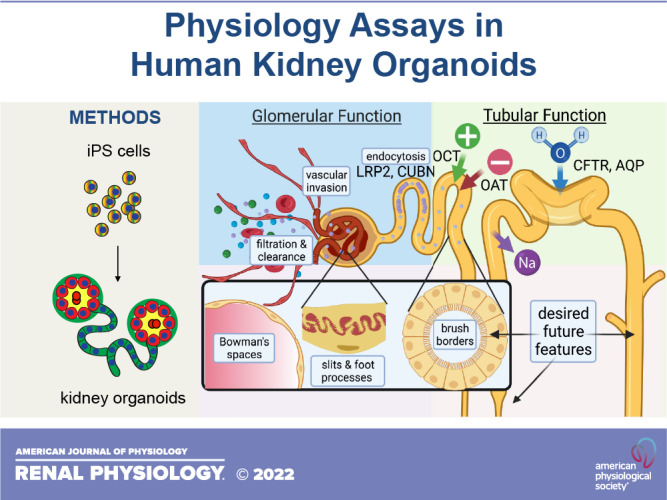
Keywords: absorption, filtration, hormone, kidney-on-chip, nephrotoxicity
Abstract
Kidney organoids derived from human pluripotent stem cells constitute a novel model of disease, development, and regenerative therapy. Organoids are human, experimentally accessible, high throughput, and enable reconstitution of tissue-scale biology in a petri dish. Although gene expression patterns in organoid cells have been analyzed extensively, less is known about the functionality of these structures. Here, we review assays of physiological function in human kidney organoids, including best practices for quality control, and future applications. Tubular structures in organoids accumulate specific molecules through active transport, including dextran and organic anions, and swell with fluid in response to cAMP stimulation. When engrafted into animal models in vivo, organoids form vascularized glomerulus-like structures capable of size-selective filtration. Organoids exhibit metabolic, endocrine, injury, and infection phenotypes, although their specificity is not yet fully clear. To properly interpret organoid physiology assays, it is important to incorporate appropriate negative and positive controls, statistical methods, data presentation, molecular mechanisms, and clinical data sets. Improvements in organoid perfusion, patterning, and maturation are needed to enable branching morphogenesis, urine production, and renal replacement. Reconstituting renal physiology with kidney organoids is a new field with potential to provide fresh insights into classical phenomena.
THE IMPORTANCE OF STUDYING PHYSIOLOGY IN ORGANOIDS
“What I cannot create, I cannot understand.”
—Richard Feynman, 20th century quantum physicist and Nobel laureate
The study of renal physiology–the normal functions of the kidneys–encompasses a wide variety of areas including fluid and electrolyte regulation, acid-base balance, clearance and reabsorption, and endocrine roles. This rich tapestry has for decades provided a fascinating framework in which to decipher fundamental biological mechanisms across a wide spectrum of model organisms. Understanding renal physiology is also important for the development of renal replacement therapies, as these must approximate normal renal function.
Recently, it has become possible to differentiate primitive nephron-like structures, known as kidney organoids, from human pluripotent stem cells (hPSCs), using a variety of distinct protocols (1–5). Such organoids constitute a new model system and raise new possibilities for studies of human renal physiology. Before delving into the details, we might ask ourselves: why is it important to study physiology in these structures? After all, there are already many established systems in which to investigate kidney physiology, with bona fide organs that may be considered more sophisticated and functional than organoids. While acknowledging the value inherent in model organisms, we would ask the reader to consider the following reasons for pursuing physiology studies in this particular model:
Species specificity: The organoids we will discuss are human and express genes and proteins whose sequences are encoded in the human genome. Thus, they may be advantageous for studies of molecules that specifically interact with human cells, genes, or proteins. The specificity of the organoid system for human studies is a unique advantage, particularly as humans in vivo typically cannot serve as subjects of experimental investigations to assess physiology.
Reconstitution: Organoids can be used to determine which types of functionality can be reconstituted in vitro and under which conditions. In doing so, the investigator has the opportunity to fulfill Dr. Feynman’s dictum and gain a more comprehensive understanding of the physiological mechanisms necessary and sufficient to produce functions. This is a unique advantage over model organisms which arrive “fully built.”
Flexibility: Organoids derived from hPSCs, which are a stable and immortal cell type, can be generated from individuals with interesting physiological features, even if the underlying mechanisms are not yet known. hPSCs can also be subjected to gene editing to introduce a wide variety of mutations, including those not found in the natural world or that would be lethal in a living organism.
Experimental accessibility: Organoids can be readily observed through a microscope for time-lapse imaging or perturbed by adding a substance to the culture media. Systematic substitution approaches can be taken to dissect mechanisms or reveal phenotypes (6). The accessibility of these structures to a wide variety of experimental approaches enables mechanistic interrogation and detailed analysis. This includes methodologies that would be difficult to conceive in vivo.
Scalability: Organoids are miniaturizeable and automatable, which means that organoids can be simultaneously interrogated with numerous compounds or cargoes to investigate physiology (7, 8). There is also potential for genetic screening approaches.
Novelty: Because the field is relatively new, organoids offer unique glimpses into areas of physiology that have been previously studied in other systems. There is much left to learn with organoids and many discoveries yet to be made. This is a strength of the system compared with other models.
In the years since these organoids were first described, much progress has been made in the descriptive analysis of the cell types within these cultures, resulting in stunning images and large single-cell RNA sequencing data sets. Yet, the fundamental functionality of these structures has remained largely uninvestigated. We still do not clearly understand how a kidney organoid compares functionally with kidney tissue. This concise review focuses on the known physiology of human kidney organoids and the criteria needed to answer this question.
DEFINITION OF KIDNEY ORGANOIDS
It is important to briefly define the key features of kidney organoids at a descriptive level that may be most relevant to their physiology. For the purposes of brevity, we will focus primarily on the “proximal nephron” kidney organoids derived from hPSCs that are currently in common use and best characterized. These kidney organoids contain epithelial structures that resemble the derivatives of nephron progenitor cells found in the metanephric mesenchyme in vivo. In the embryo, such nephron progenitor cells differentiate into a spectrum of proximal nephron cell types ranging from podocytes (most proximal) to the connecting tubule (most distal). They do not naturally give rise to the collecting ducts, which derive from a separate stem cell pool, the ureteric bud (UB) (9–12). Rather, the UB sustains and balances the nephron progenitor cell pool via mutually inductive interactions to produce branching morphogenesis of an arborized ductal network (13). Although kidney organoids can be derived from mice, most studies are of human origin and that will be our focus here.
The kidney organoid epithelium comprises contiguous segments of podocytes, proximal tubules, and distal tubules in proximal to distal order, with cell morphology and marker expression similar to in vivo. This descriptive feature, which can be discerned with immunofluorescence, is the strongest identifying hallmark of kidney organoids, common to many differentiation protocols (1–5). The “distal tubule” segment in organoids may express markers of both the distal convoluted tubule and loops of Henle (5, 8, 14). Such organoids are generally not believed to contain the UB or collecting ducts (2, 15). Although one early study suggested that these are present (4), this notion has been subsequently corrected in later publications (14, 16). Generation of putative UB-like organoids from hPSCs has also been reported, in a distinct set of differentiation protocols, but these are less established than proximal nephron-like kidney organoids (15–20). Thus, for the remainder of this article the term “kidney organoid” will refer specifically to the proximal nephron-like organoids that differentiate from hPSCs.
QUALITY CONTROL FOR KIDNEY ORGANOIDS
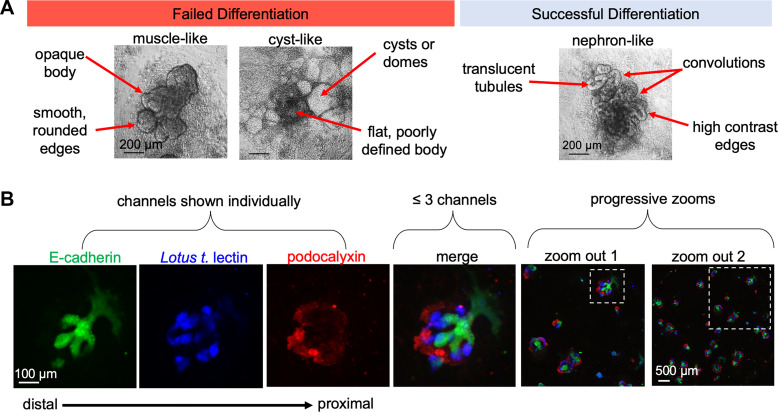
Because organoids take weeks to derive, there is substantial batch-to-batch variability and even organoid-to-organoid variability within the same batch; therefore, internal quality control is very important (7, 21, 22). Partially differentiated cultures frequently contain epithelial structures, which can be readily mistaken for kidney organoids by the untrained eye (Fig. 1A). It is therefore critical to include strong quality control data demonstrating that the organoids express basic identifying hallmarks, such as the formation of podocytes, proximal tubules, and distal tubules in the proper segmental order (Fig. 1B). These populations should be clearly identified based not only on the presence of markers specific for each segment but also by the unique morphology of the cells and structures (Table 1). Individual fluorescence channels should be shown separately in primary colors, with no more than three colors combined into the merged image, and alongside “zoomed-out” views of the larger microscopic field (Fig. 1B). These presentation practices enable the eye to readily discern overlap between the different channels and to assess the reproducibility and efficiency of differentiation. This is all the more important when modifications have been made to established differentiation protocols, in which case the effects of the modification should be clearly shown in side-by-side comparisons with the original.
Figure 1.
Quality controls improve organoid data sets. A: representative images depicting morphology of kidney organoid cultures in failed versus successful differentiations. B: “channel panel” demonstrating segment identity in organoids, with zoom-out images to show reproducibility within the culture.
Table 1.
Nephron segment markers in organoids
| Segment | Morphology | Location | Immunofluorescence Markers |
|||||
|---|---|---|---|---|---|---|---|---|
| NPHS1 | SYNPO | PODXL | CUBN | LTL | CDH1 | |||
| Podocyte | Bulbous | Periphery | + | + | + | − | − | − |
| Proximal tubule | Straight tubular | Middle | − | − | +/− | + | + | +/− |
| Distal tubule | Branching tubular | Center | − | − | − | − | +/− | + |
NPHS1, nephrin; SYNPO, synaptopodin; PODXL, podocalyxin; CUBN, cubilin; LTL, Lotus tetragonolobus lectin; CDH1, E-cadherin.
Given that a single batch of differentiation may produce numerous organoids, and there is some degree of heterogeneity between these, how many batches are needed to demonstrate reproducibility? In general, to demonstrate reproducibility of any result, the minimum number of independent biological replicates generally accepted by the scientific community is three. In the case of organoids, the following guidelines are strongly recommended as a minimal basis for reproducibility:
Statistical analysis should only be performed on data sets consisting of at least three independent biological replicates. Independent biological replicates can be three distinct batches of the same cell line (differentiations initiated on a different day) or a single batch consisting of three distinct cell lines (e.g., three different mutants of the same gene). Three different organoids from the same cell line and produced in the same batch would be considered technical replicates, not biological replicates, as these were produced under identical conditions and are all part of the same “body,” as it were (no independent variable).
For the purposes of statistical analysis, any appropriate unit may constitute a single n value for quantification, but these should be pooled from no less than three independent experiments.
More than one genotype (founder cell line) should be used as a starting point for organoid experiments. In particular, at least one male and one female hPSC line should be evaluated.
For instance, to quantify organoid size, each individual organoid may constitute a single n and its area measured as a source of statistical data. If 3 batches of organoids are generated, with each batch containing 10 organoids, then n = 30 organoids pooled from 3 independent experiments. If 2 cell lines were used in each of these batches (each line producing 10 organoids/batch), then n = 60 organoids pooled from 6 independent experiments. Either of these would constitute acceptable scenarios for statistical analysis. If, however, a single batch of 100 organoids was produced from a single cell line, these would all be technical replicates and it would be inappropriate to conduct statistical analysis from a single experiment.
Even more qualitative types of data (e.g., immunofluorescence channel panels) should be representative of multiple independent trials and ideally multiple genotypes of organoids. One approach to reduce variability is to aliquot and cryopreserve large batches of hPSCs from a single passage and then thaw a separate vial for use before each differentiation experiment (23). Whether such experiments should be considered biological replicates or technical replicates is not yet clear and needs to be determined by the scientific community. Whether separate batches or single batches of hPSCs were used as starting material for organoid differentiation should be clearly described in the figures and methods section of a publication. In general, use of multiple batches of hPSCs and multiple cell lines is to be encouraged. These practices will help to prevent errors due to small sample sizes and ensure that the published literature reflects what is not only possible but practical and reproducible.
It is acknowledged that investigators who are new to the field may find it challenging to follow the published protocols to produce well-differentiated cultures, due to the inherent heterogeneity of these cultures and batch-to-batch variability of reagents obtained from different commercial vendors. Successful differentiation can be a challenge even in laboratories with substantial expertise in generating kidney organoids and all the more so for new investigators hoping to join the field. In this regard, STEMCELL Technologies has recently released a commercially available kit to produce human kidney organoids, with high internal rigor and quality control (24). This may be a good starting point for the organoid initiate.
Currently, quality control for kidney organoids is largely limited to nephron marker analysis to demonstrate heterocellularity in proper segment order. As the field evolves, however, it will be important to complement such descriptive controls with a set of standard functional assays. Given the qualitative and descriptive nature of nephron marker analysis, physiology assays could provide a more quantitative and functional assessment of organoid quality. Establishing such assays is therefore important not only for understanding physiology but also as a means of standardizing these cultures and producing high-quality results.
FEATURES OF ORGANOIDS RELEVANT TO PHYSIOLOGY
Epithelial cells in kidney organoids have specific characteristics that are highly relevant to physiology studies. In addition to proximal-to-distal patterning, the epithelium exhibits apicobasal polarization, comprising linear arrays of cells that are aligned laterally and connected by apical junctions and basal lamina that are readily distinguished (2, 25). Related to this, organoids endogenously express transporters and receptors relevant to kidney physiology, such as organic anion transporter (OAT)1, megalin, and cubilin, that are appropriately segregated to either apical or basal membranes (7). Organoid tubular cells exhibit a columnar morphology and form elongated cylinders that branch or spiral, whereas podocytes exhibit a more bulbous cell shape, owing to their separation from one another along the lateral membranes, and form tight clusters that are roughly spheroid (25, 26). When purified out of the native organoids and replated onto an adherent substrate, epithelial cells within these segments migrate outward to form a monolayer of flatter epithelial cells with increased surface area and reduced three-dimensionality (6, 26).
Kidney organoids can adopt disease states, which can be genetic or acquired. For instance, organoids with mutations in genes associated with polycystic kidney disease undergo tubular swelling, which produces large cysts (2, 6). Although disease modeling in organoids is largely outside the scope of this review, which focuses on normal physiology, we will consider cases where disease states reveal physiological features. Similarly, new kidney organoid differentiation protocols are often presented through the lens of embryonic kidney development. As the focus here is on physiology, we will consider the developmental biology aspect only as it relates to functional differences.
With these considerations in mind, Table 2 shows a summary of physiology assays currently in use in kidney organoids. The following sections provide a detailed description of each of these.
Table 2.
Physiology assays in human kidney organoids
| Function | Molecular Agent | Nephron Segment(s) | References |
|---|---|---|---|
| Accumulation | Dextran | Tubules | Freedman et al. (2); Takasato et al. (4); Low et al. (27) |
| Fluorescein Methotrexate | Tubules | Freedman et al. (2) | |
| Fluorescein | Tubules | Shankar et al. (28) | |
| 6-Carboxyfuorescein | Tubules | Przepiorski et al. (29) | |
| Swelling | Forskolin | Tubules | Cruz et al. (6); Low et al. (27); Shimizu et al. (30); Kuraoka et al. (31) |
| 8-Bromo-cAMP | Tubules | Cruz et al. (6); Low et al. (27) | |
| Filtration | Blood | Endothelium | Sharmin et al. (32); van den Berg et al. (33); Nam et al. (34);Kumar Gupta et al. (35) |
| Dextran | Endothelium, podocytes | van den Berg et al. (36); Kumar Gupta et al. (35) | |
| Endocrine | Renin | Stroma | Shankar et al. (28) |
| Vitamin D | Undetermined | Shankar et al. (37) | |
| Injury | Cisplatin | Tubules, stroma | Freedman et al. (2); Morizane et al. (3); Takasato et al. (4); Czerniecki et al. (7); Digby et al. (38); Gupta et al. (39) |
| Gentamicin | Tubules | Freedman et al. (2); Morizane et al. (3); Lawrence et al. (40) | |
| Hemin | Tubules | Przepiorski et al. (29) | |
| SARS-CoV-2 | Tubules | Helms et al. (41); Jansen et al. (42) |
SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
CARGO ACCUMULATION IN ORGANOID TUBULES
A well-established “physiology assay” for human kidney organoids is their ability to specifically accumulate certain fluorescent molecules. In these experiments, fluorescent molecules are added to the organoid media for a period (pulse), after which the media is exchanged with fresh media lacking these molecules, and accumulation of fluorescence is assessed in the structures for a second period (chase) (2). It is difficult to monitor the fluorescent molecules during the pulse period because the media contains abundant nonspecific signals; therefore, the fate of the molecules is interpreted based on their localization patterns during the chase. Organoids tend to accumulate certain molecules in these experiments but not others. To date, a rather limited subset of cargoes has been tested, but the findings suggest that organoids exhibit a specificity that partially mimics tubules microdissected from kidneys in vivo. Specific examples are described below in this section.
Dextrans
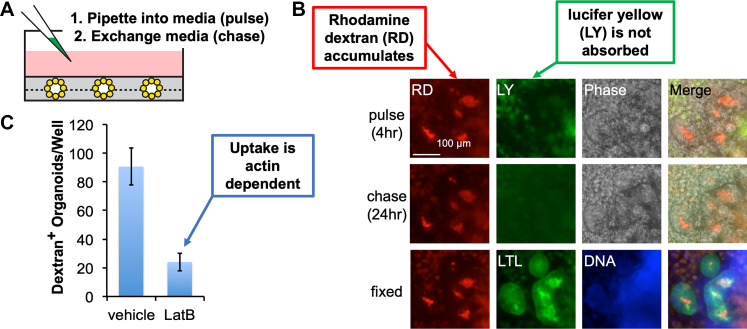
Several groups have now demonstrated that kidney organoid tubules tend to accumulate fluorophore-conjugated dextrans, branched chains of polysaccharides that are generally inert and freely soluble. Dextrans are commercially available in a wide range of molecular weights (3–2,000 kDa) and in fluorescence-tagged forms. Accumulation of 10-kDa dextran is observed in ∼80% of organoids after 4 h of pulse, and the signal remains visible for 24 h afterward in live cultures (Fig. 2A) (2). After fixation, immunofluorescence analysis indicates that dextran specifically accumulates in organoid proximal tubules [Lotus tetragonolobus lectin (LTL)+], close to the apical surface inside the tubules (Fig. 2B). Cotreatment of organoids with latrunculin B, which inhibits actin polymerization, inhibits dextran accumulation, suggesting that the mechanism depends on active transport (Fig. 2C) (2). Organoids accumulate dextrans up to 70 kDa in molecular weight, but not 2,000-kDa dextrans, in 4-h pulse-chase assays (27). Another study, utilizing a distinct protocol for kidney organoid differentiation, has similarly reported that 1 h of pulse is insufficient while 24 h results in dextran accumulation (4). Accumulation of dextran in organoids has been suggested to resemble its accumulation in isolated rabbit proximal tubules or mouse embryonic kidney explants (2, 4, 43).
Figure 2.
Kidney organoids accumulate fluorescent dextran. A: schematic depicting the addition of dextran. Organoids are depicted as bouquet structures sandwiched in Matrigel (gray) at the bottom of the culture dish. B: wide-field immunofluorescence images showing pulse chase of rhodamine-labeled dextran (RD) versus lucifer yellow (LY) in kidney organoids. Organoids are fixed at 24 h, enabling colocalization with proximal tubules [Lotus tetragonolobus lectin (LTL)]. C: quantification of dextran accumulation in the presence or absence of latrunculin B (LatB). [Images were adapted from Freedman et al. (2) via CCBY 4.0 license.]
Organic Anions
Kidney organoids can accumulate certain organic anions when added to the media. Compared with dextran accumulation, organic anion transport in organoids is less studied, with individual molecules typically limited to a single report. Organoids can accumulate fluorescein methotrexate, a fluorescent dye of molecular weight 949 Da (2). In organoids, fluorescein methotrexate accumulates with a similar distribution to dextran, which can be visualized simultaneously if the dextran is conjugated to a red fluorophore. During the chase period, fluorescein methotrexate appears to dissipate within 24 h; thus, its accumulation appears to be more reversible compared with dextran (2). Organoid tubules also accumulate fluorescein unconjugated to methotrexate after 1 h of treatment. Incubation of organoids at 4°C impairs their ability to accumulate fluorescein (28). 6-Carboxyfluorescein similarly appears to be taken up by tubular cells within organoids (29). The ability of organoids to accumulate fluorescein methotrexate resembles microdissected killifish proximal tubules, which transport this cargo into their lumens in vitro, a process believed to be mediated by multidrug resistance protein 2 (44). However, lucifer yellow, a green fluorescent organic anion, does not accumulate in organoids, even though it accumulates in killifish tubules (2, 45).
In summary, the ability of organoids to accumulate certain fluorescent molecules has been established and is perhaps the most common “physiology assay” for organoids in use today. The full range of cargoes, however, and the specific mechanisms underlying their transport remain to be elucidated.
TUBULAR SWELLING
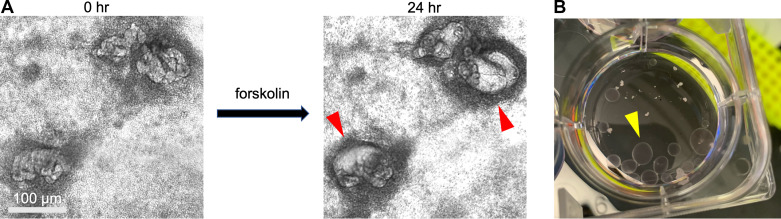
Tubular swelling is a dynamic assay that can be observed by bright-field microscopy. When treated with compounds that stimulate cAMP, tubular structures in organoids accumulate fluid and swell rapidly in size (Fig. 3A) (6, 27). This response occurs with both forskolin and 8-bromo-cAMP, suggesting that it is specific to the cAMP pathway (6, 27). Swelling is evident by 24 h after treatment and can continue for days after that. When forskolin is removed, the swelling recedes (6). While expanding in size, swollen tubules generally maintain their original shape, although they may also deform into cysts during this process (6, 27).
Figure 3.
Tubular swelling assays in kidney organoids. A: phase-contrast images of kidney organoid structures before and after treatment with forskolin, showing swelling effects (red arrowheads). B: photo showing one well of a six-well dish, containing polycystin 2 (PKD2)−/− organoids after several months of culture (yellow arrowhead).
The swelling observed in response to cAMP is similar in phenotype to the swelling of organoids carrying mutations in genes associated with polycystic kidney disease (PKD) (2, 6). Although the focus of this review is on normal physiological function, we will digress briefly to address this finding, as experiments in PKD organoids may shed light on the effects of forskolin in control organoids and vice versa. Organoids with loss-of-function mutations in genes associated with PKD, including polycystin 1 (PKD1), polycystin 2 (PKD2), and fibrocystin (PKHD1), naturally form cysts from kidney tubules, even in the absence of forskolin (2, 6, 7, 16, 27). These cysts can grow quite large over time, becoming macroscopic (Fig. 3B). Such cysts are not observed at a significant rate in isogenic control organoids, which are differentiated side by side with the PKD mutants (2, 6, 7, 16, 27). These organoids are quite distinct from other laboratory models that require forskolin to produce cysts, such as primary kidney epithelial cells (46, 47).
In organoids, only a subset of tubular structures swell, and different tubules swell at different rates, although the mechanism underlying this heterogeneity remains to be explored (2, 6, 27). Under standard differentiation conditions, the rate of cystogenesis is rather low, typically ∼15% of nephron structures (2). This cystogenesis rate increases dramatically when organoids are transferred from adherent to suspension cultures (6). Using some differentiation protocols, PKD organoids are reportedly unable to naturally form cysts, and forskolin is required to elicit cyst formation (30, 31). Some reports have suggested that, when treated with forskolin, PKD organoids form cysts at a higher rate than isogenic controls (27, 30, 31), whereas other reports indicated that these form cysts at a similar rate (6). Differences in organoid differentiation protocol, cell lines, or timing of treatments may account for these discrepancies, but this has not been tested in a formal way.
cAMP activates protein kinase A, which can phosphorylate cystic fibrosis transmembrane conductance regulator (CFTR) and promote its ability to transport Na+ and Cl− through cell membranes. When coadministered with forskolin, the CFTR inhibitor CFTRinh172 reduced tubular swelling of PKD organoids (27). These findings suggest that forskolin causes tubular swelling by increasing ion transport through CFTR. Such a mechanism would involve water transport as a follower of Cl− transport. This parallels an assay used in intestinal organoids, which also swell in response to forskolin in a CFTR-mediated process (48). It should be noted, however, that recent genetic experiments indicate that CFTR is unnecessary for the PKD phenotype in vivo, as inducible Cftr−/−Pkd1−/− mice still manifest PKD (49). Interestingly, thapsigargin, which inhibits the endoplasmic reticulum Ca2+-ATPase, also reduces cyst formation in PKD organoids, although the mechanism underlying this is less clear (27). Swollen cysts do not appear capable of accumulating dextran, compared with nonswollen tubules (27).
Historically, swelling or transport activities in isolated tubules or PKD cysts have often been coupled with electrophysiology assays, which provide useful correlative readouts of ion channel activity and transport processes. The development of corresponding electrophysiology assays for kidney organoids would add a dimension to our understanding of these structures. A relatively simple assay would be the assessment of transepithelial electrical resistance across layers of organoid cells, as is frequently assessed by electrode in monolayer cultures of epithelial cells grown on transwell plates. Undifferentiated hPSCs, which are epithelial cells with tight junctions, can produce substantial transepithelial electrical resistance readings once they reach confluence, which provides a useful control for such studies (2). In the case of derived organoids, which are multilayer structures with substantial heterogeneity, further methodological developments are required to determine the appropriate context in which to apply such an electrode.
More sophisticated electrophysiological readouts could be obtained using a patch-clamp, which can be applied to individual cells to trace changes in membrane potential as channel gates open and close. For instance, patch-clamp techniques have been previously applied to complement dye uptake assays in cyst-lining epithelia dissected from rat kidneys (50). These could be readily adapted for the macroscopic, centimeter-diameter cysts that emerge from PKD organoids (6). Small-molecule channel blockers, such as benzamil or amiloride, which inhibit epithelial Na+ channel activity, could then be used to generate hypotheses regarding the specific mechanisms underlying conductance events. These hypotheses could be formally tested in organoids derived from gene-edited hPSCs harboring mutations that knock out specific channels or their modulators of channel activity. Such an assay could be applied, for instance, to evaluate the role of “with no lysine” kinase 1 on large-conductance K+ channels in human cells, to complement studies in other model systems (51).
GLOMERULAR FILTRATION
In renal terminology, a glomerulus is a cluster of capillaries at the proximal terminus of kidney nephrons, with specialized features that enable filtration of blood to form the urine. Although kidney organoids in vitro have resident endothelial cells, these do not form functional blood vessels or filtration-competent glomeruli (2, 4). Endothelial cells do not substantially invade the clusters of podocytes in these structures to form glomerular basement membranes, and this deficiency is obvious when comparing the structures with human kidneys in vivo (7). Thus, our ability to monitor glomerular function in organoids in vitro remains quite limited.
In contrast, when organoids are implanted into animals in vivo, human podocytes become internally vascularized with host mouse blood vessels, expanding and flattening around endothelial cells to form bouquets (32–35). As these resemble glomeruli structurally, but are likely immature and have uncertain functional properties, we will refer to them as “glomerulus-like structures.” Although most studies have been performed in mice, organoid grafts into the allantoic membranes of the chick embryo can also produce podocytes in bouquet arrangements surrounding laminin-lined lacunae, suggestive of glomerulus-like structures (52). A subpopulation of the cells on the interior of these structures expressed markers of the human vasculature, suggesting that they derived from the graft rather than the host, whereas other cells were of uncertain identity (52).
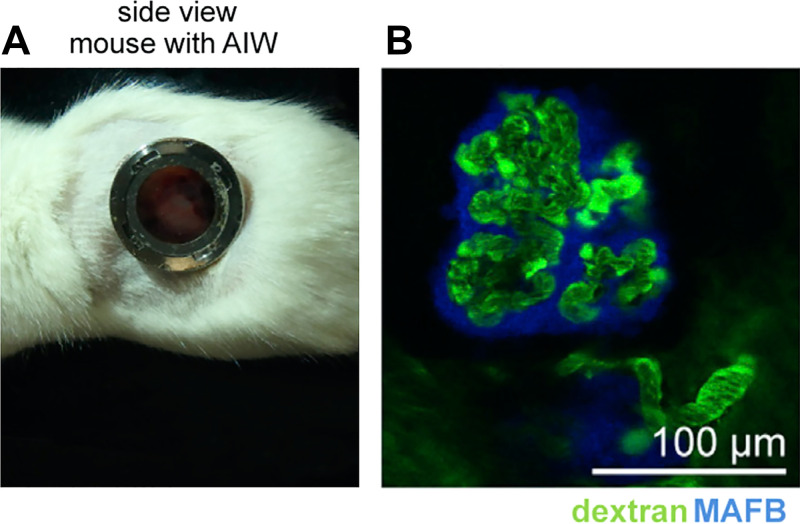
The extent to which glomerulus-like structures in such grafts in vivo can function or filter remains an area of active investigation. The capillaries have been observed to contain red blood cells, suggesting that there is some degree of vascular supply (32–35, 52). In the mouse, the placement of an abdominal window for intravital imaging has enabled live visualization of these glomerulus-like structures (Fig. 4A) (33). When fluorescent dextran is injected into the mouse bloodstream, it is immediately observed in the blood vessels of glomerulus-like structures (Fig. 4B) (33). When coinjected, dextrans of >40 kDa are retained in blood vessels, whereas dextrans of <40 kDa accumulate in tubular spaces immediately adjacent to the blood vessels and associated podocytes (36). These experiments are facilitated by incorporation of podocytes expressing a blue fluorescent marker, which makes it possible to identify dextran signal distal to the podocyte epithelial layer surrounding the capillaries. Filtration occurs in ∼40% of glomerulus-like structures, and fluorescent albumin (66 kDa) is selectively filtered out, similar to dextrans of >40 kDa (36).
Figure 4.
Human glomerulus-like structures in grafts are vascularized. A: abdominal imaging window (AIW) in a mouse, enabling microscopic analysis of subcapsular kidney organoid grafts. B: live fluorescence image showing FITC-dextran (green) in vascular structures in organoid grafts, acquired through the abdominal imaging window. Organoid podocytes express a blue fluorescent reporter and can be faintly discerned surrounding the vascular structures. [Images were reproduced without modification from van den Berg et al. (53) via CCBY-NC-ND license.]
Although these findings are certainly encouraging, it remains difficult to perceive exactly how sieving effects are being accomplished mechanistically in these structures, which is understandable given the technical challenges of performing these types of experiments in living animals. Although the simplest explanation is that the glomerulus-like structures can filter molecules in a size-selective manner, it is also conceivable that dextran accumulation represents leakiness of the capillaries, which could also be somewhat size-selective over the relatively short timescale of the experiments (minutes). Over such timescales, endothelial vessel structures may preferentially leak lower molecular weight dextrans (54). Longer timescales would allow for leakage to be detected more readily and present a more stringent test of size selectivity. It is noted, however, that nonspecific accumulation of dextran was not observed elsewhere in the graft or in the tubules, which would suggest that the endothelia were not particularly leaky (36). In addition to size selectivity, charge or shape could also contribute to the observed filtration phenotype, which has not yet been explored.
Besides dextran uptake, which is limited by the necessity of placing an abdominal window, a more comprehensive view of the kidneys can be gleaned using microcomputed tomography in live mice (35). In this technique, a contrast agent is first injected and the mouse is imaged on a microcomputed tomography scanner to detect uptake into the kidneys and associated subcapsular grafts. This reveals focal differences and microheterogeneities within the grafts, perhaps reflecting differential vascularization or blood flow (35).
In the above experiments, the native mouse kidney was far superior for filtration than the neighboring human graft, with brighter signal throughout its tubules, whereas graft accumulation of dextran or contrast agent was more difficult to observe (35, 36). This is understandable as well, as the grafts receive only a small fraction of the renal blood flow, and the glomerulus-like structures are not as well organized as actual glomeruli. As the tubules in the grafts are not connected to collecting ducts, there is no drainage mechanism, and this may also limit flow through these glomerulus-like structures. One “classic” physiology method that has not yet been attempted in these structures is micropuncture, which could be used to sample the nephron filtrate in different segments and determine its composition and pressure (53). The heterogeneity of organoids in grafts, their variable perfusion, and the lack of an appropriate outlet for the perfused liquid all represent formidable challenges in this regard. All in all, the findings indicate that glomerulus-like structures formed in vivo have a functional capacity to filter plasma components in a size-selective manner, which remains limited compared with bona fide glomeruli but is nonetheless an impressive accomplishment.
Limitations of the microenvironment in vivo include restricted access for experimentation and imaging as well as added heterogeneity due to the complex surgical procedures required. An alternative approach is to generate organoids in microfluidic devices that mimic the properties of grafts to enable studies of filtration and glomerular development. In one study, the passage of fluid over organoids was shown to increase the extent of vascular endothelial cell differentiation within these cultures and contacts between these endothelial cells and podocyte clusters, suggestive of increased invasion (55). The particular differentiation protocol used for this study does not generally produce a substantial population of endothelial cells under standard conditions (3); thus, microfluidics was necessary to increase endothelial differentiation. High flow in this microfluidic chip was considered to be 0.035 dyn/cm2, which is ∼30-fold lower than the rate of flow through nephrons in vivo.
Although a direct comparison between these chips and organoid grafts has yet to be performed, the structures produced in these chips did not appear to substantially resemble the glomerulus-like structures found in organoid grafts. Specifically, graft structures are more reminiscent of capillary loop stage glomeruli during embryonic development in vivo and exhibit an expansion and flattening of the podocyte layer around the endothelial capillaries, which was not apparent in chips in vitro (32–35). In other differentiation protocols, contacts between endothelial cells and podocytes, including a degree of invasion, have also been noted even in the absence of flow (2, 4, 27). It is thus generally recognized that cultures in vitro have not yet achieved the type of invasion observed in kidney organoid grafts in vivo, much less the level of function that occurs in actual kidney tissue. Production of organoids in vitro capable of forming glomerulus-like structures that achieve a substantial degree of selective filtration thus remains a future goal for the field.
METABOLISM AND ENDOCRINE FUNCTIONS IN ORGANOIDS
In addition to excretion, the kidneys play important metabolic and endocrine functions. The capacity of organoids to recapitulate these functions is beginning to be explored. Like many cell cultures, kidney organoids exhibit a capacity for mitochondrial respiration, with an oxygen consumption rate that can be measured in Seahorse instruments (52). The preference for oxidative metabolism arises during kidney organoid differentiation, as the undifferentiated pluripotent stem cell cultures tend to be more glycolytic based on transcriptional and metabolomic analysis (56). As the media and growth conditions are quite different before and after differentiation, and can clearly affect metabolism (52), it is difficult to know how much of the metabolic state can be attributed to cell state. The degree to which the metabolic state of kidney organoids is specific to the renal lineage, compared with other organ lineages, and how this state compares with kidney cells in vivo, which have high energy requirements, has not been clearly established.
Kidney organoid cultures have recently been shown to produce renin, a hormone that is naturally synthesized in vivo by the juxtaglomerular apparatus (28, 57). In organoids, renin expression is enriched in a stromal cell population, thought to represent pericytes, and to a lesser degree in epithelial cells. Levels of renin are increased 20-fold by the addition of forskolin or parathyroid hormone to the cultures (28). As there is a wide mixture of different cell types in kidney organoids, some that are specific to the kidney and some that are not, it is not yet clear whether these organoid renin-producing cells are the same as juxtaglomerular cells of renin lineage. Organoids also express angiotensin II receptors, and addition of recombinant angiotensin II during the time course of organoid differentiation can affect renal gene expression patterns (57). It is unclear whether kidney organoids naturally produce other hormones associated with the kidneys, such as erythropoietin. It is also important to establish whether organoids are capable of physiologically relevant production responses, for instance when transplanted into an appropriate context in vivo.
Metabolism of vitamin D is a physiological role of renal proximal tubules. Based on mRNA levels (quantitative PCR), organoid cultures express substantial quantities of the cytochrome P-450 24A1 and 27B1 (CYP24A1 and CYP27B1, respectively) enzymes involved in vitamin D metabolism and show a capacity to deplete 1,25(OH)2D3 from the culture media (37). Although these results suggest that organoids may have the potential to recapitulate renal functions, in neither of these studies were the cultures clearly shown to contain nephron-like structures comprising proximal tubules, distal tubules, and podocytes in proper proximal-to-distal contiguity.
The preceding sections summarize many of the organoid assays currently in use today, and particularly those that have been shown to work in more than one laboratory. In this catalog of organoid physiology assays, the scope has been intentionally limited to methodologies that have already been published. As this is a fertile and dynamic area of research, compendia of organoid functional assays are beginning to be developed as online resources. For instance, the National Institutes of Health-sponsored ReBuilding a Kidney Consortium has recently released a handbook of functional physiological assays suitable for organoids, cell culture, and model systems, including unpublished ones (https://www.rebuildingakidney.org/functional-assays/). Online resources, such as toolkits produced by consortium efforts or individual laboratories, may also include detailed protocols or data sets that can be probed and reanalyzed in different ways.
INJURY AND INFECTION
Kidney organoids are susceptible to injury with a variety of toxic agents, including clinically relevant nephrotoxicants such as cisplatin and gentamicin (2–4). At high doses (50 µM cisplatin or 5 mg/mL gentamicin), these factors cause deterioration of the structures by phase-contrast microscopy and expression of biomarkers associated with acute kidney injury, most prominently kidney injury molecule-1 (2, 3, 7, 38). Cisplatin treatment induces DNA damage, as reflected by γ-histone 2AX immunofluorescence and induction of homology-directed repair gene expression signatures (3, 38, 39). In addition to acute treatment at a high dose, repeated treatments at lower doses have cumulative effects on the cultures, which ultimately produce organoid deterioration (38, 39). Hemin, the oxidized form of heme, also induces DNA damage and apoptosis responses in organoids and impedes the accumulation of 6-carboxyfluorescein into tubules, suggesting that the damage has physiological consequences (29).
An important question is the degree to which such injury responses are specific to the kidneys as a whole and to specific types of cells within the kidneys. Undifferentiated hPSCs also disintegrate when exposed to gentamicin and cisplatin and indeed show greater sensitivity to cisplatin than derived organoids (2). While some experiments have determined that cisplatin specifically injures organoid proximal tubules (4), others have indicated that interstitial cells are the most severely affected (38). A blinded study of a small panel of compounds suggested that organoids exhibit sensitivity to compounds associated with clinical nephrotoxicity, as detected by a fluorescence readout of oxidative stress, although they were not perfect predictors and segment-specific effects were not detected (40). Assessment of segment specificity is also complicated by the absence of perfusion, which may contribute physiologically to uptake by the proximal tubules in vivo (40). Another concern is that organoid proximal tubules may lack appropriate organic cation transporter 2 (SLC22A2) expression for cisplatin accumulation, at least under certain growth conditions (38, 40). This underscores the importance of considering physiological mechanisms in nephrotoxicity analysis. Such mechanistic analysis would include not only assessment of expression level to verify the presence of the proposed target but also functional analysis (e.g., genetic knockout) to demonstrate the target’s importance.
The study of injury in organoids now extends beyond chemical treatments, to infectious agents. As the COVID-19 pandemic spread, it became clear that mice were not naturally susceptible to severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection, necessitating the use of human cellular models. Kidney organoids, in particular, demonstrated a strong tendency to become infected with the virus (41,42, 58). Infection results in distorted cell morphology and changes in gene expression suggestive of an injured state (41, 42). In the setting of PKD, apoptosis can be detected in infected cyst-lining epithelial cells derived from organoid proximal tubules (41). While these findings indicate a modicum of injury after infection, overt tubular necrosis of the cultures has not been observed, at least within the doses tested.
CONTROLS FOR FUNCTIONAL ASSAYS IN ORGANOIDS
Because the mechanisms underlying these functionalities have not yet been investigated in detail, it is important to avoid confirmation bias in interpreting what they mean. For instance, while it is tempting to attribute organic anion accumulation to the expression of OAT family transporters in kidney organoids, such a mechanism cannot be assumed. In this section, we will consider the types of controls that can be helpful in narrowing down the fidelity of organoid functional assays to their counterparts in organs.
Negative Controls
To demonstrate that an assay is kidney-specific, it is helpful to incorporate an alternative tissue lineage that is not expected to produce the same function. A particularly convenient “negative control” for organoid functions is undifferentiated hPSCs. These have two critical advantages:
They are polarized epithelial cells with tight junctions and the ability to form hollow spheroids (epiblast spheroids) in three-dimensional culture settings.
They are of identical lineage to the organoids from which they derive, which eliminates concerns regarding the genetic makeup of the cells.
Undifferentiated hPSCs have been used as controls for several of the assays described above. For instance, epiblast spheroids accumulate lucifer yellow in their lumens but exclude 10-kDa dextran, which is the opposite of what is observed in kidney organoids. (2). hPSCs also do not express renin, even in response to forskolin (28).
Undifferentiated hPSCs may not be an ideal control for every scenario, and there are caveats to using them, including the requirement for speciality media formulations that can affect functional outcomes. Another valuable negative control is nonkidney cells differentiated from the same hPSCs. For instance, simply lowering the concentration of CHIR99021 treatment can produce beating cardiomyocytes instead of kidney organoids (2). Such cardiomyocytes are of mesodermal origin and can grow in the same media as kidney organoids and thus might also be a useful negative control for certain types of experiments.
Ultimately, any cell type of nonkidney origin could serve as a negative control, depending on the specific feature of renal physiology that one seeks to model. The important consideration is the following: is the function observed specific to kidney organoids? Or is it a more general property, e.g., specific to epithelial cells? The use of an appropriate negative control enables the investigator to address this question at an early stage.
Positive Controls
Having a positive control for the assay under study is also very helpful. First, it establishes feasibility, i.e., it identifies conditions that can be used to ascertain whether the reagents and methods used to detect an effect are working properly. An appropriate positive control also trains the eye of the observer to understand what the physiology readout “looks like,” for instance, the distribution patterns and dynamic range of a signal. This is important because few readouts are truly binary and there can be significant “background noise” for any assay.
For a kidney physiology assay, the ideal positive control is actual kidney tissue in vivo or explants thereof (in cases where observation in vivo would be challenging). In the experiments described above, precedent from the literature suggests the use of certain models. These may include proximal tubules isolated from rabbits or killifish for assays of absorption or secretion (44), fetal kidney tissue used for engraftment beneath the kidney capsule or the neighboring kidney tissue itself (59), or metanephric explants from mouse kidneys for assays of branching, patterning, or differentiation.
Inclusion of a positive control can make the difference between correctly and incorrectly diagnosing kidney organoids. For instance, collecting ducts can be readily distinguished in human kidney tissue using Dolichos biflorus agglutinin lectin and aquaporin-2 staining, but these markers are negligible by comparison in kidney organoids, indicating that collecting ducts do not form (7). Parietal epithelial cells are also readily identified in human tissues, which led to the use of the marker combination PAX8 + LTL−PODXL− to identify corresponding cells in between proximal tubules and podocytes in organoids (7). In another example, kidney organoids were recognized early on to contain endothelial cells, but these were present only as a minor population and failed to successfully vascularize the podocytes to form glomeruli (2, 4). Comparative analysis of organoids with kidney explants derived from transgenic mice with fluorescent vasculature revealed that endothelial cell regression is a common phenomenon in these culture systems (60). Explants were also useful as positive controls for dextran uptake, providing a benchmark that was far stronger than the organoids cultured side by side (4).
Primary cell cultures of adult kidneys can also produce tubular or cystic organoids, sometimes called “tubuloids,” when grown in three-dimensional matrixes or suspensions (61, 62). Such tubuloids are thought to derive from terminally differentiated kidney epithelial cells rather than from nephron progenitor cells. As such, nondividing cells such as podocytes are not represented in tubuloid cultures. For certain characteristics, such as determining the general specificity of an antibody for immunofluorescence in tubular epithelial cells, adult-derived tubuloids and hPSC-derived organoids may serve as useful controls for one another. Ultimately, however, both types of organoids are relatively novel and must be benchmarked back to actual tissue (or at least explants) to clearly establish the physiological relevance of the characteristic in question. Selection of the ideal positive control depends on the assay and a comprehensive review of the options is beyond our scope. In general, however, the closer the positive control can come to actual tissue, the better.
To the extent possible, the experimental organoids should be compared with the positive control using the same assay. This comparison would ideally include both qualitative features (e.g., localization patterns) and quantitative features (signal intensity). This will enable the investigator to determine the extent to which results in organoids mimic results in tissues. To date, such experiments in the literature have been few and far between.
Cross-Validation With Human Clinical Data
To establish confidence in the organoid system and its relevance to human physiology, researchers are increasingly incorporating clinical data sets from human patients as a comparative source of information to their experiments in cell culture. Cross-validation of organoid data with biopsy transcriptomic and urinary proteomics from patients with clinically diagnosed COVID-19 further substantiated the physiological relevance of these findings, suggesting that SARS-CoV-2 directly impacts the kidneys and that this may contribute to the high rates of mortality observed in patients with COVID with renal disease (41, 42). As the organoid field matures, such cross-validation strategies are likely to become more commonplace, bridging research activities from bench to bedside and reducing the need for animal models.
Mechanism Controls
Ultimately, understanding the molecular mechanism underlying a particular assay is an important piece in validating the assay, particularly when trying to prove nephron segment specificity. In the case of SARS-CoV-2, the importance of the angiotensin-converting enzyme 2 receptor for SARS-CoV-2 infection of organoid proximal tubules was first surmised based on its expression pattern and the effects of targeted therapeutics (58, 63) and subsequently demonstrated formally by genetic knockout experiments (41).
Going back to the example described at the beginning of this section, what types of evidence would we seek to demonstrate or disprove that anion transport is mediated by OATs? Here are a couple of considerations:
Expression of OATs in the organoid nephron segments that show transporter activity. If the transporter is not expressed, it is unlikely to be involved in the functional process; thus, this would disprove the hypothesis. On the other hand, evidence that OATs are expressed at the right place and the right time is merely circumstantial; it is insufficient to prove that they are involved.
Functional interference with the specific transporters. This methodology enables the demonstration of the necessity for the function of interest. For instance, if genetic knockout of OAT1 ameliorates the function, then OAT1 is necessary and likely to be involved (either directly or indirectly). Pharmacological treatments (e.g., inhibitors) may also be helpful if they are sufficiently specific for the target.
LIMITATIONS OF ORGANOIDS
Organoids still have several substantial limitations. These can primarily be broken down into three categories: perfusion, maturation, and patterning.
Perfusion
The absence of perfusion through organoids in vitro remains a significant challenge in modeling physiologically relevant characteristics and clinical endpoints. Culture conditions (e.g., immersion in media) can also present a challenge for modeling physiology in organoids. Kidney-on-chip devices incorporating organoids have been proposed to provide a way to further improve these structures. To date, however, no organoid-on-chip system has come into common use or demonstrated to overcome these major challenges. For instance, a kidney organoid-on-chip device recently described is limited to superfusion external to the organoids rather than perfusion through the organoid interior (55). Moreover, such devices are substantially more complicated and must be carefully compared with static cultures and grafts in vivo.
Maturation
Although kidney organoids are differentiated structures, they currently lack several critical hallmarks of a well-differentiated nephron. For cargo transport functions, organoids may lack expression of the requisite transporter in sufficient levels, subcellular localization pattern, or polarity. For instance, the tubular structures in organoids lack strong aquaporin-1 expression in a brush border pattern (34). Transplantation of organoids does not rescue this phenotype. Three kidneys arise during embryonic development: the pronephros, mesonephros, and metanephros. It is not yet clear which of these the organoids represent. Thus, the precise correlation of these organoids in the physiological organism is uncertain. Single-cell RNA-sequencing analysis also suggests that there is a wide range of cell states within any given “cell type” in organoids, and there are also partially differentiated cells present (7). Maturation is a challenge for all organ lineages derived from induced pluripotent stem cells, which are thought to adopt a more “fetal” phenotype compared with primary cells. This is not the only roadblock to maturation, as even adult primary cultures tend to dedifferentiate, compared with native tissue from which they derive, due to our inability to sufficiently mimic the growth conditions of these cells in vivo.
Patterning
It is obvious that kidney organoids lack the type of fine patterning observed in nephrons in vivo. Anatomic structures such as the loops of Henle and macula densa are missing. Although nephron segments arise, they lack the fine patterning observed in vivo, for instance, the difference between segments S1, S2, and S3 of the proximal tubule. Although there are some suggestions that a layer of parietal epithelial cells exists between proximal tubules and podocytes, the reproducibility of “Bowman's capsule”-like structures in vitro has not been established (7). One reasonable hypothesis is that patterning of organoids is driven primarily by the differential adhesion of the differentiating cell types and therefore lacks many of the essential microenvironmental inducers and cues necessary to properly pattern the embryonic kidney. In this regard, it is notable that no UB exists in these organoids to signal and shape nephron development. Sequential waves of branching morphogenesis do not occur, nor do the nephrons align in arrays to drain into a fractal collecting duct tree. The interstitium may be unable to properly modulate organoid differentiation due to its inherent immaturity or the presence of off-target cell populations. The impact of these patterning defects on physiology is readily apparent, given that the nephron is exquisitely patterned. For instance, it is difficult to envision how a countercurrent exchange function could arise in the absence of Henle's loop.
THE FUTURE OF ORGANOID PHYSIOLOGY ASSAYS
The significant limitations described above are a moving target and may be overcome with sufficient time and energy. As we move toward more functional structures, it is worthwhile to assemble a “wish list” of physiology assays that we would desire to see manifest in kidney organoids:
Collecting Ducts and Branching Morphogenesis
The iterative process of branching morphogenesis, which occurs during kidney development, can be partially reconstituted in embryonic kidney explants in vivo (13). This process would be an excellent “physiology assay” to indicate that bona fide UB and nephron progenitor cells exist in organoid cultures and undergo mutually inductive interactions. Over the past decade, numerous publications have claimed differentiation of the UB from hPSCs, based primarily on descriptive qualities (marker analysis) (15–20). The first such publication claimed that a 4-day course of directed differentiation was sufficient to induce the UB, relying on cytokeratin 8 as a marker, but this protocol has never been further validated or reproduced (17). In 2015, it was claimed that the UB can give rise to collecting ducts in nephron-like organoids, but subsequent papers by this group and others indicated that these were actually distal tubules (4, 14, 16). More recently, several publications have reported distinct protocols in which UB-like organoids can be differentiated from hPSCs in the absence of nephron progenitor cell pools (15, 18–20). When embedded in the extracellular matrix, these hPSC-derived UB-like organoids extend tubular processes that express the RET receptor tyrosine kinase at their tips, consistent with a UB identity (15, 16, 18–20). Immunofluorescence patterns commonly associated with mature collecting ducts, however, such as robust aquaporin-2 expression and binding of Dolichos biflorus agglutinin along the length of the ductal stalks, remain less convincing.
Beyond descriptive markers, the functionality and physiological relevance of UB-like structures remain poorly characterized. While UB-like structures derived from hPSCs have some capacity to recombine with nephron-like organoids to form “higher order” structures, none of the protocols to date has clearly demonstrated the capacity for extensive branching morphogenesis (15, 18–20). Unlocking the potential of hPSCs to differentiate into a UB capable of inducing branching morphogenesis remains an important goal, the achievement of which would raise the possibility of more advanced physiology assays involving filtrate collection, water and electrolyte balance, and diuresis. PKD is another area of interest, as cysts are generally thought to affect collecting ducts more severely than proximal tubules. However, hPSC-derived UB-like structures with PKD1 mutations form cysts only with the addition of forskolin (31), a secretagogue with effects that are not wholly specific to PKD, whereas nephron-like organoids (proximal and distal tubules) can form PKD cysts without forskolin (2, 6, 7, 27). Notably, compared with hPSC-derived equivalents, mouse pluripotent stem cells produce organoids that appear to be closer in expression pattern and morphology to a bona fide UB, particularly when mixed with stromal cells, providing a useful positive control (64).
Urine Production and Concentration
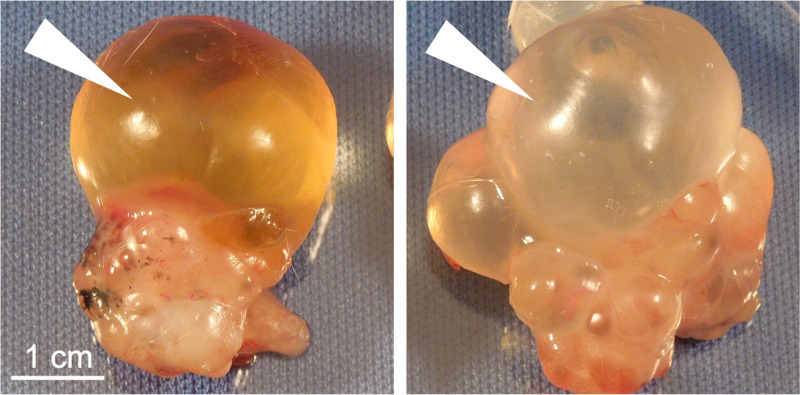
The formation of urine is the major function of the kidneys; thus, the potential of organoids to produce urine is worth considering. Although urine is readily identified in the setting of a living organism, what constitutes urine in the setting of organoids or grafts is more difficult to define. Grafts derived from fetal kidney tissues have been shown to accumulate fluid in cyst-like structures, proposed to represent urine (65, 66). Similar fluid, however, is observed to accumulate in various nonkidney growths, such as teratomas (dermoid cysts) derived from human pluripotent stem cells (Fig. 5). Thus, accumulation of an ascites-like fluid, even if yellow in color, is not unusual in grafts and is not indicative of urine production. Moreover, as described above, organoids in vitro can accumulate fluid of various sorts, which may vary in composition from the media in which they grow, as seen with the exclusion of phenol red dye in macroscopic cysts grown from organoids with PKD mutations (6). Few would suggest that such fluid constitutes urine.
Figure 5.
Teratoma growths accumulate urine-like fluid in vivo. Representative examples of human pluripotent stem cell-derived teratomas excised from beneath the neck scruff of immunodeficient mice are shown. Arrowheads indicate fluid-filled cyst structures.
Establishing criteria in advance for what is to be considered “urine” in organoid experiments is a valuable exercise. To be credible and convincing, claims that a fluid represents “organoid urine” should fulfill the following minimal criteria:
Tracing the source of the fluid to the stem cell-derived graft, as opposed to any native renal or collecting tissues of the host.
Demonstrating a composition of the fluid that reflects filtration, reabsorption, and secretion processes specific to the kidneys and comparable with actual urine.
Coupling of fluid production with an appropriate physiological benchmark of kidney function, e.g., prolongation of life following nephrectomy.
With regard to the composition of the urine, reabsorption and secretion processes will require functional demonstration of not only a proximal tubule but also an associated peritubular capillary network, with which solute and water exchange can occur. Successful achievement would demonstrate that absorbed molecules such as glucose and phosphate are substantially reabsorbed by the tubules into the blood supply, while cleared molecules such as inulin are fully removed. This will require careful consideration of the composition of not only the urinary output but also the vascular input. As a negative control, a similar vascular network associated with nonkidney organoids could be used.
Loops of Henle normally form during mammalian nephrogenesis and serve to concentrate the urine through a countercurrent multiplier system. Loop markers, such as Na+-K+-2Cl− cotransporter (SLC12A1) or uromodulin (UMOD), may be expressed in the “distal tubule” segment of kidney organoids (5, 8, 14). The characteristic shape and width of Henle’s loop structures, however, and the descending-to-ascending series of markers specific to each limb (e.g., specific aquaporins) are largely missing in organoids, even at a descriptive level. Reconstitution of Henle’s loops and the countercurrent multiplier system remains an important goal, which would be valuable for demonstrating a urine production mechanism.
Renal Replacement
A major advance for organoid physiology would be to successfully substitute for native kidneys in a transplantation model in vivo. Such an assay would constitute a therapeutic rescue, such as the ability of organoid grafts to prolong life of an animal following nephrectomy. A variety of physiological characteristics and metrics could be assessed, including levels of uremic solutes in the blood. While such experiments have not yet been demonstrated with induced pluripotent stem cell-derived kidney organoids, metanephroi and embryonic kidney fragments can grow substantially after transplantation into animals (59). Although the resultant nephrons become vascularized from the host animal, functional measurements of metanephric grafts are substantially inferior to organ transplants (67). As kidney organoids are inferior to metanephroi with respect to patterning, it may still be too early to expect such experiments to produce successful outcomes. Even if improvements in renal function are observed, it is critical to distinguish between bona fide function of the graft versus paracrine effects (68). Kidney organ transplantation should be used as a positive control for renal replacement therapy and as a gold-standard comparator.
Breakthroughs are needed in each of these areas, which would likely impact not only kidney organoids but also organoids in general. The perfect, however, should not become the enemy of the good. Organoids, as they exist today, offer valuable opportunities for investigating physiology, and the findings made will be informative for years into the future, just as pioneering physiology assays in other model organisms guide our understanding in the present day.
GRANTS
This work was supported by National Institutes of Health Grants 5UC2DK126006, R01DK117914, U01DK127553, U01HL152401, UG3TR002158, and UG3TR003288, Department of Defense Awards W81XWH-21-1-0006 and W81XWH-21-1-0007, the Cystinosis Research Foundation, the Allen Institute for Cell Science, the Lara Nowak Macklin Research Fund, Novo-Nordisk, Eloxx Pharmaceuticals, Aeovian Pharmaceuticals, the United States-Israel Binational Science Foundation, start-up funds from the University of Washington, and a gift from the Northwest Kidney Centers to the Kidney Research Institute.
DISCLOSURES
B.S.F. is an inventor on patents and patent applications related to the generation and use of kidney organoids, including one licensed to STEMCELL Technologies to produce a kidney organoid differentiation kit.
AUTHOR CONTRIBUTIONS
B.S.F. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
Biorender.com was used to illustrate parts of the graphical abstract. I thank the members of the RBK Consortium’s Functional Assays subgroup and Jonathan Himmelfarb for helpful discussions.
REFERENCES
- 1.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14: 53–67, 2014. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, Bonventre JV. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6: 8715, 2015. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015. doi: 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526: 564–568, 2015. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 5.Przepiorski A, Sander V, Tran T, Hollywood JA, Sorrenson B, Shih J-H, Wolvetang EJ, McMahon AP, Holm TM, Davidson AJ. A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Reports 11: 470–484, 2018. doi: 10.1016/j.stemcr.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill AJ, Kim YK, Winston K, Tran LM, Diaz MA, Fu H, Finn LS, Pei Y, Himmelfarb J, Freedman BS. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater 16: 1112–1119, 2017. doi: 10.1038/nmat4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, Martins TJ, Pippin JW, Fu H, Kretzler M, Shankland SJ, Himmelfarb J, Moon RT, Paragas N, Freedman BS. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22: 929–940.e4, 2018. doi: 10.1016/j.stem.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawlor KT, Vanslambrouck JM, Higgins JW, Chambon A, Bishard K, Arndt D, Er PX, Wilson SB, Howden SE, Tan KS, Li F, Hale LJ, Shepherd B, Pentoney S, Presnell SC, Chen AE, Little MH. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat Mater 20: 260–271, 2021. doi: 10.1038/s41563-020-00853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mugford JW, Sipilä P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol 324: 88–98, 2008. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosines E, Sampogna RV, Johkura K, Vaughn DA, Choi Y, Sakurai H, Shah MM, Nigam SK. Staged in vitro reconstitution and implantation of engineered rat kidney tissue. Proc Natl Acad Sci USA 104: 20938–20943, 2007. doi: 10.1073/pnas.0710428105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25: 5214–5228, 2006. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cebrian C, Asai N, D'Agati V, Costantini F. The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment. Cell Rep 7: 127–137, 2014. doi: 10.1016/j.celrep.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23: 869–881.e8, 2018. doi: 10.1016/j.stem.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taguchi A, Nishinakamura R. Higher-Order Kidney Organogenesis from Pluripotent Stem Cells. Cell Stem Cell 21: 730–746.e6, 2017. doi: 10.1016/j.stem.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Howden SE, Wilson SB, Groenewegen E, Starks L, Forbes TA, Tan KS, Vanslambrouck JM, Holloway EM, Chen Y-H, Jain S, Spence JR, Little MH. Plasticity of distal nephron epithelia from human kidney organoids enables the induction of ureteric tip and stalk. Cell Stem Cell 28: 671–684.e6, 2021. doi: 10.1016/j.stem.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu M-Z, Dubova I, Esteban CR, Montserrat N, Campistol JM, Izpisua Belmonte JC. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol 15: 1507–1515, 2013. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- 18.Mae S-I, Ryosaka M, Toyoda T, Matsuse K, Oshima Y, Tsujimoto H, Okumura S, Shibasaki A, Osafune K. Generation of branching ureteric bud tissues from human pluripotent stem cells. Biochem Biophys Res Commun 495: 954–961, 2018. doi: 10.1016/j.bbrc.2017.11.105. [DOI] [PubMed] [Google Scholar]
- 19.Uchimura K, Wu H, Yoshimura Y, Humphreys BD. Human pluripotent stem cell-derived kidney organoids with improved collecting duct maturation and injury modeling. Cell Rep 33: 108514, 2020. doi: 10.1016/j.celrep.2020.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Z, Huang B, Parvez RK, Li Y, Chen J, Vonk AC, Thornton ME, Patel T, Rutledge EA, Kim AD, Yu J, Grubbs BH, McMahon JA, Pastor-Soler NM, Hallows KR, McMahon AP, Li Z. Generation of patterned kidney organoids that recapitulate the adult kidney collecting duct system from expandable ureteric bud progenitors. Nat Commun 12: 3641, 2021. doi: 10.1038/s41467-021-23911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phipson B, Er PX, Combes AN, Forbes TA, Howden SE, Zappia L, Yen H-J, Lawlor KT, Hale LJ, Sun J, Wolvetang E, Takasato M, Oshlack A, Little MH. Evaluation of variability in human kidney organoids. Nat Methods 16: 79–87, 2019. doi: 10.1038/s41592-018-0253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz NM, Freedman BS. Differentiation of human kidney organoids from pluripotent stem cells. Methods Cell Biol 153: 133–150, 2019. [DOI] [PubMed] [Google Scholar]
- 23.Takasato M, Er PX, Chiu HS, Little MH. Generation of kidney organoids from human pluripotent stem cells. Nat Protoc 11: 1681–1692, 2016. doi: 10.1038/nprot.2016.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedman BS. (Re)Building a Kidney. A Commercially Available Kit to Generate Human Kidney Organoids (Online). https://www.rebuildingakidney.org/id/17-EBYM.
- 25.Kim YK, Refaeli I, Brooks CR, Jing P, Gulieva RE, Hughes MR, Cruz NM, Liu Y, Churchill AJ, Wang Y, Fu H, Pippin JW, Lin LY, Shankland SJ, Vogl AW, McNagny KM, Freedman BS. Gene-edited human kidney organoids reveal mechanisms of disease in podocyte development. Stem Cells 35: 2366–2378, 2017. doi: 10.1002/stem.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hale LJ, Howden SE, Phipson B, Lonsdale A, Er PX, Ghobrial I, Hosawi S, Wilson S, Lawlor KT, Khan S, Oshlack A, Quinlan C, Lennon R, Little MH. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun 9: 5167, 2018. doi: 10.1038/s41467-018-07594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, Lian MM, Liu M, Aizawa E, Rodriguez Esteban C, Yong KSM, Chen Q, Campistol JM, Fang M, Khor CC, Foo JN, Izpisua Belmonte JC, Xia Y. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 25: 373–387.e9, 2019. doi: 10.1016/j.stem.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankar AS, Du Z, Mora HT, van den Bosch TPP, Korevaar SS, Van den Berg-Garrelds IM, Bindels E, Lopez-Iglesias C, Clahsen-van Groningen MC, Gribnau J, Baan CC, Danser AHJ, Hoorn EJ, Hoogduijn MJ. Human kidney organoids produce functional renin. Kidney Int 99: 134–147, 2021. [Erratum in Kidney Int 100:1346–1347, 2021]. doi: 10.1016/j.kint.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Przepiorski A, Vanichapol T, Espiritu EB, Crunk AE, Parasky E, McDaniels MD, Emlet DR, Salisbury R, Happ CL, Vernetti LA, MacDonald ML, Kellum JA, Kleyman TR, Baty CJ, Davidson AJ, Hukriede NA. Modeling oxidative injury response in human kidney organoids. Stem Cell Res Ther 13: 76, 2022. doi: 10.1186/s13287-022-02752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu T, Mae S-I, Araoka T, Okita K, Hotta A, Yamagata K, Osafune K. A novel ADPKD model using kidney organoids derived from disease-specific human iPSCs. Biochem Biophys Res Commun 529: 1186–1194, 2020. doi: 10.1016/j.bbrc.2020.06.141. [DOI] [PubMed] [Google Scholar]
- 31.Kuraoka S, Tanigawa S, Taguchi A, Hotta A, Nakazato H, Osafune K, Kobayashi A, Nishinakamura R. PKD1-dependent renal cystogenesis in human induced pluripotent stem cell-derived ureteric bud/collecting duct organoids. J Am Soc Nephrol 31: 2355–2371, 2020. doi: 10.1681/ASN.2020030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharmin S, Taguchi A, Kaku Y, Yoshimura Y, Ohmori T, Sakuma T, Mukoyama M, Yamamoto T, Kurihara H, Nishinakamura R. Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J Am Soc Nephrol 27: 1778–1791, 2016. doi: 10.1681/ASN.2015010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, Howden SE, Takasato M, Little MH, Rabelink TJ. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports 10: 751–765, 2018. doi: 10.1016/j.stemcr.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam SA, Seo E, Kim JW, Kim HW, Kim HL, Kim K, Kim T-M, Ju JH, Gomez IG, Uchimura K, Humphreys BD, Yang CW, Lee JY, Kim J, Cho DW, Freedman BS, Kim YK. Graft immaturity and safety concerns in transplanted human kidney organoids. Exp Mol Med 51: 1–13, 2019. [Erratum in Exp Mol Med 52:180, 2020]. doi: 10.1038/s12276-019-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar Gupta A, Sarkar P, Wertheim JA, Pan X, Carroll TJ, Oxburgh L. Asynchronous mixing of kidney progenitor cells potentiates nephrogenesis in organoids. Commun Biol 3: 231, 2020. doi: 10.1038/s42003-020-0948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Berg CW, Koudijs A, Ritsma L, Rabelink TJ. In vivo assessment of size-selective glomerular sieving in transplanted human induced pluripotent stem cell-derived kidney organoids. J Am Soc Nephrol 31: 921–929, 2020. doi: 10.1681/ASN.2019060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shankar AS, van den Berg SAA, Tejeda Mora H, Du Z, Lin H, Korevaar SS, van der Wal R, van den Bosch TPP, Clahsen-van Groningen MC, Gribnau J, Hoorn EJ, Baan CC, Hoogduijn MJ. Vitamin D metabolism in human kidney organoids. Nephrol Dial Transplant 37: 190–193, 2021. doi: 10.1093/ndt/gfab264. [DOI] [PubMed] [Google Scholar]
- 38.Digby JLM, Vanichapol T, Przepiorski A, Davidson AJ, Sander V. Evaluation of cisplatin-induced injury in human kidney organoids. Am J Physiol Renal Physiol 318: F971–F978, 2020. doi: 10.1152/ajprenal.00597.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta N, Matsumoto T, Hiratsuka K, Garcia Saiz E, Galichon P, Miyoshi T, Susa K, Tatsumoto N, Yamashita M, Morizane R. Modeling injury and repair in kidney organoids reveals that homologous recombination governs tubular intrinsic repair. Sci Transl Med 14: eabj4772, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence ML, Elhendawi M, Morlock M, Liu W, Liu S, Palakkan A, Seidl LF, Hohenstein P, Sjögren AK, Davies JA. Human iPSC-derived renal organoids engineered to report oxidative stress can predict drug-induced toxicity. iScience 25: 103884, 2022. doi: 10.1016/j.isci.2022.103884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helms L, Marchiano S, Stanaway IB, Hsiang TY, Juliar BA, Saini S, Zhao YT, Khanna A, Menon R, Alakwaa F, Mikacenic C, Morrell ED, Wurfel MM, Kretzler M, Harder JL, Murry CE, Himmelfarb J, Ruohola-Baker H, Bhatraju PK, Gale M Jr, Freedman BS. Cross-validation of SARS-CoV-2 responses in kidney organoids and clinical populations. JCI Insight 6: e154882, 2021. doi: 10.1172/jci.insight.154882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen J, Reimer KC, Nagai JS, Varghese FS, Overheul GJ, de Beer M, et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 29: 217–231.e8, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz GJ, Al-Awqati Q. Carbon dioxide causes exocytosis of vesicles containing H+ pumps in isolated perfused proximal and collecting tubules. J Clin Invest 75: 1638–1644, 1985. doi: 10.1172/JCI111871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masereeuw R, Terlouw SA, van Aubel RA, Russel FG, Miller DS. Endothelin B receptor-mediated regulation of ATP-driven drug secretion in renal proximal tubule. Mol Pharmacol 57: 59–67, 2000. doi: 10.1124/mol.59.6.1433. [DOI] [PubMed] [Google Scholar]
- 45.Masereeuw R, Moons MM, Toomey BH, Russel FG, Miller DS. Active lucifer yellow secretion in renal proximal tubule: evidence for organic anion transport system crossover. J Pharmacol Exp Ther 289: 1104–1111, 1999. [PubMed] [Google Scholar]
- 46.Neufeld TK, Douglass D, Grant M, Ye M, Silva F, Nadasdy T, Grantham JJ. In vitro formation and expansion of cysts derived from human renal cortex epithelial cells. Kidney Int 41: 1222–1236, 1992. doi: 10.1038/ki.1992.184. [DOI] [PubMed] [Google Scholar]
- 47.Magenheimer BS, St John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na+,K+,2Cl− co-transporter-dependent cystic dilation. J Am Soc Nephrol 17: 3424–3437, 2006. doi: 10.1681/ASN.2006030295. [DOI] [PubMed] [Google Scholar]
- 48.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJC, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19: 939–945, 2013. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 49.Talbi K, Cabrita I, Kraus A, Hofmann S, Skoczynski K, Kunzelmann K, Buchholz B, Schreiber R. The chloride channel CFTR is not required for cyst growth in an ADPKD mouse model. FASEB J 35: e21897, 2021. [DOI] [PubMed] [Google Scholar]
- 50.Pavlov TS, Ilatovskaya DV, Palygin O, Levchenko V, Pochynyuk O, Staruschenko A. Implementing patch clamp and live fluorescence microscopy to monitor functional properties of freshly isolated PKD epithelium. J Vis Exp 103: 53035, 2015. doi: 10.3791/53035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ray EC, Carrisoza-Gaytan R, Al-Bataineh M, Marciszyn AL, Nkashama LJ, Chen J, Winfrey A, Griffiths S, Lam TR, Flores D, Wu P, Wang W, Huang C-L, Subramanya AR, Kleyman TR, Satlin LM. L-WNK1 is required for BK channel activation in intercalated cells. Am J Physiol Renal Physiol 321: F245–F254, 2021. doi: 10.1152/ajprenal.00472.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garreta E, Prado P, Tarantino C, Oria R, Fanlo L, Marti E, Zalvidea D, Trepat X, Roca-Cusachs P, Gavalda-Navarro A, Cozzuto L, Campistol JM, Izpisua Belmonte JC, Hurtado Del Pozo C, Montserrat N. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat Mater 18: 397–405, 2019. doi: 10.1038/s41563-019-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomson SC, Vallon V. Effects of SGLT2 inhibitor and dietary NaCl on glomerular hemodynamics assessed by micropuncture in diabetic rats. Am J Physiol Renal Physiol 320: F761–F771, 2021. doi: 10.1152/ajprenal.00552.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Phan DTT, Sobrino A, George SC, Hughes CCW, Lee AP. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 16: 282–290, 2016. doi: 10.1039/c5lc01050k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16: 255–262, 2019. doi: 10.1038/s41592-019-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q, Xiong Y, Zhang S, Sui Y, Yu C, Liu P, Li H, Guo W, Gao Y, Przepiorski A, Davidson AJ, Guo M, Zhang X. The dynamics of metabolic characterization in iPSC-derived kidney organoid differentiation via a comparative omics approach . Front Genet 12: 632810, 2021. doi: 10.3389/fgene.2021.632810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanofsky SM, Dugas CM, Katsurada A, Liu J, Saifudeen Z, El-Dahr SS, Satou R. Angiotensin II biphasically regulates cell differentiation in human iPSC-derived kidney organoids. Am J Physiol Renal Physiol 321: F559–F571, 2021. doi: 10.1152/ajprenal.00134.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181: 905–913.e7, 2020. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, Rechavi G, Friedman N, Kaminski N, Passwell JH, Reisner Y. Human and porcine early kidney precursors as a new source for transplantation. Nat Med 9: 53–60, 2003. doi: 10.1038/nm812. [DOI] [PubMed] [Google Scholar]
- 60.Ryan AR, England AR, Chaney CP, Cowdin MA, Hiltabidle M, Daniel E, Gupta AK, Oxburgh L, Carroll TJ, Cleaver O. Vascular deficiencies in renal organoids and ex vivo kidney organogenesis. Dev Biol 477: 98–116, 2021. doi: 10.1016/j.ydbio.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buzhor E, Harari-Steinberg O, Omer D, Metsuyanim S, Jacob-Hirsch J, Noiman T, Dotan Z, Goldstein RS, Dekel B. Kidney spheroids recapitulate tubular organoids leading to enhanced tubulogenic potency of human kidney-derived cells. Tissue Eng Part A 17: 2305–2319, 2011. doi: 10.1089/ten.TEA.2010.0595. [DOI] [PubMed] [Google Scholar]
- 62.Schutgens F, Rookmaaker MB, Margaritis T, Rios A, Ammerlaan C, Jansen J, Gijzen L, Vormann M, Vonk A, Viveen M, Yengej FY, Derakhshan S, de Winter-de Groot KM, Artegiani B, van Boxtel R, Cuppen E, Hendrickx APA, van den Heuvel-Eibrink MM, Heitzer E, Lanz H, Beekman J, Murk JL, Masereeuw R, Holstege F, Drost J, Verhaar MC, Clevers H. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol 37: 303–313, 2019. doi: 10.1038/s41587-019-0048-8. [DOI] [PubMed] [Google Scholar]
- 63.Wysocki J, Ye M, Hassler L, Gupta AK, Wang Y, Nicoleascu V, Randall G, Wertheim JA, Batlle D. A novel soluble ACE2 variant with prolonged duration of action neutralizes SARS-CoV-2 infection in human kidney organoids. J Am Soc Nephrol 32: 795–803, 2021. doi: 10.1681/asn.2020101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanigawa S, Tanaka E, Miike K, Ohmori T, Inoue D, Cai C-L, Taguchi A, Kobayashi A, Nishinakamura R. Generation of the organotypic kidney structure by integrating pluripotent stem cell-derived renal stroma. Nat Commun 13: 611, 2022. doi: 10.1038/s41467-022-28226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lanza RP, Chung HY, Yoo JJ, Wettstein PJ, Blackwell C, Borson N, Hofmeister E, Schuch G, Soker S, Moraes CT, West MD, Atala A. Generation of histocompatible tissues using nuclear transplantation. Nat Biotechnol 20: 689–696, 2002. doi: 10.1038/nbt703. [DOI] [PubMed] [Google Scholar]
- 66.Yokote S, Matsunari H, Iwai S, Yamanaka S, Uchikura A, Fujimoto E, Matsumoto K, Nagashima H, Kobayashi E, Yokoo T. Urine excretion strategy for stem cell-generated embryonic kidneys. Proc Natl Acad Sci USA 112: 12980–12985, 2015. doi: 10.1073/pnas.1507803112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rogers SA, Hammerman MR. Prolongation of life in anephric rats following de novo renal organogenesis. Organogenesis 1: 22–25, 2004. doi: 10.4161/org.1.1.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toyohara T, Mae S-I, Sueta S-I, Inoue T, Yamagishi Y, Kawamoto T, Kasahara T, Hoshina A, Toyoda T, Tanaka H, Araoka T, Sato-Otsubo A, Takahashi K, Sato Y, Yamaji N, Ogawa S, Yamanaka S, Osafune K. Cell therapy using human induced pluripotent stem cell-derived renal progenitors ameliorates acute kidney injury in mice. Stem Cells Transl Med 4: 980–992, 2015. doi: 10.5966/sctm.2014-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]