Abstract
Background
Immune checkpoint inhibitors (ICIs) are increasingly used to treat advanced malignancies. However, they are associated with the development of multiple gastrointestinal immune-related adverse events (GI-irAEs). We aimed to evaluate the types and severity of GI-irAEs associated with ICI therapy, to identify potential risk factors for developing GI-irAEs and to determine the relationship of GI-irAEs development to tumor responsiveness and overall survival.
Methods
All patients who received ICIs for advanced malignancies at our center were included. Medical records were reviewed, and data extraction included: baseline demographic characteristics, immunotherapy regimens, development of GI-irAEs, response to treatment, and overall survival. Overall survival was calculated from the date of treatment initiation and estimated by the Kaplan-Meier method.
Results
Five hundred sixty-seven patients received ICI therapy for stage IV malignancies. Forty-one (7%) patients experienced at least one GI-irAE. Among those experiencing GI-irAEs, 23 (56%) developed hepatitis, 17 (42%) developed colitis, four (10%) developed pancreatitis, and two (5%) developed gastritis. Patients who developed GI-irAEs experienced a better response to ICI therapy compared to patients who did not develop GI-irAEs (41% vs. 27%, P = 0.003). The 2-year overall survival rate of stage IV cancer patients who developed GI-irAEs was 62% (95% confidence interval (CI): 49 - 79) and 36% for those who did not develop GI-irAEs (95% CI: 32 - 41) (P = 0.002). The median follow-up time of surviving patients was 28 months. Twelve (29%) of the patients receiving dual ICI therapy developed GI-irAEs.
Conclusion
Hepatitis, colitis, and pancreatitis were the most commonly encountered GI-irAEs with ICI therapy. Development of these GI-irAEs was associated with superior tumor responsiveness and better overall survival.
Keywords: Immune checkpoint inhibitors, Gastrointestinal toxicities, Immune-related adverse events, Cancer, Tumor response, Survival
Introduction
Their use is well established for treating metastatic melanoma and non-small cell lung cancer, and an expanding body of research has recently revealed promising results in a plethora of other malignancies refractory to traditional treatments [1-3]. Immune checkpoint inhibitors (ICIs) emerged as a novel class of antineoplastic medications that specifically target cell checkpoints to stimulate an antitumor immune response back in 2011 [4]. Co-inhibitory receptors control the activity and intensity of the adaptive immune response and therefore function as immune checkpoints. These immune checkpoints induce exhaustion of T lymphocytes which is crucial to avoid exacerbated immune responses and autoimmunity. ICIs inhibit the downregulation of the immune system by blocking these checkpoints and activate immune cell function via different pathways to subsequently result in increased activation of T cells against the tumor antigens. More recently, ICIs have yielded unparalleled and substantial responses in a significant percentage of cancer patients leading to the approval of six checkpoint inhibitors by the US Food and Drug Administration (FDA) [1]. These monoclonal antibodies (mAbs) have proven to be effective in treating melanoma, non-small cell lung cancer, urothelial carcinoma, renal cell carcinoma, and Hodgkin lymphoma.
Ongoing studies are evaluating their use in the treatment of other malignancies. Among the three classes of ICIs, mAbs targeting two of the immune checkpoints (cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1)) are the two most widely used in clinical practice. The function of CTLA-4 and PD-1 in enhancing antitumor responses is largely distinct. CTLA-4 is believed to regulate T-cell proliferation during the initial phase of an immune response in the lymph nodes while PD-1 suppresses T cells in the latter part of the immune at the level of peripheral tissues. The clinical profiles of immuno-oncology agents inhibiting these two checkpoints therefore vary based on their mechanistic differences. Their use is well established for treating metastatic melanoma and non-small cell lung cancer, and an expanding body of research has revealed promising results in a plethora of other malignancies refractory to traditional treatments [1-3].
Despite the impressive response and survival outcomes experienced by patients treated with ICIs [5], many immune-related adverse events (irAEs), affecting almost all organ systems, have been reported. The etiology of irAEs has been hypothesized to stem from the blockade of immune inhibitory mechanisms and activation of the body’s immune system, which is the same immunologic mechanism involved in the therapeutic action of the ICIs [6, 7]. Only second to dermatological side effects, gastrointestinal irAEs (GI-irAEs) are relatively common with enterocolitis, hepatitis, pancreatitis, and celiac disease being the most frequently reported [8-10]. The expertise in the management of GI-irAEs is restricted due to the lack of prospective clinical data often leading to delayed diagnosis and inadequate treatments [11]. In fact, a study showed that treatment was discontinued in 38% of the included patients due to the development of adverse events during the induction phase [12]. Interestingly, colitis (10%) was the most frequent irAE which led to treatment discontinuation followed by elevated aminotransferases (9%).
As such, despite the high incidence, the impact of developing GI-irAEs on treatment response and survival remains largely unknown. Although several studies have identified a trend towards enhanced tumor response in individuals who develop irAEs, none of these studies were specific to GI toxicities [13-17]. Hence, we aimed to evaluate the incidence, severity, and types of GI-irAEs associated with various types of ICIs. In addition, we studied potential risk factors associated with GI-irAEs development. Finally, we explored the effects of developing these toxicities on cancer response to treatment with ICI and overall survival (OS) in patients with various metastatic malignancies.
Materials and Methods
Study design
This was a retrospective observational study conducted over several sites within the Cleveland Clinic healthcare system. The detailed study objectives, design and methodology were reviewed and approved by the Cleveland Clinic’s Institutional Review Board (IRB) prior to initiation of this study. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with Helsinki Declaration.
Inclusion criteria
Male and female adult patients (age ≥ 18 years old), with a confirmed diagnosis of metastatic cancer who received ICIs therapy between May 1, 2013 and February 28, 2019 were included in this study. ICI agents included in the study were anti-PD-1 (nivolumab and pembrolizumab), anti-PD-L1 (atezolizumab and avelumab) and anti-CTLA-4 (ipilimumab).
Exclusion criteria
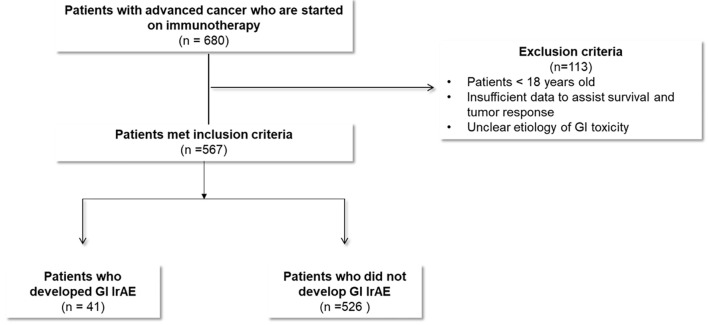
Patients in whom the etiology of GI toxicity was unclear and potential confounders existed, were excluded from this study. For example, some patients with new or progressing liver metastasis presented with acute hepatitis picture but were also being treated with ICI. When liver biopsy was not done for different reasons, the accurate distinction of hepatitis etiology was difficult (Fig. 1).
Figure 1.
Study flow diagram.
Data collection methods
The Cleveland Clinic pharmacy database provided the authors with a full list of all adult patients who were treated with these ICIs within the above time frame. A fully integrated electronic medical record (EPIC) was utilized to screen patients’ eligibility based on the above inclusion criteria. A total of 567 eligible patients were identified. A detailed chart review was conducted for baseline demographics; underlying malignancy type and stage; specific ICI therapy with start date, end date, and clinical response of cancer to immunotherapy; details of GI-irAEs including how it was diagnosed, dominant symptoms, severity grades, treatment of GI toxicity; and survival data. Authors utilized a secure Red Cap research software to design a data collection sheet that included all of the above variables. Authors who worked on data collection and data analysis were provided a secure access to the Red Cap data collection sheet and patient information. All relevant data obtained from the electronic medical records were entered into the Red Cap data collection sheets where each patient had a separate sheet. Collected data were analyzed to compare patients who developed GI toxicity to patients without GI toxicity.
Diagnosing GI-irAEs
Based on chart review, patients were educated about potential adverse events by the time ICIs were initiated. Patients were encouraged to report any new symptoms to their oncology clinic. In addition, GI system symptoms were reviewed in each oncology follow-up visit. Almost all patients had a complete metabolic panel (including alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and total bilirubin) at least at baseline prior to starting ICIs, at 4 - 6 weeks and at 10 - 12 weeks after starting ICI. Lipase was only checked in patients with suggestive symptoms or as clinically indicated. Further advanced tests like colonoscopy, biopsies or imaging studies were done on a case-by-case basis as clinically indicated.
Definitions
GI-irAEs were defined as evidence of new GI toxicity after starting ICIs with no alternative explanation (as a diagnosis of exclusion) or as clearly documented by oncologists or gastroenterologist to be caused by ICIs.
Immunotherapy-Response Evaluation Criteria In Solid Tumors (i-RECIST) guidelines were used by oncologists to define disease progression, partial response, complete response, and stable disease [18-21]. This includes the following: 1) Immune-related complete response defined as complete resolution of all measurable and non-measurable lesions, with no new lesions, on two occasions at least 4 weeks apart. 2) Immune-related partial response defined as a decrease in the total tumor burden of 50% or more compared with baseline, on two occasions at least 4 weeks apart. This category allows for the inclusion of progression of some lesions or the appearance of new lesions as long as the total tumor burden meets the response criterion. 3) Immune-related progressive disease: an increase in the total tumor burden of 25% or more relative to the minimum recorded tumor burden. This must be confirmed by a second, consecutive assessment no fewer than 4 weeks after the initial documentation of an increase in tumor. 4) Immune-related stable disease: not meeting the criteria for either a partial or complete response or for progressive disease.
As this was a retrospective study, we relied on the treating oncologist’s opinion and documentation to define disease progression, partial response, complete response and stable disease. To simplify our documentation and analysis, we classified patients based on their response into two main categories: 1) patients who had disease progression, we referred to as “Patients with no response to treatment”. On the other hand, patients who did not meet criteria for disease progression (including patients who had partial response, complete response or stable disease), we referred to as “patients with favorable response to treatment”. Some patients did not have sufficient documentation on their response status, and we referred to this group as “patients with unknown response”.
GI adverse events were graded using the US National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. These criteria generally grade irAEs on a scale of 1 - 5 (1 = mild event/reaction, 2 = moderate event/reaction, 3 = severe event/reaction, 4 = life-threatening event/reaction, 5 = death) [22].
Statistical analysis
Baseline characteristics, type of metastatic cancer treated, the response of cancer to treatment with ICI, and OS were compared between patients who developed GI-irAEs versus those who did not develop GI-irAEs. Using a two-sample t-test for continuous variables such as age and body mass index, Chi-square test and Fisher’s exact test were used for categorical variables including gender, race, malignancy, types/courses of ICI therapy and response to ICI therapy. OS was calculated from the initiation of ICI therapy, estimated by the Kaplan-Meier method, and compared by the log-rank test. The cumulative incidence of adverse events was calculated from initiation of ICI therapy with discontinuation of immunotherapy and death as competing risks and compared with Gray’s test. We performed multivariable Cox hazard analysis to assess whether GI adverse events are independent predictors of OS after adjusting for age (per 1-year increase) and immunotherapy (mono- vs. dual-therapy). A P value of less than 0.05 was considered statistically significant. All statistical calculations were made using R statistical software version 3.4.0 (R-Foundation for Statistical Computing, Vienna, Austria).
Results
Five hundred sixty-seven patients were identified who received ICI therapy for stage 4 malignancies and 41 (7%) patients experienced GI-irAEs. Non-small cell lung cancer (44%), melanoma (17%), and renal cell carcinoma (13%) were the most common underlying malignancies. The four most frequently used ICI therapy regimens were nivolumab (used in 306 (50%) patients), pembrolizumab (used in 142 (23%) patients), atezolizumab (used in 62 (10%) patients), and nivolumab/ipilimumab combination (used in 44 (7%) of patients). Combination ICI therapy was most commonly used in patients with breast cancer (29%), melanoma (19%), ovarian cancer (11%), and renal cell cancer (9%). The patient and treatment characteristics are summarized in Table 1. Sixty-five percent of our patients were males. The median age and body mass index at the initiation of ICI therapy were 66 years (range: 20 - 94) and 26 kg/m2 (range: 14 - 51), respectively.
Table 1. Gastrointestinal Toxicity and Baseline Characteristics.
| Characteristics | All patients, n (%) | GI adverse event, n (%) | No GI adverse event, n (%) | P value |
|---|---|---|---|---|
| Number of patients | 567 (100) | 41 (100) | 526 (100) | - |
| Age, years | ||||
| Median (range) | 66 (20 - 94) | 67 (20 - 94) | 64 (34 - 81) | 0.41 |
| > 60 | 377 (66.5) | 28 (68.3) | 349 (65.8) | 0.80 |
| Male gender | 369 (65.1) | 30 (73.2) | 339 (64.4) | 0.26 |
| White race | 510 (89.9) | 41 (100) | 469 (89.2) | 0.026 |
| BMI, kg/m2, median (range) | 26 (14 - 51) | 26 (17 - 40) | 26 (14 - 51) | 0.34 |
| Malignancy | ||||
| Melanoma | 96 (16.9) | 18 (43.9) | 78 (14.8) | < 0.0001 |
| Non-small cell lung cancer | 250 (44.1) | 9 (22.0) | 241 (45.8) | 0.003 |
| Renal cell carcinoma | 76 (13.4) | 7 (17.1) | 69 (13.1) | 0.47 |
| Breast | 7 (1.2) | 2 (4.9) | 5 (1.0) | 0.028 |
| Bladder/urothelial cancer | 49 (8.6) | 1 (2.4) | 48 (9.1) | 0.14 |
| Small cell lung cancer | 19 (3.4) | 1 (2.4) | 18 (3.4) | 0.74 |
| Lymphoma/multiple myeloma | 19 (3.4) | 1 (2.4) | 18 (3.4) | 0.74 |
| Ovarian | 9 (1.6) | 1 (2.4) | 8 (1.5) | 0.65 |
| Other | 22 (3.9) | 1 (2.4) | 21 (4.0) | 0.62 |
| Head and neck | 20 (3.5) | 0 (0) | 20 (3.8) | 0.20 |
| No of cycles of immunotherapy | 608 | 41 | 567 | - |
| Response to immunotherapya | 0.003 | |||
| Yes | 167 (27.5) | 17 (41.4) | 150 (26.5) | |
| No | 334 (54.9) | 12 (29.3) | 322 (56.8) | |
| Unknown | 107 (17.6) | 12 (29.3) | 95 (16.7) | |
| Intensity of each immunotherapy cyclea | < 0.0001 | |||
| Dual immunotherapy | 55 (9.0) | 12 (29.3) | 43 (7.6) | |
| Mono-immunotherapy | 553 (91.0) | 29 (70.7) | 524 (92.4) | |
| Immunotherapy regimensa | ||||
| Nivolumab | 306 (50.3) | 13 (31.7) | 293 (51.7) | 0.014 |
| Pembrolizumab | 142 (23.4) | 9 (22.0) | 133 (23.5) | 0.83 |
| Ipilimumab | 29 (4.8) | 3 (7.3) | 26 (4.6) | 0.43 |
| Atezolizumab | 62 (10.2) | 2 (4.9) | 60 (10.6) | 0.24 |
| Avelumab | 8 (1.3) | 2 (4.9) | 6 (1.1) | 0.038 |
| Durvalumab | 6 (1.0) | 0 (0) | 6 (1.1) | 0.51 |
| Nivolumab and ipilimumab | 44 (7.2) | 11 (26.8) | 33 (5.8) | < 0.0001 |
| Pembrolizumab and ipilimumab | 5 (0.8) | 1 (2.4) | 4 (0.7) | 0.24 |
| Nivolumab and atezolizumab | 3 (0.5) | 0 (0) | 3 (0.5) | 0.64 |
| Nivolumab and pembrolizumab | 1 (0.2) | 0 (0) | 1 (0.2) | 0.79 |
| Pembrolizumab and atezolizumab | 1 (0.2) | 0 (0) | 1 (0.2) | 0.79 |
| Pembrolizumab and avelumab | 1 (0.2) | 0 (0) | 1 (0.2) | 0.79 |
aDominator is number of total immunotherapy cycles, not number of patients. GI: gastrointestinal; BMI: body mass index.
Among the 41 patients who developed GI-irAEs, hepatitis developed in 23 (56%) patients. Sixty-five percent of the hepatitis patients were asymptomatic and diagnosed based on abnormal laboratory tests only. Colitis was diagnosed in 17 (42%) patients and diarrhea was the most prevalent symptom in patients with colitis (82%) and patients with pancreatitis (50%). Pancreatitis occurred in four (10%) of patients with GI toxicity, and two (5%) developed gastritis (Table 2). ICI therapy-induced colitis, hepatitis, gastritis, and pancreatitis were most commonly observed in patients who received the nivolumab/ipilimumab combination (Table 3).
Table 2. Features and Severity of Gastrointestinal Toxicity Subtypes.
| Characteristics | All GI adverse events, n (%) | Colitis, n (%) | Hepatitis, n (%) | Gastritis, n (%) | Pancreatitis, n (%) |
|---|---|---|---|---|---|
| Number of patients | 41 (100) | 17 (100) | 23 (100) | 2 (100) | 4 (100) |
| Severity grade | |||||
| 1 or 2 | 23 (56.1) | 7 (41.2) | 16 (72.7) | 2 (100) | 2 (50.0) |
| 3 or more | 18 (43.9) | 10 (58.8) | 6 (27.3) | 0 (0) | 2 (50.0) |
| 1 | 9 (22.0) | 2 (11.8) | 8 (34.8) | 1 (50.0) | 0 (0) |
| 2 | 14 (34.1) | 5 (29.4) | 9 (39.1) | 1 (50.0) | 2 (50.0) |
| 3 | 14 (34.1) | 8 (47.0) | 4 (17.4) | 0 (0) | 2 (50.0) |
| 4 | 3 (7.3) | 2 (11.8) | 1 (4.3) | 0 (0) | 0 (0) |
| 5 | 1 (2.4) | 0 (0) | 1 (4.3) | 0 (0) | 0 (0) |
| Dominant symptoms | |||||
| Laboratory abnormalities only | 16 (39.0) | 0 (0) | 15 (65.2) | 0 (0) | 1 (25.0) |
| Diarrhea | 13 (31.7) | 12 (70.6) | 2 (8.7) | 1 (50.0) | 2 (50.0) |
| Abdominal pain | 7 (17.1) | 2 (11.8) | 4 (17.4) | 0 (0) | 1 (25.0) |
| Abdominal pain and diarrhea | 2 (4.9) | 2 (11.8) | 1 (4.3) | 0 (0) | 0 (0) |
| Severe fatigue | 2 (4.9) | 1 (5.9) | 1 (4.3) | 0 (0) | 0 (0) |
| Dyspepsia | 1 (2.4) | 0 (0) | 0 (0) | 1 (50.0) | 0 (0) |
| Treated with steroid | |||||
| Yes (> 4 weeks) | 27 (65.9) | 10 (58.8) | 18 (78.3) | 1 (50.0) | 1 (25.0) |
| Yes (< 4 weeks) | 8 (19.5) | 5 (29.4) | 2 (8.7) | 1 (50.0) | 1 (25.0) |
| No | 3 (7.3) | 0 (0) | 2 (8.7) | 0 (0.0) | 1 (25.0) |
| Unknown | 3 (7.3) | 2 (11.8) | 1 (4.3) | 0 (0.0) | 1 (25.0) |
GI: gastrointestinal.
Table 3. Gastrointestinal Toxicity by Immunotherapy Regimen.
| Characteristics | Nivolumab, n (%) | Pembrolizumab, n (%) | Ipilimumab, n (%) | Atezolizumab, n (%) | Avelumab, n (%) | Nivolumab and ipilimumab, n (%) | Pembrolizumab and ipilimumab, n (%) |
|---|---|---|---|---|---|---|---|
| No. of total cycles | 306 (100) | 142 (100) | 29 (100) | 62 (100) | 8 (100) | 44 (100) | 5 (100) |
| Severity grade | |||||||
| 1 or 2 | 9 (2.9) | 10 (7.0) | 1 (3.4) | 1 (1.6) | 2 (25) | 7 (15.9) | 0 (0) |
| 3 or more | 4 (1.0) | 4 (2.8) | 2 (6.9) | 1 (1.6) | 0 (0) | 6 (13.6) | 1 (20) |
| 1 | 4 (1.3) | 2 (1.4) | 1 (3.4) | 0 (0) | 1 (12.5) | 2 (4.5) | 0 (0) |
| 2 | 5 (1.6) | 4 (2.8) | 0 (0) | 1 (1.6) | 1 (12.5) | 5 (11.4) | 0 (0) |
| 3 | 2 (0.7) | 4 (2.8) | 1 (3.4) | 1 (1.6) | 0 (0) | 5 (11.4) | 1 (20) |
| 4 | 1 (0.3) | 0 (0) | 1 (3.4) | 0 (0) | 0 (0) | 1 (2.3) | 0 (0) |
| 5 | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Type of adverse event | |||||||
| Colitis | 3 (1.0) | 3 (2.1) | 2 (6.9) | 1 (1.6) | 0 (0) | 7 (15.9) | 1 (20) |
| Hepatitis | 9 (2.9) | 7 (4.9) | 1 (3.4) | 0 (0) | 2 (25) | 4 (9.1) | 0 (0) |
| Gastritis | 0 (0) | 0 (0) | 0 (0) | 1 (1.6) | 0 (0) | 1 (2.3) | 0 (0) |
| Pancreatitis | 1 (0.3) | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 2 (4.5) | 0 (0) |
Grade 3 or greater GI adverse events developed in 18 (44%) patients with GI toxicity. Grade 3 or higher GI-irAEs were identified in patients who received pembrolizumab/ipilimumab (20%), nivolumab/ipilimumab (14%), ipilimumab (7%), pembrolizumab (3%), atezolizumab (2%), and nivolumab (1%). In patients who developed significant GI-irAEs, 27 (66%) were treated with steroid therapy for longer than 4 weeks and eight (20%) patients were treated with steroids for less than 4 weeks.
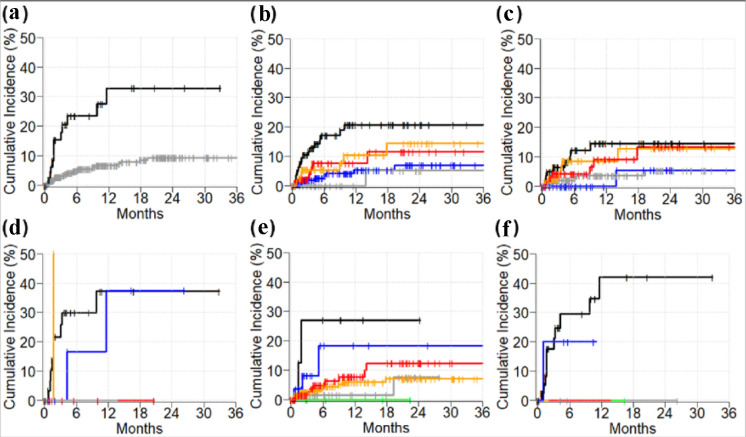
A favorable tumor response was documented in 41% of patients who developed ICI-associated GI-irAEs compared to a 27% favorable response rate in patients who did not develop GI-irAEs (P = 0.003). Dual immunotherapy was used in 12 (29%) and 43 (8%) patients with and without GI-irAEs, respectively (P < 0.0001). Median follow-up time of alive patients was 28 months. Figure 2 depicts the cumulative incidence of GI-IrAEs in several subgroups. Two-year cumulative incidences of GI-irAEs in dual- and mono-ICI cohorts were 33% (95% confidence interval (CI): 16 - 50) and 9% (95% CI: 6 - 13), respectively (P < 0.0001) (Fig. 2a). Two-year cumulative incidence of GI-irAEs in patients with melanoma (21%, 95% CI: 12 - 30) was higher compared to non-small cell lung carcinoma (7%, 95% CI: 2 - 12, P = 0.0002) and urothelial/bladder (5%, 95% CI: 5 - 15, P = 0.028). However, the 2-year cumulative incidence of GI-irAEs in renal cell carcinoma (14%, 95% CI: 4 - 25, P = 0.12), and all other cancers (11%, 95% CI: 2 - 21, P = 0.06) was not statistically significant.
Figure 2.
Cumulative incidence of immunotherapy-induced gastrointestinal adverse events. (a) Dual-immunotherapy (black) vs. mono-immunotherapy (gray). Cancer types in all patients (b), in monotherapy cohort (c), and in dual-immunotherapy cohort (d); melanoma (black), renal cell carcinoma (orange), other (red), non-small cell lung cancer (blue), bladder/urothelial (gray). (e) Drug names in mono-immunotherapy cohort: avelumab (black), ipilimumab (blue), pembrolizumab (red), atezolizumab (gray), nivolumab (orange), and durvalumab (green). (f) Drug names in dual-immunotherapy cohort: nivolumab and ipilimumab (black), pembrolizumab and ipilimumab (blue).
Among patients who received mono-immunotherapy, 2-year cumulative incidence of GI-irAEs in the avelumab cohort (27%, 95% CI: 17 - 59) was higher compared to atezolizumab (8%, 95% CI: 6 - 19, P = 0.020), and nivolumab (7%, 95% CI: 3 - 11, P = 0.022) cohorts. However, the 2-year cumulative incidence of GI-irAEs in ipilimumab (18%, 95% CI: 11 - 40, P = 0.44), and pembrolizumab (12%, 95% CI: 4 - 21, P = 0.08) was comparable to the avelumab cohort. No patients had durvalumab-induced GI adverse event (Fig. 2e).
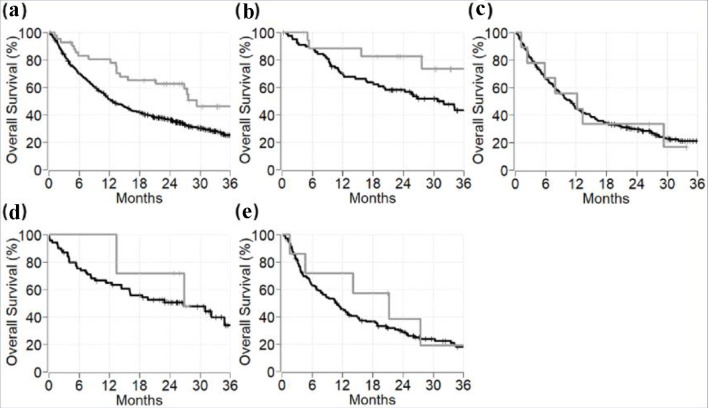
The 2-year OS of stage IV cancer patients who developed or who did not develop GI-irAEs was 62% (95% CI: 49 - 79) and 36% (95% CI: 32 - 41), respectively (P = 0.002) (Fig. 3a). The 2-year OS of patients experiencing GI-irAEs compared to the survival of patients not experiencing GI-irAEs for melanoma was 82% (95% CI: 66 - 100) vs. 58% (95% CI: 48 - 70), P = 0.08; for non-small-cell lung cancer was 33% (95% CI: 13 - 84) vs. 29% (95% CI: 24 - 36), P = 0.8; and for renal cell cancer was 71% (95% CI: 45 - 100) vs. 50% (95% CI: 40 - 64), P = 0.3 (Fig. 3). On multivariable analysis, GI adverse events were significantly associated with improvement in OS among all patients (hazard ratio (HR) 0.53, 95% CI: 0.33 - 0.84, P = 0.008) and among melanoma patients (HR 0.34, 95% CI: 0.12 - 0.96, P = 0.042) (Table 4).
Figure 3.
Overall survival of metastatic cancer patients with versus without immunotherapy-induced gastrointestinal adverse events among different cancer types. Gray line represents patients with immunotherapy-induced gastrointestinal adverse events. Black line represents patients without immunotherapy-induced gastrointestinal adverse events. (a) All malignancies. (b) Melanoma. (c) Non-small cell lung carcinoma. (d) Renal cell carcinoma. (e) All other cancer types.
Table 4. Multivariable Cox Proportional Hazards Analysis of Overall Survival.
| Variables | Multivariable analysisa |
|
|---|---|---|
| Hazard ratio (95% CI) | P value | |
| Gastrointestinal adverse events (yes vs. no) | ||
| All patients | 0.528 (0.331 - 0.844) | 0.008 |
| Cancer subtypes | ||
| Melanoma | 0.339 (0.119 - 0.962) | 0.042 |
| Lung cancer | 1.072 (0.519 - 2.212) | 0.85 |
| Renal cell carcinoma | 0.665 (0.202 - 2.185) | 0.50 |
| All other cancers | 0.656 (0.240 - 1.793) | 0.41 |
aAnalysis was adjusted for age (per 1-year increase) and immunotherapy (mono- vs. dual-therapy). CI: confidence interval.
Discussion
This large study evaluating the toxicities of ICI therapy on the GI tract of patients with metastatic malignancies found hepatitis and colitis to be the most common adverse events attributable to ICI therapy. Combination ICI therapy was associated with more GI-irAEs, and this study confirmed the enhanced anti-tumor responses in patients who experienced GI-irAEs. Incidence of GI-irAEs was higher in patients with melanoma that consequently seemed to result in better OS in these patients. Interestingly, patients with melanoma also had a higher proportion of combination immunotherapy compared to other malignancies. Adverse events occurred at a higher rate in patients receiving combination immunotherapy in our study that corroborates with the study findings of a recent systematic review by Mearns et al [23]. These findings indirectly highlight the better tumor response and OS in patients receiving combination immunotherapy over patients who receive monotherapy. Another possible explanation for a higher tumor response in melanoma patients could be a higher sample size of included patients. The small number of patients with other malignancies could have limited the ability of our study to detect an effect, if any.
Although the discovery of ICIs was a significant breakthrough in the treatment of multiple solid organ and hematologic malignancies, the incidence of irAEs associated with these agents has increased substantially over the past several years. In fact, the incidence of GI-irAEs is second only to dermatologic adverse events in individuals receiving ICI therapy. Multiple randomized controlled trials (RCTs) have identified GI toxicity as one of the leading irAEs associated with ICIs, often resulting in treatment cessation or discontinuation [24-31]. However, there is a considerable disparity in the reported incidence of GI-irAEs among these trials due to the significant heterogeneity with regards to the indications and agents used for treatment. Abdel-Rahman et al [32] conducted a systematic review in 2015, evaluating data from various RCTs and analyzed the overall risk of GI toxicities associated with ICI therapy. This meta-analysis included 4,891 patients and identified a significant risk of diarrhea and colitis with ICI therapy (relative risk of 1.64 and 10.35, respectively). However, there was no comparison of the incidence and implications of GI toxicities among the different ICI agents. Downey et al [13] evaluated prognostic factors related to improved anti-tumor response. They evaluated 139 patients with metastatic melanoma who received ipilimumab. Among high-grade (grade 3 or above) irAEs, enterocolitis was the most common. Hepatitis was identified in 1.4% of the patients as compared to 3.4% in the current study. Also, no cases of gastritis or pancreatitis were identified in the Downey study. Due to its ipilimumab-only cohort, the overall incidence of ICI-associated GI-irAEs was higher in the Downey study compared to the current study (22% vs. 10%). Their study reported that the occurrence of any irAE had an increased likelihood of an enhanced clinical response, which is similar to the finding of improved tumor response in a heavily anti-PD-1/L1 treated cohort. Objective durable tumor response was observed in 26% of the patients who developed any grade irAE compared to only 2% in patients who did not develop an irAE (P = 0.0004). Given a similar association of GI-irAE to tumor response despite type of ICI in the two studies, these data sets suggest the common mechanism leading to GI-irAEs rather than a drug-specific effect is associated with tumor response.
Similarly, Rogado et al [14] conducted an observational study evaluating the association between the incidence of irAEs and efficacy of ICI therapy. One hundred six patients with various advanced malignancies who received treatment with either nivolumab or pembrolizumab were included, with a median follow-up of 6 months. The incidence of ICI-associated hepatitis and colitis in that study was similar to the current study (2.8% and 1%, respectively). Identical to the current study, there was an association between nivolumab monotherapy and the development of GI-irAEs. They also reported enhanced clinical responses in patients treated with anti-PD1-antibodies who experienced irAEs with an odds ratio of 23.5. Nonetheless, the results of this study are also confined to a particular type of ICI therapy, i.e., anti-PD-1 antibodies with no definite evaluation of the GI toxicities from different classes or combinations of immunotherapy. Another study by Wolchok et al [15] evaluated OS with combination therapy (nivolumab and ipilimumab) in advanced melanoma patients. The 3-year OS was 58% with combination therapy compared to only 52% and 34% with nivolumab and ipilimumab monotherapy, respectively. The striking effect of combination therapy did not seem to wane off despite discontinuation due to adverse events as 67% of the involved patients were alive at 3 years. Similar to the current study, the most common GI-irAE reported in this study was abnormal aminotransferases, followed by colitis. However, the investigated ICIs were used in only 10% of patients. Moreover, these findings were also limited to patients affected with a single type of malignancy (melanoma) and the study was not designed to assess the effects of GI-irAEs on patient’s survival. More recently, Wang and colleagues [33] evaluated the impact of ICI-induced diarrhea or colitis on overall tumor response. They reported that diarrhea was an independent predictor of improved survival regardless of the treatment required. Interestingly, despite the higher number of patients included in our study (567 vs. 327), the percentage of patients with GI-irAEs was higher in that study compared to ours. While the prior study was limited to diarrhea/colitis, our larger study suggests that other GI-irAEs also contribute to improved survival.
Unlike most previous studies, the current “real world” study was not limited to patients with a specific malignancy or limited to a particular ICI agent. Additionally, we have observed that the enhanced tumor responsiveness was most pronounced in patients with melanoma and patients treated with combination ICI therapy, identifying them as potential factors to predict GI-irAEs development. A recent editorial highlighted that colons of patients with ICI colitis had significantly higher number of tissue resident memory cells (TRMS) and pronounced expression of various cytokines when compared to patients without ICI colitis. The ensuing increase in inflammatory cytokines, particularly interferon (IFN)-γ, has a well-established role in mediating effective antitumor response [34-36]. They further reported that expression of TRMS was also increased in other luminal organs, thus highlighting a shared mechanism across different immune-mediated toxicities. These findings serve as potential explanations to the enhanced survival in our patients with irAEs.
Given the relatively recent availability of the ICIs, there is a paucity of literature studying the association of GI-irAEs development with tumor response to ICI. This study identifies outcome and survival advantages in patients with GI-irAEs. Objective assessment has confirmed superior tumor responses to treatment with ICI in patients who experienced GI-irAEs compared to patients who did not develop GI-irAEs. These results are consistent with previous studies and emphasize the close relationship between autoimmunity and tumor immunity. Development of GI-irAEs may be a surrogate indicator of immune system activation, and thus may encourage clinicians to continue, rather than discontinue, ICI therapy in non-life-threatening GI toxicities as tolerated. Furthermore, in this study, almost two-thirds of the patients with hepatitis presented with asymptomatic aminotransferase or bilirubin elevation. Hence, we emphasize the importance of routine monitoring of liver function tests before and during immunotherapy for early diagnosis and management.
The generalizability of the current study is limited by design, given the inherent potential flaws of retrospective studies including but not limited to selection bias, as well as unstructured data. Also, certain factors that could have directly or indirectly influenced OS in our patients may have been inevitably not captured owing to the retrospective nature of our study. Although significantly improved clinical responses were identified in patients who developed GI-irAEs, the study was not powered to achieve the statistical significance for each individual underlying type of malignancy separately. Only a small proportion of patients in our study underwent biopsy for diagnosis of ICI-induced colitis (4%) or hepatitis (2%). Our study was also limited by the total number of cases with ICI GI adverse events (only 41), as well as the lack of complete data recorded in the electronic health records. Future work directed towards expanding the cohort with more data on other malignancies and other ICI regimens of which we only had a few cases (i.e., atezolizumab, durvalumab) are needed to understand the benefit of GI-irAEs in this subset of patients. Additionally, our study was only able to establish an association but was unable to elucidate the molecular mechanisms that lead to improved survival in patients with GI-irAEs. GI adverse events occurred at a higher rate and greater severity in patients treated with ipilimumab plus nivolumab, but the humanistic and economic burden of these adverse events with combination ICI therapy remains unexplored in the current study. Our study could not delineate the mechanistic differences between development of irAEs and effective antitumor immunity that needs to be investigated in future studies to aid in developing more tailored next line therapies. Larger prospective studies are required to address these knowledge gaps and further elucidate the association of GI toxicity development and better tumor response in patients with advanced malignancies.
Acknowledgments
We would like to acknowledge the Cleveland Clinic’s pharmacy department for providing authors with a comprehensive list of potential study candidates based on our inclusion criteria.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Mohammad Alomari and Suleiman Al Ashi as first co-authors have equally contributed to this work, including literature review, study conception and design, recruiting research team members, IRB application, designing data collection sheet on Red cap software, data collection and assembly, data interpretation, manuscript writing and revision and providing administrative support. Shrouq Khazaaleh and Pravallika Chadalavada contributed to study design, data interpretation, manuscript writing and administrative support. Fahrettin Covut contributed to literature review, data assembly and data analysis including statistical analysis and creating tables and graphs. Laith Al Momani and Ahmed Elkafrawy contributed to data collection, assembly and analysis. Vinay Padbidri contributed to literature review and data collection. Pauline Funchain and Donald Campbell contributed to supervision and data interpretation. Carlos Romero-Marrero contributed to supervision and data interpretation as primary investigator (PI). All authors contributed to paper writing, manuscript revision and approved the submitted version of this manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
- ICIs
immune checkpoint inhibitors
- GI-irAEs
gastrointestinal immune-related adverse events
- irAEs
immune-related adverse events
- GI
gastrointestinal
- FDA
Food and Drug Administration
- PD-1
programmed cell death-1
- PD-L1
programmed cell death-ligand-1
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- IRB
Institutional Review Board
- ALT
alanine transaminase
- AST
aspartate aminotransferase
- i-RECIST
Immunotherapy-Response Evaluation Criteria In Solid Tumors
- NCI
National Cancer Institute
- CTCAE
Common Terminology Criteria for Adverse Events
- OS
overall survival
- CI
confidence interval
- HR
hazard ratio
- RCT
randomized controlled trial
References
- 1.Inthagard J, Edwards J, Roseweir AK. Immunotherapy: enhancing the efficacy of this promising therapeutic in multiple cancers. Clin Sci (Lond) 2019;133(2):181–193. doi: 10.1042/CS20181003. [DOI] [PubMed] [Google Scholar]
- 2.Aerts JG, Lievense LA, Hoogsteden HC, Hegmans JP. Immunotherapy prospects in the treatment of lung cancer and mesothelioma. Transl Lung Cancer Res. 2014;3(1):34–45. doi: 10.3978/j.issn.2218-6751.2013.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ventola CL. Cancer immunotherapy, Part 1: current strategies and agents. P T. 2017;42(6):375–383. [PMC free article] [PubMed] [Google Scholar]
- 4.Ventola CL. Cancer immunotherapy, Part 2: efficacy, safety, and other clinical considerations. P T. 2017;42(7):452–463. [PMC free article] [PubMed] [Google Scholar]
- 5.Ghahremanloo A, Soltani A, Modaresi SMS, Hashemy SI. Recent advances in the clinical development of immune checkpoint blockade therapy. Cell Oncol (Dordr) 2019;42(5):609–626. doi: 10.1007/s13402-019-00456-w. [DOI] [PubMed] [Google Scholar]
- 6.Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther. 2017;6:73–82. doi: 10.2147/ITT.S126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samaan MA, Pavlidis P, Papa S, Powell N, Irving PM. Gastrointestinal toxicity of immune checkpoint inhibitors: from mechanisms to management. Nat Rev Gastroenterol Hepatol. 2018;15(4):222–234. doi: 10.1038/nrgastro.2018.14. [DOI] [PubMed] [Google Scholar]
- 9.Di Giacomo AM, Danielli R, Guidoboni M, Calabro L, Carlucci D, Miracco C, Volterrani L. et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58(8):1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentile NM, D'Souza A, Fujii LL, Wu TT, Murray JA. Association between ipilimumab and celiac disease. Mayo Clin Proc. 2013;88(4):414–417. doi: 10.1016/j.mayocp.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ. et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35(34):3807–3814. doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US. et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22 Pt 1):6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogado J, Sanchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Levi A, Arranz R. et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019;109:21–27. doi: 10.1016/j.ejca.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 16.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD. et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, Balleyguier C. et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi: 10.1016/j.ejca.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Zheng B, Shin JH, Li H, Chen Y, Guo Y, Wang M. Comparison of radiological tumor response based on iRECIST and RECIST 1.1 in metastatic clear-cell renal cell carcinoma patients treated with programmed cell death-1 inhibitor therapy. Korean J Radiol. 2021;22(3):366–375. doi: 10.3348/kjr.2020.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai YC, Chang WC, Chen CB, Wang CL, Lin YF, Ho MM, Cheng CY. et al. Response evaluation for immunotherapy through semi-automatic software based on RECIST 1.1, irRC, and iRECIST criteria: comparison with subjective assessment. Acta Radiol. 2020;61(7):983–991. doi: 10.1177/0284185119887588. [DOI] [PubMed] [Google Scholar]
- 20.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU. et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed January 5, 2020.
- 22.Mearns ES, Bell JA, Galaznik A, Puglielli SM, Cichewicz AB, Boulanger T, Garcia-Ribas I. Gastrointestinal adverse events with combination of checkpoint inhibitors in advanced melanoma: a systematic review. Melanoma Manag. 2018;5(1):MMT01. doi: 10.2217/mmt-2017-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C. et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 25.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 28.Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C. et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M. et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 30.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C. et al. Ipilimumab+Dacarbazine vs Dacarbazine for met melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Rahman O, ElHalawani H, Fouad M. Risk of gastrointestinal complications in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Immunotherapy. 2015;7(11):1213–1227. doi: 10.2217/imt.15.87. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, Lum P. et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6(1):37. doi: 10.1186/s40425-018-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ. et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175(4):998–1013.e1020. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dougan M. Immune Checkpoint Inhibitor Colitis: Resident Memory Unleashed. Gastroenterology. 2021;161(4):1106–1108. doi: 10.1053/j.gastro.2021.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.