Abstract
Introduction
Hemoglobin A1C (HbA1c) is an important marker for diabetes care management. With the increasing use of new technologies such as continuous glucose monitoring (CGM) and point-of-care testing (POCT), patients and their physicians have been able to monitor and continuously check their blood glucose levels in an efficient and timely manner. This study aimed to investigate the level of agreement between the standard laboratory test for HbA1c (Lab-HbA1c) with point-of-care testing (POCT-HbA1c) and glucose monitoring index (GMI) derived by intermittently scanned CGM (isCGM) or estimated average glucose (eAG) derived by conventional self-monitored blood glucose (SMBG) devices.
Methods
A cross-sectional study was conducted at the Diabetes Treatment Center, Prince Sultan Military Medical City, Saudi Arabia, between May and December 2020 with 81 patients with diabetes who used the isCGM system (n = 30) or conventional finger-pricking SMBG system (n = 51). At the same visit, venous and capillary blood samples were taken for routine HbA1c analysis by the standard laboratory and POCT methods, respectively. Also, for isCGM users, the GMI data for 28 days (GMI-28) and 90 days (GMI-90) were obtained, while for SMBG users, eAG data for 30 days (eAG-30) and 90 days (eAG-90) were calculated. The limits of agreement in different HbA1c measurements were evaluated using a Bland-Altman analysis. Pearson correlation and multivariate linear regression analyses were also performed.
Results
Based on the Bland-Altman analysis, HbA1c levels for 96.7% and 96.1% of the patients analyzed by the POCT and the standard laboratory methods were within the range of the 95% limit of agreement in both isCGM and conventional SMBG users, respectively. About 93.3% of the GMI measurements were within the 95% limit of agreement. Also, about 94.12% of the eAG-30 and 90.2% of the eAG-90 measurements were within the 95% limit of agreement. Moreover, the correlation analysis revealed a statistically significant positive correlation and linear regression among Lab-HbA1c, POCT-HbA1c, GMI, and eAG in both conventional SMBG and isCGM users (all p < 0.001). These positive results persisted significantly after adjusting for different factors (all p < 0.001).
Conclusion
GMI derived by isCGM or eAG derived by conventional SMBG systems, as well as the POCT-HbA1c measurements, showed a high level of agreement; therefore, we recommend them as potential methods for diabetes monitoring, especially when a rapid result is needed or with patients with uncontrolled diabetes or on intensive insulin therapy.
Keywords: Diabetes, HbA1c, isCGM, POCT, Saudi Arabia
Key Summary Points
| HbA1c levels measured by POCT device showed a high level of agreement with standard HbA1c laboratory tests in both isCGM and conventional SMBG users. |
| The GMI measured by the isCGM showed a high level of agreement with HbA1c levels measured by the POCT device. |
| The eAG measured by the conventional SMBG systems showed a high level of agreement with HbA1c levels measured by the POCT device. |
| For uncontrolled DM patients, isCGM provides more personalized detailed glycemic data for a better diabetes care management plan. |
| Measuring HbA1c levels with a POCT device is a crucial method for diabetes screening and monitoring in daily clinic visits, especially when a rapid result is needed. |
Introduction
Diabetes mellitus (DM) is one of the major public health challenges worldwide that burdens global public health [1, 2]. The disease prevalence has increased considerably in both developed and developing countries over the past decades [3–5].
Current global data indicate that this condition affects 537 million people, which is expected to increase to 783 million by 2045 [6]. The Middle East and North African (MENA) region has the highest regional prevalence at 16.2% and the second-highest expected increase (86%) in diabetic patients, reaching a predicted 136 million by 2045. Also, the MENA region has the highest percentage (24.5%) of diabetes-related deaths [6]. The prevalence of DM in Saudi Arabia was estimated to be 14.4% by a World Health Organization (WHO) report [7].
Glycated hemoglobin A1c (HbA1c) in the laboratory is the primary technique for assessing glycemic control in diabetic patients but requires multiple patient visits, which delay important treatment modification or intensification and reduce long-term patient adherence [8].
HbA1c is an important biomarker that guides blood glucose management plans to avoid the risk of developing diabetic complications. HbA1c indicates the average blood sugar value, according to a recent study; thus, HbA1c is a measure of average blood sugar (i.e., HbA1c of 6.5% indicates average daily glucose of 140 mg/dl) [9].
In 2021, the American Diabetes Association (ADA) standards of medical care recommended the use of patient point-of-care testing (POCT) for HbA1c to clinicians, nurses, and diabetes educators to provide patients with well-timed treatment changes for better glycemic control [10].
High HbA1c levels are seen in 50–60% of diabetic patients, indicating that they either are not receiving proper care or are not adhering to their treatment regimen [8]. Furthermore, delayed therapeutic escalation is the primary cause of diabetes management failure after treatment intensification [11]. Therefore, addressing inadequate glycemic control is recognized as a significant issue in successful diabetes care [8].
Nowadays, for most patients with type 1 (T1DM) and type 2 diabetes mellitus (T2DM) globally, self-monitored blood glucose (SMBG) has become an essential component of modern diabetic therapy to achieve the target level of glycemic control and to prevent hypoglycemia [12]. However, SMBG just gives snapshots of blood glucose concentrations. Furthermore, many patients may not conduct frequent SMBG as required because of pain and inconvenience [13]. Patients now have an alternative to conventional SMBG in the form of continuous glucose monitoring (CGM) systems that can be intermittently scanned (isCGMS), intermittently viewed (ivCGMS), or used in real time (rtCGMS) [14]. The CGM system can record glucose concentrations in interstitial fluid subcutaneously by sensors based on enzymatic amperometric electrodes and provide many glycemic data, glucose trends, and expected future glycemic status, especially for T1DM patients [13, 15]. Furthermore, the CGM technique eliminates the restrictions of the present method of monitoring glucose levels, which involves frequent and painful finger pricks. In Saudi Arabia, the commercially available CGMS is the FreeStyle Libre® (Abbott Diabetes Care, Inc.) flash glucose monitoring (FGM) system [16].
Both SMBG and CGM have significant evidence supporting their efficacy in promoting better long-term glycemic control [17]. However, previous reports have shown a discordance between the glucose monitoring index (GMI) derived by isCGM and estimated average glucose (eAG) derived by conventional SMBG system with the standard laboratory HbA1c [18–21]. The potential discrepancies between laboratory and GMI derived by CGM devices are caused by estimated HbA1c being calculated through the average glucose and correlated with time in range, which is the relative time spent in a range of normal blood glucose [22]. Moreover, studies showed that POCT values of HbA1c can be varied from the laboratory HbA1c [23]. Thus, the potential disagreement/discrepancies between HbA1c and estimated HbA1c may be the source of therapeutic errors. As CGM usage grows, it is critical to comprehend and utilize its data and biomarkers [22].
Therefore, this study aimed to provide real-world data on the limits of agreement and correlation between the HbA1c measured by standard laboratory test or POCT and the GMI derived by the isCGM system or the eAG derived by a conventional SMBG device.
Methods
Study Design and Procedures
We conducted a cross-sectional study at the Department of Endocrinology and Diabetes, Diabetes Treatment Center, Prince Sultan Military Medical City (PSMMC), Riyadh. Diabetic patients who used the conventional finger-pricking self-monitoring of blood glucose (SMBG) or intermittently scanned continuous glucose monitoring (isCGM) to self-test their glucose levels between May and December 2020 were included in this study. This research was conducted following the Declaration of Helsinki, and the study protocol was approved by the Research and Ethics Committee of PSMMC, Riyadh, Saudi Arabia (IRB approval no. 1486). After explaining the objectives and research methodology clearly, all participants provided oral and written informed consent before completing the study measurement.
This study was part of the PSMMC local experience to assess the potential discrepancies between laboratory HbA1c and GMI by isCGM using FreeStyle Libre, a sensor-based flash glucose monitoring system (Abbott Laboratories, Chicago, IL, USA) and POCT for HbA1c using cobas b 101 HbA1c analyzer, a point-of-care device by Roche to achieve significant control in HbA1c and glycemic parameters for diabetic patients [24–26]. Conventional SMBG was performed using Abbott FreeStyle Optium Neo® blood glucose meter, while the laboratory HbA1c was performed using cobas integra 400 plus/800 analyzers.
Study Population
Consecutive patients with diabetes having blood samples drawn for routine laboratory analysis of HbA1c at PSMMC between May and December 2020 were asked to provide a capillary blood sample for same-visit testing with the cobas b 101 POC-HbA1c.
Patients were eligible for inclusion if they (1) were adult patients with DM and agreed to participate in the study and signed the informed consent form and (2) tested their blood glucose at least twice a day (for conventional finger-pricking SMBG users) and (3) if they had at least 70% time sensor activity (for isCGM users). Patients were excluded if they were (1) pregnant females, (2) severe or unstable medical disorders, (4) severe hypoglycemia, (6) diabetic ketoacidosis, or (7) hyperosmolar hyperglycemic state. For isCGM users, patients with inadequate use of the FGM system (percentage time sensor active < 70%) were excluded from the isCGM cohort.
Study Endpoints and Data Collection
The primary endpoint of this study was to assess the limit of agreement between the point-of-care testing for HbA1c (POCT-HbA1c) using the cobas b 101 POC-HbA1c system analyzer with a standard laboratory measurement method (Lab-HbA1c) and the GMI derived by isCGM [28 days (GMI-28) and 90 days (GMI-90)] or estimated average glucose (eAG) derived by conventional SMBG device [30 days (eAG-30) and 90 days (eAg-90)].
The secondary endpoint was to assess the correlation between POCT-HbA1c with Lab-HbA1c and GMI-28/GMI-90 derived by isCGM or eAG-30/eAG-90 derived by conventional SMBG to investigate any potential discordance between GMI, Lab-HbA1c, and/or POCT-HbA1c.
To achieve these endpoints, the following data were collected:
Patient socio-demographic and clinical characteristics (age, gender, type of diabetes, treatment modality, diabetes duration, Lab-HbA1c, and POCT-HbA1c).
For the patients who used the conventional SMBG, 30- and 90-day averages on blood glucose meter readings were also extracted.
For isCGM users, ambulatory glucose profile (AGP) was downloaded for GMI, derived from isCGM mean glucose (previously known as estimated A1C), including 28- and 90-day average on the blood glucose meter, glucose variability, percentage of sensor data, and FreeStyle Libre (FSL) scanning frequency per day.
The eA1c derived by conventional SMBG was calculated using the eAG/A1C conversion calculator by the American Diabetes Association (ADA) [9, 27]. GMI data were obtained through Libreview online platform.
Statistical Analysis
The data were analyzed using SPSS version 25 software. All categorical variables were presented in frequency and percentage, whereas the continuous variables were presented with descriptive statistics [mean, standard deviation (SD), and range]. Correlations among laboratory HbA1c, POCT-HbA1c, GMI, and eAG were performed using the Pearson correlation coefficient. p < 0.05 was considered to be statistically significant.
The limits of agreement in different HbA1c measurements were evaluated using a Bland-Altman analysis (GraphPad Prism 7.04, MD, USA). The Bland-Altman analysis provided the 95% confidence interval (95% CI) limits of agreement between both methods of measuring the HbA1c (HbA1c by POCT and HbA1c by slandered laboratory method, for example) in the same patients. The National Glycohemoglobin Standardization Program reported that the limits of agreement (LoA) must fall within 0.75% HbA1c [28], and the coefficient of variation for POCT HbA1c and laboratory HbA1c was not statistically significant above 3% [28]. Univariate linear regression analysis was used to fit the association between laboratory HbA1c, POCT-HbA1c, and estimated HbA1c. Multivariable linear regression analysis was performed to examine the relationship between Lab-HbA1c as a dependent variable and POCT-HbA1c and estimated HbA1c after adjusting for potential clinical factors including age, gender, duration of diabetes, treatment modality, and type of diabetes. For the isCGM group, we add glucose variability, percentage of available sensor data, and FSL scanning frequency per day to the regression model.
Results
Characteristics of the Included Patients
This study included a total of 81 adult patients with DM. Of them, 30 patients were isCGM users, and 51 used the conventional SMBG. The demographics and clinical characteristics of the study population are shown in Table 1. All of the included T1DM patients were using isCGM, while most T2DM patients were using conventional SMBG (n = 51). Participants had a mean ± SD age of 49.43 ± 7.65 and 29.13 ± 11.4 years, with a diabetes duration of 8.0 ± 5.0 and 9.0 ± 4.0 years for the SMBG and isCGM groups, respectively. The mean ± SD of Lab-HbA1c was 8.4 ± 0.63% for the SMBG group and 8.45 ± 0.88% for the isCGM group, while the mean ± SD of POCT-HbA1c were 8.13 ± 0.57% for SMBG group, 8.03 ± 0.8% for isCGM group (Fig. 1). The percentage of available sensor data was 91.73%, with a scanning frequency of 7.7 (± 1.49) per day for isCGM users. Meanwhile, the frequency of self-testing among the conventional SMBG users was 2.49 times/day.
Table 1.
Baseline demographic and clinical characteristics of the study population
| Variables | Groups | Values |
|---|---|---|
| Age in years | ||
| Mean (± SD), range | Conventional SMBG group | 49.43 (± 7.65), [33—69] |
| isCGM group | 29.13 (± 11.4), [18—49] | |
| Gender, N (%) | ||
| Female | Conventional SMBG group | 25 (49.02%) |
| isCGM group | 14 (46.67%) | |
| Male | Conventional SMBG group | 26 (50.98%) |
| isCGM group | 16 (53.33%) | |
| Type of diabetes, N (%) | ||
| T1DM | Conventional SMBG group | 0 (0.0%) |
| isCGM group | 21 (70.00%) | |
| T2DM | Conventional SMBG group | 51 (100.00%) |
| isCGM group | 9 (30.00%) | |
| Diabetes treatment, N (%) | ||
| MDI | Conventional SMBG group | 10.0 (19.61%) |
| isCGM group | 18.0 (60.00%) | |
| Oral alone | Conventional SMBG group | 17.0 (33.33%) |
| isCGM group | 1.0 (3.33%) | |
| Combination | Conventional SMBG group | 24.0 (47.06%) |
| isCGM group | 6.0 (20.00%) | |
| Insulin pump | Conventional SMBG group | 0.0 (0.0%) |
| isCGM group | 5.0 (16.67%) | |
| DM duration, years | ||
| Mean (± SD), range | Conventional SMBG group | 8.0 (± 5.0), [1.0—22.0] |
| isCGM group | 9.0 (± 4.0), [2.0—19.0] | |
| HbA1c (laboratory), % | ||
| Mean (± SD), range | Conventional SMBG group | 8.4 (± 0.63), [7.2—10.2] |
| isCGM group | 8.45 (± 0.88), [7—11.3] | |
| HbA1c (POCT), % | ||
| Mean (± SD), range | Conventional SMBG group | 8.13 (± 0.57), [7—9.9] |
| isCGM group | 8.03 (± 0.8), [6.9—10.5] | |
| Estimated HbA1c % [28 or 30 days] | ||
| Mean (± SD), range | Conventional SMBG group (eAG-30) | 8.08 (± 0.54), [7.1—9.8] |
| isCGM group (GMI-28) | 8.07 (± 1.05), [6.7—11.2] | |
|
Estimated HbA1c % [90 days] |
||
| Mean (± SD), range | Conventional SMBG group (eAG-90) | 8.09 (± 0.52), [7.3—10] |
| isCGM group (GMI-90) | 7.97 (± 0.67), [6.9–9.8] | |
| Glucose variability, % | ||
| Mean (± SD), range | Conventional SMBG group | – |
| isCGM group | 38.36 (± 11.06), [24–62.8] | |
| Percentage of sensor data, % | ||
| Mean (± SD), range | Conventional SMBG group | – |
| isCGM group | 91.73 (± 3.9), [86 – 98] | |
| FSL scanning frequency per day | ||
| Mean (± SD), range | Conventional SMBG group | – |
| isCGM group | 7.7 (± 1.49), [5–10] | |
| Frequency of self-testing (times/day) | ||
| Mean (± SD), range | Conventional SMBG group | 2.49 (± 0.64), [1–4] |
| isCGM group | – |
eAG: estimated average glucose, GMI: glucose monitoring index, iscCGM, intermittently scanned continuous glucose monitoring; FSL, FreeStyle Libre; MDI, multiple daily injection; POCT, point-of-care resting; SD, standard deviation, SMBG, self-monitoring of blood glucose
Fig. 1.
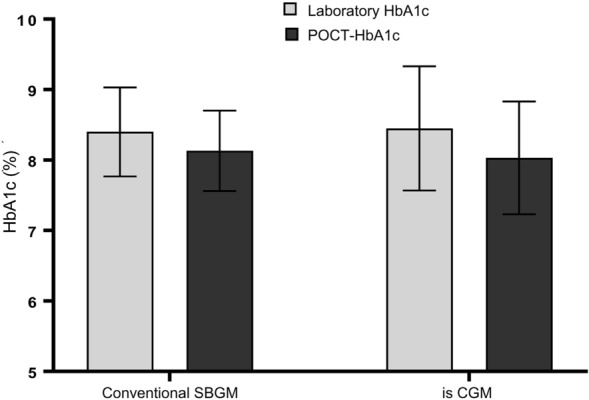
Means of laboratory HbA1c and POCT-HbA1c in isCGM and conventional SMBG users
Agreement Between POCT-HbA1c with Lab HbA1c and Estimated HbA1c by isCGM or Conventional SMBG
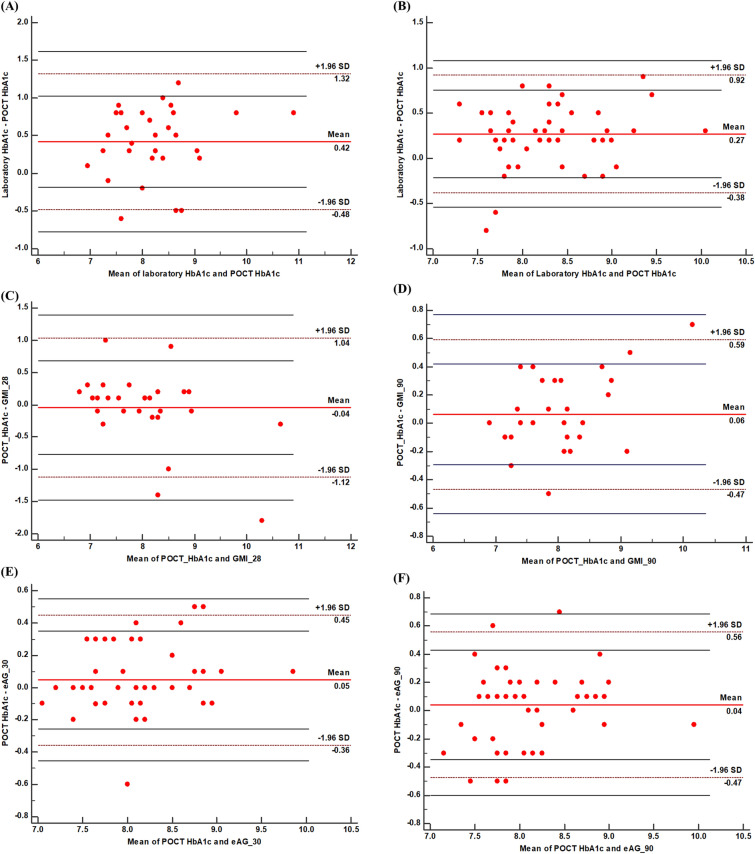
According to the Bland-Altman plot analysis, HbA1c levels of 29 out of 30 patients (96.7%) analyzed with POCT and the standard laboratory testing in the isCGM group were within the range of agreement limits (95% CI − 0.48% to 1.32%, Fig. 2A). For the conventional SMBG group, HbA1c levels of 49/51 (96.1%) patients analyzed with POCT and the standard laboratory testing were within the range of agreement limits (95% CI − 0.38% to 0.92%, Fig. 2B).
Fig. 2.
Bland-Altman plot for the agreement between POCT-HbA1c with Lab HbA1c and estimated HbA1c by isCGM or conventional SMBG. A Limit of agreement between the laboratory HbA1c and the POCT-HbA1c in isCGM users. B Limit of agreement between the laboratory HbA1c and the POCT-HbA1c in Conventional SMBG users; C Limit of agreement between the POCT-HbA1c and GMI-28 in isCGM users. D Limit of agreement between the POCT-HbA1c and GMI-90 in isCGM users. E Limit of agreement between the POCT-HbA1c and eAG-30 in conventional SMBG users. F Limit of agreement between the POCT-HbA1c and eAG-90 in conventional SMBG users
This indicates that 96.7% (in the isCGM group) and 96.1% (in the SMBG group) of the HbA1c measurements by the POCT are in line with the standard laboratory method for HbA1c testing.
The GMI-28s of 28/30 (93.3%) patients analyzed with the isCGM system and POCT were within the range of agreement limits (95% CI − 1.12% to 1.04%, Fig. 2C), while the GMI-29s of 28/30 (93.3%) patients were within the range of agreement limits (95% CI − 0.47% to 0.59%, Fig. 2D).
The eAG-30 levels of 48/51 (94.12%) patients analyzed with the conventional SMBG system and POCT were within the range of agreement limits (95% CI − 0.36% to 0.45%, Fig. 2E), while the eAG-90s of 46/51 (90.2%) patients were within the range of agreement limits (95% CI − 0.47 to 0.56%, Fig. 2F).
Correlation analysis
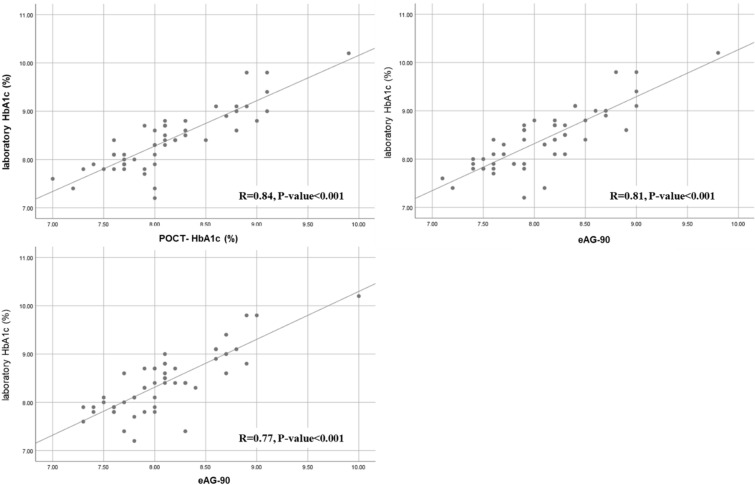
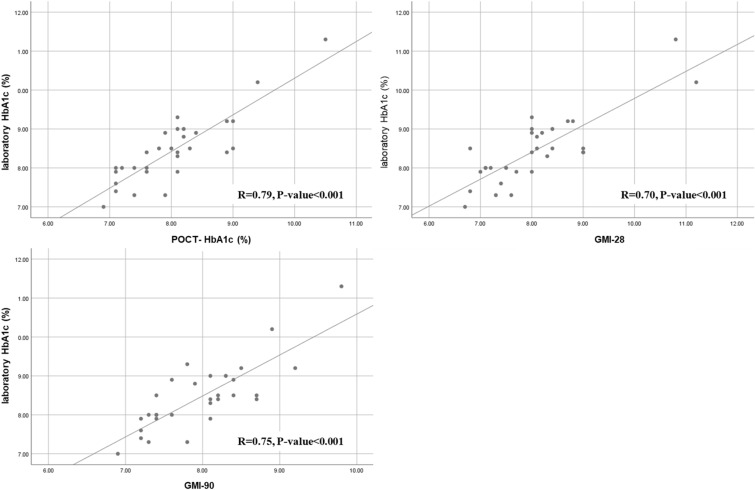
Person’s correlation analysis revealed statistically significant positive correlations between Lab-HbA1c and POCT-HbA1c (r = 0.84, p < 0.001), laboratory HbA1c and eAG-30 (r = 0.81, p < 0.001), and laboratory HbA1c and eAG-90 (r = 0.77, p < 0.001) in the conventional SMBG group (Fig. 3). The same significant positive correlations were observed among the laboratory HbA1c with POCT-HbA1c (r = 0.79, p < 0.001), GMI-28 (r = 0.70, p < 0.001), and GMI-90 (r = 0.75, p < 0.001) in the isCGM group (Fig. 4).
Fig. 3.
Correlation among the laboratory HbA1c, POCT-HbA1c, eAG-30, and eAG-90 in conventional SMBG users
Fig. 4.
Correlation among the laboratory HbA1c, POCT-HbA1c, estimated HbA1c (28 days), and estimated HbA1c (90 days) in isCGM users
Regression analysis
isCGM group
The univariate linear regression analysis revealed a significant linear relationship between Lab-HbA1c and POCT-HbA1c in the isCGM group (R = 0.86, R2 = 0.740, p < 0.001). The same significant linear relationships were observed between Lab-HbA1c and estimated GMI-28 (R = 0.82, R2 = 0.672, p < 0.001) or GMI-90 (R = 0.81, R2 = 0.656, p < 0.001) in the isCGM group. In the multivariable linear regression model, the linear relationship among Lab-HbA1c, POCT-HbA1c, GMI, and eAG persisted significantly after adjusting for age and gender duration of diabetes, type of diabetes, and diabetes treatment (Table 2).
Table 2.
Linear regression analyses for the association among laboratory HbA1c, POCT- HbA1c, and GMI in isCGM users
| Model | Parameters | R | R2 | Adj R2 | Standardize β | B (95% CI) | p value |
|---|---|---|---|---|---|---|---|
| Unadjusted | GMI-28 | 0.82 | 0.672 | 0.66 | 0.82 | 2.87 (1.45–4.37) | < 0.001 |
| GMI-90 | 0.81 | 0.656 | 0.64 | 0.81 | 1.05 (0.75–1.35) | < 0.001 | |
| POCT-HbA1c | 0.86 | 0.740 | 0.72 | 0.86 | 0.94 (0.72–1.16) | < 0.001 | |
| Adjusted Module 1 | GMI-28 | 0.84 | 0.706 | 0.69 | 0.83 | 0.70 (0.51–0.88) | < 0.001 |
| GMI-90 | 0.83 | 0.689 | 0.66 | 0.83 | 1.08 (0.78–1.37) | < 0.001 | |
| POCT-HbA1c | 0.86 | 0.740 | 0.71 | 0.85 | 0.93 (0.71–1.16) | < 0.001 | |
| Adjusted Module 2 | GMI-28 | 0.86 | 0.740 | 0.69 | 0.79 | 0.66 (0.47–0.85) | < 0.001 |
| GMI-90 | 0.84 | 0.706 | 0.64 | 0.84 | 1.19 (0.76–1.44) | < 0.001 | |
| POCT-HbA1c | 0.86 | 0.740 | 0.68 | 0.84 | 0.93 (0.67–1.19) | < 0.001 | |
| Adjusted Module 3 | GMI-28 | 0.87 | 0.757 | 0.68 | 0.74 | 0.62 (0.41–0.83) | < 0.001 |
| GMI-90 | 0.91 | 0.828 | 0.73 | 0.80 | 1.1 (0.73–1.39) | < 0.001 | |
|
POCT-1 HbA1c |
0.91 | 0.828 | 0.73 | 0.79 | 0.87 (0.61–1.12) | < 0.001 |
Dependent variable is laboratory HbA1c, and independent variables are GMI-28, GMI-90, and POCT- HbA1c
Module 1 was adjusted for age and gender. Module 2 was adjusted for all variables in Model 1 plus duration of DM, type of diabetes, and treatment. Module 3 was adjusted for all variables in Model 2 plus glucose variability, percentage of sensor data, and FSL scanning frequency per day
Conventional SMBG group
The univariate linear regression analysis revealed a significant linear relationship between Lab-HbA1c and POCT- HbA1c conventional SMBG (R = 0.83, R2 = 0.689, p < 0.001) users. The same significant linear relationships were observed between Lab-HbA1c and eAG-30 (R = 0.83, R2 = 0.689, p < 0.001) and eAG-90 (R = 0.81, R2 = 0.656, p < 0.001) in conventional SMBG group. In the multivariable linear regression model, the linear relationship between Lab-HbA1c, POCT-HbA1c, GMI, and eAG persisted significantly after adjusting for age and gender, duration of diabetes, type of diabetes, and diabetes treatment (Table 3).
Table 3.
Linear regression analyses for the association between laboratory HbA1c, POCT-HbA1c, and estimated HbA1c (30 days), and estimated HbA1c (90 days) in conventional SMBG users
| Model | HbA1c parameter | R | R2 | Adj R2 | Standardize β | B (95% CI) | p value |
|---|---|---|---|---|---|---|---|
| Unadjusted | eAG-30 | 0.83 | 0.689 | 0.69 | 0.83 | 0.97 (0.79–1.16) | < 0.001 |
| eAG-90 | 0.81 | 0.656 | 0.65 | 0.81 | 0.99 (0.79–1.21) | < 0.001 | |
| POCT-HbA1c | 0.83 | 0.689 | 0.72 | 0.85 | 0.94 (0.77–1.11) | < 0.001 | |
| Adjusted Module 1 | eAG-30 | 0.84 | 0.706 | 0.68 | 0.83 | 0.98 (0.76–1.17) | < 0.001 |
| eAG-90 | 0.81 | 0.656 | 0.64 | 0.81 | 0.99 (0.78–1.19) | < 0.001 | |
| POCT-HbA1c | 0.86 | 0.740 | 0.72 | 0.86 | 0.95 (0.77–1.12) | < 0.001 | |
| Adjusted Module 2 | eAG-30 | 0.84 | 0.706 | 0.67 | 0.98 | 0.98 (0.78–1.17) | < 0.001 |
| eAG-90 | 0.81 | 0.656 | 0.63 | 0.81 | 0.99 (0.77–1.20) | < 0.001 | |
| POCT-HbA1c | 0.87 | 0.757 | 0.73 | 0.87 | 0.96 (0.79–1.13) | < 0.001 |
Dependent variable is laboratory HbA1c, and independent variables are eAG-30, eAG-90, and POCT- HbA1c
Module 1 was adjusted for age and gender, and module 2 was adjusted for all variables in Model 1 plus duration of DM, type of diabetes, and treatment
Discussion
This observational study is the first in the MENA (Middle East and North Africa) region to assess the agreements and correlations between the GMI derived by isCGM and eAG derived by a conventional SMBG device with the POCT for HbA1c or the standard laboratory HbA1c testings. Our results demonstrated that the agreement between the Lab-HbA1c and POCT-HbA1c in both conventional BGM or CGM users meet the clinical standers of accuracy. Furthermore, there are significant positive correlations between laboratory HbA1c, POCT-HbA1c, GMI, and eAG in conventional SMBG and isCGM users. These significant correlations were supported by the results of univariate and multivariate regression modules with multiple adjustments and demonstrated the same significant relationships.
Patients living with diabetes and needing close monitoring for their blood glucose now have an alternative to conventional laboratory or SMBG in the form of CGM systems that can be isCGMS, ivCGMS, or rtCGMS [14]. In Saudi Arabia, the FreeStyle Libre® FGM system is commercially available. PSMMC hospital had recently disseminated isCGM using the FreeStyle Libre device to all patients with T1DM.
Measuring the time to glycemic target range or hypoglycemia, glucose management indicators, and changes in blood glucose levels over hours or days using CGM devices provides a more personalized approach to diabetes management, and it may contribute to overcoming diabetes-related complications [29]. Thus, ADA currently considers CGM a valuable part of diabetes self-management and the standard of care for diabetic patients treated with intensive insulin therapy [30]. However, reports have shown a discordance between the HbA1c data measured by CGM compared to the standard laboratory HbA1c [18–21]. The exact reasons for this discrepancy are unknown [31]. Bergenstal et al. reported a possible explanation for the potential variation between the laboratory and GMI. The average glucose and hence the GMI values will be higher than a laboratory HbA1c (tested at the same time) during periods of hyperglycemia because the laboratory test represents glucose levels predominantly during the last 2–3 months [19]. Beck et al. reported that a laboratory HbA1C of 8.0% could be associated with a CGM-measured mean estimated HbA1C of 7.0% to 8.5% [32]. This result is in agreement with the results of the ADAG study [9]. As a result, relying solely on laboratory HbA1c or GMI to measure glycemic control may mislead clinical judgments. On the other hand, Liu et al. assessed the impact of glycemic variability on the relationship between GMI and laboratory HbA1c. They observed a discrepancy between the GMI (measured by the iProTM2 system) and the laboratory HbA1c in T1DM patients (mainly in patients with high hemoglobin glycation index). This result is similar to those highlighted by a previous study by Hempe et al., who linked this discrepancy to the individual variations in laboratory HbA1c [33].
Although several studies have assessed the link between mean glucose and laboratory HbA1c, the data show inconsistent results. Given that most of these studies employed SMBG data to calculate glycemic variability and mean glucose, the findings revealed that glycemic variability did not affect the link between mean glucose and laboratory HbA1c [9, 34]. Contrarily, using CGM data rather than SMBG data, researchers discovered that glycemic variability impacted the relationship between mean glucose and laboratory HbA1c [35].
Nevertheless, the controversy over the estimated HbA1c derived by the CGM systems and its agreement with the actual HbA1c measured by the standard laboratory techniques is still a concern for the users. Diabetologists have reported these potential discrepancies between laboratory and estimated HbA1c; however, there were insufficient data to support this observation. The recently published French case report showed that the patient had poor glycemic control measured by the CGM device (estimated HbA1c = 9%) compared to 7.4% by the laboratory HbA1c test and confirmed at 7.7% by HPLC [22].
However, implementing POCT devices in the outpatient diabetes clinic helps physicians provide patients with well-timed treatment changes for better glycemic control [8]. Our results showed that POCT HbA1c measurements have a high level of agreement with the laboratory HbA1c (0.48% to 1.32% for the isCGM group and -0.38% to 0.92% for conventional SMBG users). The same results were obtained by Berbudi et al., where the Bland-Altman plot analysis showed that the HbA1c by POCT and the standard laboratory method of HbA1c were within the range of agreement limits (− 1.67 to 1.3). They concluded that POCT-HbA1c is a potential method for diabetes screening and monitoring, especially when a rapid result is needed [36]. Moreover, the study by Raalten et al. demonstrated high limits of agreement between the POCT HbA1c and the central laboratory HbA1c tests in non-diabetic, obese patients in the preoperative outpatient setting [37]. On the other hand, the recently published local experience of PSMMC hospital showed that POCT for HbA1c improved the patient’s adherence to the physician’s recommendations for HbA1c testing and higher patient satisfaction [26].
The agreement between the laboratory HbA1c, GMI by CGM, or the eAG by conventional SMBG is uncertain, and this study contributes valuable data to resolve these discrepancies. However, our study was limited by the relatively small sample size of patients from a single site, which might limit the generalizability of the study results. Therefore, a larger multicenter study is warranted. The relatively small range of HbA1c (almost all patients were within a range between 7.5 and 9%) may have influenced the study findings because the lack of lower HbA1c data can bias the level of the agreement since these patients often show a high variation of glucose values with unrecognized hypoglycemic events. Another limitation was that most patients with T2DM were treated with oral medication alone or BOT; only 20% were treated with MDI. Furthermore, there were relatively few blood glucose measurements. This may have led to a selection bias of patients with stable glucose values and a better level of agreement between SMBG and HbA1c.
Conclusions
HbA1c is used to guide blood glucose management plans and is an important biomarker of the risk of developing diabetic complications. Because of the high level of agreement between the GMI measured by the isCGM or eAG measured by the conventional SMBG systems with the POCT and the standard laboratory test, as well as the advantages of isCGM over the other methods of glucose monitoring, we believe that estimated HbA1c and other parameters provided by the isCGM provide more personalized detailed glycemic data for a better diabetes care management plan, especially with uncontrolled patients or on intensive insulin therapy. Moreover, POCT-HbA1c measurements showed a high level of agreement and correlation with the standard laboratory test. Therefore, POCT-HbA1c is a potential method for diabetes screening and monitoring in daily clinic visits, especially when a rapid result is needed. However, for the initial diagnosis of DM, HbA1c level measured using the standard laboratory method is still recommended.
Acknowledgements
The authors thank the study participants for their participation. We also thank RAY-CRO for the medical writing assistance, which was funded by Roche Diagnostics Saudi Arabia.
Funding
No funding or sponsorship was received for this study. The journal’s Rapid Service Fee was funded by the authors.
Editorial and Medical Writing Assistance
The authors thank RAY-CRO for the assistance and editorial support when developing this manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Ayman Al Hayek, Samia H Sobki, Abdulghani H Al-Saeed, Wael M Alzahrani, and Mohamed A Al Dawish contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by Ayman Al Hayek, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Ayman Al Hayek, Samia H Sobki, Abdulghani H Al-Saeed, Wael M Alzahrani, and Mohamed A Al Dawish confirm that they have nothing to disclose.
Compliance with Ethics Guidelines
The protocols and the subject information/informed consent forms were reviewed and approved by the Research and Ethics Committee of PSMMC (IRB approval no. 1486). Our study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All participants provided oral and written informed consent before completing the study measurement. For patients ≤ 18 years of age, verbal consent from the patients and written informed consent from their parents/caregivers were obtained.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Al-Lawati JA. Diabetes mellitus: a local and global public health emergency! Oman Med J. 2017;32:177–179. doi: 10.5001/omj.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelhaleem IA, Salamah HM, Alsabbagh FA, Eid AM, Hussien HM, Mohamed NI, et al. Efficacy and safety of imeglimin in patients with type diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials. Diabetes Metab Syndr. 2019;15:102323. doi: 10.1016/j.dsx.2021.102323. [DOI] [PubMed] [Google Scholar]
- 3.Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia. 2019;62:408–417. doi: 10.1007/s00125-018-4763-3. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA J Am Med Assoc. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwyerindgren L, Mackenbach JP, Van Lenthe FJ, Flaxman AD. Mokdad AH (2016) Diagnosed and undiagnosed diabetes prevalence by County in the U.S. 1999-2012. Diabetes Care. American Diabetes Association Inc. 2016;39:1556–1562. doi: 10.2337/dc16-0678. [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation. IDF Diabetes Atlas, 7th Edition. 2021
- 7.World Health Organization. Saudi Arabia—Diabetes country profile.
- 8.Schnell O, Crocker JB, Weng J. Impact of HbA1c testing at point of care on diabetes management. J Diabetes Sci Technol SAGE Publications Inc.; 2017; 611–7. [DOI] [PMC free article] [PubMed]
- 9.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Glycemic targets: Standards of medical care in diabetes—2021. Diabetes Care. American Diabetes Association. 2021;44:73–84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 11.Lelanc ES, Rosales AG, Kachroo S, Mukherjee J, Funk KL, Schneider JL, et al. Provider beliefs about diabetes treatment have little impact on glycemic control of their patients with diabetes. BMJ Open Diabetes Res Care BMJ; 2015;3:62. [DOI] [PMC free article] [PubMed]
- 12.Gracia TR. Structured SMBG in early management of T2DM: Contributions from the St Carlos study. World J Diabetes. 2014;5:471. doi: 10.4239/wjd.v5.i4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruttomesso D, Laviola L, Avogaro A, Bonora E, Del Prato S, Frontoni S, et al. The use of real time continuous glucose monitoring or flash glucose monitoring in the management of diabetes: A consensus view of Italian diabetes experts using the Delphi method. Nutr Metab Cardiovasc Dis. 2019;29:421–431. doi: 10.1016/j.numecd.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Hohendorff J, Gumprecht J, Mysliwiec M, Zozulinska-Ziolkiewicz D, Malecki MT. Intermittently scanned continuous glucose monitoring data of polish patients from real-life conditions: more scanning and better glycemic control compared to worldwide data. Diabetes Technol Ther. 2021;23:577–585. doi: 10.1089/dia.2021.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown SA, Basu A, Kovatchev BP. Beyond HbA 1c: using continuous glucose monitoring metrics to enhance interpretation of treatment effect and improve clinical decision-making. Diabet Med. 2019;36:679–687. doi: 10.1111/dme.13944. [DOI] [PubMed] [Google Scholar]
- 16.Al Hayek AA, Robert AA, Al Dawish MA. Evaluation of freestyle libre flash glucose monitoring system on glycemic control, health-related quality of life, and fear of hypoglycemia in patients with type 1 diabetes. Clin Med Insights Endocrinol Diabetes. 2017;10:117955141774695. doi: 10.1177/1179551417746957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone JY, Bailey TS. Benefits and limitations of continuous glucose monitoring in type 1 diabetes. Expert Rev Endocrinol Metab. 2020;15:41–49. doi: 10.1080/17446651.2020.1706482. [DOI] [PubMed] [Google Scholar]
- 18.Johnson ML, Martens TW, Criego AB, Carlson AL, Simonson GD, Bergenstal RM. Utilizing the ambulatory glucose profile to standardize and implement continuous glucose monitoring in clinical practice. Diabetes Technol Ther. 2019;21:S217–S225. doi: 10.1089/dia.2019.0034. [DOI] [PubMed] [Google Scholar]
- 19.Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41:2275–2280. doi: 10.2337/dc18-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nayak AU, Singh BM, Dunmore SJ. Potential clinical error arising from use of HbA1c in diabetes: effects of the glycation gap. Endocr Rev. 2019;40:988–999. doi: 10.1210/er.2018-00284. [DOI] [PubMed] [Google Scholar]
- 21.Wright JJ, Hu J-R, Shajani-Yi Z, Bao S. Use of continuous glucose monitoring leads to diagnosis of hemoglobin C trait in a patient with discrepant hemoglobin A1C and self-monitored blood glucose. AACE Clin Case Rep. 2019;5:e31–e34. doi: 10.4158/ACCR-2018-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mialon F, Catargi B, Rami L, Barat P, Bedel A, Lacape G, et al. Biomarkers in diabetes mellitus: contributions and discrepancies of new technologies. A case report. Ann Biol Clin (Paris). 2021;79:445–451. doi: 10.1684/abc.2021.1680. [DOI] [PubMed] [Google Scholar]
- 23.Lenters-Westra E, Slingerland RJ. Three of 7 hemoglobin A1c point-of-care instruments do not meet generally accepted analytical performance criteria. Clin Chem. 2014;60:1062–1072. doi: 10.1373/clinchem.2014.224311. [DOI] [PubMed] [Google Scholar]
- 24.Imrit C, Neergheen-Bhujun VS, Joonas N. Evaluation of the Roche Cobas b 101 glycosylated hemoglobin point-of-care analyzer. Point Care J Near Patient Test Technol. 2017;16:135–137. doi: 10.1097/POC.0000000000000142. [DOI] [Google Scholar]
- 25.Al Hayek AA, Al Dawish MA. The potential impact of the freestyle libre flash glucose monitoring system on mental well-being and treatment satisfaction in patients with type 1 diabetes: a prospective study. Diabetes Ther. 2019;10:1239–1248. doi: 10.1007/s13300-019-0616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Hayek AA, Al-Saeed AH, Alzahrani WM, Al Dawish MA. Assessment of patient satisfaction with on-site point-of-care hemoglobin A1c testing: an observational study. Diabetes Ther. 2021;12:2531–2544. doi: 10.1007/s13300-021-01126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes Association. eAG/A1C Conversion Calculator.
- 28.Little RR. Glycated hemoglobin standardization—national glycohemoglobin standardization program (NGSP) perspective. Clin Chem Lab Med. 2003;41 [DOI] [PubMed]
- 29.Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18:S23–S213. doi: 10.1089/dia.2015.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Diabetes Association Diabetes technology: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:71–80. doi: 10.2337/dc19-S007. [DOI] [PubMed] [Google Scholar]
- 31.Oriot P, Hermans MP. “Mind the gap please…”: estimated vs. measured A 1 c from continuous measurement of interstitial glucose over a 3-month period in patients with type 1 diabetes. Acta Clin Belg. 2020;75:109–115. doi: 10.1080/17843286.2018.1561780. [DOI] [PubMed] [Google Scholar]
- 32.Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40:994–999. doi: 10.2337/dc17-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hempe JM, Gomez R, McCarter RJ, Chalew SA. High and low hemoglobin glycation phenotypes in type 1 diabetes. J Diabetes Complicat. 2002;16:313–320. doi: 10.1016/S1056-8727(01)00227-6. [DOI] [PubMed] [Google Scholar]
- 34.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 35.Kuenen JC, Borg R, Kuik DJ, Zheng H, Schoenfeld D, Diamant M, et al. Does glucose variability influence the relationship between mean plasma glucose and HbA1c levels in type 1 and type 2 diabetic patients? Diabetes Care. 2011;34:1843–1847. doi: 10.2337/dc10-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Performance of point-of-care testing compared with the standard laboratory diagnostic test in the measurement of HbA1c in Indonesian diabetic and nondiabetic subjects. J Diabetes Res. 2020;2020:1–6. doi: 10.1155/2020/2037565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Raalten F, Hiemstra YL, Keulen N, van Duivenvoorde Y, Stoecklein K, Verhagen EA, et al. Level of agreement of point-of-care and laboratory HbA1c measurements in the preoperative outpatient clinic in non-diabetic patients who are overweight or obese. J Clin Monit Comput. 2019;33:1139–1144. doi: 10.1007/s10877-019-00255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.