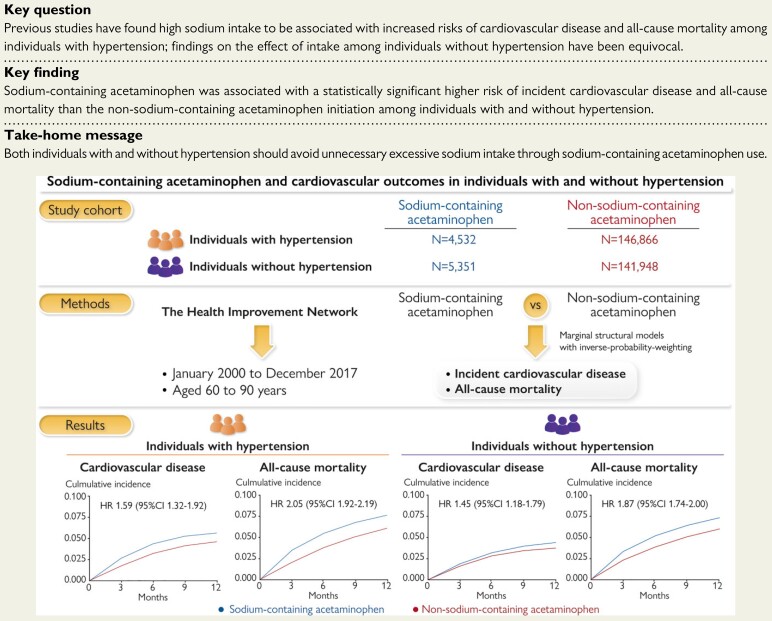
Abstract
Aims
Previous studies have found high sodium intake to be associated with increased risks of cardiovascular disease (CVD) and all-cause mortality among individuals with hypertension; findings on the effect of intake among individuals without hypertension have been equivocal. We aimed to compare the risks of incident CVD and all-cause mortality among initiators of sodium-containing acetaminophen with the risk of initiators of non-sodium-containing formulations of the same drug according to the history of hypertension.
Methods and results
Using The Health Improvement Network, we conducted two cohort studies among individuals with and without hypertension. We examined the relation of sodium-containing acetaminophen to the risk of each outcome during 1-year follow-up using marginal structural models with an inverse probability weighting to adjust for time-varying confounders. The outcomes were incident CVD (myocardial infarction, stroke, and heart failure) and all-cause mortality. Among individuals with hypertension (mean age: 73.4 years), 122 CVDs occurred among 4532 initiators of sodium-containing acetaminophen (1-year risk: 5.6%) and 3051 among 146 866 non-sodium-containing acetaminophen initiators (1-year risk: 4.6%). The average weighted hazard ratio (HR) was 1.59 [95% confidence interval (CI) 1.32–1.92]. Among individuals without hypertension (mean age: 71.0 years), 105 CVDs occurred among 5351 initiators of sodium-containing acetaminophen (1-year risk: 4.4%) and 2079 among 141 948 non-sodium-containing acetaminophen initiators (1-year risk: 3.7%), with an average weighted HR of 1.45 (95% CI 1.18–1.79). Results of specific CVD outcomes and all-cause mortality were similar.
Conclusion
The initiation of sodium-containing acetaminophen was associated with increased risks of CVD and all-cause mortality among individuals with or without hypertension. Our findings suggest that individuals should avoid unnecessary excessive sodium intake through sodium-containing acetaminophen use.
Keywords: Sodium, Acetaminophen, Cardiovascular disease, Mortality
Structured Graphical Abstract
Sodium-containing acetaminophen was associated with increased risks of CVD and all-cause mortality among individuals with or without hypertension.
See the editorial comment for this article ‘The sodium hidden in medication: a tough pill to swallow’, by A.E. Schutte and B. Neal, https://doi.org/10.1093/eurheartj/ehab888.
Introduction
Excessive sodium intake is a major public health concern globally.1,2 Numerous studies have found high sodium intake to be associated with increased risks of cardiovascular disease (CVD) and all-cause mortality among patients with hypertension,3–7 but findings among individuals without hypertension have been equivocal.3–12 Although several randomized controlled trials have evaluated the effect of reduction in sodium intake,13–15 no trial has been conducted to assess the effect of extra sodium intake on the risks of CVD and mortality owing to ethical considerations.
In addition to dietary sodium intake, sodium-containing drugs are another source of sodium intake as it is widely used in drug preparations for enhancing solubility or disintegration.16,17 In 2018, the proportion of sodium-containing medication users was 169.9/10 000 inhabitants in the UK and was even higher among women and increased with age.18 Acetaminophen, a commonly used pain-relief medication, consists of both sodium-containing (e.g. the effervescent or soluble) and non-sodium-containing formulations.16,19 The effervescent and soluble formulations of 0.5 g acetaminophen contain 0.44 and 0.39 g of sodium, respectively; thus, the intake of maximum daily dose (i.e. 4 g/day) of sodium-containing acetaminophen corresponds to the ingestion of 3.5 and 3.1 g of sodium,20 a dose that alone exceeds the recommended total daily sodium intake allowance of the World Health Organization (i.e. 2 g/day).2 Thus, the aim of this study was to compare the risks of incident CVD and all-cause mortality among initiators of sodium-containing acetaminophen (i.e. an incident exposure of extra sodium intake) with that among initiators of non-sodium-containing formulations of the same drug according to the history of hypertension.
Methods
Data source
The Health Improvement Network (THIN) is an electronic medical record database of records of general practitioners (GPs) in the UK and represents the UK population in demographics and medical conditions. THIN contains anonymized medical records from 790 general practices with approximately 17 million patients. Health care information is recorded at each practice on socio-demographics, anthropometrics, lifestyle factors, visits to GPs, diagnoses from specialists and hospital admissions, and laboratory test results.21 The Read classification system is used to code specific diagnoses22 and the Multilex classification system based on the British National Formulary and Anatomical Therapeutic Chemical code is used for medications.23 Previous study has demonstrated the validity of THIN database in clinical and epidemiological research.21 The scientific review committee for THIN database and the institutional review board at the Xiangya Hospital, Central South University, China, approved this study, with a waiver of informed consent.
Study design and cohort definition
We conducted two cohort studies to examine the initiation of sodium-containing acetaminophen in relation to the risks of incident CVD and all-cause mortality. Eligible participants in the first study were 60–90 years of age, were registered for at least 1 year of continuous enrollment with a general practice during January 2000–December 2017, had a diagnosis of hypertension at any time prior to the index date, and had been prescribed neither sodium-containing acetaminophen nor non-sodium-containing formulations of acetaminophen at least 1 year before entering the study. Because the use of over-the-counter acetaminophen was not recorded in THIN, to minimize the potential exposure misclassification we restricted participants to those aged ≥60 years as the National Health Service of the UK provides healthcare with most services free for those participants. The date of the initial prescription of acetaminophen was the index date for the follow-up. Participants were excluded if they had a history of cancer or CVD (myocardial infarction [MI], stroke, or heart failure) before the index date. We took the same approach in a second cohort study that was conducted among individuals without hypertension at baseline.
Assessment of exposure and comparator
We identified individuals who initiated either sodium-containing acetaminophen (i.e. in effervescent or soluble formulation) or non-sodium-containing acetaminophen (i.e. in tablet, oral suspension, or capsule formulation) based on the ATC code (N02BE01) and the formulation recorded in THIN.16,20 We excluded individuals (16 694 with and 9095 without a history of hypertension) who were prescribed compound acetaminophen (e.g. acetaminophen 0.5 g/dihydrocodeine 30 mg tablet), which accounted for 4.3% of the total acetaminophen use (see the full list in Supplementary material online, Table S1).
Assessment of outcomes
The primary outcomes were incident CVD and all-cause mortality, hereafter referred to as mortality. As approximately 90% of subjects took acetaminophen for ≤1 year in the current study, we focused on outcomes during the 1-year follow-up period after initial acetaminophen prescription to minimize a potential selection bias from the loss to follow-up.24 CVD outcomes (MI, stroke, and heart failure) were identified by Read codes.25–27 Date of death was recorded in THIN through linkage to the National Health Service.28 To verify the robustness of our findings, we examined the association of initiation of sodium-containing acetaminophen with incident hypertension, identified using Read codes.29–31
Assessment of covariates
Socio-demographic and anthropometric characteristics, as well as lifestyle factors, were assessed using the nearest available data prior to the index date; comorbidities and medication use were assessed at any time before the index date; and healthcare utilization was ascertained during the 1-year period before the index date (Table 1).
Table 1.
Baseline characteristics according to the hypertension status among patients initiating sodium-containing or non-sodium-containing acetaminophen
| Variable list | With a history of hypertension | Without a history of hypertension | ||||||
|---|---|---|---|---|---|---|---|---|
| Sodium-containing acetaminophen (n = 4532) | Non-sodium-containing acetaminophen (n = 146 866) | Standard difference before IPWa | Standard difference after IPWa | Sodium-containing acetaminophen (n = 5351) | Non-sodium-containing acetaminophen (n = 141 948) | Standard difference before IPWa | Standard difference after IPWa | |
| Demographics | ||||||||
| Age, mean (SD), years | 74.3 (7.9) | 73.3 (7.7) | 0.119 | 0.077 | 72.1 (8.0) | 70.8 (7.6) | 0.155 | 0.080 |
| Socioeconomic Deprivation Index, mean (SD)b | 2.7 (1.4) | 2.7 (1.3) | 0.057 | 0.011 | 2.7 (1.4) | 2.6 (1.3) | 0.065 | 0.013 |
| Female (%) | 70.7 | 61.0 | 0.205 | 0.014 | 67.7 | 58.8 | 0.185 | 0.004 |
| BMI, mean (SD), kg/m2 | 27.4 (5.5) | 28.4 (5.5) | 0.182 | 0.053 | 25.6 (4.9) | 26.5 (4.8) | 0.183 | 0.062 |
| Region (%) | 0.069 | 0.021 | 0.094 | 0.050 | ||||
| England | 69.6 | 67.9 | 69.7 | 68.3 | ||||
| Northern Ireland | 5.0 | 4.1 | 6.0 | 4.4 | ||||
| Scotland | 16.3 | 18.4 | 16.3 | 18.5 | ||||
| Wales | 9.2 | 9.6 | 8.0 | 8.8 | ||||
| Ethnicity (%) | 0.073 | 0.044 | 0.104 | 0.027 | ||||
| White | 36.0 | 39.0 | 33.9 | 38.9 | ||||
| Non-White | 1.6 | 2.0 | 1.6 | 1.7 | ||||
| Missing | 62.4 | 59.0 | 64.5 | 59.5 | ||||
| Lifestyle factors | ||||||||
| Drinking (%) | 0.165 | 0.012 | 0.142 | 0.017 | ||||
| None | 30.9 | 23.9 | 27.2 | 21.5 | ||||
| Past | 3.4 | 2.8 | 3.0 | 2.5 | ||||
| Current | 65.7 | 73.3 | 69.8 | 76.1 | ||||
| Smoking (%) | 0.077 | 0.015 | 0.097 | 0.014 | ||||
| None | 58.8 | 55.3 | 57.3 | 52.5 | ||||
| Past | 30.0 | 33.5 | 26.7 | 30.3 | ||||
| Current | 11.2 | 11.3 | 16.0 | 17.2 | ||||
| Comorbidity (%) | ||||||||
| Other ischaemic heart disease | 14.8 | 15.0 | 0.006 | 0.021 | 8.5 | 8.6 | 0.006 | 0.020 |
| Other cerebrovascular accident | 5.0 | 4.4 | 0.029 | 0.024 | 2.6 | 2.1 | 0.031 | 0.005 |
| Atrial fibrillation | 7.1 | 7.1 | 0.001 | 0.004 | 3.5 | 4.0 | 0.030 | 0.007 |
| Venous thromboembolism | 3.4 | 3.6 | 0.011 | 0.005 | 3.6 | 3.2 | 0.024 | 0.022 |
| Peripheral vascular disease | 2.3 | 2.6 | 0.020 | 0.017 | 1.3 | 1.3 | 0.004 | 0.009 |
| Hyperlipidaemia | 17.4 | 19.8 | 0.062 | 0.021 | 8.1 | 9.8 | 0.058 | <0.001 |
| Diabetes | 18.7 | 20.3 | 0.042 | 0.001 | 7.6 | 8.6 | 0.035 | <0.001 |
| Peptic ulcer disease | 7.3 | 7.1 | 0.010 | 0.003 | 7.7 | 7.0 | 0.026 | 0.017 |
| Gastrointestinal bleeding | 2.7 | 2.3 | 0.024 | 0.008 | 2.4 | 2.0 | 0.031 | 0.015 |
| Gastrooesophageal reflux disease | 15.6 | 13.9 | 0.048 | 0.010 | 14.5 | 13.3 | 0.035 | 0.036 |
| Gastritis | 21.1 | 19.6 | 0.038 | 0.009 | 20.1 | 19.1 | 0.024 | 0.044 |
| Chronic obstructive pulmonary disease | 7.6 | 6.3 | 0.050 | 0.006 | 7.5 | 7.2 | 0.012 | 0.039 |
| Pneumonia or infection | 7.2 | 6.7 | 0.021 | 0.001 | 8.0 | 6.9 | 0.041 | 0.007 |
| Chronic kidney disease | 14.4 | 15.4 | 0.027 | 0.024 | 3.7 | 4.0 | 0.017 | 0.009 |
| Liver disease | 2.4 | 2.3 | 0.004 | 0.013 | 2.1 | 1.7 | 0.026 | 0.084 |
| Alcohol abuse | 1.8 | 2.1 | 0.019 | 0.015 | 2.4 | 2.3 | 0.008 | 0.010 |
| Fall | 15.3 | 14.3 | 0.029 | 0.004 | 14.0 | 12.3 | 0.049 | 0.020 |
| Fracture | 2.9 | 2.1 | 0.050 | 0.005 | 2.7 | 1.9 | 0.052 | 0.013 |
| Gout | 5.8 | 8.0 | 0.085 | 0.042 | 2.6 | 3.6 | 0.057 | <0.001 |
| Osteoarthritis | 33.0 | 36.5 | 0.074 | 0.069 | 26.9 | 32.9 | 0.130 | 0.006 |
| Rheumatoid arthritis | 2.5 | 2.2 | 0.022 | 0.025 | 2.4 | 2.2 | 0.013 | 0.008 |
| Depression | 11.5 | 10.9 | 0.020 | 0.007 | 11.6 | 11.7 | 0.004 | 0.036 |
| Medication (%) | ||||||||
| Anticoagulants | 7.9 | 8.0 | 0.005 | 0.001 | 5.4 | 5.4 | <0.001 | 0.012 |
| Beta receptor inhibitors | 48.1 | 50.0 | 0.040 | 0.001 | 16.9 | 16.9 | 0.001 | 0.013 |
| Angiotensin receptor blocker | 20.6 | 22.4 | 0.043 | 0.013 | 1.1 | 1.5 | 0.030 | 0.009 |
| ACE inhibitors | 52.1 | 57.5 | 0.109 | 0.020 | 6.1 | 7.0 | 0.035 | 0.008 |
| Calcium channel blockers | 54.3 | 58.6 | 0.086 | 0.014 | 8.7 | 8.8 | 0.002 | 0.020 |
| Antihypertensive medicine | 95.4 | 96.3 | 0.045 | 0.001 | 37.3 | 34.7 | 0.053 | 0.008 |
| Potassium-sparing diuretics | 12.1 | 9.8 | 0.073 | 0.041 | 6.3 | 4.8 | 0.062 | 0.019 |
| Thiazide-like diuretic | 11.0 | 10.7 | 0.011 | 0.008 | 0.5 | 0.6 | 0.005 | 0.028 |
| Thiazide diuretics | 65.3 | 66.5 | 0.026 | 0.025 | 9.6 | 8.3 | 0.047 | 0.003 |
| Loop diuretics | 24.4 | 21.8 | 0.062 | 0.040 | 15.2 | 12.3 | 0.085 | 0.010 |
| Nitrates | 13.6 | 14.4 | 0.022 | 0.028 | 9.0 | 9.5 | 0.019 | 0.016 |
| Insulin | 3.3 | 3.4 | 0.007 | 0.003 | 1.1 | 1.3 | 0.018 | 0.013 |
| Antidiabetic medicine | 13.1 | 14.7 | 0.045 | 0.006 | 4.9 | 6.0 | 0.047 | 0.016 |
| PPIs | 48.0 | 47.9 | 0.001 | 0.006 | 45.0 | 45.8 | 0.016 | 0.031 |
| H2 blockers | 24.1 | 20.5 | 0.085 | 0.029 | 22.6 | 20.1 | 0.061 | 0.054 |
| Glucocorticoids | 23.3 | 21.4 | 0.046 | 0.019 | 21.8 | 20.7 | 0.028 | 0.042 |
| Oestrogen | 13.5 | 13.1 | 0.015 | 0.009 | 15.2 | 16.4 | 0.033 | 0.005 |
| NSAIDs | 76.2 | 81.0 | 0.117 | 0.008 | 72.8 | 81.1 | 0.199 | 0.020 |
| Opioids | 36.5 | 40.9 | 0.090 | 0.027 | 32.5 | 38.5 | 0.127 | 0.067 |
| DMARDs | 3.4 | 3.3 | 0.003 | 0.017 | 3.2 | 3.5 | 0.014 | 0.013 |
| Bisphosphonates | 10.5 | 9.4 | 0.035 | 0.007 | 10.6 | 9.4 | 0.039 | 0.023 |
| Antiepileptic medicine | 8.9 | 8.3 | 0.020 | 0.016 | 8.7 | 8.4 | 0.010 | 0.002 |
| Healthcare utilization, mean (SD)c | ||||||||
| Hospitalizations | 0.6 (1.5) | 0.5 (1.1) | 0.078 | 0.026 | 0.5 (1.2) | 0.4 (1.0) | 0.059 | 0.047 |
| General practice visits | 7.7 (7.2) | 7.1 (6.5) | 0.090 | 0.016 | 6.2 (6.3) | 5.9 (5.8) | 0.061 | 0.026 |
| Specialist referrals | 0.6 (1.0) | 0.6 (1.0) | 0.018 | 0.017 | 0.5 (1.0) | 0.5 (0.9) | 0.001 | 0.008 |
BMI, body mass index; SD, standard deviation; NSAID, non-steroidal anti-inflammatory drug; ACE, angiotensin-converting enzyme; PPIs, proton pump inhibitors; IPW, inverse probability weighting; DMARD, disease-modifying antirheumatic drug.
IPW was calculated based on propensity scores (PS) for each individual’s probability of receiving a specific treatment, given the confounders (i.e. 1/PS for participants who initiated sodium-containing acetaminophen and 1/(1 − PS) for the participants who initiated non-sodium-containing acetaminophen).
The Socioeconomic Deprivation Index was measured by the Townsend Deprivation Index, which was grouped into quintiles from 1 (least deprived) to 5 (most deprived).
Frequency during the past 1 year.
Statistical analysis
Person-years of follow-up for each participant were calculated as the amount of time from the index date to the first of the following events: incident CVD, death, age of 90 years, transferring out of THIN GP practice, the end of the 1-year follow-up period, or 31 December 2018 when the study was closed.
To account for time-varying exposures and confounders, we divided the follow-up time into four 3-month time blocks starting from the index date and used a marginal structural model with an inverse probability weighting (IPW)32–34 to estimate the average weighted hazard ratio (HR) of incident CVD and mortality for sodium-containing acetaminophen.35 Time-varying exposures and confounders were updated every 3 months. Weights were calculated based on propensity scores (PSs) for each individual’s probability of receiving a specific treatment, given the confounders.32–34 The weights were 1/PS for participants who initiated sodium-containing acetaminophen and 1/(1 − PS) for the participants who initiated non-sodium-containing acetaminophen.33,34 We fitted a pooled logistic regression model to obtain the relative estimates. The odds ratio (OR) generated from this model approximated the HR because the outcome is rare. We also estimated the absolute 1-year risk and risk difference (RD) of CVD by fitting the pooled logistic models with product terms between the ‘initiation of sodium-containing acetaminophen’ indicator and the 3 months of follow-up variables. The models’ predicted values were then used to estimate the cumulative incidence of CVD from baseline.36 The cumulative incidence curves were standardized to the baseline variables.37 We used a non-parametric bootstrap with 100 samples to compute the 95% confidence interval (CI) for RD. We used the same approach to examine the relation of sodium-containing acetaminophen to mortality and to the risk of incident hypertension. In addition, we took the same approach to compare the risks of incident CVD and mortality among initiators of sodium-containing ibuprofen or ranitidine with that among initiators of non-sodium-containing formulations of the same drugs according to the history of hypertension.
We conducted several sensitivity analyses. First, since 35% of participants had missing values for acetaminophen dose, we limited the analyses to participants who initiated acetaminophen 3–4 g/day. Second, to account for missing values of four important potential confounding variables (i.e. body mass index, alcohol drinking, smoking, and the Socioeconomic Deprivation Index), we took a sequential regression approach to impute the missing value based on a set of covariates as predictors. To minimize random error, we imputed 20 datasets and used the PROC MIANALYZE in the SAS to combine estimates from these datasets.38 Third, we restricted the analyses to a homogeneous population, i.e. participants with osteoarthritis, who are likely to have similar indications for acetaminophen use. Fourth, to further minimize residual confounding bias (i.e. potential unbalanced distribution of iatrogenic sodium intake between two comparison groups), we excluded participants who had a history of using other sodium-containing medications.16 Fifth, we conducted a quantitative sensitivity analysis to evaluate the minimum unmeasured confounding effect that would explain away an association observed in the primary analyses. Finally, as a previous study showed that there was a potential interaction between acetaminophen and warfarin on the anticoagulant effect of the latter,39 we assessed the association between sodium-containing acetaminophen and the risk of CVD or mortality stratified by the use of warfarin.
We conducted a nested case–control study to assess the dose–response relationship between the number of prescriptions of sodium-containing acetaminophen and the risk of incident CVD. For each case of CVD, we created a risk set that included up to 10 controls who were alive and free of CVD when a CVD case occurred and matched by sex, year of entry into the study, and age of entry into the study. The number of prescriptions of sodium-containing acetaminophen was calculated from the date of sodium-containing acetaminophen initiation to the date of the case (i.e. CVD) and matched controls (assigned the same date as their matched case) were identified. We divided the number of prescriptions of sodium-containing acetaminophen into the following four categories: none, 1, 2–4, and ≥5. We examined the relation of the number of sodium-containing acetaminophen prescriptions to the risk of CVD using conditional logistic regression and tested a dose–response relationship by entering the number of prescriptions into the regression model. We took the same approach to evaluate the dose–response relationship between sodium-containing acetaminophen prescriptions and the risk of mortality.
All P-values were two-sided and P < 0.05 was considered significant for all tests. All statistical analyses were performed with the SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Overall, 151 398 individuals with hypertension and 147 299 individuals without hypertension were analyzed. The flowcharts depicting the selection process of participants are shown in Supplementary material online, Figures S1 and S2. Supplementary material online, Table 1 shows the baseline characteristics of participants. After adjustment for IPW, all covariates were well balanced (all standardized differences <0.1) (Table 1).
Incident cardiovascular disease
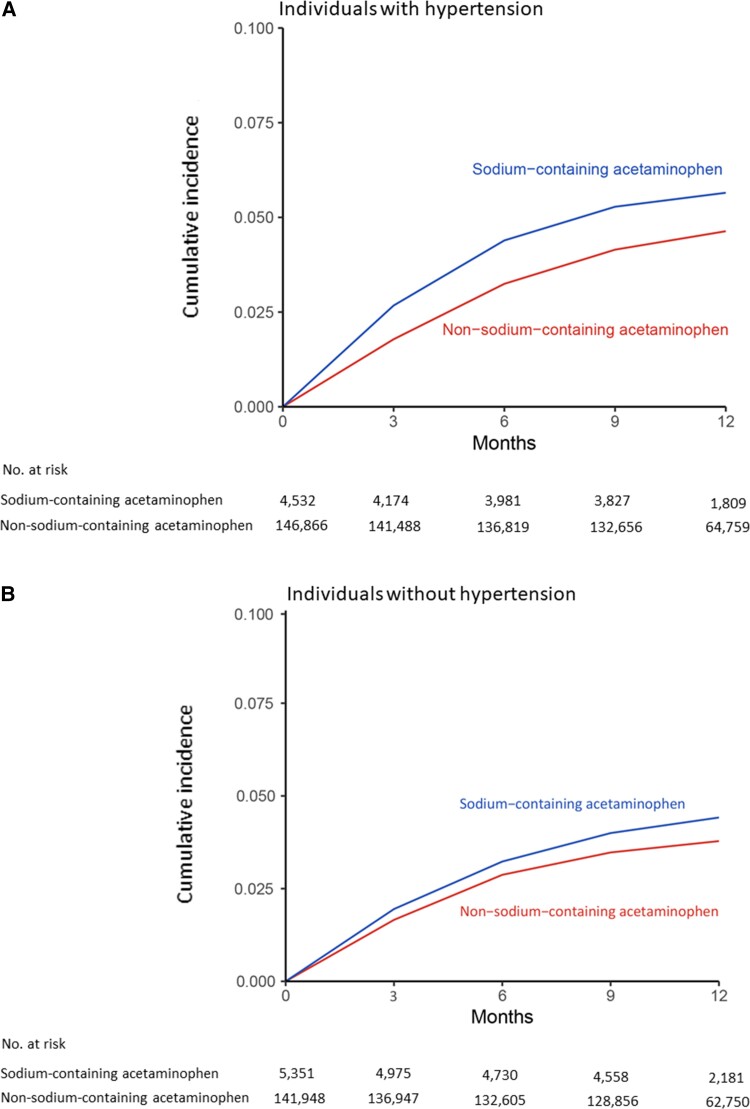
The risk of incident CVD was higher among sodium-containing acetaminophen initiators than among non-sodium-containing acetaminophen initiators regardless of hypertension (Figure 1). Among individuals with hypertension, 122 cases of CVD (1-year risk: 5.6%) occurred in the sodium-containing acetaminophen group and 3051 (1-year risk: 4.6%) in the non-sodium-containing acetaminophen group during the 1-year follow-up period (Table 2). The RD of CVD was 1.0% (95% CI 0.9–1.1%). The average weighted HR of CVD was 1.59 (95% CI 1.32–1.92). The average weighted HRs were 1.41 (95% CI 1.28–1.56) for MI, 1.59 (95% CI 1.20–2.09) for stroke, and 1.44 (95% CI 1.13–1.83) for heart failure, respectively. Among individuals without hypertension, 105 cases of CVD (1-year risk: 4.4%) occurred in the sodium-containing acetaminophen initiators and 2079 (1-year risk: 3.7%) in the non-sodium-containing acetaminophen initiators (Table 2). Compared with non-sodium-containing acetaminophen, the RD and HR of CVD for sodium-containing acetaminophen were 0.7% (95% CI 0.6–0.8%) over 1 year and 1.45 (95% CI 1.18–1.79), respectively. Sodium-containing acetaminophen was associated with higher risks of MI (HR 1.27; 95% CI 1.17–1.38), stroke (HR 1.43; 95% CI 1.08–1.91), and heart failure (HR 1.30; 95% CI 1.14–1.57). Based on the smaller numbers than for acetaminophen-containing drugs, the risk of incident CVD was also higher among sodium-containing ibuprofen or ranitidine initiators than among initiators of non-sodium-containing formulations of the same drug regardless of history of hypertension (see Supplementary material online, Table S2).
Figure 1.
Time to cardiovascular disease for patients initiating sodium-containing acetaminophen or non-sodium-containing acetaminophen among individuals: (A) with prevalent hypertension and (B) without prevalent hypertension.
Table 2.
Incident cardiovascular disease according to the hypertension status within 1 year among patients initiating sodium-containing or non-sodium-containing acetaminophen
| With a history of hypertension | Without a history of hypertension | |||
|---|---|---|---|---|
| Sodium-containing acetaminophen | Non-sodium-containing acetaminophen | Sodium-containing acetaminophen | Non-sodium-containing acetaminophen | |
| Composite cardiovascular disease | ||||
| Participant, no. | 4532 | 146 866 | 5351 | 141 948 |
| Event no. | 122 | 3051 | 105 | 2079 |
| Mean follow-up (years) | 0.89 | 0.93 | 0.89 | 0.94 |
| One-year risk, % | 5.6 | 4.6 | 4.4 | 3.7 |
| IPW RD (95% CI), % | 1.0 (0.9–1.1) | 0.0 (reference) | 0.7 (0.6–0.8) | 0.0 (reference) |
| Average weighted HR (95% CI) | 1.59 (1.32–1.92) | 1.00 (reference) | 1.45 (1.18–1.79) | 1.00 (reference) |
| Restricting to intake of 3–4 g/day | 1.71 (1.61–1.81) | 1.00 (reference) | 1.42 (1.32–1.52) | 1.00 (reference) |
| Missing data imputation | 1.23 (1.19–1.27) | 1.00 (reference) | 1.16 (1.13–1.20) | 1.00 (reference) |
| Restricting to osteoarthritis | 1.82 (1.65–2.00) | 1.00 (reference) | 1.26 (1.17–1.37) | 1.00 (reference) |
| Excluding other sodium-containing medications | 1.47 (1.40–1.54) | 1.00 (reference) | 1.22 (1.15–1.29) | 1.00 (reference) |
| Myocardial infarctiona | ||||
| Participant, no. | 5281 | 165 556 | 5819 | 150 971 |
| Event no. | 32 | 1019 | 29 | 680 |
| Mean follow-up (years) | 0.88 | 0.93 | 0.89 | 0.94 |
| One-year risk, % | 2.2 | 1.8 | 1.7 | 1.5 |
| IPW RD (95% CI), % | 0.4 (0.3–0.5) | 0.0 (reference) | 0.2 (0.1–0.3) | 0.0 (reference) |
| Average weighted HR (95% CI) | 1.41 (1.28–1.56) | 1.00 (reference) | 1.27 (1.17–1.38) | 1.00 (reference) |
| Strokea | ||||
| Participant, no. | 5228 | 167 761 | 5816 | 156 102 |
| Event no. | 57 | 1302 | 47 | 936 |
| Mean follow-up (years) | 0.89 | 0.94 | 0.89 | 0.94 |
| One-year risk, % | 2.6 | 1.9 | 2.0 | 1.4 |
| IPW RD (95% CI), % | 0.7 (0.6–0.8) | 0.0 (reference) | 0.6 (0.4–0.8) | 0.0 (reference) |
| Average weighted HR (95% CI) | 1.59 (1.20–2.09) | 1.00 (reference) | 1.43 (1.08–1.91) | 1.00 (reference) |
| Heart failurea | ||||
| Participant, no. | 5462 | 170 725 | 5946 | 156 135 |
| Event no. | 70 | 1889 | 46 | 1074 |
| Mean follow-up (years) | 0.88 | 0.93 | 0.89 | 0.94 |
| One-year risk, % | 2.6 | 2.3 | 1.7 | 1.5 |
| IPW RD (95% CI), % | 0.3 (0.2–0.4) | 0.0 (reference) | 0.2 (0.1–0.3) | 0.0 (reference) |
| Average weighted HR (95% CI) | 1.44 (1.13–1.83) | 1.00 (reference) | 1.30 (1.14–1.57) | 1.00 (reference) |
HR, hazard ratio; RD, risk difference; IPW, inverse probability weighting; CI, confidence interval.
When we estimated the risk of a specific cardiovascular disease outcome (e.g. myocardial infarction), participants were excluded if they had a recorded history of this specific cardiovascular disease (e.g. myocardial infarction), and the recorded history of the other two cardiovascular diseases (e.g. stroke and heart failure) were considered as covariates.
The results from all sensitivity analyses were consistent with those from the main analyses (Table 2). To completely nullify the observed associations (e.g. HRs of 1.59 and 1.45 for the smallest effect estimate among hypertensive and non-hypertensive individuals, respectively), the OR of residual confounder(s) with either sodium-containing acetaminophen or with CVD must be ≥2.56 among hypertensive participants and ≥2.26 among non-hypertensive participants, respectively. Such strong residual confounder(s) seems unlikely given that many known confounders have been accounted for in the analysis. In addition, sodium-containing acetaminophen was associated with a higher risk of CVD regardless of warfarin use, although the association was stronger among participants with a warfarin prescription (P for interaction = 0.001) (see Supplementary material online, Table S3).
Mortality
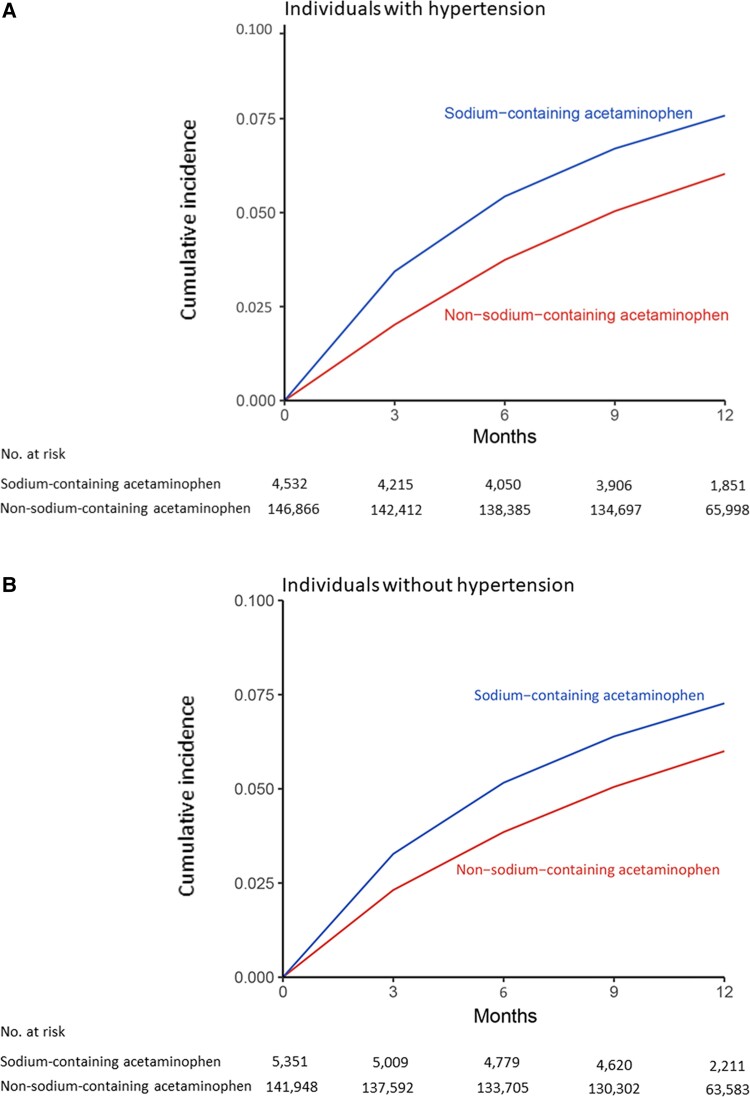
Mortality was higher in sodium-containing acetaminophen initiators than in non-sodium-containing acetaminophen initiators regardless of hypertension (Figure 2). As shown in Table 3, during the 1-year follow-up period, 404 deaths (1-year risk: 7.6%) occurred in the sodium-containing acetaminophen initiators and 5510 (1-year risk: 6.1%) in the non-sodium initiators among individuals with hypertension. The RD of mortality was 1.6% (95% CI 1.5–1.7%) over 1 year. The average weighted HR was 2.05 (95% CI 1.92–2.19). Similar associations were observed among individuals without hypertension. The mortality was higher among initiators of sodium-containing ibuprofen or ranitidine than among initiators of non-sodium-containing formulations of the same drug regardless of hypertension (see Supplementary material online, Table S4).
Figure 2.
Time to death for patients initiating sodium-containing acetaminophen or non-sodium-containing acetaminophen among individuals: (A) with prevalent hypertension and (B) without prevalent hypertension.
Table 3.
All-cause mortality according to the hypertension status within 1 year among patients initiating sodium-containing or non-sodium-containing acetaminophen
| With a history of hypertension | Without a history of hypertension | |||
|---|---|---|---|---|
| Sodium-containing acetaminophen | Non-sodium-containing acetaminophen | Sodium-containing acetaminophen | Non-sodium-containing acetaminophen | |
| Participant, no. | 4532 | 146 866 | 5351 | 141 948 |
| Event no. | 404 | 5510 | 517 | 5190 |
| Mean follow-up (years) | 0.90 | 0.94 | 0.90 | 0.94 |
| One-year risk, % | 7.6 | 6.1 | 7.3 | 5.9 |
| IPW RD (95% CI), % | 1.6 (1.5–1.7) | 0.0 (reference) | 1.4 (1.2–1.6) | 0.0 (reference) |
| Average weighted HR (95% CI) | 2.05 (1.92–2.19) | 1.00 (reference) | 1.87 (1.74–2.00) | 1.00 (reference) |
| Restricting to intake of 3–4 g/day | 2.67 (2.19–3.27) | 1.00 (reference) | 2.20 (1.90–2.54) | 1.00 (reference) |
| Missing data imputation | 2.26 (2.21–2.31) | 1.00 (reference) | 2.12 (2.07–2.18) | 1.00 (reference) |
| Restricting to osteoarthritis | 2.07 (1.56–2.75) | 1.00 (reference) | 1.96 (1.83–2.10) | 1.00 (reference) |
| Excluding other sodium-containing medications | 2.49 (2.41–2.57) | 1.00 (reference) | 2.22 (2.15–2.30) | 1.00 (reference) |
HR, hazard ratio; RD, risk difference; IPW, inverse probability weighting; CI, confidence interval.
Sensitivity analyses did not change the results materially (Table 3). In addition, to completely nullify the observed associations, the OR of residual confounder(s) with either sodium-containing acetaminophen or with mortality must be ≥3.52 among hypertensive participants and ≥3.15 among non-hypertensive participants, respectively. In addition, sodium-containing acetaminophen was associated with a higher risk of mortality regardless of warfarin use, although its effect was larger among warfarin users than non-users of warfarin (P for interaction = 0.001) (see Supplementary material online, Table S3).
Dose–response relationship
As shown in Supplementary material online, Table S5, there was a dose–response relationship between the number of sodium-containing acetaminophen prescriptions and the risk of CVD. Compared with non-sodium-containing acetaminophen, the ORs of CVD for 1, 2–4, and ≥5 prescriptions of sodium-containing acetaminophen were 1.26, 1.33, and 1.45, respectively (P for trend = 0.034), and the corresponding ORs of mortality were 2.77, 3.02, and 3.64, respectively (P for trend <0.001) among individuals with hypertension. Similar findings were observed among individuals without hypertension (see Supplementary material online, Table S5).
Incident hypertension
The risk of incident hypertension was higher in the sodium-containing acetaminophen initiators than in the non-sodium-containing acetaminophen initiators (see Supplementary material online, Figure S3). As shown in Supplementary material online, Table S6, 246 cases of hypertension (1-year risk: 4.4%) occurred among the sodium-containing acetaminophen initiators and 5941 (1-year risk: 3.6%) among the non-sodium-containing acetaminophen initiators over the 1-year follow-up period [RD 0.8% (95% CI 0.6–1.0%) and HR 1.37 (95% CI 1.22–1.54)].
Discussion
In this large population-based cohort study, sodium-containing acetaminophen was associated with a statistically significant higher risk of incident CVD and mortality than non-sodium-containing acetaminophen initiation among individuals with and without hypertension. The risk of CVD and mortality increased as the duration of sodium-containing acetaminophen increased (Structured Graphical Abstract). Our findings were consistent in several sensitivity analyses, indicating that observed associations were robust.
Comparison with previous studies
Many studies have examined the association of habitual sodium intake (e.g. urinary sodium excretion) and risks of CVD and mortality, but evidence among non-hypertensive individuals has been conflicting.3–12 Observational studies of the effect of habitual sodium intake on the risk of CVD or mortality are susceptible to potential survival bias, especially in studies conducted among older adults. To overcome this difficulty, we conducted a cohort study mimicking a randomized controlled trial40 to assess an incident exposure of extra sodium intake and provide real-world evidence that extra high sodium intake increases the risks of CVD and mortality among non-hypertensive individuals.
Notably, a nested case–control study using the UK Clinical Practice Research Datalink (CPRD) database found that exposure to sodium-containing medicines was associated with significantly increased odds of adverse cardiovascular events compared with standard formulations of those same drugs.16 By uncovering such iatrogenic sodium load, the findings are of public health importance.16 However, owing to the limitations of case–control studies,41,42 the absolute risk of CVD or mortality according to the exposure to sodium-containing medicines among non-hypertensive individuals remains unknown. In the current study, we used incident exposure to minimize potential selection bias and an active comparator as well as IPW (see PS distribution in Supplementary material online, Table S7) to reduce potential confounding effects.40 The cohort study design allowed us to estimate the risk of CVD and mortality according to the use of sodium-containing acetaminophen and the RD between sodium-containing acetaminophen and its comparator. These measures have potential public health implications that a case–control study is unable to provide.
Biological mechanism
Numerous studies have shown that a high sodium intake increases the risk of hypertension.43,44 Since hypertension is one of the strongest risk factors for CVD,45 it is plausible that the sodium-containing acetaminophen would increase the risks of CVD and mortality among normotensive individuals through increased blood pressure. Indeed, results from a crossover randomized controlled trial found that the sodium-containing acetaminophen intervention resulted in an increase of 24 h ambulatory blood pressure [5.04 mmHg (95% CI 1.80–8.28)] compared with the non-sodium-containing acetaminophen intervention during the 3-week period.17 Nevertheless, the effervescent tablet of sodium-containing acetaminophen contained sodium bicarbonate, not sodium chloride, and findings of the effect of sodium bicarbonate on blood pressure are inconclusive.46 In addition, our study reported a higher risk of CVD from sodium-containing acetaminophen among individuals with hypertension (HR 1.59, RD 10.4%) than that among individuals without hypertension (HR 1.45, RD 7.1%), suggesting that individuals with a history of hypertension may be more susceptible to sodium detrimental effect. This result is consistent with the findings that hypertension is a major and recognized trait associated with salt sensitivity of blood pressure.47,48 Thus, future observational studies should consider genetic traits or salt sensitivity as major cofounders or effect measure modifiers when assessing the effect of salt intake on the risk of CVD or mortality.
Several other mechanisms have also been postulated. First, the role of inflammatory mechanisms in mediating the damage of salt on the endothelium is increasingly recognized and the suppressive effect of salt on the endothelial function has been demonstrated to be independent of blood pressure.49–51 Second, a high salt intake can undermine the course and balance of the immune response by promoting the development of macrophage and T cells with proinflammatory functions.52,53 It induces pathogenic interleukin 17, thereby decreasing bioavailable nitric oxide, impairing vasodilation, and increasing vascular stiffness, resulting in endothelial dysfunction and elevations in systematic vascular resistance.54,55 Third, the gut microbiome has been recently proposed as a key moderator of the effect of salt on intermediate mechanisms (e.g. inflammation) and health outcomes (e.g. CVD).56,57
Limitations
Potential limitations of our study include the lack of urinary sodium excretion or dietary sodium intake data. Second, although we controlled for many potential confounders, residual confounding (e.g. genetic traits)47,48,58 cannot be ruled out in an observational study. Third, because the use of over-the-counter acetaminophen was not recorded in THIN, our exposure assessment is susceptible to misclassification bias. However, such bias, if it occurred, was likely to be non-differential and dilute the observed association. In addition, the sensitivity analysis restricting to participants aged ≥60 years did not change the results materially. Fourth, as the cause of death was not recorded in THIN, we could not assess the association between sodium-containing acetaminophen and cause-specific mortality. Fifth, physician-ordered prescriptions may not reflect the actual medication use by patients. Sixth, using Read codes to diagnose hypertension might miss some hypertension cases. Finally, restricting participants with individuals who had been prescribed acetaminophen may limit the generalizability of our findings.
Clinical implications
Sodium-containing drugs are an important source of sodium intake that could be easily overlooked. Acetaminophen is one of the most widely used analgesics worldwide and can be purchased over-the-counter.16,19 Although the US Food and Drug Administration requires all over-the-counter medications to label the sodium content, to our knowledge, the potentially detrimental effect of sodium-containing acetaminophen on the risks of hypertension, CVD, and mortality has not been issued a warning. Given that the pain-relief of non-sodium-containing acetaminophen is similar to that of sodium-containing acetaminophen,17,59 our results suggest re-visiting the safety profile of effervescent and soluble acetaminophen use. Large observational studies mimicking a randomized controlled trial,40 such as the present study, can provide real-world empirical evidence for public health and clinical care in the absence of clinical trials.
Conclusions
In this population-based cohort study, the initiation of sodium-containing acetaminophen was associated with increased risks of CVD and mortality among individuals with or without hypertension. Our findings suggest that individuals should avoid unnecessary excessive sodium intake through sodium-containing acetaminophen use.
Authors’ contributions
Y.Z. and G.L. had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. G.L. and Y.Z. are joint corresponding authors. All authors have read, provided critical feedback on intellectual content, and approved the final manuscript. G.L., Y.Z., and C.Z. conceptualized and designed the study. C.Z., L.R., X.L., L.D., J.W., G.L., and Y.Z. are involved in acquisition, analysis, or interpretation of the data. C.Z., G.L., and Y.Z. drafted the manuscript, and provided administrative, technical, or material support. L.R., X.L., L.D., J.W., G.L., and Y.Z. critically revised the manuscript for important intellectual content. X.L., J.W., and Y.Z. are involved in statistical analysis of the data. C.Z., J.W., and G.L. obtained funding.. G.L. and Y.Z. supervised the study.
Supplementary Material
Acknowledgements
Everyone who contributed significantly to the work has been listed.
Contributor Information
Chao Zeng, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China; Division of Rheumatology, Allergy, and Immunology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; The Mongan Institute, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Lynn Rosenberg, Slone Epidemiology Center at Boston University, Boston, MA, USA.
Xiaoxiao Li, Hunan Key Laboratory of Joint Degeneration and Injury, Changsha, China.
Luc Djousse, Division of Aging, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Jie Wei, Division of Rheumatology, Allergy, and Immunology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; The Mongan Institute, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Health Management Center, Xiangya Hospital, Central South University, Changsha, China.
Guanghua Lei, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China; Hunan Key Laboratory of Joint Degeneration and Injury, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
Yuqing Zhang, Division of Rheumatology, Allergy, and Immunology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; The Mongan Institute, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the National Natural Science Foundation of China (81772413, 81930071, 81902265, and 82072502), the National Key Research and Development Project (2018YFB1105700), the Project Program of National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, 2020LNJJ03), the Key Research and Development Program of Hunan Province (2018SK2070 and 2018SK2071), and the Science and Technology Program of Hunan Province (2019RS2010). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
none declared.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for the design or implementation of the study. No patients were asked to advice on interpretation or writing up of results. Dissemination of the findings to participants is not possible owing to the use of an anonymized data set.
Role of the funder/sponsor
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Ethical approval
This study received approval from the Medical Ethical Committee at the Xiangya Hospital, Central South University, China, with a waiver of informed consent.
Scientific approval
This study was approved by THIN Scientific Review Committee (20SRC026).
Statement
THIN is a registered trademark of Cegedim SA in the UK and other countries. Reference made to THIN database is intended to be descriptive of the data asset licensed by the IQVIA. This work uses de-identified data provided by patients as a part of their routine primary care.
Disclaimer
The interpretation of these data is the sole responsibility of the authors.
Transparency
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014;371:624–634. [DOI] [PubMed] [Google Scholar]
- 2. Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013;3:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014;371:612–623. [DOI] [PubMed] [Google Scholar]
- 4. Joosten MM, Gansevoort RT, Mukamal KJ, Lambers Heerspink HJ, Geleijnse JM, Feskens EJ, et al. Sodium excretion and risk of developing coronary heart disease. Circulation 2014;129:1121–1128. [DOI] [PubMed] [Google Scholar]
- 5. Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw K-T. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur J Heart Fail 2014;16:394–402. [DOI] [PubMed] [Google Scholar]
- 6. Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet 2016;388:465–475. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, Oparil S, Whelton PK, McKee M, Dominiczak A, Luft FC, et al. The technical report on sodium intake and cardiovascular disease in low- and middle-income countries by the joint working group of the World Heart Federation, the European Society of Hypertension and the European Public Health Association. Eur Heart J 2017;38:712–719. 10.1093/eurheartj/ehw549. [DOI] [PubMed] [Google Scholar]
- 8. Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med 2009;169:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 2014;129:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook NR, Appel LJ, Whelton PK. Sodium intake and all-cause mortality over 20 years in the trials of hypertension prevention. J Am Coll Cardiol 2016;68:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao X, Yang X, Zhang X, Li Y, Zhao X, Ren L, et al. Dietary salt intake and coronary atherosclerosis in patients with prehypertension. J Clin Hypertens 2014;16:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lelli D, Antonelli-Incalzi R, Bandinelli S, Ferrucci L, Pedone C. Association between sodium excretion and cardiovascular disease and mortality in the elderly: a cohort study. J Am Med Dir Assoc 2018;19:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang L, Tian M, Yu J, Li Q, Liu Y, Yin X, et al. Interim effects of salt substitution on urinary electrolytes and blood pressure in the China Salt Substitute and Stroke Study (SSaSS). Am Heart J 2020;221:136–145. [DOI] [PubMed] [Google Scholar]
- 15. Neal B, Wu Y, Feng X, Zhang R, Zhang Y, Shi J, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med 2021;385:1067–1077. [DOI] [PubMed] [Google Scholar]
- 16. George J, Majeed W, Mackenzie IS, Macdonald TM, Wei L. Association between cardiovascular events and sodium-containing effervescent, dispersible, and soluble drugs: nested case-control study. BMJ 2013;347:f6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benitez-Camps M, Morros Padrós R, Pera-Pujadas H, Dalfó Baqué A, Bayó Llibre J, Rebagliato Nadal O, et al. Effect of effervescent paracetamol on blood pressure: a crossover randomized clinical trial. J Hypertens 2018;36:1656–1662. [DOI] [PubMed] [Google Scholar]
- 18. Ju C, Wei L, Mackenzie IS, MacDonald TM, George J. Changes in prescribing rates of sodium-containing medications in the UK from 2009 to 2018: a cross-sectional study with interrupted time series analysis. BMJ Open 2021;11:e043566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perrin G, Berdot S, Thomas F, Pannier B, Danchin N, Durieux P, et al. Evaluation of exposure to effervescent drugs in a large health check-up population in France: a cross-sectional study. BMJ Open 2018;8:e022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. UK Medicines Information . What is the sodium content of medicines? https://www.sps.nhs.uk/articles/about-ukmi-medicines-qas/ (18 May 2018.).
- 21. Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007;16:393–401. [DOI] [PubMed] [Google Scholar]
- 22. Chisholm J. The Read clinical classification. BMJ 1990;300:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. First Databank. Multilex. https://www.fdbhealth.co.uk/solutions/multilex-clinical-decision-support (20 February 2017).
- 24. Choi HK, Nguyen U-S, Niu J, Danaei G, Zhang Y. Selection bias in rheumatic disease research. Nat Rev Rheumatol 2014;10:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herrett E, Gadd S, Jackson R, Bhaskaran K, Williamson E, van Staa T, et al. Eligibility and subsequent burden of cardiovascular disease of four strategies for blood pressure-lowering treatment: a retrospective cohort study. Lancet 2019;394:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gamble J-M, Chibrikov E, Twells LK, Midodzi WK, Young SW, MacDonald D, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017;5:43–52. [DOI] [PubMed] [Google Scholar]
- 27. Wright AK, Kontopantelis E, Emsley R, Buchan I, Mamas MA, Sattar N, et al. Cardiovascular risk and risk factor management in Type 2 diabetes mellitus. Circulation 2019;139:2742–2753. [DOI] [PubMed] [Google Scholar]
- 28. Zeng C, Dubreuil M, LaRochelle MR, Lu N, Wei J, Choi HK, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA 2019;321:969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Serumaga B, Ross-Degnan D, Avery AJ, Elliott RA, Majumdar SR, Zhang F, et al. Effect of pay for performance on the management and outcomes of hypertension in the United Kingdom: interrupted time series study. BMJ 2011;342:d108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, et al. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol 2017;70:1429–1437. [DOI] [PubMed] [Google Scholar]
- 31. Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L. Bariatric surgery in the United Kingdom: a cohort study of weight loss and clinical outcomes in routine clinical care. PLoS Med 2015;12:e1001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Webster-Clark M, Sturmer T, Wang T, Man K, Marinac-Dabic D, Rothman KJ, et al. Using propensity scores to estimate effects of treatment initiation decisions: state of the science. Stat Med 2021;40:1718–1735. [DOI] [PubMed] [Google Scholar]
- 33. Mansournia MA, Altman DG. Inverse probability weighting. BMJ 2016;352:i189. [DOI] [PubMed] [Google Scholar]
- 34. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 35. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–570. [DOI] [PubMed] [Google Scholar]
- 36. Emilsson L, García-Albéniz X, Logan RW, Caniglia EC, Kalager M, Hernán MA. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol 2018;4:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Danaei G, García Rodríguez LA, Cantero OF, Logan RW, Hernán MA. Electronic medical records can be used to emulate target trials of sustained treatment strategies. J Clin Epidemiol 2018;96:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 39. Mahé I, Caulin C, Bergmann J-F. Does paracetamol potentiate the effects of oral anticoagulants?: a literature review. Drug Saf 2004;27:325–333. [DOI] [PubMed] [Google Scholar]
- 40. Dickerman BA, García-Albéniz X, Logan RW, Denaxas S, Hernán MA. Avoidable flaws in observational analyses: an application to statins and cancer. Nat Med 2019;25:1601–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schneeweiss S, Suissa S. Discussion of Schuemie, et al: “A plea to stop using the case-control design in retrospective database studies”. Stat Med 2019;38:4209–4212. [DOI] [PubMed] [Google Scholar]
- 42. Dickerman BA, Garcia-Albeniz X, Logan RW, Denaxas S, Hernan MA. Emulating a target trial in case-control designs: an application to statins and colorectal cancer. Int J Epidemiol 2020;49:1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mente A, O’Donnell M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet 2018;392:496–506. [DOI] [PubMed] [Google Scholar]
- 44. Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 2014;371:601–611. [DOI] [PubMed] [Google Scholar]
- 45. Nabel EG, Guttmacher AE, Collins FS. Cardiovascular disease. N Engl J Med 2003;349:60–72. [DOI] [PubMed] [Google Scholar]
- 46. Uzoigwe OF. Too early to attach blanket health warnings to all drugs containing sodium, irrespective of conjugate anion. BMJ 2014;348:g1404. [DOI] [PubMed] [Google Scholar]
- 47. Kotchen TA, Cowley AW Jr, Frohlich ED. Salt in health and disease—a delicate balance. N Engl J Med 2013;368:1229–1237. [DOI] [PubMed] [Google Scholar]
- 48. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 49. Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol 2012;590:5519–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, et al. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 2013;31:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matthews EL, Brian MS, Ramick MG, Lennon-Edwards S, Edwards DG, Farquhar WB. High dietary sodium reduces brachial artery flow-mediated dilation in humans with salt-sensitive and salt-resistant blood pressure. J Appl Physiol (1985) 2015;118:1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013;496:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, et al. Sodium chloride inhibits the suppressive function of FOXP3 + regulatory T cells. J Clin Invest 2015;125:4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013;496:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 2015;116:1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci 2018;21:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017;551:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 59. Møller PL, Norholt SE, Ganry HE, Insuasty JH, Vincent FG, Skoglund LA, et al. Time to onset of analgesia and analgesic efficacy of effervescent acetaminophen 1000 mg compared to tablet acetaminophen 1000 mg in postoperative dental pain: a single-dose, double-blind, randomized, placebo-controlled study. J Clin Pharmacol 2000;40:370–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.