Abstract
Cu,Zn superoxide dismutase (Sod1) is required for insusceptibility of Saccharomyces cerevisiae to oxytetracycline (OTC). Here we report that Sod1 is also required for insusceptibility to doxycycline (DOX). Furthermore, among a range of antioxidant and redox balance mutants, mac1 and ctr1 deletion strains exhibited marked sensitization to OTC and DOX. Certain mutants exhibited a slight sensitivity to methacycline and minocycline. Addition of copper suppressed antibiotic sensitivity. Thus, intracellular copper as well as superoxide dismutase can be critical for eukaryotic tolerance of several tetracycline antibiotics.
The tetracyclines are broad-spectrum antibiotics that block bacterial protein synthesis by inhibition of aminoacyl-tRNA binding to the ribosomal A site (5). As with any useful prokaryote-specific antibiotics, eukaryotic insusceptibility to tetracyclines is a prerequisite for successful chemotherapy. However, adverse reactions to antibiotics are common, arising in around 5 to 10% of patients to whom they are prescribed (11). Reported side effects of tetracyclines include hypersensitivity, photosensitivity, neurotoxicity, hepatotoxicity, teratogenicity, and nephrotoxicity (17). The underlying causes for these effects are unknown in most cases.
With the Saccharomyces cerevisiae yeast model, a single gene was recently identified that is required for eukaryotic insusceptibility to oxytetracycline (OTC) (3). Thus, sod1Δ cells (lacking Cu,Zn-superoxide dismutase) were sensitive to OTC, exhibiting a >95% reduction in colony-forming ability at OTC concentrations that had no effect on the wild type. Sod1p was required for protection against a novel oxidative mode of OTC action. This action was manifested as OTC-induced lipid peroxidation, protein oxidation, and cytotoxicity in sod1Δ cells only (3). Our main objective in the present study was to test whether these findings pertained specifically to Sod1p and OTC or whether they reflected a broader requirement for eukaryotic antioxidant functions in protection against a range of tetracyclines.
S. cerevisiae strains were derived from the parental background BY4741 and are available as the Y00000 series from Euroscarf (Frankfurt, Germany). The gpx1/2/3Δ triple mutant was constructed by short-flanking homology PCR (24) using the URA3, His3MX6, and kanMX6 markers for gene disruption. Organisms were cultured for experimental purposes in liquid yeast extract-peptone-dextrose (YEPD) medium, as described previously (14). To test for antibiotic susceptibility, mid- to late-exponential-phase cultures were diluted to optical densities at 600 nm of ∼2.5, 0.25, 0.025, 0.0025, and 0.00025 for each strain. Samples (4 μl) from each dilution were spotted on YEPD agar supplemented with filter-sterilized antibiotic where specified (filter-sterilized antibiotic stocks were dissolved in water). All antibiotics (as hydrochlorides) were obtained from Sigma, except methacycline-HCl, which was from US Pharmacopoeial Convention (Rockville, Md.).
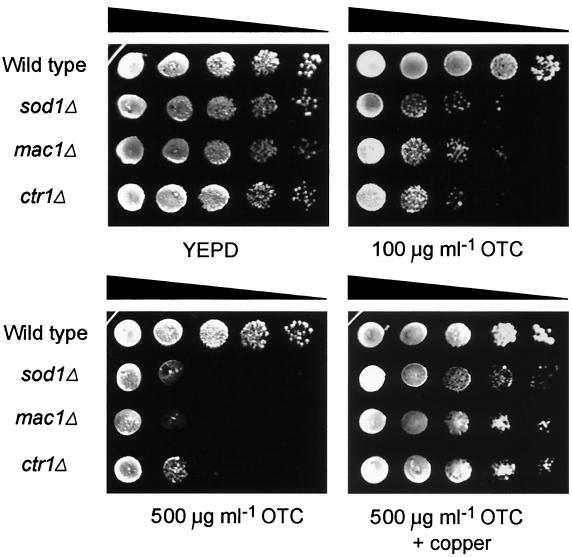
To determine whether antioxidant proteins other than Sod1p might be required for insusceptibility to OTC, we examined a range of S. cerevisiae mutants deficient in the following components of the oxidative stress response and/or maintenance of cellular redox balance: Sod2p (mitochondrial Mn-superoxide dismutase), Ctt1p (cytosolic catalase), Gsh1p (γ-glutamyl cysteine synthetase), Ogg1p (8-oxoguanine DNA glycosylase), PHGpx1p, PHGpx2p, PHGpx3p (phospholipid hydroperoxide glutathione peroxidases), Mxr1p (methionine sulfoxide reductase), Yap1p (oxidative stress response transcription factor), and Mac1p (copper metalloregulatory transcription factor). Of these strains, only mac1Δ S. cerevisiae was found to exhibit a growth defect on OTC similar to that in sod1Δ cells (Fig. 1). The growth of mac1Δ and sod1Δ cells was inhibited at an OTC concentration of 100 μg ml−1, and this effect was accentuated at an OTC concentration of 500 μg ml−1, where growth was almost fully abolished; the same concentrations had no effect on growth of the wild type or the other mutants tested (not shown) (note that some inhibition of sod1Δ S. cerevisiae is detectable at OTC concentrations as low as 10 μg ml−1 [3]). Two of the key genes under Mac1p-control in S. cerevisiae are CTR1, encoding a high-affinity Cu(I) transporter, and FRE1, encoding a cell surface Cu(II)/Fe(III) reductase. Ctr1p is limiting for cellular Cu uptake (7), whereas Fre1p activity affects copper uptake by ∼50% (13) but is limiting for ferric iron uptake (6). To help establish the downstream determinant of susceptibility to OTC in mac1Δ S. cerevisiae, ctr1Δ and fre1Δ mutants were examined for inhibition by OTC. Whereas the growth of fre1Δ S. cerevisiae cells was unaffected by OTC (not shown), ctr1Δ cells exhibited a marked sensitivity to OTC, similar to that of mac1Δ and sod1Δ cells (Fig. 1). Therefore, in addition to Sod1p, Mac1p and Ctr1p are required for yeast insusceptibility to OTC.
FIG. 1.
Susceptibilities of antioxidant-deficient and redox balance-deficient S. cerevisiae mutants to OTC. Dilutions of decreasing cell concentration were spotted from left to right on each plate. S. cerevisiae sod2Δ, ctt1Δ, gsh1Δ, ogg1Δ, gpx1/2/3Δ, mxr1Δ, yap1Δ, and fre1Δ mutants were unaffected by OTC (not shown). Typical results from one of three independent experiments are shown.
Diminished Ctr1p-dependent Cu uptake in mac1Δ or ctr1Δ strains could exacerbate OTC-dependent oxidative damage in two principal ways: (i) via diminished Sod1p activity, since free copper is strictly limited in S. cerevisiae (22) and active Sod1p requires Cu (8, 18), or (ii) via diminished direct antioxidant activity of Cu (19) (note that although loss of Ctr1p function also affects iron accumulation [7], a role for Fe seems unlikely here considering that fre1Δ cells were not sensitized to OTC). We sought to test the first possibility by introducing the SOD1-bearing multicopy plasmid YEp600, described by Nishida et al. (20), to mac1Δ S. cerevisiae. We observed partial suppression of the OTC susceptibility phenotype in these SOD1-overexpressing cells (data not shown) (copper limitation would preclude full suppression with excess Sod1p), indicating that diminished Sod1p activity may at least partly account for the mutant's OTC sensitivity. To test whether cellular Cu could contribute to OTC insusceptibility independently of Sod1p (ii), we examined the OTC susceptibility of sod1Δ S. cerevisiae treated with 50 μM Cu(NO3)2 (note that exogenous complexation with cationic metals would not adversely affect cellular uptake of tetracyclines [23]). Inhibition of growth of the sod1Δ strain (as well as the mac1Δ and ctr1Δ strains) by OTC was suppressed in the presence of copper (Fig. 1), confirming that Cu can act independently of Sod1p in conferring insusceptibility to OTC. Therefore, a combination of the two mechanisms listed above likely accounts for the sensitivity of the mac1Δ and ctr1Δ S. cerevisiae strains to OTC.
Mitochondrial protein synthesis could be a target of tetracyclines, and it is known that deletion of SOD1 and MAC1 results in respiratory deficiency (9, 16). To test whether forced dependency on mitochondrial function might sensitize wild-type S. cerevisiae to tetracyclines, we examined the cells' ability to grow on YEPG medium supplemented with a 500-μg ml−1 concentration of OTC or tetracycline (TET); YEPG contains glycerol and ethanol as respiratory carbon sources (12). As on YEPD, the growth of wild-type S. cerevisiae on YEPG was unaffected by these antibiotics (data not shown). Furthermore, some limited respiratory growth of the sod1Δ strain that was discernible on YEPG was abolished by OTC (not shown). Therefore, the results evident during forced respiratory growth were similar to our original findings using YEPD, suggesting that the observed effects are not directly linked to mitochondrial function.
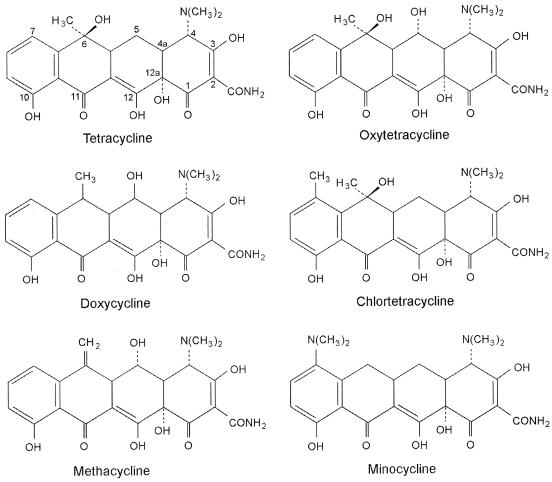
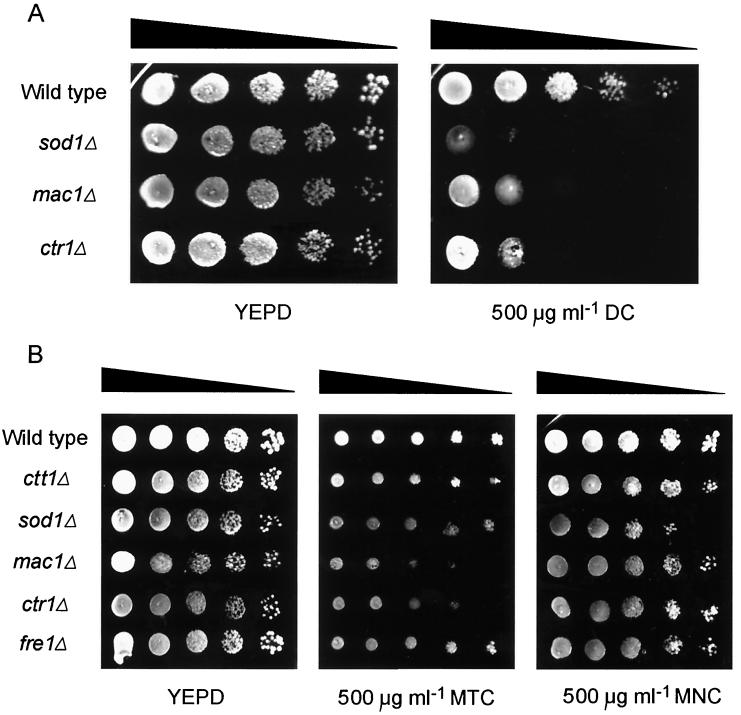
Previously, Sod1p appeared to be required for insusceptibility to OTC specifically, since growth with TET was unaffected by SOD1 deletion (3). The presence of a hydroxyl group at the C-5 position distinguishes OTC from TET (see Fig. 2). An -OH group occurs at the same position also in doxycycline (DOX). Therefore, we tested sod1Δ S. cerevisiae for growth in the presence of DOX (Fig. 3A). The mutant exhibited a marked sensitivity to DOX, similar to that for OTC; as with OTC, wild-type cells grew normally up to a DOX concentration of 500 μg ml−1. The growth of the mac1Δ and ctr1Δ deletion strains, but not the fre1Δ strain (data not shown), was also strongly inhibited by DOX (Fig. 3A). Therefore, these single gene products establish the insusceptibility of yeast to DOX as well as OTC.
FIG. 2.
Structures of the tetracyclines.
FIG. 3.
Susceptibilities of antioxidant-deficient and redox balance-deficient S. cerevisiae mutants to a range of tetracyclines. Dilutions of decreasing cell concentration were spotted from left to right on each plate. Results are shown for doxycycline (DC) (A) and for methacycline (MTC) and minocycline (MNC) (B). Note that spots were smaller with MTC due to the surface tension imparted by the antibiotic. The mutants were not affected by TET or CTC (not shown). Typical results from one of three independent experiments are shown.
To test further the specificity for particular tetracycline antibiotics, the growth of some key mutants was examined in the presence of a range of tetracyclines: chlortetracycline (CTC), methacycline (MTC), minocycline (MIN), and TET (OTC and DOX served as routine positive controls). To ensure that any moderate susceptibility was not missed, 500 μg of antibiotic ml−1 was used for tests. All of the strains tested (wild type, ctt1Δ, sod1Δ, mac1Δ, ctr1Δ, and fre1Δ) exhibited full tolerance of TET and CTC. MTC was of particular interest, since it has an -OH group at the C-5 position (see above). However, the sod1Δ mutant grew normally in the presence of MTC (Fig. 3B), eroding the model in which the -OH functional group plays a key role in susceptibility. A very slight but consistent sensitization to MTC was apparent in the mac1Δ and ctr1Δ strains and also in sod1Δ S. cerevisiae grown in the presence of MIN. All of the other strains tested grew normally at a MIN concentration of 500 μg ml−1.
One property that could account for oxidative stress generated by tetracyclines is the high metal-binding affinities of these antibiotics (10, 21); certain complexed metals can act as foci for redox cycling activity and/or free radical generation (2). Although the metal-binding affinities of OTC are very similar to those of TET and CTC (10), OTC and DOX are distinctive in having greater polarity than the other tetracyclines (4). Thus, one possibility could be that OTC and DOX complexes partition more readily into (polar) subcellular milieus that favor reactions to which Sod1- or copper-deficient cells might be susceptible. Note that total cellular OTC uptake is not affected by SOD1 deletion (3).
This report underscores the potentially fragile nature of antibiotic insusceptibility in eukaryotes. Cellular copper homeostasis and superoxide dismutase activity are critical determinants of yeast insusceptibility to both OTC and DOX. Since the mechanisms for handling oxidative stress and regulating copper homeostasis are quite similar in higher eukaryotes and S. cerevisiae (1, 15), the insusceptibility of higher eukaryotes to tetracyclines may well also rely on these functions.
Acknowledgments
This work was supported by an award from the University of Nottingham Research Committee.
Edith B. Gralla (University of California, Los Angeles) kindly supplied the plasmid YEp600.
REFERENCES
- 1.Askwith C, Kaplan J. Iron and copper transport in yeast and its relevance to human disease. Trends Biochem Sci. 1998;123:135–138. doi: 10.1016/s0968-0004(98)01192-x. [DOI] [PubMed] [Google Scholar]
- 2.Avery S V. Metal toxicity in yeasts and the role of oxidative stress. Adv Appl Microbiol. 2001;49:111–142. doi: 10.1016/s0065-2164(01)49011-3. [DOI] [PubMed] [Google Scholar]
- 3.Avery S V, Malkapuram S, Mateus C, Babb K S. Cu/Zn superoxide dismutase is required for oxytetracycline resistance of Saccharomyces cerevisiae. J Bacteriol. 2000;182:76–80. doi: 10.1128/jb.182.1.76-80.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwood R K, English A R. Structure-activity relationships in the tetracycline series. In: Perlman D, editor. Structure-activity relationships among the semisynthetic antibiotics. London, United Kingdom: Academic Press; 1977. pp. 397–426. [Google Scholar]
- 5.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dancis A, Klausner R D, Hinnebusch A G, Barriocanal J G. Genetic evidence that ferric reductase is required for iron uptake in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:2294–2301. doi: 10.1128/mcb.10.5.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dancis A, Yuan D S, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner R D. Molecular characterization of a copper transport protein in Saccharomyces cerevisiae—an unexpected role for copper in iron transport. Cell. 1994;76:393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 8.Dancis A, Haile D, Yuan D S, Klausner R D. The Saccharomyces cerevisiae copper transport protein (Ctr1p)—biochemical characterization, regulation by copper, and physiological role in copper uptake. J Biol Chem. 1994;269:25660–25667. [PubMed] [Google Scholar]
- 9.De Freitas J M, Liba A, Meneghini R, Valentine J S, Gralla E B. Yeast lacking Cu-Zn superoxide dismutase show altered iron homeostasis. J Biol Chem. 2000;275:11645–11649. doi: 10.1074/jbc.275.16.11645. [DOI] [PubMed] [Google Scholar]
- 10.Duluisio J T, Martin A N. Metal complexation of the tetracycline hydrochlorides. J Med Chem. 1963;6:16–20. doi: 10.1021/jm00337a003. [DOI] [PubMed] [Google Scholar]
- 11.Finch R G. Adverse reactions to antibiotics. In: Greenwood D, editor. Antimicrobial chemotherapy. 4th ed. Oxford, United Kingdom: Oxford University Press; 2000. pp. 200–211. [Google Scholar]
- 12.Fox T D, Folley L S, Mulero J J, McMullin T W, Thorsness P E, Hedin L O, Costanzo M C. Analysis and manipulation of mitochondrial genes. Methods Enzymol. 1991;194:149–165. doi: 10.1016/0076-6879(91)94013-3. [DOI] [PubMed] [Google Scholar]
- 13.Hassett R, Kosman D J. Evidence for Cu(II) reduction as a component of copper uptake by Saccharomyces cerevisiae. J Biol Chem. 1995;270:128–134. doi: 10.1074/jbc.270.1.128. [DOI] [PubMed] [Google Scholar]
- 14.Howlett N G, Avery S V. Induction of lipid peroxidation during heavy metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation. Appl Environ Microbiol. 1997;63:2971–2976. doi: 10.1128/aem.63.8.2971-2976.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamieson D J. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast. 1998;14:1511–1527. doi: 10.1002/(SICI)1097-0061(199812)14:16<1511::AID-YEA356>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Jungmann J, Reins H A, Lee J, Romeo A, Hassett R, Kosman D, Jentsch S. MAC1, a nuclear regulatory protein related to Cu-dependent transcription factors is involved in Cu/Fe utilization and stress resistance in yeast. EMBO J. 1993;12:5051–5056. doi: 10.1002/j.1460-2075.1993.tb06198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kucers A, Crowe S M, Grayson M L, Hoy J F. The use of antibiotics: a clinical review of antibacterial, antifungal and antiviral drugs. 5th ed. Oxford, United Kingdom: Butterworth-Heinemann; 1997. [Google Scholar]
- 18.Labbe S, Zhu Z W, Thiele D J. Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J Biol Chem. 1997;272:15951–15958. doi: 10.1074/jbc.272.25.15951. [DOI] [PubMed] [Google Scholar]
- 19.Liu X F, Culotta V C. The requirement for yeast superoxide dismutase is bypassed through mutations in BSD2, a novel metal homeostasis gene. Mol Cell Biol. 1994;14:7037–7045. doi: 10.1128/mcb.14.11.7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishida C R, Gralla E B, Valentine J S. Characterization of 3 yeast copper zinc superoxide dismutase mutants analogous to those coded for in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 1994;91:9906–9910. doi: 10.1073/pnas.91.21.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinlan G J, Gutteridge J M C. Hydroxyl radical generation by the tetracycline antibiotics with free radical damage to DNA, lipids, and carbohydrate in the presence of iron and copper salts. Free Radic Biol Med. 1988;5:341–348. doi: 10.1016/0891-5849(88)90106-2. [DOI] [PubMed] [Google Scholar]
- 22.Rae T D, Schmidt P J, Pufahl R A, Culotta V C, O'Halloran T V. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 23.Schnappinger D, Hillen W. Tetracyclines: antibiotic action, uptake, and resistance mechanisms. Arch Microbiol. 1996;165:359–369. doi: 10.1007/s002030050339. [DOI] [PubMed] [Google Scholar]
- 24.Wach A, Brachat A, Alberti-Segui C, Rebischung C, Philippsen P. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast. 1997;13:1065–1075. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1065::AID-YEA159>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]