Abstract
Purpose of the Review
Consensus on the effects of soft tissue surgical intervention in the management of brachial plexus birth injury (BPBI) sequalae is lacking. The purpose of this review is to examine the available literature on the functional and structural outcomes following soft tissue surgical management of BPBI sequalae.
Recent Findings
EMBASE, PubMed, and MEDLINE were searched for related literature from the point of database inception until April 2021. Relevant papers were screened by two reviewers independently and in duplicate. A meta-analysis was performed using a random effects model. A total of 25 studies (852 patients) were included in the review, with the number included in each meta-analysis varying based on outcome of interest. There were significant improvements from pre- to post-operative time points for the following measures: Mallet aggregate scores (5.0 points, p<0.0001), active external rotation in adduction (48.9°, p=0.003), passive external rotation in adduction (64.6°, p< 0.00001), active abduction (46.2°, p<0.00001), glenoid version (14.4°, p< 0.00001), and percentage of the humeral head anterior to the scapular line (17.53°, p< 0.00001). Furthermore, data revealed an overall complication rate of 9.3% (79/852 patients) and a major complication rate of 0.47% (4/852 patients).
Summary
Patients with BPBI sequela experience statistically significant improvements in functional, structural, and range of motion outcomes of the GH joint following soft tissue surgical management. Understanding the ideal indications for each procedure and age of surgical management with future prospective studies will help to optimize surgical management of these patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12178-022-09747-6.
Keywords: Brachial plexus neuropathies, Orthopaedics, Shoulder joint
Introduction
Brachial plexus birth injury (BPBI) is an injury with an incidence of 0.9 cases per 1000 live births [1], thought to be a result of lowering of the shoulder with opposite inclination of the cervical spine, often during challenging births [2,3]. While the majority of patients recover without surgery, a considerable number of cases are left with persistent deficits [3–6]. Common structural sequelae of the injury occurs at the glenohumeral (GH) joint as an internal rotation (IR) contracture [3,7], as well as varying degrees of glenoid fossa flattening, glenoid retroversion, and posterior subluxation and/or dislocation [8–12]. BPBI also precipitates functional impairments of the GH joint, often including deficits with external rotation (ER), abduction (ABD), and impairments in patients bringing their hand to their neck and/or mouth [10,13,14,15••].
Many patients with residual impairments are referred to an orthopaedic surgeon for surgical management of their GH deformities. The surgical approach to managing these patients typically focuses on soft tissue structures of the GH joint, as it includes extra-articular musculotendinous lengthening, tendon transfers, and/or articular release [6,14,16•,17•,18•,19]. Osseous approaches do exist, but are often reserved for cases of severe glenoid deformity, where soft tissue approaches are thought to be insufficient in correcting joint morphology [6,7,17,20,21]. There exists literature investigating surgical management of the BPBI sequalae with soft tissue approaches, with minimal consensus on its efficacy.
Thus, the purpose of this review is to systematically examine the available literature focusing on the functional and structural outcomes following surgical interventions involving the soft tissues of the GH joint, in the management of BPBI sequalae. We seek to investigate if patients who undergo soft tissue surgical management of BPBI sequalae experience significant improvements in functional and structure measures of the GH joint following surgery. Furthermore, we seek to examine the complication rate of these surgical interventions in this population.
Materials and Methods
Search Strategy
This systematic review was performed according to the methods outlined in the Cochrane handbook and reported according to the PRISMA guidelines [22]. Three online databases (EMBASE, PubMed, and MEDLINE) were searched for literature from database inception until April 3rd, 2021. The search terms “Brachial Plexus”, “Shoulder”, “glenohumeral”, and “birth”/“neonatal”/“obstetric” were used (Appendix Table 1).
Study Screening
The titles, abstracts, and full-text articles were screened by two reviewers independently and in duplicate. Disagreements during title and abstract screening moved onto the next stage for more in-depth review. Any disagreements were discussed between reviewers, and a senior author was consulted for any remaining discrepancies. The references of the included studies were subsequently manually screened for additional articles that may have eluded the initial search strategy.
Assessment of Study Eligibility
The research question and eligibility criteria were determined a priori. The inclusion criteria were (1) English-language studies, (2) studies that reported outcomes following surgical management of soft tissues (with either capsule release or tendon transfers) for glenohumeral deformity in brachial plexus birth injury, and (3) therapeutic studies with levels of evidence of I to IV. The exclusion criteria were (1) studies assessing bony procedures such as osteotomies, arthroplasty, or arthrodesis; (2) cadaveric studies; (3) animal studies; (4) conference papers; (5) book chapters; (6) review articles; and (7) case reports with < 4 patients.
Quality Assessment
The methodological quality of the included studies was assessed using the Methodological Index for Non-Randomized Studies (MINORS) instrument. This tool was designed to assess the methodological quality of comparative and non-comparative, non-randomized surgical studies [23]. Using the MINORS checklist, non-comparative studies are assigned a maximum score of 16, and comparative studies can achieve a maximum score of 24. Publication bias was assessed by analysis of the symmetry of the individual funnel plots.
Data Abstraction and Statistical Analysis
Inter-reviewer agreement was assessed by the kappa (κ) statistic for the title, abstract, and full-text screening stages. Agreement was categorized a priori as follows: κ of 0.61 or greater was considered substantial agreement; κ of 0.21 to 0.60, moderate agreement; and κ of 0.20 or less, slight agreement [24]. Data was abstracted and recorded in a Microsoft Excel spreadsheet (Version 2007, Microsoft Co., Redmond, WA, USA). Data regarding authors, year of publication, study design, level of evidence [25], sample size, age, sex, follow-up, clinical and radiographic diagnosis, surgical techniques, functional outcomes, range of motion, and complications were recorded. In papers where only individual data from all patients was reported for an outcome, means and standard deviations were calculated by the review authors when possible.
A meta-analysis was conducted to determine the pooled mean outcomes reported across the included studies where possible. We anticipated heterogeneity in the post-operative data given the variability in the duration of follow-up. Therefore, the data at final follow-up was used for post-operative data from each study. The mean difference between pre- and post-operative values and their respective variance reported across studies was combined using the DerSimonian–Laird random-effects model (to incorporate the anticipated heterogeneity) [26]. The pooled mean values are all presented with 95% confidence intervals (CI). P values < 0.05 were considered the threshold for statistical significance. Studies were only included in the pooled mean values and meta-analysis if they reported data for pre-operative and post-operative means and standard deviations for the outcome of analysis. The I2 test was used to assess statistical heterogeneity. For other variables, where results were presented in a non-uniform nature across studies, the results are presented in narrative summary fashion. Descriptive statistics were calculated including means, standard deviations, counts, proportions, and ranges. Calculations were conducted using StatsDirect statistical software (Version 3.2.7, StatsDirect software, Cheshire, UK). Forest plots were developed using the Review Manager software (RevMan) 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2012).
Results
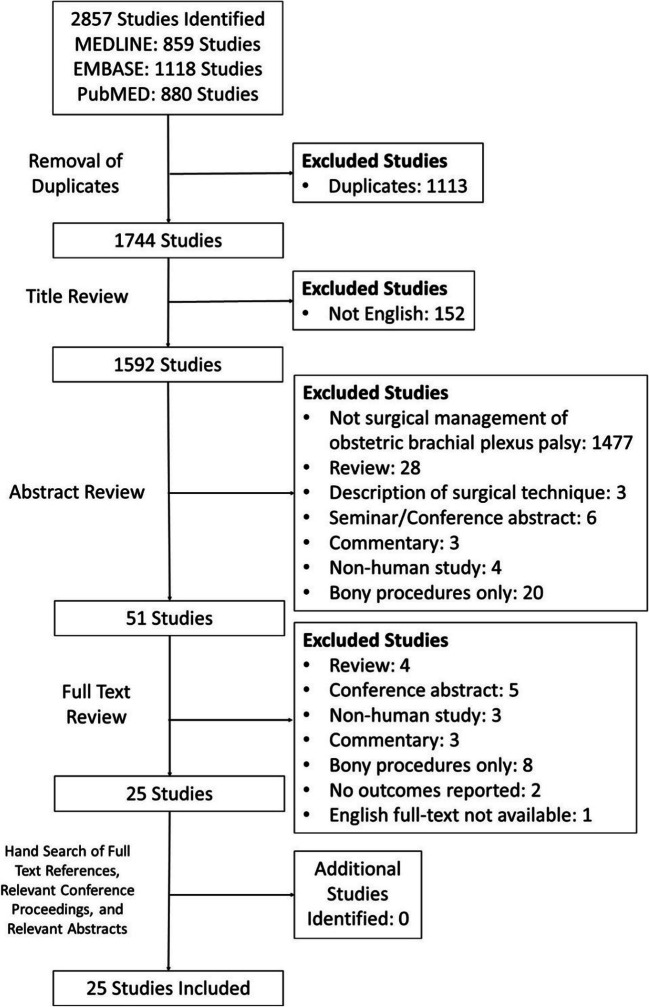
The initial search resulted in 2,857 studies. A systematic screening and assessment identified 25 full-text articles (Figure 1). The reviewers reached substantial agreement on the titles (κ = 0.869; 95% CI, 0.818-0.920), abstracts (κ = 0.882; 95% CI, 0.821 to 0.943), and full-texts (κ =1.00).
Figure 1.
PRISMA flow diagram demonstrating the systematic review of the literature for the surgical management of soft tissues for glenohumeral deformity and contracture in brachial plexus birth injury
The 25 included studies included were non-comparative. These studies had a mean MINORS score of 10.8/16 (range: 8–12), and all displayed a fair degree of quality (Table 1). There was no evidence of publication bias on review of the individual funnel plots. The review includes 852 patients (47% female, 46% male, 7% unreported; 5 to 150 per study). The mean age at operation was 4.38 years (range: 5 months to 17 years), with a mean FU duration of 4.48 years (range: ≤5 weeks to 42 years). All BPBI were involving C5-C6 or more. Eleven studies reported a total of 169 previous procedures (Table 2).
Table 1.
Study characteristics
| Authors (year) | Study design (level of evidence) | MINORS score | Number of patients | % female | Mean age at operation (range), years | Mean follow-up duration (range), years |
|---|---|---|---|---|---|---|
| Abid et al. (2012) | Prospective case series | 8 | 6 (14 at start) | 35.7% of original 14 | 1.9 (1.17–4.5) | 5 (3.5–6) |
| Ahmed and Hashmi (2006) | Descriptive case series | 10 | 10 | 70% | 6.25 (3–9.75) | 2.08 (0.58–6.58) |
| Andres-Cano et al. (2015) | Retrospective case series | 11 | 5 | 80% | 2.8 (1.4–4.0) | 1.66 (1.07–3.33) |
| Breton et al. (2012) | Retrospective case series | 12 | 18 | 72.22% | 4.17 (1–11) | 4.5 (1–7) |
| Burnier et al. (2019) | Retrospective case series | 12 | 40 | 37.5% | 4.17 (1.33–12.83) | 1.92 (1–4.08) |
| Cohen et al. (2010) | Retrospective case series | 12 | 32 | 43.75% | 2.5 (0.9–9.2) | 9.5 (2–23) |
| El-Gammal et al. (2019) | Prospective case series | 11 | 26 | 34.62% | 3.01 (1.0–10.0) | 8.23 (5.0–16) |
| Greenhill et al. (2019) | Retrospective case series | 12 |
14CLTT 14ITMTT |
50% CLTT 71.43% ITMTT |
CLTT: 2.5 (0.6–4.4) ITMTT: 1.9 (0.8–3.3) |
CLTT: 3.1 (1.0–7.7) ITMTT: 1.5 (1.0–3.2) |
| Hoffer and Phipps (1998) | Prospective case series | 10 | 8 | 62.50% | 2.33 (r: 1.17– 3.92) | 3.08 (2–5) |
| Hui and Torode (2003) | Prospective case series | 11 | 23 | N/A | 2.42 (0.67–6.58) | 3.58 (1.83–5.5) |
| Jonsson et al. (2019) | Prospective case series | 10 |
<2years: 20 2–5 years: 33 >5 years: 8 |
65.57% | 3.2 (0.67–15) | 10.2 (7–16) |
| Kirkos et al. (2005) | Retrospective case series | 11 | 10 | 10% | 6 (5–9) | 30 (25–42) |
| Kozin et al. (2010) | Retrospective case series | 11 | 44 | 63.64% | 2.7 (0.9–8.4) |
Fxn: 1.0 (±0.3) MRI:1.2 (±0.3) |
| Kozin et al. (2010) (2) | Retrospective chart review | 12 | 24 | 58.33% | 5.0 (±1.8) |
Fxn:1.1 (±0.3), 2.9 (±0.7) MRI:1.1 (±0.2), 3.2 (±0.1) |
| Mehlman et al. (2011) | Retrospective case series | 12 | 50 | 54% | 5.1 (0.83–11.8) |
Fxn:2.5 (2–5.42) Radiological: 2 (0.92–3.5) |
| Ozben et al. (2011) | Retrospective case series | 11 | 26 | 53.85% | 7.19 (1–17) | 4.25 (0.5–16) |
| Ozturk et al. (2010) | Prospective case series | 10 | 30 | 40% | 9 (4–15) | 3.98 (0.75–7) |
| Pearl et al. (2006) | Prospective case series | 9 | 33 (19 RLx; 14 RLxT; 4 repeat RLxT) | 54.55% |
3.7 (0.83–12) (RLx:1.4; RLxT: 6.7; repeat RLxT: 1.8 and 3.0) |
(2–2.58) |
| Pedowitz et al. (2007) | Prospective case series | 8 | 22 (7 RLx; 15 RLxT) | 36.36% | 3.9 (1.6–8.3) | ≤5 weeks |
| Ruyer et al. (2018) | Prospective observational study | 11 | 28 (35 at start) | 51% of original 35 | 6.3 (2.1–14.5) | 2 |
| Sarac et al. (2020) | Retrospective case series | 12 |
112 RLx: 37 RLxT: 68 T only: 7 |
47.3% |
RLx: 3 (+/−2.6) RLxT: 5 (+/−3/4) T: 8(+/−4/5) |
5.3 years (+/− 2.7) |
| Shah et al. (2019) | Retrospective case series | 12 | 20 | 40% | 6.5 (1.3–18) | 2.53 (2–4.08) |
| Thatte et al. (2011) | Prospective case series | 11 | 150 | 46% | 3.33 (1.25–8) | 4 (2.5–8) |
| Van Heest et al. (2010) | Retrospective-case controlled series | 11 | 26 | 69.23% | 3.67 (0.83–11.17) | 1.42 (0.83–3.83) |
| Vuillermin et al. (2020) | Prospective case series therapeutic | 11 | 20 | 60% | 2.4 (0.58–4.2) |
Radiographic: 4.2 (2–6) Fxn:4.5 (1.92–7) |
CLTT, conjoined latissimus dorsi tendon transfer; ITMTT, isolated teres major tendon transfer; Fxn, functional follow-up; MRI, MRI follow-up; RLx, release surgery; RLxTx, release and tendon transfer surgery
Table 2.
Patient characteristics
| Authors (year) | % right shoulder affected | Type of brachial plexus injury | Prior surgical procedures | Indications for inclusion and surgical intervention |
|---|---|---|---|---|
| Abid et al. (2012) | 64.3% of original | 10 C5C6 root damage, 4 C5-C7 root damage | 1 case of nerve graft | Shoulder IR contracture secondary to BPBI. Surgical release was indicated when pER was ≤0. |
| Ahmed and Hashmi (2006) | 70% | Erb’s palsy | No prior procedures | Patients identified by medical records maintained by the Health Information Management System to have Erb’s palsy. |
| Andres-Cano et al. (2015) | 60% | C5C6 brachial plexus injury | 2 cases of neurotisation; 1 case of open reduction of GH joint. | BPBI surgically treated or spontaneously recovered with acceptable shoulder functionality and a marked limitation of ER and ABD. Mild-to-moderate GH dysplasia (Water’s grades I to IV) according to ultrasound and MRI. Adequate family support. |
| Breton et al. (2012) | 9 C5C6, 4 C5-C7 and 5 Complete brachial plexus injuries | pER with elbow at side of less than 10°, secondary to BPBI, all with bicep function that allowed for elbow flexion. Excluded patients with secondary osteotomy procedures. | ||
| Burnier et al. (2019) | 62.5% | 27 C5C6 and13 C5-C7 brachial plexus injuries | No prior shoulder surgeries. |
Inclusion criteria: ≥1 year and ER with arm at side <0° and palpable and irreducible posterior displacement of the humeral head or evidence of osseous deformities of GH joint as noted on imaging. Exclusion: Patients without imaging evidence of dysplasia of the glenoid process or GH incongruity and patients with prior procedures or treatment of their shoulder other than PT. |
| Cohen et al. (2010) | 46.875% | 26 C5C6, 2 C5-C7, 2 C5-C8 and 2 C5-C8D1 brach plexus injuries | Surgical indications: pER and aER deficits despite treatment with nocturnal bracing in ER and PT, all with regard to BPBI. | |
| El-Gammal et al. (2019) | 61.54% | C5C6 or C5-C7 (Narakas types I and II) |
Inclusion: Spontaneous recovered BPBI, with persistent ER weakness with or without IR contractures, a nondysplastic GH joint and functioning TM. All had shoulder Abd of ≥45° pre-operative. Exclusion: Patients with total palsy, brachial plexus exploration, and reconstruction. Patients with other tendon transferred or humeral osteotomy. Patients followed for <5 years were excluded. |
|
| Greenhill et al. (2019) | C5C6 brachial plexus injuries | 2 cases of nerve graft/transfer (1 per group) |
Inclusion: shoulder abduction of ~90°, limited shoulder ER and adequate midline function. Choice between tendon transfer was by virtue of the surgeon. Exclusion: no pre-op Mallet score ≤1 month of operation, <12 months FU, poor compliance with post-op protocols, persistent C7 or lower trunk dysfunction, pre-op humeral osteotomy, any shoulder or microsurgical procedure ≤8 months prior to tendon transfer, upper extremity surgery within specified FU period, recurrent post-op GH subluxation confirmed by US or MRI, or incomplete modified Mallet score suggesting noncompliance with PT at >12 months FU. |
|
| Hoffer and Phipps (1998) | 87.50% | 6 C5C6 and 2 C5-C7 brachial plexus injuries | Surgery performed on children with shoulder dislocation identified on radiograph, secondary to BPBI. | |
| Hui and Torode (2003) | 47.83% | 21 Upper BPBP and 2 whole brachial plexus injuries |
Surgical indication: Shoulder subluxation or dislocation that required open reduction and tendon lengthening. Inclusion in data: ≥22 months FU. |
|
| Jonsson et al. (2019) | 59 Erb-type Palsy and 2 C5-T1 brachial plexus injuries | 5 cases of nerve reconstruction |
Indications for surgery: (1) aER of ≤0 or (2) aER of <20° w/ positive trumpet sign and have an aIR >70°. Exact surgery type is based on meeting following criteria. Indications for isolated transfer: above conditions, plus age >2 years, pER≥50°. Indications for release and transfer: above conditions, age >2 years, pER ≤0° or ≤40° w/ trumpet sign, and weak aER. Indications for isolated release: above, plus age ≤2 years, and pER≤10° or palpable dorsal head displacement. Exclusion: Patient too young for MRI without sedation. |
|
| Kirkos et al. (2005) | 80% | 8 C5C6 and 2 C5-T1 brachial plexus injuries | 1 case of transfer of the flexor carpi ulnaris to the extensor tendons of the fingers and thumb | Criteria for surgery: Beyond the age at which any spontaneous recovery could be expected. Had radiological appearance of the GH joint showing only minor osseous changes with no flattening or deformity of the humeral head and no evidence of subluxation or dislocation. Power of the LT and TM must be 4+ or 5 on the Medical Research Council Scale. |
| Kozin et al. (2010) | 54.55% | 36 C5C6 and 8 C5-C7 brachial plexus injuries | 6 cases of nerve grafting/transfers |
Criteria for release: Dysplastic GH joint and impaired motion secondary to BPBI in children <3 years old. Criteria for release and tendon transfer: Dysplastic GH joint and impaired motion secondary to BPBI in child >3 years old or parents saw additional surgery as unacceptable or family had difficulty returning to hospital. Patients must have had pre-op and post-op imaging and clinical measurements. |
| Kozin et al. (2010) (2) | 45.83% | 19 C5C6 and 5 C5-C7 brachial plexus injuries | 3 cases of nerve grafts, 1 case of anterior capsule and pectoralis major release | Candidates for tendon transfer surgery were children who failed to attain adequate active Abd and aER. |
| Mehlman et al. (2011) | 54% | 38 C5-C7 (Narakas I (31) or II (7)) and 12 complete (Narakas III (9) or IV (3)) brachial plexus injuries | Surgical indications: ≥18 months of age with IR contracture of the shoulder (≤3 on ER Mallet score) and variable amounts of shoulder ABD deficit or select younger children who demonstrated radiographic evidence of significant GH dysplasia. The decision to pursue arthroscopic release alone versus arthroscopic release and open LT tendon transfer was made according to review of physical and EMG evidence. Favourable EMG characteristics of the muscles innervated by the suprascapular nerve underwent release alone. Unfavourable EMG findings underwent release and transfer. If ABD and ER deficits coexist, the LT tendon was transferred to the posterosuperior greater tuberosity. If deficit was more isolated to ER, the LT tendon was transferred to the posterolateral proximal humeral shaft. Every child had to have a minimum of 24 months of FU. | |
| Ozben et al. (2011) | 61.54% | 13 C5C6 and 13 C5-C7 brachial plexus injuries | Inclusion: Reasonable elbow and hand function, GH changes ≤III (Water’s scoring), ABD and ER could be maximized by passive motion, and deltoid function was ≥3 (British Medical Research Council Scale for muscle strength). Exclusion: humeral osteotomy, subscapularis release or capsular plication was necessary during surgery or if the patient was lost to FU or operated on ≤6 months before the onset of the study or pan-plexus injury or if the deltoid power ≤2. | |
| Ozturk et al. (2009) | 60% | 9 C5C6, 14 C5-C7 and 7 C5-T1 brachial plexus injuries | Prior procedures noted, but not specified. | Inclusion: unable to use the involved extremity in daily activities due to loss of ER and ABD with an IR deformity, all resultant of a BPBI. To be eligible for a tendon transfer, the patients had to have sufficient deltoid muscle strength (M3 or M4 according to the British Medical Research Council Grading System). |
| Pearl et al. (2006) | 26 C5C6 and 7 C5-C7 brachial plexus injuries | Inclusion for study: IR contracture secondary to BPBI treated with arthroscopic release. Surgical indications: Failure to respond to 2–3 months of supervised stretching exercises as well as ER <0° with the arm at the side or ER was sufficiently restricted as to impair the child’s ability to reach overhead, as indicated by a Burglar’s position. Further indication was palpable posterior displacement of the humeral head that did not reduce with attempted ER. Isolated contracture release was recommended for children who were <4 years old and additional LT tendon transfer was for older children. Tendon transfer was also indicated when families expressed desire to avoid a future operation in younger children. Minimum of 2 years FU. | ||
| Pedowitz et al. (2007) | 68.18% | Brachial plexus birth palsies | 5 cases of previous tendon transfer that failed to reduce the GH joint (all in isolated release group) | Inclusion: Children with GH subluxation as a result of BPBI. Selected for surgery when patients were found to have the presence of an IR contracture and MRI studies that should GH joint deformity. Additional LT and TM tendon transfers were performed on patients based on age, degree of deformity, capacity for continued nerve regeneration and family education. |
| Ruyer et al. (2018) | 60% | 9 C5C6, 22 C5-C7 and 3 C5-T1 brachial plexus injuries | 10 cases of primary nerve repair (7 C5-C7; 3 C5-T1) |
Inclusion: Children >2 years with limited ROM of the shoulder, secondary to BPBP. Patients had to have aER with the elbow at the side of ≤30° and/or their range of active anterior elevation had to be ≤90°. Exclusion: Secondary shoulder surgery before or during FU or parents and/or child refused surgery. |
| Sarac et al. (2020) | 58.9% | 67 C5-C6, 37 C5-C7, 4 C5-C8, and 4 C5-T1 brachial plexus injuries |
70 cases of nerve reconstruction 8 cases of neurolysis |
Inclusion to study: Obstetric brachial plexus injuries that underwent treatment with internal contracture release and/or tendon transfer, a maximum age of 18 years at the time of surgery, and a minimum follow-up period of 2 years. Selection criterion for surgical intervention, was based on pre-operative values of passive and active ER. Children with limited pER and good function of aER or those who had a chance of recovery, underwent only contracture release—if not recovered 4 weeks after the release, an additional Tendon transfer was performed. Children with no aER and pER in ADD of less than 20° received anterior release and tendon transfer. Patients with pER greater than 20° in ADD with no aER underwent a tendon transfer without soft-tissue release. |
| Shah et al. (2019) | 19 Narakas-1 and 1 Narakas-3 brachial plexus injuries |
Surgical indications: Passive IR of <30° and no aER. Study required a FU ≥2 years. Exclusion: non-congruous GH joints on axial MRI. |
||
| Thatte et al. (2011) | 52% | 80 C5C6, 34 C5-C7, 32 C5-T1 and 4 C5-T1+Horner’s sign, brachial plexus injuries | 62 cases of exploration, neurotisation and nerve grafting (18 C5C6, 15C5-C7, 25 C5-T1, 4 C5-T1 w/ Horner’s sign) | Indications: Children with BPBI with shoulder deformities and contractures. All patients had shoulder ABD and ER weakness with a concomitant IR contracture. |
| Van Heest et al. (2010) | 57.69% | Brachial plexus birth injuries |
Inclusion for study: documented shoulder IR contracture with loss of aER that lead to tendon transfer surgery. Required patients to have had pre-op and 1 year post-op 3D imaging of the shoulder. Exclusion: Diagnosis other than BPBP as cause of GH dysplasia, humeral osteotomies at time of surgery, or tendon transfers other than LT and TM. Surgical indications: absent aER with loss of pER. |
|
| Vuillermin et al. (2020) | N/A | Brachial plexus birth injuries |
Surgical indications: made on the basis of neurological status, muscle strength, soft-tissue contractures, and underlying GH joint deformity—all had an IR contracture and ER weakness. Those with mild-to-moderate GH joint deformity and a joint reducible with surgery were managed with the operation. Study inclusion required patients to have radiological FU ≥2 years. |
BPBI, brachial plexus birth injury; GH, glenohumeral; PT, physiotherapy; pER, passive external rotation; aER, active external rotation; IR, internal rotation; ER, external rotation; TM, teres major; ABD, abduction; ADD, adduction; cLT, conjoined tendon; iTM, isolated teres major; FU, follow-up; LT, latissimus dorsi; ROM, range-of-motion; US, ultrasound; MRI, magnetic resonance imaging; N/A, not available
Surgical approaches within cohorts were not always consistent, with different approaches used in different patients. Overall, surgical approaches included (n=number of studies that used the approach at least once) capsular ligament release (n=14), muscular release/tenotomy (n=14), tendon lengthening (n=9), tendon transfer (n=21), GH reduction (n=9), and bony procedure (n=3). Tendon transfers consisted of the relocation of the latissimus dorsi (n=20) and/or teres major tendon (n=17) to an external rotator position on the humeral head. Overall, 4 studies used only an anterior release in all patients, 10 studies utilized tendon transfer and release in all patients, 1 study used only tendon transfer in all patients, and 10 studies used a mix of tendon transfer and/or anterior release in their cohort, depending on indications (Appendix Table 2).
Functional Outcomes
Seven studies were included in pooled mean Mallet aggregate score [27,28], displaying a significant improvement. Pooled mean Mallet scores pre-operatively: 12.8 (294 patients, 95% CI = 11.6 to 14.0, I2=95%); post-operatively: 17.8 (289 patients, 95% CI = 16.6 to 19.1, I2=96%); pooled mean improvement: 5.0 (95% CI = 2.8 to 7.3, p<0.0001, I2=97%) (Figure 2).
Figure 2.
Forest plot of the mean difference (95% CI) in Mallet scores from pre- to post-operative
Six studies were included in pooled mean global ABD scores, rendering scores of 3.4 (144 patients, 95% CI = 3.2 to 3.7, I2=94.6%) pre-operatively and 4.0 (139 patients, 95% CI = 3.8 to 4.2, I2=87.1%) at FU. Six studies were included in pooled mean global ER scores rendering scores of 2.3 (144 patients, 95% CI = 2.1 to 2.5, I2=79.8%) pre-operatively and 3.7 (139 patients, 95% CI = 3.4 to 3.9, I2=83.3%) at FU. Four studies were included in pooled mean hand-to-neck scores, rendering scores of 2.4 (68 patients, 95% CI = 2.0 to 2.9, I2=81.3%) pre-operatively and 3.9 (63 patients, 95% CI = 3.6 to 4.3, I2=85.4%) at FU. Five studies were included in pooled mean Mallet hand-to-mouth scores, rendering scores of 2.3 (94 patients, 95% CI = 2.0 to 2.5, I2=75.5%) pre-operatively and 3.6 (89 patients, 95% CI = 3.2 to 4.0, I2=92.6%) at FU.
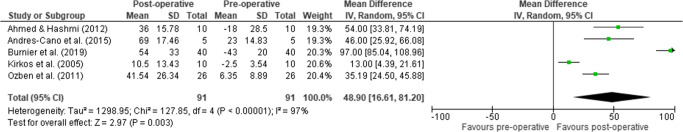
Five studies were included in pooled mean active ER, rendering means of −0.4° (91 patients, 95% CI = −23.0 to 22.2, I2=77.6%) pre-operatively and 41.7° (91 patients, 95% CI = 22.8 to 60.6, I2=98.4%) at FU, displaying a significant pooled mean improvement of 48.9 (95% CI = 16.6 to 81.2, p=0.003, I2=97%) (Figure 3).
Figure 3.
Forest plot of the mean difference (95% CI) in active ER with the arm in adduction from pre- to post-operation
Seven studies were included in pooled mean passive ER, rendering means of −6.2° (289 patients, 95% CI = −15.4 to 2.9, I2=97.7%) pre-operatively and 55.7° (281 patients, 95% CI = 45.1 to 66.7, I2=98.4%) at FU, displaying a significant pooled mean improvement of 64.6° (95% CI = 57.5 to 71.6, p< 0.00001, I2=90%) (Figure 4).
Figure 4.
Forest plot of the mean difference (95% CI) in passive ER with the arm in adduction from pre- to post-operative
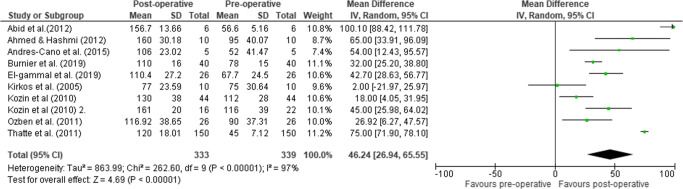
Ten studies were included in pooled mean active ABD, rendering means of 78.9° (339 patients, 95% CI = 63.5 to 94.3, I2=98.4%) pre-operatively and 124.9° (333 patients, 95% CI = 112.7 to 137, I2=95.1%) at FU, displaying a significant pooled mean improvement of 46.2° (95% CI = 26.9 to 65.6, p<0.00001, I2=97%) (Figure 5). Functional and ROM data can be found in the appendix Tables 3 and 4, respectively.
Figure 5.
Forest plot of the mean difference (95% CI) in active ABD from pre- to post-operative
Structural Outcomes
Nine studies were included in pooled mean glenoid version, rendering means of −28.8° (237 patients, 95% CI = −32.4 to −25.3°, I2=82%) pre-operatively and −14.3° (229 patients, 95% CI = −16.8 to −11.8, I2=77%) at FU, displaying a significant pooled mean improvement of 14.4° (95% CI = 9.47 to 19.31, p< 0.00001, I2=83%). Mean glenoid version in children less than 8 years old without GH pathology has been reported as −4.2° (standard deviation: 6.2°; range: −23 to 8°) [29]
Eight studies were included in pooled mean percentage of the humeral head anterior to the scapular line (PHHA), rendering means of 20.8% (214 patients, 95% CI = 15.7% to 25.8%, I2=85.7%) pre-operatively and 38.7% (206 patients, 95% CI = 34.4% to 42.9%, I2=85%) at FU, displaying a significant pooled mean improvement of 17.53% (95% CI = 10.93% to 24.14%, p< 0.00001, I2=84%) (Figure 6). Average PHHA in shoulders not effected by BPBI has been reported to be 47.4% (range: 41.5% to 54.8%) [30]. Structural data can be found in appendix Table 5.
Figure 6.
Forest plot of the mean difference (95% CI) in PHHA measures from pre- to post-operative
Complications
Eleven studies reported one or more complications, for a total of 79 complications (79/852 patients; 9.3%) [7, 15••, 36•, 16•, 18•, 21, 31, 32, 33••, 34, 35] [7, 15••, 18•, 31–35] [7, 15••, 18•, 31–35] [7, 15••, 18•, 31–35] [7, 15••, 18•, 31–35] [7, 15••, 18•, 31–35] [7, 15••, 18•, 30–32, 33••, 34]. Four complications were deemed major (i.e. nerve palsy or nerve injury; 4/852 patients; 0.47%) [7, 31, 33••, 35]. Nine studies reported a total of 33 cases requiring a secondary procedure [7, 15••, 16•, 18•, 21, 32, 33••, 34, 36•] (Table 3).
Table 3.
Complications and failures
| Authors (year) | Complications of surgery |
|---|---|
| Burnier et al. (2019) |
-1 case of recurrent contracture (pER less than −20° at 3 months); patients underwent repeat anterior release. -7 cases of weak ER; patients underwent additional surgery for transfer of the latissimus dorsi -1 case of weak ABD; patient underwent additional surgery for transfer of the latissimus dorsi |
| Cohen et al. (2010) | -6 patients had surgical revisions; 2 patients were from the non-tendon transfer group of the study with one receiving LT and TM transfers while the other received the same tendon transfer procedure along with humeral derotation osteotomy; 4 other patients had humeral derotation osteotomies |
| Greenhill et al. (2019) |
-35.7% of patients in the cLTT group and 14.3% in the iTMTT group (p=0.378) had a loss of midline function defined by a modified Mallet IR score that decrease to less than 3; this totalled to 9 cases of loss of midline function. -4 patients underwent subsequent humeral osteotomies. |
| Kirkos et al. (2005) | -1 case experienced hypoaesthesia in the area of the superficial radial nerve. |
| Mehlman et al. (2011) |
-4 patients had a decrease in shoulder IR. -2 patients required a repeat release procedure for persistent IR contracture. |
| Pearl et al. (2006) |
-1 patient failed to achieve ≥45° passive ER at the time of surgery and this resulted in an open release through an extended deltopectoral approach to release the anterior capsule. -4 patients required a repeat arthroscopic release after not retaining the range of ER. -1 patient lost 40° of active elevation; thought to be due to partial axillary nerve injury or another mechanical compromise of the shoulder from loss of subscapular function. -In the isolated release group, the average loss of IR was 37°, and in the release and transfer group the average loss of IR was 42°. -4 patients had negative post-op IR values in 90° of shoulder abduction. -1 patient who was lost to follow-up had a repeat contracture. |
| Ruyer et al. (2018) |
-No perioperative complications. -7 patients had poor outcomes in terms of active ER in ADD. -1 patient had regional pain syndrome with stiffness of the shoulder at 6 months. -2 patients had recurrence of shoulder contracture. -4 patients had negative active anterior elevation. -2 patients had decreased aggregate Mallet scores at 2 years post-op. -1 patient saw no change in Mallet score at 2 years post-op. -1 patient was excluded from the study as they required a secondary arthroscopic release during the FU period. |
| Sarac et al. (2020) |
-4 patients developed Keloid scars; 1 was surgically corrected. -1 patients had transient post-op ulnar nerve compression from casting. -4 patients following tendon transfer had ER contractures that limited IR after; only 2 of these patients require additional surgery that included release of the posterior capsule and the transferred latissimus dorsi and teres major tendons. |
| Shah et al. (2019) |
-2 patients developed subcutaneous hematomas along the entry site for the periosteal elevator, but resolved spontaneously. -1 patient experienced inadvertent penetration of the scapular body in surgery. -4 patients in the anterior release procedure group developed ER contracture; 2 of these patients required internal rotation humeral osteotomy. |
| Van Heest et al. (2010) |
-2 patients experienced superficial skin irritations associated with cast immobilization. -1 patient experienced partial radial nerve motor palsy that resolved within 4 weeks without treatment. |
| Vuillermin et al. (2020) | -2 patients required secondary external rotational osteotomies after 2-year FU. |
ER, external rotation; IR, internal rotation; LT, latissimus dorsi; TM, teres major; FU, follow-up; cLTT, conjoint latissimus dorsi and teres major tendon transfer; iTMTT, isolated teres major tendon transfer; ABD, abduction; GH, glenohumeral
Discussion
The most impactful findings of this review are the significant improvements in functional, range-of-motion and structural measures following surgical intervention of the soft tissues of the GH joint in the management of BPBI sequalae. Specifically, the results suggest that these patients often experience significant improvements in Mallet aggregate score, active ER in shoulder adduction, passive ER in shoulder adduction, active ABD, glenoid version, and PHHA. Moreover, the review of the literature revealed a low rate of complications following surgery, with even lower rates of major complications.
Several areas of debate arise from examination of the literature. One area of debate is in regard to surgical intervention, with two contrasting management plans including an isolated release of the GH joint (capsular release, tenotomy and/or muscular lengthening) or a release of the GH joint with the addition of a tendon transfer (latissimus dorsi, teres major, or both). The application of a tendon transfer was exercised in 21 studies [7, 13, 34, 35, 36•, 37–38, 39•, 40–43, 14, 44, 15••, 17•, 19, 21, 31, 32, 33••]; however, 7 of these studies only utilized a tendon transfer in some of their patients [7, 21, 32, 33••, 37, 39•, 40]. In Cohen et al. (2010), tendon transfers were utilized in 24 of the 32 cases where patients displayed an active ER deficit pre-operatively. In the 8 patients who did not receive the transfer, 6 were found to be below mean scores of the cohort for both Mallet and ER measures, and 2 required secondary surgery that involved a tendon transfer. Kozin et al. (2010) [40] utilized a tendon transfer in patients older than 3 years old, and found that the transfer was associated with better active elevation only if the patient had a pre-operative ABD range of >100°. In a series by Pearl et al. (2006), tendon transfers were added to release procedures on the basis of patients wishing to avoiding future surgery or being older than the age of 4, and outcomes were found to be similar between the release only cohort and the release plus transfer cohort. However, 4 cases of isolated releases from this series did go on to receive further surgical intervention that included a tendon transfer. Mehlman and colleagues showed the use of tendon transfer offered little benefit in outcomes, as only measures of Mallet hand-to-neck score rendered a significant difference between the two approaches [32]. Interestingly, Mehlman et al. elected to include tendon transfers in the release procedure of patients who demonstrated poor subscapular nerve function rather than basing the decision on age—further highlighting a discrepancy in the indications for a tendon transfer. Conversely, Sarac et al. (2019) completed tendon transfers and release in patients when indicated by poor active ER strength and passive ER of less than 20° in adduction, compared to only release in patients who displayed good active ER but limited passive ER. These authors went on to report that 17 of 37 patients who received an isolate release initially required a secondary tendon transfer as a result of insufficient ER. Interestingly, these authors reported 2 cases of limited IR after tendon transfer, pointing to a potential downfall of the tendon transfer procedure. Thus, more research needs to be done in regard to investigating both the added efficacy and the indications for tendon transfers.
A further inconsistency exists with tendon transfers, as authors who elect to complete tendon transfers do not do so homogeneously. Of these authors, 12 elect to transfer the tendons of both the latissimus dorsi and teres major [14,15••,40,41,43,44,19,21,31,32,34,35,36•,38], whereas others elect to transfer the tendons of just the teres major [15••,33••], just the latissimus dorsi [7,13,32,37,39•], or of the teres major and pectoralis major [17•]. This only furthers highlights the need of additional investigation into the use of tendon transfers in the surgical management of BPBI sequelae.
Further inconsistencies in the literature involved indications to operate, as the review of these articles exposed a lack of concrete indications for electing to operate in this patient population. The indications for surgeries were inconsistent, as criteria between the authors required differing levels of severity in differing injury markers. Authors that used ER measures had various cutoffs that included indicators such as passive ER ≤0° [7,13,16•], passive ER <10° [45], active ER of ≤ 30° [18•], an ER Mallet score of ≤3 [32], or ill-defined limitations in ER [15••,36•,37,44]. Similarly, joint morphology was inconsistently used as an indication to surgery. For instance, authors Andres-cano et al. (37) required mild-to-moderate GH dysplasia with a Water’s score of I–IV, whereas Ozben et al. [19] required a Water’s score ≤III and Vuillermin et al. [36•] require an ill-defined mild-to-moderate deformity.
Keeping with inconsistencies, the age at time of operation was widespread, ranging from 5 months to 17 years. The factor of age may be an important factor in the long-term success of the operation, as Van Heest et al. [35] showed that patients who are operated on at ages 2 and younger had significantly better improvements in both PHHA and glenoid version when compared with older surgical patients. This relationship is yet to be fully understood, as another study found no difference in these changes between those operated on at <4 years and those operated on at ≥4 years [16•], while ANOVA analysis in another series failed to highlight age at operation as a significant factor for changes in injury markers [39•]. Reports on effect of age at surgery can be seen in appendix table 6. In short, there is a clear need for evidence-based surgical indications in this patient population, as well as clarity on the impact age at operation has on the outcomes of these procedures.
Based on the aforementioned controversies within the literature, future prospective studies should seek to determine the optimal surgical indications for children with BPBI sequala of the shoulder. Namely, such studies should focus on the ideal age for surgical management and the indications for including tendon transfers, as well as indications for each type of tendon transfer.
This review is not without its limitations, as it is comprised of mostly retrospective case series with limited sample sizes. With this lack of high-quality evidence, it is difficult to conclude that the surgical management of these patients is superior to other non-operative, conservative approaches. Notably, many studies featured post-operative physical therapy, making it difficult to discern if the positive improvements in the patients are directly a result of surgical interventions. Additionally, the studies had a large variation in follow-up duration as well as extent of brachial plexus injury, further limiting the results. Lastly, the vast heterogeneity of the surgical approaches utilized by the various surgical teams limits our understanding of whether these positive outcomes are a result of several different approaches, or a select few. All in all, the vast heterogeneity in the above factors, as well as in outcome measures and statistics reported, limited inclusion of studies in pooled statistical analysis and the ability to perform subgrouping analysis. However, a random effects model was used to pool the data given the heterogeneity identified.
Conclusion
Based upon the literature available from observational studies, patients with BPBI sequela who undergo soft tissue surgical management of the GH joint experience statistically significant improvements in functional, structural, and range of motion outcomes of the shoulder. Understanding the ideal indications and age of surgical management with future prospective studies will further help to optimize surgical management in these patients.
Supplementary Information
(DOCX 56 kb)
Declarations
Not applicable.
Conflict of Interest
The authors declare that they have no conflicts of interest to disclose. The authors declare they have no funding to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Outcomes in Research in Orthopedics
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sean R. McKellar, Email: sean.mckellar@mail.utoronto.ca
Jeffrey Kay, Email: jeffrey.kay@medportal.ca.
Muzammil Memon, Email: muzammil.memon@medportal.ca.
Nicole Simunovic, Email: simunon@mcmaster.ca.
Waleed Kishta, Email: waleed.kishta@medportal.ca.
Olufemi R. Ayeni, Email: ayenif@mcmaster.ca
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Defrancesco CJ, Shah DK, Rogers BH, Shah AS. The epidemiology of brachial plexus birth palsy in the United States: declining incidence and evolving risk factors. J Pediatr Orthop. 2019;39(2):E134–E140. doi: 10.1097/BPO.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert A, Brockman R, Carlioz H. Surgical treatment of brachial plexus birth palsy. Clin Orthop Relat Res. 1991;264:39–47. doi: 10.1097/00003086-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 3.O’Berry P, Brown M, Phillips L, Helen ES. Obstetrical brachial plexus palsy. Curr Probl Pediatr Adolesc Health Care [Internet] 2017;47(7):151–155. doi: 10.1016/j.cppeds.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Boome R, Kaye J. Obstetric traction injuries of the brachial plexus. Natural history, indications for surgical repair and results. J Bone Joint Surg Br. 1988;70(4):571–576. doi: 10.1302/0301-620X.70B4.3403599. [DOI] [PubMed] [Google Scholar]
- 5.Wall LB, Mills JK, Leveno K, Jackson G, Wheeler LC, Oishi SN, Ezaki M. Incidence and prognosis of neonatal brachial plexus palsy with and without clavicle fractures. Obstet Gynecol. 2014;123(6):1288–1293. doi: 10.1097/AOG.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 6.Hale HB, Bae DS, Waters PM. Current concepts in the management of brachial plexus birth palsy. J Hand Surg Am [Internet] 2010;35(2):322–331. doi: 10.1016/j.jhsa.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Pearl ML, Edgerton BW, Kazimiroff PA, Burchette RJ, Wong K. Arthroscopic release and latissimus dorsi transfer for shoulder internal rotation contractures and glenohumeral deformity secondary to brachial plexus birth palsy. J Bone Jt Surg. 2006;88-A(3):564–574. doi: 10.2106/JBJS.D.02872. [DOI] [PubMed] [Google Scholar]
- 8.Sibinski M, Woźniakowski B, Drobniewski M, Synder M. Secondary gleno-humeral joint dysplasia in children with persistent obstetric brachial plexus palsy. Int Orthop. 2010;34(6):863–867. doi: 10.1007/s00264-010-0965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearl M, Edgerton B. Glenohumeral deformity secondary to brachial plexus birth palsy. J Bone Jt Surg -. 1998;80-A(5):659–667. doi: 10.2106/00004623-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Waters PM, Smith GR, Jaramillo D. Glenohumeral deformity secondary to brachial plexus birth palsy. J Bone Jt Surg - Am. 1998;80(5):668–677. doi: 10.2106/00004623-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Bhardwaj P, Burgess T, Sabapathy SR, Venkataramani H, Ilayaraja V. Correlation between clinical findings and CT scan parameters for shoulder deformities in birth brachial plexus palsy. J Hand Surg Am. 2013;38(8):1557–1566. doi: 10.1016/j.jhsa.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Crouch DL, Plate JF, Li Z, Saul KR. Computational sensitivity analysis to identify muscles that can mechanically contribute to shoulder deformity following brachial plexus birth palsy. J Hand Surg Am. 2014;39(2):303–311. doi: 10.1016/j.jhsa.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Abid A, Accadbled F, Louis D, Kany J, Knörr J, Cahuzac JP, de Gauzy JS. Arthroscopic release for shoulder internal rotation contracture secondary to brachial plexus birth palsy: Clinical and magnetic resonance imaging results on glenohumeral dysplasia. J Pediatr Orthop Part B. 2012;21(4):305–309. doi: 10.1097/BPB.0b013e328353688e. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed S. Hashmi p. Restoration of glenohumeral motion in Erb’s palsy by tendon transfers. J Ayub Med Coll Abbottabad. 2006;18(2):44–49. [PubMed] [Google Scholar]
- 15••.Greenhill DA, Smith WR, Ramsey FV, Kozin SH, Zlotolow DA. Double versus single tendon transfers to improve shoulder function in brachial plexus birth palsy. J Pediatr Orthop. 2019;39(6):328–334. doi: 10.1097/BPO.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 16•.Burnier M, Le Hanneur M, Cambon-Binder A, Belkheyar Z. Isolated open anterior shoulder release in brachial plexus birth palsy. J Shoulder Elb Surg. 2019;28(7):1347–1355. doi: 10.1016/j.jse.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 17•.El-Gammal TA, Ali AE-S, Kotb MM, Saleh WR, El-Gammal YT. Long-term evaluation of teres major to infraspinatus transfer for treatment of shoulder sequelae in obstetrical brachial plexus palsy. Ann Plast Surg. 2020;84(5):565–569. doi: 10.1097/SAP.0000000000002288. [DOI] [PubMed] [Google Scholar]
- 18•.Ruyer J, Grosclaude S, Lacroix P, Jardel S, Gazarian A. Arthroscopic isolated capsular release for shoulder contracture after brachial plexus birth palsy: clinical outcomes in a prospective cohort of 28 children with 2 years’ follow-up. J Shoulder Elb Surg. 2018;27(8):e243–e251. doi: 10.1016/j.jse.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Ozben H, Atalar AC, Bilsel K, Demirhan M. Transfer of latissmus dorsi and teres major tendons without subscapularis release for the treatment of obstetrical brachial plexus palsy sequela. J Shoulder Elb Surg. 2011;20(8):1265–1274. doi: 10.1016/j.jse.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Waters PM, Bae DS. Effect of tendon transfers and extra-articular soft-tissue balancing on glenohumeral development in brachial plexus birth palsy. J Bone Jt Surg - Am. 2005;87(2):320–325. doi: 10.2106/00004623-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Cohen G, Rampal V, Aubart-Cohen F, Seringe R, Wicart P. Brachial plexus birth palsy shoulder deformity treatment using subscapularis release combined to tendons transfer. Orthop Traumatol Surg Res. 2010;96(4):334–339. doi: 10.1016/j.otsr.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 25.Wright JG. Levels of evidence and grades of recommendations. AAOS Bull. 2005.
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Mallet J. Obstetrical paralysis of the brachial plexus. II. Therapeutics. Treatment of sequelae. Must transplants be performed? Rev Chir Orthop Reparatrice Appar Mot. 1972;58(suppl 1):186. [PubMed] [Google Scholar]
- 28.Clarke HM, Curtis CG. An approach to obstetrical brachial plexus injuries. Hand Clin. 1995;11(4):563–580. doi: 10.1016/S0749-0712(21)00264-X. [DOI] [PubMed] [Google Scholar]
- 29.Mintzer CM, Waters PM, Brown DJ. Glenoid version in children. J Pediatr Orthop. 1996;16(5):563–566. doi: 10.1097/01241398-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 30.van de Bunt F, Pearl ML, Lee EK, Peng L, Didomenico P. Analysis of normal and dysplastic glenohumeral morphology at magnetic resonance imaging in children with neonatal brachial plexus palsy. Pediatr Radiol. 2017;47(10):1337–1344. doi: 10.1007/s00247-017-3882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkos JM, Kyrkos MJ, Kapetanos GA, Haritidis JH. Brachial plexus palsy secondary to birth injuries. J Bone Jt Surg - Br. 2005;87(2):231–235. doi: 10.1302/0301-620X.87B2.14739. [DOI] [PubMed] [Google Scholar]
- 32.Mehlman CT, DeVoe WB, Lippert WC, Michaud LJ, Allgier AJ, Foad SL. Arthroscopically assisted Sever-L’Episcopo procedure improves clinical and radiographic outcomes in neonatal brachial plexus palsy patients. J Pediatr Orthop. 2011;31(3):341–351. doi: 10.1097/BPO.0b013e31820cada8. [DOI] [PubMed] [Google Scholar]
- 33••.Sarac C, Amghar H, Nieuwenhuijse MJ, Nagels J, Buitenhuis SM, Wolterbeek R, et al. What range of motion is achieved 5 years after external rotationplasty of the shoulder in infants with an obstetric brachial plexus injury? Clin Orthop Relat Res. 2020;478(1):114–123. doi: 10.1097/CORR.0000000000000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah MM, Naik N, Patel T, Gupta G, Makadia A. Minimally invasive subscapularis release: a novel technique and results. J Pediatr Orthop. 2019;00(00):1–7. doi: 10.1097/BPO.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 35.Van Heest A, Glisson C, Ma H. Glenohumeral dysplasia changes after tendon transfer surgery in children with birth brachial plexus injuries. J Pediatr Orthop. 2010;30(4):371–378. doi: 10.1097/BPO.0b013e3181d8d34d. [DOI] [PubMed] [Google Scholar]
- 36•.Vuillermin C, Bauer AS, Kalish LA, Lewine EB, Bae DS, Waters PM. Follow-up study on the effects of tendon transfers and open reduction on moderate glenohumeral joint deformity in brachial plexus birth injury. J Bone Jt Surg. 2020;00-A(00):1–10. doi: 10.2106/JBJS.19.00685. [DOI] [PubMed] [Google Scholar]
- 37.Andrés-Cano P, Toledo MÁ, Farrington DM, Gil JJ. Arthroscopic treatment for internal contracture of the shoulder secondary to brachial plexus birth palsy: report of a case series and review of the literature. Eur J Orthop Surg Traumatol. 2015;25(7):1121–1129. doi: 10.1007/s00590-015-1670-x. [DOI] [PubMed] [Google Scholar]
- 38.Hoffer MM, Phipps GJ. Closed reduction and tendon transfer for treatment of dislocation of the glenohumeral joint secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 1998;80(7):997–1001. doi: 10.2106/00004623-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 39•.Jönsson K, Werner M, Roos F, Hultgren T. Development of the glenohumeral joint after subscapular release and open relocation in children with brachial plexus birth palsy: long-term results in 61 patients. J Shoulder Elb Surg. 2019;28(10):1983–1990. doi: 10.1016/j.jse.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Kozin SH, Boardman MJ, Chafetz RS, Williams GR, Hanlon A. Arthroscopic treatment of internal rotation contracture and glenohumeral dysplasia in children with brachial plexus birth palsy. J Shoulder Elb Surg. 2010;19(1):102–110. doi: 10.1016/j.jse.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Kozin SH, Chafetz RS, Shaffer A, Soldado F, Filipone L. Magnetic resonance imaging and clinical findings before and after tendon transfers about the shoulder in children with residual brachial plexus birth palsy: a 3-year follow-up study. J Pediatr Orthop. 2010;30(2):154–160. doi: 10.1097/BPO.0b013e3181cfce09. [DOI] [PubMed] [Google Scholar]
- 42.Öztürk K, Bülbül M, Demir BB, Büyükkurt CD, Ayanoǧlu S, Esenyel CZ. Reconstruction of shoulder abduction and external rotation with latissimus dorsi and teres major transfer in obstetric brachial plexus palsy. Acta Orthop Traumatol Turc. 2010;44(3):186–193. doi: 10.3944/AOTT.2010.2332. [DOI] [PubMed] [Google Scholar]
- 43.Pedowitz DI, Gibson B, Williams GR, Kozin SH. Arthroscopic treatment of posterior glenohumeral joint subluxation resulting from brachial plexus birth palsy. J Shoulder Elb Surg. 2007;16(1):6–13. doi: 10.1016/j.jse.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Thatte MR, Agashe MV, Rao A, Rathod CM, Mehta R. Clinical outcome of shoulder muscle transfer for shoulder deformities in obstetric brachial plexus palsy: a study of 150 cases. Indian Journal of Plastic Surgery. 2011;44:21–28. doi: 10.4103/0970-0358.81441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breton A, Mainard L, De Gaspéri M, Barbary S, Maurice E, Dautel G. Arthroscopic release of shoulder contracture secondary to obstetric brachial plexus palsy: retrospective study of 18 children with an average follow-up of 4.5 years. Orthop Traumatol Surg Res. 2012;98(6):638–644. doi: 10.1016/j.otsr.2012.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 56 kb)