Abstract
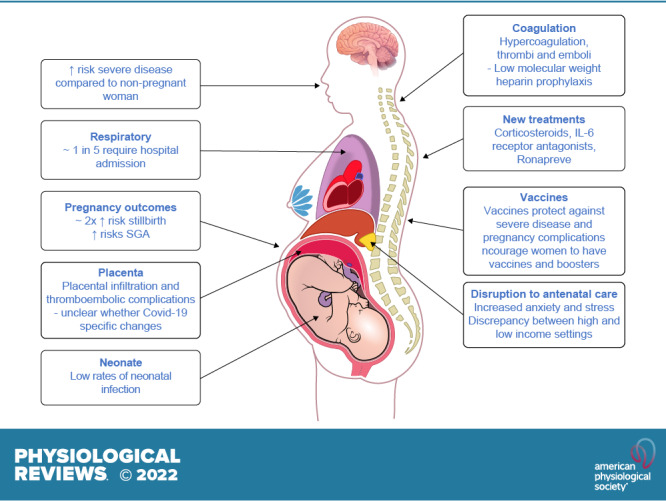
1. INTRODUCTION
Eighteen months ago we reviewed the existing literature about COVID-19 in pregnancy and highlighted key evidence gaps for further research to minimize its impact for pregnant women (1). Since then considerable progress has been made in understanding the clinical course of the disease in the general population, together with major advances in novel treatments and a rollout of vaccination programs. With greater than 5,000 publications cited on PubMed under the search terms “COVID-19 and pregnancy,” it is timely to reflect on the new knowledge gained about the relevance and importance of this disease for pregnant women. With emergence of the new rapidly transmissible SARS-CoV-2 B.1.1.529 (Omicron) variant (2) when many pregnant women remain unvaccinated, with potentially low seroprevalence rates (3), we consider the ongoing uncertainties for pregnant women and areas where more research is still urgently needed as the pandemic continues.
2. ARE PREGNANT WOMEN AT INCREASED RISK OF SEVERE COVID-19?
Even early in the pandemic there were signals that the risk of severe COVID-19 during pregnancy may be higher than in the general population (1). This observation has been confirmed with increasing data accrual. A “living review” including data on 67,271 pregnant and recently pregnant women attending or admitted to the hospital for any reason, and diagnosed as having suspected or confirmed COVID-19, confirms that although symptoms and clinical presentation are similar to the general population, pregnant women with COVID-19 are more likely to have severe disease than nonpregnant women of a similar age (4). The data also demonstrate a 2-fold increase in rates of admission to the intensive care unit (4) and a 1.3-fold increase in maternal death in pregnant women with COVID-19 compared with those without disease (5). The US Centers for Disease Control and Prevention (CDC) COVID-19 data tracker also demonstrates the high rates of hospital admission (∼1 in 5) in pregnant women with COVID-19, many of whom go on to receive intensive respiratory support. The available data from January 22, 2020 to March 7, 2022 includes 187,751 cases of COVID-19 in pregnancy, of whom 293 (0.2%) died. Of the 150,920 with available data, 30,327 (20.1%) were hospitalized. Of these, 689 of 18,293 (3.8%) were admitted to the intensive care unit, 139 of 13,303 (1.0%) required invasive ventilation, and 47 of 12,462 (0.4%) required additional advanced life support with extracorporeal membrane oxygenation (6). Risk factors associated both with being infected and hospitalized with COVID-19 during pregnancy include ethnic minority backgrounds, body mass index above 25 kg/m2, prepregnancy comorbidity (e.g., diabetes or hypertension), maternal age of 35 yr or older, increased socioeconomic deprivation, and working in healthcare or other public-facing occupations. There are no data yet specifically reporting on exposure to the Omicron variant in pregnancy. While this variant may be associated with less severe disease than the delta variant in the general population, it is more infectious and so likely to be associated with adverse maternal and neonatal outcomes, especially in pregnant women who are unvaccinated.
3. HAS THE PANDEMIC IMPACTED PREGNANCY OUTCOMES?
The broader impact of COVID-19 on pregnancy outcomes remains challenging to dissect and needs to be considered in light of the widespread changes in maternity service provision (7). There has been a global reduction in maternity healthcare seeking and healthcare provision during the pandemic, including reductions in antenatal clinic attendance and face-to-face consultations (7). Furthermore, the mitigation strategies introduced, including the national lockdowns, likely had a wider impact on pregnancy through additional pathways and exposures known to impact pregnancy outcomes, such as “stress” (8) and air pollution (9).
A striking example of where there have been inconsistencies in the literature are the data about preterm birth, stillbirth, and fetal growth restriction (TABLE 1). With increasing available data synthesized in systematic reviews, evidence now supports an approximately doubled risk of stillbirth in women with COVID-19 (5, 10, 12, 14–16). This is confirmed with recent data from the United States demonstrating that women with COVID-19 were at increased risk for stillbirth compared with women without [adjusted relative risk: 1.90, 95% confidence interval (CI): 1.69 to 2.15] among 1,249,634 delivery hospitalizations during March 2020–September 2021 (12). Notably, the magnitude of association was higher during the period of SARS-CoV-2 B.1.617.2 (Delta) variant predominance than during the pre-Delta period. Reports of two to three times higher rates of preterm birth (birth less than 37 wk of gestation) compared with background population data in symptomatic women are primarily medically indicated preterm births (4). In the most comprehensive systematic review and meta-analysis including data from 40 studies from Jan 1, 2020, to Jan 8, 2021, preterm births before 37 wk of gestation were not significantly changed overall (pooled odds ratio, 95% CI: 0.94, 0.87 to 1.02) (5). Notably, preterm births were decreased in high-income countries (0.91, 0.84 to 0.99), where spontaneous preterm birth was also decreased (0.81, 0.67 to 0.97), highlighting a concerning disparity between outcomes in high and low income settings (5). Few studies have reported both stillbirth and preterm birth in the same cohort, which is necessary to ascertain whether the cost of a reduction in preterm birth was an increase in stillbirth (17). Findings also need to be interpreted with caution due to risk of publication bias and multiple comparisons (11, 13). Newer data, from systematic reviews and a large multinational study, now also suggest that COVID-19 is associated with an increased incidence of small-for-gestational age babies (10, 18). However, overall outcomes for neonates appear positive, with very low rates of neonatal infection (4, 19).
Table 1.
Key systematic reviews and large observational studies published since the reviews
| Reviews/Studies/Authors | Country | Study Design | Participants | Preterm Birth Outcomes | Stillbirth Outcomes | Comments |
|---|---|---|---|---|---|---|
| Key systematic reviews | ||||||
| Wei et al. 2021 (10) | Systematic review and meta-analysis | 42 studies | Compared with no SARS-CoV-2 infection in pregnancy, COVID-19 was associated with preterm birth (OR: 1.82; 95% CI: 1.38 to 2.39) | Compared with no SARS-CoV-2 infection in pregnancy, COVID-19 was associated with stillbirth (OR: 2.11; 95% CI: 1.14 to 3.90) | ||
| Up to Jan. 29, 2021 | 438,548 people who were pregnant | Compared with mild COVID-19, severe COVID-19 was strongly associated preterm birth (OR: 4.29; 95% CI: 2.41 to 7.63) | ||||
| Chmielewska et al. 2021 (5) | Systematic review and meta-analysis | 40 studies | Preterm births before 37 wk of gestation were not significantly changed overall (pooled OR: 0.94; 95% CI: 0.87–1.02; I2 = 75%; 15 studies, 170,640 and 656,423 pregnancies) but were decreased in high-income countries (pooled OR: 0.91; CI: 0.84–0.99; I2 = 63%; 12 studies, 159,987 and 635,118 pregnancies), where spontaneous preterm birth was also decreased (pooled OR: 0.81; CI: 0.67–0.97; 2 studies, 4,204 and 6,818 pregnancies) | Significant increases in stillbirth (pooled OR: 1.28; 95% CI: 1.07–1.54; I2 = 63%; 12 studies, 168.295 pregnancies during and 198,993 before the pandemic) | Retrospective design of the included studies, as well as the heterogeneity of the study populations and the definitions and ways of measuring outcomes | |
| Jan 1, 2020 to Jan 8, 2021 | ||||||
| Yang et al. 2022 (11) | One study was from a low-income country, 17 were from middle-income countries, and 35 were from high-income countries | Living systematic review and meta-analysis | 52 studies | There was significant reduction in unadjusted estimates of preterm birth [43 studies, unadjusted odds ratio (uaOR): 0.95; 95% CI: 0.93–0.98], but not in adjusted estimates [5 studies, adjusted OR (aOR): 0.94; 95% CI: 0.74–1.19] | There was no difference in the odds of stillbirth between the pandemic and prepandemic time periods (32 studies, uaOR: 1.07; 95% CI: 0.97–1.18; and 3 studies, aOR: 1.18; 95% CI: 0.86–1.63) | There was significant publication bias for the outcome of preterm birth. |
| Data up to November 20, 2021 | 2,372,521 pregnancies during the pandemic period | There was reduction in spontaneous preterm birth (9 studies, uaOR: 0.91; 95% CI: 0.88–0.94) but not in induced preterm birth (8 studies, uaOR: 0.90; 95% CI: 0.79–1.01) | ||||
| 28,518,300 pregnancies during the prepandemic period | ||||||
| Large observational studies published since systematic reviews | ||||||
| DeSisto et al. 2021 (12) | United States | Hospital-based administrative database | 1,249,634 births | Not reported | The adjusted risk for stillbirth was higher in deliveries with COVID-19 (273/21,653, 1.26%) compared with deliveries without COVID-19 (7,881/1,227,981 (0.64%), adjusted relative risk: 1.90; 95% CI: 1.69–2.15 | Retrospective study, biases of routine data |
| March 2020 to September 2021 | ||||||
| Gurol-Urganci et al. 2022 (13) | England | National observational study of singleton births in English National Health Service hospitals | 948,020 singleton births | Preterm birth was slightly lower during the pandemic 6.0% vs. 6.1%, aOR: 0.96; 95% CI: 0.94–0.97; P < 0.001) | Stillbirth rates remained similar (0.36% pandemic vs. 0.37% prepandemic, P = 0.16) | Findings should be interpreted with caution, because differences were small and many comparisons were made |
| Compared births during the COVID-19 pandemic period (March 23, 2020 to February 22, 2021) with births during the corresponding calendar period 1 yr earlier | 451,727 births occurred during the defined pandemic period | |||||
| Litman et al. 2022 (14) | United States | Observational cohort study using national dataset | 683,905 women | Compared with women without COVID-19, women with COVID-19 were more likely to experience early preterm birth 3.2% vs. 2.2%, aOR: 1.38; 95% CI: 1.1–1.7 and late preterm birth 9.0% vs. 5.8%, aOR: 1.62; 95% CI: 1.3–1.7 | No significant difference in the prevalence of stillbirth between women with (n = 16/2,708) and without (n = 174/39,562) COVID-19 (0.6% vs. 0.5%, aOR: 1.46; 95% CI: 0.8–2.4) | This study is unable to differentiate iatrogenic preterm birth from spontaneous preterm birth, which may be an important driver of preterm birth |
| January 1, 2019 to May 31, 2021 | During the prepandemic period, 271,444 women were hospitalized for childbirth | Women diagnosed with COVID-19 within 30 days before hospitalization were more likely to experience early preterm birth (4.0% vs. 2.4%, aOR: 1.7; 95% CI: 1.1–2.6) | The prevalence of stillbirths occurring in women diagnosed with COVID-19 within the previous 30 days was significantly greater compared with women diagnosed with COVID-19 31–60 days, 60–120 days, or greater than 120 days before hospitalization (11/16 (68.8%), 0/16 (0 %), 3/16 (18.7%), 2/16 (12.5%), respectively, P < 0.001) | |||
| During the pandemic, 308,532 women were hospitalized for childbirth and 2,708 had COVID-19 | ||||||
| Stock et al. (15) | Scotland | National prospective cohort | 18,457 pregnant women; 2,364 babies born to women who had SARS-CoV-2 infection in pregnancy | 2,353 live births, of which 241 were preterm births (<37 of wk gestation; preterm birth rate 10.2%; 95% CI: 9.1−11.6) | 11 stillbirths (deaths in utero ≥ 24 wk of gestation) and 8 neonatal deaths (death within 28 days of birth), giving an extended perinatal mortality rate of 8.0 per 1,000 births following SARS-CoV-2 infection at any point in pregnancy (19 out of 2,364, 95% CI: 5.0−12.8). 10 of the stillbirths and 4 neonatal deaths occurred in babies born within 28 days of the onset of maternal infection, giving an extended perinatal mortality rate of 22.6 per 1,000 births (14 out of 620, 95% CI: 12.9−38.5) in this population. All perinatal deaths following SARS-CoV-2 infection in pregnancy occurred in women who were unvaccinated at the time of SARS-CoV-2 infection. | No access to detailed clinical records to assess whether COVID-19 directly or indirectly contributed to the preterm births and deaths seen following maternal infection |
| December 8, 2020 to October 31, 2021 | 610 of the live births and 101 of the preterm births occurred within 28 days of the date of onset of the mother’s SARS-CoV-2 infection, giving a preterm birth rate among babies born within 28 days of SARS-CoV-2 infection of 16.6% (95% CI: 13.7−19.8) | |||||
Studies are listed in chronological order. CI, confidence interval; OR, odds ratio; I2, I2 statistic.
These studies have all the limitations of observational studies and the caveats of using routinely collected data with key variables missing such as the gestation of infection, inclusion of asymptomatic cases detected on routine screening, unknown vaccination status, and lack of consideration of seasonal trends in patterns of preterm birth, among others. It is also impossible to determine which of the many sociodemographic, medical, obstetric, fetal, psychosocial, and environmental factors implicated in the etiology of preterm birth and stillbirth are the key drivers. Outcomes of ongoing multicountry collaborative initiatives such as the international Perinatal Outcomes in the Pandemic Study are eagerly awaited (20).
4. HAVE WE ADVANCED KNOWLEDGE ABOUT UNDERLYING MECHANISMS?
In our previous review we speculated on potential mechanisms whereby SARS-CoV-2 might impact placental (dys)function including binding and internalization of SARS-CoV-2 via the angiotensin-converting enzyme 2 (ACE2) receptor on endothelial cells with subsequent endothelial dysfunction and microthrombi (1). Two systematic reviews and meta-analyses, with data on almost 1,500 placentas, have reported a full spectrum of histopathological changes in the placenta of women with COVID-19 in pregnancy (21, 22). Less than a fifth of placentas had no abnormalities (21). Both vascular and inflammatory lesions consistent with placental hypoperfusion and inflammation were common. This has led to interest in whether SARS-CoV-2 placental invasion and infection is linked to development of preeclampsia (23, 24) and other complications of COVID-19 in pregnancy including fetal growth restriction. However, the more stringent systematic review, which aimed to avoid bias by only including publications with 10 or more cases and excluding cases that were reported only because of pathological findings, concluded that that there were no COVID-19-specific placenta changes and the incidence of vascular and inflammatory lesions was comparable to that of non-COVID-19 pregnancies (22). Further research is needed to separate the impact of acute maternal illness with maternal-fetal hypoxia in the context of pneumonia rather than viral-specific effects. While SARS-CoV-2 virus is detected in the placenta (25), it is still not known if this translates to fetal infection. Indeed, with inconsistencies in the literature as to whether the placenta coexpresses ACE2 and transmembrane protease serine 2 (TMPRSS2), it remains unclear as to whether transmission of virus from mother to fetus is even possible, particularly in the second and third trimesters (26).
5. WHAT ADVANCES IN TREATMENT ARE AVAILABLE FOR PREGNANT WOMEN WITH COVID-19?
Concerns about short- and long-term effects of drugs on the fetus have led to a reluctance of including pregnant women in clinical trials of new therapeutics for COVID-19 (27, 28). There is thus a paucity of studies on the treatment of pregnant women with COVID-19, with available data only from case series and studies with small sample sizes (29, 30). Both the WHO COVID-19 SOLIDARITY therapeutics trial (ISRCTN18066414) and the RECOVERY trial (ISRCTN50189673) include pregnant women, but recruitment rates are low. The RECOVERY trial enabled pregnant women to participate in evaluation of dexamethasone treatment, by substitution with prednisolone, but the number of pregnant women included was too small to draw pregnancy-specific conclusions (31). One clinical trial is currently investigating intervention with hydroxycholoroquine specifically for COVID-19 in pregnancy (NCT04410562) but another, using this intervention (NCT04365231), has been withdrawn, despite this drug having an established safety record in pregnancy. Importantly, national guidelines recommend that women should continue their scheduled antenatal care appointments, with particular consideration given to their mental health, as depression and anxiety have increased during the pandemic (5, 16). Pregnant women admitted to the hospital with confirmed or suspected COVID-19 should be offered prophylactic low-molecular-weight heparin for prevention of venous thromboembolism, unless birth is expected within 12 h or there is significant risk of hemorrhage (16). In case of clinical deterioration, treatment with corticosteroids, and consideration of tocilizumab (interleukin-6 receptor antagonist) or ronapreve (casirivimab and imdevimab) monoclonal antibodies, are recommended, despite the lack of direct evidence (16). The oral antiviral therapy molnupiravir is not recommended in pregnancy until further studies have established its effectiveness and safety (16). The conclusion of our previous review still holds: “Researchers should be urged to consider inclusion of pregnant women and other underrepresented groups to create a balanced and informed evidence base with data from a representative population” (1).
6. THE IMPORTANCE OF VACCINATION FOR PREGNANT WOMEN
More than 275,000 women in the United Kingdom and United States have had a COVID-19 vaccine in pregnancy with no concerning safety signals (32). Data from linked vaccine safety surveillance systems run by the CDC and US Food and Drug Administration have shown levels of pregnancy and neonatal outcomes in vaccinated populations similar to background (33, 34). Preclinical (animal) studies also show no adverse effects on reproduction or pregnancy outcomes.
Pregnant women were excluded from the premarketing trials of vaccines but postmarketing surveillance shows vaccines are as effective in pregnant women as in nonpregnant populations (35). For example, vaccine effectiveness against confirmed SARS-CoV-2 at ≥28 days following first vaccination was 78%, in line with that in clinical trials and observational studies in nonpregnant populations (35). There is also strong evidence that evidence of transplacental transfer of antibodies to the fetus following maternal COVID-19 vaccination in pregnancy, indicating vaccines have benefits for both women and babies (36–38). The international Pfizer vaccine trial (NCT04754594) and the Preg-CoV trial (ISRCTN15279830) on dosing schedules in pregnant women are underway, while a Jansenn vaccine trial (NCT04765384) is planned.
Policies on vaccination worldwide vary (39), but there is increasing strong guidance that vaccines are protective of severe disease and pregnancy complications (16, 40–42). Despite this, there remains low uptake of the COVID-19 vaccine among pregnant women in many parts of the world. Notably, severe disease and baby deaths are predominantly now in unvaccinated populations in high-income settings, with 98% of women admitted to the hospital and getting severe infection having not had the vaccine (15, 32). Pregnant women are recognized as a vulnerable group who are more at risk of severe COVID-19 disease (40) and increasing vaccine uptake is important in view of the emergence of new variants and the need for boosters in view of waning antibodies. It is essential to improve vaccine confidence among pregnant women and healthcare professionals and widen access to COVID-19 vaccines, as this appears the most promising way to optimize outcomes for women and their babies.
GRANTS
We acknowledge Medical Research Council Center for Reproductive Health Grant MR/N022556/1 and the support of the British Heart Foundation (RE/18/5/34216) and Tommys. S. J. Stock and J. A. Maybin are supported by Wellcome Trust Clinical Career Development Fellowships 209560/Z/17/Z and 209589/Z/17/Z, respectively.
DISCLOSURES
H. O. D. Critchley has clinical research support for laboratory consumables and staff from Bayer AG (paid to Institution) and provides consultancy advice (but with no personal remuneration) for Bayer AG, PregLem SA, Gedeon Richter, Vifor Pharma UK, AbbVie, and Myovant Sciences. H. O. D. Critchley receives royalties from UpToDate for an article on abnormal uterine bleeding. S. J. Stock has received research grants paid to the institution from Wellcome Trust, Scottish Chief Scientist Office, National Institute of Healthcare Research, and Tommys during the course of this study. No conflicts of interest, financial or otherwise, are declared by any of the other authors.
AUTHOR CONTRIBUTIONS
R.M.R. drafted manuscript; R.M.R., S.J.S., F.C.D., J.A.M., and H.O.D.C. edited and revised manuscript; R.M.R., S.J.S., J.A.M., and H.O.D.C. approved final version of manuscript.
REFERENCES
- 1.Wastnedge EA, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, Critchley HO. Pregnancy and COVID-19. Physiol Rev 101: 303–318, 2021. doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupferschmidt K, Vogel G. How bad is Omicron? Some clues are emerging. Science 374: 1304–1305, 2021. doi: 10.1126/science.acx9782. [DOI] [PubMed] [Google Scholar]
- 3.McAuley A, Gousias P, Hasan T, Rashid L, Richardson C, Reid G, Templeton K, McGuire J, Wise H, McVicar L, Jenks S, Gunn R, Dickson E, Stock SJ, Stockton A, Waugh C, Wood R, McMenamin J, Robertson C, Goldberg DJ, Palmateer NE. National population prevalence of antibodies to SARS-CoV-2 among pregnant women in Scotland during the second wave of the COVID-19 pandemic: a prospective national serosurvey. Public Health 199: 17–19, 2021. doi: 10.1016/j.puhe.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D, Balaji R, Lee SI, Qiu X, Yuan M, Coomar D, Sheikh J, Lawson H, Ansari K, van Wely M, van Leeuwen E, Kostova E, Kunst H, Khalil A, Tiberi S, Brizuela V, Broutet N, Kara E, Kim CR, Thorson A, Oladapo OT, Mofenson L, Zamora J, Thangaratinam S; for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 370: m3320, 2020. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol-Urganci I, O’Brien P, Morris E, Draycott T, Thangaratinam S, Le Doare K, Ladhani S, von Dadelszen P, Magee L, Khalil A. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Global Health 9: e759–e772, 2021. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#pregnant-population [2022 Mar 12].
- 7.Townsend R, Chmielewska B, Barratt I, Kalafat E, van der Meulen J, Gurol-Urganci I, O’Brien P, Morris E, Draycott T, Thangaratinam S, Doare KL, Ladhani S, Dadelszen PV, Magee LA, Khalil A. Global changes in maternity care provision during the COVID-19 pandemic: a systematic review and meta-analysis. EClinicalMedicine 37: 100947, 2021. doi: 10.1016/j.eclinm.2021.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobel CJ, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol 51: 333–348, 2008. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- 9.Bekkar B, Pacheco S, Basu R, DeNicola N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open 3: e208243, 2020. doi: 10.1001/jamanetworkopen.2020.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 193: E540–E548, 2021. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, D’Souza R, Kharrat A, Fell DB, Snelgrove JW, Shah PS. COVID-19 pandemic and population-level pregnancy and neonatal outcomes in general population: a living systematic review and meta-analysis (Update#2: November 20, 2021). Acta Obstet Gynecol Scand 101: 273–292, 2022. doi: 10.1111/aogs.14318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, Ellington SR. Risk for stillbirth among women with and without COVID-19 at Delivery Hospitalization—United States, March 2020–September 2021. MMWR Morb Mortal Wkly Rep 70: 1640–1645, 2021. doi: 10.15585/mmwr.mm7047e1external. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurol-Urganci I, Waite L, Webster K, Jardine J, Carroll F, Dunn G, Frémeaux A, Harris T, Hawdon J, Muller P, van der Meulen J, Khalil A. Obstetric interventions and pregnancy outcomes during the COVID-19 pandemic in England: a nationwide cohort study. PLoS Med 19: e1003884, 2022. doi: 10.1371/journal.pmed.1003884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litman EA, Yin Y, Nelson SJ, Capbarat E, Kerchner D, Ahmadzia HK. Adverse perinatal outcomes in a large US birth cohort during the COVID-19 Pandemic: adverse perinatal outcomes during COVID-19. Am J Obst Gynecol MFM 4: 100577, 2022. doi: 10.1016/j.ajogmf.2022.100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stock SJ, Carruthers J, Calvert C, Denny C, Donaghy J, Goulding A, Hopcroft LE, Hopkins L, McLaughlin T, Pan J, Shi T, Taylor B, Agrawal U, Auyeung B, Katikireddi SV, McCowan C, Murray J, Simpson CR, Robertson C, Vasileiou E, Sheikh A, Wood R. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med 28: 504–512, 2022. doi: 10.1038/s41591-021-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19), Pregnancy and Women’s Health. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy [2022 Mar 12].
- 17.Hedley PL, Hedermann G, Hagen CM, Bækvad-Hansen M, Hjalgrim H, Rostgaard K, Laksafoss AD, Hoffmann S, Jensen JS, Breindahl M, Melbye M, Hviid A, Hougaard DM, Krebs L, Lausten-Thomsen U, Christiansen M. Preterm birth, stillbirth and early neonatal mortality during the Danish COVID-19 lockdown. Eur J Pediatr 181: 1–10, 2021. doi: 10.1007/s00431-021-04297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr 175: 817–826, 2021. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvatore CM, Han JY, Acker KP, Tiwari P, Jin J, Brandler M, Cangemi C, Gordon L, Parow A, DiPace J, DeLaMora P. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health 4: 721–727, 2020. doi: 10.1016/S2352-4642(20)30235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stock SJ, Zoega H, Brockway M, Mulholland RH, Miller JE, Been JV, et al. The international Perinatal Outcomes in the Pandemic (iPOP) study: protocol. Wellcome Open Res 6: 21, 2021. doi: 10.12688/wellcomeopenres.16507.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Girolamo R, Khalil A, Alameddine S, D’Angelo E, Galliani C, Matarrelli B, Buca D, Liberati M, Rizzo G, D’Antonio F. Placental histopathology after SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 3: 100468, 2021. doi: 10.1016/j.ajogmf.2021.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suhren JT, Meinardus A, Hussein K, Schaumann N. Meta-analysis on COVID-19-pregnancy-related placental pathologies shows no specific pattern. Placenta 117: 72–77, 2022. doi: 10.1016/j.placenta.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabre M, Calvo P, Ruiz-Martinez S, Peran M, Oros D, Medel-Martinez A, Strunk M, Benito Ruesca R, Schoorlemmer J, Paules C. Frequent placental SARS-CoV-2 in patients with COVID-19-associated hypertensive disorders of pregnancy. Fetal Diagn Ther 48: 801–811, 2021. doi: 10.1159/000520179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosier H, Farhadian SF, Morotti RA, Deshmukh U, Lu-Culligan A, Campbell KH, et al. SARS-CoV-2 infection of the placenta. J Clin Invest 130: 4947–4953, 2020. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, Benachi A, De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 11: 3572, 2020. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol 226: 177–186, 2022. doi: 10.1016/j.ajog.2021.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor MM, Kobeissi L, Kim C, Amin A, Thorson AE, Bellare NB, Brizuela V, Bonet M, Kara E, Thwin SS, Kuganantham H, Ali M, Oladapo OT, Broutet N. Inclusion of pregnant women in COVID-19 treatment trials: a review and global call to action. Lancet Glob Health 9: e366–e371, 2021. doi: 10.1016/S2214-109X(20)30484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costantine MM, Landon MB, Saade GR. Protection by exclusion: another missed opportunity to include pregnant women in research during the coronavirus disease 2019 (COVID-19) pandemic. Obstet Gynecol 136: 26–28, 2020. doi: 10.1097/AOG.0000000000003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arco-Torres A, Cortés-Martín J, Tovar-Gálvez MI, Montiel-Troya M, Riquelme-Gallego B, Rodríguez-Blanque R. Pharmacological treatments against COVID-19 in pregnant women. J Clin Med 10: 4896, 2021. doi: 10.3390/jcm10214896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giesbers S, Goh E, Kew T, Allotey J, Brizuela V, Kara E, Kunst H, Bonet M, Thangaratinam S, PregCOV-19 Group. Treatment of COVID-19 in pregnant women: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 267: 120–128, 2021. doi: 10.1016/j.ejogrb.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19. New Engl J Med 384: 693–704, 2021. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.UK Obstetric Surveillance System. Key Information on COVID-19 in Pregnancy. https://www.npeu.ox.ac.uk/ukoss/news/2172-covid-19-in-pregnancy [2022 March 12].
- 33.Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, Ellington SR, Burkel VK, Smoots AN, Green CJ, Licata C, Zhang BC, Alimchandani M, Mba-Jonas A, Martin SW, Gee JM, Meaney-Delman DM, CDC v-safe COVID-19 Pregnancy Registry Team. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med 384: 2273–2282, 2021. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zauche LH, Wallace B, Smoots AN, Olson CK, Oduyebo T, Kim SY, Peterson EE, Ju J, Beauregard J, Wilcox AJ, Rose CE, Meaney-Delman D, Ellington SR. Receipt of mRNA COVID-19 vaccines preconception and during pregnancy and risk of self-reported spontaneous abortions, CDC v-safe COVID-19 Vaccine Pregnancy Registry 2020-21 (Preprint). Res Sq rs.3.rs-798175, 2021. doi: 10.21203/rs.3.rs-798175/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, Segal Y. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA 326: 728–735, 2021. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collier AY, McMahan K, Yu J, Tostanoski LH, Aguayo R, Ansel J, Chandrashekar A, Patel S, Apraku Bondzie E, Sellers D, Barrett J, Sanborn O, Wan H, Chang A, Anioke T, Nkolola J, Bradshaw C, Jacob-Dolan C, Feldman J, Gebre M, Borducchi EN, Liu J, Schmidt AG, Suscovich T, Linde C, Alter G, Hacker MR, Barouch DH. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA 325: 2370–2380, 2021. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prabhu M, Murphy EA, Sukhu AC, Yee J, Singh S, Eng D, Zhao Z, Riley LE, Yang YJ. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet Gynecol 138: 278–280, 2021. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beharier O, Plitman Mayo R, Raz T, Nahum Sacks K, Schreiber L, Suissa-Cohen Y, Chen R, Gomez-Tolub R, Hadar E, Gabbay-Benziv R, Moshkovich YJ, Biron-Shental T, Shechter-Maor G, Farladansky-Gershnabel S, Yitzhak Sela H, Benyamini-Raischer H, Sela ND, Goldman-Wohl D, Shulman Z, Many A, Barr H, Yagel S, Neeman M, Kovo M. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest 131: e150319, 2021. doi: 10.1172/JCI150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berman Institute of Bioethics & Center for Immunization Research, Johns Hopkins University. Covid-19 Maternal Immunization Tracker (COMIT). https://www.comitglobal.org/ [2022 Mar 12].
- 40.Boelig RC, Aagaard KM, Debbink MP, Shamshirsaz AA, SMFM Research Committee. Society for maternal-fetal medicine special statement: COVID-19 research in pregnancy: progress and potential. Am J Obstet Gynecol 225: B19–B31, 2021. doi: 10.1016/j.ajog.2021.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Royal Australian and New Zealand College of Obstetricians and Gynaecologists. COVID-19 Vaccination in Pregnant and Breastfeeding Women and Those Planning Pregnancy. https://ranzcog.edu.au/statements-guidelines/covid-19-statement/covid-19-vaccination-information [2022 Mar 12].
- 42.World Health Organization. Information for the Public: COVID-19 Vaccines. https://www.who.int/westernpacific/emergencies/covid-19/information-vaccines [2022 Mar 12].


