Abstract
Aims
The prevalence of chronic limb-threatening ischaemia (CLTI) is increasing and available data often derive from cohorts with various selection criteria. In the present study, we included CLTI patients and studied sex-related differences in their risk profile, vascular procedures, and long-term outcome.
Methods and results
We analysed 199 953 unselected patients of the largest public health insurance in Germany (AOK: Local healthcare funds), hospitalized between 2010 and 2017 for a main diagnosis of CLTI. A baseline period of 2 years before index hospitalization to assess comorbidities and previous procedures, and a follow-up period until 2018 were included. Female CLTI patients were older (median 81.4 vs. 73.8 years in males; P < 0.001) and more often diagnosed with hypertension, atrial fibrillation, chronic heart failure, and chronic kidney disease. Male patients suffered more frequently from diabetes mellitus, dyslipidaemia, smoking, cerebrovascular disease, and chronic coronary syndrome (all P < 0.001). Within hospitalized CLTI patients, females represent the minority (43% vs. 57%; P < 0.001) and during index hospitalization, women underwent less frequently diagnostic angiographies (67 vs. 70%) and revascularization procedures (61 vs. 65%; both P < 0.001). Moreover, women received less frequently guideline-recommended drugs like statins (35 vs. 43%) and antithrombotic therapy (48 vs. 53%; both P < 0.001) at baseline. Interestingly, after including age and comorbidities in a Cox regression analysis, female sex was associated with increased overall-survival (OS) [hazard ratio (HR) 0.95; 95% confidence interval (CI) 0.94–0.96] and amputation-free survival (AFS) (HR 0.84; 95% CI 0.83–0.85; both P < 0.001).
Conclusion
Female patients with CLTI were older, underwent less often vascular procedures, and received less frequently guideline-recommended medication. Nevertheless, female sex was independently associated with better OS and AFS during follow-up.
Keywords: Overall-survival, Amputation-free-survival, LEAD, CLTI, Sex differences, Outcome research
Structured Graphical Abstract
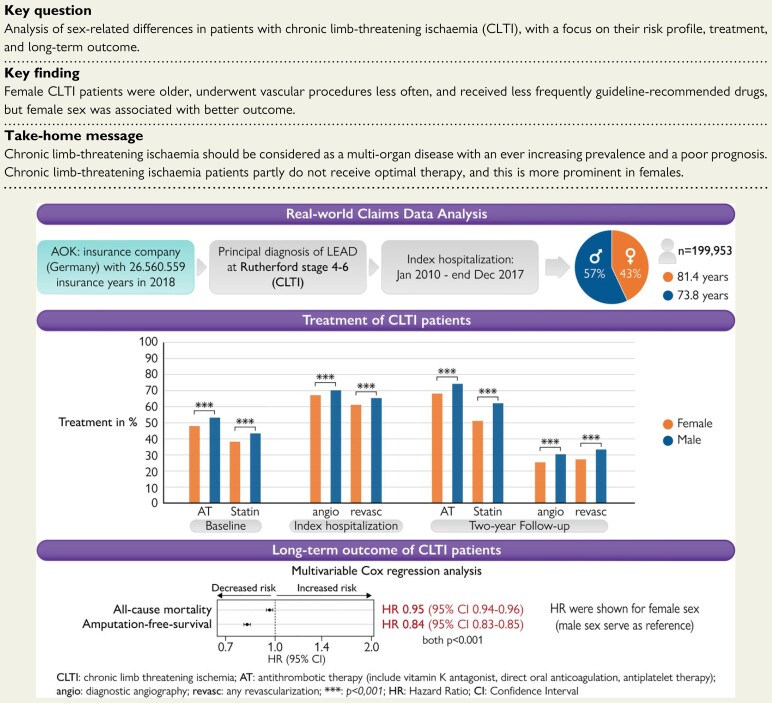
An analysis of 199 953 unselected CLTI patients of the largest public health insurance in Germany (AOK) showed that female CLTI patients were older and display the minority of the hospitalized CLTI patients. During index hospitalization and during follow-up period, women underwent less frequently any vascular procedure (diagnostic angiography or revascularization procedures) and, moreover, received less frequently guideline-recommended drugs like statins or antithrombotic therapy. Interestingly, after including age and comorbidities in a Cox regression analysis, female sex was associated with increased OS and AFS.
See the editorial comment for this article ‘Sex equality or sex equity: what are we looking for in treating limb-threatening ischaemia?’, by David Kingsmore and Julie Brittenden, https://doi.org/10.1093/eurheartj/ehac047.
Introduction
The prevalence of lower extremity artery disease (LEAD) is increasing and affecting approximately over 200 million people worldwide.1 LEAD is caused by atherosclerotic-induced narrowing or occlusion of the arteries in the lower limbs (LL) leading to impaired blood flow.2 Symptoms can vary from intermittent claudication (IC) to chronic limb-threatening ischaemia (CLTI) with rest pain, ulcerations or gangrene.3
In 2009, it was recorded that 3% of all hospitalizations in Germany were due to LEAD, while accounting for 4.8% of all expenses,4 impressively demonstrating the high financial burden for health systems. The Heinz-Nixdorf recall study showed a prevalence of LEAD in men of 3% in the age group 45–49 years, increasing to 18.2% in patients between 70 and 75 years (for women: 2.7% in patients 45–49 years old and 10.8% in patients between 70 and 75 years), this points out that age is at least one risk indicator for LEAD.5 Furthermore, several cardiovascular risk factors (CVRFs) like hypertension, dyslipidaemia, smoking, or diabetes mellitus (DM)6–10 are more common in patients with LEAD. Beyond that, LEAD patients have a three- to six-fold higher risk for the development of atherosclerotic diseases like chronic coronary syndrome (CCS) and cerebrovascular disease (CVD),11,12 since the prevalence of CCS is up to 90% in CLTI patients, while CVD occurs in 60% of the patients.13 Furthermore, the fact that most LEAD patients present with at least one or more other cardiovascular manifestations increases the risk for major adverse cardiovascular events (MACE).13–15 Moreover, LEAD patients have a poor prognosis in terms of amputation of the LL and mortality rates. While the mortality rate of IC patients at the Rutherford stage (RF) 1–3 is ∼20% 4 years after diagnosis,16,17 the rate is rising to 40.1% in RF 4, 55.0% in RF 5, and 68.5% in RF 6.16,18 The 4-year amputation rate of the LL in patients with IC (RF 1–3) is ∼5% and is also increasing in CLTI patients (12.1, 35.3, and 67.3% in LEAD patients at RF 4, 5, and 6).16 These data indicate the high psychological strain in this patient group, especially in patients with CLTI.
It is assumed that the prevalence of LEAD, based on the ankle-brachial index (ABI), is equal in both sexes. This is probably caused due to the fact that women more often present with borderline ABI and longer symptom-free periods compared with their male counterparts.19–21 In IC patients, approximately two-thirds of the patients are male,22,23 while in CLTI patients, the prevalence is only marginal lower in women compared with men.16,24–26 Various studies have shown that women are older23,27,28 and older patients, as well as women, receive less often guideline-recommended medication.29 In addition, women receive less often any revascularization, while endovascular approaches were more often in female patients. In contrast, atherectomy, the use of stents and open surgical revascularization was more common in men.23,24,30
Most clinical trials include only small patient cohorts and, moreover, women are often underrepresented in these trials.29,31 Different groups focused on secondary data analysis with mostly other inclusion criteria. For example, there exist secondary data analyses, which include outpatient data of LEAD patients,32 hospitalized LEAD patients with the performance of vascular procedures,27,28,30 or include only the index hospitalization without any follow-up period.33 This leads to limited knowledge about the impact of sex on diagnosis, therapy, and outcome in CLTI patients. For a better understanding of these special groups, we analysed health claims data of the largest public German insurance.
Methods
Anonymized patient data were retrieved from the AOK (Allgemeine Ortskrankenkasse) Local healthcare funds (in the following described as AOK); a system of 11 regional health care funds in Germany, with more than 26 million insured persons (corresponding to 26 560 559 insurance years; data from 2018). Enrolment in the AOK is open to any inhabitant regardless of region, profession, income, age, or health status. Data of 199 953 patients with an index hospitalization, due to a principal diagnosis of CLTI (RF 4–6), between 1 January 2010 until 31 December 2017 were included. The data included all in- and outpatient data 2 years prior index hospitalization and a follow-up period until 31 December 2018 (see Supplementray material online, Figure S1). For analyses of main and secondary diagnosis, the ICD-10 German Modification (ICD-10-GM) was used. For analyses of vascular procedures, the German procedure classification system (OPS) was considered. For analyses of prescribed pharmaceuticals, the anatomical therapeutic chemical classification system (ATC) was used (for distinct codes, see Supplementary material online, Table S1; for further details, see Reinecke et al.16). The institutional review board has been informed and approved unreserved usage of the retrospective anonymized data sets provided by the research institute of the AOK (WIdO; file reference: 2019-212-f-S; ethics committee Muenster, Germany).
Patient cohort
All patients aged ≥18 years, with a principal diagnosis of LEAD at RF 4–6 in the index period from 1 January 2010 to 31 December 2017, were included for further analyses. If a patient was repeatedly hospitalized during the index period, only the first hospitalization was used as index hospitalization. Patients with implausible or incomplete data, like implausible entry or discharge dates, implausible date of death or birth, a gap of the insurance coverage during baseline, implausible sex in their patient file during baseline, or index hospitalization were excluded. If incomplete data were noticed during follow-up, the patient was censored from this time point ongoing.
Baseline characteristics for each selected patient were requested 2 years prior index hospitalization including CVRFs and comorbidities, as well as vascular procedures were included, if encoded at least once during in- or outpatient area. This proceeding was also applied to the index period and follow-up. Prescribed medication at baseline was recognized, if it was encoded at least two times in two different quartiles. During follow-up, one prescription during index or follow-up period was counted for the distinct medication.
As primary endpoints, the overall-survival (OS), amputation-free-survival (AFS), and freedom from amputation were defined. Moreover, complications including acute myocardial infarction (AMI), bleeding, infection or acute renal failure, as well as the need for vascular procedures and prescription of guideline-recommended medication were used as secondary endpoints.
Data availability
The authors confirm that the data utilized in this study cannot be made available in the manuscript, the Supplementary material online, or in a public repository due to German data protection laws (‘Bundesdatenschutzgesetz’, BDSG). Therefore, they are stored on a secure drive in the WIdO, to facilitate replication of the results. Generally, access to data of statutory health insurance funds for research purposes is possible only under the conditions defined in German Social Law (SGB V § 287). Requests for data access can be sent as a formal proposal specifying the recipient and purpose of the data transfer to the appropriate data protection agency. Access to the data used in this study can only be provided to external parties under the conditions of the cooperation contract of this research project and after written approval by the sickness fund. For assistance in obtaining access to the data, contact wido@wido.bv.aok.de.
Statistics
The endpoints OS, AFS, and freedom from amputation were analysed using multivariable time-dependent Cox regression models in a full model (all patients) and with sex-interaction terms for each risk factor. The models for freedom from amputation considered death as a competing risk in contrast to the AFS models, which included death in the endpoint. For this purpose, we used Fine and Gray's method to estimate sub-distributional hazard ratios (HRs) in the AFS model.
The models included risk profiles of patients at baseline and additionally time-dependent occurrence of comorbidities or procedures in the follow-up period. As our primary objective, differences in the features of the outcomes between female and male sex were analysed. Thus, we evaluated the interaction of sex with all variables in the Cox regression models. Two-sided P-values for the test of interaction of the two models were jointly adjusted using the Benjamini–Hochberg procedure34 to control the false discovery rate (FDR) with respect to the multiple testing problem. False discovery rate-corrected P-values—denoted with P int—will be discussed and presented in the Supplementary material online. Additionally, we will quote the unadjusted two-sided interaction P-values for all models in the Supplementary material. All presented confidence intervals (CI) are standard unadjusted and all P-values relating to non-sex-interaction terms, i.e. the main effects in the full models or the effects in the subgroups, are two-sided, purely descriptive and unadjusted. We also calculated a sex-specific HR, which summarizes the sex-interaction terms, by a joint full model Cox regression with all comorbidities, but without additionally gender-interaction terms.
We also performed various secondary, explorative analysis. The 30-day mortality, amputation rate, and mortality or amputation were analysed using multivariable logistic regression models.
The 2 year event rates of vascular procedures were estimated with competing risk models by calculating the cumulative incidence, where death was considered as a competing risk.
OS and AFS rates were estimated with a Kaplan–Meier estimator and the freedom from amputation rate was estimated with a cumulative incidence estimator, where death was considered as a competing risk for several time points (30 days, 1, 2, 5 years).
Furthermore, we calculated for these time points risk ratios with 95% confidence intervals based on 10 000 parametric bootstrap samples obtained from the Gaussian distribution of the respective estimated rates. Descriptive qualitative data were tested via two-sided χ 2 test and quantitative data were tested using a two-sided Wilcoxon test. All secondary P-values of the test procedures described above are purely descriptive and unadjusted.
Hazard ratio and unadjusted 95% CI for all features in the subgroups are shown in the graphs. Inferential statistics are intended to be exploratory (hypotheses-generating), not confirmatory, and are interpreted accordingly.
As a further sensitivity analysis, we repeated the above analyses with a matched cohort by age and RF. Patients were first stratified into subgroups defined by integer age in years and RF. Within each separate subgroup, an Optimal Full Matching algorithm was applied, accounting for the Euclidean distance of the patients' exact age in days.35 Thus, all matched partners have the same RF, and the age of matched partners differs by maximal 1 year. Statistical analyses were performed using the R version 3.6.0 (2019-04-26), R Foundation, Vienna, Austria.
Results
We identified 199 953 patients, who were hospitalized due to a main diagnosis of CLTI between 1 January 2010 and 31 December 2017. We analysed baseline characteristics 2 years prior to index hospitalization and included a follow-up period until 31 December 2018. The median follow-up was 5.3 years, with an interquartile range (IQR) of 4.1 years.
In the entire cohort, more male patients were hospitalized due to CLTI (43% female vs. 57% male), while female CLTI patients were older (median: 81.4 vs. 73.8 years, P < 0.001). The analysis of CVRFs and comorbidities showed that male CLTI patients were more often diagnosed with DM, dyslipidaemia, smoking, CVD, CCS, as well as previous AMI, and stroke. Female CLTI patients showed higher prevalence of hypertension, chronic heart failure (CHF), atrial fibrillation (AF) and flutter (AFI), and chronic kidney disease (CKD) in the unadjusted cohort (Table 1). A matched analysis showed higher prevalence of all cardiovascular risk factors and comorbidities in male patients, except hypertension, and obesity (see Supplementary material online, Table S2). Previously performed diagnostic angiographies had been applied in one-quarter of the patients, and previously performed revascularizations only in 17% of CLTI patients, both more often in male than in female CLTI patients. Moreover, the prescription of lipid-lowering drugs (statins) were present in nearly 40% of CLTI patients, while a little more than half of the patients received antithrombotic therapy (AT) and around 70% an angiotensin-converting-enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) before index hospitalization. Both first-line recommended drugs (statin and AT) were significantly more common in male compared with female CLTI patients, while ACEi or ARB were more often prescribed in female patients (Table 1, see Supplementary material online, Table S2).
Table 1.
Baseline characteristics
| Total | Females | Males | P-value | |
|---|---|---|---|---|
| Patients, n (%) | 199 953 (100.0) | 85 923 (43.0) | 114 030 (57.0) | |
| Age, years, median (IQR) | 76.9 (15.4) | 81.4 (12.8) | 73.8 (15.2) | <0.001 |
| Cardiovascular risk factors, n (%) | ||||
| DM | 106 177 (53.1) | 44 649 (52.0) | 61 528 (54.0) | <0.001 |
| Dyslipidaemia | 114 896 (57.5) | 46 914 (54.6) | 67 982 (59.6) | <0.001 |
| Hypertension | 178 651 (89.3) | 78 877 (91.8) | 99 774 (87.5) | <0.001 |
| Smoking | 43 509 (21.8) | 11 766 (13.7) | 31 743 (27.8) | <0.001 |
| Obesity | 44 971 (22.5) | 19 625 (22.8) | 25 346 (22.2) | 0.001 |
| Cardiovascular comorbidities, n (%) | ||||
| AF/AFl | 64 634 (32.3) | 29 556 (34.4) | 35 078 (30.8) | <0.001 |
| CVD | 35 465 (17.7) | 13 214 (15.4) | 22 251 (19.5) | <0.001 |
| CCS | 94 647 (47.3) | 37 089 (43.2) | 57 558 (50.5) | <0.001 |
| CHF | 87 447 (43.7) | 40 184 (46.8) | 47 263 (41.4) | <0.001 |
| CKD | 94 188 (47.1) | 43 190 (50.3) | 50 998 (44.7) | <0.001 |
| Previous AMI | 20 965 (10.5) | 7413 (8.6) | 13 552 (11.9) | <0.001 |
| Previous stroke | 36 107 (18.1) | 15 158 (17.6) | 20 949 (18.4) | <0.001 |
| Other comorbidities, n (%) | ||||
| Malignancies | 34 299 (17.2) | 12 894 (15.0) | 21 405 (18.8) | <0.001 |
| Previous vascular procedures, n (%) | ||||
| Prior any diagnostic angiography of the LL | 52 729 (26.4) | 19 961 (23.2) | 32 763 (28.7) | <0.001 |
| Prior any revascularization of the LL | 34 326 (17.2) | 12 771 (14.9) | 21 555 (18.9) | <0.001 |
| Prior EVR of the LL | 23 058 (11.5) | 8 585 (10.0) | 14 473 (12.7) | <0.001 |
| Prior vascular surgery of the LL | 16 919 (8.5) | 6 161 (7.2) | 10 758 (9.4) | <0.001 |
| Medication at baseline, n (%) | ||||
| Any statin | 78 908 (39.5) | 29 858 (34.8) | 49 050 (43.0) | <0.001 |
| Any AT (VKA, NOAC, AP) | 102 035 (51.0) | 41 262 (48.0) | 60 773 (53.3) | <0.001 |
| Any ACEi or ARB | 139 563 (69.8) | 62 590 (72.8) | 76 973 (67.5) | <0.001 |
The qualitative data were tested via two-sided χ 2 test and the quantitative data were tested using a two-sided Wilcoxon test.
IQR, interquartile range; DM, diabetes mellitus; AF, atrial fibrillation; AFl, atrial flutter; CVD, cerebrovascular disease; CCS, chronic coronary syndrome; CHF, chronic heart failure; CKD, chronic kidney disease; AMI, acute myocardial infarction; LL, lower limbs; EVR, endovascular revascularization; AT, antithrombotic therapy; VKA, vitamin K antagonist; NOAC, new oral anticoagulant; AP, antiplatelet; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
In-hospital treatment and outcomes during index hospitalization
At admission to index hospitalization, 28% of all CLTI patients suffered from rest pain (RF 4), while 34% had minor (RF 5) and 38% major (RF 6) tissue loss. More women were diagnosed at RF 5 (36% female vs. 32% male), while more men were diagnosed at RF 4 (27 vs. 29%) and RF 6 (37 vs. 39%, all P < 0.001). In total, 69% underwent a diagnostic angiography and 63% received a revascularization of the LL, both more frequently in male CLTI patients. Interestingly, endovascular revascularization (EVR) of the LL was equal between the sexes (42 vs. 41%), while vascular surgery of the LL was more common in males (23 vs. 30%, both P < 0.001). A matched analysis showed no difference in the performance of any endovascular procedure, but female patients received more often endovascular procedure, while vascular surgery were performed more frequently in men (Table 2, see Supplementary material online, Table S2).
Table 2.
Treatment and outcome during index hospitalization
| Total | Females | Males | P-value | |
|---|---|---|---|---|
| Primary diagnosis leading to index hospitalization, n (%) | ||||
| RF 4 | 55 985 (28.0) | 23 255 (27.1) | 32 730 (28.7) | <0.001 |
| RF 5 | 67 687 (33.9) | 31 175 (36.3) | 36 512 (32.0) | <0.001 |
| RF 6 | 76 281 (38.1) | 31 493 (36.7) | 44 788 (39.3) | <0.001 |
| Vascular procedures during index hospitalization, n (%) | ||||
| Any diagnostic angiography of the LL | 136 890 (68.5) | 57 116 (66.5) | 79 774 (70.0) | <0.001 |
| Any revascularization of the LL | 126 590 (63.3) | 52 024 (60.6) | 74 566 (65.4) | <0.001 |
| EVR of the LL | 82 842 (41.4) | 36 027 (41.9) | 46 815 (41.1) | <0.001 |
| Vascular surgery of the LL | 53 567 (26.8) | 19 647 (22.9) | 33 920 (29.8) | <0.001 |
| Any amputation of the LL | 38 352 (19.2) | 14 382 (16.7) | 23 970 (21.0) | <0.001 |
| Complications during index hospitalization, n (%) | ||||
| 30-day mortality | 14 172 (7.1) | 7513 (8.7) | 6659 (5.8) | <0.001 |
| Acute renal failure | 6916 (3.5) | 3062 (3.6) | 3854 (3.4) | 0.026 |
| AMI | 3100 (1.6) | 1243 (1.5) | 1857 (1.6) | 0.001 |
| Ischaemic stroke | 1897 (1.0) | 856 (1.0) | 1041 (0.9) | 0.057 |
| Infections/sepsis | 5268 (2.6) | 1973 (2.3) | 3295 (2.9) | <0.001 |
| Bleeding | 37 522 (18.8) | 17 195 (20.0) | 20 327 (17.8) | <0.001 |
| Reimbursement and length of stay | ||||
| Reimbursement (€), mean (SD) | 7205 (7.380) | 6765 (6.373) | 7536 (8.041) | <0.001 |
| Length of stay (days), median (IQR) | 15.2 (15.4) | 14.6 (14.4) | 15.6 (16.0) | <0.001 |
The qualitative data were tested via two-sided χ 2 test and the quantitative data were tested using a two-sided Wilcoxon test.
RF, Rutherford stage; LL, lower limbs; EVR, endovascular revascularization; AMI, acute myocardial infarction; SD, standard deviation; IQR, interquartile range.
The complication rates during index hospitalization showed no significantly noticeable sex-related differences for acute renal failure or AMI, while both were more common in male patients after age and RF adjustment. However, infections/sepsis was slightly more frequent in male CLTI patients, while bleeding events occurred more often in female CLTI patients. The amputation rate of the LL during index hospitalization was at 19.2%, and more frequent in male CLTI patients. Moreover, the 30-day mortality rate was at 7.1% and significantly noticeable higher for female CLTI patients (Table 2). However, in the age- and RF-matched cohort as well as after adjustment for age and comorbidities by logistic regression analysis, the 30-day mortality showed no significantly noticeable differences between the sexes. In addition, female sex was associated with decreased risk of amputation of the LL and the combined endpoint of amputation of the LL or death (see Supplementary material online, Table S2 and Table S3).
Both the reimbursement of index hospitalization and length of stay were significantly noticeable smaller in female CLTI patients compared with males, while the length of stay was equal in the age- and RF-adjusted cohort (Table 2, see Supplementary material online, Table S2).
Treatment during follow-up and long-term outcomes
Two years after hospital discharge, approximately one-third of the entire cohort needed subsequent diagnostic angiography and/or a revascularization procedure, both less often performed in female CLTI patients compared with male patients. Moreover, 57% of the patients received a statin and 71% AT 2 years after index hospitalization. Both prescription rates were significantly noticeable higher in male CLTI patients than in female CLTI patients. The prescription rate 1 year after index hospitalization was increasing from 2010 to 2017 for statins (41–51%) and AT (63–73%) and lower in female patients. The prescription rate of ACEi/ARB was around 60% and was notably higher in women only in the age- and RF-matched cohort (see Supplementary material online, Figure S2). Furthermore, at 2-year follow-up, the rate of LL amputations was around 18% and lower for women. Moreover, the secondary endpoints acute renal failure and AMI were slightly higher prevalent in male CLTI patients, while no sex-related differences were observed for the endpoint ischaemic stroke (Table 3). All trends were present in the age- and RF-matched cohort, too (see Supplementary material online, Table S2).
Table 3.
Outcomes at the 2-year follow-up
| Total | Females | Males | P-value | |
|---|---|---|---|---|
| Vascular procedures during the 2-year follow-up, n (%) | ||||
| Any diagnostic angiography of the LL | 55 980 (28.0) | 21 456 (25.0) | 34 524 (30.3) | <0.001 |
| Any revascularization of the LL | 61 325 (30.7) | 23 344 (27.2) | 37 981 (33.3) | <0.001 |
| EVR of the LL | 40 988 (20.5) | 16 029 (18.7) | 24 959 (21.9) | <0.001 |
| Vascular surgery of the LL | 32 397 (16.2) | 11 678 (13.6) | 20 719 (18.2) | <0.001 |
| Medication at 2-year follow-up (inclusive baseline), n (%) | ||||
| Any statin | 114 111 (57.1) | 43 659 (50.8) | 70 452 (61.8) | <0.001 |
| Any AT (VKA, NOAC, AP) | 142 026 (71.0) | 58 196 (67.7) | 83 830 (73.5) | <0.001 |
| Any ACEi or ARB | 157 196 (78.6) | 69 051 (80.4) | 88 145 (77.3) | <0.001 |
| Outcomes at a 2-year follow-up, n (%) | ||||
| Amputation of the LL, total | 36 585 (18.3) | 12 215 (14.2) | 24 370 (21.4) | <0.001 |
| Minor amputation of the LL | 24 440 (12.2) | 7286 (8.5) | 17 154 (15.0) | <0.001 |
| Major amputation of the LL | 18 014 (9.0) | 6659 (7.8) | 11 355 (10.0) | <0.001 |
| Acute renal failure | 23 984 (12.0) | 9649 (11.2) | 14 335 (12.6) | <0.001 |
| AMI | 21 531 (10.8) | 7547 (8.8) | 13 984 (12.3) | <0.001 |
| Ischaemic stroke | 27 588 (13.8) | 11 674 (13.6) | 15 914 (14.0) | 0.008 |
Data were estimated with competing risk models via cumulative incidence function, where death was considered as a competing risk.
LL, lower limbs; EVR, endovascular revascularization; AT, antithrombotic therapy; VKA, vitamin K antagonist; NOAC, new oral anticoagulant; AP, antiplatelet; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AMI, acute myocardial infarction.
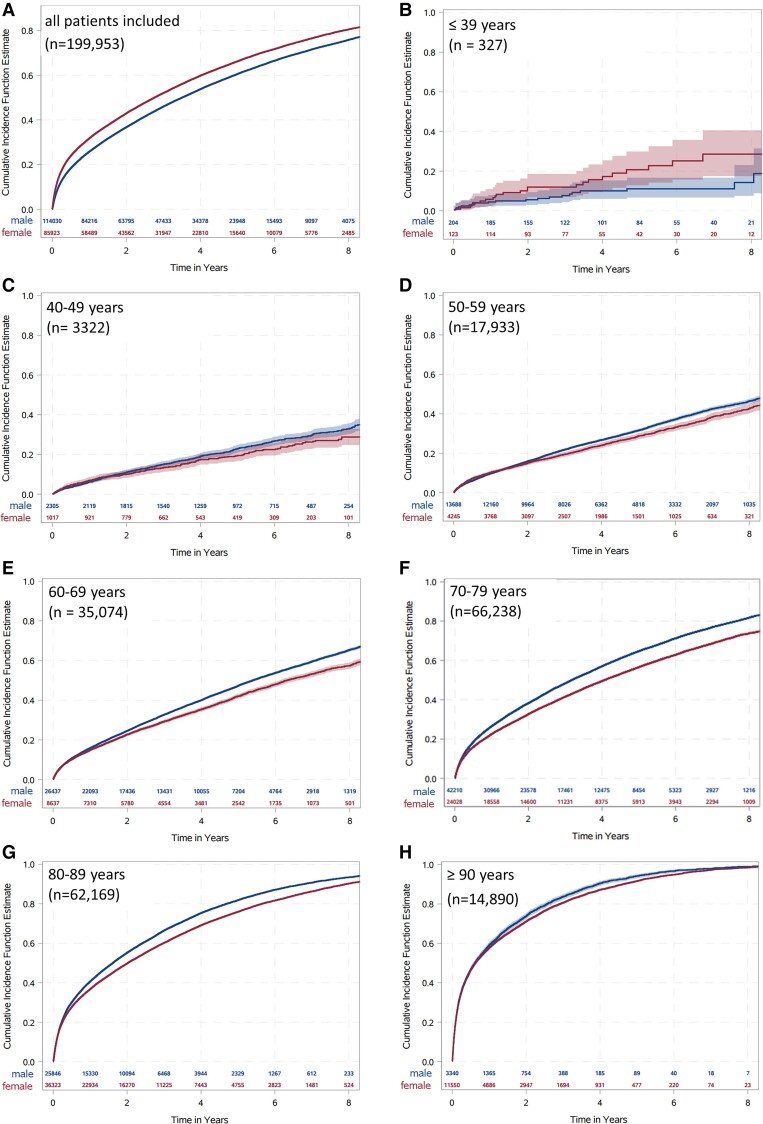
The unstratified Kaplan–Meier estimators showed that OS was around 72% 1 year after index hospitalization, decreasing with longer follow-up (61% at 2-year and 37% at 5-year follow-up). Female CLTI patients showed lower survival rates compared with male patients (Figure 1A and see Supplementary material online, Table S4) and the impression that the mortality was higher in females was supported by the Kaplan–Meier curves independently of the RF (see Supplementary material online, Figure S3A–D). But, when the entire cohort was classified in different age groups (≤39, 40–49, 50–59, 60–69, 70–79, 80–89, and ≥90 years), female CLTI patients showed a decreased mortality rate in all age groups (Figure 1B–H). The Kaplan–Meier estimators of the age- and RF-matched cohort showed higher mortality rates for men (see Supplementary material online, Table S4). Moreover, almost one-sixth underwent an amputation of the LL during the first year after index hospitalization with rising values and the probability for amputation was lower in female CLTI patients compared with men (see Supplementary material online, Table S4 and Figure S3E–H). In addition, age distribution and analysis of the age- and RF-matched cohort showed the same trend between the sexes (see Supplementary material online, Figure S4 and Table S4). The Kaplan–Meier estimators of the combined endpoint of amputation of the LL or death related to 40% of the patients, without any major differences between the sexes during 1-year follow-up (see Supplementary material online, Table S4 and Figure S3I–L). If the entire cohort was divided into different age groups (≤39, 40–49, 50–59, 60–69, 70–79, 80–89, and ≥90 years) or matched for age and RF, female CLTI patients were at lower risk for the combined endpoint amputation of the LL or death (see Supplementary material online, Figure S5 and Table S4).
Figure 1.
The cumulative incidence estimates (event rates) for death. The event rate is shown over time (in years) divided for female (red) and male (blue) patients in the entire cohort (A) and in different age groups (B–H). Data were estimated with the Kaplan–Meier estimates.
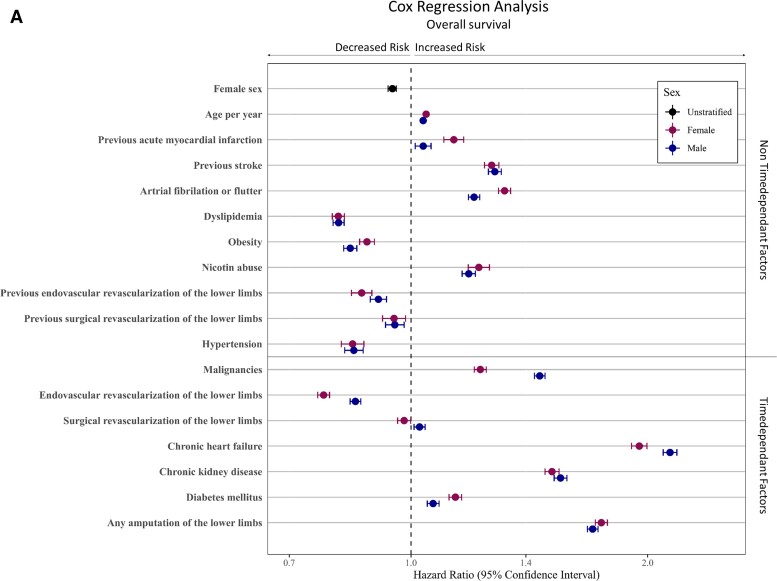
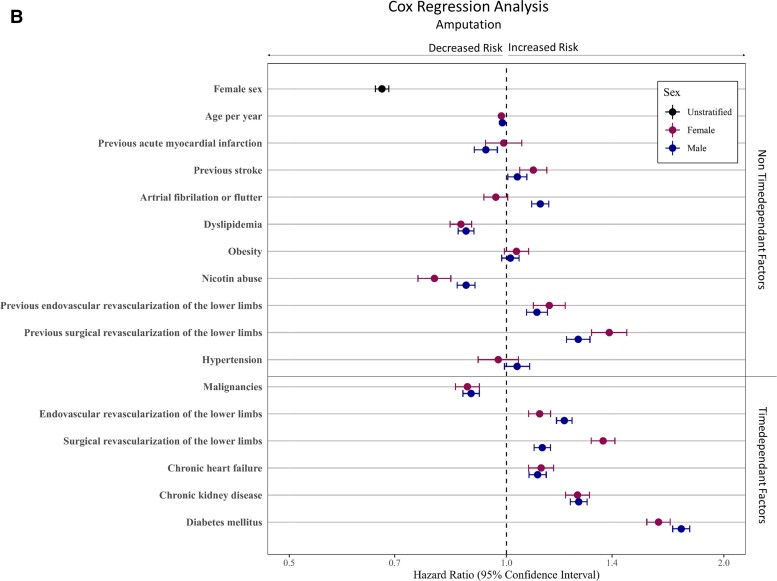
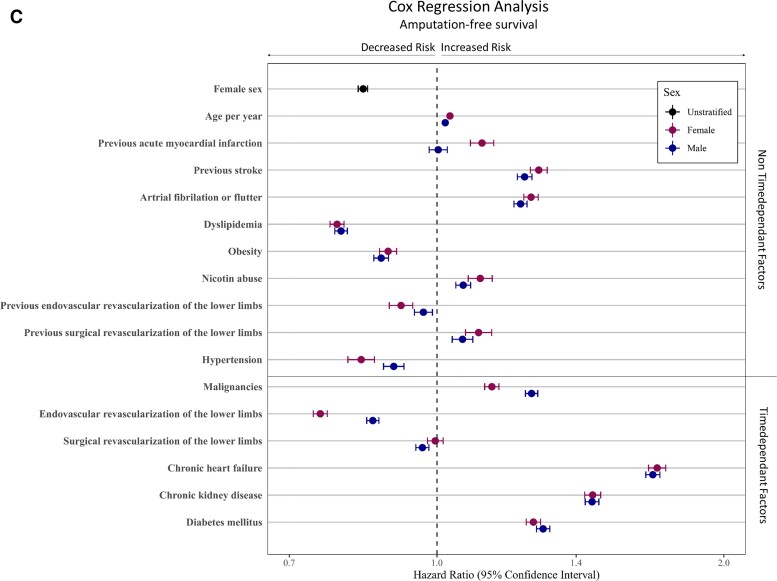
A multivariable Cox regression analysis including age, non-time-dependent risk factors (i.e. previous AMI, previous stroke, AF/AFI, dyslipidaemia, obesity, nicotine abuse, previous revascularization procedure and hypertension), as well as time-dependent factors (i.e. malignancies, revascularization procedure, CHF, CKD, DM, and amputation of the LL) displayed the sex-specific impact of these factors on long-term outcome. After adjustment to age and patient comorbidities, female sex was associated with improved long-term outcome, resulting in decreased hazard (HR 0.95; 95% CI 0.94–0.96, P < 0.001). Furthermore, in women, the probability to reach the endpoints amputation of the LL (HR 0.67; 95% CI 0.66–0.69, P < 0.001) and the combined endpoint AFS (HR 0.84; 95% CI 0.83–0.85, P < 0.001) was lower (Figure 2; see Supplementary material online, Table S5). Moreover, the multivariable Cox regression analysis showed that a diagnosis of previous stroke, AF, nicotine abuse, CHF, CKD, DM, and amputation of the LL was associated with a higher mortality rates in both sexes (all P < 0.001). Interestingly, the presence of previous AMI, AF, or DM increases the hazards for mortality more in female CLTI patients compared with men, while malignancies or CHF showed lower mortality rates in female CLTI patients than in male patients. The performance of EVRs had a beneficial effect on OS in both sexes, while this advantage was more evident in female CLTI patients compared to male patients (all P int ≤ 0.001, see Supplementary material online, Table S6 and Figure 2). Amputation-free survival as a combined endpoint of amputation of the LL or death showed HRs < 1 in both sexes in the presences of previous stroke, AF, nicotine abuse, CHF, CKD, and DM (all P < 0.001). The effect of prior AMI on AFS was significantly noticeable worse in women (P int < 0.001, see Supplementary material online, Table S6 and Figure 2).
Figure 2.
Multivariable Cox regression analyses for predictors of long-term outcomes. The Cox regression analysis was done for the adjusted endpoints overall survival (A), freedom from amputation (B), and amputation-free-survival (C). Divided for male and female patients. Cofactors were age, non-time-dependent risk factors (e.g. previous acute myocardial infarction, previous stroke, atrial fibrillation or flutter, dyslipidaemia, obesity, nicotine abuse, previous revascularization procedure, and hypertension), as well as time-dependent factors (e.g. malignancies, revascularization procedure, chronic heart failure, chronic kidney disease, diabetes mellitus, and amputation of the lower limbs). The models for freedom from amputation considered death as a competing risk in contrast to the amputation-free survival models, which included death in the endpoint. We used Fine and Gray's method to estimate sub-distributional hazard ratios in the amputation-free survival model.
Discussion
Different clinical trials and other studies demonstrated the poor prognosis of patients with LEAD and especially with CLTI and in most cardiovascular trials, LEAD was understudied.36 Interestingly, the results on sex-related differences in LEAD patients and the proportion of female patients are controversial and are probably depending on methodological approaches and differences in inclusion/exclusion criteria.29,31,37 One major point could be that women have longer symptom-free periods and the initial diagnosis in female patients is often diagnosed at higher age compared with male patients.38,39 Especially with regard to the high number of CLTI patients, more knowledge is necessary to analyse sex-related differences in this patient group. In the present study, we assessed sex-related differences in an unselected cohort of 199 953 patients, hospitalized for a main diagnosis of CLTI, as indicated by RF 4–6, from 2010 to 2017. We included a 2-year pre-treatment period before an index hospitalization due to CLTI and a follow-up period of up to 9 years. In contrast to many other analyses, we include all LEAD patients, who were hospitalized with and without performance of any vascular procedure.27,28,30 In our defined cohort, all patients had treatment required symptoms and the reason why patients did not receive a vascular procedure can be manifold (higher age, severe multi-morbid patients, or patient request to proceed conservatively). Nevertheless, consistent with other studies,16,17 we found that CLTI patients had a poor prognosis in terms of all-cause mortality and amputation of the LL. While unadjusted mortality was higher in women, after adjustment for age and patient risk profile, male sex was associated with higher all-cause mortality and amputation of the LL during the 9-year follow-up period. In line with other studies, the prevalence of CLTI was increasing with age and female CLTI patients were older compared with male patients.5,10 Furthermore, the number of men hospitalized with CLTI was higher compared with women as found by others.16,24–26 Additionally, most CLTI patients suffered from one or more typical CVRFs (e.g. hypertension, dyslipidaemia, or DM) and/or cardiovascular comorbidities (e.g. CCS, CHF, CVD, or AF) which was also consistent with other reports4,16,22 (Structured Graphical Abstract).
Female CLTI patients were almost 8 years older than men, resulting in higher probability of diagnosis and distinct treatments at baseline for women. Contrary to this assumption, female CLTI patients had undergone less frequently treatment required hospitalization, vascular procedures and had received less often prescriptions of guideline-recommended medication, such as statins or ATs, compared with male CLTI patients. Reasons for this lower application rates may be mainfold and complex: due to higher age, women could be more fragile, suffer often from impaired psychomental status thereby unfit for interventional procedures and presenting contraindications to some of the pharmacological agents.
Furthermore, it is known that female LEAD patients with IC are present more often with borderline ABI and longer symptom-free periods compared with male patients,1,19,20 leading to significant delayed diagnosis of LEAD or less intensive resource use patterns. It may also be possible that for a greater proportion of female patients, the index hospitalization is the first diagnosis of LEAD. Moreover, male patients were more often co-diagnosed with other atherosclerotic diseases such as CCS or CVD, displaying that men suffer more often from polyvascular diseases. Therefore, male CLTI patients were probably more often already under medical treatment with guideline-recommended medication because of the presence of other cardiovascular diseases. Nevertheless, one more reason for this low prescription rates of urgently required medications is probably that women are still not recognized in the same degree to be vascular high-risk patients as men are.
With reference to the secondary medical supply of CLTI patients, 2 years after index hospitalization, only just over half of the patients received a statin and almost three-quarter any type of AT. In addition, we included time trends of prescription rates and we could show that the rate for ATs and statins is increasing from 2010 to 2017 as shown by others,32 but were still more common in men. Interestingly, this underuse of both first-line recommended medication was more prominent in female CLTI patients compared with male CLTI patients. Several trials, like the randomized controlled EUCLID trial, did not report different prescription rates of guideline-recommended medication.40 This is contrary to data published from other real-world cohorts23 and probably due to the fact that patients included in randomized trials display a highly selected patient group with higher medical care.37 In consequence, these findings are alarming, since it was shown that a statin therapy as well as the usage of AT is associated with a reduction of MACE, amputations, and all-cause mortality in high-risk patients.41,42 Therefore, these drugs are strongly recommended for these patients in all current guidelines.43,44 Further analyses are needed to identify sex-related differences in pharmacotherapy and the effect on outcome parameters.
Unstratified Kaplan–Meier models of death and amputation of the LL assumed higher mortality rates in female CLTI patients, while male CLTI patients underwent more often LL amputation. Interestingly, if we divide the entire cohort into different age groups or analyse an age- and RF-matched cohort, the trend of higher mortality rates in women was reversed and in each separated group, male sex was associated with higher mortality. This statistical phenomenon is known as Simpson's paradox45 and is caused by the fact that the sex effect is dominated by effect of the age difference between men and woman in our cohort, when using the unstratified Kaplan–Meier model. Since age was reported to be a relevant risk indicator for worse outcome in cardiovascular diseases,5,24,46 we included a multivariable Cox regression analysis, adjusted for age and patients risk constellation. These we found that women had a significantly noticeable better OS and AFS compared with men.
Despite all the progress in conservative and interventional therapeutic strategies in the last decades, CLTI patients remain a very high-risk cohort as illustrated by 39% death and 18% LL amputation rate during a 2-year follow-up period. The presence of cardiovascular comorbidities like CCS, CVD, CHF, CKD, or DM increases all-cause mortality and amputation of the LL events in LEAD patients,16,18 clearly indicating that CLTI patients are highly morbid with a markedly increased risk of cardiovascular events. In the entire cohort, DM, CVD, and CCS were significantly noticeable higher co-prevalent in male CLTI patients. Moreover, the rate of AF, CHF, and CKD in the age- and RF-matched cohort was higher in males too. Therefore, the higher frequency of these cardiovascular comorbidities in men is probably an important factor, which contributes to the poor outcome observed in male CLTI patients.
Interestingly, it was shown that patients co-prevalent with DM have an increased likelihood to underwent LL amputation, but only marginal increased probability of all-cause mortality compared with CLTI patients without DM.18,47 In addition, CHF clearly negatively influences the mortality rate in both sexes. We observed higher prevalence of CHF in women, while the impact on mortality by CHF was stronger in male CLTI patients compared with women. Usually, CLTI is interpreted as an advanced form of LEAD; however, it should not be understood just as a bad disease of the peripheral vessels. Especially CLTI patients are often co-diagnosed with other concomitant diseases such as CHF, CKD, or impairment of other organs. This fact demonstrates that CLTI should be understood as a multi-organ disease, and the diagnosis of CLTI and the appearance of symptoms such as rest pain, ulcers, and necrosis is in most patients the transition to a multi-organ failure. This multimorbidity is the reason why the prognosis of CLTI patients is much worse compared with other diseases.44 In line with Mustapha et al.,18 we found also a protective effect on all-cause mortality in the presence of hypertension. This is probably due to the better medical supply in this patient group, since these patients may visit more often the family doctor or specialist and in the entire cohort, women were more often co-prevalent with hypertension.
Finally, the performance of an EVR procedure was associated with higher survival rates, while there was a markedly negative impact of LL amputation independently of sex. As shown before,23,24 endovascular approaches are more common in women, while vascular surgery is more frequent in men. Currently, the endovascular first approach is the therapy of choice43,44 because of its high effectiveness together with less invasiveness. The performance of open vascular surgery might refer to patients with a more advanced stage of LEAD but also point to centres that are not capable of endovascular therapy as the state of the art. This may contribute to the more frequent unfavourable endpoints observed in male CLTI patients.
Strengths and limitations
We included almost 200 000 LEAD patients, who were all insured by the AOK Health Insurance Fund, which covers almost 32% patients in Germany. The AOK consist of 11 independent regional Health Insurance Funds, which cover healthcare nationwide in Germany. Nevertheless, the AOK insured patients have a lower socioeconomic status and higher migration background and presence of cardiovascular risk factors, found in all regions compared with other health insurances. These differences, probably leading to different healthcare supply depending on the regional Health Insurance Fund, can influence the data but demonstrate a nationwide healthcare supply in a heterogeneous patient population. In contrast to randomized trials, observational studies, and registries, the studied health claims data are not subject to selection by the sponsor or implementer. Patients were included until 2017 and the follow-up phase was until 2018, displaying the current care situation under current guideline recommendations with a long follow-up time up to 9 years. A very low loss to follow-up events was determined, since the change in the health insurance is rather rare, especially in older age groups. The analysis presented here has general limitations in the use of health claims data, mentioned as missing information on clinical status and parameters (e.g. laboratory parameters, questionnaires on quality of life, or results of medical examinations like ABI), the success or failure of interventions, or patient compliance. In addition, the basis of our analysed data were the diagnostic codes, used for validation reasons. This means non-billable diagnoses were often not present and thus not included in our analyses. However, health claims data are validated with regard to cardiovascular events (like myocardial infarction) or survival. Furthermore, the factors influencing an event during follow-up can be identified and statistically assessed. The basis of our requested data were the ICD-10-GM, OPS, and ATC codes, important for correct billing process. Diagnoses of not accounting relevant data were probably not specified and by this could not be included in our analyses. Furthermore, differences depending on biological sex (e.g. hormone status), socioeconomic, financial, or health educational aspects cannot be included in this analysis. Finally, medical adherence of patients can only be derived from the fulfilled prescriptions, but we do not know whether patients take their prescribed drugs or additionally take non-prescription drugs.
Authors’ contributions
L.M.: conception, interpretation and design of the data, drafting the initial manuscript. J.K. and J.G.: statistical analysis, interpretation of the data. C.E., L.K., A.J.F., N.M., and SA.L.: interpretation of the data, critical revision for important intellectual content. P.D., T.R., and C.G. data retrieval and routine data structure, advisory support in project planning. E.F.: financial support, conception, interpretation, and design of the data. H.R. and E.F. supervision, critically revised the draft. J.F.: statistical analysis, interpretation and processing of the data, critically revised the draft. All authors read and approved the final manuscript.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Contributor Information
Lena Makowski, Department of Cardiology I—Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Cardiol, Albert Schweitzer Campus 1, A1, 48149 Muenster, Germany.
Jeanette Köppe, Institute of Biostatistics and Clinical Research, University of Muenster, Muenster, Germany.
Christiane Engelbertz, Department of Cardiology I—Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Cardiol, Albert Schweitzer Campus 1, A1, 48149 Muenster, Germany.
Leonie Kühnemund, Department of Cardiology I—Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Cardiol, Albert Schweitzer Campus 1, A1, 48149 Muenster, Germany.
Alicia J Fischer, Department of Cardiology III—Adult Congenital and Valvular Heart Disease, University Hospital Muenster, Cardiol, Albert Schweitzer Campus 1, A1, 48149 Muenster, Germany.
Stefan A Lange, Department of Cardiology I—Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Cardiol, Albert Schweitzer Campus 1, A1, 48149 Muenster, Germany.
Patrik Dröge, AOK Research Institute (WIdO), Berlin, Germany.
Thomas Ruhnke, AOK Research Institute (WIdO), Berlin, Germany.
Christian Günster, AOK Research Institute (WIdO), Berlin, Germany.
Nasser Malyar, Department of Cardiology I—Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Cardiol, Albert Schweitzer Campus 1, A1, 48149 Muenster, Germany.
Joachim Gerß, Institute of Biostatistics and Clinical Research, University of Muenster, Muenster, Germany.
Eva Freisinger, Department of Cardiology I—Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Cardiol, Albert Schweitzer Campus 1, A1, 48149 Muenster, Germany.
Holger Reinecke, Department of Cardiology I—Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Cardiol, Albert Schweitzer Campus 1, A1, 48149 Muenster, Germany.
Jannik Feld, Institute of Biostatistics and Clinical Research, University of Muenster, Muenster, Germany.
Funding
The study was conducted within the framework of the GenderVasc project (Gender-specific real care situation of patients with arteriosclerotic cardiovascular diseases) funded by The Federal Joint Committee, Innovation Committee (G-BA, Innovationsfond, number 01VSF18051). GenderVasc is a cooperation project with the AOK Research Institute of the AOK (WIdO).
Conflict of interest
L.M. and C.E. report travel support from Bayer Vital and Abbott, outside the submitted work. J.K., L.K., A.J.F., P.D., T.R., C.G., J.G., and J.F. nothing to disclose. SA.L. report travel support from Daiichi Sankyo and Bayer Vital, outside the submitted work. N.M. reports speaker honoraria and travel support from BARD and Bayer Vital GmbH, outside the submitted work. E.F. reports non-financial support from Vascuros, outside the submitted work. H.R. reports personal fees from Daiichi, grants from BMS/Pfizer, personal fees from MedUpdate, personal fees from DiaPlan, personal fees from NeoVasc, grants and personal fees from Pluristem, grants from Bard, grants from Biotronik, personal fees from NovoNordisk, personal fees from StreamedUp, personal fees from Corvia, all outside the submitted work.
References
- 1. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 2. Shu J, Santulli G. Update on peripheral artery disease: epidemiology and evidence-based facts. Atherosclerosis 2018;275:379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duff S, Mafilios MS, Bhounsule P, Hasegawa JT. The burden of critical limb ischemia: a review of recent literature. Vasc Health Risk Manag 2019;15:187–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malyar N, Fürstenberg T, Wellmann J, Meyborg M, Lüders F, Gebauer K, et al. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: a nationwide population-based analysis. Eur Heart J 2013;34:2706–2714. [DOI] [PubMed] [Google Scholar]
- 5. Kröger K, Stang A, Kondratieva J, Moebus S, Beck E, Schmermund A, et al. Prevalence of peripheral arterial disease - results of the Heinz Nixdorf recall study. Eur J Epidemiol 2006;21:279–285. [DOI] [PubMed] [Google Scholar]
- 6. Cimminiello C, Kownator S, Wautrecht JC, Carvounis CP, Kranendonk SE, Kindler B, et al. The PANDORA study: peripheral arterial disease in patients with non-high cardiovascular risk. Intern Emerg Med 2011;6:509–519. [DOI] [PubMed] [Google Scholar]
- 7. Meijer WT, Grobbee DE, Hunink MG, Hofman A, Hoes AW. Determinants of peripheral arterial disease in the elderly: the Rotterdam study. Arch Intern Med 2000;160:2934–2938. [DOI] [PubMed] [Google Scholar]
- 8. Ding N, Sang Y, Chen J, Ballew SH, Kalbaugh CA, Salameh MJ, et al. Cigarette smoking, smoking cessation, and long-term risk of 3 major atherosclerotic diseases. J Am Coll Cardiol 2019;74:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45:S5–S67. [DOI] [PubMed] [Google Scholar]
- 10. Diehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange S, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis 2004;172:95–105. [DOI] [PubMed] [Google Scholar]
- 11. Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol 2016;19:91–95. [DOI] [PubMed] [Google Scholar]
- 12. Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg 2010;51:230–241. [DOI] [PubMed] [Google Scholar]
- 13. Gallino A, Aboyans V, Diehm C, Cosentino F, Stricker H, Falk E, et al. Non-coronary atherosclerosis. Eur Heart J 2014;35:1112–1119. [DOI] [PubMed] [Google Scholar]
- 14. Klevsgård R, Risberg BO, Thomsen MB, Hallberg IR. A 1-year follow-up quality of life study after hemodynamically successful or unsuccessful surgical revascularization of lower limb ischemia. J Vasc Surg 2001;33:114–122. [DOI] [PubMed] [Google Scholar]
- 15. Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess 2010;14:1–210, iii–iv. [DOI] [PubMed] [Google Scholar]
- 16. Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Lüders F, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J 2015;36:932–938. [DOI] [PubMed] [Google Scholar]
- 17. Olinic DM, Spinu M, Olinic M, Homorodean C, Tataru DA, Liew A, et al. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int Angiol 2018;37:327–334. [DOI] [PubMed] [Google Scholar]
- 18. Mustapha JA, Katzen BT, Neville RF, Lookstein RA, Zeller T, Miller LE, et al. Determinants of long-term outcomes and costs in the management of critical limb ischemia: a population-based cohort study. J Am Heart Assoc 2018;7:e009724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Higgins JA. Epidemiology of peripheral arterial disease in women. J Epidemiol 2003;13:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prasada S, Shah SJ, Michos ED, Polak JF, Greenland P. Ankle-brachial index and incident heart failure with reduced versus preserved ejection fraction: the Multi-Ethnic Study of Atherosclerosis. Vasc Med 2019;24:501–510. [DOI] [PubMed] [Google Scholar]
- 21. Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation 2009;120:2053–2061. [DOI] [PubMed] [Google Scholar]
- 22. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–1526. [DOI] [PubMed] [Google Scholar]
- 23. Makowski L, Feld J, Köppe J, Illner J, Kühnemund L, Wiederhold A, et al. Sex related differences in therapy and outcome of patients with intermittent claudication in a real-world cohort. Atherosclerosis 2021;325:75–82. [DOI] [PubMed] [Google Scholar]
- 24. Freisinger E, Malyar NM, Reinecke H, Unrath M. Low rate of revascularization procedures and poor prognosis particularly in male patients with peripheral artery disease—a propensity score matched analysis. Int J Cardiol 2018;255:188–194. [DOI] [PubMed] [Google Scholar]
- 25. Lo RC, Bensley RP, Dahlberg SE, Matyal R, Hamdan AD, Wyers M, et al. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg 2014;59:409–418.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mentias A, Vaughan-Sarrazin M, Saad M, Girotra S. Sex differences in management and outcomes of critical limb ischemia in the medicare population. Circ Cardiovasc Interv 2020;13:e009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heidemann F, Kuchenbecker J, Peters F, Kotov A, Marschall U, L’Hoest H, et al. A health insurance claims analysis on the effect of female sex on long-term outcomes after peripheral endovascular interventions for symptomatic peripheral arterial occlusive disease. J Vasc Surg 2021;74:780–787.e7. [DOI] [PubMed] [Google Scholar]
- 28. Behrendt CA, Sigvant B, Kuchenbecker J, Grima MJ, Schermerhorn M, Thomson IA, et al. Editor’s Choice - International variations and sex disparities in the treatment of peripheral arterial occlusive disease: a report from VASCUNET and the international consortium of vascular registries. Eur J Vasc Endovasc Surg 2020;60:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jelani Q, Petrov M, Martinez SC, Holmvang L, Al-Shaibi K, Alasnag M. Peripheral arterial disease in women: an overview of risk factor profile, clinical features, and outcomes. Curr Atheroscler Rep 2018;20:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramkumar N, Suckow BD, Brown JR, Sedrakyan A, MacKenzie T, Stone DH, et al. Role of sex in determining treatment type for patients undergoing endovascular lower extremity revascularization. J Am Heart Assoc 2019;8:e013088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mayor JM, Preventza O, McGinigle K, Sr MJ, Montero-Baker M, Gilani R, et al. Persistent under-representation of female patients in United States trials of common vascular diseases from 2008 to 2020. J Vasc Surg 2022;75:30–36. [DOI] [PubMed] [Google Scholar]
- 32. Rammos C, Steinmetz M, Lortz J, Mahabadi AA, Petrikhovich O, Kirsch K, et al. Peripheral artery disease in Germany (2009–2018): prevalence, frequency of specialized ambulatory care and use of guideline-recommended therapy—a population-based study. Lancet Reg Health Eur 2021;5:100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kühnl A, Knipfer E, Lang T, Bohmann B, Trenner M, Eckstein H-H. Hospital incidence, in-patient care and outcome of peripheral arterial occlusive disease and arterial thrombosis/embolism in Germany, 2005–2018. Gefässchirurgie 2020;25:433–445. [Google Scholar]
- 34. Benjamin Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat 1995;57:289–300. [Google Scholar]
- 35. Hansen BB, Klopfer SO. Optimal full matching and related designs via network flows. J Comput Graph Stat 2006;15:609–627. [Google Scholar]
- 36. Vyas MV, Mrkobrada M, Donner A, Hackam DG. Underrepresentation of peripheral artery disease in modern cardiovascular trials: systematic review and meta-analysis. Int J Cardiol 2013;168:4875–4876. [DOI] [PubMed] [Google Scholar]
- 37. Abtan J, Bhatt DL, Elbez Y, Sorbets E, Eagle K, Reid CM, et al. Geographic variation and risk factors for systemic and limb ischemic events in patients with symptomatic peripheral artery disease: insights from the reach Registry. Clin Cardiol 2017;40:710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71:510–515. [DOI] [PubMed] [Google Scholar]
- 39. Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) collaborative research group. Circulation 1993;88:837–845. [DOI] [PubMed] [Google Scholar]
- 40. Haine A, Kavanagh S, Berger JS, Hess CN, Norgren L, Fowkes FGR, et al. Sex-specific risks of major cardiovascular and limb events in patients with symptomatic peripheral artery disease. J Am Coll Cardiol 2020;75:608–617. [DOI] [PubMed] [Google Scholar]
- 41. Ramos R, García-Gil M, Comas-Cufí M, Quesada M, Marrugat J, Elosua R, et al. Statins for prevention of cardiovascular events in a low-risk population with low Ankle Brachial index. J Am Coll Cardiol 2016;67:630–640. [DOI] [PubMed] [Google Scholar]
- 42. Antithrombotic Trialists’ Collaboration . Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deutsche Gesellschaft für Angiologie-Gesellschaft für Gefäßmedizin, S3-Leitlinie zur Diagnostik, Therapie und Nachsorge der peripheren arteiellen Verschlusskrankheit. https://www.dga-gefaessmedizin.de/uploads/media/S3_PAVK_15-11-30.pdf. (15 December 2021, date last accessed).
- 44. Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO) The task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 45. Knapp TR. Instances of simpson’s paradox. Coll Math J 1985;16:209–211. [Google Scholar]
- 46. Vaccarezza M, Papa V, Milani D, Gonelli A, Secchiero P, Zauli G, et al. Sex/gender-specific imbalance in CVD: could physical activity help to improve clinical outcome targeting CVD molecular mechanisms in women? Int J Mol Sci 2020;21:1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Freisinger E, Malyar NM, Reinecke H, Lawall H. Impact of diabetes on outcome in critical limb ischemia with tissue loss: a large-scaled routine data analysis. Cardiovasc Diabetol 2017;16:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data utilized in this study cannot be made available in the manuscript, the Supplementary material online, or in a public repository due to German data protection laws (‘Bundesdatenschutzgesetz’, BDSG). Therefore, they are stored on a secure drive in the WIdO, to facilitate replication of the results. Generally, access to data of statutory health insurance funds for research purposes is possible only under the conditions defined in German Social Law (SGB V § 287). Requests for data access can be sent as a formal proposal specifying the recipient and purpose of the data transfer to the appropriate data protection agency. Access to the data used in this study can only be provided to external parties under the conditions of the cooperation contract of this research project and after written approval by the sickness fund. For assistance in obtaining access to the data, contact wido@wido.bv.aok.de.