Abstract
Introduction Awareness of SARS-CoV-2 infection in pregnant women and the potential risk for infection of their neonates is increasing. The aim of this study was to examine the immune status of affected women and evaluate the dynamics of placental antibody transfer.
Materials and Methods The study included 176 women with SARS-CoV-2 infection during pregnancy who delivered between April 2020 and December 2021 at eight obstetric maternity sites. Demographic data, maternal and neonatal characteristics were summarized. Antibody testing for IgA and IgG in maternal blood sera and umbilical cord samples was evaluated and IgG transfer ratios were calculated. Values were related to the time of infection during pregnancy and birth.
Results The percentage of IgG positive women increased from 29.0% (95% CI 23.8 – 37.8) at presentation with a positive PCR test result to 75.7% (95% CI 71.6 – 79.8), the percentage of IgG positive umbilical cord blood samples increased from 17.1% (95% CI 13.0 – 21.3) to 76.4% (95% CI 72.2 – 80.7) at more than six weeks after infection. Regression lines differed significantly between maternal and fetal IgG responses (p < 0.0001). Newborns react with a latency of about one week; umbilical cord blood antibody concentrations are highly correlated with maternal concentration levels (ρ = 0.8042; p < 0.0001). IgG transplacental transfer ratios were dependent on infection-to-birth interval. Two of the umbilical cord blood samples tested positive for IgA.
Conclusions These findings confirm vertical SARS-CoV-2 transmission is rare; however, antibodies are transferred to the fetus soon after infection during pregnancy. Since transplacental antibody transfer might have a protective value for neonatal immunization this information may be helpful when counseling affected women.
Key words: antibody, COVID-19, pregnancy, SARS-CoV-2
Zusammenfassung
Einleitung Das Wissen um die Auswirkungen von SARS-CoV-2-Infektionen in schwangeren Frauen und um das potenzielle Risiko einer Infektion ihrer neugeborenen Kinder wächst. Ziel dieser Studie war es, den Immunstatus von betroffenen Frauen und die Wechselwirkungen des plazentären Transfers von Antikörpern zu untersuchen.
Material und Methoden Es wurden 176 Frauen, die während ihrer Schwangerschaft mit SARS-CoV-2-infiziert wurden und zwischen April 2020 und Dezember 2021 in einer von 8 Geburtskliniken entbanden, in die Studie aufgenommen. Demografische Daten und Charakteristika von Müttern und Neugeborenenen wurden aufgenommen. Die Ergebnisse von IgA- und IgG-Antikörpertests im mütterlichen Blut und Nabelschnurblut wurden evaluiert und die IgG-Transfer-Quotienten wurden berechnet. Die Werte wurden dem jeweiligen Zeitpunkt der Infektion während der Schwangerschaft und der Geburt zugeordnet.
Ergebnisse Der Prozentsatz an IgG-positiven Frauen erhöhte sich von 29,0% (95%-KI 23,8 – 37,8) bei Vorliegen eines positiven PCR-Tests auf 75,7% (95%-KI 71,6 – 79,8), der Prozentsatz an IgG-positiven Blutproben von Nabelschnüren erhöhte sich von 17,1% (95%-KI 13,0 – 21,3) auf 76,4% (95%-KI 72,2 – 80,7) mehr als 6 Wochen nach der Infektion. Es gab signifikante Unterschiede zwischen den jeweilligen Regressionslinien der mütterlichen und der fötalen Immunantwort auf IgG (p < 0,0001). Neugeborenene reagierten mit eine Latenzzeit von ungefähr 1 Woche; Antikörperkonzentrationen im Nabelschnurblut korrelierten sehr stark mit den mütterlichen Konzentrationen (ρ = 0,8042; p < 0,0001). Der transplazentäre IgG-Transfer-Quotient hing von dem Zeitintervall zwischen Infektion und Geburt ab. Zwei der mittels Nabelschnurpunktion gewonnenen Blutproben waren IgA-positiv.
Schlussfolgerungen Diese Ergebnisse bestätigen, dass vertikale Transmission von SARS-CoV-2 selten vorkommt; kurz nach der mütterlichen Infektion während der Schwangerschaft werden aber Antikörper zum Fötus transferiert. Da ein transplanzentärer Transfer von Antikörpern einen gewissen Schutz bei der Immunisierung des Neugeborenen bietet, könnte das eine nützliche Information bei der Beratung betroffener Frauen sein.
Schlüsselwörter: Antikörper, COVID-19, Schwangerschaft, SARS-CoV-2
Abbreviations
- COVID-19
coronavirus disease 2019
- ICU
intensive care unit
- NICU
neonatal intensive care unit
- RT-PCR
reverse transcription-polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus-2
Introduction
Since the outbreak of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in Wuhan, China, in December 2019, the coronavirus disease 2019 (COVID-19) pandemic has been pushing healthcare systems to their limits. There is limited knowledge about the clinical course of COVID-19 in pregnant women, although the level of information has been substantially increasing 1 . The majority of pregnant women experience mild to moderate symptoms such as fever, cough, dyspnea, shortness of breath, fatigue, myalgia, or anosmia 2 , 3 , 4 , 5 . Systematic reviews and case series are indicating a low rate of maternal mortality 3 , 5 , 6 , 7 , 8 , 9 . However, pregnant women appear to be at a higher risk of admission to intensive care units (ICUs) and needing mechanical ventilation support in comparison with nonpregnant patients 6 , 10 . The risk of vertical transmission to the fetus is still not entirely clear. There have only been case reports suggesting a possible infection of the fetus in utero 4 , 11 , 12 , 13 , and most reviews and case series have reported negative testing of neonates 3 , 14 – 19 .
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of nasopharyngeal samples remains the gold standard among the direct detection methods for diagnosing SARS-CoV-2 infection. The development of serological assays allows epidemiological assessments or vaccine trials and is essential for evaluating the protection of neonates due to maternally derived, transplacentally acquired antibodies 20 , 21 , 22 . The time from infection and potential onset of the disease to the development of antibodies varies. Some authors have also detected a correlation between the severity of the disease and antibody concentrations 23 . Immunoglobulins A and M (IgA and IgM) are markers for acute infection and tend to be detectable between days 5 and 11, whereas immunoglobulin G (IgG) develops between days 12 and 14 21 , 24 . IgG antibodies can be transferred to the fetus actively through the placenta, leading to protection from infection 12 , 13 , 25 , 26 . Currently, there are only small case series reporting on transplacental transfer of maternal SARS-CoV-2-specific antibodies 12 , 13 , 22 .
The aim of the present study was to determine the incidence and dynamics of antibodies in symptomatic and asymptomatic women with confirmed SARS-CoV-2 infection and in cord blood at the time of delivery. The results should make it possible to estimate the potential for neonatal protection via transplacental transfer of antibodies and the risk of vertical transmission.
Materials and Methods
The COVID-19-Related Obstetric and Neonatal Outcome Study (CRONOS) is a multicenter prospective observational registry study, established by the German Society of Perinatal Medicine (DGPM) in April 2020, to rapidly provide data for counseling women with SARS-CoV-2 infection during pregnancy. Information about the study is available at www.dgpm-online.org and from the German Clinical Trials Register (DRKS00021208); some of the study results have been recently published 27 . A reporting form was developed using a cloud-based electronic data capture platform available from the service provider castoredc.com (Amsterdam, Netherlands). Women who had a positive result on SARS-CoV-2 nucleic acid testing using quantitative RT-PCR assay of maternal nasopharyngeal swab specimens or serum antibody testing were included. In addition to COVID-19-specific symptoms and treatments, pregnancy and birth-specific events and neonatal outcomes were documented. As of first January 2022, data were entered by 117 hospitals after patients had given informed consent. Among these, five university hospitals with eight obstetric sites in Germany and Austria frequently analyzed both maternal and umbilical cord-blood anti-SARS-CoV-2 serum antibody concentrations as part of routine care or for research purposes. The analyses were done immediately peripartum or postpartum using commercially available methods and in accordance with standard procedures in the participating hospitalsʼ clinical laboratories.
Between April 2020 and December 2021, the participating study sites included 364 patients in the CRONOS registry; 325 women gave birth to a living neonate after 24 weeks of gestation. For the present study, medical records were reviewed retrospectively for the presence or absence of antibodies against SARS-CoV-2 in maternal and umbilical cord sera. Positive and negative values were indicated as above or below a defined threshold value, in accordance with the manufacturersʼ instructions. Since the analytical methods used differed between hospitals, a ratio of the respective patientʼs individual value and the lower reference value of the method used was calculated to normalize for interassay and interlaboratory differences. In total, 176 mother-and-child pairs were identified, with at least one measurement of the IgG subclass in both maternal and umbilical cord blood. Borderline results were considered positive 28 . The transfer ratio was calculated in 68 mother-and-child pairs with a positive IgG test result for which quantitative concentration data were additionally available as the umbilical cord IgG concentration divided by the maternal IgG concentration. Additionally, qualitative results were obtained for the IgA subclass in 156 mother-and-child pairs. Values were related to clinical parameters such as week of gestation at infection or birth, and used to compare symptomatic with asymptomatic women. Multiple pregnancies (n = 3) were treated as single pregnancies.
Statistical analysis
Nominal data are presented as absolute and relative frequencies. Medians and interquartile ranges (IQRs) were computed for ordinal and nonnormal metric variables. Spearmanʼs rank test was used to calculate the correlation coefficient of maternal and umbilical cord blood IgG values. Third order polynomial (cubic) non-linear regression analyses were used to model regression lines and corresponding 95% confidence intervals for qualitative assessments of IgG and IgA measurements, as well as the transfer ratios. Fisherʼs exact test was used to test for the presence or absence of antibodies in accordance with symptoms and an infection-to-birth interval of up to versus more than six weeks, respectively. Mann–Whitney U tests were conducted to compare infection-to-birth intervals of symptomatic versus asymptomatic women. The level of significance was set at 0.05. The software package Prism 8.3.0 (GraphPad Software, San Diego, USA) was used for statistical analysis.
Ethical approval
Ethical approval was obtained from Schleswig-Holstein University Hospital (UKSH), Campus Kiel (D 451/20) and Johannes Kepler University Linz (EK – 1074/2020), and from the local ethics committee in each participating hospital.
Role of the funding source
Sabine Enengl and Peter Oppelt received a grant for COVID-19 research from Johannes Kepler University Linz. The funders had no role in the study design, the collection, analysis, and interpretation of the data, the writing of the report, or the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Clinical data are presented in Table 1 . The median maternal age was 30 years, and the median body mass index (BMI) at booking was 24.5 kg/m 2 . Pre-existing medical conditions were reported in 41% of the women, with diabetes mellitus/gestational diabetes being the most common (17.9%) in this cohort. Four women (2.3%) smoked during pregnancy. Infection occurred at a median of 36 weeks of gestation. Symptoms related to COVID-19 were reported in 97 cases (55.1%; values are missing for five women). Asymptomatic women had a lower infection-to-birth interval (median = 0, IQR 0 – 2.25 weeks) than symptomatic women (median = 6, IQR 1 – 15 weeks, p < 0.0001). Eleven women (6.3%) were hospitalized for COVID-19, seven (3.4%) received oxygen therapy, and two (1.1%) were admitted to the ICU, one of them for critical illness requiring invasive mechanical ventilation.
Table 1 Maternal and neonatal clinical characteristics.
| Variable | N or Median | IQR or % | Missing (%) |
|---|---|---|---|
| Absolute (n) and relative frequencies (%) are provided for as nominal and ordinal variables for the total study population. It should be noted that valid observations are used to
calculate percentage values. Medians and interquartile ranges are calculated for metric variables. “Missing (%)” indicates missing values in the register. BMI: body mass index, CPAP: continuous positive airway pressure, ICU: intensive care unit, IQR: interquartile range, N/A: not applicable, NICU: neonatal intensive care unit, PCR: polymerase chain reaction. | |||
| Total number of patients (n) | 176 | ||
| Maternal age (years; median, IQR) | 30 | 27 – 39 | 0.0 |
| Maternal BMI at booking (median, IQR) | 24.5 | 21 – 28 | 4.5 |
| Maternal comorbidities (n, %) | 72 | 40.9 | 0.0 |
| Diabetes mellitus, gestational diabetes (n, %) | 31 | 17.6 | |
| Hypertension, gestational hypertension (n, %) | 4 | 2.3 | |
| Lung disease, bronchial asthma (n, %) | 2 | 1.1 | |
| Primiparity (n, %) | 59 | 33.5 | 0.0 |
| Weeks of gestation at infection (median, IQR) | 36 | 28 – 39 | 1.1 |
| COVID-19 symptoms (n, %) | 97 | 55.1 | 3.4 |
| Hospitalization for COVID-19 (n, %) | 13 | 7.4 | 0.0 |
| Oxygen treatment (n, %) | 7 | 4.0 | 0.6 |
| ICU admission (n, %) | 2 | 1.1 | 0.6 |
| Weeks of gestation at birth (median, IQR) | 40 | 38 – 41 | 0.0 |
| Birth mode: cesarean section (n, %) | 65 | 37.1 | 0.6 |
| Iatrogenic delivery due to COVID-19 (n, %) | 3 | 1.7 | 0.6 |
| Preterm birth (< 37 weeks; n, %) | 27 | 15.3 | 0.0 |
| Birth weight percentile < 10 P (n, %) | 14 | 8.0 | 0.6 |
| 5-min Apgar < 7 (n, %) | 4 | 2.3 | 0.6 |
| NICU admission (n, %) | 28 | 16.0 | 0.6 |
| Neonatal breathing support (CPAP or more; n, %) | 13 | 7.4 | 0.6 |
| Neonatal SARS-CoV-2 as diagnosed by PCR (n, %) | 4 | 7.4 | N/A |
| Neonatal death (n, %) | 1 | 0.6 | 0.6 |
Preterm delivery occurred in 15.3% of the patients, but when the analysis was restricted to those with infection before 37 weeks of gestation (n = 101), preterm delivery was observed in 25.7%. Twenty-eight neonates (15.9%) were admitted to the neonatal intensive care unit (NICU) for various reasons, none of which included maternal or neonatal COVID-19. One neonate suffered from nonimmunologic hydrops fetalis of unknown origin, diagnosed at 29 weeks of gestation after the mother developed COVID-19 with mild symptoms at 25 weeks of gestation. SARS-CoV-2 PCR from amniotic fluid obtained via amniocentesis was negative. The baby was delivered at 33 weeks due to progressive findings. After eight weeks, the neonate was discharged in a healthy and well condition. Another child was born at 34 weeks of gestation with a lung tumor. The mother had been infected at 31 weeks of gestation. The malformation was diagnosed antenatally before SARS-CoV-2 infection. The child died on day 6 of life, most likely due to a postoperative bleeding complication. The exact cause could not be determined. Although both mothers were positive for IgG and IgA, only IgG antibodies were detected in the neonates. Placental histology was unspecific in both cases. Ultimately, maternal COVID-19 could not be excluded as a cause of adverse events in the neonates, but it remains unlikely according to the clinical documentation.
SARS-CoV-2 virus was detected by PCR in nasopharyngeal swabs of four neonates (2.3%) on the first or second day after birth. The mothers of these neonates tested positive for SARS-CoV-2 less than two weeks before birth. None of the neonates and only one of the mothers were positive for IgG and IgA antibodies, indicating acute peripartum infection in all four cases, with viral transmission taking place most likely during labor or immediately postnatally. The neonates were discharged in a healthy condition.
Qualitative assessment of anti-SARS-CoV-2 antibodies
IgG anti-SARS-CoV-2 antibodies were found to be positive in 97 women (55.1%) and 82 neonates (46.6%). On nonlinear regression analysis, the percentage of IgG-positive women increased from 29.0% (95% CI 23.8 – 37.8) at presentation with a positive PCR test, reaching 75.7% (95% CI 71.6 – 79.8) at six weeks after infection (R 2 = 0.5813). Similarly, the proportion of IgG-positive neonates increased from 17.1% (95% CI 13.0 – 21.3) to 76.4% (95% CI 72.2 – 80.7) at six weeks after infection (R 2 = 0.7113). Regression lines differed significantly between the maternal and fetal IgG responses (comparison of fits: p < 0.0001, F = 8.958; dfn = 4, dfd = 340) ( Fig. 1 ). Similarly, maternal IgA antibodies were found to be positive in 78 (50.0%) women and two umbilical cord blood samples (1.3%). The percentage of IgA-positive women increased from 23.5% (95% CI 17.3 – 29.8) at presentation with a positive PCR test result to a mean value of 65.4% (95% CI 60.5 – 70.5) at six weeks after infection. The two neonates positive for anti-SARS-CoV-2 IgA antibodies were born three and eight weeks, respectively, after the mother had been infected.
Fig. 1.
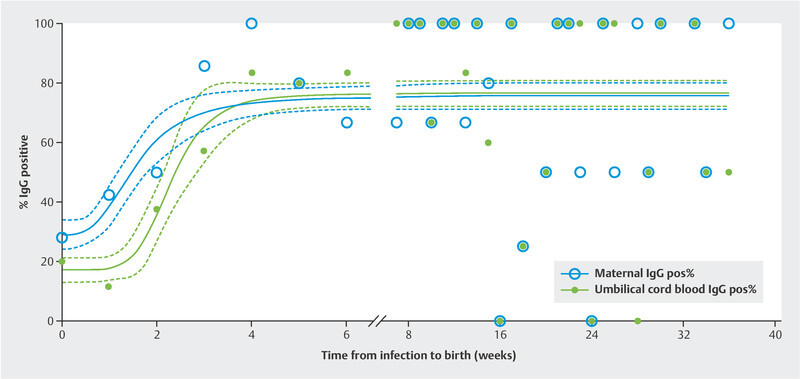
Regression lines, with 95% confidence intervals, for maternal (R 2 = 0.5813) and umbilical cord blood (R 2 = 0.7113) IgG seropositivity relative to the infection-to-birth interval (weeks). Blue circles indicate percentages (Y axis) of mothers, while green dots represent percentages of umbilical cord blood testing positive for anti-SARS-CoV-2 IgG for the indicated weeks between infection and birth (X axis). The regression lines differed significantly (comparison of fits: p < 0.0001, F = 8.958; dfn = 4, dfd = 340). Note the delayed response in seroconversion by one week between mothers and their offspring early after infection.
Symptomatic women were more likely to be IgG-positive within six weeks of infection in comparison with asymptomatic women (59.6% versus 34.4%, p = 0.0087). Similarly, neonates of symptomatic women were more likely to be IgG-positive than neonates of asymptomatic women (42.3% versus 23.4%, p = 0.0446). After six weeks from infection, no differences in IgG status were observed between symptomatic and asymptomatic women (71.1% versus 80.0%, p = 0.7100) or their neonates (73.3% versus 80.0%, p > 0.9999).
Quantitative assessment of SARS-CoV-2 antibodies
In IgG-positive cases, a strong positive correlation was observed between maternal and umbilical IgG concentrations (n = 68, ρ = 0.8042; p < 0.0001; Fig. 2 ). The mean IgG transfer ratio was 1.124 (SD = 0.5298). Transfer ratios showed a mean of less than 1.0 when the infection-to-birth interval was less than six weeks, but the mean transfer ratios were above 1.0 when the interval was six weeks or more after infection ( Fig. 3 ). The transfer ratios did not differ between symptomatic women (mean 1.109, SD 0.5478) and asymptomatic women (mean 1.102, SD 0.5068; adjusted p = 0.2177).
Fig. 2.
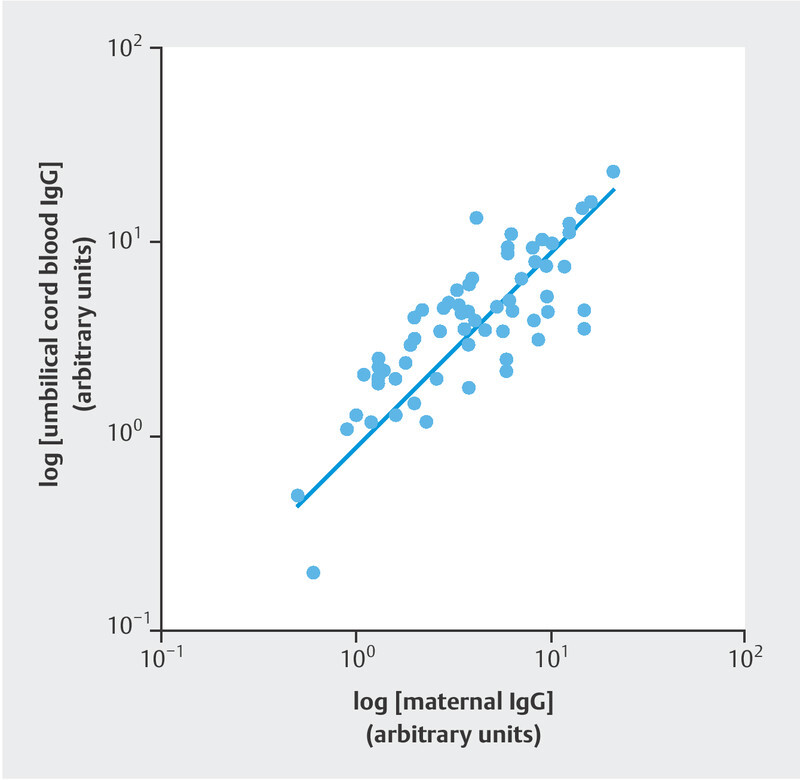
Scatter plot and regression line for logarithmic maternal and logarithmic umbilical cord IgG concentrations. Each dot represents a mother–child pair, both of whom tested positive for anti-SARS-CoV-2 IgG antibodies (n = 68, ρ = 0.8042; p < 0.0001).
Fig. 3.
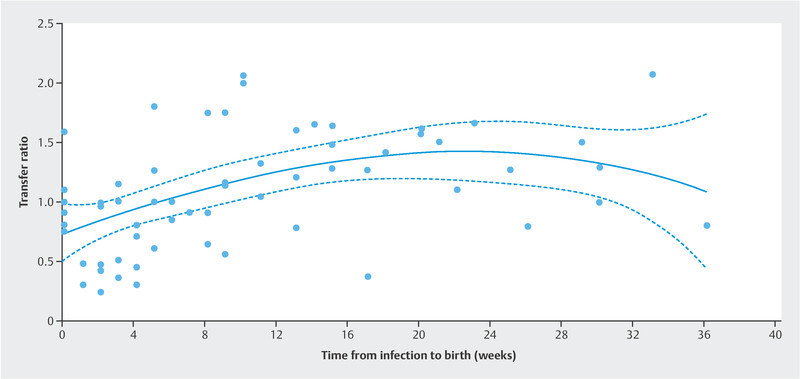
Third-order cubic polynomial regression line for maternofetal anti-SARS-CoV-2 IgG antibody transfer ratios (c [umbilical cord blood IgG]/c [maternal blood IgG]) relative to the infection-to-birth interval. Note that the ratios increase to more than 1 after six weeks from infection (R 2 = 0.2440).
At a follow-up visit, anti-SARS-CoV-2 antibody concentrations were assessed further in one neonate seven weeks after birth. The mother had developed infection with fever and malaise but no dyspnea at 32 weeks of gestation. She recovered within seven days and delivered a healthy neonate at 39 weeks. IgG was detected in the neonateʼs umbilical cord blood and in venous blood in the seventh week of life, but the concentration levels decreased by 64% from 5.6 to 2.0 (arbitrary units/mL; lower reference cut-off: 0.8), although they remained positive.
Discussion
One of the most important concerns of women suffering from SARS-CoV-2 infection during pregnancy is whether the child will also be affected and whether it will receive protection via maternal antibodies transmitted via the placenta. As there is a risk of the neonate being infected from a SARS-CoV-2-positive mother, there is a high level of interest in the potential development of protective neonatal immunity 22 . To answer the question whether fetal immunocompetence can be passively acquired from the mother, we analyzed maternal and cord blood antibodies against SARS-CoV-2 in order to quantify transplacental IgG antibody transfer ratios. A strong correlation was observed between maternal and fetal anti-SARS-CoV-2 IgG antibodies in women suffering from SARS-CoV-2 infection during pregnancy. The maternal immune response precedes fetal IgG positivity by about one week. Consistent with this, the IgG transfer ratio increased as the time interval between infection and birth increased, and the IgG concentration in the fetus exceeded the maternal IgG concentration at more than six weeks after infection.
The present findings also support previous evidence that vertical transmission of SARS-CoV-2 is rarely observed, as only two neonates were positive for IgA, and PCR-confirmed SARS-CoV-2 was observed in four neonates 3 , 14 , 15 , 16 , 17 , 18 , 19 . Transplacental immunization via antibody transfer may play a pivotal role in neonatal immunization. Although there was a significant correlation in IgG levels between maternal blood and cord blood, and maternal IgG transfer was commonly observed, previous studies have shown evidence of reduced transfer ratios for SARS-CoV-2 IgG in comparison with influenza, pertussis, and measles 29 . In line with data reported by other groups, the overall seropositivity rates for the mothers and in cord blood were 55% and 47%, respectively, in the present study 30 . The rather low incidence of IgG-positive women is probably due to the high proportion of women who were infected shortly before giving birth. These women were identified by screening in hospital, often presenting with a mild or asymptomatic course. Symptomatic women and their neonates had positive IgG tests significantly more often than asymptomatic cases. A positive correlation between anti-SARS-CoV-2 IgG titers and the severity of COVID-19 has been reported in a recent study of 473 survivors. One year after infection, 74.4% of recovered asymptomatic carriers had negative anti-SARS-CoV-2 IgG test results, which would additionally explain the lower IgG seropositivity in asymptomatic cases in the present study 31 . However, some case series also show evidence that placental positivity for SARS-CoV-2 might have severe consequences for the fetus or the newborn. One case report detected viral proteins and RNA in multiple fetal villous cellular subsets of the placenta, leading to pneumonia and severe respiratory distress of the newborn soon after birth 32 . Also, SARS-CoV-2 infection can result in significant placental lesions with a higher risk of intrauterine fetal death 33 . Interestingly, another case series found that SARS-CoV-2 was undetected in the placenta even though it induced unique maternal immune and fetal stromal responses in the extra- placental membranes 34 .
It is very likely that the transfer of antibodies through the placenta to the fetus provides neonatal protection. However, the strength of this effect and the percentage by which the probability of infection or an unfavorable course is reduced remain unclear. It is also unclear how long circulating maternal antibodies persist in the newborn. In one case, we found a decrease in antibody concentration of 64% within seven weeks in the present study. It can therefore be assumed that the passive immunoprotection provided by these antibodies declines over time. Studies by other groups suggest that maternally derived passive immunity may persist in infants for up to six months of life 30 , 35 . Fortunately, other mechanisms may additionally protect the newborn during the first year of life. Studies indicate that antibodies associated with prior SARS-CoV-2/COVID-19 infection are present in breast milk 36 . In vitro, these antibodies were able to neutralize SARS-CoV-2 activity 37 . In a recently published study, mechanisms were uncovered whereby the neonate can imitate IgA antibodies supplied by the motherʼs breast milk, and that these antibodies can be found in the newbornʼs saliva weeks after infection of the mother. Maternal infection during the peripartum period thus appears not only to passively protect the newborn via breast milk secretory IgA, but also to actively stimulate and train the neonatal immune system via breast milk immune complex activity 38 .
Fetal and neonatal protection may also be achieved via vaccination of the mother. Vaccination campaigns against COVID-19 have started worldwide, and an understanding of antibody dynamics also plays an important role in vaccine trials. Recent studies suggest that the BNT162b2 mRNA vaccine elicits a strong maternal humoral IgG response (anti-S receptor-binding domain) that crosses the placental barrier and approaches maternal titers in the fetus within 15 days after the first dose, and that the resulting maternal to neonatal anti-COVID-19 antibody ratios are similar to those after infection 39 , 40 . Usually, there is minimal transfer of antibodies before 16 weeks, but transfer increases throughout the second trimester and peaks in the third trimester, especially in the last four weeks of pregnancy. This may lead to higher antibody titers in the baby than in the mother – a finding that is in line with the present study, in that transfer ratios exceed 1 with an increasing time interval from infection to birth 41 , 42 , 43 . However, as some studies show impaired antibody transfer in patients with SARS-CoV-2 infection during the third trimester of pregnancy, vaccination is recommended during the second trimester, leading to a rise in IgG titers and effective transfer across the placenta 29 , 39 , 44 .
The strengths of this study include the multicenter involvement of a large number of confirmed SARS-CoV-2-positive parturients to evaluate the immune status in pregnant women and their newborns. One limitation of the study is that a high percentage of the patients were asymptomatic and tested positive incidentally at admission for birth. This explains the relatively favorable outcome in this cohort of women with SARS-CoV-2 infection during their pregnancy. There was a short period between testing and birth, but the onset of infection could have been much earlier. The mean maternal IgG concentration was therefore commonly lower in the asymptomatic group.
Conclusion
These findings add valuable information to our understanding of the mechanisms of fetoplacental protection from SARS-Cov-2 infection. One to two weeks after clinical detection of SARS-CoV-2 infection, more than 50% of women are positive for anti-SARS-CoV-2 IgG antibodies. Newborns react with a latency period of about one week, and neonatal antibody concentrations are strongly related to maternal concentration levels. Since transplacental antibody transfer may play a protective value in neonatal immunization, this information may be helpful for counseling affected women and for birth planning. Further studies, with repeated scheduled testing during pregnancy, may contribute to a better understanding of the mechanisms involved.
Ethical Approval
Ethics approval was obtained from Schleswig-Holstein University Hospital (UKSH), Campus Kiel (D 451/20), Johannes Kepler University Linz (EK – 1074/2020), and the local ethics committees of each participating hospital.
Informed Consent
Written informed consent was obtained from all patients.
Funding
Research for this paper was supported by a grant from Johannes Kepler University Linz, Austria. The funders had no role in the design and conduct of the study.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authorsʼ Contributions
S. Enengl: protocol development, data management, manuscript writing; U. Pecks: protocol development, data management, statistical analysis, manuscript writing; P. Oppelt: protocol development, manuscript editing; P. Stelzl: data management; P. S. Trautner: data management; O. Shebl: protocol development, manuscript editing; B. Lamprecht: manuscript editing; A. C. Longardt: data management; C. Eckmann-Scholz: data management; C. Keil: data management; N. Mand: data management; C. S. von Kaisenberg: data management; M. Jegen: data management; S. Doppler: data collection; P. Hermann: statistical analysis, manuscript editing; J. Lastinger: data management, manuscript writing.
Acknowledgements
We are grateful to Helga Wagner and Philipp Hermann for their valuable comments. Sabine Enengl and Peter Oppelt received a grant for COVID-19 research from Johannes Kepler University Linz.
Footnotes
Conflict of Interest Sabine Enengl and Peter Oppelt received a grant for COVID-19 research from Johannes Kepler University Linz. All other authors declare that they have no competing interests.
References
- 1.Thompson J L, Nguyen L M, Noble K N. COVID-19-related disease severity in pregnancy. Am J Reprod Immunol. 2020;84:e13339. doi: 10.1111/aji.13339. [DOI] [PubMed] [Google Scholar]
- 2.Juan J, Gil M M, Rong Z. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Guo J, Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99:823–829. doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dashraath P, Wong J LJ, Lim M XK. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allotey J, Stallings E, Bonet M. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrazzi E, Frigerio L, Savasi V. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. BJOG Int J Obstet Gynaecol. 2020;127:1116–1121. doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz D A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal–fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020;144:799–805. doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Wang M, Zhu Z. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med. 2020 doi: 10.1080/14767058.2020.1759541. [DOI] [PubMed] [Google Scholar]
- 10.Zambrano L D, Ellington S, Strid P. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status – United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alzamora M C, Paredes T, Caceres D. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37:861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong L, Tian J, He S. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng H, Xu C, Fan J. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stumpfe F M, Titzmann A, Schneider M O. SARS-CoV-2 Infection in Pregnancy – a Review of the Current Literature and Possible Impact on Maternal and Neonatal Outcome. Geburtshilfe Frauenheilkd. 2020;80:380–390. doi: 10.1055/a-1134-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullins E, Evans D, Viner R M. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55:586–592. doi: 10.1002/uog.22014. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, Guo J, Fan C. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:1110–1.11E16. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu N, Li W, Kang Q. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Guo L, Chen L. A case report of neonatal 2019 coronavirus disease in China. Clin Infect Dis. 2020;71:853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Zhou Y-H, Yang H-X. Intrauterine vertical transmission of SARS-CoV-2: what we know so far. Ultrasound Obstet Gynecol. 2020;55:724–725. doi: 10.1002/uog.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padoan A, Sciacovelli L, Basso D. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin Chim Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jääskeläinen A J, Kekäläinen E, Kallio-Kokko H. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Euro Surveill. 2020;25:2.000603E6. doi: 10.2807/1560-7917.ES.2020.25.18.2000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flannery D D, Gouma S, Dhudasia M B. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021;175:594–600. doi: 10.1001/jamapediatrics.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okba N MA, Müller M A, Li W. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo L, Ren L, Yang S. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler P F, Farr R S. Elevation of cord over maternal IgG immunoglobulin: evidence for an active placental IgG transport. Nature. 1966;210:1070–1071. doi: 10.1038/2101070a0. [DOI] [PubMed] [Google Scholar]
- 26.Machado A P, Gonçalves G, Barros H. [Mother–child transmission of immunoglobulins G] Acta Med Port. 1995;8:81–85. [PubMed] [Google Scholar]
- 27.Pecks U, Kuschel B, Mense L. Pregnancy and SARS-CoV-2 infection in Germany – the CRONOS registry. Dtsch Arztebl Int. 2020;117:841–842. doi: 10.3238/arztebl.2020.0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beavis K G, Matushek S M, Abeleda A PF. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA and IgG antibodies. J Clin Virol. 2020;129:104468. doi: 10.1016/j.jcv.2020.104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edlow A G, Li J Z, Collier A Y. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020;3:e2030455. doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song D, Prahl M, Gaw S L. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: prospective cohort study. BMJ Open. 2021;11:e053036. doi: 10.1136/bmjopen-2021-053036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan X, Chen G, Jin Z. Anti-SARS-CoV-2 IgG levels in relation to disease severity of COVID-19. J Med Virol. 2022;94:380–383. doi: 10.1002/jmv.27274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Facchetti F, Bugatti M, Drera E. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine. 2020;59:102951. doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubucs C, Groussolles M, Ousselin J. Severe placental lesions due to maternal SARS-CoV-2 infection associated to intrauterine fetal death. Hum Pathol. 2022 doi: 10.1016/j.humpath.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Flores V, Romero R, Xu Y. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun. 2022;13:320. doi: 10.1038/s41467-021-27745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangat C, Milosavljevic N. BNT162b2 Vaccination during Pregnancy Protects Both the Mother and Infant: Anti-SARS-CoV-2 S Antibodies Persistently Positive in an Infant at 6 Months of Age. Case Rep Pediatr. 2021;2021:6.901131E6. doi: 10.1155/2021/6901131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu F, Zozaya C, Zhou Q. SARS-CoV-2 genome and antibodies in breastmilk: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2021;106:514–521. doi: 10.1136/archdischild-2020-321074. [DOI] [PubMed] [Google Scholar]
- 37.Pace R M, Williams J E, Järvinen K M. Characterization of SARS-CoV-2 RNA, Antibodies, and Neutralizing Capacity in Milk Produced by Women with COVID-19. mBio. 2021;12:e03192-20. doi: 10.1128/mBio.03192-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conti M G, Terreri S, Piano Mortari E. Immune Response of Neonates Born to Mothers Infected With SARS-CoV-2. JAMA Netw Open. 2021;4:e2132563. doi: 10.1001/jamanetworkopen.2021.32563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beharier O, Plitman Mayo R, Raz T. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131:e150319. doi: 10.1172/JCI150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mithal L B, Otero S, Shanes E D. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol. 2021;225:192–194. doi: 10.1016/j.ajog.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saji F, Samejima Y, Kamiura S. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod. 1999;4:81–89. doi: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- 42.Wilcox C R, Holder B, Jones C E. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front Immunol. 2017;8:1294. doi: 10.3389/fimmu.2017.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calvert A, Jones C E. Placental transfer of antibody and its relationship to vaccination in pregnancy. Curr Opin Infect Dis. 2017;30:268–273. doi: 10.1097/QCO.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 44.Atyeo C, Pullen K M, Bordt E A. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184:628–6.42E12. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


