Keywords: early-life adversity, GLUT2, metabolic disease, porcine intestine, SGLT1
Abstract
Early-life adversity (ELA) is linked with the increased risk for inflammatory and metabolic diseases in later life, but the mechanisms remain poorly understood. Intestinal epithelial glucose transporters sodium-glucose-linked transporter 1 (SGLT1) and glucose transporter 2 (GLUT2) are the major route for intestinal glucose uptake but have also received increased attention as modulators of inflammatory and metabolic diseases. Here, we tested the hypothesis that early weaning (EW) in pigs, an established model of ELA, alters the development of epithelial glucose transporters and coincides with elevated markers of metabolic inflammation. The jejunum and ileum of 90-day-old pigs previously exposed to EW (16 days wean age), exhibited reduced SGLT1 activity (by ∼ 30%, P < 0.05) than late weaned (LW, 28 days wean age) controls. In contrast, GLUT2-mediated glucose transport was increased (P = 0.003) in EW pigs than in LW pigs. Reciprocal changes in SGLT1- and GLUT2-mediated transport coincided with transporter protein expression in the intestinal brush-border membranes (BBMs) that were observed at 90 days and 150 days of age. Ileal SGLT1-mediated glucose transport and BBM expression were inhibited by the β-adrenergic receptor (βAR) blocker propranolol in EW and LW pigs. In contrast, propranolol enhanced ileal GLUT2-mediated glucose transport (P = 0.015) and brush-border membrane vesicle (BBMV) abundance (P = 0.035) in LW pigs, but not in EW pigs. Early-weaned pigs exhibited chronically elevated blood glucose and C-reactive protein (CRP) levels, and adipocyte hypertrophy and upregulated adipogenesis-related gene expression in visceral adipose tissue. Altered development of intestinal glucose transporters by EW could underlie the increased risk for later life inflammatory and metabolic diseases.
NEW & NOTEWORTHY These studies reveal that early-life adversity in the form of early weaning in pigs causes a developmental shift in intestinal glucose transport from SGLT1 toward GLUT2-mediated transport. Early weaning also induced markers of metabolic inflammation including persistent elevations in blood glucose and the inflammatory marker CRP, along with increased visceral adiposity. Altered intestinal glucose transport might contribute to increased risk for inflammatory and metabolic diseases associated with early-life adversity.
INTRODUCTION
Early-life stress or adversity (ELA) is a well-established risk factor for the later life inflammatory and metabolic diseases and reduced lifespan (1–5). The mechanisms linking ELA and later disease risk remain poorly understood. The gastrointestinal system is particularly sensitive to the long-lasting programming impacts of ELA as evidenced by the link between ELA and later life adversity and later life gastrointestinal (GI) disease, such as irritable bowel syndrome (6, 7). Moreover, the GI system is increasingly recognized for its role as a driver of systemic inflammatory and metabolic diseases and neurodevelopmental disorders.
The mechanisms by which ELA impacts GI and systemic disease risk remain poorly understood. The GI system exhibits a high level of plasticity in early postnatal life, where host biology and environmental cues (e.g., psychological, neuroendocrine, antigenic, and microbial cues) interact to shape the developmental trajectories of critical GI systems and functions (e.g., epithelial barrier function, mucosal immune development and tolerance, enteric nervous system, etc.). Thus, stress or adversity (ELA) during the critical early-life period can alter the developmental trajectory of gut functions potentially driving increased disease risk. Early weaning (EW) in pigs is a significant early life stressful event involving multitude of psychosocial and environmental stressors including maternal separation, disruption of littermate social hierarchy, and abrupt changes in environment and food source. Moreover, EW-associated stressors occur during a critical period of postnatal development when GI functions are established and programmed for life-long function (8). Although the objective of EW in piglets is to improve reproductive efficiency in sows and reduce vertical transmission of select pathogens from the dam to piglets, some unintended long-term consequences have been realized including increased mortality and suboptimal performance and feed efficiency (9–11) that persists into adulthood. Given the nature of the stressors that EW pigs face during an early developmental window and the increased risk for later-life disease, EW in piglets represents a large animal model of ELA-associated disease risk in people (8). Understanding, how EW leads to lifetime disease risk will be critical in identifying targets for mitigation of disease risk with dual relevance to animals and as a model for humans.
As mentioned earlier, animal models of ELA have demonstrated alterations in the developmental programming of adult GI functions, with the majority of research focusing on the development of intestinal barrier, nervous system, and immune systems (8). A major GI function that has received relatively little attention in the context of ELA is intestinal glucose transport. Intestinal epithelial glucose transporters represent the major route of glucose uptake into the body for subsequent energy metabolism by cells and storage. Although studied mainly in the context of postprandial glucose absorption and water absorption, growing evidence indicates that intestinal glucose transporters play important roles in host immune defense and metabolic inflammatory disorders. Expression and (or) activity of intestinal sodium-glucose-linked cotransporter 1 (SGLT1) and facilitated glucose transporter 2 (GLUT2) has been shown to be increased during infectious challenges (12, 13). Glucose uptake, via intestinal epithelial SGLT1, was shown to exert antiinflammatory effects during in vitro and in vivo LPS challenge (14). Intestinal epithelial cell-specific GLUT2 is increased in human patients with obesity, and intestinal epithelial-specific knockdown of GLUT2 demonstrated a critical role for epithelial GLUT2 in obesity-induced intestinal permeability and susceptibility to infection in a rodent model (15). Furthermore, intestinal glucose transporter activity and expression are altered in response to the psychological and environmental stressors, independent of food intake, and across different species including rodents (16) and pigs (17, 18). Together, these studies highlight the dynamic expression of intestinal glucose transporters in response to various stressors, and the diverse roles these glucose transporters might play in inflammatory and metabolic diseases. In the present study, we tested the hypothesis that EW in piglets alters the long-term development of intestinal epithelial glucose transporter function, which will coincide with increased markers of chronic inflammation and altered glucose and adipose tissue homeostasis.
METHODS
Animals
The animal experimental protocols were approved by Institutional Animal Care and Use Committee (IACUC) at Michigan State University. A total 48 female piglets from 10 litters (Yorkshire-Hampshire cross) were used in this study. Female piglets within each litter were weighed and then randomly assigned to one of two weaning groups. Piglets were weaned from their sow at 15 days of age (early weaning; EW) or 28 days of age (late weaning; LW). Weaned pigs were housed in standard nursery pens and then were moved to raised pens at 6 wk of age. All pigs were offered ad libitum access to water, and the same phase diet was formulated to meet or exceed the nutrient requirements of all pigs at each stage of growth. Environment temperatures were monitored continuously and visual physical exams on pigs were performed no less than twice daily. Individual pig body weight was measured weekly.
Tissue Sample Collections
Euthanasia and tissue samples collections were performed in two cohorts of EW and LW pigs based on age (10 wk and 20 wk of age; Fig. 1). Sample collections (blood and tissue) from cohort 1 (n = 36 pigs; 18 EW, 18 LW pigs) were performed at 10 wk of age for blood and tissue analyses and Ussing chamber experiments (see below)and from cohort 2 (6 EW and 6 LW pigs) for blood and tissue analyses. Euthanasia of EW and LW littermates was conducted in a staggered time schedule to facilitate rapid collection and processing of tissues. All 36 pigs were euthanized within 6 continuous days. To minimize stress, pigs were sedated first with a combination of Telazol (2.5 mg/kg), ketamine (11 mg/kg), and xylazine (1.5 mg/kg) at a dose of 0.03 mL/kg body wt. After animals were sedated, pigs were immediately euthanized by intracardiac injection of pentobarbital sodium (Euthasol; 85.9 mg/kg pentobarbital sodium). Immediately after euthanasia, 10 cm of midjejunum (10 m proximal from the end of the continuous ileal Peyer’s patches) and distal ileum (10 cm proximal to the ileocecal junction) were harvested and placed in cold oxygenated (95% O2–5% CO2) Ringer solution for Ussing chamber analyses, as described previously (18). A 40-cm section of jejunum proximal to the midjejunum samples described earlier was collected for intestinal epithelial cell isolation. An additional 30-cm section of jejunum and ileum was harvested, cut open longitudinally, and rinsed generously with 0.87% saline to remove any digesta before sample collection. Jejunal and ileal mucosal scrapings were performed with a glass slide and immediately flash-frozen in liquid N2 and stored at −80°C until brush-border membrane vesicle (BBMV) isolation and gene/protein analysis. The rest of the intestinal segments were fixed in 10% formaldehyde for histological analysis. Retroperitoneal adipose tissue was isolated from each pig and either fixed in 10% formalin or flash-frozen in liquid nitrogen and stored at −80°C for RNA isolation.
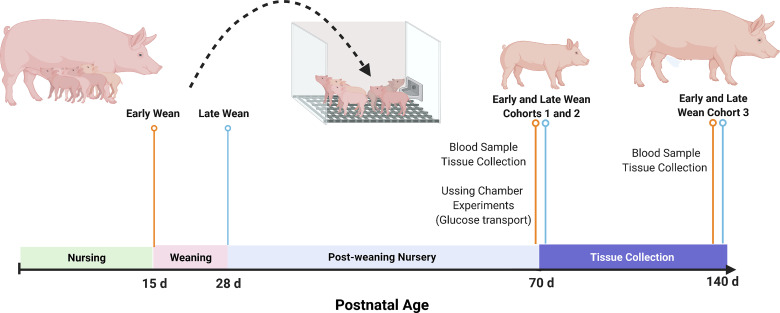
Figure 1.
Experimental design. Piglets were weaned from their dam either at 15 days of age (early weaned, EW) or at 28 days of age (late weaned, LW). Three independent animal cohort were used. At 70 days of age (cohort 1; n = 16 pigs and cohort 2; n = 16 pigs), blood and tissue (jejunum, ileum, and retroperitoneal adipose tissue) samples were harvested. Jejunal and ileal mucosa were prepared for Ussing chamber analyses for intestinal glucose transport experiments. Cohort 3 (n = 12 pigs) was euthanized at 140 days of age for blood and tissue sampling (jejunum and ileum). Image created with BioRender and published with permission.
Blood Glucose and C-Reactive Protein
Blood samples were collected and then centrifuged at 4°C for 30 min. Serum glucose level was measured by using commercial Amplex Red Glucose/Glucose Oxidase Assay kit (Invitrogen, Ltd., Paisley, UK), and C-reactive protein (CRP) was measured by using commercially available ELISA kit (Life Diagnostics, Inc., Cat. No. CRP-9, West Chester, PA).
Sequential Isolation of Epithelial Cells
Immediately after midjejunum segments were dissected, sequential isolation of pig small intestinal epithelial cells along the crypt-villus axis was performed, according to the protocol described by Xiong et al. (19). After the fluid contents of the intestinal segments were drained, the intestinal segments were then filled with 45 mL of the oxygenated warm isolation buffer (1.5 mM Na2EDTA, 0.2 mM PMSF, 0.5 mM DTT, 2.5 mM d-glucose, 2.5 mM l-glutamine, 0.25% BSA, and 0.5 mM dl-hydroxybutyrate sodium salt) for sequential isolation of three epithelial cell fractions (upper villus, middle villus, and upper crypt) from the villus tip to the crypt in total 60 min of incubation. Isolated cell fractions were also pooled for each piglet. Collected cell fractions were washed twice with an oxygenated cell suspension buffer (155 mM KCl at pH 7.4), and the cells were retained through centrifugation at 400 g for 4 min at 4°C. The washed cells were immediately frozen by liquid nitrogen and stored at −80°C for further use.
Intestinal Morphology
Jejunal and ileal tissues were taken immediately after euthanasia and were stored in 10% neutral buffered formalin until processing for routine histological evaluation as described before. Briefly, paraffin-embedded intestinal samples were sectioned (5 µm) and stained with hematoxylin and eosin for histological analysis under a light microscope. Photomicrographs were acquired with ×20 and ×40 magnification at a resolution of 1,360 × 1,024 using imaging software (Olympus DP2-BSW v. 2.2), a high-resolution digital camera (Olympus DP72) affixed to a clinical light microscope (Olympus BX45). Villi were measured for villus height and crypt depth by using the ×20 objective. For each histological slide prepared for each pig, four different areas were located on the slide within the ×20 field view that contained at least three, well-oriented villi based on the criteria that 1) the entire crypt and villi were captured in cross section and 2) the central lacteal was present. Therefore, a minimum of 12 individual villi measurements per pig were taken and then averaged to derive the mean villi height for each pig. All histopathology data were measured in micrometer unit and analyzed as n = 8 pigs/treatment.
Ussing Chamber Experiments
Immediately after euthanasia, segments of the middle jejunum and distal ileum were prepared for Ussing chamber experiments as described previously (18). Briefly, the mucosa was stripped from the seromuscular layer in oxygenated (95% O2–5% CO2) Ringer solution. Tissues were then mounted in 0.71-cm2 aperture Ussing chambers (Physiologic Instruments, San Diego, CA). Tissues were bathed on the serosal and mucosal sides with 10 mL oxygenated Ringer solution maintained at 37°C. The spontaneous potential difference (PD) was measured with the use of Ringer-agar bridges connected to calomel electrodes, and the PD was short-circuited through Ag-AgCl electrodes with the use of a voltage clamp that corrected for fluid resistance. Tissues were maintained in the short-circuited state, except for brief intervals to record the open-circuit PD. Transepithelial electrical resistance (TER; Ω · cm2) was calculated from the spontaneous PD and short-circuit current (Isc), as previously described (18). After a 20-min equilibration period in Ussing chambers, TER and Isc were recorded over a 10-min period and then averaged to derive the basal Isc and basal TER values for a given pig. To measure Na+-dependent glucose transport, a dose of 10 mM or 100 mM glucose (Sigma, St. Louis, MO) was added to mucosal side of Ussing chamber and osmotically balanced on the serosal side with equimolar amounts of mannitol. Electrogenic glucose transport was determined as the change in ΔIsc in response of glucose addition over a 20-min period. To test the tissue capability of overall glucose transport, a fluorescent-labeled glucose tracer, 2-NBD [2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose]-glucose (NBDG, Cayman Chemical, Ann Arbor, MI), was premixed in 100 mM glucose dose before addition. The supernatant samples from both sides of Ussing chambers were collected after 3 and 23 min post-NBDG dose addition. The NBDG level was measured by microplate reader for fluorescent signal detection. The amount of mucosal NBDG disappearance was calculated for indication of overall glucose transport.
Pharmacological Modulation of Glucose Uptake in Ussing Chambers
To investigate the high-dose glucose-induced Na+-independent glucose transport, the inhibitor of SGLT1, phlorizin, was added to the mucosal side in a final concentration of 0.2 mM, 20 min before glucose addition. Propranolol (2 µM) was added to the mucosal and serosal chambers to evaluate the contribution of β-adrenergic receptors (βARs) to intestinal glucose transport and transporter expression. The neural inhibitor tetrodotoxin (TTX) 10 µM was added to the serosal side of tissue 20 min before glucose stimulation. At the end of glucose uptake study, tissue samples on the Ussing chamber were rinsed in Ringer solution and snap frozen in liquid nitrogen for later BBMV isolation.
Preparation of Brush-Border Membrane Vesicles
Brush-boarder membrane (BBM) vesicles (BBMVs) from jejunum mucosal scrapings were prepared by Mg2+-precipitation (20). Briefly, frozen mucosa scrapings (1 g) were homogenized on ice in homogenization buffer (20 mL; 50 mM d-mannitol, 10 mM HEPES, and 0.2 mM PMSF; pH = 7.4) using a polytron homogenizer (Brinkmann Instruments, Inc., Westbury, NY) for three 1-min cycles. The resulting homogenate was collected for protein analysis, and the remaining homogenate was centrifuged at 2,000 g for 15 min at 4°C. The supernatant was saved and added with MgCl2 to reach 10 mM of [Mg2+], then gently shaded on ice for 15 min. The resulting precipitates were discarded after 2,400 g centrifuge for 15 min at 4°C. The crude BBM portion of the supernatant was collected by ultracentrifuge at 19,000 g for 30 min at 4°C. The resulting pellet was suspended in vesicle preloading buffer (150 mM KSCN, 10 mM mannitol, and 5 mM HEPES; pH = 7.4). Final BBMV samples were collected by ultracentrifuging for an additional 30 min at 39,000 g and 4°C. The BBMV isolation was confirmed by the enrichment of sucrase and maltase activity between BBMV and lysate (Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14842446.v1), and then aliquoted at 5 mg/mL protein concentration and stored in −80°C for further Western blot analysis.
Sucrase and Maltase Activities
Sucrase and maltase activities were estimated according to James et al. (21) Briefly, 20 µg samples of mucosal lysate and BBMV were incubated with 100 mM sucrose or maltose for 60 min at 37°C in buffer of 90 mM NaCl and 4 mM disodium succinate, pH = 6.0. Released glucose was measured by Amplex Red Glucose/Glucose Oxidase Assay kit (Invitrogen, Ltd, Paisley, UK). Enzymatic activities were defined as millimeter glucose released per minute per milligram of protein under described reaction conditions.
Gene Expression
Total RNA samples were isolated from frozen tissues, mucosal scrapings, and isolated epithelial cells using the Qiagen RNeasy Mini kit. First-strand cDNA was synthesized from 3.5 µg of RNA using Thermo Scientific Maxima First Strand cDNA Synthesis Kit for RT-qPCR with ds DNase (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer’s instructions. Semiquantitative, real-time PCR was used to determine the relative quantities of transcripts of the genes of interest. The genes encoding the 60S ribosomal subunit (RPL4) and β-actin (ACTB) were selected and validated as suitable internal reference genes (22, 23). The relative gene expressions of transporters sodium-glucose-linked transporter 1 (SGLT1), glucose transporter 1, 2, and 4 (GLUT1, GLUT2, and GLUT4), fructose transporter (GLUT5); primer sequences for all genes are provided in the Supplemental Material. All PCR reactions were subjected to a melt curve analysis to validate the absence of nonspecific products. The data are presented as 2−ΔΔCT in gene expression relative to control group, normalized to the geometric mean of RPL4 and ACTB (22, 23) before statistical analysis.
Western Blotting Analyses of Nutrient Transporter
The same amount of protein in BBMV isolated from jejunal scraping or jejunal tissue on Ussing chamber were boiled in 2× SDS loading buffer (250 mM Tris·HCl, 500 mM β-mercaptoethanol, 2% SDS, 0.1% phenol blue, 10% glycerol at pH 6.8) at 70°C for 10 min. The same amounts of sample aliquots (30 μg or 15 μg protein, respectively) were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). Total protein of loading on gel was activated by UV exposure before being transferred. The membranes were blocked at room temperature for 1 h with 5% bovine serum albumin dissolved in 1× Tris-buffered saline (TBS; 25 mM Tris·HCl, 0.15 M NaCl at pH 7.4), and then incubated at 4°C with a rabbit anti-pig SGLT1 polyclonal antibody (ab14686, 1 μg/mL; Abcam, Cambridge, MA) or rabbit anti-pig GLUT2 polyclonal antibody (LS-C40343, 0.8 μg/mL; LSBio, Seattle, WA) or rabbit anti-pig GLUT5 polyclonal antibody (LS-C129290, 1 μg/mL; LSBio, Seattle, WA). After the overnight incubation at 4°C, membranes were washed with TBS with 0.1% Tween-20 (TBS-T) and incubated at room temperature for 1 h with secondary anti-rabbit horseradish peroxidase (HRP)-conjugated IgG (Santa Cruz Biotechnology, Santa Cruz, CA), washed with TBS-T, and incubated in Clarity Western ECL substrate (Bio-Rad). Blots were visualized using ChemiDoc MP Imager (Bio-Rad), and the Image Lab software (v. 4.1; Bio-Rad) was used for densitometry analysis. Protein level of transporters were calculated relative to total protein (20–150 kDa). Western blotting analyses were all performed in duplicate for each sample. Data were presented as relative level to the average expression on each blot, as an arbitrary unit.
Statistical Analysis
Data are presented as means ± SE. All the comparison between EW and LW groups were analyzed by Student’s t test using GraphPad Prism8 software (San Diego, CA). To investigate the neural blockade effects, comparisons were done using a two-way ANOVA for weaning age and drug effect. Data were presented as relative value to the nontreated tissue. In all instances, P values ≤ 0.05 were considered statistically significant.
RESULTS
Early Weaning Induces Long-Term Reductions in Intestinal SGLT1-Mediated Glucose Transport
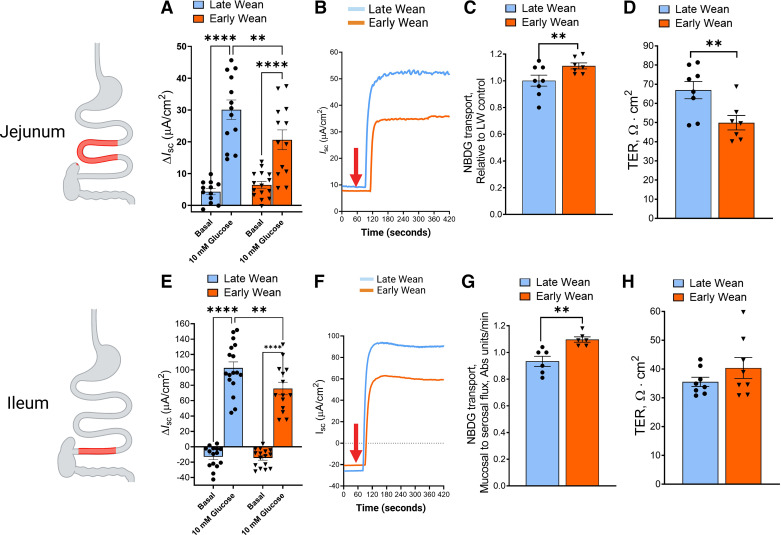
Jejunal and ileal mucosa obtained from 10-wk-old pigs from EW and LW experimental groups, were mounted on Ussing chambers. Epithelial SGLT1 transport was measured as the change in transepithelial Isc (ΔIsc) after addition of 10 mM glucose to the mucosal side of tissues as described in methods. Basal Isc levels before glucose addition were similar between EW and LW pig jejunum and ileum (Fig. 2, A and E). Compared with LW controls, glucose-stimulated ΔIsc was lower in EW jejunum (by 32%; P < 0.05; Fig. 2, A and B), and ileum (by 27%; P < 0.05; Fig. 2, E and F) overall indicating a reduced small intestinal SGLT1 transport activity in the EW pigs. We next evaluated whether glucose transport via GLUT2 was impacted by EW by measuring the mucosal-to-serosal flux of labeled glucose (NBDG) in the presence of the SGLT1 inhibitor phlorizin (PZ) in jejunum from EW and LW pigs. Pretreatment of jejunal mucosa with PZ reduced glucose-stimulated Isc responses by ∼90% in both LW and EW jejunum confirming the inhibition of SGLT1 by PZ (Supplemental Fig. S2). Pilot experiments also demonstrated that the PZ-insensitive NBDG transport was inhibited by the GLUT2 inhibitor, phloretin, thus validating the measurement of GLUT2-mediated transport using this approach (Supplemental Fig. S3). Using this approach, relative to LW controls, GLUT2-mediated glucose transport was greater (P < 0.05) in EW jejunal (Fig. 2C) and ileal mucosa (Fig. 2G). Jejunum from EW pigs exhibited a lower transepithelial electrical resistance (TER, Fig. 2D) than LW jejunum that indicates a persistent increase in intestinal permeability in EW pigs, which we have demonstrated in previous publication in EW pigs (24, 25). However, ileal mucosal TER was not different between EW and LW pigs in this study (Fig. 2H), despite reduced SGLT1 and increased GLUT2-mediated glucose transport, suggesting that TER might not be directly related to shift in intestinal glucose transporters in EW pigs.
Figure 2.
Early weaning induces a development shift in small intestinal epithelial SGLT1 and GLUT2 transport. Jejunal and ileal mucosa obtained from 70-day-old female pigs that were previously weaned from their dam at 28 days of age (late wean; blue) or 15 days of age (early wean; orange) were mounted on Ussing chambers. The peak change in transepithelial Isc (ΔIsc) was calculated following addition of mucosal d-glucose (10 mM) in jejunum (A) and ileum (E). Representative Isc tracings of jejunum (B) and ileum (F) after addition of glucose (indicated by red arrow). GLUT2-mediated transport was measured in the jejunum (C) and ileum (G) as determined by NBD-glucose flux across in the presence of the SGLT1 inhibitor phlorizin. Basal transepithelial electrical resistance (TER) was measured in jejunum (D) and ileum (H). Values represent means ± SE for n = 8–16 pigs/weaning age group. Glucose-induced Isc was performed on two independent cohorts of animals (cohort 1; n = 16 pigs and cohort 2; n = 16 pigs), and data from both cohorts is combined within respective wean age groups. NBDG flux and TER measurements reflect one cohort (cohort 2) of animals. **P < 0.01, **** P < 0.0001, LW vs. EW, Student’s t test. EW, early wean; GLUT2, glucose transporter 2; Isc, short-circuit current; LW, late wean; SGLT1, sodium-glucose-linked transporter 1. Image created with BioRender and published with permission.
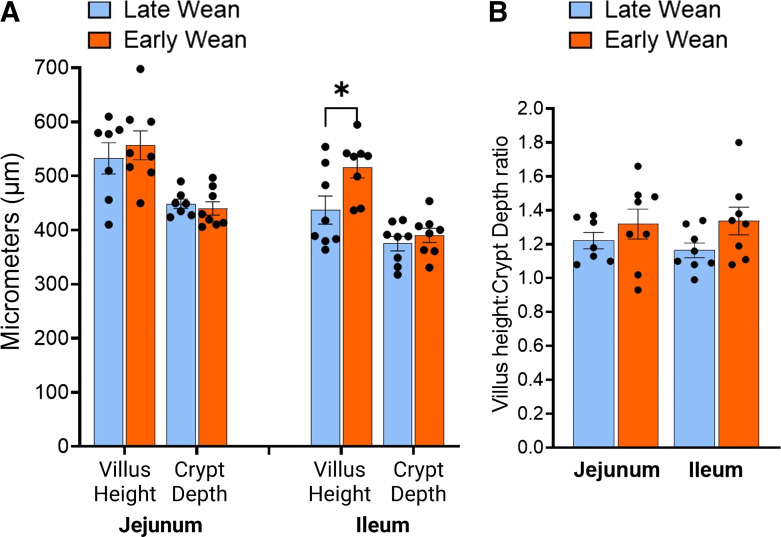
To determine whether divergent alterations in intestinal SGLT1 and GLUT2 function were associated with morphological changes of the intestinal mucosa in EW pigs, villus height and crypt depth in the jejunum and ileum were measured. There were no differences in jejunal villus height or crypt depth observed between EW and LW pigs at 10 or 20 wk of age (Fig. 3A). In the ileum, villus height was greater in EW pigs than in LW pigs (P < 0.05; Fig. 3A). There were no differences in villus-to-crypt ratios between wean age groups (Fig. 3B). Expression of ATP1A1 gene, which encodes for the basolateral Na+-K+-ATPase pump that drives the electrochemical Na+ gradient required for SGLT1 cotransport, was not influenced by EW (Supplemental Fig. S4), Together, these data suggest that alterations in SGLT1 and GLUT2-transport induced by EW in pigs reflect changes in apical transport function and not associated with morphological changes or transcriptional regulation of ATP1A1 expression.
Figure 3.
Jejunal and ileal villus morphology is not altered by early weaning. Jejunum and ileum samples were fixed in 10% neutral buffered formalin (NBF) and processed and stained with H&E. A: villus height and crypt depth were averaged across 12 intact villi per pig tissue section. B: the ratio of VH:CD was calculated. Values represent means ± SE for n = 7 or 8 pigs/weaning age group. *P < 0.05, Student’s t test. CD, crypt depth; H&E, hematoxylin-eosin; VH, villus height.
SGLT1 and GLUT2 Localization to the Intestinal Epithelial Brush-Border Membranes
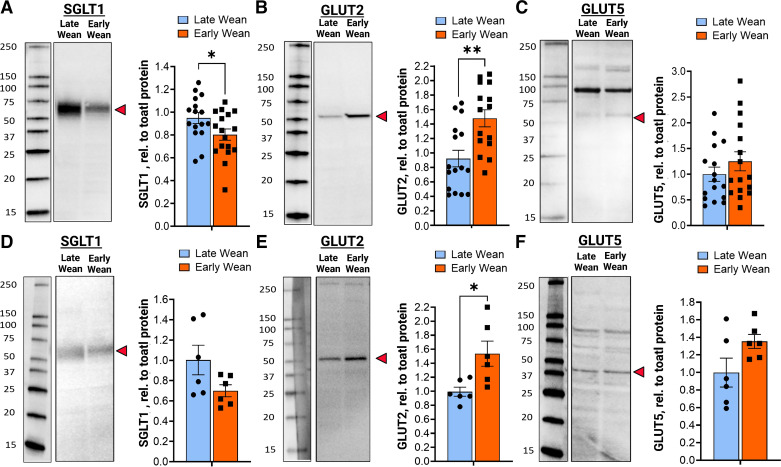
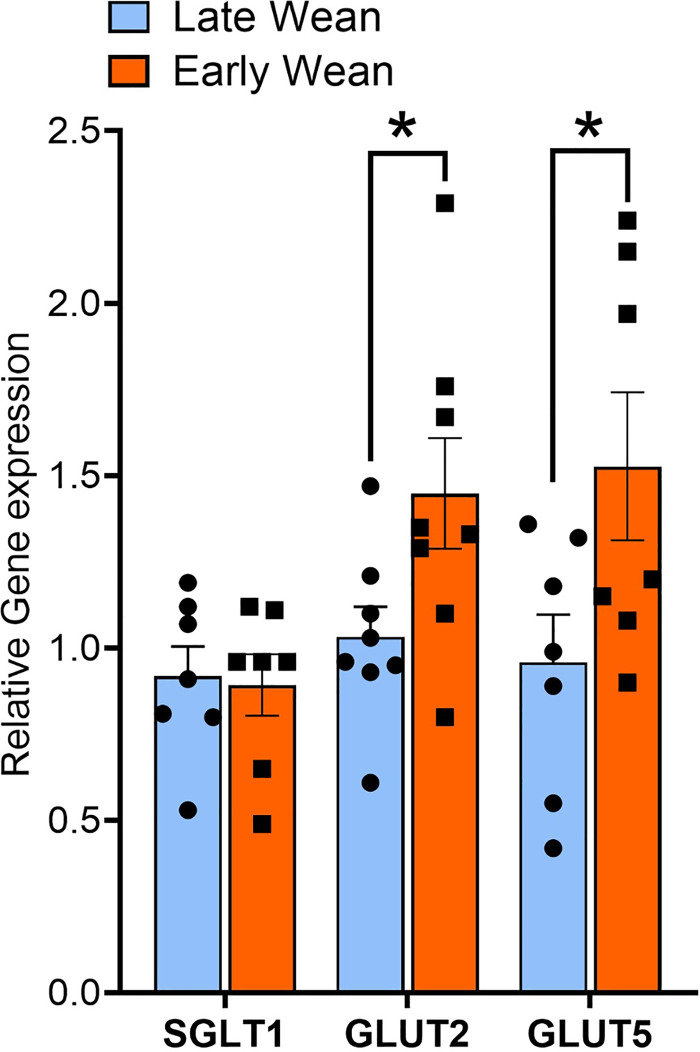
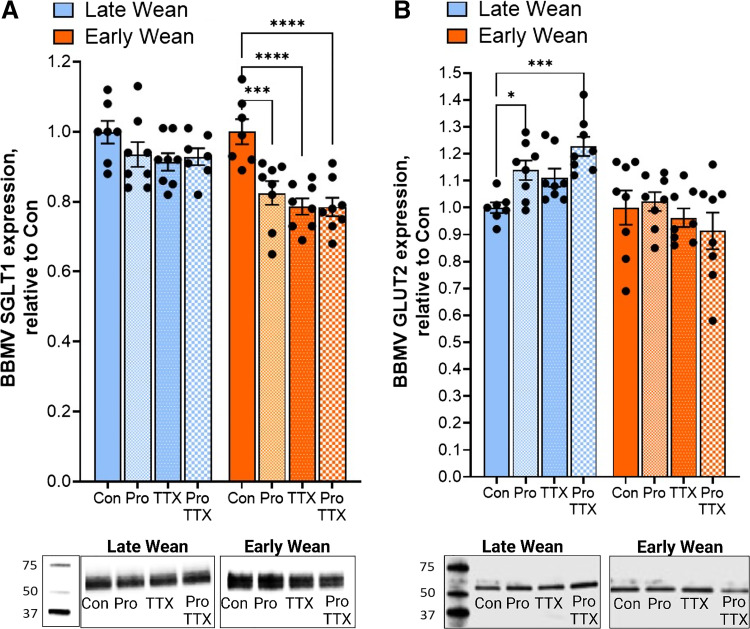
Intestinal SGLT1 and GLUT2-mediated glucose transport is dependent on trafficking and insertion of transporters into the apical BBM of intestinal epithelial cells. We, therefore, investigated if EW-induced alterations in SGLT1 and GLUT2 transport were associated with differential expression and abundance of the transporters in the BBM. In 10-wk-old EW pigs, SGLT1 protein abundance in BBMs was lower (P < 0.05) compared with LW pigs (Fig. 4A). In contrast and in line with glucose transport data, jejunal GLUT2 BBM abundance was significantly greater (P < 0.01) in EW pigs than in LW pigs (Fig. 4B). Greater BBM GLUT2 expression was also observed in jejunum from 20-wk-old EW pigs (Fig. 4E), demonstrating a long-term upregulation of GLUT2 BBM abundance. The mean expression value for BBM SGLT1 was lower in 20-wk-old EW pigs but not statistically significant (P = 0.08), compared with LW pigs. Abundance of GLUT5, which is the major fructose transporter, was not different between EW and LW pigs at 10 wk or 20 wk of age (Fig. 4, C and F). There were no differences in intestinal epithelial cell SGLT1 mRNA (Fig. 5A) between EW and LW pigs, indicating that the EW-associated reductions in BBM SGLT1 protein abundance were not associated with downregulated transcription/translation of SGLT1, and thus likely due to decreased trafficking and(or) recycling to the BBM. In contrast, GLUT2 and GLUT5 mRNA were significantly upregulated (Fig. 5A) in EW pigs indicating that GLUT2 and GLUT5 are transcriptionally regulated by EW. In summary, these data show that EW induces long-term functional alterations in intestinal glucose transport characterized by a developmental shift toward reduced SGLT1 and increased GLUT2 function, which coincided with reciprocal changes in SGLT1 and GLUT2 abundance in the BBM.
Figure 4.
Early weaning induces a long-term shift in SGLT1 and facilitated GLUT2 and GLUT5 transporters in small intestinal brush-border membranes. Jejunal brush-border membrane vesicles (BBMVs) were isolated from the female pigs that were previously weaned from their dam on day 28 (late wean) or 15 days (early wean) of age for SGLT1, GLUT2, and GLUT5 protein expression via Western blot analysis. Expression level of glucose transporters was determined using densitometric analysis and relative to total blot protein, as described in the methods. Values in A–C (70-day-old pigs) represent the combined data from two independent experimental cohorts (n = 16 pigs/cohort), where the same response was observed. Values in D–F (140-day-old pigs) represent one experimental cohort of pigs (n = 16). Representative Western blot images: molecular weight markers from each blot were from the same blot as protein bands, but not adjacent to the lanes shown (indicated by the line borders). *P < 0.05, **P < 0.01, Student’s t test. GLUT5, fructose transporter; GLUT2, glucose transporter 2; SGLT1, sodium-glucose-linked transporter 1.
Figure 5.
Early weaning induces a long-term upregulation of GLUT2 and GLUT5 but not SGLT1 mRNA expression in isolated jejunal epithelial cells. Jejunum epithelial cells were isolated from 70-day-old female pigs (n = 7 or 8 pigs/wean age group) that were previously weaned from their dam at 28 days of age (late weaned) or 15 days of age (early weaned). Relative gene expression of SGLT1, GLUT2, and GLUT5 were quantified as described in the methods. *P < 0.05, LW vs. EW, Student’s t test. EW, early wean; GLUT5, fructose transporter; GLUT2, glucose transporter 2; LW, late wean; SGLT1, sodium-glucose-linked transporter 1.
β-Adrenergic Receptor Regulation of SGLT1 and GLUT2 Glucose Transport and BBM Abundance
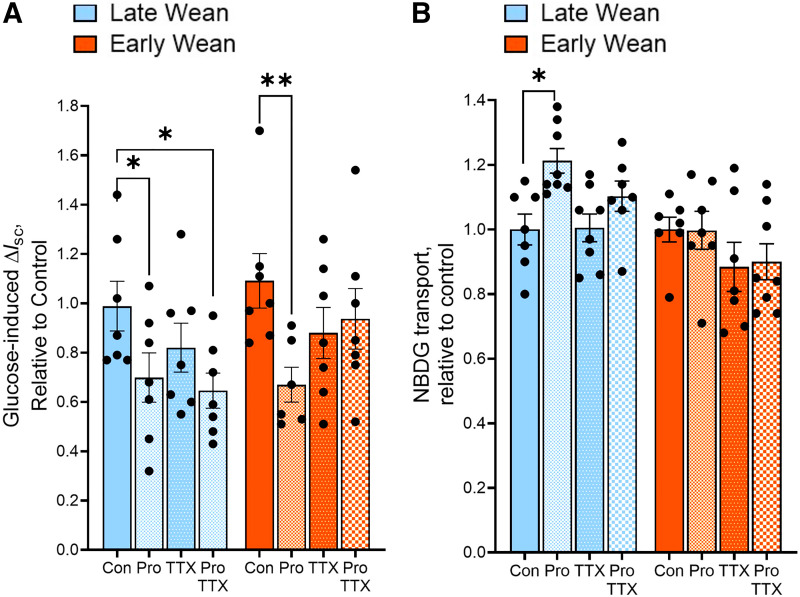
The sympathetic nervous system (SNS) plays a central role in the stress response via release of catecholamines (norepinephrine and epinephrine), which bind to α- and β-adrenergic receptors (βARs) on target cells to mediate their actions. Catecholamines via βARs have been shown to directly modulate intestinal glucose transport in multiple species and in different regions of the GI tract (20, 26). Given the link between stress and regulation of intestinal glucose transport by βAR signaling, we investigated whether regulation of glucose transporters by βAR differed between LW and EW pigs. Given our previously published studies demonstrating the long-term effects of EW on ileal enteric nervous system (27), we focused on βAR regulation in the ileum in these experiments. Ileal mucosa from EW and LW pigs were pretreated with the βAR inhibitor propranolol, and SGLT1- and GLUT2-mediated transport was measured as described earlier. Propranolol decreased SGLT1-mediated glucose transport (determine by glucose-induced Isc responses) in both LW ileum (by 29%; P = 0.044) and EW ileum (by 39%; P = 0.006; Fig. 6A), indicating that endogenous βAR signaling promotes SGLT1-mediated glucose transport, independent of wean age. In both LW and EW pig ileum, the neuronal inhibitor TTX alone or in combination with propranolol had no significant effect on SGLT1-mediated transport (Fig. 6A). In contrast to SGLT1, propranolol-enhanced GLUT2-mediated glucose uptake (by 1.2-fold; P = 0.01) in ileum from LW pigs; however, this effect was not observed in EW pig ileum (Fig. 6B). There was no effect of TTX on GLUT2-mediated transport. The altered sensitivity to propranolol-mediated SGLT1 and GLUT2 regulation between LW and EW pigs did not coincide with different expression levels of βARs (Supplemental Fig. S5). Overall, these data indicate that SGLT1-mediated glucose transport is positively regulated by enteric βARs in EW and LW pigs. In contrast, βAR signaling negatively regulates GLUT2-mediated glucose ileal transport in ileum from LW pigs, but this inhibitory control was absent in EW pigs intestine.
Figure 6.
Differential regulation of SGLT1- and GLUT2-mediated glucose transport by β-adrenergic receptors in late-weaned and early-weaned pigs. Ileal mucosa tissues from EW and LW pigs at day 70 of age were mounted on Ussing chamber. Tissues from each pig were pretreated with Ringer (control, con), propranolol (pro), TTX, or Pro + TTX for 20 min before luminal addition of 100 mM d-glucose mixed with NBD-glucose (NBDG). Blockade effect of treated tissues were presented relative to the controls within each pig. A: SGLT1 activity was measured as glucose-induced ΔIsc. B: GLUT2-mediated transport was determined by NBD-G flux in the presence of the SGLT1 inhibitor phlorizin. Values represent means ± SE for n = 8 pigs/weaning age group from one experimental cohort of pigs (cohort 2). *P < 0.05, **P < 0.01, LW vs. EW, two-way ANOVA. EW, early weaned; GLUT2, glucose transporter 2; LW, late weaned; SGLT1, sodium-glucose-linked transporter 1; TTX, tetrodotoxin.
To determine if the effects of propranolol on SGLT1 and GLUT2 activity corresponded to changes in the transporter localization to the BBM, SGLT1 and GLUT2 protein abundance in BBM fractions was measured following experimental treatments with propranolol and TTX (Fig. 7). Neither propranolol nor TTX treatment significantly affected SGLT1 BBM abundance in ileum from LW pigs (Fig. 7A). However, propranolol and TTX treatments significantly reduced (P < 0.0001) the abundance of BBM SGLT1 in EW pig ileum. Given that the combined treatment of ileal tissues with propranolol and TTX did not induce further reductions in BBM SGLT1 abundance suggests that propranolol and TTX are acting via a common pathway. The level of BBM GLUT2 expression was increased by propranolol in LW ileum (Fig. 7B), which is line with the propranolol-associated increased in GLUT2-mediated transport. In line with GLUT2-mediated transport, propranolol had no effect on BBM GLUT expression in EW pigs. Together, these data demonstrate that endogenous intestinal βAR signaling has opposing actions on SGLT1 and GLUT2 function and localization to the BBM. Furthermore, these data suggest that the inhibitory regulation of ileal GLUT2 function and BBM abundance by βAR is absent in EW pigs.
Figure 7.
Differential regulation of SGLT1- and GLUT2-mediated BBM localization by β-adrenergic receptors in late-weaned and early-weaned pigs. Brush-border membrane vesicles (BBMVs) were isolated ileal mucosa mounted on Ussing chamber tissues that were previously treated with propranolol (pro) and tetrodotoxin (TTX) described in Fig. 6 and in methods. Protein abundance (relative to total protein on blot) of SGLT1 (A) and GLUT2 (B) in BBM fractions were quantified via Western blot analysis and densitometric analysis. Effects of Pro and TTX presented as relative densitometry values compared with controls within each pig. *P < 0.05, ***P < 0.001, ****P < 0.0001, LW vs. EW, two-way ANOVA. BBM, brush-border membrane; EW, early weaned; GLUT2, glucose transporter 2; LW, late weaned; SGLT1, sodium-glucose-linked transporter 1.
Blood Glucose and CRP Levels Are Elevated in EW Pigs
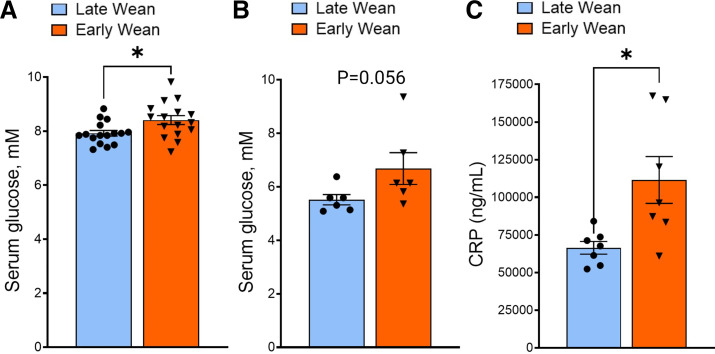
As discussed in the introduction, exposure to ELA is a risk factor for later life development of chronic inflammatory and metabolic disorders including Type 2 diabetes (T2D) and obesity. Furthermore, increased expression of intestinal epithelial glucose transporters has been reported in human patients with T2D and obesity (23, 28) and was shown to be important in obesity-induced intestinal permeability, inflammation, and infection risk in a rodent model (15). Therefore, given the relationship between ELA and inflammatory and metabolic disorders, and the current findings on enhanced GLUT2-mediated glucose transporter function and expression in EW pigs, we investigated whether EW pigs also exhibit elevated systemic and tissue markers of glucose dysregulation and indices associated with metabolic inflammation. Basal serum glucose concentrations were greater (P < 0.05) in 70-day-old EW pigs (Fig. 8A) and tended (P = 0.056) to be greater at 140-day-old pigs (Fig. 8B). Early-weaned pigs also exhibit increased blood levels of the inflammatory marker, CRP (P = 0.05; Fig. 8C), a marker of low-grade systemic inflammation.
Figure 8.
Long-term effects of early weaning on basal serum glucose and CRP concentrations. Serum glucose level in early- and late-weaned pigs at day 70 (A) and day 150 (B) of age. C: serum CRP level at day 70 of age. Values represent means ± SE for n = 6–8 pigs/wean age group. *P < 0.05, LW vs. EW, Student’s t test. CRP, C-reactive protein; EW, early weaned; LW, late weaned.
Visceral Adipose Tissue Histology and Gene Expression
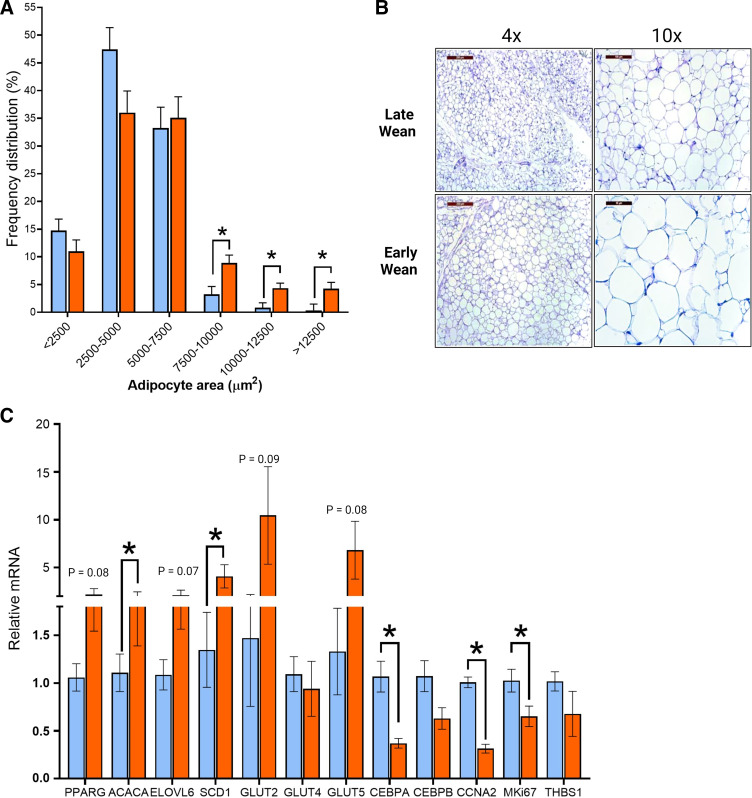
A strong association exists between visceral adiposity, metabolic inflammation, and risk for T2D (29). Therefore, we investigated whether differences existed in visceral adipose tissue (AT) histology and gene expression between EW and LW pigs. Histological analysis of visceral adipose tissue (perirenal adipose) revealed a shift in adipocyte size distributions in EW pigs toward increased adipocyte hypertrophy, compared with LW pigs (Fig. 9, A and B). Specifically, visceral adipose tissue from EW pigs showed increased number of large size (>7,500 µm2) and reduced count of midsize (2,500–5,000 µm2) adipocytes than LW (Fig. 9A). In support of this finding, adipose tissue from EW pigs also exhibited upregulation of lipogenic genes including those involve in fatty acid synthesis (ACACA, P < 0.05) and desaturation (SCD1, P = 0.04). In contrast, genes associated with differentiation and proliferation of adipocytes (CEBPA, CCNA2, and MKi67) were all significantly downregulated in EW pig visceral adipose tissue compared with LW pigs (Fig. 9C), indicating a hypertrophic response in perirenal AT. In summary, these data support that EW pigs exhibit enhanced visceral adiposity characterized by adipocyte hypertrophy.
Figure 9.
Increased adipocyte size and decreased proliferation in visceral adipose tissue from early-weaned pigs. A: H&E staining of perirenal-abdominal adipose tissue from early- and late-weaned pigs at day 70 of age. Frequency distribution (percentage) of adipocyte area. B: representative histological images of perirenal adipose tissue at ×4 and ×10 magnifications. C: relative gene expression of adipose tissue. Values represent means ± SE for n = 6–8 pigs/weaning age group. *P < 0.05, LW vs. EW, Student’s t test. EW, early weaned; H&E, hematoxylin-eosin; LW, late weaned.
DISCUSSION
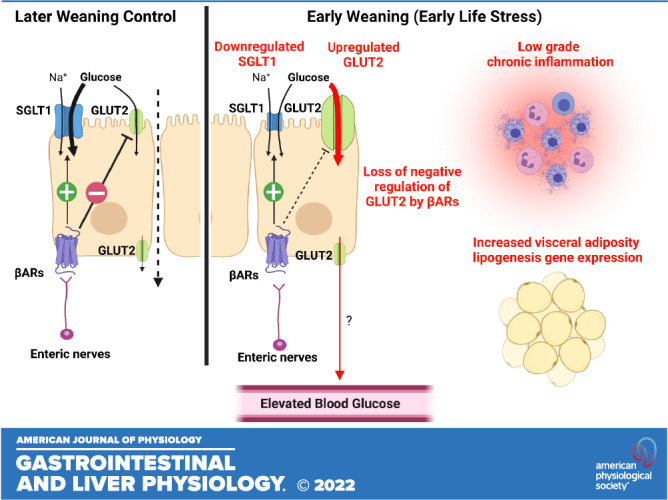
Early-life adversity is a significant risk factor for later life risk for chronic inflammatory disease and metabolic disorders such as obesity and T2D (5, 30, 31); however, the mechanisms linking ELA and long-term disease risk remain poorly understood. The link between ELA and negative later life health outcomes have been demonstrated across several wild, domesticated and research animal species including shortened lifespan (32), and increased susceptibility for inflammatory and metabolic disease in research animal models of early-life stress (24, 29, 33, 34). The mechanisms linking ELA and lifetime disease risk remain poorly understood. Intestinal epithelial glucose transporters, SGLT1 and GLUT2, are the primary route for luminal glucose uptake. Although glucose intestinal epithelial transporters are well established in monosaccharide absorption following a meal, they have more recently implicated in the pathophysiology of inflammatory, infectious, and metabolic disorders (15, 28, 35). The risk of these diseases is also increased in individuals with a history of ELA; however, very little is known about the impact of ELA on the development and function of intestinal glucose transporters. Here, we investigated the impact of EW in pigs, an established large animal model of ELA, on the developmental expression and function of intestinal glucose transporters and markers of metabolic inflammation. We showed that EW induced a developmental shift in small intestinal epithelial glucose transport toward reduced SGLT1 and increased GLUT2 activity, which coincided with their localization to the intestinal BBM. Epithelial SGLT1 and GLUT2 transporter activity and BBM localization were shown to also be reciprocally regulated by the enteric βAR system and the enteric nervous system (ENS) in the porcine intestine and that this regulatory mechanism was altered by EW. Specifically, the negative regulation of GLUT2 by βAR observed in ileum from LW pigs was absent in EW pig ileum. Early-weaned pigs also exhibited systemic markers of metabolic disease characterized by elevated CRP and glucose, and visceral adipocyte hypertrophy lipogenic gene expression.
The long-term reciprocal regulation of SGLT1- and GLUT2-mediated intestinal transport induced by EW in pigs, suggests a developmental programming of intestinal epithelial glucose transport systems. However, the physiological and health implications for such as shift remains to be fully elucidated. Intestinal epithelial SGLT1 and GLUT2 differ significantly in their energetic dependence, sensitivity, and capacity for glucose transport, which may provide insight into the role for the reciprocal changes in SGLT1 and GLUT2 in EW pigs. SGLT1 operates as a secondary transporter that requires a Na+ electrochemical gradient established by the basolateral Na+-K+-ATPase pump and thus requires cellular energy in the form of ATP. In contrast, GLUT2 is a facilitated passive transporter. Therefore, SGLT1 represents a more energy-consuming transport process compared with the energy-independent diffusive/passive glucose transport pathway mediated via GLUT2. Furthermore, compared with SGLT1, GLUT2 has a three to four times higher transport capacity for glucose transport (36). Thus, a shift toward reduced SGLT1 and increased GLUT2 represents a more energy-efficient mechanism for basal intestinal glucose transport (37, 38). However, several major questions remain to be answered regarding this shift in glucose transporters including first, does this represent an allostatic response to ELA to meet increased energetic demands? Given that the body weights of LW and EW pigs were similar in the present study, increased efficiency of glucose uptake does not align with increased efficiency or demand for lean muscle gain, although body composition was not assessed. The immune and nervous systems have large requirements for energy, primarily via glucose, which is further increased upon immunological challenges and activation of neuroendocrine system by stress (39–41). Our previous studies showed that EW pigs exhibit persistent upregulation of ENS activity (27) and mast cell number and activation (25) compared with LW pigs. Furthermore, in the present study, we demonstrated that EW pigs have persistent elevations in blood CRP levels, indicative of a chronic, low-grade inflammatory state in the EW pigs. Elevated CRP has also been shown in humans who have a history of ELA and thus is a biomarker of chronic inflammation associated with ELA and disease risk (4, 42). Thus, given the increased energetic demands of the immune and nervous system, favoring GLUT2 over SGLT1 may represent an adaptive mechanism to maintain the metabolic requirements of a persistently upregulated nervous and immune systems in EW pigs than in LW pigs. However, studies that investigate the direct connection between metabolic requirements of the neuroimmune system in EW pigs would be needed to prove this hypothesis.
What are the mechanisms by which ELA alters the development programming of intestinal glucose transport toward reduced SGLT1 and increased GLUT2? In the present study, we investigated the role of the sympathetic nervous system due to its central role, the stress response, and its regulation of immune system, inflammation, and glucose transport. Specifically, the catecholamines epinephrine/norepinephrine have been shown to regulate intestinal glucose transport via activation of βARs (20, 26). In the present study, Ussing chamber experiments using the βAR inhibitor propranolol showed that endogenous intestinal βAR signaling is a positive regulator of SGLT1 BBM localization and SGLT1-mediated glucose transport in EW and LW pigs. In agreement with our findings, infusion of the ΒAR ligand norepinephrine into rat intestinal loops increased SGLT1 BMM abundance and increased glucose transport (20). ΒAR agonists have also been shown to increase SGLT1-mediated uptake in sheep rumen epithelium (26). Oyebola et al. (43) showed that the small intestinal glucose uptake induced by epinephrine administration to rabbits was blocked with propranolol, similar to the current study. In contrast to SGLT1, GLUT2 was shown to be negatively regulated by ΒAR signaling, but this negative regulation was only observed in LW pigs not observed in the EW intestine. This loss of negative regulation of GLUT2 by ΒAR pathways in EW ileum is particularly intriguing given the persistent upregulation of GLUT2 function and BBM localization in EW pigs. However, whether this loss of negative regulation effect is contributing to the increased GLUT2 abundance and activity in EW pigs remains to be determined. Given that the SNS activation is a common pathway activated in response to a diverse array of psychological, immunological, and infectious stimuli, these findings could have important implications to the understanding how intestinal glucose transport is regulated by stress and its role in stress-related disease risk. Although the current study focused on enteric βAR pathways, it is important to consider that SGLT1 and GLUT2 can be regulated by a diverse array of neuronal, inflammatory, and hormonal mediators (e.g., insulin, leptin, and neurokinin; 44), which could play important roles in the differences observed between EW and LW pigs. Thus, a more fundamental understanding of how βAR signaling negatively regulates GLUT2 in LW pigs and whether these pathways are altered in EW pigs will be important.
What are the health implications for a shift in epithelial glucose transporters in EW pigs? There is increasing evidence in animal models and in humans for a role of intestinal glucose transport in inflammatory and metabolic diseases. Intestinal GLUT2 and GLUT5 mRNA expression were increased in patients with obesity (45) and patients with T2D (28). Furthermore, intestinal organoids derived from human patients with obesity showed a positive relationship between body mass index (BMI) and epithelial organoid glucose uptake an increased expression of SGLT1 and GLUT2 (35). Experimental animal models of diabetes have also reported elevated expression of intestinal glucose transporters (46). However, the direct link between intestinal glucose transport and metabolic and inflammatory disease risk remains to be fully elucidated. Given the elevated expression of glucose transporter in patient with T2D and patient with obesity, enhanced glucose uptake may be associated with immune activation and inflammation as discussed earlier and visceral adiposity. In the present study, adipose tissue of EW pigs had increased expression of genes related to the fatty acid synthesis and lipogenesis, suggesting enhanced adipocyte capacity for triglyceride accumulation. The lipogenic response to ELA in the present paper did not support an adipogenesis response as markers of adipocyte progenitor proliferation and differentiation were reduced in EW pigs. Thus, these findings suggest an adipose hypertrophic response in EW pigs. We showed a trend for elevated GLUT2 and GLUT5 in adipose tissue, reflecting increased uptake and transport of glucose and fructose, respectively, in EW pigs. The significance of the increased visceral adiposity in the EW pig remains to be elucidated. Although the increased energy uptake and capacity for synthesis and storage of lipids has perceived benefits for survival, as a readily available fuel source during times of stress or scarce nutrient resources, such a system may pose problems when nutrients are readily available, such as the case in pigs and in Western human populations, resulting in increased accumulation of adipose tissue, which contributes to metabolic disease and chronic inflammation.
Although increased intestinal GLUT2 and GLUT5, elevated blood glucose levels, inflammation and adiposity observed in the EW pig are observed in people with a higher risk for metabolic and cardiovascular diseases, the present studies do not provide evidence for a direct link between EW and metabolic disease risk. Furthermore, important hallmarks of metabolic disease such as insulin resistance and cardiovascular disease were not assessed. However, it is important to note that alterations in blood glucose, CRP, and adipose tissue induced by EW were observed in pigs under basal, unstressed conditions, and with diets that contained a relatively low-fat content (1.3% dietary fat). This demonstrates that EW alone was enough to induced long-term changes in metabolic status. Future studies investigating the impact of EW under conditions, which promote development of metabolic disease (e.g., high-fat/high-sugar diet, chronic inflammation, metabolic stress, etc.) could reveal a more definitive connection between EW and metabolic disease risk in this model.
In summary, the present studies show that EW, a common early life stressor in pigs, initiates an altered developmental trajectory in intestinal glucose transporters, characterized by a lasting programmed shift from SGLT1 toward GLUT2 transport and expression. This coincided with elevated markers of metabolic disease in EW pigs and differential regulation by intestinal ΒARS. Given the central importance of intestinal glucose uptake in energy homeostasis and support of growth performance as well as the functions of critical systems such as nervous and immune functions, these findings have potential significantly implications to understating performance reductions and increased disease risk in EW animals. Furthermore, given that the risk for metabolic disease is greatly increased in individuals who have a history of ELA, this may represent a valid, large animal model to investigate the link between ELA and later life risk for metabolic disorders. A more fundamental understanding of how early-life stressors, such as EW, initiate and maintain an altered intestinal glucose transporter system, and its implications on, positive or negative, on disease risk and productivity may reveal new therapeutic targets or dietary strategies to mitigate lifetime disease risk in pigs and people.
SUPPLEMENTAL MATERIAL
Supplemental Figs. S1–S5: https://doi.org/10.6084/m9.figshare.17132414.v1.
GRANTS
This study was supported by National Institutes of Health Grant HD072968 and National Institute of Food and Agriculture Grant 2019-67015-29483 to A. J. Moeser.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L., R.N., M.R., N.L.T., G.A.C., and A.J.M. conceived and designed research; Y.L., K.M.T., K.M.F., R.N., M.F., M.R., and A.J.M. performed experiments; Y.L., K.M.T., R.N., M.F., M.R., G.A.C., and A.J.M. analyzed data; Y.L., R.N., M.R., G.A.C., and A.J.M. interpreted results of experiments; Y.L., M.R., and A.J.M. prepared figures; Y.L., R.N., and A.J.M. drafted manuscript; Y.L., K.M.T., K.M.F., R.N., M.R., N.L.T., G.A.C., and A.J.M. edited and revised manuscript; Y.L., K.M.T., K.M.F., R.N., M.F., M.R., N.L.T., G.A.C., and A.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
Images in Figs. 1 and 2 and the Graphical Abstract were created with BioRender and are published with permission. Present addresses: Y. Li, Dept. of Animal and Food Sciences, University of Delaware, Newark, Delaware; K. M. Fernández, ENDURE program Michigan State University, University of Puerto Rico, Ponce, Puerto Rico; M. Rajput, Dept. of Biology, University of Dayton, Dayton, Ohio; N. L. Trottier, Dept. of Animal Science, Cornell University, Ithaca, New York.
REFERENCES
- 1.Chen E, Turiano NA, Mroczek DK, Miller GE. Association of reports of childhood abuse and all-cause mortality rates in women. JAMA Psychiatry 73: 920–927, 2016. doi: 10.1001/jamapsychiatry.2016.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan A, Bernstein CN, Graff LA, Patten SB, Sareen J, Fisk JD, Bolton JM, Hitchon C, Marriott JJ, Marrie RA; CIHR Team in Defining the Burden and Managing the Effects of Immune-mediated Inflammatory Disease. Childhood maltreatment and psychiatric comorbidity in immune-mediated inflammatory disorders. Psychosom Med 84: 10–19, 2022. doi: 10.1097/PSY.0000000000001025. [DOI] [PubMed] [Google Scholar]
- 3.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) study. Am J Prev Med 14: 245–258, 1998.doi: 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 4.Lin JE, Neylan TC, Epel E, O’Donovan A. Associations of childhood adversity and adulthood trauma with C-reactive protein: a cross-sectional population-based study. Brain Behav Immun 53: 105–112, 2016. doi: 10.1016/j.bbi.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas C, Hyppönen E, Power C. Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics 121: e1240–e1249, 2008. doi: 10.1542/peds.2007-2403. [DOI] [PubMed] [Google Scholar]
- 6.Ju T, Naliboff BD, Shih W, Presson AP, Liu C, Gupta A, Mayer EA, Chang L. Risk and protective factors related to early adverse life events in irritable bowel syndrome. J Clin Gastroenterol 54: 63–69, 2020.doi: 10.1097/MCG.0000000000001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SH, Videlock EJ, Shih W, Presson AP, Mayer EA, Chang L. Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil 28: 1252–1260, 2016. doi: 10.1111/nmo.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pohl CS, Medland JE, Moeser AJ. Early-life stress origins of gastrointestinal disease: animal models, intestinal pathophysiology, and translational implications. Am J Physiol Gastrointest Liver Physiol 309: G927–G941, 2015. doi: 10.1152/ajpgi.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faccin JEG, Tokach MD, Allerson MW, Woodworth JC, DeRouchey JM, Dritz SS, Bortolozzo FP, Goodband RD. Relationship between weaning age and antibiotic usage on pig growth performance and mortality. J Anim Sci 98: skaa363, 2020. doi: 10.1093/jas/skaa363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Main RG, Dritz SS, Tokach MD, Goodband RD, Nelssen JL. Increasing weaning age improves pig performance in a multisite production system. J Anim Sci 82: 1499–1507, 2004. doi: 10.2527/2004.8251499x. [DOI] [PubMed] [Google Scholar]
- 11.McLamb BL, Gibson AJ, Overman EL, Stahl C, Moeser AJ. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS One 8: e59838, 2013. doi: 10.1371/journal.pone.0059838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai L, Hu WW, Xia L, Xia M, Yang Q. Transmissible gastroenteritis virus infection enhances SGLT1 and GLUT2 expression to increase glucose uptake. PLoS One 11: e0165585, 2016. doi: 10.1371/journal.pone.0165585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia JS, Byrd JA, Wong EA. Expression of nutrient transporters and host defense peptides in Campylobacter challenged broilers. Poult Sci 97: 3671–3680, 2018. doi: 10.3382/ps/pey228. [DOI] [PubMed] [Google Scholar]
- 14.Palazzo M, Gariboldi S, Zanobbio L, Selleri S, Dusio GF, Mauro V, Rossini A, Balsari A, Rumio C. Sodium-dependent glucose transporter-1 as a novel immunological player in the intestinal mucosa. J Immunol 181: 3126–3136, 2008. [Erratum in J Immunol 181: 7428, 2008]. doi: 10.4049/jimmunol.181.5.3126. [DOI] [PubMed] [Google Scholar]
- 15.Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359: 1376–1383, 2018. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 16.Boudry G, Cheeseman CI, Perdue MH. Psychological stress impairs Na+-dependent glucose absorption and increases GLUT2 expression in the rat jejunal brush-border membrane. Am J Physiol Regul Integr Comp Physiol 292: R862–R867, 2007. doi: 10.1152/ajpregu.00655.2006. [DOI] [PubMed] [Google Scholar]
- 17.Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, Rhoads RP, Baumgard LH, Gabler NK. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One 8: e70215, 2013. doi: 10.1371/journal.pone.0070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Song Z, Kerr KA, Moeser AJ. Chronic social stress in pigs impairs intestinal barrier and nutrient transporter function, and alters neuro-immune mediator and receptor expression. PLoS One 12: e0171617, 2017. doi: 10.1371/journal.pone.0171617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong X, Yang H, Tan B, Yang C, Wu M, Liu G, Kim SW, Li T, Li L, Wang J, Wu G, Yin Y. Differential expression of proteins involved in energy production along the crypt-villus axis in early-weaning pig small intestine. Am J Physiol Gastrointest Liver Physiol 309: G229–G237, 2015. doi: 10.1152/ajpgi.00095.2015. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa Y, Eguchi T, Ishida H. Mechanism of β-adrenergic agonist-induced transmural transport of glucose in rat small intestine. Regulation of phosphorylation of SGLT1 controls the function. Biochim Biophys Acta 1357: 306–318, 1997. doi: 10.1016/S0167-4889(97)00043-8. [DOI] [PubMed] [Google Scholar]
- 21.James PS, Smith MW, Tivey DR, Wilson TJ. Epidermal growth factor selectively increases maltase and sucrase activities in neonatal piglet intestine. J Physiol 393: 583–594, 1987. doi: 10.1113/jphysiol.1987.sp016842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Stahl CH. Dietary calcium deficiency and excess both impact bone development and mesenchymal stem cell lineage priming in neonatal piglets. J Nutr 144: 1935–1942, 2014. doi: 10.3945/jn.114.194787. [DOI] [PubMed] [Google Scholar]
- 23.Ait-Omar A, Monteiro-Sepulveda M, Poitou C, Le Gall M, Cotillard A, Gilet J, Garbin K, Houllier A, Château D, Lacombe A, Veyrie N, Hugol D, Tordjman J, Magnan C, Serradas P, Clément K, Leturque A, Brot-Laroche E. GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes 60: 2598–2607, 2011. doi: 10.2337/db10-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith F, Clark JE, Overman BL, Tozel CC, Huang JH, Rivier JEF, Blikslager AT, Moeser AJ. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol 298: G352–G363, 2010. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pohl CS, Medland JE, Mackey E, Edwards LL, Bagley KD, DeWilde MP, Williams KJ, Moeser AJ. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol Motil 29: e13118, 2017. doi: 10.1111/nmo.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aschenbach JR, Borau T, Gäbel G. Glucose uptake via SGLT-1 is stimulated by β2-adrenoceptors in the ruminal epithelium of sheep. J Nutr 132: 1254–1257, 2002. doi: 10.1093/jn/132.6.1254. [DOI] [PubMed] [Google Scholar]
- 27.Medland JE, Pohl CS, Edwards LL, Frandsen S, Bagley K, Li Y, Moeser AJ. Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterol Motil 28: 1317–1329, 2016. doi: 10.1111/nmo.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 282: G241–G248, 2002. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- 29.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM, de Lemos JA. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 21: E439–E447, 2013. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tosato S, Bonetto C, Lopizzo N, Cattane N, Barcella M, Turco G, Ruggeri M, Provasi S, Tomassi S, Dazzan P, Cattaneo A. Childhood and adulthood severe stressful experiences and biomarkers related to glucose metabolism: a possible association? Front Psychiatry 12: 629137, 2021. doi: 10.3389/fpsyt.2021.629137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huffhines L, Noser A, Patton SR. The link between adverse childhood experiences and diabetes. Curr Diab Rep 16: 54, 2016. doi: 10.1007/s11892-016-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tung J, Archie EA, Altmann J, Alberts SC. Cumulative early life adversity predicts longevity in wild baboons. Nat Commun 7: 11181, 2016. doi: 10.1038/ncomms11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy MO, Herald JB, Wills CT, Unfried SG, Cohn DM, Loria AS. Postnatal treatment with metyrapone attenuates the effects of diet-induced obesity in female rats exposed to early-life stress. Am J Physiol Endocrinol Physiol 312: E98–E108, 2017. doi: 10.1152/ajpendo.00308.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lennon EM. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10−/− mice. Inflamm Bowel Dis 19: 712–719, 2013. doi: 10.1097/MIB.0b013e3182802a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasan NM, Johnson KF, Yin J, Baetz NW, Fayad L, Sherman V, Blutt SE, Estes MK, Kumbhari V, Zachos NC, Kovbasnjuk O. Intestinal stem cell-derived enteroids from morbidly obese patients preserve obesity-related phenotypes: elevated glucose absorption and gluconeogenesis. Mol Metab 44: 101129, 2021. doi: 10.1016/j.molmet.2020.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr 28: 35–54, 2008.doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- 37.Krimi RB, Letteron P, Chedid P, Nazaret C, Ducroc R, Marie J-C. Resistin-like molecule-β inhibits SGLT-1 activity and enhances GLUT2-dependent jejunal glucose transport. Diabetes 58: 2032–2038, 2009. doi: 10.2337/db08-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J 385: 485–491, 2005. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med 267: 543–560, 2010. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 40.McKenna MC. Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem Res 37: 2613–2626, 2012. doi: 10.1007/s11064-012-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kvidera SK, Horst EA, Mayorga EJ, Sanz-Fernandez MV, Abuajamieh M, Baumgard LH. Estimating glucose requirements of an activated immune system in growing pigs. J Anim Sci 95: 5020–5029, 2017. doi: 10.2527/jas2017.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger E, Castagné R, Chadeau-Hyam M, Bochud M, d'Errico A, Gandini M, Karimi M, Kivimäki M, Krogh V, Marmot M, Panico S, Preisig M, Ricceri F, Sacerdote C, Steptoe A, Stringhini S, Tumino R, Vineis P, Delpierre C, Kelly-Irving M. Multi-cohort study identifies social determinants of systemic inflammation over the life course. Nat Commun 10: 773, 2019. doi: 10.1038/s41467-019-08732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oyebola DDO, Taiwo EO, Idolor GO, Alada ARA, Owoeye O, Isehunwa GO. Effect of adrenaline on glucose uptake in the rabbit small intestine. Afr J Med Med Sci 40: 225–233, 2011. [PubMed] [Google Scholar]
- 44.Koepsell H. Glucose transporters in the small intestine in health and disease. Pflugers Arch 472: 1207–1248, 2020. doi: 10.1007/s00424-020-02439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritze Y, Bárdos G, D’Haese JG, Ernst B, Thurnheer M, Schultes B, Bischoff SC. Effect of high sugar intake on glucose transporter and weight regulating hormones in mice and humans. PLoS One 9: e101702, 2014. doi: 10.1371/journal.pone.0101702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita Y, Kojima H, Hidaka H, Fujimiya M, Kashiwagi A, Kikkawa R. Increased intestinal glucose absorption and postprandial hyperglycaemia at the early step of glucose intolerance in Otsuka Long-Evans Tokushima fatty rats. Diabetologia 41: 1459–1466, 1998. doi: 10.1007/s001250051092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S5: https://doi.org/10.6084/m9.figshare.17132414.v1.