Abstract
Agrimonia pilosa Ledeb., which belongs to Agrimonia and Rosaceae, is used in traditional Chinese medicine. It exhibits excellent medicinal properties and has been used to treat various diseases, such as tumors, trichomoniasis, vaginitis, diarrhea, and dysentery. Phytochemical studies have revealed that Agrimonia has over 100 secondary metabolites that can be categorized into six classes, i.e., flavonoids, isocoumarins, triterpenes, phloroglucinol derivatives, tannins, and organic acids. This review summarizes recently published literature on the chemical structures of 90 bioactive compounds that have been identified in A. pilosa and examines their pharmacological properties, including their antitumor, anti-inflammatory, antioxidant, antibacterial, and antidiabetic properties, as well as the potential development of parasitic resistance to these chemicals. This review highlights existing knowledge gap and serves as a basis for developing novel preparations of A. pilosa with medicinal value.
1. Introduction
Agrimonia pilosa Ledeb, also known as agrimony, Agrimoniae herba, or hairyvein agrimony, belongs to Rosaceae. It is a perennial herb that grows in east Asia, central Europe, and the former Soviet Union. It is 50–100 cm long and covered with white pilose; it has a cylindrical (diameter of 4–6 mm) red brown lower part and a square columnar, slightly concave on all sides, and also has a green brown upper part with longitudinal grooves, ridge lines, and knots. It has a light, hard, easy-to-break, and hollow stem. It also has odd pinnate compound leaves that show alternate, dark green, wrinkled, and curled structures. Its leaves are brittle and fragile and have two sizes, alternating on the leaf axis. Top leaflets are large, and complete leaflets are oval or long oval after flattening. The apex is sharp, the base has a wedge shape, and the edge is serrated. Leaves have two amplexicaul and obliquely ovate stipules. The raceme is slender, the lower part of the calyx is tubular, the upper part of the calyx tube has barbs, the apex is five lobed, and petals are yellow. The fruit is 0.7–0.8 cm long and 0.3–0.4 cm in diameter and tastes slightly bitter.
In traditional Chinese medicine (TCM), A. pilosa is considered to have hemostasis-regulating, antimalarial, antidiarrheal, detoxification, and complement deficiency-restoring effects. It is often used to treat tumors, vaginal trichomoniasis, diarrhea, and dysentery. “ZhongHuaBenCao” (Chinese Materia Medica) recorded that the compatibility of A. pilosa with Arborvitae leaves and lotus root can treat hemoptysis and hematemesis. Rhizoma Imperatae and Jiaoshanzhi are used for gingival bleeding, and Daji, Mutong, and Rhizoma Rhizoma are administered for hematuria. “National Compendium of Chinese Herbal Medicine” documents that the double concentrate of the whole plant is externally used to treat Trichomonas vaginalis infection. Phytochemical studies have revealed that flavonoids, isocoumarins, triterpenes, tannins, organic acids, and phloroglucinol derivatives constitute the main classes of active ingredients in A. pilosa and might be responsible for its antioxidant, antifatigue, antitumor, hypoglycemic, cardioprotective, and hepatocyte protection effects [1–5].
Several reviews have been published over the past 20 years. Table 1 summarizes all the reviews related to A. pilosa found in the scientific literature.
Table 1.
Previous reviews.
| Year of publication | Main topic | Years surveyed | Limitations | References |
|---|---|---|---|---|
| 2003 | Pharmacology and ethnomedicine | NS | Short review | [6] |
| 2004 | Pharmacology and ethnomedicine | NS | Short review | [7] |
| 2006 | Phytochemistry and pharmacology | NS | Short review | [8] |
| 2008 | Phytochemistry and pharmacology | NS | [9] | |
| 2008 | Phytochemistry and pharmacology | NS | Short review | [10] |
| 2009 | Phytochemistry and pharmacology | NS | Only the antitumor activity was discussed | [11] |
| 2011 | Botany, phytochemistry and pharmacology | NS | Only the antitumor activity was discussed | [12] |
| 2011 | Phytochemistry and pharmacology | NS | Only the antioxidant activity was discussed | [13] |
| 2015 | Phytochemistry and pharmacology | NS | Short review | [14] |
| 2016 | Phytochemistry and pharmacology | NS | [15] | |
| 2017 | Phytochemistry and pharmacology | NS | The phytochemical part is briefly presented | [16] |
| 2018 | Pharmacology | NS | Only the antitumor activity was discussed | [17] |
| 2020 | Phytochemistry and pharmacology | NS | [18] | |
| 2021 | Ethnomedicine | NS | Only the antitumor activity was discussed and the review is based mostly on A. pilosa preparation | [19] |
We noted that the existing reviews related to A. pilosa in major databases are mostly summaries of a single component or relatively brief short reviews, but an analysis of pharmacological activities of specific monomers and a discussion of related mechanisms are lacking. In this paper, to further understand the current research status of A. pilosa and provide justification for in-depth investigation and comprehensive application, we review the phytochemistry and pharmacological activities of A. pilosa and its possible mechanisms of action.
2. Methodology
The literature was reviewed by consulting scientific databases, including Web of Science, Springer, PubMed, ScienceDirect, and China National Knowledge Infrastructure. Plant taxonomy was confirmed via The Plant List. The query was supplemented by searching the reference lists of papers included in the first selection. The search terms were as follows: “Agrimonia pilosa” alone or in combination with “chemistry,” “pharmacology,” and “toxicity.” For this review, articles written in English or Chinese were taken into consideration.
3. Bioactive Compounds
Series bioactive compounds, including triterpenes and their glycosides, phloroglucinol derivatives, flavonoids and their glycosides, tannins, organic acids, and isocoumarins, have been isolated from A. pilosa. The two main classes include triterpenes and their glycosides and phloroglucinol derivatives. In this part, we summarized information about the main natural products isolated from A. pilosa over the past decade.
3.1. Flavonoids
Flavonoids identified in the A. pilosa extract mainly include quercetin, isoquercitrin, quercitrin, rutin, hyperoside, pilosanol A–C, and agriflavone (see Table 2 for details); these flavonoids exhibit significant antitumor, hepatocyte protection, free radical-scavenging, antioxidant, and immunoregulatory effects.
Table 2.
Flavonoids isolated from Agrimonia pilosa Ledeb.
| No | Compounds | Molecules | Molecular weight | Plant part | References |
|---|---|---|---|---|---|
| 1 | Quercetin 
|
C15H10O7 | 302 | Aerial parts | [15] |
| 2 | Isoquercitrin | C21H20O12 | 464 | Aerial parts | [20] |
| 3 | Quercitrin | C21H20O11 | 448 | Aerial parts | [20] |
| 4 | Rutin | C27H30O16 | 610 | Aerial parts | [20] |
| 5 | Hyperin | C21H20O12 | 464 | Aerial parts | [20] |
| 6 | Kaempferol 
|
C15H10O6 | 386 | Aerial parts | [22] |
| 7 | Kaempferol-3-O-β-d-glucopyranoside | C21H20O11 | 448 | Aerial parts | [23] |
| 8 | Kaempferol-3-o-α-l-rhamnopyranoside | C21H20O10 | 432 | Aerial parts | [23] |
| 9 | Tiliroside | C30H26O13 | 594 | Aerial parts | [23] |
| 10 | Kaempferide | C16H12O6 | 300 | Aerial parts | [24] |
| 11 | Kaempferide-3-O-α-L-rhamnopyranoside | C22H22O10 | 446 | Aerial parts | [24] |
| 12 | Kaempferol-3-O-rutinoside | C27H30O15 | 594 | Aerial parts | [24] |
| 13 | Apigenin 
|
C15H10O5 | 270 | Aerial parts | [25] |
| 14 | Apigenin-7-O-β-d-glucopyranoside | C21H20O10 | 432 | Aerial parts | [15] |
| 15 | Apigenin-7-O-β-D-methylglucuronate | C22H20O11 | 460 | Aerial parts | [25] |
| 16 | Apigenin-7-O-β-D-butylglucuronate | C25H26O11 | 502 | Aerial parts | [25] |
| 17 | Luteolin-7-O-sophoroside 
|
C27H30O16 | 610 | Aerial parts | [15] |
| 18 | Luteolin-7-O-(6-O-acetyl)-D-glucopyranoside | C23H22O11 | 474 | Aerial parts | [15] |
| 19 | Luteolin | C15H10O6 | 286 | Aerial parts | [22] |
| 20 | Luteolin-7-O-β-D-glucopyranoside | C21H20O11 | 448 | Aerial parts | [15] |
| 21 | Wogonin 
|
C15H12O5 | 272 | Aerial parts | [25] |
| 22 | (+)-Catechin 
|
C15H14O6 | 290 | Whole plant | [26] |
| 23 | Pilosanol A 
|
C29H32O10 | 540 | Root | [27] |
| 24 | Pilosanol B | C28H30O10 | 526 | Root | [27] |
| 25 | Pilosanol C | C28H30O10 | 526 | Root | [27] |
| 26 | (2R, 3R)-(+)-Taxifolin 
|
C15H12O7 | 304 | Whole plant | [20] |
| 27 | (2R, 3R)-(+)-Taxifolin-3-O-β-D-glucopyranoside | C21H22O12 | 466 | Aerial parts | [28] |
| 28 | (2S, 3S)-(−)-Taxifolin 
|
C15H12O7 | 304 | Whole plant | [26] |
| 29 | (2S, 3S)–(−)-Taxifolin-3-O-β-D-glucopyranoside | C21H22O12 | 466 | Aerial parts | [28] |
| 30 | (−)-Aromadendrin-3-O-β-D-glucopyranoside | C21H22O11 | 450 | Aerial parts | [23] |
| 31 | Dehydrodicatechin A 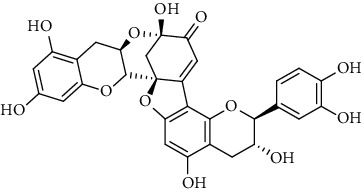
|
C30H24O12 | 576 | Whole plant | [26] |
| 32 | Agriflavone 
|
C27H28O15 | 593 | Aerial parts | [29] |
| 33 | Vitexin 
|
C21H20O10 | 432 | Aerial parts | [30] |
| 34 | Isovitexin 
|
C21H20O10 | 432 | Aerial parts | [30] |
| 35 | Dihydrodehydro-diconiferyl alcohol 9′-O-3-D-glucoside 
|
C26H34O11 | 522 | Aerial parts | [29] |
| 36 | Dihydrokaempferol 3-O-β-D-glucoside 
|
C21H22O11 | 450 | Aerial parts | [31] |
| 37 | (2S, 3R)-dihydrokaempferol 3-O-β-D-glucoside 
|
C21H22O11 | 450 | Aerial parts | [31] |
3.2. Isocoumarins
Isocoumarins identified in A. pilosa extracts mainly include agrimonolide, agrimonolide-6-O-β-D-glucopyranoside, and desmethylagrimonolide (Table 3). They exhibit hepatocyte protection, anti-inflammatory, and antitumor activities; they also regulate blood glucose and reduce insulin resistance (IR) [35–37].
Table 3.
Isocoumarins isolated from Agrimonia pilosa Ledeb.
| No | Compounds | Molecules | Molecular weight | Plant part | References |
|---|---|---|---|---|---|
| 1 | Agrimonolide 
|
C18H18O5 | 314 | Root | [32] |
| 2 | Agrimonolide-6-O-β-D-glucopyranoside | C24H28O10 | 476 | Root | [23] |
| 3 | Desmethylagrimonolide-6-O-β-D-glucopyranoside | C23H26O10 | 462 | Aerial parts | [23] |
| 4 | Desmethylagrimonolide | C17H16O5 | 300 | Whole plant | [4] |
| 5 | (3S)-Agrimonolide-6-O-α-L-arabinofuranosyl-(1 ⟶ 6)-β-D-glucopyranoside 
|
C29H36O14 | 609 | Whole plant | [33] |
| 6 | (3S, 4R)-4-Hydroxyagrimonolide-6-O-β-D-glucopyranoside | C24H28O11 | 493 | Whole plant | [33] |
| 7 | (3S)-Desmethylagrimonolide-4ʹ-O-β-D-glucopyranoside | C23H26O10 | 462 | Whole plant | [33] |
| 8 | (3S)-Agrimonolide-6-(60ʹ-galloyl)-O-b-D-glucopyranoside 
|
C31H32O14 | 629 | Aerial parts | [34] |
3.3. Triterpenes
Triterpenes are the main chemical constituents of A. pilosa. Several bioactive monomers, such as corosolic acid, euscaphic acid, ursolic acid, and pomolic acid, are also found in A. pilosa (Table 4). Most of A. pilosa triterpenes promote insulin sensitivity, improve glucose metabolism, and reduce oxidative stress in vitro, indicating that they have potential for the development of antidiabetic drugs [41].
Table 4.
Triterpenes isolated from Agrimonia pilosa Ledeb.
| No | Compounds | Molecules | Molecular weight | Plant part | References |
|---|---|---|---|---|---|
| 1 | Corosolic acid 
|
C30H48O4 | 472 | Aerial parts | [38] |
| 2 | Euscaphic acid | C30H48O5 | 488 | Aerial parts | [15] |
| 3 | Ursolic acid 
|
C30H48O3 | 456 | Aerial parts | [38] |
| 4 | Pomolic acid | C30H48O4 | 472 | Aerial parts | [38] |
| 5 | Ziyu-glucoside II | C35H56O8 | 604 | Aerial parts | [39] |
| 6 | 3-O-acetyl pomolic acid | C32H50O5 | 514 | Aerial parts | [39] |
| 7 | Rosamultin 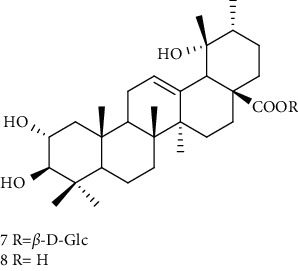
|
C36H58O10 | 650 | Aerial parts | [38] |
| 8 | Tormentic acid | C30H48O5 | 488 | Aerial parts | [38] |
| 9 | 1β, 2α, 3β, 19α-Terahydroxyurs-12-en-28-oic acid 
|
C30H48O6 | 504 | Aerial parts | [39] |
| 10 | 1β, 2α, 3β, 19α-Terahydroxyurs-12-en-28-oic acid | C30H48O6 | 504 | Whole plant | [40] |
| 11 | 27-Hydroxy-α-amyrin 
|
C30H50O2 | 442 | Whole plant | [40] |
3.4. Phloroglucinol Derivatives
Several phloroglucinol derivatives have been extracted from A. pilosa. For example, agrimol A, B, C, D, and E were isolated from A. pilosa petroleum ether extract by the Shanghai Institute of Materia Medica and the Shanghai Fourteenth Pharmaceutical Factory (1975) [42]. The phenolic compounds agrimol F and G were isolated from A. pilosa ethyl ether extract by Yamaki et al. [43]. Agrimophol and pseudoaspidin were isolated from the petroleum ether extract of A. pilosa rhizomes by Pei et al. [44]. Agripinol A–C were isolated from A. pilosa and named by Tang et al. [45] (Table 5).
Table 5.
Phloroglucinol derivatives isolated from Agrimonia pilosa Ledeb.
| No | Compounds | Molecules | Molecular weight | Plant part | References |
|---|---|---|---|---|---|
| 1 | Agrimol A 
|
C37H46O12 | 682 | Whole plant | [43] |
| 2 | Agrimol B | C37H46O12 | 682 | Whole plant | [43] |
| 3 | Agrimol C | C36H44O12 | 668 | Whole plant | [43] |
| 4 | Agrimol D | C35H42O12 | 654 | Whole plant | [43] |
| 5 | Agrimol E | C33H38O12 | 626 | Whole plant | [43] |
| 6 | Agrimol F | C34H40O12 | 640 | Whole plant | [46] |
| 7 | Agrimol G | C36H44O12 | 668 | Whole plant | [43] |
| 8 | Pilosanol N 
|
C26H26O10 | 498 | Foliage | [47] |
| 9 | Agrimophol 
|
C25H32O8 | 460 | Root | [44] |
| 10 | Pseudoaspidin | C24H30O8 | 446 | Root | [44] |
| 11 | Agripinol A 
|
C25H31O8 | 459 | Aerial parts | [45] |
| 12 | Agripinol B 
|
C26H33O8 | 473 | Aerial parts | [45] |
| 13 | Agripinol C 
|
C26H33O8 | 473 | Aerial parts | [45] |
| 14 |

|
C25H38O14 | 563 | Aerial parts | [48] |
| 15 | 3,5-Dimethyl-a-methylbutyrylphloroglucinol-2,4-O-b-D-diglucopyranoside 
|
C25H38O14 | 563 | Aerial parts | [48] |
| 16 |

|
C25H38O14 | 563 | Aerial parts | [48] |
| 17 |

|
C16H24O10 | 376 | Aerial parts | [48] |
| 18 | Agrimone A 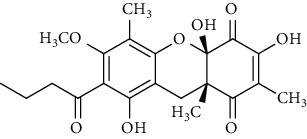
|
C21H25O8 | 405 | Whole plant | [49] |
| 19 | Agrimone B 
|
C21H25O8 | 405 | Whole plant | [49] |
| 20 | Agrimone C 
|
C26H34O8 | 475 | Whole plant | [49] |
| 21 | Agrimone D 
|
C26H34O8 | 475 | Whole plant | [49] |
| 22 | Agrimone E 
|
C27H36O8 | 489 | Whole plant | [49] |
3.5. Tannins and Organic Acids
Tannins and organic acids in A. pilosa mainly include potentillin, pedunculagin, casuarinin, isovanillic acid, and protocatechuic acid (Table 6), which exhibit antitumor, anti-inflammatory, and free radical-scavenging activities [51–53]. However, studies on the pharmacological activities of agrimony tannins have mostly focused on their components rather than specific monomers.
Table 6.
Tannins and organic acids isolated from Agrimonia pilosa Ledeb.
| No | Compounds | Molecules | Molecular weight | Plant part | References |
|---|---|---|---|---|---|
| 1 | Potentillin 
|
C41H28O25 | 920 | Root | [50] |
| 2 | Pedunculagin | C34H24O22 | 784 | Root | [50] |
| 3 | Casuarinin 
|
C41H28O26 | 936 | Root | [50] |
| 4 | Alagrimonic A 
|
C47H39O31 | 1099 | Root | [50] |
| 5 | Alagrimonic B 
|
C47H39O32 | 1115 | Root | [50] |
| 6 | Agrimoniin 
|
C82H54O52 | 1871 | Root | [50] |
| 7 | Gallic acid 
|
C7H6O5 | 170 | Aerial parts | [20] |
| 8 | Isovanillic acid | C8H8O4 | 168 | Aerial parts | [26] |
| 9 | Protocatechuic acid | C7H6O4 | 154 | Aerial parts | [26] |
| 10 | Protocatechuic aldehyde 
|
C7H6O3 | 138 | Aerial parts | [26] |
| 11 | Agritannin 
|
C27H22O18 | 657 | Aerial parts | [29] |
| 12 | Ellagic acid 
|
C14H6O8 | 302 | Aerial parts | [29] |
4. Pharmacological Activity
For about 100 hundred years, A. pilosa has been used in China for treating cancers, bleeding, diarrhea, and parasitic infections [54]. In this section, we summarize the pharmacological activities of A. pilosa (see Table 7, Table 8, Table 9, Table 10, Table 11, and Table 12 for details).
Table 7.
Antitumor activities of components from Agrimonia pilosa Ledeb.
| A. pilosa extract | Experimental model | Test dose range | Contrast | Route of administration | Pharmacological action | Mechanism of action | References |
|---|---|---|---|---|---|---|---|
| Quercetin | AGS cells | 6.25, 12.5, 25, 50, and 100 μM | SN-38 (6.25, 12.5, 25, 50, and 100 μM) | NS | Sensitize AGS to SN-38 | NS | [55] |
| Quercetin | AGS inoculate BALB/c nude mice | 20 mg/kg, three times a week + IRI; 10 mg/kg, once a week | IRI; 10 mg/kg, once a week 20 mg/kg, three times a week | i.p. | Sensitize AGS to SN-38 | NS | [55] |
| Agrimonolide | AGS cells | 5, 10, 20, and 40 μM | Negative control | NS | Apoptosis-inducing | Bcl-2/Bax↑, p-p38↑ and caspase-3 protease activation | [56] |
| Beta-carotene | AGS cells | 0, 20, 50, and 100 μmol/L | Negative control | NS | Apoptosis-inducing and DNA fragmentation | Bcl-2/Bax↑,p-53↑ | [1] |
| Agrimol B | PC-3 cells | 0, 6.25, 12.5, and 25 μmol/L | Negative control | NS | Arrest cancer cells at G0 phase | p27↑, SKP2↓,cMYC↓ | [57] |
| Agrimol B | A549 cells | 0, 6.25, 12.5, and 25 μmol/L | Negative control | NS | Arrest cancer cells at G0 phase | SKP2↓, cMYC↓, SPT16↓ and SSRP1↓, p27↑ | [57] |
| Ellagic acid | PC-3 cells | 0, 6.25, 12.5, and 25 μmol/L | Negative control | NS | Arrest cancer cells at G0 phase | c-MYC↓, SKP2↓, SPT16↓, SSRP1↓, p27↑ | [57] |
| Ellagic acid | A549 cells | 0, 6.25, 12.5, and 25 μmol/L | Negative control | NS | Arrest cancer cells at G0 phase | c-MYC↓, SKP2↓, SPT16↓, SSRP1↓, CRM1↓, p27↑ | [57] |
| Agrimol B | PC-3 inoculated male BALB/c nude mice | 10 mg/kg | Negative control | p.o. | Tumor growth reducing | NS | [57] |
| Apigenin | PC-3 cells | 20, 40 μM | Negative control | NS | Apoptosis inducing | HDACs↓, especially HDAC1↓ and HDAC3↓ | [58] |
| Apigenin | PC-3 xenografts in athymic nude mice | 20 and 50 μg/mouse/day | Negative control | p.o. | Tumor growth reducing | HDACs↓ p21/waf1↑, Bax/bcl2↑ | [58] |
| Quercetin | MCF-7 cells | 0–100 μg/ml, IC50 = 0.87 μg/mL | Negative control | NS | Cytotoxicity | May be related to the presence of 2,3-double bond in ring C, carbonyl group at C-4 and ortho-hydroxylation in ring B | [59] |
| Agrimophol | K562 cells | 0.1, 1, 10 mg/ml | Vincristine (50 IU/ml) | NS | Apoptosis inducing | NS | [60] |
| Agripinol A | HCT-116, MDA-MB-231 and PC-3 cells | (IC50 = 12.34 ± 0.93, 5.44 ± 0.35, 9.47 ± 0.70, 14.29 ± 1.24 μg/ml, respectively) | Fluorouracil | NS | Cytotoxicity | NS | [45] |
| Agripinol B | HCT-116, MDA-MB-231 and PC-3 cells | (IC50 = 12.34 ± 0.93, 5.44 ± 0.35, 9.47 ± 0.70, 14.29 ± 1.24 μg/ml, respectively) | Fluorouracil | NS | Cytotoxicity | NS | [45] |
| Agripinol C | HCT-116, MDA-MB-231 and PC-3 cells | (IC50 = 12.63 ± 1.40, 2.12 ± 0.16, 7.50 ± 0.86, 9.85 ± 1.08 μg/ml, respectively) | Fluorouracil | NS | Cytotoxicity | NS | [45] |
| Agrimoniin | MM2 inoculated C3H/H e and BALB/ c mice | 1, 3, 10, 30 mg/kg | Negative control | i.p. | Prolonged the life span of mice bearing MM2 | Direct inhibit tumor cell activity and increased the number of peripheral white blood cells and the ratio of monocytes | [61] |
| Quercetin and hyperoside in combination (1 : 1 ratio) | 786-O renal cancer cells | 3.8–60 μg/ml | Negative control | NS | Cancer cell proliferation inhibition | ZBTB10↑ Sp1, Sp3, and Sp4 mRNA↓ | [62] |
Table 8.
Free radical-scavenging and antioxidant activities of components from A. pilosa.
| A. pilosa extract | Experimental model | Test dose range | Contrast | Route of administration | Pharmacological action | Mechanism of action | References |
|---|---|---|---|---|---|---|---|
| A. pilosa flavonoids (36.45 mg/ml) | FRAP working fluid | FRAP = 56.87mg−1 | Vit C (FRAP = 45.47 mg−1) | NS | Antioxidant activities | NS | [63] |
| A. pilosa flavonoids (316.53 ± 6.37 mg/g) | 100 μL sample in methanol was mixed with 1.9 mL of 0.1 mM DPPH in ethanol | 0.25, 0.5, 2.5, 5.0, 25.0, 50.0, 100.0 μg/mL | 2,6-Di-tert-butyl-4-methylphenol | NS | DPPH scavenging activity | NS | [64] |
| A. pilosa flavonoids 316.53 ± 6.37 mg/g | 0.75 mM 1,10-phenanthroline and 0.75 mM FeSO4 were prepared in 0.05 M phosphate buffer (pH 7.4) and mixed thoroughly (method described by De Avellar and jin) | 5.0, 10.0, 50.0, 100.0, 500.0, 1000.0, 2000.0 μg/mL | Negative control | NS | Hydroxyl radical scavenging activity | NS | [64] |
| A. pilosa aqueous extract | Low immunity mice | 100, 300, 1000 mg/kg | Negative control | p.o. | Antioxidant | MDA↑、SOD↑ | [15] |
| Protocatechuic acid | The method of Brand-Williams et al. | 15 μM | Negative control | NS | DPPH free radical scavenging | Providing hydrogen atoms or electron donation | [65] |
| Protocatechuic acid | Generated by the deoxyribose method (Halliwell 1987) | 15 μM | Negative control | NS | Superoxide radical (O2-) scavenging | NS | [65] |
| A. pilosa flavonoids 316.53 ± 6.37 mg/g | Supercoiled plasmid pBR322 DNA | 0.1 mM, 1.0 mM | Negative control | NS | Against DNA oxidative damage | NS | [3] |
| Protocatechuic acid | Male albino rats of Wistar strain | 10, 20 mg/kg | Negative control | p.o. | Protects damaged rat liver cells | Enhancing antioxidant capacity and enhancing stage II enzyme activity through the Nrf-2 pathway | [66] |
Table 9.
Anti-inflammatory activity of components from A. pilosa.
| A. pilosa extract | Experimental model | Test dose range | Contrast | Route of administration | Pharmacological action | Mechanism of action | References |
|---|---|---|---|---|---|---|---|
| Agrimonolide | LPS-induced RAW 264.7 cells | 0, 20, 40, 60, 80 μg/ml | Negative control | NS | Anti-inflammatory | NO scavenging, COX-2/inos↓, NF-κB ↓, MAPKs↓, JAK-STATs↓ | [35] |
| Agrimonolide-6-O-β-D-glucopyranoside | LPS-induced RAW 264.7 cells | 50, 100, 200 μg/ml | 4-Ethyl-2-hydroxyamino-5-nitro-3-hexenamide (200 μM) | NS | NO scavenging | NS | [47] |
| Agrimonolide-6-O-β-D-glucopyranoside | LPS-induced RAW 264.7 cells | 25, 50, 100 μg/ml | Negative control | NS | NO production decreasing | NS | [35] |
| Pilosanol N | LPS/IFN-γ-induced RAW264.7 macrophages | 25, 50, 100 μg/ml | NOR3 (200 μM) | NS | NO scavenging | May contribute to the catechol group (3′, 4′-OH) of the B ring in the structure | [67] |
| Pilosanol N | LPS/IFN-γ-induced RAW 264.7 macrophages | 25, 50, 100 μg/ml | IFN-γ and l-arginine | NS | NO production decreasing | iNOS↑and may also contribute to NF-κB/NO signaling disrupting | [67] |
Table 10.
Antidiabetic activity and lipid metabolism regulation effects of components from A. pilosa.
| A. pilosa extract | Experimental model | Test dose range | Contrast | Route of administration | Pharmacological action | Mechanism of action | References |
|---|---|---|---|---|---|---|---|
| Apigenin 7-O-β-D-glucuronide | NS | IC50 = 7.14 ± 1.75 μM | Ursolic acid (IC50 = 9.43 ± 0.14 μM) | NS | Improve insulin resistance | PTP1B inhibition | [29] |
| Ellagic acid | NS | IC50 = 7.73 ± 0.24 μM | Ursolic acid (IC50 = 9.43 ± 0.14 μM) | NS | Improve insulin resistance | PTP1B inhibition | [29] |
| Quercetin | NS | IC50 = 28.7 ± 1.2 μM | Acarbose (IC50 = 45.2 ± 1.2 μM) | NS | Glycogen decomposition and glucose regulation | Competitively α-glucosidase inhibition | [30] |
| Agrimonolide | NS | IC50 = 24.2 μM | Acarbose (IC50 = 45.2 ± 1.2 μM) | NS | Glycogen decomposition and glucose regulation | Noncompetitively α-glucosidase inhibition | [30] |
| Desmethylagrimonolide | NS | IC50 = 37.4 μM | Acarbose (IC50 = 45.2 ± 1.2 μM) | NS | Improve insulin resistance of HepG2 cells | Non-competitively α-glucosidase inhibition | [30] |
| Agrimonolide | Insulin-resistance HepG2 cells | IC50 = 8.3 ± 0.6 μM | Metformin (IC50 = 18.6 ± 0.8 μM) | NS | Improve insulin resistance of HepG2 cells | Phosphoenolpyruvate carboxykinase inhibition | [68] |
| Agrimonolide | Insulin-resistance HepG2 cells | IC50 = 11.6 ± 0.8 μM | Metformin (IC50 = 12.4 ± 1.6 μM) | NS | Improve insulin resistance of HepG2 cells | Hepatic glucose-6-phosphatase inhibition | [68] |
| Total triterpenoids of Agrimonia pilosa Ledeb (415.97 ± 5.15 mg/g) | 3T3-L1 cells | 1, 5, 25, and 125 μg/ml | Pioglitazone (10 μM) | NS | Insulin sensitization effects with low lipid formation effects | PPARγ↑SREBP-1↑C/EBPα↑, thus upregulating adiponectin and GLUT4 mRNA expression | [69] |
| Total triterpenoids of Agrimonia pilosa Ledeb (the content is not clear) | High sugar concentration-induced IR-HepG2 cells | 5, 25, 50, 15, 100, 125 μg/ml | Rosig (30 μM) | NS | Regulation of lipid metabolism | Improving oxidative stress and regulating the JNK and IRS pathways, thus improved glucose metabolism in IR-HepG2 cells | [41] |
| Agrimol B | 3T3-L1 cells | 3, 10 μg/ml IC50 = 3.35 ± 0.32 μM | Resveratrol (50 μM) and berberine (10 μM) | NS | Regulation of lipid metabolism | PPAR↓、C/EBPα↓、FAS↓、UCP-1 and apoE↓, thus inhibited 3T3-L1 adipocyte differentiation | [70] |
Table 11.
Anthelmintic activity of components from A. pilosa.
| A. pilosa extract | Experimental model | Test dose range | Contrast | Route of administration | Pharmacological action | Mechanism of action | References |
|---|---|---|---|---|---|---|---|
| Agrimophol | Cysticercus cellulosae with intact cyst in vitro | 2 × 10−4, 5 × 10−4, 2 × 10−5, 2 × 10−6, 2 × 10−7 μg/ml | Negative control | NS | Antitapeworm | Aerobic and anaerobic metabolism inhibition through direct contact with insects | [71] |
|
| |||||||
| Agrimol G | Adult H. contortus parasites | 150, 300, 600, and 1200 μg/ml | Albendazole (380 μg/ml) Ivermectin (500 μg/ml) | Coincubation | Antitapeworm | Microtubule aggregation inhibition | [72] |
Table 12.
Hepatocyte protection activity of components from A. pilosa.
| A. pilosa extract | Experimental model | Test dose range | Contrast | Route of administration | Pharmacological action | Mechanism of action | References |
|---|---|---|---|---|---|---|---|
| Agrimonolide | Tacrine-induced human liver-derived hep G2 cells | 1–100 μM EC50 = 88.2 ± 2.8 μM | Silybin | NS | Hepatocyte protection effects | NS | [5] |
|
| |||||||
| Agrimonolide | Tert-butyl hydroperoxide-induced rat primary hepatocytes | 1–100 μM EC50 = 37.7 ± 1.6 μM | Silybin | NS | Hepatocyte protection effects | NS | [5] |
|
| |||||||
| Agrimonolide | H2O2 induced HepG2 cells | 50, 100, 200 μM | Negative control | NS | Oxidative stress reducing and hepatocyte protection effects | Inducing heme oxygenase-1 and Nrf2 expression and inhibiting Kelch-like ECH-associated protein 1 expression | [73] |
|
| |||||||
| Desmethylagrimonolide | H2O2 induced HepG2 cells | 50, 100, 200 μM | Negative control | NS | Oxidative stress reducing and hepatocyte protection effects | Inducing heme oxygenase-1 and Nrf2 expression and inhibiting Kelch-like ECH-associated protein 1 expression | [73] |
4.1. Antitumor Activity
Total flavonoids derived from A. pilosa exhibit antitumor effects in a concentration-dependent manner against several tumor cell lines, including MKN-45 human gastric cancer cells, HepG2 human hepatoma cells, U266 human multiple myeloma cells, MCF-7 human breast cancer cells, A549 human non-small cell lung cancer cells, and HeLa cells, with an IC50 of 127.50, 53.31, 202.10, 206.80, 54.17, and 170.40 μg/mL, respectively [63, 74]. Catechin, luteolin, quercetin, quercitrin, hyperoside, rutin, and luteolin 7-O-β-glucoside exhibit significant 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical-scavenging activity, with an IC50 of 5.06, 7.29, 4.36, 7.12, 6.34, 6.36, and 8.12 μM, respectively [3].
Gastric cancer is one of the most common malignant tumors. Studies have shown that quercetin, a flavonoid isolated from A. pilosa, can sensitize human gastric adenocarcinoma cell lines (AGS) to SN-38, a DNA topoisomerase I inhibitor; the cell viability and apoptosis rates induced by the combination of quercetin and low-dose SN-38 are similar to those obtained with a high-dose SN-38 alone. In vivo, the combined activity of quercetin and SN-38 induces the downregulation of the concentrations of the vascular endothelial growth factor (VEGF)-A and the VEGF-receptor 2; it also decreases the percentage of Tie2-expressing monocytes in AGS xenograft mice compared to control mice [55]. In addition, agrimonolide exhibits a dose-dependent apoptosis-inducing effect in AGS cells with an IC50 of 25.9 μmol/L; its underlying mechanism involves the B-cell lymphoma-2 (Bcl-2)/Bcl-2-associated X (Bax) and mitogen-activated protein kinase (MAPK) pathways and occurs through the regulation of Box Bcl-2/Bax and protein kinase 1/2 expression, p38 phosphorylation, and caspase-3 protease activation, thereby promoting apoptosis in AGS cells [56]. At 100 μmol/L, beta-carotene induces apoptosis and DNA fragmentation in AGS cells by promoting p53 and Bcl-2 expression [1, 2].
Agrimol B, a phloroglucinol derivative isolated from A. pilosa, causes the arrest of prostate and A549 lung cancer cells at the G0 phase by decreasing cellular myelocytomatosis viral oncogene (c-MYC) and SKP2 expression, promoting p27 expression, and downregulating SPT16 and SSRP1 expression. Oral administration of agrimol B (10 mg/kg) inhibits tumor growth in mice injected with human prostate cancer cells, but it does not significantly modify their body weight [57]. Ellagic acid also arrests A549 cells at the G0 phase [57]. Prostate cancer is the second-most frequent cancer and the fifth leading cause of cancer-related deaths in men [75]. Agrimol B efficiently inhibits the proliferation of prostate cancer cells. It also decreases c-MYC and SKP2 expression and increases p27 expression in PC-3 cells, thereby inhibiting mitosis in these cells [57]. Moreover, ellagic acid arrests PC-3 cells at the G0 phase [57]. Apigenin induces apoptosis in PC-3 cells and significantly reduces tumor size by inhibiting class I histone deacetylases (HDACs), especially HDAC1 and HDAC3 [58, 76].
Among flavonoids, quercetin shows the strongest cytotoxicity to MCF-7 breast cancer cells [59]. Agrimophol elicits a concentration-dependent inhibitory effect against K562 human chronic myelogenous leukemia cells; particularly, 10 mg/mL agrimophol induces cytotoxicity similar to 50 IU/mL vincristine (P > 0.05) [60]. Agripinol A–C have more significant cytotoxic effects on HCT-116, MDA-MB-231, and PC-3 cells than 5-fluorouracil [45].
Total A. pilosa tannins exhibit antitumor activities against HeLa, MCF7, and SPC-A-1 human lung adenocarcinoma cells [77]. In vitro, tannin extracts induce apoptosis in Bel-7402 and HepG2 cells by regulating free Ca2+ level overloading and increasing reactive oxygen species levels [78]. In vivo, the total tannin in A. pilosa extract inhibits the growth of S180 sarcoma [78]. The methanolic extract of A. pilosa can inhibit the invasion and metastasis of HT1080 cells by inhibiting the expression activity of MMP-2 and MMP-9 through ERK, JNK, and AKT-1 inactivation [79]. Methanolic A. pilosa extract shows obvious cytotoxicity at 10 μM, and by 34% at 20 μM in IMR90 cells, higher than the cytotoxicity to HT1080 cells at the same concentration. Therefore, the pure alcohol extract is limited by poor selectivity and strong cytotoxicity. Active components should be further elaborated and subjected to in vivo experiments. Notably, 10 μg/ml alcohol extract of A. pilosa shows obvious cytotoxicity (P < 0.001), and 20 μg/mL alcohol extract of A. pilosa shows a cytotoxicity of 34% against IMR90 cells, higher than the cytotoxicity against HT1080 cells at the same concentration. Similar to pure alcohol extract, alcohol extracts at different concentrations are limited by poor selectivity and strong cytotoxicity; therefore, the active components of A. pilosa extracts should be further elucidated. Ellagic acid inhibits proliferation and metastasis and induces apoptosis in several tumor cells, thereby eliciting broad-spectrum antitumor effects [80]. Agrimoniin, a tannin found in A. pilosa, exhibits antitumor activity against ascite- and solid-type rodent tumors in mice, and the underlying mechanism may involve the enhancement of the host's immune response [61]. The combination of quercetin and hyperoside at a 1 : 1 ratio inhibits 786‐O renal cancer cell proliferation by upregulating the expression of zinc finger and BTB domain containing 10 (ZBTB10) and downregulating the mRNA expression of Sp1, Sp3, and Sp4 [62].
In summary, A. pilosa extracts elicit inhibitory effects against several tumor cell types, and this finding provides a theoretical basis for developing A. pilosa-based antitumor therapies. The combination of A. pilosa constituents and classical chemotherapeutic agents may be potential treatment strategies against tumors.
4.2. Free Radical Scavenging and Antioxidant Activities
Metabolic processes lead to the production of large amounts of active oxygen free radicals. Free radicals in humans have been linked to the deterioration of chronic diseases, such as diabetes, tumors, and Alzheimer's disease (AD).
The methanolic extract of A. pilosa leaves protect nonlipid oxidative damage from various model systems, including liposome oxidation, deoxyribose oxidation, protein oxidation, metal ion oxidation, and hydrogen peroxide oxidation models [81]. Tannin extracts of A. pilosa roots exhibit dose-dependent DPPH free radical-scavenging and liposome peroxidation inhibitory effects in vitro [15]. Total A. pilosa flavonoids elicit dose-dependent antioxidant effects, with a ferric reducing antioxidant potential assay value of 56.87 mmol/L FeSO4 [63]. In addition, A. pilosa flavonoids exhibit significant scavenging effects against DPPH, 2ʹ-azinobis-(3-ethylbenzthiazoline-6-sulphonate), and hydroxyl radicals [47, 64]. The free radical-scavenging activities of these flavonoid extracts may be attributed to the presence of quercetin and hypericin, and one of the possible mechanisms underlying this effect is the activation of the Sonic hedgehog signaling pathway [82]. Tannins from A. pilosa also scavenge DPPH free radicals in a dose-dependent manner and inhibit liposome peroxidation activity. Protocatechuic acid exhibits significant free radical-scavenging activity, especially against DPPH• and O2 [65]. A DNA nicking assay has revealed that taxifolin, catechin, hyperoside, quercitrin, and rutin protect against oxidative DNA damage. Based on the structure-function relationship provided by quantum chemistry theory, glycosylation at C-6 enhances the antioxidant activity of flavonoids by rendering a uniform distribution of spin density and improving free radical stability. These findings may serve as a theoretical basis for designing and developing antioxidant preparations [3]. Antioxidant activity is one of the main activities of A. pilosa tannins and organic acids. In D-galactose-induced subacute aging mice, A. pilosa tannin extract elicits an antioxidant activity by increasing superoxide dismutase activity and decreasing malondialdehyde (MDA) activity in blood [15]. This antioxidant activity may be due to protocatechuic acid, protocatechuic aldehyde, and gallic acid as the main monomers. Protocatechuic acid protects damaged rat liver cells through menadione by enhancing its antioxidant capacity and stage II enzyme activity through the Nrf-2 pathway [66]. In H9c2 cell exposed to hypoxia-induced oxidative stress, agrimonolide maintains mitochondrial homeostasis, thereby reducing oxidative stress damage to mitochondria. Moreover, agrimonolide promotes cell proliferation by regulating the cell cycle and inhibits H9c2 apoptosis by reducing caspase 3 and Bax and promoting Bcl 2. Autodock software predicts that Tom20 protein may be a potential target of agrimonolide, but the precise mechanism of agrimonolide and Tom20 interaction needs further research [83].
4.3. Anti-Inflammatory Activity
A. pilosa isocoumarins play a beneficial anti-inflammatory role by scavenging intracellular nitric oxide (NO), inhibiting cyclooxygenase-2 (COX-2) and inducible NO synthase (iNOS) transcription and translation, and reducing the expression of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin (IL) 6. These activities are correlated with the presence of agrimonolide and agrimonolide-6-O-β-D-glucopyranoside, especially with that of agrimonolide [33, 35, 47, 55]. In lipopolysaccharide (LPS) induced RAW 264.7 macrophages, agrimonolide inhibits NO release in a dose-dependent manner by reducing IL-1, IL-6, and TNF-α levels and inhibiting iNOS activity [47]. The mechanisms underlying the anti-inflammatory activity of agrimonolide involve three signaling pathways. First, it inactivates nuclear factor-kappa B by inhibiting p65 transcription and phosphorylation and preventing LPS-induced IκBα degradation; second, pretreatment with agrimonolide prevents LPS-induced P38 MAPK, C-Jun n-terminal kinase (JNK), and extracellular regulated protein kinase phosphorylation; and third, it reduces the LPS-induced production of phosphorylation proteins, such as Janus kinase 1, signal transducer and activator of transcription (STAT)-1, and STAT-3, thereby blocking inflammatory signaling cascades (Figure 1) [35, 84, 85].
Figure 1.

The ethanol extract of agrimony can inhibit xylene-induced ear edema in mice and carrageenan-induced paw edema in rats, and tiliroside has been proved to be the main active ingredient. Moreover, A. pilosa tiliroside significantly inhibits the overproduction of NO, downregulates the LPS-induced overexpression of iNOS and COX-2, and inhibits the phosphorylation of JNK and p38 proteins in LPS-activated RAW 264.7 macrophages; these results suggest that the anti-inflammatory mechanism includes the downregulation of iNOS and COX-2 protein and the inactivation of mitogen-activated protein kinase (MAPK)/JNK, in addition to the MAPK/p38 signaling pathway [86].
Phloroglucinol derivatives also exhibit anti-inflammatory activities [67]. In RAW 264.7 macrophages, pilosanol N inhibits NO production by inhibiting the expression of iNOS and the expression of COX-2; it also induces the elimination of NO and nitrogen free radicals generated by the NO donor 4-ethyl-2-hydroxyamino-5-nitro-3-hexenamide.
The mixed extract from A. pilosa and Salvia miltiorrhiza Bunge alleviates gouty arthritis [87]. In terms of analgesic effects, one off administration and one-week treatment reduce the pain threshold in a dose-dependent manner (from 10 mg/kg to 100 mg/kg) in a mono-iodoacetate (MIA) induced osteoarthritis (OA) model. In terms of anti-inflammatory activity, the mixed extract reduces plasma TNF-α, IL-6, and CRP levels in MIA-induced osteoarthritis and ameliorates the progress of 2.5% croton oil-induced ear edema in mice. In LPS-stimulated RAW 264.7 cells, the mixed extract inhibits the release of NO, PGE2, LTB4, and IL-6 and increases PPARγ phosphorylation of proteins in a concentration-dependent manner (from 1 μg/mL to 100 μg/mL). In most experiments, the effects induced by the mixed extract are almost equal to or higher than those induced by Perna canaliculus powder. However, the limitation of this study is that the major active ingredient in the mixed extract is unknown, and the potential mechanism of its analgesic effect should be further analyzed.
4.4. Antidiabetic Activity
T2DM is characterized by insulin and leptin resistance. Insulin levels are regulated by protein tyrosine phosphatase (PTP) 1B, a key member of the PTP family, which decreases insulin sensitivity [88]. PTP1B is considered an important target for the treatment of T2DM and obesity. Nguyen et al. [29] isolated agriflavone and kaempferol-3-O-((S)-3-hydroxy-3-methylglutaryl(1 ⟶ 6))-β-d-glucoside, which are two new flavonoid glycosides, and 16 known compounds—kaempferol 7-O-β-D-glucoside, kaempferol 7-O-β-D-glucuronide, kaempferol 3-O-β-D-glucoside, apigenin, apigenin 7-O-β-D-glucoside, apigenin 7-O-β-D-glucuronide, quercetin, quercetin 3-O-β-D-glucoside, quercetin 3ʹ-O-β-D-glucoside, luteolin 7-O-β-D-glucoside, luteolin 7-O-β-D-glucuronide, luteolin 7-O-β-D-glucuronide methyl ester, luteolin 7-O-β-D glucuronide butyl ester, luteolin 3ʹ-O-β-D-glucoside, ellagic acid, and dihydrodehydro-diconiferyl alcohol 9ʹ-O-3-D-glucoside—from the aerial parts of A. pilosa and evaluated their inhibitory effects on PTP1B. They found that apigenin 7-O-β-D-glucuronide and ellagic acid inhibit the PTP1B activity with IC50 of 7.14 ± 1.75 and 7.73 ± 0.24 μM, respectively [29]. However, PTP1B inhibitors are limited by their high anionic charge that prevents their binding affinities; the bioavailability of apigenin 7-O-β-D-glucuronide, which has a carboxylic acid group, can be improved by using its methyl or ethyl derivative; therefore, it is a potential natural T2DM inhibitor.
Postprandial hyperglycemia is closely related to T2DM progression [64, 89, 90]. In a clinical study, A. pilosa powder reduces the incidence T2DM-related complications by reducing high postprandial hyperglycemia, showing its potential for use in T2DM treatment [36]. Flavonoids and isocoumarins present in A. pilosa may be responsible for such postprandial hyperglycemia-reducing effects.
α-Glycosidase is involved in glycogen decomposition and glucose regulation. Total A. pilosa flavonoids inhibit α-glucosidase activity, and the active components are luteolin, quercetin, vitexin, and isovitexin; among them, quercetin has the highest activity and noncompetitively inhibits α-glucosidase [30, 64]. In addition, four isocoumarins, agrimonoide, agrimonolide-6-O-β-D- glucopyranoside, desmethylagrimonolide, and desmethylagrimonolide-6-O-β-D-glucopyranoside, were found to be α-glucosidase inhibitors. Moreover, the endogenous glucose-inhibitory activity of agrimonolide is related to the inhibition of phosphoenolpyruvate carboxykinase, which is the rate-limiting enzyme in the gluconeogenesis pathway (IC50, 8.3 μmol/L) [68]. In insulin-resistant cells, agrimonolide improves insulin sensitivity and promotes insulin-mediated glycogen synthesis. Agrimonolide significantly improves glucose uptake in IR cells and exhibits the highest hypoglycemic activity; glucose consumption in HepG2 cells is 62.3% lower than that of 3 mM at a concentration of 20 μM agrimonolide, which is not significantly different from that obtained with metformin (70.5%) [37].
4.5. Lipid Metabolism Regulation Activity
Obesity is significantly associated with the pathogenesis of IR, metabolic syndrome, and T2DM. Lipid metabolism and adipose tissue inflammation are partly responsible for the development of obesity-induced IR [91]. Thiazolidinediones, which are A. pilosa triterpenoids, have better insulin sensitization effects and lower lipid formation effects on 3T3-L1 cells than classical hypoglycemic drugs; they elicit these effects by regulating adiponectin and GLUT4 mRNA expression through the upregulation of upstream genes, such as peroxisome proliferator-activated receptor γ (PPARγ), SREBP-1, and C/EBPα. These findings indicate that A. pilosa triterpenoids may be potential natural drugs for the treatment of IR and T2DM [69] and improve fat metabolism.
In high-sugar-concentration-induced IR-HepG2 cells, A. pilosa triterpenes improve glucose metabolism [41], decrease reactive oxygen species levels, promote superoxide dismutase release, reduce malondialdehyde content, and activate the nuclear factor-E2-related factor 2 (Nrf2) antioxidative response element signaling pathway, thereby ameliorating oxidative stress in these cells. Moreover, they reduce JNK expression and phosphorylation and promote insulin receptor substrate-1 (IRS-1) Ser 307 expression in these cells. Therefore, A. pilosa triterpenes ameliorate hyperglycemia in IR cells by improving oxidative stress and regulating the JNK and IRS pathways [41].
Silent mating-type information regulation 2 homolog 1 (SIRT1) is a key regulator of obesity-related metabolic pathways, and its deletion leads to obesity, metabolic dysregulation, and IR [92, 93]. Agrimol B inhibits adipogenesis in 3T3-L1 adipocytes at the early differentiation stage, with an IC50 of 3.35 μM, and this effect is partly due to the stimulation of SIRT1 expression and the induction of the cytoplasm-to-nucleus SIRT1 shuttle. Agrimol B also inhibits 3T3-L1 adipocyte differentiation by inhibiting PPARγ, C/EBPα, FAS, UCP-1, and apoE expression [70, 91].
A. pilosa aqueous extract (5.000 g/kg diet) can improve the glucose and lipid metabolism of ovariectomized rats under a high-fat diet and the degree of hepatic steatosis in rats. The aqueous extract of A. pilosa has no effect on the increase in body weight and food intake of rats, but it improves the postprandial high-glucose state of ovariectomized rats, reduces the level of serum total cholesterol and low-density lipoprotein, and improves the weight of liver and the degree of fatty liver. A. pilosa extract also improves insulin resistance and glucose metabolism by increasing adiponectin levels without affecting the expression of adiponectin receptor. Moreover, it increases serum insulin levels, but the molecular mechanism of promoting insulin secretion is unclear. It also inhibits the synthesis of fatty acids and cholesterol, inhibits the increase of liver quality induced by high-fat diet, and improves the degree of fatty liver by downregulating fat formation-related genes, such as fatty acid synthase, acetyl-coenzyme A carboxylase alpha, and 3-hydroxy-3-methylglutaryl-coenzyme; furthermore, A. pilosa extract inhibits the synthesis of fatty acids and cholesterol. In addition, the improvement of fatty liver is related to the increase in adiponectin levels and the improvement of insulin resistance [94]. However, a limitation of the present study is that estrogen-like activities of A. pilosa in postmenopausal metabolic syndrome models are not analyzed. In addition, the specific concentration and effective components of A. pilosa aqueous extract are not described. Therefore, studies should aim to isolate and characterize the functionality of each compound derived from A. pilosa.
4.6. Anthelmintic Activity
A. pilosa shows an anthelmintic activity. Agrimophol inhibits glycogen decomposition in tapeworm by directly coming in contact with the tapeworm's body, thereby inhibiting tapeworm aerobic and anaerobic metabolism [71]. Agrimol G destroys the parasite cuticle when it is incubated with adult Haemonchus parasites for 3 h. Microtubule degeneration and the presence of electron-dense and electron-lucent bodies around microtubules are not observed in A. pilosa and albendazole or ivermectin cotreatment; therefore, agrimol G elicits a killing effect on Haemonchus parasites by inhibiting microtubule aggregation [72]. Agrimonia essential oil (at concentrations of 10, 50, and 100 μg/mL) shows a dose-dependent inhibitory activity on Leishmania promastigote and intracellular amastigote forms in vitro. Agrimonia essential oil at different concentrations have no toxic effects on host cells. The active chemical components of essential oil should be analyzed and purified [95].
Most antiparasitic drugs, such as chloroquine and albendazole, cause evident side effects, including fetal malformation. A. pilosa extracts, as natural products, have a strong antiparasitic effect with relatively low toxicity. Therefore, the effects of A. pilosa extracts on embryonic development and pharmacokinetics should be studied to provide a basis for developing safe antiparasitic drugs for pregnant women. In addition, the in vivo immune stress mechanism of A. pilosa to prevent and treat parasitic infection should be evaluated.
4.7. Others
4.7.1. Anti-Alzheimer's Disease Activity
The mechanism underlying AD development is closely associated with amyloid-β aggregation and neurotic plaque formation, which causes neurotoxicity and accumulation of neurotic plaques in the brain. In β-amyloid-infused rats, the administration of 2% A. pilosa lyophilized aqueous extracts in a high-fat diet (43% energy as fat) induces a reduction in neuro-inflammation, prevents hippocampal amyloid-β accumulation, and enhances hippocampal insulin signaling, thereby effectively preventing cognitive dysfunction and improving hippocampal IR [96]. The loss of brain cholinergic function causes memory impairment in patients with AD, and AchE is involved in the termination of the cholinergic signal by playing an important role in acetylcholine hydrolysis. Sixteen flavonoids extracted from the aerial parts of A. pilosa exhibit moderate inhibitory effects against AchE in vitro, suggesting that flavonoids from A. pilosa may be natural agents for AD treatment [29]. Among the 10 flavonoid glycosides (1–10) isolated from the part of crane grassland [31], compounds 1 and 4 have no activity, and the other compounds show a moderate acetylcholinesterase inhibitory activity. IC50 ranges from 76.59 ± 1.16 μM to 97.53 ± 1.64 μM, which supports the above conclusion.
4.7.2. Hepatocyte Protection Activity
The aqueous extract of A. pilosa improves the development of fatty liver in a high-fat diet model [90, 94]. A high-fat diet increases the expression of inflammatory cytokines in the adipose tissue and liver of rats, whereas the aqueous extract of A. pilosa (0.1%) supplement inhibits the increase of liver weight and improves the degree of the fatty liver of rats. A. pilosa aqueous extract also improves the impaired glucose tolerance of rats caused by high-fat diet and reduces the blood glucose level of rats, suggesting that A. pilosa aqueous extract can improve insulin resistance. The specific mechanism is related to the inhibition of liver and adipose tissue inflammation and the improvement of insulin resistance by reducing the expression of the rat liver inflammation-related genes G6PD and IL1B and the levels of the serum inflammatory cytokines IL-6 and TNF-α [90].
Isocoumarinic compounds may be responsible for the hepatocyte protection activity of A. pilosa as they improve oxidative stress. Agrimonolide, the main active isocoumarin in A. pilosa, protects rat primary hepatocytes by inhibiting oxidative stress induced by tacrine and tert-butyl hydrogen peroxide [5]. Agrimonolide and demethylated agrimonolide reduces oxidative stress in HepG2 cells by inducing heme oxygenase-1 and Nrf2 expression and inhibiting Kelch-like ECH-associated protein 1 expression [73]. Recently, five new dimeric phloroglucinol derivatives, namely, agrimones A−E, have been isolated from the whole plant of A. pilosa. Among them, 10 μM agrimones A, D, and E show a moderate liver protective activity in p-n-acetyl-p-aminophenol-induced HepG2 cell and increase the cell viability from 62.09% to 70.66%, 67.21%, and 69.21%, respectively [48].
A. pilosa ethanol extract exerts the protective effect on LPS-induced cell damage in human HepG2 hepatocytes through antioxidant and anti-inflammatory activities. The mechanism involves A. pilosa extract (100 and 200 μg/mL) that dose dependently reduces the production of intracellular reactive oxygen species stimulated by LPS to the basal level, reverses the expression of glutathione peroxidase gene and protein inhibited by LPS, and has no cytotoxic effect at the experimental dose. However, only in vitro experiments have been performed; although the content and proportion of various components in the extract are determined, the components mainly related to the above hepatocyte protective activity remain unknown [42].
4.7.3. Antimicrobial Activity
Several phloroglucinol derivatives present in A. pilosa have antibacterial activities. For example, agrimol C, agrimol F, agrimol G, and agrimophol completely inhibit the growth of methicillin-resistant Staphylococcus aureus, Bacillus cereus, and Gardnerella species [46, 97]. However, experiments on the antibacterial activity of phenolic components of A. pilosa were performed in 1988, and the specific mechanism has not been clarified. Considering the possibility of bacterial variation and drug resistance, the antibacterial activity of phenolic compounds in A. pilosa should be further investigated (see Table 13).
Table 13.
Antimicrobial activity of components from A. pilosa.
| A. pilosa extract | Experimental model | Test dose range | Contrast | Route of administration | Pharmacological action | Mechanism of action | References |
|---|---|---|---|---|---|---|---|
| Agrimol C | MIC against Staphylococcus aureus 2o9P, Bacillus cereus var. mycoides, and Nocardia gardneri. Method described by takagi et al. | MIC = 50, 25, 100 μM, respectively | Negative control | NS | Antimicrobial activity | NS | [46] |
|
| |||||||
| Agrimol F | MIC against Staphylococcus aureus 2o9P, Bacillus cereus var. mycoides, and Nocardia gardneri. Method described by Takagi et al. | MIC = 25, 25, 100 μM, respectively | Negative control | NS | Antimicrobial activity | NS | [46] |
|
| |||||||
| Agrimol G | MIC against Staphylococcus aureus 2o9P, Bacillus cereus var. mycoides, and Nocardia gardneri. Method described by Takagi et al. | MIC = 12.5, 50, 100 μM, respectively | Negative control | NS | Antimicrobial activity | NS | [46] |
|
| |||||||
| Agrimophol | MIC against Staphylococcus aureus 2o9P, Bacillus cereus var. mycoides, and Nocardia gardneri. Method described by Takagi et al. | MIC = 3.13,6.25,100 μM, respectively | Negative control | NS | Antimicrobial activity | NS | [46] |
4.7.4. Antiviral Activity
The mixture of A. pilosa and gallnut extract (APRG64) at a 6 : 4 ratio significantly inhibits the expression of HCV core 1b and NS5A proteins at a concentration of 5 μg/mL in vitro. Further experiments have shown that 14 compounds isolated from the mixture inhibit the expression of these two proteins; the experimental concentration of 5 μg/mL has no obvious cytotoxicity, but the inhibitory activity of luteolin is the most significant (P < 0.01) [98]. However, only in vitro experiments have been conducted, and experimental results have shown that the antiviral activity of a single A. pilosa extract is significantly weaker than that of gallnut and PRG64. Considering that all 14 compounds isolated from APRG64 can also be obtained from Agrimonia, this situation may be related to the relatively low content of anti-HCV active components in Agrimonia extract. The same group of researchers also studied the inhibitory effects of APRG64 on SARS-CoV-2 [99]. They found that ARGP64 strongly inhibits SARS-CoV-2 by interfering virus entry and replication. Further studies have revealed that the active components in the mixture are ursolic acid, quercetin, and 1,2,3,4,6-penta-o-gallol-β-D-glucose). These compounds (purity > 97%) show strong antiviral activities (reduction rate of 21.05% at 25 μg/mL) against SARS-CoV-2; in particular, 1,2,3,4,6-penta-o-gallol-β-D-glucose entirely suppresses the formation of plaques at 1 μg/mL and exhibits a potent antiviral activity at lower concentrations (0.125, 0.25, and 0.5 μg/mL). Molecular docking analysis has shown that these components bind potently to the spike receptor-binding-domain (RBD) of SARS-CoV-2 and its variant B.1.1.7. These findings indicate A. pilosa and APRG64 as potent drug candidates for treating SARS-CoV-2 and its variants.
4.8. Estrogen-Like Effect
The aqueous extract of A. pilosa shows an estrogen-like activity in vitro. Its specific performance is described as follows. In a competitive binding experiment, apigenin hexose, luteolin glucosidic acid, and apigenin glucosidic acid in the aqueous extract of A. pilosa can bind to estrogen receptors (ERs) and display E2-bound ERα and ERβ. In an E-SCREEN assay using MCF-7 cells, A. pilosa significantly stimulates MCF-7 cell proliferation at concentrations of 1 and 10 μG/mL (P < 0.001) and does not show an antagonistic activity against E2 in MCF-7 cells when they are co-treated with E2. A. pilosa-stimulated proliferation is blocked by the addition of the estrogen antagonist ICI 182780. A. pilosa increases the mRNA expression of the estrogen response genes PS 2 and PR (P < 0.05) [100]. However, no animal experiments have been performed, and a single cell model was used. Another study [94] has presented a supporting conclusion via experiments on ovariectomized rats cultured on a high-fat diet although more direct and persuasive experiments, such as effects of A. pilosa on estrogen receptors and the uterus in postmenopausal syndrome models, have not been performed.
4.9. Analgesic Activity
The analgesic properties of A. pilosa extract have been examined in ICR mice. In tail flick (P < 0.05), hot plate tests and esthetic acid-induced writing test, 200 mg/kg A. pilosa extract elicits different degrees of pain relief. As for the test on nociceptive behavior induced by substance P (0.7 μg/5 μL), 200 mg/kg A. pilosa extract administered orally for 30 min prior to the substance P intrathecal injection significantly reduces the cumulative nociceptive response time of mice. A. pilosa also elicits an analgesic effect on yohimbine (α2-adrenergic receptor antagonist) that decreases during intraperitoneal pretreatment. It is not affected by naloxone (opioid receptor antagonist) or mexiletine (5-HT serotonergic receptor antagonist), suggesting that this analgesic effect may be mediated by α2-adrenergic receptor but not by an opioid receptor or serotonergic receptor [101]. However, the specific extraction method of A. pilosa extract has not been described, and the concentration has not been specified. Therefore, only qualitative experiments can be performed, and the analgesic effect intensity of Agrimonia extract cannot be determined.
The tannin component (0.375 g/kg) in the water extract of A. pilosa regulates rhythm in a desynchronosis model. This chronic effect is determined by the lithium dose, corresponding lithium concentration in the brain, and nature of lithium carriers; a dose-dependent effect is clearly observed, but lithium-depleted acute extract with a high dose (10 times) is not absorbed in the rat intestine and does not show rhythm mediation, suggesting that lithium ions in A. pilosa tannin are more easily absorbed by the intestine and can pass through the blood-brain barrier to regulate rhythm. However, the pharmacokinetic mechanism of lithium ion in A. pilosa tannin remains to be analyzed. Further research may provide ideas for the development of wide treatment windows and highly selective psychotropic drugs [102].
5. Conclusions and Prospects
In China, A. pilosa has been applied to treat diseases for hundreds of years. Although systematic toxicology research has not been performed, no obvious toxic reactions caused by A. pilosa have been reported in the cases of clinical application of A. pilosa formula. In all reported animal experiments, oral administration or injection of agrimony extract does not cause weight loss in experimental animals compared with that in control animals.
Numerous in vitro or animal experiments on the pharmacological activities of A. pilosa have been conducted, but the clinical application of A. pilosa preparation is mostly described in simple case reports; and systematic case-control studies, clinical control experiments, or cohort studies have not been conducted. Among pharmacological activities that have been reported, the strong sensitizing effect of quercetin on irinotecan should be investigated. The combination of quercetin and irinotecan may become one of the effective means to reduce the serious adverse reactions caused by large irinotecan doses. A. pilosa extract has great potential for regulating lipid metabolism and treating T2DM. As an edible medicinal plant, A. pilosa can be conveniently applied with oral hypoglycemic agents to treat patients with obesity and T2DM. The high safety of A. pilosa has also ensured its application in the treatment of T2DM without any additional adverse reactions. The effects of A. pilosa on fetal teratogenesis and development should also be analyzed to provide a basis for developing antiparasitic drugs for pregnant women. Considering the emergence of multidrug-resistant bacteria due to the widespread use of antibiotics, experiments on antibacterial active components from A. pilosa against common drug-resistant bacteria are also needed.
Although A. pilosa has been extensively studied, further research should be conducted to clarify the accurate correlation between phytochemical and pharmacological profiles and evaluate the pharmacokinetic and pharmacodynamic interactions of active components. This review summarized the available information on A. pilosa and provided evidence of activity; therefore, it may contribute to the development of new medicinal formulations.
Acknowledgments
Thanks are due to Peng Fang for kindly assisting in the preparation of figures. This work was supported by the Beijing Municipal Natural Science Foundation (grant no. 7172030).
Abbreviations
- TCM:
Traditional Chinese medicine
- HDAC:
Histone deacetylase
- Bcl-2:
B-cell lymphoma-2
- T2DM:
Type 2 diabetes mellitus
- PTP:
Protein tyrosine phosphatase
- IL:
Interleukin
- TNF-α:
Tumor necrosis factor-α
- AchE:
Acetylcholinesterase
- Nrf2:
Nuclear factor-E2-related factor 2
- MAPK:
Mitogen-activated protein kinase
- NO:
Nitric oxide
- iNOS:
Inducible nitric oxide synthase
- COX-2:
Cyclooxygenase-2
- LPS:
Lipopolysaccharide
- JNK:
C-Jun n-terminal kinase
- STAT:
Signal transducer and activator of transcription
- IR:
Insulin resistance
- Bax:
Bcl-2-associated X
- IRS:
Insulin receptor substrate
- PPARγ:
Peroxisome proliferator-activated receptor γ
- SIRT1:
Silent mating-type information regulation 2 homolog 1
- c-MYC:
Cellular-myelocytomatosis viral oncogene
- DPPH:
2,2-Diphenyl-1-picrylhydrazyl
- MDA:
Malondialdehyde
- ERs:
Estrogen receptors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Jang S. H., Lim J. W., Kim H. Mechanism of β-carotene-induced apoptosis of gastric cancer cells: involvement of ataxia-telangiectasia-mutated. Annals of the New York Academy of Sciences . 2009;1171(1):156–162. doi: 10.1111/j.1749-6632.2009.04711.x. [DOI] [PubMed] [Google Scholar]
- 2.Rauf A., Imran M., Khan I. A., et al. Anticancer potential of quercetin: a comprehensive review. Phytotherapy Research . 2018;32(11):2109–2130. doi: 10.1002/ptr.6155. [DOI] [PubMed] [Google Scholar]
- 3.Zhu L., Chen J., Tan J., Liu X., Wang B. Flavonoids from agrimonia pilosa ledeb: free radical scavenging and DNA oxidative damage protection activities and analysis of bioactivity-structure relationship based on molecular and electronic structures. Molecules . 2017;22(3):p. 195. doi: 10.3390/molecules22030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen S., Zhong S., Lu H., Huang W., Xiao G. A meta-analysis of lymphatic vessel invasion correlated with pathologic factors in invasive breast cancer. Journal of Cancer Therapy . 2015;6(4):p. 315. doi: 10.4236/jct.2015.64034. [DOI] [Google Scholar]
- 5.Park E.-J., Oh H., Kang T.-H., Sohn D.-H., Kim Y.-C. An isocoumarin with hepatoprotective activity in Hep G2 and primary hepatocytes from Agrimonia pilosa. Archives of Pharmacal Research . 2004;27(9):944–946. doi: 10.1007/bf02975848. [DOI] [PubMed] [Google Scholar]
- 6.Yang X. B. Modern pharmacological research progress and clinical application of Agrimony spp. Shizhen Guoyi Guoyao . 2003;12:p. 780. in Chinese. [Google Scholar]
- 7.Zhang D., Wu G. D., Zhang S. Y. The new progress of pharmacology and clinical research of Agrimony. Chinese Pharmaceutical . 2004;6:79–80. in Chinese. [Google Scholar]
- 8.Jin Z. J. Chemical constituents and clinical research progress of agrimony spp. West China Journal of Pharmaceutical . 2006;5:468–471. in Chinese. [Google Scholar]
- 9.Wu X. D., Jin Z. X. Research progress of agrimony. Proceedings of the First National Academic Conference of Traditional Chinese Medicine Commodities; 2008; Yiwu, China. China Commodity Society; pp. 302–309. [Google Scholar]
- 10.Hong G., Dai Y. H., Liu P. X., Shen X., Wei Y. Y., Li G. Research progress on chemical constituents and pharmacological effects of Agrimony spp. Pharmacy Service Research . 2008;5:362–366. in Chinese. [Google Scholar]
- 11.Feng L., Jia X. B., Chen Y., Li X., Gao C. L. Research progress on chemical constituents and antitumor activity of Agrimony spp. China Pharmaceutical . 2009;20(6):465–467. in Chinese. [Google Scholar]
- 12.Ba X. Y., He Y. Z., Lu F., Shi L. L. Research progress of agrimony. Journal of Liaoning University Traditional Chinese Medicine . 2011;13(5):258–261. in Chinese. [Google Scholar]
- 13.Wang B. Q., Jin Z. X. Research progress on chemical constituents and antioxidants of Agrimony spp. Northern Horticulture . 2011;10:167–169. in Chinese. [Google Scholar]
- 14.Jin M., Liu S. Y. Research progress of agrimony. Anhui Agricultural Science . 2015;43(19):78–115. in Chinese. [Google Scholar]
- 15.Liu W. J., Liang J. Y., Sun J. B., Feng F. Research progress of the Agrimonia pilosa Ledeb. on the chemical constituents and aharmacological activity. Strait Pharmaceutical Jounal . 2016;2:1–7. doi: 10.3969/j.issn.1006-3765.2016.02.001. [DOI] [Google Scholar]
- 16.Huang X., Wang Z., Wang B. H. Research progress on the physical effects and clinical application of crane herbs. Shandong Journal of Traditional Chinese Medicine . 2017;36(2):172–176. in Chinese. [Google Scholar]
- 17.Zhu Y., Huang S. Y., Wang J., Lin S. Y. A review of the anti-tumor mechanism and clinical application of Agrimony spp. World Science and Technology-Modernization of Traditional Medicine . 2018;20(12):2196–2201. in Chinese. [Google Scholar]
- 18.Li J., Yang J. Research progress on the main chemical constituents and pharmacological effects of Agrimony. China Wild Plant Resources . 2020;39(4):54–60. in Chinese. [Google Scholar]
- 19.Li L., Liu H. B. Research progress on the anti-tumor effect of traditional Chinese medicine Agrimony. Modern Medicine Health . 2021;37(24):4141–4145. in Chinese. [Google Scholar]
- 20.Wang X., Zhang K., Chen Y. S. Extraction and isolation of lowering blood sugar substances from the Agrimony. Chinese Journal of Experimental Traditional Medical Formulae . 2010;16:85–87. doi: 10.13422/j.cnki.syfjx.2010.06.054. [DOI] [Google Scholar]
- 21.Zhang J. H., Chen Y. S. Studies on the lowering blood sugar substances from Agrimony. China Journal of Chinese Material Medica . 2009;24(10):1537–1539. doi: 10.13863/j.issn1001-4454.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y., Liu H. X., Zhuang Y. L., Ding L. Q., Chen L. X., Qiu F. Studies on isolation and identification of flavonoids in herbs of Agrimonia pilosa. China Journal of Chinese Material Medica . 2008;33:2925–2928. doi: 10.3321/j.issn:1001-5302.2008.24.014. [DOI] [PubMed] [Google Scholar]
- 23.Kato H., Li W., Koike M., Wang Y., Koike K. Phenolic glycosides from Agrimonia pilosa. Phytochemistry . 2010;71(16):1925–1929. doi: 10.1016/j.phytochem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Bilia A. R., Palme E., Marsili A., Pistelli L., Morelli I. A flavonol glycoside from Agrimonia eupatoria. Phytochemistry . 1993;32(4):1078–1079. doi: 10.1016/0031-9422(93)85262-p. [DOI] [Google Scholar]
- 25.Lu F., Ba X., He Z. Chemical constituents of Agrimoniae herba. Chinese Traditional and Herbal Drugs . 2012;5:851–855. [Google Scholar]
- 26.Liu H. X., Liu Z. X., Jiang Q. H., Ding L. Q., Chen L. X., Qiu F. Phenolic constituents of whole plant of Agrimonia pilosa Ledeb. Journal of Shenyang Pharmaceutical University . 2010;4(27):286–289. doi: 10.14066/j.cnki.cn21-1349/r.2010.04.009. [DOI] [Google Scholar]
- 27.Shizuo K., Sayaka W., Jun K., Satoshi T., Junya M. Antimicrobial catechin derivatives of Agrimonia pilosa. Pergamon . 1992;31(3):787–789. doi: 10.1016/0031-9422(92)80015-7. [DOI] [Google Scholar]
- 28.Li X., Ye M., Yu X., He W., Li R. Studies on chemical constituents of agrimonia pilosa L. Journal of Beijing Medical University . 1995;27(1):p. 60. [Google Scholar]
- 29.Nguyen D. H., Seo U. M., Zhao B. T., et al. Ellagitannin and flavonoid constituents from Agrimonia pilosa Ledeb. with their protein tyrosine phosphatase and acetylcholinesterase inhibitory activities. Bioorganic Chemistry . 2017;72:293–300. doi: 10.1016/j.bioorg.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Park M. J., Kang Y.-H. Isolation of isocoumarins and flavonoids as α-glucosidase inhibitors from agrimonia pilosa L. Molecules . 2020;25(11):p. 2572. doi: 10.3390/molecules25112572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo U. M., Nguyen D. H., Zhao B. T., Min B. S., Woo M. H. Flavanonol glucosides from the aerial parts of Agrimonia pilosa Ledeb. and their acetylcholinesterase inhibitory effects. Carbohydrate Research . 2017;445:75–79. doi: 10.1016/j.carres.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Yamato M. On the chemical structure of agrimonolide, a new constituent of agrimonia pilosa LEDEB. I. Yakugaku Zasshi . 1958;78(10):1086–1089. doi: 10.1248/yakushi1947.78.10_1086. [DOI] [Google Scholar]
- 33.Woo K. H., Junha P., Bin K. K., et al. Acylphloroglucinolated catechin and phenylethyl isocoumarin derivatives from agrimonia pilosa. Journal of Natural Products . 2016;79(9):2376–2383. doi: 10.1021/acs.jnatprod.6b00566. [DOI] [PubMed] [Google Scholar]
- 34.Li H. R., Li Y. K., Xiao J., et al. Secondary metabolites isolated from Agrimonia pilosa Ledeb. Natural Product Research . 2021;36(1):263–270. doi: 10.1080/14786419.2020.1779263. [DOI] [PubMed] [Google Scholar]
- 35.Chen L., Teng H., Fang T., Xiao J. Agrimonolide from Agrimonia pilosa suppresses inflammatory responses through down-regulation of COX-2/iNOS and inactivation of NF-κB in lipopolysaccharide-stimulated macrophages. Phytomedicine . 2016;23(8):846–855. doi: 10.1016/j.phymed.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Guo X. H., Zhang X. H. Control study of clinical effect in Agrimony hypoglycemic powder treating type 2 diabetes. China Modern Medicine . 2015;22:157–160. [Google Scholar]
- 37.Huang Q., Chen L., Teng H., Song H., Wu X., Xu M. Phenolic compounds ameliorate the glucose uptake in HepG2 cells’ insulin resistance via activating AMPK. Journal of Functional Foods . 2015;19:487–494. doi: 10.1016/j.jff.2015.09.020. [DOI] [Google Scholar]
- 38.An R. B., Kim H. C., Jeong G. S., Oh S. H., Oh H. C., Kim Y. C. Constituents of the aerial parts of Agrimonia pilosa. Natural Product Sciences . 2005;11(4):196–198. [Google Scholar]
- 39.Liu J., Huiying L., Miao G. Experimental study on antitumor activities and immunity regulation of hedyotis diffusa willd injection. Chinese Journal Information Traditional Chinese Medicine . 2008;15(S1):22–23. [Google Scholar]
- 40.Isao K., Naosuke B., Yumiko O., Nobusuke K. Triterpenoids from agrimonia pilosa. Pergamon . 1988;27(1):297–299. [Google Scholar]
- 41.Chen J. Q. Study on the IR Improvement Mechanism of Activity Components of Agrimonia Pilosa Basing on the Nrf2-ARE Signaling Pathway . Chongqing, China: Chongqing university; 2017. [Google Scholar]
- 42.Shanghai Institute of Materia Medica. Shanghai fourteenth pharmaceutical factory, study, extraction and separation of the effective components of hairyvein agrimony and the structure and synthesis of agrimophol c. Acta Pharmaceutica Sinica B . 1975:25–35. [Google Scholar]
- 43.Medica S. I. O. M., Factory S. F. P. Study, extraction and separation of the effective components of hairyvein agrimony and the structure and synthesis of agrimophol C. Acta Pharmaceutica Sinica B . 1975;(01):25–35. [Google Scholar]
- 44.Pei Y. H., Li X., Zhu T. R. Study on the structure of new isocoumarin in in the root bud of hairyvein agrimony. Acta Pharmaceutica Sinica B . 1989;24:837–840. [PubMed] [Google Scholar]
- 45.Tang L., Fu L., Lu C., Hou X., Shan W., Zhan Z. New cytotoxic phloroglucinol derivatives from Agrimonia pilosa. Fitoterapia . 2017;118:69–72. doi: 10.1016/j.fitote.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Yamaki M., Kashihara M., Ishiguro K., Takagi S. Antimicrobial principles of Xian he cao (Agrimonia pilosa) Planta Medica . 1989;55(2):169–170. doi: 10.1055/s-2006-961915. [DOI] [PubMed] [Google Scholar]
- 47.Taira J., Nanbu H., Ueda K. Nitric oxide-scavenging compounds in Agrimonia pilosa Ledeb on LPS-induced RAW264.7 macrophages. Food Chemistry . 2009;115(4):1221–1227. doi: 10.1016/j.foodchem.2009.01.030. [DOI] [Google Scholar]
- 48.Zhang J., Yang Y.-N., Jiang J.-S., et al. New dimeric phloroglucinol derivatives from Agrimonia pilosa and their hepatoprotective activities. Bioorganic Chemistry . 2021;116 doi: 10.1016/j.bioorg.2021.105341.105341 [DOI] [PubMed] [Google Scholar]
- 49.Zhang J., Yang Y.-N., Jiang J.-S., et al. The discovery of new phloroglucinol glycosides from Agrimonia pilosa and the mechanism of oxidative dearomatization of the methyl-substituted phloroglucinol derivatives. RSC Advances . 2021;11(36):22273–22277. doi: 10.1039/d1ra03588f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okuda T., Yoshida T., Kuwahara M., Memon M. U., Shingu T. Tannins of rosaceous medicinal plants. I. Structures of potentillin, agrimonic acids A and B, and agrimoniin, a dimeric ellagitannin. Chemical and Pharmaceutical Bulletin . 1984;32(6):2165–2173. doi: 10.1248/cpb.32.2165. [DOI] [Google Scholar]
- 51.Erukainure O. L., Hafizur R. M., Choudhary M. I., et al. Anti-diabetic effect of the ethyl acetate fraction of clerodendrum volubile: protocatechuic acid suppresses phagocytic oxidative burst and modulates inflammatory cytokines. Biomedicine & Pharmacotherapy . 2017;86:307–315. doi: 10.1016/j.biopha.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 52.Inoue M., Suzuki R., Sakaguchi N., et al. Selective induction of cell death in cancer cells by gallic acid. Biological and Pharmaceutical Bulletin . 1995;18(11):1526–1530. doi: 10.1248/bpb.18.1526. [DOI] [PubMed] [Google Scholar]
- 53.Wei G., Guan Y., Yin Y., et al. Anti-inflammatory effect of protocatechuic aldehyde on myocardial ischemia/reperfusion injury in vivo and in vitro. Inflammation . 2013;36(3):592–602. doi: 10.1007/s10753-012-9581-z. [DOI] [PubMed] [Google Scholar]
- 54.Du L., Lin Q. Progress in the clinical application of Agrimonia pilosa L. International Journal of Traditional Chinese Medicine . 2012;9:848–849. [Google Scholar]
- 55.Lei C.-S., Hou Y.-C., Pai M.-H., Lin M.-T., Yeh S.-L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. The Journal of Nutritional Biochemistry . 2018;51:105–113. doi: 10.1016/j.jnutbio.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Teng H., Huang Q., Chen L. Inhibition of cell proliferation and triggering of apoptosis by agrimonolide through MAP kinase (ERK and p38) pathways in human gastric cancer AGS cells. Food & Function . 2016;7(11):4605–4613. doi: 10.1039/c6fo00715e. [DOI] [PubMed] [Google Scholar]
- 57.Hnit S. S. T., Ding R., Bi L., et al. Agrimol B present in Agrimonia pilosa Ledeb impedes cell cycle progression of cancer cells through G0 state arrest. Biomedicine & Pharmacotherapy . 2021;141 doi: 10.1016/j.biopha.2021.111795.111795 [DOI] [PubMed] [Google Scholar]
- 58.Pandey M., Kaur P., Shukla S., Abbas A., Fu P., Gupta S. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: in vitro and in vivo study. Molecular Carcinogenesis . 2012;51(12):952–962. doi: 10.1002/mc.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed H., Moawad A., Owis A., AbouZid S., Ahmed O. Flavonoids of calligonum polygonoidesand their cytotoxicity. Pharmaceutical Biology . 2016;54(10):2119–2126. doi: 10.3109/13880209.2016.1146778. [DOI] [PubMed] [Google Scholar]
- 60.Yin Y. L., Li P., Li J., Li S. J. Inhibitory effect of agrimol on K562 leukemia cell. Medicinal Plants . 2011;39(22):13417–13418. [Google Scholar]
- 61.Miyamoto K., Kishi N., Koshiura R. Antitumor effect of agrimoniin, a tannin of Agrimonia pilosa Ledeb., on transplantable rodent tumors. The Japanese Journal of Pharmacology . 1987;43(2):187–195. doi: 10.1016/s0021-5198(19)43538-5. [DOI] [PubMed] [Google Scholar]
- 62.Li W., Liu M., Xu Y.-F., et al. Combination of quercetin and hyperoside has anticancer effects on renal cancer cells through inhibition of oncogenic microRNA-27a. Oncology Reports . 2014;31(1):117–124. doi: 10.3892/or.2013.2811. [DOI] [PubMed] [Google Scholar]
- 63.Cheng Y. G., Tan J. Y., Ye W. C., et al. Optimization of extraction technology for total flavonoids from Agrimonia pilosa Ledeb by Plackett-Burman design combined with Box-Behnken response surface methodology and study on its antioxidant and antitumor activities. Chinese Arch Traditional Chinese Medicie . 2018;36:2414–2419. doi: 10.13193/j.issn.1673-7717.2018.10.028. [DOI] [Google Scholar]
- 64.Liu X., Zhu L., Tan J., et al. Glucosidase inhibitory activity and antioxidant activity of flavonoid compound and triterpenoid compound from Agrimonia Pilosa Ledeb. BMC Complementary and Alternative Medicine . 2014;14(1):12–126. doi: 10.1186/1472-6882-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathew S., Abraham T. E., Zakaria Z. A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. Journal of Food Science and Technology . 2015;52(9):5790–5798. doi: 10.1007/s13197-014-1704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ibitoye O. B., Ajiboye T. O. Protocatechuic acid protects against menadione-induced liver damage by up-regulating nuclear erythroid-related factor 2. Drug and Chemical Toxicology . 2018;43(6):1–7. doi: 10.1080/01480545.2018.1523187. [DOI] [PubMed] [Google Scholar]
- 67.Taira J., Ohmine W., Ogi T., Nanbu H., Ueda K. Suppression of nitric oxide production on LPS/IFN-γ-stimulated RAW264.7 macrophages by a novel catechin, pilosanol N, from Agrimonia pilosa Ledeb. Bioorganic & Medicinal Chemistry Letters . 2012;22(4):1766–1769. doi: 10.1016/j.bmcl.2011.12.086. [DOI] [PubMed] [Google Scholar]
- 68.Teng H., Chen L., Song H. The potential beneficial effects of phenolic compounds isolated from A. pilosa Ledeb on insulin-resistant hepatic HepG2 cells. Food & Function . 2016;7(10):4400–4409. doi: 10.1039/c5fo01067e. [DOI] [PubMed] [Google Scholar]
- 69.Guo T., Zhu L., Tan J., et al. Promoting effect of triterpenoid compound from Agrimonia pilosa Ledeb on preadipocytes differentiation via up-regulation of PPARγ expression. Pharmacognosy Magazine . 2015;11(41):219–225. doi: 10.4103/0973-1296.149741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S., Zhang Q., Zhang Y., et al. Agrimol B suppresses adipogenesis through modulation of SIRT1-PPAR gamma signal pathway. Biochemical and Biophysical Research Communications . 2016;477(3):454–460. doi: 10.1016/j.bbrc.2016.06.078. [DOI] [PubMed] [Google Scholar]
- 71.Feng Y. S., Yuan W. J., Gui L. H., Han Y. J. Study on the detaenia function and its principle of agrimophol. Chinese Traditional and Herbal Drugs . 1978;1:32–36. [Google Scholar]
- 72.Adamu M., Mukandiwa L., Awouafack M. D., Ahmed A. S., Eloff J. N., Naidoo V. Ultrastructure changes induced by the phloroglucinol derivative agrimol G isolated from Leucosidea sericea in Haemonchus contortus. Experimental Parasitology . 2019;207 doi: 10.1016/j.exppara.2019.107780.107780 [DOI] [PubMed] [Google Scholar]
- 73.Hu Y., Gu R., Zhao J., et al. Prognostic significance of Ki67 in Chinese women diagnosed with ER+/HER2− breast cancers by the 2015 St. Gallen consensus classification. BMC Cancer . 2017;17(1):p. 28. doi: 10.1186/s12885-016-3021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian L. L., Bao Y. R., Wang S., Li T. J., Meng X. S. Study on extraction and purification of total flavonoids from hairyvein agrimony and its anti-hepatic tumor efficacy in vitro. Journal of Chinese Medicinal Materials . 2019;42:380–384. doi: 10.13863/j.issn1001-4454.2019.02.031. [DOI] [Google Scholar]
- 75.Hyuna S., Jacques F., Rebecca S. L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians . 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 76.Shukla S., Fu P., Gupta S. Apigenin induces apoptosis by targeting inhibitor of apoptosis proteins and Ku70-Bax interaction in prostate cancer. Apoptosis . 2014;19(5):883–894. doi: 10.1007/s10495-014-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan J., Wang Y. X. Inhibitory effect of Agrimonia pilosa tannin on human tumor cells in vitro. Chinese Journal Traditional Medical Science Technology . 2000;7:378–379. [Google Scholar]
- 78.Wu X. D. Study on Infrared Spectrum Analysis and Antitumor Effect of Tannin in Agaricum Sinensis . Harbin, China: Northeast Forestry University; 2010. [Google Scholar]
- 79.Eom S. Y., Kim M. M. The inhibitory effect of Agrimonia Pilosa methanolic extract on matrix metalloproteinases in HT1080 cells. Journal of Food Biochemistry . 2021;45(9):1–11. doi: 10.1111/jfbc.13894. [DOI] [PubMed] [Google Scholar]
- 80.Zhao J. L., Li G. D., Liu M. Mechanism of anticarcinogenic function of ellagic acid in gastrointestinal cancers. International Journal of Oncology . 2016;43(06):472–474. [Google Scholar]
- 81.Hah D.-S., Kim C.-H., Kim E.-K., Kim J.-S. Evaluation of antioxidative activity of agrimonia pilosa-ledeb leaves on non-lipid oxidative damage. Toxicological Research . 2009;25(4):243–251. doi: 10.5487/tr.2009.25.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X., Fan G., Wei F., Bu Y., Huang W. Hyperoside protects rat ovarian granulosa cells against hydrogen peroxide-induced injury by sonic hedgehog signaling pathway. Chemico-Biological Interactions . 2019;310 doi: 10.1016/j.cbi.2019.108759.108759 [DOI] [PubMed] [Google Scholar]
- 83.Wang C., Qi C., Liu M., et al. Protective effects of agrimonolide on hypoxia-induced H9c2 cell injury by maintaining mitochondrial homeostasis. Journal of Cellular Biochemistry . 2021;123(2):306–321. doi: 10.1002/jcb.30169. [DOI] [PubMed] [Google Scholar]
- 84.Diehl S., Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Molecular Immunology . 2002;39(9):531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 85.Hirano T., Ishihara K., Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene . 2000;19(21):2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 86.Jin X., Song S., Wang J., Zhang Q., Qiu F., Zhao F. Tiliroside, the major component of Agrimonia pilosa Ledeb ethanol extract, inhibits MAPK/JNK/p38-mediated inflammation in lipopolysaccharide-activated RAW 264.7 macrophages. Experimental and Therapeutic Medicine . 2016;12(1):499–505. doi: 10.3892/etm.2016.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng J.-H., Kim H.-Y., Sim S.-M., et al. The anti-inflammatory and the antinociceptive effects of mixed agrimonia pilosa ledeb. and salvia miltiorrhiza bunge extract. Plants . 2021;10(6):1234–1238. doi: 10.3390/plants10061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson T. O., Ermolieff J., Jirousek M. R. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nature Reviews Drug Discovery . 2002;1(9):696–709. doi: 10.1038/nrd895. [DOI] [PubMed] [Google Scholar]
- 89.Maheshwari N., Karthikeyan C., Trivedi P., Moorthy N. S. H. N. Recent advances in protein tyrosine phosphatase 1B targeted drug discovery for type II diabetes and obesity. Current Drug Targets . 2018;19(5):551–575. doi: 10.2174/1389450118666170222143739. [DOI] [PubMed] [Google Scholar]
- 90.Jang H. H., Nam S. Y., Kim M. J., et al. Agrimonia pilosa Ledeb. aqueous extract improves impaired glucose tolerance in high-fat diet-fed rats by decreasing the inflammatory response. BMC Complementary and Alternative Medicine . 2017;17(1):p. 442. doi: 10.1186/s12906-017-1949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abdesselem H., Madani A., Hani A., et al. SIRT1 limits adipocyte hyperplasia through c-Myc inhibition. Journal of Biological Chemistry . 2016;291(5):2119–2135. doi: 10.1074/jbc.m115.675645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature . 2006;444(7121):868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 93.Chalkiadaki A., Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metabolism . 2012;16(2):180–188. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jang H.-H., Bae J. H., Kim M.-J., Park M. Y., Kim H. R., Lee Y.-M. Agrimonia pilosa Ledeb. ameliorates hyperglycemia and hepatic steatosis in ovariectomized rats fed a high-fat diet. Nutrients . 2020;12(6):1631–1638. doi: 10.3390/nu12061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dhami D. S., Pandey S. C., Shah G. C., Bisht M., Samant M. In vitro antileishmanial activity of the essential oil from Agrimonia pilosa. National Academy Science Letters . 2021;44(3):195–198. doi: 10.1007/s40009-020-00992-2. [DOI] [Google Scholar]
- 96.Park S., Kang S., Kim D. S., Moon B. R. Agrimonia pilosa Ledeb, Cinnamomum cassia Blume, and Lonicera japonica Thunb. protect against cognitive dysfunction and energy and glucose dysregulation by reducing neuroinflammation and hippocampal insulin resistance in β-amyloid-infused rats. Nutritional Neuroscience . 2017;20:77–88. doi: 10.1080/1028415X.2015.1135572. [DOI] [PubMed] [Google Scholar]
- 97.Yu Q. Screening and Study of Natural Products against Methicillin-Resistant Staphylococcus aureus (MRSA) Chongqing, China: Southwest University; 2012. [Google Scholar]
- 98.Kwon J. E., Lee Y. G., Kang J. H., et al. Anti-viral activity of compounds from Agrimonia pilosa and galla rhois extract mixture. Bioorganic Chemistry . 2019;93 doi: 10.1016/j.bioorg.2019.103320. [DOI] [PubMed] [Google Scholar]
- 99.Lee Y. G., Kang K. W., Hong W., et al. Potent antiviral activity of Agrimonia pilosa, galla rhois, and their components against SARS-CoV-2. Bioorganic & Medicinal Chemistry . 2021;45:1–11. doi: 10.1016/j.bmc.2021.116329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee Y. M., Kim J. B., Bae J. H., et al. Estrogen-like activity of aqueous extract from Agrimonia pilosa Ledeb. in MCF-7 cells. BMC Complementary and Alternative Medicine . 2012;12(1):260–268. doi: 10.1186/1472-6882-12-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park S.-H., Sim Y.-B., Kang Y.-J., Lee J.-K., Lim S.-S., Suh H.-W. Effect of Agrimonia pilosa Ledeb Extract on the antinociception and mechanisms in mouse. Korean Journal of Physiology and pharmacology . 2012;16(2):119–123. doi: 10.4196/kjpp.2012.16.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krasnov E. A., Otmakhov V. I., Zamoshchina T. A., Reshetov Y. E., Kuskova I. S., Petrova E. V. Rhythm-modulating activity of fractions of aqueous extract of agrimonia pilosa. Pharmaceutical Chemistry Journal . 2021;54(11):1145–1149. doi: 10.1007/s11094-021-02333-z. [DOI] [Google Scholar]


