Atrial fibrillation (AF) remains the most common arrhythmia in the world. Recently, significant randomized controlled trials in patients with AF were published with the focus on rhythm-control therapy.1 Large randomized controlled trials help to truly assess the benefit of an intervention or treatment in a given population. Great examples include the studies showing the decrease of mortality with anticoagulation and in particular non-vitamin K antagonists (NOAC) to prevent strokes in AF.2 However, one field where randomized data are scarce is in patients who cannot take oral anticoagulation (OAC) due to recurrent bleedings. Two randomized trials, PROTECT-AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL-AF (Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation vs. Long Term Warfarin Therapy) compared interventional left atrial appendage occlusion (LAAO) to the vitamin K antagonist warfarin in patients with AF and moderate stroke risk (CHA2DS2-VASc scores of 2.2 ± 1.2 and 2.6 ± 1.0, respectively).3 In both PROTECT-AF and PREVAIL-AF, patients with contraindication for warfarin were excluded as the post-procedural OAC included 45 days of warfarin after LAAO.3 Similarly, the PRAGUE-17 trial (Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in Atrial Fibrillation) compared patients with high risk for bleeding and stroke with NOAC and non-inferiority for cardiovascular events.4
Interestingly, the most recent European Society of Cardiology guidelines cite both studies and make a IIb recommendation for LAAO in patients with contraindication to OAC, reflecting the lack of robust evidence on the efficacy of this therapy compared to anticoagulation.5 Fortunately, several outcome-powered clinical trials are ongoing, but it will take a few years until they report. As LAAO technology is available for clinical use in the European Union, this leads to uncertainty about patient selection for LAAO in clinical practice, which is difficult to handle.
The Hamburg AF Heart Team approach to LAAO
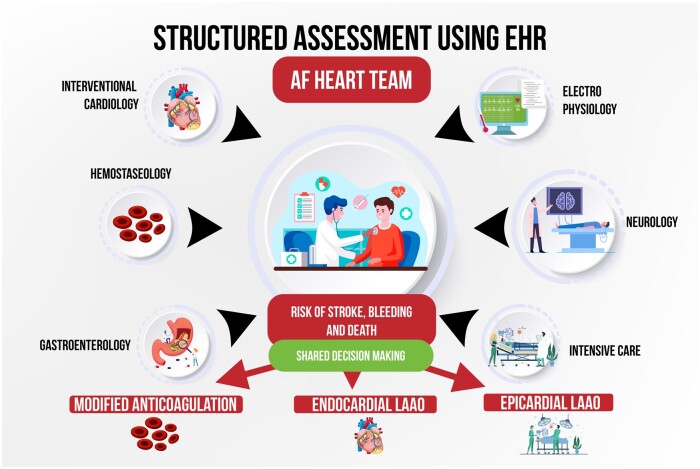
Since 2016, ‘Atrial Fibrillation Heart Teams’ have been recommended to guide therapy in view of the complex treatments possible.6 Our approach to guiding therapy in AF patients with recurrent strokes and/or contraindication to anticoagulation begins with a structured assessment using the electronic health records (EHR) (Figure 1). The physician can calculate the guideline recommended CHA2DS2-VASc score for stroke risk as well as the HAS-BLED score for bleeding risk assessment.5 In the next step, significant co-morbidities and recent events (major bleedings or strokes) are detailed. This produces visible scores and a detailed assessment in the EHR. The AF Heart Team for LAAO consists of neurologists, haemostaseologists, anticoagulation specialists, gastroenterologists, electrophysiologists, and interventional cardiologists. In certain cases, when additional expertise is needed, intensive care specialists or heart surgeons are participating. The need for additional diagnostics such as imaging of the brain or coagulation diagnostics can be discussed and information requested by each specialist. Through evaluation of each patient’s history, co-morbidities, bleeding, and stroke risk, an individualized treatment plan including a modified anticoagulation therapy or endocardial LAAO as well as the option of epicardial LAAO (either surgical or interventional ligature) is developed. Another option is to evaluate the patient for randomized controlled trials in which enrolment could be discussed, for instance the industry-independent randomized trial CLOSURE-AF, which compares endocardial LAAO with best medical therapy (including, but not limited to NOAC). Every single patient who is referred for LAAO is undergoing this process to prevent interventional treatment without exploring every option and adhere to primum non nocere.
Figure 1.
Flow-chart of an Atrial Fibrillation Heart Team approach to assessing patients who are referred for left atrial appendage occlusion. EHR, electronic health records.
The root of the problem—lack of data
Many interesting studies about stroke prevention in AF were conducted over the last years, for instance Elder-AF, which showed that a reduced dose of 15 mg edoxaban still significantly lowered strokes in very old patients compared with placebo.7 Yet, we still do not know enough and randomized trials are few. The LAAOS III (Left Atrial Appendage Occlusion Study III) trial impressively showed that LAAO in patients undergoing cardiac surgery reduces stroke or systemic embolism; however, this evidence does not exist for interventional closure.8 Large outcome studies like CLOSURE-AF will certainly help but enrolment proves to be slow. Until then, we have the duty to navigate the treatment selection as best as possible. We therefore strongly encourage the establishment of AF Heart Teams to increase individualized solutions for our patients and to screen for patients who fit the criteria for randomized trials and enrol as many patients as best as possible.
Funding
P.K. is partially supported by European Union BigData@Heart (grant agreement EU IMI 116074), British Heart Foundation (FS/13/43/30324; PG/17/30/32961 and PG/20/22/35093; AA/18/2/34218), German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK e.V., 81Z1710103), and Leducq Foundation.
Conflict of interest: PK receives research support for basic, translational, and clinical research projects from the European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last 3 years. PK is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783).
References
- 1. Kirchhof P, Camm AJ, Goette Aet al. ; EAST-AFNET 4 Trial Investigators . Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 2. Ruff CT, Giugliano RP, Braunwald Eet al. . Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 3. Reddy VY, Doshi SK, Kar Set al. ; PREVAIL and PROTECT AF Investigators . 5-Year outcomes after left atrial appendage closure. J Am Coll Cardiol 2017;70:2964–2975. [DOI] [PubMed] [Google Scholar]
- 4. Osmancik P, Herman D, Neuzil Pet al. ; PRAGUE-17 Trial Investigators . Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122–3135. [DOI] [PubMed] [Google Scholar]
- 5. Hindricks G, Potpara T, Dagres Net al. . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 6. Kirchhof P, Benussi S, Kotecha Det al. ; ESC Scientific Document Group . 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 7. Okumura K, Akao M, Yoshida Tet al. . Low-dose edoxaban in very elderly patients with atrial fibrillation. N Engl J Med 2020;383:1735–1745. [DOI] [PubMed] [Google Scholar]
- 8. Whitlock RP, Belley-Cote EP, Paparella Det al. ; LAAOS III Investigators . Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–2091. [DOI] [PubMed] [Google Scholar]