain, how does it compare against nivolumab and does it have a role in patients who have failed first line atezolizumab and bevacizumab. Further trials and guidelines will hopefully answer these questions.
Regorafenib
Regorafenib is another TKI with a mechanism of action similar to sorafenib. The RESORCE trial evaluated Regorafenib as a second-line treatment for patients with HCC whose disease progressed during sorafenib treatment11. Eligible patients had CP class A, ECOG 0–1 and BCLC B or C disease who were not candidates for resection or locoregional therapy and had disease progression according to mRECIST. Regorafenib improved overall survival with a hazard ratio of 0·63 (95% CI 0·50–0·79; one-sided p<0·0001); median survival was 10·6 months (95% CI 9·1–12·1) for regorafenib versus 7·8 months for placebo. Results from the interim analysis of the prospective, observational REFINE study (n=500) looking at the real-world data demonstrated regorafenib to be a tolerable treatment in unresectable HCC patients and it also confirmed the OS benefit observed in the RESORCE trial46. Regorafenib is generally considered as second-line therapy for patients who were able to tolerate sorafenib well but showed progression of underlying HCC. Given the availability of other better tolerated second-line options including immunotherapy, the role of regorafenib in sequential therapy is likely to be narrower.
Cabozantinib
The CELESTIAL trial evaluated another TKI, cabozantinib, in comparison with placebo in previously treated patients with advanced HCC who had disease progression per RECIST, version 1.1. The trial showed a median overall survival of 10.2 months with cabozantinib and 8.0 months with placebo (hazard ratio for death, 0.76; 95% confidence interval [CI], 0.63 to 0.92; P=0.005)47. Patients with CP class A and ECOG status of 0–1 were included in the trial. Randomization was performed according to the disease etiology (HBV, HCV, or other), geographic region (Asia or other), and evidence of extrahepatic disease and/or macrovascular invasion. The CELESTIAL trial was overall less selective in its inclusion criteria without mandating prior tolerance to sorafenib and it allowed for 2 or more prior lines of treatments. Thus, it became the first agent that had been evaluated in the third-line. In a subgroup analysis of the CELESTIAL trial of patients who had received two prior systemic therapies, cabozantinib (n=130) appeared to have some benefit over placebo (n=62). Another subgroup analysis has suggested that cabozantinib may be relatively safer even in patients older than 65 years48. A matching-adjusted indirect comparison of second-line cabozantinib (CELESTIAL trial) and regorafenib (RESORCE trial) in advanced HCC showed similar OS for cabozantinib and regorafenib49. However, median PFS appeared longer for cabozantinib than regorafenib (5.6 versus 3.1 months; p = 0.0005). As more real-world data is gathered, this will hopefully help determine the sequential order in which these second line agents should be used.
Ramucirumab
Ramucirumab is a monoclonal antibody that binds VEGFR2. Upon initial evaluation, Ramucirumab failed to show survival benefit over sorafenib in the REACH trial, but an unplanned subgroup analysis revealed that patients with AFP ≥ 400 ng/ml at baseline appeared to benefit50. This was followed up with the REACH-2 trial, which showed that Ramucirumab, in comparison with placebo, improved the median overall survival (8·5 months vs 7·3 months; HR- 0·710 [0·531–0·949] in the setting of second-line treatment for patients with HCC with AFP ≥ 400 ng/mL51. Eligible patients had BCLC B or C disease that was refractory or not amenable to locoregional therapy, CP class A, ECOG status 0–1 who were intolerant or showed disease progression on sorafenib therapy. The subsequent approval of ramucirumab makes it the first drug approved with a biomarker-stratified approach for HCC. The one advantage of ramucirumab as a second-line therapy compared to the other TKIs, regorafenib and cabozantinib, is the lack of adverse effects associated with TKI like hand-foot syndrome. So, this drug might have a special role in patients with high AFP and those who do not tolerate TKIs.
Adverse effects of molecular targeted therapy
The main side effects of tyrosine kinase inhibitors, sorafenib, lenvatinib, regorafenib and cabozantinib include hand-foot skin reaction, hypertension, diarrhea, fatigue, delayed wound healing and weight loss. If we consider the four pivotal phase 3 trials of these drugs, the overall incidence of adverse events ranges from 45–75%2,11,13,47. Most adverse effects were managed with dose reductions or drug interruptions combined with symptomatic care. However, 9–15% of the patients had to completely discontinue the treatment due to the adverse events. As mentioned earlier, the toxicity symptoms have been reported to predict better response to the drug, however this has not been consistently validated. Overall, there appears to be a mortality risk of 1–3% from life-threatening complications with TKI, like thromboembolic events, bleeding or congestive heart failure52,53. In general, the drug has to be discontinued if it leads to severe grade 3 or 4 adverse events. For grade 2 adverse events, it is reasonable to hold the TKI, manage the adverse event till the patient recovers, and then try to reintroduce the TKI under close observation. The drug can potentially be continued in patients with grade 1 toxicity alongside symptomatic management of the complication54,55. Early recognition of adverse events and institution of specific pharmacologic and non-pharmacologic management interventions can help treat TKI-induced adverse events and improve quality of life54,56. Apart from the adverse events discussed above, bevacizumab is known to have a significant risk for bleeding complications. Several meta-analyses have revealed that bevacizumab increases the risk for severe bleeding by two-fold57,58. In patients with cirrhosis and untreated esophageal or gastric varices, bevacizumab can lead to massive life-threatening hemorrhage. Hence, it’s imperative that all patients undergo upper endoscopy and appropriate treatment of varices before initiating bevacizumab.
Adverse effects of immune checkpoint inhibitors (ICIs)
Immune checkpoint inhibitors activate antitumor immune responses in the host. This desired action can unfortunately be complicated by inflammatory auto-immune-like side effects in multiple organ systems, and these reactions are called immune related adverse events (irAE). The reactions range from more common ones like colitis, rash, hepatitis and pneumonitis to less common ones like hypophysitis, encephalitis, thyroiditis, arthralgia, myalgia, and vitiligo59,60. The key to managing these irAE is to take a proactive approach to recognize them early, followed by appropriate use of steroids and supportive care. The irAE can be graded from 1- mild to grade 4-life-threatening using the American society of clinical oncology (ASCO) guidelines61. In general, grade 1 irAE can be monitored conservatively, while ICIs have to be interrupted and treatment with steroids started for grade 2 irAE. For grade 3 and 4 irAE, ICI should be discontinued, and these patients usually need high dose steroids61.
There are some liver specific concerns while using immunotherapy. All patients who are scheduled to receive ICIs should undergo hepatitis B screening prior to initiation of immunotherapy, and active viremia should be treated, if present. Patients with underlying immune disorders are considered to be at high risk for such irAE62. Thus, patients with autoimmune hepatitis have not been included in clinicals trials of immunotherapy, and it is not yet known how these patients will respond to ICI. Given that most patients with HCC have underlying hepatic dysfunction, there was significant concern that they would not tolerate ICIs. However, these drugs have been found to be relatively safe in HCC, with most patients experiencing only mild and transient elevation in liver enzymes63.
Sequential systemic treatment for advanced HCC
Currently, there are multiple first- and second-line systemic therapy options for advanced HCC. Therefore, the guidelines regarding the best sequences of therapy are still evolving. Here, we discuss our approach to the optimal use of first- or second-line therapies.
Preferred first-line systemic therapy
In general, the combination of atezolizumab with bevacizumab should be considered the preferred first-line treatment in all patients with advanced HCC with CP A liver disease. In those patients who are ineligible for anti-angiogenic agents (bevacizumab) due to increased risk for bleeding, or in patients with autoimmune diseases or organ transplantation who can have a significant risk of adverse events with checkpoint inhibitors, either sorafenib or lenvatinib can be used as first-line options, in CP class A disease. We do note that lenvatinib is non-inferior to sorafenib with regards to overall survival but has shown better ORR and PFS compared to sorafenib. In selected patients who are ineligible for both TKIs and antiangiogenic agents, nivolumab can potentially be used as first-line agent. Also, grade 3/4 treatment-related AEs are lower with nivolumab compared to sorafenib and the quality-of-life scores are more favorable for nivolumab. Moreover, nivolumab is approved for both CP class A and B7 patients.
Second-line systemic therapy upon disease progression
Within the last 4 years, the FDA approved five drugs as second-line therapies for advanced HCC. There is no data to define the best second-line treatment option in patients who progress after first-line atezolizumab with bevacizumab. Levatinib and sorafenib will likely be the good option if patients fail the atezo-bev combination due to side effects from immunotherapy. Further, current evidence supports the role of regorafenib (in sorafenib tolerant patients), cabozantinib (independent of sorafenib tolerability), or ramucirumab (when AFP level is over 400 ng/ml) as a second-line option in these specific subsets of patients who fail sorafenib. Pembrolizumab and nivolumab alone or in combination with ipilimumab are approved in second-line settings in patients who have not received checkpoint-inhibitors in the first-line treatment. Combination of nivolumab plus ipilimumab resulted in an ORR twice that of nivolumab alone (31% vs. 14%, respectively), therefore, this regimen can be used in selected patients with excellent performance status and liver function knowing that the risk of immune-related adverse events is higher upon addition of ipilimumab to nivolumab. The safety and efficacy of using another checkpoint inhibitor (anti CTLA-4) following exposure to atezolizumab or nivolumab remains unclear. In figure 2 we summarize the sequencing algorithm based on the NCCN guidelines for management of advanced HCC.
Figure 2. Options for Sequential Management of Advanced HCC.
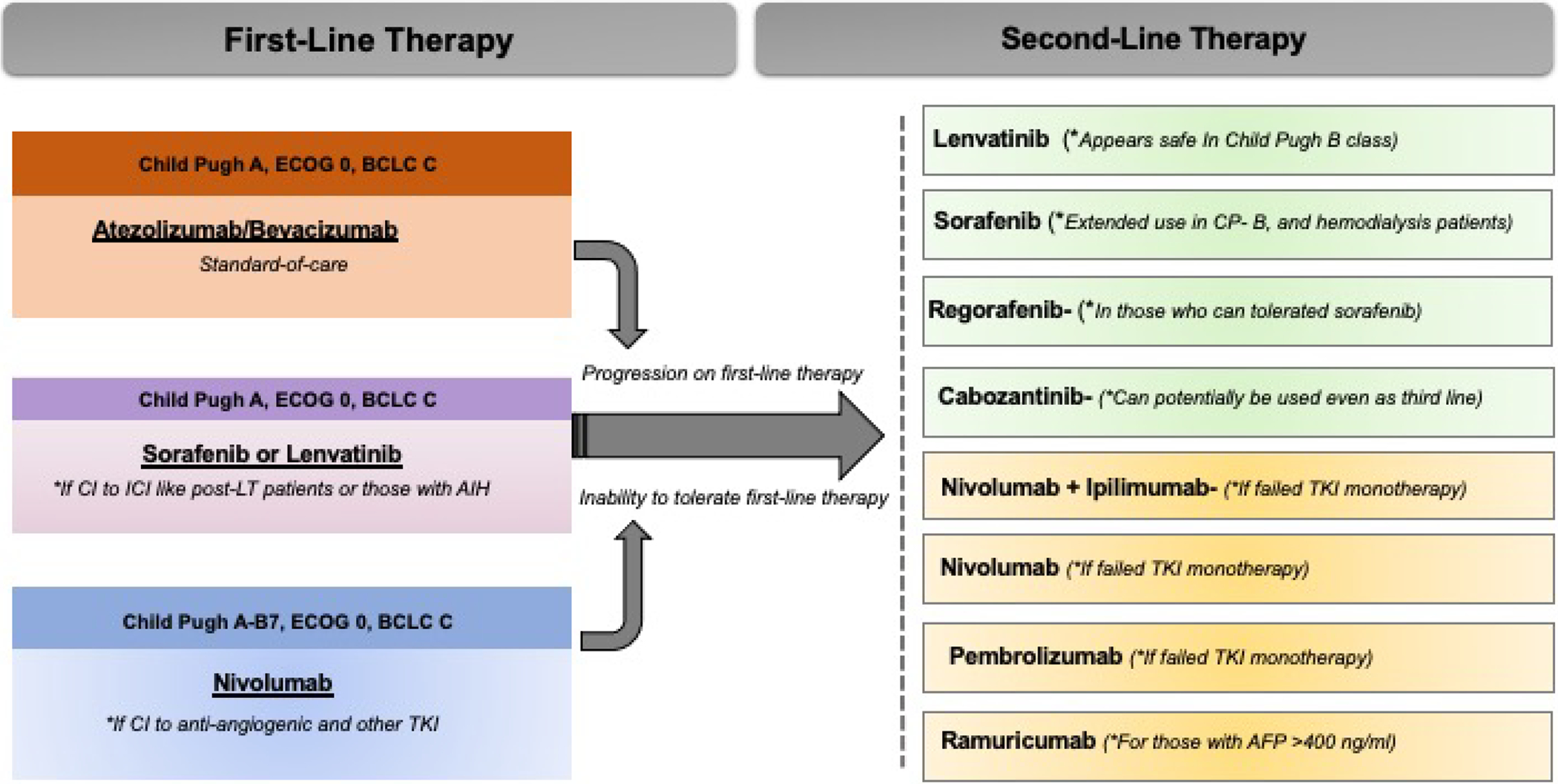
The figure presents an overview of first line and second line options available for systemic therapies for the management of advanced HCC.
ECOG- Eastern Cooperative Oncology Group (ECOG) scale for performance status. BCLC-Barcelona Clinic Liver Cancer Staging System. CP- Child Pugh Class. TKI- Tyrosine kinase inhibitors.
Ongoing trials
In this section we briefly overview some of the highly anticipated ongoing trials on systemic therapy for HCC. There are currently two general categories of ongoing trials for combination therapies, combination of different immune checkpoint inhibitors or combination of checkpoint inhibitors with molecular targeted therapy. The HIMALAYA phase III clinical trial is designed to evaluate the safety and efficacy of a combination of tremelimumab (CTLA-4 inhibitor) and durvalumab (PDL-1 inhibitor) compared to sorafenib in the first-line setting. The interim data analysis showed the best ORR of 22% in the arm that included a high dose of tremelimumab with the standard dose of durvalumab64. Similar results with the prolonged OS were seen in the other combination regimen with high dose ipilimumab (CTLA-4 inhibitor) and nivolumab (PD1 inhibitor). Another promising ongoing trial of checkpoint inhibitor combination, the checkmate-9DW trial, a randomized, multi-center, phase III study of nivolumab in combination with ipilimumab compared to sorafenib or lenvatinib as first-Line. The LEAP-002 (Lenvatinib plus pembrolizumab) and COSMIC-312 (atezolizumab plus cabozantinib) are other trials that study the combination of a checkpoint inhibitor and a TKI agent in the first-line setting compared to sorafenib. Several phase 2 trials which are evaluating the safety and tolerability of newer therapies are also in progress.
Challenges, and Future Directions
The field of systemic therapy for HCC is rapidly evolving but several important challenges remain. Most trials, as mentioned above, recruit patients with CP class A disease. Patients with underlying CP class B/C are generally excluded from clinical trials of novel systemic therapies for HCC due to decreased tolerability and increased rate of adverse events. However, selected patients with CP class B disease, can likely tolerate systemic therapy well and even benefit from them. Hence, there is a significant unmet need to identify systemic therapy options which are safe and effective for patients with CP class B disease.
Next challenge is to determine the utility and sequence of systemic therapies in intermediate stage HCC as adjuvant therapies to locoregional treatment. There are several clinical trials in progress looking at the combination of either TKIs or checkpoint inhibitors with locoregional cancer therapies based on the premise that the immunomodulation resulting from locoregional therapies can enhance the efficacy of immunotherapy for HCC. Emerald-1, for example, is an ongoing randomized phase 3 multicenter study of transarterial chemoembolization (TACE) in combination with durvalumab plus bevacizumab in patients with HCC. Similarly, Checkmate-74W is a trial that is designed to study TACE in combination with a dual checkpoint inhibitor regimen (nivolumab and ipilimumab). Once the trials are complete, we will have a better idea if immunotherapy can be used in conjunction with locoregional therapy.
Lastly, despite promising results and durable responses to the checkpoint inhibitors alone or in combination with TKIs in HCC, a significant proportion of patients do not respond to immunotherapy or unfortunately develop significant immune-related adverse events. We need circulating biomarkers which can predict responses to these drugs to better select patients who are more likely to respond. In figure 3 we summarize the major challenges that are currently being tackled by various clinical trials.
Figure 3. Future Challenges in the Management of Advanced HCC.
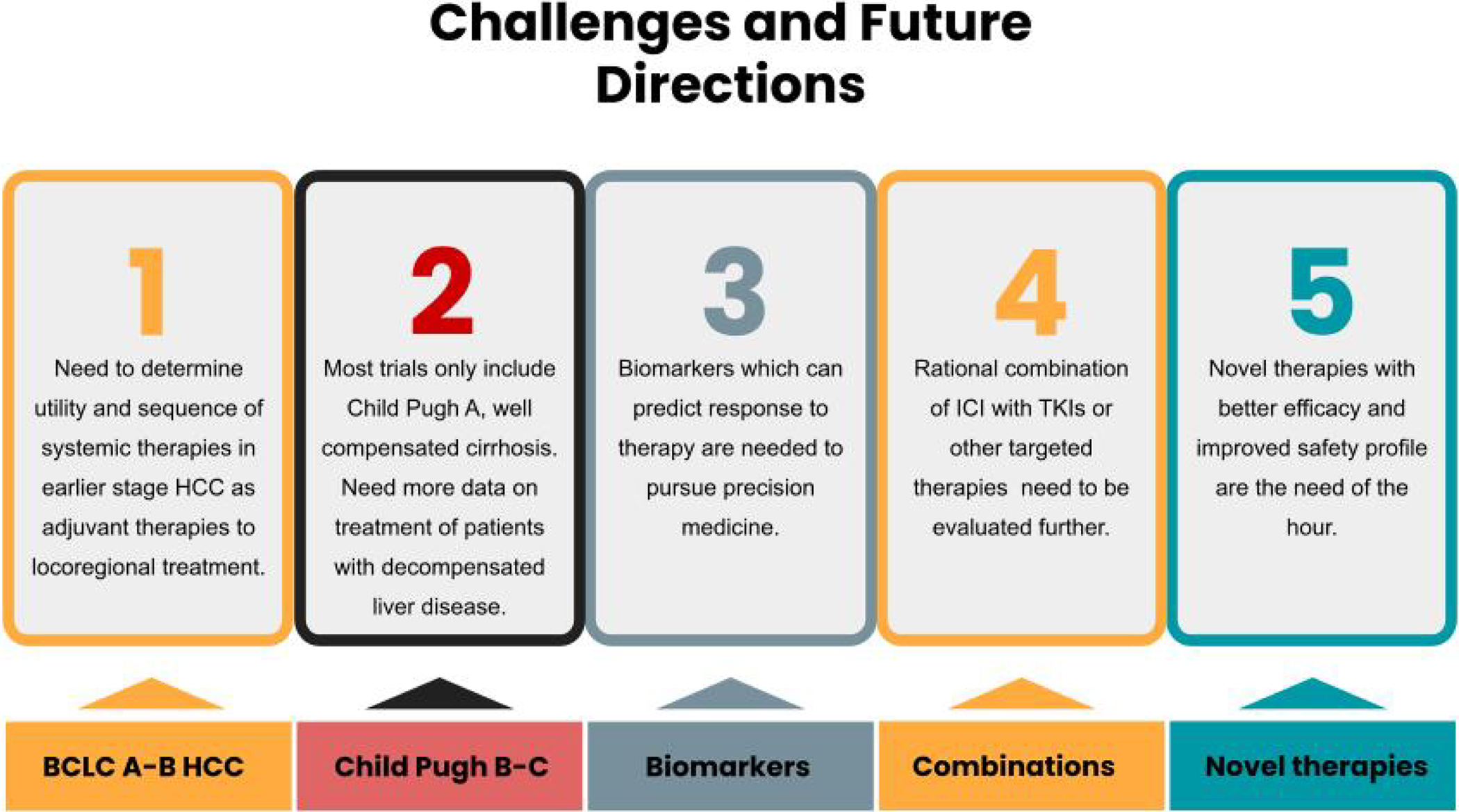
The schematic describes some of the current challenges in the management of advanced HCC. Future clinical trials are currently tackling these challenges.
Conclusions
We now have several approved systemic agents that have been shown to improve the outcome in patients with advanced HCC, both in the first- and second-line settings. The results from the phase 3 clinical IMBRAVE150 have led to approval of the combination of atezolizumab and bevacizumab as first-line therapy for advanced HCC. Multiple ongoing trials are evaluating other combinations for advanced HCC. It is critical to take a multidisciplinary approach to the management of these patients, in order to select the most effective sequence of treatment. Also, it is imperative to avoid repeated treatment with ineffective locoregional therapies, and to switch to systemic therapy before the development of liver dysfunction that might potentially lead to ineligibility to receive effective systemic therapy. Both molecular targeted therapies and immunotherapy can be associated with a wide range of multi-system adverse effects. It is important to recognize the adverse effects early, proactively manage them, and seek expert opinion from the appropriate specialists.
Thus, we have now entered a very exciting phase in the management of advanced HCC and we hope more positive results from ongoing clinical trials can lead to even better treatment options for our patients with HCC and improve their clinical outcomes.
Table 2.
Pivotal Phase 3 Clinical trials of Systemic Therapies for Management of Advanced HCC
| Systemic Therapy | Comparison | Treatment Line | Trial name | OS (months) | PFS (months) | ORR %) | Serious adverse events (%) |
|---|---|---|---|---|---|---|---|
| Bevacizumab + atezolizumab | Sorafenib | First | IMBRAVE 150 | 19.2 vs. 13.4 | 6.8 vs. 4.3 | 35 vs. 13 | 56 vs. 55 |
| Sorafenib | Placebo | First | SHARP | 10.7 vs. 7.9 | 4.1 vs. 4.9 | 2 vs. 1 | 52 vs. 54 |
| Lenvatinib | Sorafenib | First | REFLECT | 13.6 vs. 12.3 | 7.3 vs. 3.6 | 40 vs. 12 | 57 vs. 49 |
| Nivolumab | Sorafenib | First | Checkmate-459 | 16.4 vs. 14.7 | 3.7 vs. 3.8 | 15 vs. 7 | 22 vs. 49 |
| Regorafenib | Placebo | Second | RESORCE | 10.7 vs. 7.8 | 3.1 vs. 1.4 | 11 vs. 4 | 44 vs. 47 |
| Cabozantinib | Placebo | Second | CELESTIAL | 10.2 vs. 8.0 | 5.2 vs. 1.9 | 4 vs. <1 | 50 vs. 37 |
| Ramucirumab | Placebo | Second | REACH-2 | 8.5 vs. 7.3 | 2.8 vs. 1.6 | 8 vs. 1 | 35 vs. 29 |
| Pembrolizumab | Placebo | Second | KEYNOTE-240 | 13.9 vs. 10.6 | 3.0 vs. 2.8 | 18 vs. 4 | 53 vs. 46 |
The table lists and briefly describes the major clinical trials which led to approval of the respective therapies as first and second line therapies for advanced HCC.
OS-Overall Survival; PFS- Progression free survival
Footnotes
No conflicts of interest.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Cancer Today 2020. Global Cancer Observatory: International Agency for Research on Cancer. https://gco.iarc.fr/today/home. [Google Scholar]
- 2.Llovet JM et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med 359, 378–390 (2008). [DOI] [PubMed] [Google Scholar]; This trial led to the approval of sorafenib for the management of advanced HCC
- 3.Yeo W et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J. Natl. Cancer Inst 97, 1532–1538 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM et al. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology vol. 31 54–58 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Ikeda M et al. Systemic Chemotherapy for Advanced Hepatocellular Carcinoma: Past, Present, and Future. Diseases 3, 360–381 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. *.Cheng A-L et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34 (2009). [DOI] [PubMed] [Google Scholar]; *This trial led to the approval of sorafenib for the management of advanced HCC
- 7.Cheng A-L et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J. Clin. Oncol 31, 4067–4075 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Johnson PJ et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J. Clin. Oncol 31, 3517–3524 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Cainap C et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J. Clin. Oncol 33, 172–179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M et al. A randomized, double-blind, placebo-controlled phase III study of S-1 in patients with sorafenib-refractory advanced hepatocellular carcinoma (S-CUBE). Journal of Clinical Oncology vol. 33 4018–4018 (2015). [Google Scholar]
- 11. *.Bruix J et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017). [DOI] [PubMed] [Google Scholar]; *This trial led to the approval of regorafenib as second-line therapy for advanced HCC.
- 12.El-Khoueiry AB et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. *.Kudo M et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018). [DOI] [PubMed] [Google Scholar]; *The results of this trial led to the approval of lenvatinib as first-line therapy for advanced HCC.
- 14. * *.Finn RS et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med 382, 1894–1905 (2020). [DOI] [PubMed] [Google Scholar]; **Results of this early phase trial revealed that immunotherapy can be safe for patients with HCC and can potentially lead to durable responses, leading to its accelerate approval for advanced HCC
- 15.Hegde PS, Wallin JJ & Mancao C Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin. Cancer Biol 52, 117–124 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Wallin JJ et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun 7, 12624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu C-H et al. Randomised efficacy and safety results for atezolizumab (Atezo) bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Annals of Oncology vol. 30 ix187 (2019). [Google Scholar]
- 18.Finn RS et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Orthod 39, 267–267 (2021). [Google Scholar]
- 19.Bruix J et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J. Hepatol 57, 821–829 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson R, Psarelli E-E, Berhane S, Khan H & Johnson P Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. J. Clin. Oncol 35, 622–628 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Bruix J et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol 67, 999–1008 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Reig M et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J. Hepatol 61, 318–324 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Estfan B, Byrne M & Kim R Sorafenib in advanced hepatocellular carcinoma: hypertension as a potential surrogate marker for efficacy. Am. J. Clin. Oncol 36, 319–324 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Bettinger D et al. Diarrhea predicts a positive response to sorafenib in patients with advanced hepatocellular carcinoma. Hepatology vol. 56 789–790 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Arao T et al. FGF3/FGF4 amplification and multiple lung metastases in responders to sorafenib in hepatocellular carcinoma. Hepatology 57, 1407–1415 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Personeni N et al. Molecular determinants of outcome in sorafenib-treated patients with hepatocellular carcinoma. J. Cancer Res. Clin. Oncol 139, 1179–1187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrero JA et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J. Hepatol 65, 1140–1147 (2016). [DOI] [PubMed] [Google Scholar]
- 28.McNamara MG et al. Sorafenib as first-line therapy in patients with advanced Child-Pugh B hepatocellular carcinoma-a meta-analysis. Eur. J. Cancer 105, 1–9 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Abou-Alfa GK et al. Assessment of Treatment With Sorafenib Plus Doxorubicin vs Sorafenib Alone in Patients With Advanced Hepatocellular Carcinoma: Phase 3 CALGB 80802 Randomized Clinical Trial. JAMA Oncol (2019) doi: 10.1001/jamaoncol.2019.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assenat E et al. Sorafenib alone vs. sorafenib plus GEMOX as 1-line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br. J. Cancer 120, 896–902 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer T et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2, 565–575 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Lencioni R et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol 64, 1090–1098 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Shimose S et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: A multicenter cohort study using data mining analysis. Oncol. Lett 20, 2257–2265 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheon J et al. Real-World Efficacy and Safety of Lenvatinib in Korean Patients with Advanced Hepatocellular Carcinoma: A Multicenter Retrospective Analysis. Liver Cancer 9, 613–624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amaro CP et al. Efficacy and safety of lenvatinib in the real-world treatment of hepatocellular carcinoma: Results from a Canadian multicenter database (HCC CHORD). Journal of Clinical Oncology vol. 39 275–275 (2021). [Google Scholar]
- 36.Lee J et al. A Real-World Comparative Analysis of Lenvatinib and Sorafenib as a Salvage Therapy for Transarterial Treatments in Unresectable HCC. J. Clin. Med. Res 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network® (NCCN®). NCCN Guidelines for Patients® Liver Cancer. (2020).
- 38.Crocenzi TS et al. Nivolumab (nivo) in sorafenib (sor)-naive and -experienced pts with advanced hepatocellular carcinoma (HCC): CheckMate 040 study. Journal of Clinical Oncology vol. 35 4013–4013 (2017). [Google Scholar]
- 39.Yau T et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Annals of Oncology vol. 30 v874–v875 (2019). [Google Scholar]
- 40.Larkin J et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med 381, 1535–1546 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Hellmann MD et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med 381, 2020–2031 (2019). [DOI] [PubMed] [Google Scholar]
- 42.El-Khoueiry AB et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): Long-term results from CheckMate 040. J. Clin. Orthod 39, 269–269 (2021). [Google Scholar]
- 43.Zhu AX et al. Pembrolizumab (pembro) in patients with advanced hepatocellular carcinoma (HCC): KEYNOTE-224 update. J. Clin. Orthod 36, 4020–4020 (2018). [Google Scholar]
- 44.Finn RS et al. KEYNOTE investigators. Results of KEYNOTE-240: Phase 3 study of pembrolizumab (Pembro) vs. best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J. Clin. Oncol 37, 4004 (2019). [Google Scholar]
- 45.Merle P et al. Pembrolizumab (pembro) vs placebo (pbo) in patients (pts) with advanced hepatocellular carcinoma (aHCC) previously treated with sorafenib: Updated data from the randomized, phase III KEYNOTE-240 study. J. Clin. Orthod 39, 268–268 (2021). [Google Scholar]
- 46.Merle P et al. Sequential treatment with sorafenib (SOR) followed by regorafenib (REG) in patients (pts) with unresectable hepatocellular carcinoma (HCC): Interim analysis of the observational REFINE study. J. Clin. Orthod 38, e16680–e16680 (2020). [Google Scholar]
- 47.Abou-Alfa GK et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med 379, 54–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rimassa L et al. Outcomes based on age in the phase 3 CELESTIAL trial of cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC). Journal of Clinical Oncology vol. 36 4090–4090 (2018). [Google Scholar]
- 49.Kelley RK et al. Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma. Adv. Ther 37, 2678–2695 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu AX et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 16, 859–870 (2015). [DOI] [PubMed] [Google Scholar]
- 51. *.Zhu AX et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology vol. 20 282–296 (2019). [DOI] [PubMed] [Google Scholar]; *The results of this trial led to the approval of Ramucirumab for advanced sorafenib experienced HCC in patients with AFP>400 ng/ml, the first biomarker stratified treatment strategy.
- 52.Schutz FAB, Je Y, Richards CJ & Choueiri TK Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J. Clin. Oncol 30, 871–877 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Sivendran S et al. Treatment-related mortality with vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy in patients with advanced solid tumors: a meta-analysis. Cancer Treat. Rev 38, 919–925 (2012). [DOI] [PubMed] [Google Scholar]
- 54. *.Rimassa L, Danesi R, Pressiani T & Merle P Management of adverse events associated with tyrosine kinase inhibitors: Improving outcomes for patients with hepatocellular carcinoma. Cancer Treat. Rev 77, 20–28 (2019). [DOI] [PubMed] [Google Scholar]; *This paper describes the management of adverse events from systemic therapy for HCC.
- 55.Tridente G Adverse Events and Oncotargeted Kinase Inhibitors. (Academic Press, 2017). [Google Scholar]
- 56.Kim S & Abou-Alfa GK The role of tyrosine kinase inhibitors in hepatocellular carcinoma. Clin. Adv. Hematol. Oncol 12, 36–41 (2014). [PubMed] [Google Scholar]
- 57.Hang XF et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol 67, 613–623 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Hapani S, Sher A, Chu D & Wu S Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology 79, 27–38 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Myers G Immune-related adverse events of immune checkpoint inhibitors: a brief review. Curr. Oncol 25, 342–347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Postow MA & Hellmann MD Adverse Events Associated with Immune Checkpoint Blockade. The New England journal of medicine vol. 378 1165 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Brahmer JR et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol 36, 1714–1768 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naing A et al. Strategies for improving the management of immune-related adverse events. J Immunother Cancer 8, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeung H-C, Oh SE & Kim JH Immune-related Adverse Events: Overview and Management Strategies for the Use of Immune Checkpoint Inhibitors. Journal of Rheumatic Diseases vol. 26 221 (2019). [Google Scholar]
- 64.Kelley RK et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC). J. Clin. Orthod 38, 4508–4508 (2020). [Google Scholar]


