Abstract
Objectives:
OxyContin was reformulated with a polyethylene oxide matrix in August 2010 to reduce the potential for intravenous abuse and for abuse by insufflation. The objective of this study was to evaluate the impact of OxyContin’s reformulation on overdose (OD) risk for individuals dispensed OxyContin in comparison to those dispensed other opioids under regular care.
Materials and Methods:
Three national insurance databases with National Death Index linkage identified OD in individuals with any dispensing of OxyContin or a primary comparator opioid (extended release morphine, transdermal fentanyl, or methadone) between July 2008 through September 2015. A difference-in-differences design was used to compare the pre-post reformulation changes in OD rates for OxyContin versus comparators.
Results:
A total of 297,836 individuals were dispensed OxyContin and 659,673 individuals were dispensed a primary comparator across the 3 databases. Overall, there was little or no difference in the temporal change in OD incidence in comparators versus OxyContin (Medicaid: adjusted ratio-of-rate-ratios (aRoRs) ranging from 0.90 to 1.05; MarketScan/HIRD: aRoR ranging from 1.10 to 1.22). However, restriction to person-time without concomitant opioid use revealed a modestly greater reduction in OD incidence over time during OxyContin use, as the aRoRs comparing the primary comparators to OxyContin ranged from 1.06 to 1.30 in Medicaid and from 1.64 to 1.85 in MarketScan/HIRD.
Discussion:
This study did not detect an overall effect of the OxyContin reformulation on OD in insured patients under regular medical care. There is a suggestion of a modestly reduced OxyContin-associated OD risk following the reformulation but only in commercially insured individuals receiving single-opioid regimens.
Key Words: opioids, abuse-deterrent, OxyContin, reformulation, overdose
Misuse and abuse of prescription opioids are serious public health problems and place a significant burden on national health systems.1 OxyContin is a commonly used extended release (ER) oral tablet formulation of oxycodone hydrochloride. Although the original formulation of OxyContin was intended to be taken orally intact (ie, swallowing the tablet whole), similar to other opioids, it was possible to manipulate the original formulation of OxyContin for misuse and abuse to engage in unapproved routes of administration [eg, chewing, crushing/swallowing, insufflation (snorting), intravenous injection].2–4
In 2010, the US Food and Drug Administration (FDA) approved an abuse-deterrent formulation (ADF) of OxyContin tablets intended to reduce misuse and abuse of OxyContin and their consequences, including overdose (OD) and death. OxyContin was reformulated with a polyethylene oxide matrix that hardens tablets and resists syringe aspiration and subsequent injection. Reformulated OxyContin became commercially available on August 9, 2010, and, in consultation with FDA, shipments of the original formulation ceased that same month. There was no notification to the general public or prescribers, and no modification in price. By December 2010 and December 2011, 90% and 99%, respectively, of OxyContin prescriptions dispensed were for reformulated OxyContin.
In 2013, FDA approved new labeling of OxyContin describing abuse-deterrent properties (ADP), based on evidence generated from in vitro studies and short-term clinical trials regarding abuse.5–7 However, there have been no long-term randomized trials examining the effect of reformulated OxyContin on abuse of OxyContin and related outcomes. As a condition of approval,8 FDA required a postmarketing epidemiology program for reformulated OxyContin, including this study.1
Several prior publications have assessed the impact of the OxyContin reformulation in the United States9–19 including studies that used claims data which suggested a decline in OxyContin utilization after the reformulation.17,18 One of the studies suggested a decline in the rate of OD because of prescription opioids after the reformulated OxyContin entered the market,18 but did not attempt to identify which specific opioid product(s) had been dispensed before each OD. It is possible that overall estimates could miss or misstate effects in patients specifically dispensed OxyContin. Overall changes in the incidence of OD may have been impacted by co-varying temporal factors causally unrelated to the OxyContin reformulation.
To date, there had been no direct evaluation of the impact of OxyContin’s reformulation on OD risk for individuals dispensed OxyContin in comparison to those dispensed other opioids. In this study, we utilized 3 large administrative claims databases to evaluate and compare unintentional fatal and nonfatal OD rates among patients dispensed OxyContin or comparator opioids.
MATERIALS AND METHODS
Study Population and Design
This study included the national Medicaid database and 2 national commercial claims databases [IBM MarketScan Commercial and Medicare Supplemental Claims and Encounters database (MarketScan) and the HealthCore Integrated Research Database (HIRD)]. The Medicaid database, Medicaid Analytic eXtract (MAX), covers all 50 states and Washington, DC through 2012. The Medicaid population was restricted to treatment episodes that were from fee-for-service (FFS) or comprehensive managed care (CMC) plans in which the combined state, year, and basis of eligibility group demonstrated research usability (ie, continuity and connectivity between data elements following criteria defined by Li et al).20 MarketScan collects data from employers and health plans. The HIRD includes health plan members insured through Anthem. Each database was restricted to populations that were linkable to the National Death Index (NDI), a central computerized index of death record information derived from state vital statistics data.21,22 For inclusion into this study, individuals were required to be aged 16 to 74 years (16 to 64 y in Medicaid) and have ≥3 months continuous health plan enrollment before eligible opioid dispensings.
The study design was a retrospective cohort study comparing rates of OD before and after the OxyContin reformulation in users of OxyContin and in contemporaneous users of comparator opioids. The study period ranged from July 1, 2008 to June 30, 2010 as the prereformulation period (preperiod), and January 1, 2011 to September 30, 2015 for the postreformulation period for the commercial databases and through December 31, 2012 for Medicaid (postperiod) (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CJP/A873). The main analyses excluded a transition period of July 1, 2010 to December 31, 2010 when both the original and reformulated versions of OxyContin were likely used. Analyses included all users (new and prevalent) and separately restricted to new users which allowed for the application of the new user design. We compared the 5-year postperiod to the 2-year preperiod for the main analyses in the commercial insurance databases, and a 2-year postperiod and 2-year preperiod in Medicaid because of the unavailability of more recent data (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CJP/A873).
Exposures
The primary exposure evaluated in this study was OxyContin. The primary comparator opioids were ER morphine, transdermal fentanyl, and methadone tablets/capsules, selected because of similarities with OxyContin in labeled indications, long marketing histories, and large, stable market shares throughout the study period. Secondary comparator opioids consisted of single-entity ER oxymorphone, incidence rates (IR) oxycodone tablets, and IR hydromorphone tablets. Evidence showed that these products were the most preferred alternatives for abuse among prescription opioids during the time of decreasing popularity of OxyContin after it was reformulated.12
The analytic datasets were constructed using treatment episodes, with time-dependent covariates evaluated at the beginning of each treatment episode. Individuals were considered exposed to OxyContin and comparator opioids beginning on the day of dispensing and continuing through the days’ supply of the drug plus an extension period of half of the days’ supply. A treatment episode ended if there was 1 or more days of discontinuity between dispensings, and was censored if an individual discontinued any of the opioids that defined the episode, initiated another study opioid, had an OD, died, terminated their health insurance coverage, or reached the end of the preperiod, transition period, or study period. Thus, if 2 study opioids were dispensed within days of each other, the treatment episode for the first study opioid was terminated on the day of the dispensing of the second study opioid, and a new treatment episode was created at the dispensing of the second study opioid. If an individual obtained a new dispensing of an opioid agent before exhausting the days’ supply of a prior dispensing of that agent, we assumed that medication was taken starting on the day of the pharmacy dispensing.
Exposure for each treatment episode was classified in subcategories including “any use” of the drug (ie, with or without concomitant opioid use) and “only use” of the drug (ie, without concomitant opioid use). An example of treatment episode construction and exposure groups is provided in Supplemental Figure 2 (Supplemental Digital Content 1, http://links.lww.com/CJP/A873). “Only use” treatment episodes represented approximately one-third of the “any use” person-time. For comparative analyses with the primary comparators, we excluded treatment episodes with concomitant use of 2 or more primary comparators, or OxyContin with a primary comparator.
New (ie, incident) use was assessed for each treatment episode and defined as having had no recorded dispensing of any opioid study drug in the 3 months before the start of the treatment episode. This new use treatment episode plus any adjacent, continuous treatment episodes for that study drug comprised an incident use episode. An individual could have multiple incident use treatment episodes in the study if more than 1 treatment episode met the incident use criteria.
Outcomes
The primary outcome was unintentional fatal or nonfatal unintentional OD. Opioid OD was defined using a previously validated algorithm that uses the diagnostic codes associated with services in US health insurance data23,24 for events resulting in health services (both fatal and nonfatal). Further identification of fatal OD used linkage to the US NDI. A patient was classified as having experienced an OD event if they had at least one OD related ICD-9 and ICD-10 code (provided in Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CJP/A873) in any position or setting. Events were restricted to those not classified as intentional (defined through codes for suicide and other correlated factors)23,24 and those that occurred during a treatment episode.
Validation of nonfatal (insurance claims-based) OD was conducted through medical record review for 159 cases in the HIRD. Standardized data collection techniques were used to abstract information from the medical records including medical history and treatment of the possible OD event. Results from this validation study were similar to the previous validation studies of Green et al23,24 Among the 159 code-defined ODs, 135 (positive predictive value=85%) were confirmed on chart review (47 intentional and 88 unintentional). The algorithm to detect intentionality had lower accuracy than the overall OD algorithm (positive predictive value=69%), but a high sensitivity (98%). This suggests that a sizeable subset of intentional ODs were classified as unintentional with the unintentional OD algorithm. Additional methods and results for the validation study are further described in our prior publication.25
Covariates
Demographic characteristics, clinical characteristics, and other comorbidities and conditions noted in Supplemental Table 2 (Supplemental Digital Content 1, http://links.lww.com/CJP/A873) were assessed in claims for each treatment episode during a 3-month or 6-month lookback period before each treatment episode, except certain demographic characteristics that were assessed on the index date. Evaluation at each treatment episode allowed for the ability to account for potential time-varying confounding.
Statistical Analyses
Poisson regression models, which model log-linear relationships for rates, were used to calculate IRs and all comparison measures for OxyContin and comparator opioid groups. Since only the numerator of a rate (the number of events) is random, the dependent variable for each treatment category was the logarithm of the number of events. The logarithm of the denominator (person-time) was introduced into the model as an offset. The regression models were carried out using repeated-measures Generalized Estimating Equations with sandwich variance estimators and with independent covariance matrices for repeated observations because of multiple treatment episodes per patient. Approximately 10% of patients with an OD had multiple, distinct ODs during follow-up of the study. A time-varying covariate term for “prior OD event” was introduced to account for the most substantial source of within-person correlation.
Incidence rate ratios (IRRs) compared IRs between OxyContin and comparator groups within the prereformulation and postreformulation time periods. For pre-post comparisons, analyses were conducted in 3 ways in each database: (1) unadjusted for covariates; (2) adjusted for possibly time-varying covariates; and (3) adjusted for possibly time-varying covariates as well as other baseline characteristics through propensity score weighting in a new user cohort.
Postreformulation versus prereformulation rate ratios (RRs) were calculated for OxyContin and each of the comparator opioid groups. To compare pre-post reformulation changes in OD rates (ie, the RRs) for OxyContin and for comparator opioids, this study utilized a difference-in-differences design [implemented here as ratios-of-rate-ratios (RoRs)]. RRs and RoRs were also adjusted for baseline demographic and clinical covariates provided in Supplemental Table 2 (Supplemental Digital Content 1, http://links.lww.com/CJP/A873) using the Poisson regression models with Generalized Estimating Equations.
Adjusted RRs and RoRs were determined in the new user cohort using propensity score weighting (through inverse probability of treatment weighting) to match the covariate distributions of the prereformulation and postreformulation cohorts. The aim was to weight the postreformulation group to match the covariate structure of the prereformulation group in regard to demographic and clinical characteristics, in addition to other comorbidities and conditions. For OxyContin and each of the comparator opioids propensity scores were derived using logistic regression to estimate the fitted probability that a given incident use treatment episode with a specified set of covariates was from the prereformulation period as opposed to the postreformulation period. Propensity scores outside the range of overlap between the 2 time periods (prereformulation and postreformulation) were trimmed.
Sensitivity analyses included examination of the main results stratified by incident versus prevalent opioid use, fatal versus nonfatal OD status, and intentional versus unintentional OD status. We also examined results restricting the postperiod to 3 years in the commercial databases, and stratifying results by CMC versus FFS status in the Medicaid database. Analyses were conducted by HealthCore, IBM, and STATinMED using SAS.
Results from the 2 commercial databases (HIRD and MarketScan) were additionally pooled using random-effects meta-analysis.26–28 The Medicaid population was not included in the pooling given a priori differences in this population (eg, lower social economic status).
Human Participants
Institutional Review Board (IRB) approval was obtained for each site before NDI linkage and for conducting the validation of the algorithm for OD in the HIRD. No individual-level data were shared with the study sponsor.
RESULTS
Study Population
This study included 94,445 Medicaid patients, 122,254 MarketScan patients, and 81,137 HIRD patients who were dispensed OxyContin, and 367,814 Medicaid patients, 181,240 MarketScan patients, and 110,619 HIRD patients dispensed at least 1 of the primary comparator opioids. Exposed person-time per patient in each of the databases ranged from 6 to 10 months (Table 1). The dose of the dispensed OxyContin was 40 mg or more in 54% of Medicaid patients, 20% of MarketScan patients, and 36% of HIRD patients. Much of the use of OxyContin and primary comparator was concomitant with other opioids such as IR oxycodone or other nonprimary or secondary opioids (Table 1). Patient characteristics were largely similar between OxyContin treatment episodes and primary comparator opioid treatment episodes, with a few notable differences including a higher proportion of females and patients with certain pain disorders (abdominal, chronic, and neuropathic) among primary comparator opioid treatment episodes compared with OxyContin treatment episodes. Each of these personal characteristics included in the analysis had modest or no association with subsequent OD risk (all IRRs <2 and >0.5). By contrast, having experienced a prior OD was strongly associated with a subsequent OD (IRRs>14 in each database; results available upon request).
TABLE 1.
Demographic and Clinical Characteristics Summary of OxyContin and Primary Comparator Opioid Use in the Medicaid, MarketScan and HIRD Databases
| Any OxyContin Use* | Any Primary Comparator Opioid Use† | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Value | Medicaid | MarketScan | HIRD | Medicaid | MarketScan | HIRD |
| Patients | 94,445 | 122,254 | 81,137 | 367,814 | 181,240 | 110,619 | |
| Total person-time per patient in months | Mean (SD) | 7.8 (10.0) | 6.0 (10.3) | 6.1 (11.4) | 8.1 (10.3) | 8.0 (11.9) | 9.5 (13.9) |
| Treatment episodes | 522,775 | 561,703 | 378,441 | 2,039,232 | 975,389 | 654,462 | |
| Sex, n (%) | Female | 295,875 (56.6) | 285,366 (50.8) | 189,986 (50.2) | 1,241,520 (60.9) | 560,051 (57.4) | 382,769 (58.5) |
| Male | 226,900 (43.4) | 276,337 (49.2) | 188,455 (49.8) | 797,712 (39.1) | 415,338 (42.6) | 271,693 (41.5) | |
| Age (y) | Mean (SD) | 46.7 (10.5) | 53.1 (12.0) | 51.4 (12.2) | 46.9 (10.6) | 54.6 (11.6) | 53.4 (11.9) |
| DCI | Mean (SD) | 2.0 (2.8) | 2.0 (3.1) | 1.7 (2.8) | 2.0 (2.8) | 2.4 (3.3) | 2.0 (3.0) |
| Comparator use, any, n (%) | ER morphine | 0 | 0 | 0 | 964, 343 (47.3) | 383,442 (39.3) | 252, 960 (38.7) |
| TD fentanyl | 0 | 0 | 0 | 564,161 (27.7) | 441,383 (45.3) | 272,898 (41.7) | |
| Methadone | 0 | 0 | 0 | 510,728 (25.1) | 150,564 (15.4) | 128,604 (19.7) | |
| IR oxycodone | 133,497 (25.5) | 148, 267 (26.4) | 96,452 (25.5) | 290,641 (14.3) | 122,702 (12.6) | 89,639 (13.7) | |
| IR hydromorphone | 29, 378 (5.6) | 24,217 (4.3) | 17,397 (4.6) | 137,657 (6.8) | 67,264 (6.9) | 46,086 (7.0) | |
| ER oxymorphone | 4,937 (0.9) | 5,769 (1.0) | 3,602 (1.0) | 14,035 (0.7) | 10,630 (1.1) | 6,200 (0.9) | |
| Other opioid use (nonprimary/secondary comparators | 210,501 (40.3) | 229,127 (40.8) | 153,387 (40.5) | 924,121 (45.3) | 451,161 (46.3) | 290,985 (44.5) | |
| Clinical and co-morbidity characteristics‡ | Abdominal pain | 99,797 (19.1) | 80,535 (14.3) | 55,554 (14.7) | 436,472 (21.4) | 179,919 (18.4) | 120,612 (18.4) |
| Chronic pain | 104,311 (20.0) | 65,463 (11.7) | 63,456 (16.8) | 427,644 (21.0) | 143,661 (14.7) | 138,170 (21.1) | |
| Neuropathic pain | 16,857 (3.2) | 14,164 (2.5) | 10,627 (2.8) | 70,734 (3.5) | 32,678 (3.4) | 26,043 (4.0) | |
| COPD | 102,942 (19.7) | 64,556 (11.5) | 49,926 (13.2) | 401,863 (19.7) | 129,161 (13.2) | 104,775 (16.0) | |
| Major depression disorder | 88,372 (16.9) | 62,556 (11.1) | 58,692 (15.5) | 378,331 (18.6) | 128,661 (13.2) | 119,470 (18.3) | |
| History of overdose | 2657 (0.5) | 1428 (0.3) | 1110 (0.3) | 15,485 (0.8) | 3801 (0.4) | 3160 (0.5) | |
| Opioid type dependence | 30,472 (5.8) | 9560 (1.7) | 11,343 (3.0) | 119,537 (5.9) | 18,777 (1.9) | 23,706 (3.6) | |
| Nonopioid drug dependence | 32,589 (6.2) | 7963 (1.4) | 8840 (2.3) | 119,625 (5.9) | 15,083 (1.5) | 19,215 (2.9) | |
| Benzodiazepines‡ | 97,110 (18.6) | 86,631 (15.4) | 60,818 (16.1) | 368,051 (18.1) | 154,579 (15.8) | 109,074 (16.7) | |
Frequency (percent) presented unless otherwise specified.
Any use of OxyContin excluding concomitant primary comparator opioid use.
Any use of any of the primary comparators (ER morphine, TD fentanyl, or methadone) excluding concomitant OxyContin or other primary comparator use.
Each of the clinical and co-morbidity characteristics listed used a 3-month lookback period before each treatment episode for their calculation, except for the comparator use, other opioid use and benzodiazepines which were measured during the treatment episodes.
COPD indicates chronic obstructive pulmonary disease; DCI, Deyo-Charlson Index; ER, extended release; HIRD, HealthCore Integrated Research Database; IR, immediate release; TD, transdermal.
Incidence of OD During OxyContin Use
The IR for OD during OxyContin treatment episodes varied by database between 0.8 (MarketScan) and 1.6 (Medicaid) per 1000 person-months and was lower than the IR during comparator opioid use, which varied between 0.9 and 2.9 per 1000 person-months (Table 2 and Fig. 1). Over 80% of ODs were nonfatal for both OxyContin and comparator opioids. In each database, the IR for OD during any use OxyContin exposure time declined from prereformulation to postreformulation [Medicaid: postperiod vs. preperiod adjusted aRR=0.93, 95% confidence interval (CI)=0.83-1.04; MarketScan/HIRD: aRR=0.86, 95% CI=0.75-1.00; Supplemental Table 3, Supplemental Digital Content 1, http://links.lww.com/CJP/A873]. For the any use analysis, these comparisons permitted concurrent dispensing of Schedule II opioids (other than the comparators). The decline in the IR for OD over time was more pronounced during use of OxyContin when it was dispensed with no other concurrent Schedule II opioid (Medicaid: aRR=0.80, 95% CI=0.63-1.01; MarketScan/HIRD: aRR=0.57, 95% CI=0.42-0.77; Fig. 2 and Supplemental Table 3, Supplemental Digital Content 1, http://links.lww.com/CJP/A873).
TABLE 2.
Incidence Rates (IRs) of Unintentional Fatal or Nonfatal Opioid Overdose (OD) Among Any OxyContin and Any Primary Comparator Opioid (Nonoverlapping)* Use 2 Years Before and 5 Years After the Reformulation by Database
| Unintentional Fatal or Nonfatal Opioid Overdose | Period | Patients | Overdoses | Person-months | IR Per 1000 Person-months | IRR (Comp/OxyContin) |
|---|---|---|---|---|---|---|
| Medicaid† | ||||||
| OxyContin | Pre | 54,855 | 630 | 384,417 | 1.64 | — |
| ER morphine | Pre | 98,795 | 1352 | 581,045 | 2.33 | 1.42 |
| TD fentanyl | Pre | 59,597 | 780 | 337,179 | 2.31 | 1.41 |
| Methadone | Pre | 55,930 | 1201 | 421,755 | 2.85 | 1.74 |
| OxyContin | Post† | 53,161 | 569 | 349,899 | 1.63 | — |
| ER morphine | Post† | 132,902 | 1794 | 803,822 | 2.23 | 1.37 |
| TD fentanyl | Post† | 62,377 | 860 | 359,083 | 2.40 | 1.47 |
| Methadone | Post† | 60,932 | 1205 | 477,538 | 2.52 | 1.55 |
| MarketScan | ||||||
| OxyContin | Pre | 51,027 | 212 | 268,476 | 0.79 | — |
| ER morphine | Pre | 34,180 | 185 | 190,891 | 0.97 | 1.23 |
| TD fentanyl | Pre | 37,991 | 194 | 216,440 | 0.90 | 1.14 |
| Methadone | Pre | 13,262 | 100 | 102,965 | 0.97 | 1.23 |
| OxyContin | Post | 82,797 | 344 | 459,907 | 0.75 | — |
| ER morphine | Post | 60,206 | 407 | 378,948 | 1.07 | 1.44 |
| TD fentanyl | Post | 57,861 | 404 | 373,612 | 1.08 | 1.45 |
| Methadone | Post | 18,396 | 209 | 177,857 | 1.18 | 1.57 |
| HIRD | ||||||
| OxyContin | Pre | 34,740 | 156 | 176,145 | 0.89 | — |
| ER morphine | Pre | 19,523 | 128 | 121,089 | 1.06 | 1.19 |
| TD fentanyl | Pre | 21,992 | 151 | 132,254 | 1.14 | 1.29 |
| Methadone | Pre | 10,859 | 95 | 86,429 | 1.10 | 1.24 |
| OxyContin | Post | 53,217 | 256 | 314,899 | 0.81 | -- |
| ER morphine | Post | 37,153 | 302 | 293,756 | 1.03 | 1.26 |
| TD fentanyl | Post | 31,662 | 277 | 250,060 | 1.11 | 1.36 |
| Methadone | Post | 14,943 | 209 | 169,022 | 1.24 | 1.52 |
Treatment episodes that had overlapping use of more than one of the primary comparators or OxyContin at the same time were excluded.
A postperiod of 2 years was used for the Medicaid database instead of a 5-year postperiod.
ER indicates extended release; HIRD, HealthCore Integrated Research Database; IR, incidence rate; IRR, incidence rate ratio; OD, overdose; TD, transdermal.
FIGURE 1.
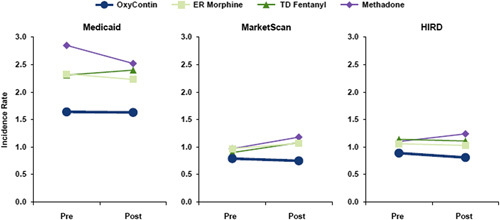
Incidence rates of unintentional fatal or nonfatal opioid overdose in the postreformulation and prereformulation period during “any use” of OxyContin and the primary comparators. Any use-concurrent use of opioids other than the study drugs was permitted. ER indicates extended release; HIRD, HealthCore Integrated Research Database; OD, opioid overdose; TD, transdermal.
FIGURE 2.
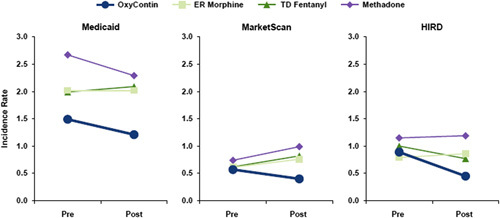
Incidence rates of unintentional fatal or nonfatal opioid overdose in the postreformulation and prereformulation period during “only use” of OxyContin and the primary comparators. Only use-no concurrent use of any opioid. ER indicates extended release; HIRD, HealthCore Integrated Research Database; OD, opioid overdose; TD, transdermal.
OxyContin Versus Primary Comparators
For “any use” exposures, the postreformulation versus prereformulation RRs of OD were similar for OxyContin and each of the primary comparators (Fig. 3). In Medicaid, the crude RoRs measuring the postreformulation versus prereformulation RRs for comparator versus OxyContin ranged from 0.89 to 1.04, with the adjusted ratio-of-rate-ratios (aRoRs) ranging from 0.90 to 1.05 (Fig. 3; aRoR>1 means that the pre-post decline was greater during use of OxyContin than during use of the comparator). In the pooled MarketScan/HIRD results, the RoRs ranged from 1.13 and 1.26 comparing the pre-post trend in each comparator to OxyContin, with the aRoRs ranging from 1.10 to 1.22 (Fig. 3). In the “only use” comparisons, which were restricted to person-time exposed only to OxyContin or only to a single primary comparator, there was evidence of trends in OD incidence in the prereformulation to postreformulation periods that were more favorable for OxyContin versus the primary comparators in the commercial databases (Medicaid: aRoRs ranged from 1.06 to 1.30 comparing primary comparators to OxyContin; MarketScan/HIRD: aRoRs ranged from 1.64 to 1.85 comparing the primary comparators to OxyContin; Fig. 3).
FIGURE 3.
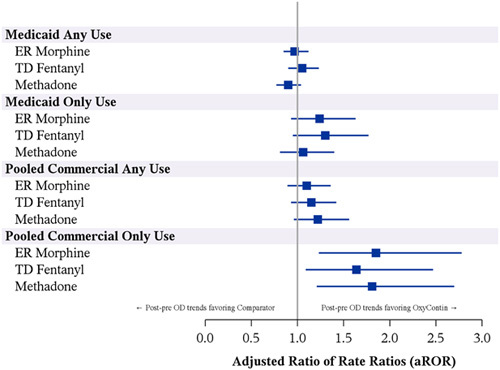
Adjusted ratio of rate ratios (aRoRs) comparing the rate ratio of unintentional opioid overdose (OD) in the postreformulation versus prereformulation period for primary comparators to the rate ratio of unintentional OD in the postreformulation versus prereformulation period for OxyContin. Any use-concurrent use of opioids other than the study drugs was permitted. Only use-no concurrent use of any opioid. ER indicates extended release; TD, transdermal.
OxyContin Versus Secondary Comparators
Results were largely similar to the primary comparator analysis for OxyContin versus the secondary comparators. There was a lack of an association when comparing any use, but there was evidence of lower risk of OD during OxyContin only use than comparator only use, particularly in the commercial databases (Table 3). There were differences between the 2 commercial databases. In the HIRD, there were relatively similar patterns in OD during any OxyContin use, while there was a stronger decline in the OD rate during OxyContin only use than during secondary comparator only use (Table 3). In MarketScan, there was evidence of a stronger decline for OxyContin in the OD rate over time than those observed in the HIRD for both analyses (any use and only use; Table 3). The pooled aRoR results for any use of each of the 3 secondary comparators relative to any use of OxyContin ranged from 1.42 to 1.74. The pooled aRoRs for only use of each of the 3 secondary comparators relative to OxyContin only ranged from 1.43 to 2.91 (data available upon request).
TABLE 3.
Rate Ratios of Unintentional Fatal or Nonfatal Overdose (OD) Among OxyContin and Secondary Comparator Opioid Use Before and After the Reformulation, by Database
| Rate Ratio for Postreformulation vs. Prereformulation Periods | Ratio of Rate Ratios for Comparator Opioids vs. OxyContin | |||||
|---|---|---|---|---|---|---|
| Adjusted Rate Ratio | 95% LCL | 95% UCL | Adjusted Ratio of Rate Ratios | 95% LCL | 95% UCL | |
| Medicaid | ||||||
| Any nonoverlapping use* | ||||||
| OxyContin | 0.85 | 0.73 | 0.98 | Ref | — | — |
| ER oxymorphone | 1.06 | 0.81 | 1.38 | 1.25 | 0.92 | 1.69 |
| IR oxycodone SE | 0.92 | 0.83 | 1.01 | 1.08 | 0.9 | 1.29 |
| IR hydromorphone | 0.90 | 0.76 | 1.07 | 1.06 | 0.85 | 1.33 |
| Use without concomitant opioids (“only use”) | ||||||
| OxyContin | 0.80 | 0.63 | 1.01 | Ref | — | — |
| ER oxymorphone | 1.01 | 0.66 | 1.54 | 1.27 | 0.79 | 2.06 |
| IR oxycodone SE | 0.94 | 0.83 | 1.05 | 1.18 | 0.90 | 1.53 |
| IR hydromorphone | 1.00 | 0.80 | 1.25 | 1.26 | 0.91 | 1.74 |
| MarketScan | ||||||
| Any nonoverlapping use* | ||||||
| OxyContin | 0.69 | 0.55 | 0.86 | Ref | — | — |
| ER oxymorphone | 1.74 | 0.94 | 3.24 | 2.54 | 1.31 | 4.92 |
| IR oxycodone SE | 1.20 | 0.94 | 1.54 | 1.75 | 1.26 | 2.44 |
| IR hydromorphone | 1.03 | 0.72 | 1.47 | 1.50 | 0.99 | 2.29 |
| Use without concomitant opioids (“only use”) | ||||||
| OxyContin | 0.64 | 0.44 | 0.94 | Ref | — | — |
| ER oxymorphone | 2.76 | 0.84 | 9.08 | 4.30 | 1.23 | 15.04 |
| IR oxycodone SE | 1.29 | 0.96 | 1.73 | 2.01 | 1.24 | 3.25 |
| IR hydromorphone | 0.83 | 0.54 | 1.25 | 1.29 | 0.73 | 2.26 |
| HIRD | ||||||
| Any nonoverlapping use* | ||||||
| OxyContin | 0.59 | 0.44 | 0.80 | Ref | — | — |
| ER oxymorphone | 0.72 | 0.41 | 1.26 | 1.21 | 0.64 | 2.28 |
| IR oxycodone SE | 0.98 | 0.71 | 1.35 | 1.65 | 1.07 | 2.53 |
| IR hydromorphone | 0.78 | 0.53 | 1.16 | 1.32 | 0.81 | 2.14 |
| Use without concomitant opioids (“only use”) | ||||||
| OxyContin | 0.48 | 0.30 | 0.78 | Ref | — | — |
| ER oxymorphone | 1.00 | 0.35 | 2.87 | 2.08 | 0.65 | 6.66 |
| IR oxycodone SE | 1.00 | 0.68 | 1.48 | 2.09 | 1.13 | 3.84 |
| IR hydromorphone | 0.80 | 0.49 | 1.28 | 1.66 | 0.86 | 3.19 |
Treatment episodes that had overlapping use of more than 1 secondary comparator or any primary comparators or OxyContin at the same time were excluded.
ER indicates extended release; HIRD, HealthCore Integrated Research Database; IR, immediate release; LCL, lower confidence limit; SE, single entity; UCL, upper confidence limit.
Sensitivity Analyses
Results were largely similar in sensitivity analyses. Results were similar when stratified by prevalent versus incident use, by fatal versus nonfatal OD, by intentional versus unintentional OD, and when restricting the postperiod in commercial databases to 3 years (data available upon request). In the Medicaid database, results appeared modestly different by FFS/CMC status. A decline in the OD rate during any use of OxyContin in the preperiod to postperiod was observed in the FFS population (aRR=0.81, 95% CI=0.69-0.95), but no change was observed in the CMC population (aRR=1.10, 95% CI=0.92-1.31). The estimates for the aRoRs were also often slightly different by population, as the OD comparing any transdermal fentanyl to any OxyContin was 1.28 (95% CI=1.03-1.59) in the FFS population, and 0.84 (95% CI=0.66-1.07) in the CMC population.
DISCUSSION
The 2010 reformulation of OxyContin to a product with physicochemical barriers to deter injection or insufflation was not associated with a decline in the incidence of OD in OxyContin users beyond what might have been expected from secular trends seen in comparator opioids. However, when analyses were restricted to person-time during which there was no use of concomitant opioids, in the commercially insured databases, the OxyContin reformulation was modestly associated with a decline in OD rates during OxyContin only use as compared with during the use of comparators. This decline was not seen among the Medicaid population in this study, generally, a higher risk population for opioid misuse and abuse.29
While this study noted a modest decline in the OD rate over time during any OxyContin use and a lower rate of OD during any OxyContin use than any comparator use, in most of the analyses, the reformulation appeared to have had minimal impact on the rate of OD among insured patients receiving medical care and dispensed OxyContin. The lack of an effect among individuals using multiple opioids concomitantly may be because the reformulated OxyContin was intended to deter abuse of OxyContin (ie, not other opioids).
The analyses that focused on OxyContin use without concomitant opioids (ie, “OxyContin only”), which represented approximately one-third of the person-time in the overall analyses, showed a decline in the incidence of OD in the postreformulation period in comparison to the prereformulation period in the commercially insured databases. Comparisons to the primary and secondary comparator opioids without concomitant opioids supported the hypothesis of improvement among OxyContin recipients (ie, greater reduction in OD rates over time). The decline may suggest that the reformulation could represent a barrier to injecting or snorting OxyContin (as suggested in other treatment and poison center studies) (FDA, 2020) but not a barrier to overdosing from poly-opioid use involving oral OxyContin. Thus, the reformulation may only have a measurable effect among OxyContin use without concomitant opioids in certain populations. Alternatively, persons using 1 opioid may tend to misuse or abuse only that drug. Thus, the direct effect of the OxyContin reformulation may be to drive people who misuse or abuse opioids to request another opioid in the place of OxyContin, or to request supplementary opioids given that the reformulation was not intended to treat opioid addiction. Either scenario would result in a decline in the OD rate during OxyContin usage after the reformulation.
In this study of insured, largely middle-aged patients in active medical treatment with opioids, the incidence of OD was between 1.0 and 2.0 nonfatal ODs per 1000 person-months (ie, 1200 to 2400 nonfatal ODs per 100,000 person-years), and between 0.1 and 0.4 fatal ODs per 1000 person-months (ie, 120 to 480 fatal ODs per 100,000 person-years). As expected, these OD IRs are considerably higher than the general US population (~5 fatal ODs from prescription opioids per 100,000 person-years),30 but are likely significantly lower than the fatal OD rates seen among patients who misuse or abuse opioids and those with opioid use disorder.31 Medical monitoring in this study’s population may have limited or prevented the emergence of prescription opioid misuse or abuse, or resulted in early treatment of misuse or abuse if it occurred. This study was unable to identify opioids obtained outside of insurance claims (eg, cash only payments).
There are a few limitations to this study. Misclassification of the exposure or outcome could bias results of this study. This study used a previously validated algorithm for OD,23,24 and results from the validation study in the HIRD suggest that this algorithm may be transportable to at least some of this study’s populations, but somewhat limited in the accuracy of intentionality of the OD. Given its observational nature, this study is also prone to confounding and there was potential for channeling of individuals in either direction. While higher risk individuals may have initiated/switched to other opioids after the OxyContin reformulation, it is also possible that individuals at higher risk of OD may have been channeled to ADFs during the postperiod, as doctors and other prescribers may have become aware of the presence and potential value of the ADF. However, the results for the unadjusted, covariate adjusted, and propensity score weighted models were all relatively similar in this study, and this overall lack of impact of the covariate control is consistent with small or absent changes in the covariates within study drug use categories; at most, there was a modest effect of each of the covariates on the OD outcome (other than recent history of OD, which was strongly associated with the outcome). Although, it should be noted that not all potential important confounders could be directly measured in this study. For example, while we included opioid abuse/dependence from claims diagnoses in propensity score weighted models, we were unable to directly measure opioid misuse or abuse or the predilection to misuse or abuse opioids or other substances. Given that the association between misuse or abuse and OD may be large, modest differences between exposure categories could have an impact of inferences even if misuse or abuse were relatively uncommon in these populations receiving active medical care.29,32 It should also be noted that this study was restricted to individuals dispensed opioids while in active medical care and may not fully capture those at greatest risk of OD from illicit use.33 Finally, despite the size of the study and robustness of the sample, power was still limited for some subpopulations.
This study also had a number of strengths. It included 3 large administrative claims databases tracked longitudinally with a high degree of certainty for the opioid exposures and linkage to NDI to ascertain fatal outcomes. The opioid(s) dispensed to the individual overlapping with the OD is identified from prescription claims database records, rather than relying on respondents’ self-report such as in-drug treatment center studies. In addition, while there were likely other ongoing secular trends and policies during the study period that could have impacted opioid use and OD incidence, this study utilized contemporaneous opioid comparators, the difference-in-differences design, regression adjustment, propensity score weighting, and numerous sensitivity analyses aiming to address confounding and other biases.
CONCLUSIONS
The results of this study of individuals medically treated with opioids suggest that for any use of OxyContin or of comparator opioids there was little or no change in the rate of OD following the reformulation of OxyContin, although there was a suggestion in commercially insured individuals receiving single-opioid regimens of a reduced OxyContin-associated OD risk following the reformulation in commercially insured individuals. However, this was not seen among the Medicaid population captured in this study.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.clinicalpain.com.
ACKNOWLEDGMENTS
The authors acknowledge Nianya Liu, MS, HealthCore Inc., Andover, MA and Shiva Krishna Vojjala, MS, HealthCore Inc., Wilmington, DE (HealthCore) for programming support, and Mark Paullin, MS, HealthCore Inc., Wilmington, DE (HealthCore) for operational support of the validation study. Sulena Shrestha, MS, STATinMED, Plano, TX, Li Wang, PhD, MS, STATinMED, Plano, TX and Lu Li, MS, STATinMED, Plano, TX formerly of STATinMED who were involved of the Medicaid database related analyses. Rachelle Rodriguez, PhD, MPH, Purdue Pharma L.P., Stamford, CT and Stacy Baldridge, PhD, RN, Purdue Pharma, L.P., Stamford, CT of Purdue Pharma L.P., also provided advice on the research. The authors also acknowledge former Purdue Pharma L.P. researchers who had a role in the early study conceptualization and design aspects.
Footnotes
Study conception/design: D.C.B., K.H., L.B., G.B., R.G., H.Y., A.M.W. Analysis: L.L., R.G., G.B., H.Y. Interpretation of data: D.C.B., K.H., L.B., G.B., R.G., L.L., H.Y., A.M.W. Draft or revision of manuscript: D.C.B., K.H., L.B., G.B., R.G., L.L., H.Y., A.M.W.
This work was supported by Purdue Pharma L.P., Stamford, CT; HealthCore Inc., Wilmington, DE, STATinMED Research, Plano, TX, IBM Watson Health, Cambridge, MA, and World Health Information Science Consultants (WHISCON), Boston, MA received funding from Purdue Pharma L.P. to conduct this study and for the development of this manuscript. This underlying study was an FDA-mandated Post Market Requirement (PMR) study presented at the “Joint Meeting of the Drug Safety and Risk Management Advisory Committee (DSaRM) and the Anesthetic and Analgesic Drug Products Advisory Committee (AADPAC)” on September 10 to 11 2020. D.C.B. and K.H. are employees of HealthCore Inc. R.G., G.B., and L.L. were employees of HealthCore Inc. at the time of the study. L.B. is an employee of IBM Watson Health. H.Y. is an employee of New York City College of Technology-CUNY, Brooklyn, NY and collaborated as an external consultant to STATinMED Research for this study. A.M.W. is an employee of WHISCON. Purdue Pharma L.P. provided funding to HealthCore Inc., STATinMED Research, IBM Watson Health, and WHISCON to conduct this study and for the development of this manuscript. H.Y. declares no conflict of interest.
Contributor Information
Daniel C. Beachler, Email: dbeachler@healthcore.com.
Kelsey Hall, Email: kelseygangemi@gmail.com.
Renu Garg, Email: renu.k.garg@gmail.com.
Geetanjoli Banerjee, Email: geetanjoli.banerjee@gmail.com.
Ling Li, Email: rgliling@hotmail.com.
Luke Boulanger, Email: lboulang@us.ibm.com.
Huseyin Yuce, Email: Hyuce@citytech.cuny.edu.
Alexander M. Walker, Email: awalker@hsph.harvard.edu.
REFERENCES
- 1.US Food & Drug Administration. September 10-11, 2020 Joint Meeting of the Drug Safety and Risk Management Advisory Committee and the Anesthetic and Analgesic Drug Products Advisory Committee Meeting Announcement. 2020. Available at: https://www.fda.gov/advisory-committees/advisory-committee-calendar/september-10-11-2020-joint-meeting-drug-safety-and-risk-management-advisory-committee-and-anesthetic. Accessed December 15, 2020.
- 2.Butler SF, Black RA, Cassidy TA, et al. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J. 2011;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hays LR. A profile of OxyContin addiction. J Addict Dis. 2004;23:1–9. [DOI] [PubMed] [Google Scholar]
- 4.Katz N, Dart RC, Bailey E, et al. Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37:205–217. [DOI] [PubMed] [Google Scholar]
- 5.Cone EJ, Giordano J, Weingarten B. An iterative model for in vitro laboratory assessment of tamper deterrent formulations. Drug Alcohol Depend. 2013;131:100–105. [DOI] [PubMed] [Google Scholar]
- 6.Harris SC, Perrino PJ, Smith I, et al. Abuse potential, pharmacokinetics, pharmacodynamics, and safety of intranasally administered crushed oxycodone HCl abuse-deterrent controlled-release tablets in recreational opioid users. J Clin Pharmacol. 2014;54:468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.OxyContin [package insert]. 2021. Available at: http://app.purduepharma.com/xmlpublishing/pi.aspx?id=o. Accessed May 11, 2019.
- 8.FDA letter dated April 05, 2010, NDA 02272, NDA approval. Silver Springs, MD: Department of Health and Human Services, 2010.
- 9.Chilcoat HD, Coplan PM, Harikrishnan V, et al. Decreased diversion by doctor-shopping for a reformulated extended release oxycodone product (OxyContin). Drug Alcohol Depend. 2016;165:221–228. [DOI] [PubMed] [Google Scholar]
- 10.Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N Engl J Med. 2012;367:187–189. [DOI] [PubMed] [Google Scholar]
- 11.Cicero TJ, Ellis MS, Kasper ZA. A tale of 2 ADFs: differences in the effectiveness of abuse-deterrent formulations of oxymorphone and oxycodone extended-release drugs. Pain. 2016;157:1232–1238. [DOI] [PubMed] [Google Scholar]
- 12.Cicero TJ, Ellis MS. Abuse-deterrent formulations and the prescription opioid abuse epidemic in the United States: lessons learned from OxyContin. JAMA Psychiatry. 2015;72:424–430. [DOI] [PubMed] [Google Scholar]
- 13.Coplan PM, Kale H, Sandstrom L, et al. Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended-release oxycodone with abuse-deterrent characteristics. Pharmacoepidemiol Drug Saf. 2013;22:1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coplan PM, Chilcoat HD, Butler SF, et al. The effect of an abuse-deterrent opioid formulation (OxyContin) on opioid abuse-related outcomes in the postmarketing setting. Clin Pharmacol Ther. 2016;100:275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dart RC, Severtson SG, Bucher-Bartelson B. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:1573–1574. [DOI] [PubMed] [Google Scholar]
- 16.Havens JR, Leukefeld CG, DeVeaugh-Geiss AM, et al. The impact of a reformulation of extended-release oxycodone designed to deter abuse in a sample of prescription opioid abusers. Drug Alcohol Depend. 2014;139:9–17. [DOI] [PubMed] [Google Scholar]
- 17.Hwang CS, Chang HY, Alexander GC. Impact of abuse-deterrent OxyContin on prescription opioid utilization. Pharmacoepidemiol Drug Saf. 2015;24:197–204. [DOI] [PubMed] [Google Scholar]
- 18.Larochelle MR, Zhang F, Ross-Degnan D, et al. Rates of opioid dispensing and overdose after introduction of abuse-deterrent extended-release oxycodone and withdrawal of propoxyphene. JAMA Intern Med. 2015;175:978–987. [DOI] [PubMed] [Google Scholar]
- 19.Sessler NE, Downing JM, Kale H, et al. Reductions in reported deaths following the introduction of extended-release oxycodone (OxyContin) with an abuse-deterrent formulation. Pharmacoepidemiol Drug Saf. 2014;23:1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Zhu Y, Chen C, et al. Internal validation of Medicaid Analytic eXtract (MAX) data capture for comprehensive managed care plan enrollees from 2007 to 2010. Pharmacoepidemiol Drug Saf. 2017;27:1067–1076. [DOI] [PubMed] [Google Scholar]
- 21.Skopp NA, Smolenski DJ, Schwesinger DA, et al. Evaluation of a methodology to validate National Death Index retrieval results among a cohort of US service members. Ann Epidemiol. 2017;27:397–400. [DOI] [PubMed] [Google Scholar]
- 22.Williams BC, Demitrack LB, Fries BE. The accuracy of the National Death Index when personal identifiers other than Social Security number are used. Am J Public Health. 1992;82:1145–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green CA, Perrin NA, Janoff SL, et al. Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol Drug Saf. 2017;26:509–517. [DOI] [PubMed] [Google Scholar]
- 24.Green CA, Perrin NA, Hazlehurst B, et al. Identifying and classifying opioid-related overdoses: a validation study. Pharmacoepidemiol Drug Saf. 2019;8:1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beachler DC, Zhou S, Gangemi K, et al. Validation of an algorithm to identify opioid overdose among US commerically insured individuals. International Conference on Pharmcoepidemiology and Therapeutic risk Mangement Meeting (ICPE 2018). Prague, Czech Republic. 2018.
- 26.Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roland CL, Lake J, Oderda GM. Prevalence of prescription opioid misuse/abuse as determined by international classification of diseases codes: a systematic review. J Pain Palliat Care Pharmacother. 2016;30:258–268. [DOI] [PubMed] [Google Scholar]
- 30.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hser YI, Mooney LJ, Saxon AJ, et al. High mortality among patients with opioid use disorder in a large healthcare system. J Addict Med. 2017;11:315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mojtabai R, Amin-Esmaeili M, Nejat E, et al. Misuse of prescribed opioids in the United States. Pharmacoepidemiol Drug Saf. 2019;28:345–353. [DOI] [PubMed] [Google Scholar]
- 33.Hedegaard H, Minino AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief. 2018;329:1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.clinicalpain.com.


