Abstract
An increase in multiresistant Enterobacteriaceae was observed at one of the departments of the University Medical Center Utrecht. Nine different integrons and 17 gene cassettes were found, including the new gene cassette aadA8. This cassette was highly related to aadA3 and aadA2. In addition, an unknown promoter sequence was found for two integrons.
In gram-negative bacteria and especially among Enterobacteriaceae, class 1 integrons are involved in antibiotic resistance (2, 4, 7, 10, 11). Hall and Collis (3) defined integrons as elements that contain the genetic determinants of the components of a site-specific recombination system that recognizes and captures mobile gene cassettes. Gene cassettes in class I integrons are composed of a single coding sequence and a so-called 59-base element, which is involved in the mobility of the gene cassettes. Most gene cassettes lack a promoter, and expression is dependent on two potential promoters called P1 and P2, which may differ in sequence and strength in different integrons (5). More than 60 gene cassettes have been described (1).
During 1995–1996 an increase of multiresistant Enterobacteriaceae was observed at the neurology and neurosurgery department of the University Medical Center Utrecht. Characterization of the increase demonstrated the involvement of class 1 integrons as determined using conserved segment PCR (CS-PCR) (6). Thirty-five of a total of 68 multiresistant Enterobacteriaceae (51%) yielded at least one PCR product. At least 11 different PCR products were obtained. DNA sequencing, using the Big Dye Terminator Cycle Sequencing kit (PE Applied Biosystems, Gouda, The Netherlands) and an Applied Biosystems ABI 377 sequencer as described by the manufacturers, showed that two CS-PCR amplification products had no homology with integrons and were considered artifacts. The nine other products analyzed revealed homology with known integrons and gene cassettes (Table 1). The majority of the gene cassettes found have been described before (6, 9) or were presented in GenBank. The aadA8 gene cassette has not been described before.
TABLE 1.
Characteristics of the CS-PCR products and integrons and GenBank accession numbers of the gene cassettes and highly related gene cassettes
| Species | Isolate no. | RFLP typea | Size of CS-PCR product (bp) | Gene cassette(s) and order | GenBank accession no. |
|---|---|---|---|---|---|
| Klebsiella oxytoca | 5 | I | 1,000 | aadA2 | X68227 |
| II | 1,450 | aadB-catB3 | U13880-U13880 | ||
| Klebsiella pneumoniae | 72 | III | 2,500 | aacA7-oxa2a-aadA8 | U13880-M95287-AF326210 |
| Klebsiella pneumoniae | 139 | IV | 3,000 | aacA4-aacC1-orf9/orf10-aadA1a | M55547-L06157-AF326211, U90945,bAJ009820c- X12870 |
| Escherichia coli | 272 | V | 1,000 | aadA1a | X12870 |
| Enterobacter aerogenes | 302 | VI | 2,200 | dfrV-ereA2 | X12868-AF326209, AF099140,dM11277e |
| Escherichia coli | 422 | VII | 1,550 | dfrIa-aadA1a | X00926-X12870 |
| Escherichia coli | 366 | VIII | 1,800 | dfrXII-orfF-aadA2 | Z21672-Z21672-X68227 |
| Citrobacter freundii | 291 | XI | 2,200 | orfD-aacA4-catB7 | U13880-M55547-AF227506 |
| Klebsiella pneumoniae | 227 | 1,250 | |||
| Klebsiella pneumoniae | 366 | 850 |
The oxa2 gene has been described before by Stokes et al. as being in an integron carrying two copies of the gene (12). However, our analysis of the published sequences showed that the oxa2 genes differ by three consecutive base pairs. The first gene in the integron is now called oxa2a and the second is called oxa2b (R. M. Hall, personal communication). The gene cassette found here was identical to oxa2a.
The ereA2 gene was nearly identical to the gene in GenBank, but the differences with the ereA1 sequence were larger and resulted in significant differences in the EreA1 N terminus, which is 62 amino acids shorter than the EreA2 N terminus. In addition, the next six amino acids were different. A single nucleotide deletion in the sequence of ereA1 is largely responsible for this large difference. It can be debated whether the difference is either the result of evolution or a sequencing error. Despite the difference in amino acid sequence, putative promoter and ribosome binding sites in front of both coding sequences have been identified, suggesting that the ereA gene cassettes can be transcribed from its own promoter sequences, but this does not exclude transcription from the promoters in the 5′-CS region.
The sequences of orf9/10 have been described as part of an integron on pACM1, and a comparison showed a one-nucleotide difference with the sequences labeled orfA and orfB (8). Seven nucleotide differences are observed in comparison with the orfX sequences. The most important difference was a deletion of one nucleotide in the orfX sequence. This deletion led to a different and longer C-terminal end for the putative product of the first open reading frame. The other nucleotide differences were observed between the orf10 and orfX sequences, all of them located downstream from the stop codon. A putative GTTRRcY consensus sequence for the end of the attC site of orf9 could not be identified unambiguously. Also, the RYYYAAC consensus sequence for the start of the 59-base element of orf10 could not be identified unambiguously. Since both open reading frames have been described only together, the possibility exists that orf9 lacks an attC site or that this element is not functional.
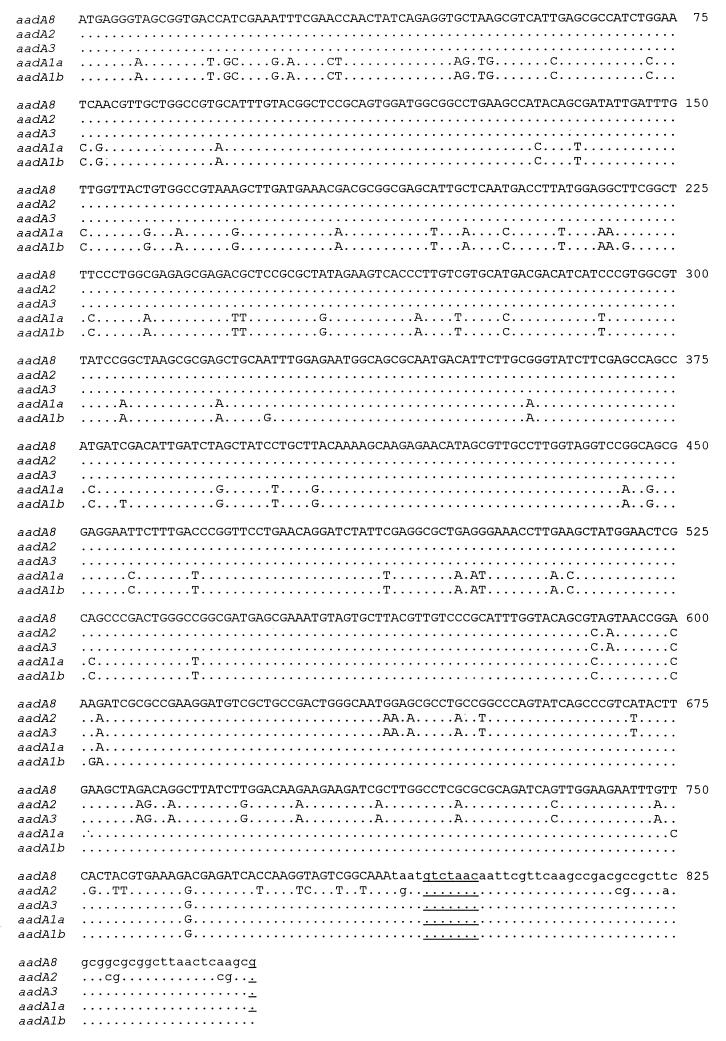
The aadA8 gene cassette was 956 nucleotides long, including a 60-nucleotide 59-base element, and encoded a 262-amino-acid adenylyltransferase. Comparison of the sequence with those of the aadA1a, aadA1b, and aadA2-aadA7 genes showed that it was closely related to aadA3 and aadA2 (Fig. 1) and may have arisen by a recombination of those genes. Direct sequence comparison of the aadA8 gene cassette with its close relatives showed only 20 nucleotide changes when compared to the closely related aadA3 gene. These differences gave rise to 11 differences in the amino acid sequences specified by the genes. A few additional differences were observed when the aadA8 gene was compared with the aadA2 gene, especially in the C-terminal part. Differences with other aadA genes were distributed across the sequences. The attC site of the aadA1a gene had the same attC site sequence as both the aadA8 and aadA3 genes, whereas the attC site of the aadA1b gene, which was more closely related to the aadA1a gene, differed by five nucleotides from the attC site of aadA8 and aadA3.
FIG. 1.
Alignment of the DNA sequences of aadA1a, aadA1b, aadA2, and aadA3 with the aadA8 DNA sequence. The homology of the sequences is 93, 91, 96, and 98% when compared with the aadA8 sequence. Non-codon sequences are in lowercase. The RYYYAAC motif and the G from the GTTRRcY motif delineating the attC site are underlined.
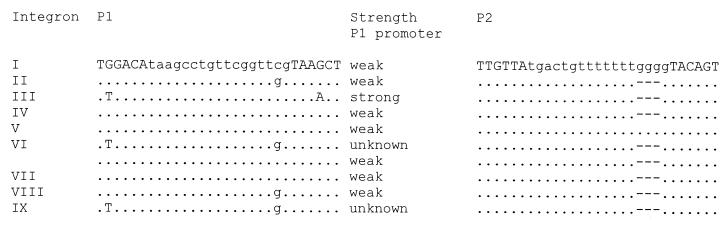
Analysis of the promoter regions of the nine integrons showed that five different P1 promoter sequences were present, but nucleotide 16, which was either a C or a G, most likely does not influence the activity of the promoter, because it is outside the consensus −35 and −10 sequences. The P1 sequence for integron VI could not fully be resolved, because two variants were present. Part of the P1 sequences, which were obtained from a single isolate, had T and G mutations compared to the rest of the P1 sequences, which had a G and a C at these positions, respectively. Seven of the 10 P1 promoter sequences for the integrons described here were weak promoters, and 1 promoter was a strong promoter, whereas 2 promoters have not been described before and their levels of activity are unknown (Fig. 2). The P2 promoter regions were identical among the nine integrons with the exception of the promoters for integron I and V, which were separated by 17 nucleotide sequences instead of 14 nucleotide sequences. Only the first P2 promoter sequences with 17 nucleotide sequences are active (5).
FIG. 2.
Alignment of P1 and P2 sequences. The −35 and −10 boxes are in capital letters, and the spacing between these two boxes is in lower case. The strength of the P1 promoters is indicated. Only the P2 promoters of integron I and V are active.
The detection of at least nine different integrons, one new gene cassette, and two unknown integron promoters isolated from multiresistant Enterobacteriaceae from a single specialty (neurology and neurosurgery) at our hospital demonstrates once more the wide distribution of these genetic elements and their potential to contribute to antibiotic resistance.
REFERENCES
- 1.Fluit A C, Schmitz F-J. Class 1 integrons, gene cassettes, mobility, and epidemiology. Eur J Clin Microbiol Infect Dis. 1999;18:761–770. doi: 10.1007/s100960050398. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez G, Sossa K, Bello H, Dominguez M, Mella S, Zemelman R. Presence of integrons in isolates of different biotypes of Acinetobacter baumannii from Chilean hospitals. FEMS Microbiol Lett. 1998;161:125–128. doi: 10.1111/j.1574-6968.1998.tb12937.x. [DOI] [PubMed] [Google Scholar]
- 3.Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 4.Jones M E, Peters E, Weersink A, Fluit A, Verhoef J. Widespread occurrence of integrons causing multiple resistance in bacteria. Lancet. 1997;349:1742–1743. doi: 10.1016/S0140-6736(05)62954-6. [DOI] [PubMed] [Google Scholar]
- 5.Levesque C, Brassard S, Lapointe J, Roy P H. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene. 1994;142:49–54. doi: 10.1016/0378-1119(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 6.Levesque C, Piche L, Larose C, Roy P H. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother. 1995;39:185–191. doi: 10.1128/aac.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Freijo P, Fluit A C, Schmitz F-J, Grek V S C, Verhoef J, Jones M E. Class I integrons in gram-negative isolates from different European hospitals and association with decreased susceptibility to multiple antibiotic compounds. J Antimicrob Chemother. 1998;42:689–696. doi: 10.1093/jac/42.6.689. [DOI] [PubMed] [Google Scholar]
- 8.Preston K E, Kacica M A, Limberger R J, Archinal W A, Venezia R A. The resistance and integrase genes of pACMI, a conjugative multiple-resistance plasmid, from Klebsiella oxytoca. Plasmid. 1997;37:105–118. doi: 10.1006/plas.1997.1284. [DOI] [PubMed] [Google Scholar]
- 9.Recchia G D, Hall R M. Gene cassettes: a new class of mobile element. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 10.Sallen B, Rajoharison A, Desvarenne S, Mabilat C. Molecular epidemiology of integron-associated antibiotic resistance genes in clinical isolates of Enterobacteriaceae. Microb Drug Resist. 1995;1:195–202. doi: 10.1089/mdr.1995.1.195. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz F-J, Martinez-Freijo P, Theis S, Fluit A C, Verhoef J, Heinz H-P, Jones M E. Prevalence of class I integrons and association with decreased antibiotic susceptibility in German gram-negative blood culture isolates. Clin Microbiol Infect. 1999;5:496–498. doi: 10.1111/j.1469-0691.1999.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 12.Stokes H W, Hall R M. The integron In1 in plasmid R46 includes two copies of the oxa2 gene cassette. Plasmid. 1992;28:225–234. doi: 10.1016/0147-619x(92)90054-e. [DOI] [PubMed] [Google Scholar]