For Pseudomonas spp., two plasmid-mediated mechanisms of resistance to rifampin have been reported: one involves an efflux pump (1), and the other involves an ADP-ribosylating transferase (3) encoded by a gene (arr-2) that is integrated as a gene cassette in a class 1 integron (10). Recently, plasmid-mediated rifampin resistance (Arr-2) was described for various Enterobacteriaceae from Southeast Asia producing the extended-spectrum β-lactamase VEB-1 (4, 6). The genes encoding these mechanisms of resistance are integron located as gene cassettes (4, 6).
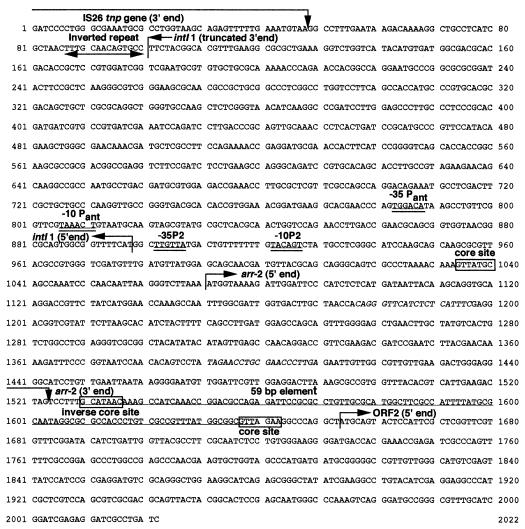
We recently described an outbreak in France of Klebsiella pneumoniae isolates that produce the ACC-1 β-lactamase and that are resistant to rifampin, the first of which (strain SLK54) was isolated from a patient previously hospitalized in Tunisia (7). Resistance to ceftazidime and rifampin was cotransferred by conjugation to Escherichia coli K-12 strain HB101 for all strains (rifampin MICs, >256 μg ml−1) except one (KP SLK55) (rifampin MIC, 16 μg ml−1). Plasmid DNA from the transconjugant E. coli HB101 of the first isolate in the outbreak (K. pneumoniae SLK54) was partially digested with Sau3A (Roche Molecular Biochemicals, Meylan, France) and ligated (T4 DNA ligase; Amersham Pharmacia Biotech, Saclay, France) into the BamHI site of the cloning vector pBK-CMV Kanr (Stratagene, La Jolla, Calif.) (8). The recombinant plasmid, pRIF-1, which harbored the smallest insert (2 kb) was sequenced (9). Analysis of the DNA sequence revealed an open reading frame of 453 nucleotides (Fig. 1) predicting a 150-amino-acid sequence. This protein was 99% identical to the rifampin ADP-ribosylating transferase Arr-2. The predicted rifampin ADP-ribosylating transferase differed from Arr-2 by a Lys98Arg substitution. In Pseudomonas aeruginosa the location of the gene encoding this protein is unclear (10), although in Enterobacteriaceae it has been found to be plasmid located (4, 6). However, in both cases, the arr-2 gene was a gene cassette located in a class 1 integron. In K. pneumoniae SLK54, the plasmid-mediated arr-2-like gene was also a gene cassette, located immediately downstream of the integrase and the promoter region (Fig. 1). The expression of the arr-2-like gene is probably driven by the Pant promoter and not the P2 promoter because the insertion of three G residues increasing the spacing between the −35 and −10 boxes to 17 was absent in the P2 promoter (Fig. 1) (2). The configuration of the Pant promoter was a hybrid combining the −35 box (TGGACA), found in weak Pant promoters, and the −10 box (TAAACT), found in strong Pant promoters, and has been shown to have intermediate strength (5). The 5′ coding sequence of the integron, corresponding to the last 214 bp (71 amino acids) of the integrase gene, was truncated by the insertion of the IS26 sequence and resulted in a nonfunctional integrase. A similar observation was made for In53, which carries the arr-2 gene cassette in E. coli (6), in which the IS26 truncated the integrase gene and especially the Pant promoter.
FIG. 1.
Nucleotide sequence of the arr-2 gene and its genetic environment. The 5′ and 3′ ends, the −35 and −10 promoter regions, the core sites and inverse core sites, and the 59-bp element are indicated.
The rifampin ADP-ribosylating transferase Arr-2 was previously detected in strains of P. aeruginosa and Enterobacteriaceae isolated from patients in Southeast Asia. These strains also produce the VEB-1 extended-spectrum β-lactamase (4, 6, 10). We now report the plasmid-mediated rifampin resistance encoded by the arr-2-like gene cassette in K. pneumoniae which also produces the plasmid-mediated cephalosporinase ACC-1 and which was isolated in France from a patient previously hospitalized in Tunisia (7).
The EMBL accession number for the nucleotide sequence reported in this paper is AJ277027.
REFERENCES
- 1.Chandrasekaran S, Lalithakumari D. Plasmid-mediated rifampicin resistance in Pseudomonas fluorescens. J Med Microbiol. 1998;47:197–200. doi: 10.1099/00222615-47-3-197. [DOI] [PubMed] [Google Scholar]
- 2.Collis C M, Hall R M. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob Agents Chemother. 1995;39:155–162. doi: 10.1128/aac.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dabbs E R, Yazawa K, Mikami Y, Miyaji M, Morisaki N, Iwasaki S, Furihata K. Ribosylation by mycobacterial strains as a new mechanism of rifampin inactivation. Antimicrob Agents Chemother. 1995;39:1007–1009. doi: 10.1128/aac.39.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girlich D, Poirel L, Leelaporn A, Karim A, Tribuddharat C, Fennewald M, Nordmann P. Molecular epidemiology of the integron-located VEB-1 extended-spectrum β-lactamase in nosocomial enterobacterial isolates in Bangkok, Thailand. J Clin Microbiol. 2001;39:175–182. doi: 10.1128/JCM.39.1.175-182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Léveque C, Brassard S, Lapointe J, Roy P H. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene. 1994;142:49–54. doi: 10.1016/0378-1119(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 6.Naas T, Mikami Y, Imai T, Poirel L, Nordmann P. Characterization of In53, a class 1 plasmid- and composite transposon-located integron of Escherichia coli which carries an unusual array of gene cassettes. J Bacteriol. 2001;183:235–249. doi: 10.1128/JB.183.1.235-249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadjar D, Rouveau M, Verdet C, Donay J L, Herrmann J L, Lagrange P H, Philippon A, Arlet G. Outbreak of Klebsiella pneumoniae producing transferable AmpC-type beta-lactamase (ACC-1) originating from Hafnia alvei. FEMS Microbiol Lett. 2000;187:35–40. doi: 10.1111/j.1574-6968.2000.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 8.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 9.Sanger T, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tribuddharat C, Fennewald M. Integron-mediated rifampin resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:960–962. doi: 10.1128/aac.43.4.960. [DOI] [PMC free article] [PubMed] [Google Scholar]