Abstract
Anti-Programmed cell Death protein 1 (Anti-PD1) or Programmed Death-Ligand 1 (PDL1) immune checkpoint inhibitors provide treatment options for advanced HCC patients with low response rates. Combination therapy is becoming a major issue to improve the unmet need. Proton beam radiotherapy (PBT) could effectively control the local tumor with a low-risk injury to peripheral liver parenchyma. We retrospectively reviewed the patients who have received PBT combined with anti-PD1/PDL1 to evaluate the efficacy and safety of the advanced HCC patients. This study reviewed 29 advanced HCC patients who have received PBT and anti-PD1/PDL1 during 2016 and 2019. All were Child-Pugh A and performance status 0-1. Seventeen patients (58.6%) had extrahepatic spreading. Concurrent PBT started during anti-PD1/PDL1 with a median of 96.6 grays equivalent dose. The PBT field covered all tumors in 13 (44.8%) patients under curative intent. Other patients (55.2%) received palliative PBT that covered only the principal tumors. All patients have completed the concurrent PBT protocol. The median anti-PD1/PDL1 duration was 3.9 months. After a median follow-up of 13.2 months, the rates of 1-year PBT infield tumor control, 1-year outfield tumor control, and overall response were 90.5%, 90.9%, and 61.5%, and 70.8%, 69.2%, and 43.8%, respectively for curative-intent and palliative-control PBT. Complete response was found in 4 (30.8%) curative-intent and 1 (6.3%) palliative-control patients. The median overall progression-free survival was 27.2 months for curative-intent patients and 15.9 months for palliative-control patients. The overall survival was non-reached for both groups. The ALBI grade and Child-Pugh score change at 3-month and 6-month after PBT initiation were nonsignificant. No unexpected adverse event occurred except nine patients (31.0%) had treatment-related adverse events higher than or equal to Grade 3, including 2 (6.9%) had a radiation-induced liver injury. PBT combined with anti-PD1/PDL1 was safe without unexpected adverse events. The concurrent therapy could effectively treat advanced HCC through sustained local tumor necrosis and effective systemic tumor control for the patients who received curative-intent or palliative-control PBT combined with anti-PD1/PDL1.
Keywords: Immunotherapy, radiotherapy, hepatocellular carcinoma, survival, adverse event
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related deaths worldwide [1]. Although tumor resection, ablation, or liver transplantation can cure the disease in its early stages, the prognosis of patients with HCC at advanced stages is dismal. Sorafenib, a multi-kinase inhibitor (MKI), was the first drug to be approved to treat HCC patients in advanced stages. However, its objective response rate (ORR) is less than 12% [2]. Recently, studies of monotherapy with immune checkpoint inhibitors (ICIs) such as nivolumab or pembrolizumab, both belonging to the programmed death receptor-1 (PD1) class of inhibitors, have shown that the ORR increases to 15-18% with a durable response [3,4]. A recent study reported that combination therapy with atezolizumab (a PDL1 inhibitor) plus bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, showed an ORR of up to 33%; thus, the combination therapy appears to confer a superior survival advantage as compared to sorafenib alone [5]. Therefore, enhancing the use of ICI for advanced HCC patients is an emerging issue.
External beam radiotherapy (EBRT) has been shown to offer excellent local tumor control with acceptable safety for intrahepatic HCC tumors [6-8]. Proton beam radiotherapy (PBT), a type of radiotherapy that uses a high-energy proton beam, enables the safe and reasonable local control of small or local advanced HCC [9,10] Compared to the conventional photon radiation technique, PBT offers dosimetry advantages, and it reduces unwanted spreading of the dose to the surrounding normal liver and adjacent organs [11]. Although EBRT was not recommended for advanced HCC by the west guidelines, curative intent EBRT could be considered for oligometastasis HCC with combination of hepatic arterial infusion chemotherapy or transarterial chemoembolization (TACE) by the guidelines in Asia [12]. However, these combinations did not have systemic tumor control. Therefore, the combination of an ICI and PBT for advanced HCC has attracted high clinical interest, but its effects have never been reported to date. Clinically, some patients with advanced HCC had chosen ICIs plus PBT for systemic anti-tumor effect and better local tumor control. We retrospectively reviewed the consecutive advanced HCC patients who received ICI therapy with concurrently receiving PBT to investigate the infield tumor control (IFTC) ability by PBT and the outfield tumor control (OFTC) effect by ICI therapy as well as the overall results, including overall response rate (ORR), progression-free survival (PFS), OS (overall survival) and safety profile after receiving this combination therapy.
Materials and methods
Patient recruitment
We retrospectively reviewed the medical records of 303 consecutive patients who received anti-PD1/PDL1 ICI treatment for liver tumors between January 2016 and October 2019 at a tertiary medical center in Taiwan. The inclusion criteria for the study were as follows: HCC diagnosis based on pathology or image criteria of the American Association for the Study of Liver Disease [13], unresectable HCC in the advanced stage according to Barcelona clinic liver cancer (BCLC) staging B or C, not amenable to locoregional therapies, received ICI therapy for HCC treatment and PBT for the majority of liver tumors, liver function assessed as Child-Pugh A, and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. The exclusion criteria were as follows: patients who had not received PBT within one month before or during the course of ICI treatment, a diagnosis of cholangiocarcinoma (CCC) or mixed type HCC-CCC by pathology, ECOG PS > 2, and Child-Pugh class B or C. HCC treatment was determined by the patients themselves and upon doctors’ recommendations. All HCC treatment strategies were thoroughly discussed in our multidisciplinary meetings with the hepatologist, surgeon, medical oncologist, radiational oncologist, radiologist, and pathologist. The study was approved by the institutional review board of Chang Gung Medical Foundation, and written informed consent was waived due to the retrospective design.
A total of 34 patients who received concurrent PBT during ICI therapy met the above-mentioned criteria, and they were all enrolled in the study. Five patients were excluded due to a diagnosis of BCLC stage D for three patients and a pathological diagnosis of CCC or HCC-CCC mixed type in two patients. Thus, 29 patients with advanced HCC were included in the final analysis (Supplementary Figure 1).
Anti-PD1/PDL1 ICI therapy
Anti-PD1/PDL1 ICI therapy consisted of anti-PD1 monotherapy or ICI combination therapy by anti-PD1/PDL1 plus MKI or VEGF inhibitor or (anti-PD1 plus cytotoxic T lymphocyte antigen-4 inhibitor. Seventeen patients (59%) received anti-PD1 monotherapy (15 nivolumab and 2 pembrolizumab), and 12 patients (41%) used combination ICI combination therapy (4 with nivolumab plus sorafenib or lenvatinib; 6 with bevacizumab combined with nivolumab or atezolizumab; and 2 with nivolumab plus ipilimumab) (Supplementary Table 1). The ICI dosage was modified according to the current recommendations or ongoing trials (NCT03382886) [14-19]. Patients received ICI therapy along with PBT, and they have prescribed an anti-HCC regimen until disease progression, development of intolerance to adverse reactions, or patient’s choice.
PBT
PBT started within the first month of the first dose of ICI therapy. All patients underwent four-dimensional simulation computed tomography at 2.5 mm intervals in the treatment position to determine the tumor motion. An intravenous contrast medium was administered to patients with creatinine values < 1.5 mg/d. Abdominal belt compression was used to reduce respiratory motion, and gating was implemented at the exhalation phase (Anzai Medical, Tokyo, Japan) in patients with liver motion > 1 cm. Magnetic resonance image simulations (Optima MR450w MR system, GE Healthcare, Chicago, IL, USA) were also obtained to determine the tumor and margin. The gross tumor volume was defined as the enhanced area on computed tomography and magnetic resonance imaging. A 0- to 5-mm expansion of gross tumor volume was used to create a clinical target volume, and the planning target volume was generated by adding a 5- to 7-mm margin.
Treatment plans were generated using the Eclipse planning system (Eclipse, version 13.0; Varian Medical System, Palo Alto, CA, USA). Proton beams were generated using a cyclotron (Sumitomo Heavy Industries, Tokyo, Japan), which were then delivered using a wobbling system. The radiation dose was calculated by multiplying the physical dose (gray) with the relative biological effectiveness of protons (1.1). The two most common dose schedules were 60-66 grays in 10 fractions for tumors with at least a distance of 1 cm from the gastrointestinal tract and porta hepatis, and 72.6 grays in 22 fractions for tumors with a distance of at least < 1 cm from the gastrointestinal tract or porta hepatis. The biological equivalent dose (BED) was calculated using an α/β value of 10 grays.
Thirteen patients (45%) received curative intent PBT to cover all the viable HCC tumors, while 16 patients (55%) underwent PBT with palliative control for the main tumor only. Classic radiation-induced liver disease (RILD) was defined as anicteric hepatomegaly and ascites with more than double the upper limit of the normal level of alkaline phosphate, whereas non-classic RILD was defined as more than 3 times the upper limit of the normal level of blood bilirubin or more than 5 times the upper limit of the normal levels of ALT or AST [20].
Statistical analysis and definitions
After the initiation of combination therapy, biochemistry tests, including liver function, renal function, serum alpha-fetoprotein, and complete blood components, were performed every 2-4 weeks. Image evaluation with computed tomography or magnetic resonance were performed every 2-3 months. The last follow-up date was cut off on March 31, 2021. All adverse events (AEs) were registered according to the Common Terminology Criteria for Adverse Events v5.0. Radiology responses were defined as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) by a radiologist according to the Modified Response Evaluation Criteria in Solid Tumors [21]. Progression-free survival (PFS) was calculated from the first day of ICI therapy to disease progression or death. Overall survival (OS) was defined from the initiation of ICI therapy to death. Time to progression (TTP) referred to the period of treatment initiation to radiology progression. Duration of response (DOR) was defined as the period of the first image response to radiology PD among the responders (CR and PR). Statistical analysis was performed using the Chi-square or Fisher exact test for comparisons of categorical variables. PFS, TTP, OS, and DOR were estimated using the Kaplan-Meier method, and a log-rank test was used to compare differences between variables between the subgroups. Predictive factors of PFS and ORR were determined using a Cox-Regression and Logistic regression model. A P value of less than 0.05 was considered statistically significant. SPSS software (release Version 22.0, IBM Corp., Armonk, NY, USA) was used for all analyses.
Results
Patient data
Baseline patient characteristics are presented in Table 1. At baseline, all patients had ECOG PS of 0-1. Child-Pugh scores of 5 and 6 were in 79% and 21% of patients, respectively. Most patients (90%) had BCLC stage C with 59% of macroscopic vascular invasion (MVI) and extrahepatic spread (EHS) at baseline for both. Additionally, 38% of the patients underwent systemic treatment and 52% of the patients underwent locoregional treatment previously. Seventeen patients (59%) received anti-PD1 monotherapy with PBT treatment. Sixteen patients (55%) showed radiologic PD events. Seven patients (24%) died during the follow-up period.
Table 1.
Baseline characteristics of the advanced hepatocellular carcinoma patients who received immune checkpoint inhibitor plus proton beam radiotherapy
| Patient characteristics | Overall N = 29 | PBT curative purpose N = 13 | PBT palliative control N = 16 |
|---|---|---|---|
| Age (years) | 61.0 ± 10.0 | 60.0 ± 10.3 | 61.5 ± 9.9 |
| > 60 y/o, n (%) | 18 (62.1) | 7 (53.8) | 11 (68.8) |
| Male, n (%) | 20 (69.0) | 9 (69.2) | 11 (68.8) |
| ECOG PS, n (%) | |||
| 0 | 16 (55.2) | 8 (61.5) | 8 (50.0) |
| 1 | 13 (44.8) | 5 (38.5) | 8 (50.0) |
| Child-Pugh score, n (%) | |||
| A5 | 23 (79.3) | 11 (84.6) | 12 (75.0) |
| A6 | 6 (20.7) | 2 (15.4) | 4 (25.0) |
| ALBI, n (%) | |||
| Grade 1 | 17 (58.6) | 9 (69.2) | 8 (50.0) |
| Grade 2 | 12 (41.4) | 4 (30.8) | 8 (50.0) |
| BCLC, n (%) | |||
| B | 3 (10.3) | 2 (15.4) | 1 (6.3) |
| C | 26 (89.7) | 11 (84.6) | 15 (93.8) |
| BMI ≥ 25 kg/m2, n (%) | 9 (31.0) | 6 (46.2) | 3 (18.8) |
| AFP ≥ 400 ng/mL, n (%) | 11 (37.9)* | 3 (23.1)* | 8 (50.0) |
| Hepatitis virus, n (%) | |||
| HBsAg positive | 15 (51.7) | 4 (30.8) | 11 (68.8) |
| Anti-HCV positive | 6 (20.7) | 3 (23.1) | 3 (18.8) |
| Alcohol, n (%) | 9 (31.0) | 3 (23.1) | 6 (37.5) |
| Macrovascular invasion, n (%) | 17 (58.6) | 8 (61.5) | 9 (56.3) |
| Extrahepatic metastasis, n (%) | 17 (58.6) | 6 (46.2) | 11 (68.8) |
| Tumor diameter ≥ 5 cm | 19 (65.5) | 7 (53.8) | 12 (75.0) |
| Previous locoregional treatment, n (%) | 15 (51.7) | 9 (69.2) | 6 (37.5) |
| Systemic treatment, n (%) | |||
| First-line | 21 (72.4) | 11 (84.6) | 9 (56.3) |
| ≥ second-line | 8 (38.1) | 2 (15.4) | 7 (43.8) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; ALBI, Albumin-Bilirubin Index; BCLC, Barcelona Clinic Liver Cancer; BMI, Body Mass Index; AFP, Alpha-Fetoprotein; HBsAg, Hepatitis B Surface Antigen; HCV, Hepatitis C Virus; PBT, Proton Beam Radiotherapy.
One patient missing baseline AFP data.
PBT infield tumor control
All patients completed the PBT protocol with a median BED 96.6 gray (range: 43.9-109.6 gray) (Supplementary Table 1). The median follow-up period was 13.2 months after initiation of therapy (range 1.0-48.1). Four patients (14%) had no accessible image after treatment. Six patients (21%) had infield tumor progression during follow-up. The median TTP for the infield tumors was not-reached, with the estimated IFTC rates at 6-, 12-, 18-, and 24-month were 86.4%, 80.4%, 65.1%, and 65.1%, respectively, Figure 1A). Although the curative-intent and palliative-control PBT groups had EHS in 46.2% and 68.8%, the 1-year IFTC rates in the curative and palliative PBT groups were 90.5% and 70.8%, respectively, including the PBT field covering the EHS tumors. Both groups had a long-term infield tumor control with non-reached TTP mediums (Figure 2A). The risk of Infield tumor progression did not associate with AFP > 400 ng/ml (P = 0.446), inclusion of EHS (P = 0.738), lines of systemic treatment in ICI combination (P = 0.518), previous locoregional therapy (P = 0.428), anti-PD1 monotherapy (P = 0.936), or purpose of PBT with palliative (P = 0.381) by univariate analysis. Univariant analysis showed MVI (P = 0.033) and PBT irradiation BED ≥ 96.6 grays (P = 0.037) had lower infield tumor progression risk, but only MVI (HR = 0.102, 95% CI = 0.010-0.981) associated with lower tumor progression in multivariate analysis. The 1-year IFTC rates in patients with MVI or not were 100% vs. 55%.
Figure 1.
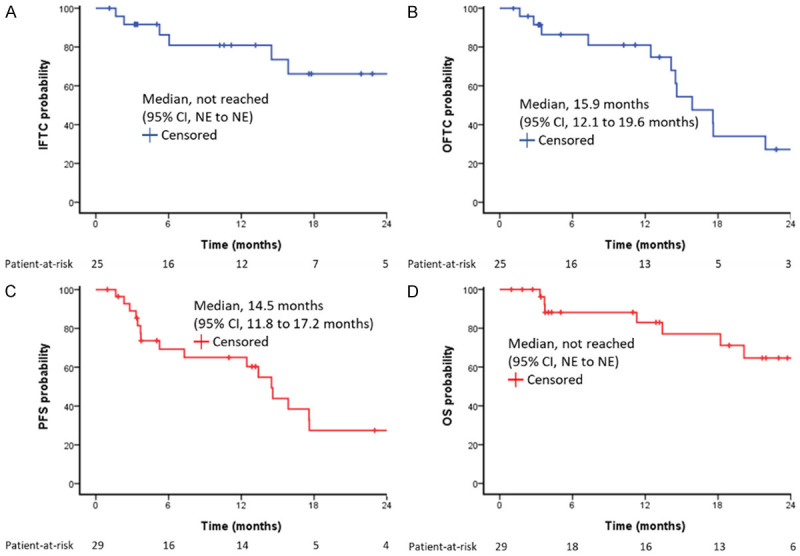
Kaplan-Meier survival function for all patients. (A) IFTC, (B) OFTC, (C) PFS, (D) OS. IFTC, infield tumor control; OFTC, outfield tumor control; PFS, progression-free survival; OS, overall survival; NE, not estimable.
Figure 2.
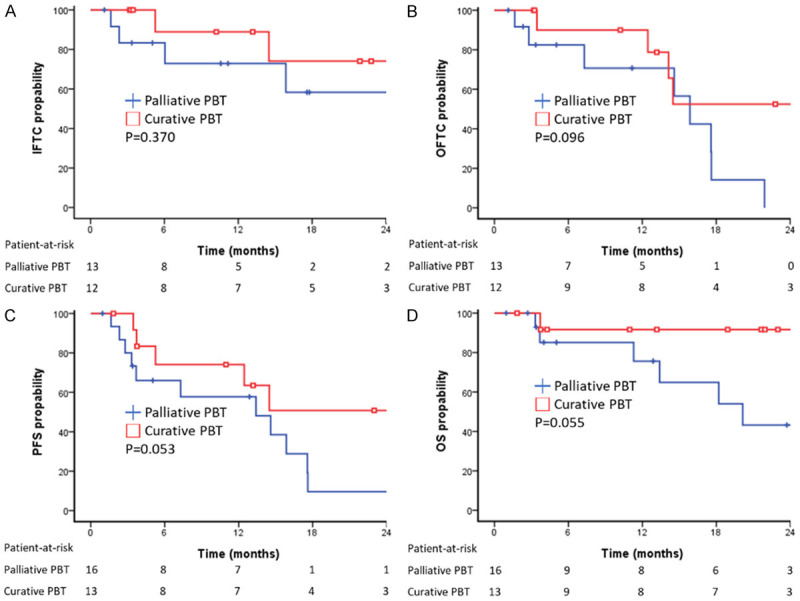
Kaplan-Meier survival function for palliative PBT vs. curative PBT. (A) IFTC, P = 0.370, (B) OFTC, P = 0.096, (C) PFS, P = 0.053, (D) OS, P = 0.055. PBT, proton beam radiotherapy; IFTC, infield tumor control; OFTC, outfield tumor control; PFS, progression-free survival; OS, overall survival; NE, not estimable.
PBT outfield tumor control
The median duration and doses of ICI treatment were 3.9 months (0.6-27.7 months) and 7 doses (2-36 doses), respectively. Outfield tumor progression was observed in 13 patients (45%) during the follow-up period. The median TTP of the tumors in the outfield was 15.9 months for all patients (95% CI, 12.1 to 19.6 months) with the estimated OFTC rates at 6-, 12-, 18-, and 24-month were 86.4%, 80.6%, 35.5% and 27.6%, respectively (Figure 1B). The outfield median TTP and the 1-year OFTC rate were 27.2 months and 90.9% in curative PBT, and 15.9 months and 69.2% in palliative PBT (P = 0.096). The risk of the outfield tumor progression was not associated with the factors of AFP > 400 ng/ml (P = 0.759), tumor number > 3 (P = 0.757), bilateral involved (P = 0.567), anti-PD1 monotherapy (P = 0.439), palliative PBT (P = 0.109). Univariate analysis showed tumor size > 5 cm increased higher risk of outfield tumor progression (P = 0.023), but PBT irradiation BED ≥ 96.6 grays (P = 0.012), systemic line 1 of ICI (P = 0.040) and MVI (P = 0.052) associated with lower risk of outfield tumor progression. Multivariate analysis showed the independent factors associated with outfield tumor progression were main tumor size > 5 cm (HR = 4.957, 95% CI = 1.191-20.639) and MVI (HR = 0.140, 95% CI = 0.028-0.694).
Overall outcomes
ORR and survival outcomes are summarized in Table 2. As the best response by modified RECIST criteria, CR, PR, SD, and PD were respectively found in 5 (17%), 10 (35%), 6 (21%), and 4 (14%) patients. The ORR was 51.7% and the disease control rate was 72.4%. The median DOR was 14.1 months (95% CI, 9.1 to 19.1 months) for the 15 responders (Supplementary Figure 2). The median overall PFS was 14.5 months (95% CI, 11.8 to 17.2 months) for all patients (Figure 1C). The cumulative overall PFS rates at 6, 12, 18, and 24 months were 69.8%, 65.3%, 30.1%, and 30.1%, respectively. The median OS was not achieved (Figure 1C). Cumulative OS rates at 6, 12, 18, and 24 months were 88.0%, 83.0%, 77.4%, and 62.7%, respectively. Curative-intent PBT had a trend of better ORR, medium overall PFS, and medium OS than those of the patients received palliative-control PBT (ORR: 61.5% vs. 43.8%, P = 0.340; medium overall PFS: 27.2 months vs. 15.9 months, P = 0.118; medium OS: not reached vs. 20.1 months, P 0.052, Figure 2C and 2D). ICI withdrawal reasons and subsequent anti-tumor therapy are summarized in Supplementary Tables 2 and 3. Among the five CR patients, four patients survived until the last follow-up (DOR: 15.1 to 45.8 months); two of these patients maintained their CR status to the last follow-up and two showed only small intrahepatic HCC recurrences, whereas one patient died due to liver failure under CR status.
Table 2.
Therapeutic outcomes after immune checkpoint inhibitor plus proton beam radiotherapy
| Therapeutic outcomes | Overall N = 29 | PBT curative purpose N = 13 | PBT palliative control N = 16 | P value |
|---|---|---|---|---|
| ORR, n (%) | 15 (51.7) | 8 (61.5) | 7 (43.8) | 0.340 |
| Best overall response, n (%) | ||||
| CR | 5 (17.2) | 4 (30.8) | 1 (6.3) | |
| PR | 10 (34.5) | 4 (30.8) | 6 (37.5) | |
| SD | 6 (20.7) | 3 (23.1) | 3 (18.8) | |
| PD | 4 (13.8) | 1 (7.7) | 3 (18.8) | |
| Not accessible | 4 (13.8) | 1 (7.7) | 3 (18.8) | |
| DCR, n (%) | 21 (72.4) | 11 (84.6) | 10 (62.5) | 0.238 |
| DOR for responders, months | ||||
| median | 14.1 | 25.0 | 10.7 | 0.054 |
| 95% CI | 9.1 to 19.1 | NE to 56.6 | 0.5 to 21.0 | |
| DOR ≥ 6 months, n | 10 | 6 | ||
| PFS, months | ||||
| Median | 14.5 | 27.2 | 13.4 | 0.053 |
| 95% CI | 11.8 to 17.2 | 10.0 to 44.5 | 2.9 to 23.9 | |
| Patient with events | 18 (62.1) | 6 (46.2) | 12 (75.0) | |
| PD | 16 (55.2) | 6 (46.2) | 10 (62.5) | |
| Death | 2 (6.9) | 1 (7.7) | 1 (6.3) | |
| PFS rate, % | ||||
| 6 months | 69.8 | 75.0 | 65.5 | |
| 12 months | 65.3 | 75.0 | 57.3 | |
| 18 months | 30.1 | 51.9 | 13.2 | |
| 24 months | 30.1 | 51.9 | 13.2 | |
| Infield TTP, months | ||||
| Median | NR | NR | NR | 0.370 |
| 95% CI | NE to NE | NE to NE | NE to NE | |
| IFTC, % | ||||
| 6 months | 86.4 | 90.5 | 82.6 | |
| 12 months | 80.4 | 90.5 | 70.8 | |
| 18 months | 65.1 | 76.6 | 53.1 | |
| 24 months | 65.1 | 76.6 | 53.1 | |
| Outfield TTP, months | ||||
| Median | 15.9 | 27.2 | 15.9 | 0.096 |
| 95% CI | 12.1 to 19.6 | 11.7 to 42.7 | 12.7 to 19.0 | |
| OFTC, % | ||||
| 6 months | 86.4 | 90.9 | 81.8 | |
| 12 months | 80.6 | 90.9 | 69.2 | |
| 18 months | 35.5 | 54.6 | 13.9 | |
| 24 months | 27.6 | 54.6 | 0 | |
| OS, months | ||||
| Median | NR | NR | 20.1 | 0.055 |
| 95% CI | NE to NE | NE to NE | 10.5 to 29.8 | |
| Death | 7 (24.1) | 1 (7.7) | 6 (37.5) | |
| OS rate, % | ||||
| 6 months | 88.0 | 91.3 | 85.2 | |
| 12 months | 83.0 | 91.3 | 75.7 | |
| 18 months | 77.4 | 91.3 | 65.6 | |
| 24 months | 62.7 | 91.3 | 41.8 |
Abbreviations: ORR, Objective Response Rate; CR, Complete Response; PR, Partial Response; SD, Stable Disease; PD, Progressive Disease; DCR, Disease Control Rate; DOR, Duration of Response; PFS, Progression-free Survival; TTP, Time-to-progression; IFTC, Infield Tumor Control; OFTC, Outfield Tumor Control; OS, Overall Survival; PBT, Proton Beam Radiotherapy; NE, Not Estimable; NR, Not Reached.
Table 3 showed the univariate and multivariate analyses of the independent factors for PFS. Only the MVI associated with better PFS (HR = 0.158, 95% CI = 0.038-0.658). ORR of the patients with MVI and non-MVI were 64.7% and 33.3% (P = 0.096). Overall PFS was significantly better in the patients with MVI than non-MVI (17.6 months vs. 7.3 months, P = 0.046). Figure 3 showed a 62-year-old male having a 9.2-cm intrahepatic HCC in the right lobe of liver with the right main portal vein thrombosis and lung metastasis. The patient received PBT with a total radiation dose of 72.6 grays in 22 fractions plus nivolumab monotherapy. Infield tumor total necrosis of and lung metastasis significantly reducing the tumor size were observed in the 6th month follow-up image. Finally, at the 18th month follow-up image showed overall tumors complete response.
Table 3.
Predictors of progression-free survivals in patients who received immune checkpoint inhibitor plus proton beam radiotherapy
| Variable | Number | Median PFS (95% CI) (months) | Crude HR (95% CI) | P value | Adjust HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| < 60 | 11 | 14.5 (2.8 to 26.2) | Reference | 0.588 | ||
| ≥ 60 | 18 | 14.6 (7.1 to 22.1) | 0.771 (0.300-1.980) | |||
| ECOG PS | ||||||
| 0 | 16 | 14.5 (12.7 to 16.3) | Reference | 0.334 | ||
| 1 | 13 | 25.2 (NE to 67.2) | 0.589 (0.201-1.723) | |||
| ALBI grade | ||||||
| 1 | 17 | 15.9 (11.2 to 20.5) | Reference | 0.127 | ||
| 2 | 12 | 3.7 (NE to 7.5) | 2.074 (0.813-5.290) | |||
| AFP* | ||||||
| < 400 | 17 | 14.5 (9.0 to 20.0) | Reference | 0.788 | ||
| ≥ 400 | 11 | 14.6 (2.8 to 26.4) | 1.150 (0.416-3.180) | |||
| Macrovascular invasion | ||||||
| No | 12 | 7.3 (1.6 to 12.9) | Reference | 0.054 | Reference | 0.011 |
| Yes | 17 | 17.6 (12.8 to 22.3) | 0.366 (0.132-1.016) | 0.158 (0.038-0.658) | ||
| Extrahepatic spreading | ||||||
| No | 12 | 12.5 (2.0 to 23) | Reference | 0.296 | ||
| Yes | 17 | 15.9 (11.3 to 20.4) | 0.608 (0.239-1.545) | |||
| Tumor size | ||||||
| < 5 cm | 10 | 25.2 (6.5 to 43.9) | Reference | 0.143 | ||
| ≥ 5 cm | 19 | 13.4 (10.5 to 16.3) | 2.119 (0.776-5.785) | |||
| PBT BED | ||||||
| < 96.6 | 8 | 7.3 (NE to 15.4) | Reference | 0.079 | Reference | 0.431 |
| ≥ 96.6 | 21 | 14.6 (9.6 to 19.6) | 0.370 (0.122-1.123) | 0.431 (0.388-9.186) | ||
| PBT goal | ||||||
| Curative-intent | 13 | 27.2 (10.0 to 44.5) | Reference | 0.062 | Reference | 0.069 |
| Palliative-intent | 16 | 13.4 (2.9 to 23.9) | 2.731 (0.949-7.859) | 3.837 (0.899-16.377) | ||
| Systemic line | ||||||
| 1st line | 21 | 14.6 (9.7 to 19.5) | Reference | 0.057 | Reference | 0.147 |
| ≥ 2nd line | 8 | 7.3 (NE to 18.8) | 2.693 (0.970-7.476) | 2.602 (0.715-9.469) | ||
| ICIs regiments | ||||||
| Single-agent | 17 | 14.6 (3.2 to 26.0) | Reference | 0.725 | ||
| Combo-agents | 12 | 13.4 (9.6 to 17.3) | 0.838 (0.312-2.246) |
Abbreviations: PFS, Progression Free Survival; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ALBI, Albumin-Bilirubin Index; AFP, Alpha-fetoprotein; PBT, Proton Beam Radiotherapy; BED, Biological Effective Dose; ICI, Immune Check Point Inhibitor; NE, Not Estimable; NR, Not Reached.
One patient missing baseline AFP data.
Figure 3.
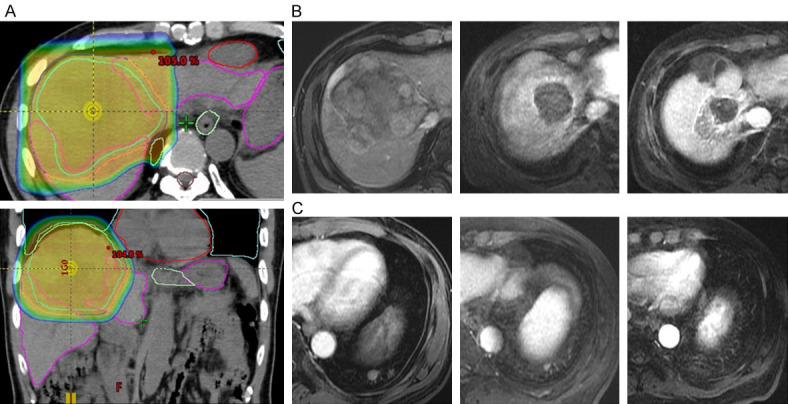
An example case having a 9.2-cm right lobe HCC with the right main portal vein thrombosis and lung metastasis received PBT with a total radiation dose of 72.6 grays in 22 fractions plus nivolumab monotherapy. (A) PBT dose distribution, (B) Pre-treat, 6th, and 18th month image for PBT infield tumor control in right lobe HCC, (C) Pre-treat, 6th, and 18th month image for PBT outfield tumor control in lung metastasis. HCC, hepatocellular carcinoma; PBT, proton beam radiotherapy.
Complications
Twenty-eight patients (97%) experienced at least one AE. The most frequent AEs of any grade were dermatitis (83%), anemia (52%), anorexia or nausea (48%), and thrombocytopenia (34%). Severe (equal or higher than Grade 3) AEs occurred in 9 patients (31%) (Grade 3, 28% [n = 8]; Grade 4, 7% [n = 2]; Grade 5, 10% [n = 3]) (Table 4). Dermatitis induced by immune therapy or radiation burns was self-limiting or well-controlled with topical agents. Only one patient with Grade 3 bullous pemphigoid received systemic steroid treatment in the hospital. Four patients (14%) suffered from severe gastrointestinal bleeding, including 2 were variceal bleeding during ICI plus bevacizumab, and 2 were peptic ulcers potentially PBT-related. All were Grade 3 and successfully controlled after endoscopic hemostasis therapy. Three patients suffered a severe liver injury with Grade 3 or Grade 4 of AST, ALT, or total bilirubin elevation. One of them subsided after steroid therapy and was considered immune-related. The other two were probably PBT-related due to biliary stricture or no response to steroids. The risk of the AE higher or equal to Grade 3 was the same (31%) in the patients receiving PBT with curative or palliative purposes. But the patients who received combo-agent ICI probably had a higher risk of severe AE (≥ Grade 3) than those who received anti-PD1 monotherapy (42% vs. 24%).
Table 4.
Treatment-related adverse events
| Adverse events | Any grade | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| Dermatitis | 24 (83) | 1 (3) | 0 | 0 |
| Anemia | 15 (52) | 1 (3) | 0 | 0 |
| Anorexia or nausea | 14 (48) | 0 | 0 | 0 |
| Thrombocytopenia | 9 (31) | 1 (3) | 0 | 0 |
| Aspartate aminotransferase increased | 7 (24) | 3 (10) | 0 | 0 |
| Leukopenia/Neutropenia | 7 (24) | 1 (3) | 0 | 0 |
| Alanine aminotransferase increased | 6 (21) | 2 (7) | 0 | 0 |
| Gastrointestinal bleeding | 5 (17) | 4 (14) | 0 | 0 |
| Blood bilirubin increased | 5 (17) | 1 (3) | 1 (3) | 0 |
| Fatigue | 5 (17) | 0 | 0 | 0 |
| Pneumonitis | 4 (14) | 0 | 0 | 0 |
| Diarrhea | 3 (10) | 0 | 0 | 0 |
| Hepatic failure | 2 (7) | 0 | 0 | 2 (7) |
| Alkaline phosphatase increased | 2 (7) | 0 | 0 | 0 |
| Duodenal perforation | 1 (3) | 0 | 0 | 1 (3) |
| Biliary stricture | 1 (3) | 0 | 1 (3) | 0 |
| Biliary tract infection | 1 (3) | 1 (3) | 0 | 0 |
| Lung infection | 1 (3) | 1 (3) | 0 | 0 |
| Skin infection | 1 (3) | 0 | 0 | 0 |
Post-PBT ALBI grade and Child-Pugh score evaluations at 3-month and 6-month after initiation of PBT therapy are presented in Figure 4. Overall, no statistically significant change was observed (ALBI grade: pre-treat vs. 3-month, P = 0.261, pre-treat vs. 6-month P = 0.563; Child-Pugh score: pre-treat vs. 3-month, P = 0.162, pre-treat vs. 6-month P = 0.321). No classic RILD occurred in our study. Two patients (6.9%) suffered from non-classic RILD. One patient with a large liver tumor with portal vein thrombosis and portal hypertension received extensive PBT irradiation and died of liver failure under CR status. The other patient had hepatobiliary toxicity leading to liver failure due to a history of photon radiotherapy. The 3 grade 5 AE (10%) were considered PBT-related, including the 2 non-classic RILD and one duodenal perforation.
Figure 4.
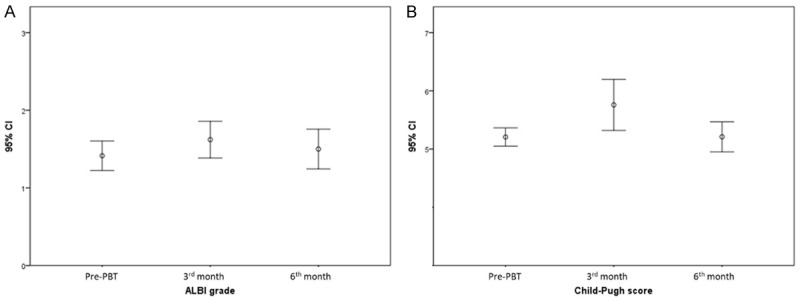
Error bars for post-PBT liver function evaluation. (A) Pre-PBT, 3rd, and 6th month ALBI grades, (B) Pre-PBT, 3rd, and 6th month Child-Pugh scores. PBT, proton beam radiotherapy; ALBI, albumin-bilirubin index.
Discussion
Through historical comparison with the pivotal phase 3 trials (IMbrave 150, SHARP, and REFLECT) [2,5,22], concurrent PBT and anti-PD1/PDL1 may improve the unmet need for systemic treatment of advanced HCC. The current recommendation for advanced HCC is atezolizumab plus bevacizumab for the firstline therapy [23]. Sorafenib or lenvatinib is an alternative. However, the current unmet need in the guidelines is a relatively low ORR up to 27% and a short overall PFS of 6.8 months. In this small cohort study, we included the patients with similar clinical baseline data to the pivotal phases 3 studies. In addition, we enrolled 38% of patients who experienced systemic therapy failure. The concurrent PBT plus anti-PD1/PDL1 can achieve 51.7% ORR, 17.2% CR rate, and overall PFS to 14.5 months. Moreover, under curative-intent PBT, ORR can increase to 61.5%, and PFS improve to 27.2 months. The reasons for the combination of PBT with ICI having better results for advanced HCC patients probably resulted from the principal tumor sustained control by PBT irradiation and the excellent systemic anti-tumor effects by ICI therapies.
Although Western guidelines do not recommend EBRT, consensus guidelines in the Asia-Pacific region can consider high-dose radiotherapy with potential curative purposes for locally advanced HCC patients with oligometastatic tumors [12]. Recent studies have shown that high-dose radiotherapy can effectively control the principal tumors locally. In a phase I/II study, 201 patients with locally advanced HCC received a total dose of 24-54 grays by stereotactic body radiotherapy (SBRT) to obtain the 1-year local tumor control rate reaching 87% [7]. PBT can provide higher energy transmission efficiency to the tumor than conventional photon therapy to achieve the anti-tumor ablative dose of the BED dose higher than 100 grays without increasing the RILD [9,10,24]. The local tumor control rate can be maintained at 95% on 2-year after PBT radiotherapy [9]. In our study, we also showed the PBT having an excellent sustained local control ability. Under a median BED dose of 96.6 grays, most tumors in the irradiation field achieved a sustained control with 1-year IFTC up to 80.4% for all patients. In addition, the 1-year IFTC could reach 90.5% for the curative-intent patients despite the inclusion of more than 40% of extrahepatic metastasis at baseline in the irradiation field. This study showed that PBT could effectively control the tumors in the liver or at peripheral oligo-extrahepatic metastasis in the irradiation field.
High-dose radiotherapy alone may not be adequate for advanced HCC due to the lack of systemic anti-tumor effect. New tumor recurrence or enlarging tumor volume outside the irradiation field is the main reason for treatment failure. The outfield tumor progression limits the results of overall PFS and OS [25]. Therefore, concurrent systemic disease control is warranted [7]. TACE has been used to combine with SBRT because the combination therapy is well tolerated and provides an anti-tumor effect for the outfield intrahepatic areas. TACE combined with SBRT has successfully improved ORR, TTP, and OS for advanced HCC [26]. However, this combination therapy only benefited the patients without extrahepatic metastasis. One recent preliminary study reported that anti-PD1/PDL1 could safely use with palliative photon radiotherapy with a dose of up to 60 grays [27]. This combination therapy can achieve a relatively high ORR of 40% in patients with extrahepatic metastasis. In this study, we used PBT instead of SBRT in combination with ICI to treat advanced HCC. We achieved an 80.6% 1-year OFTC rate and 51.7% ORR for all patients. Since PBT irradiation could not cover all tumors in advanced HCC, such as bilateral involvement or distant extrahepatic tumor spread, 16 patients (55%) received only palliative control PBT for the principal tumors. Curative-intent and palliative-control PBT combined with ICI therapy both can obtain 1-year OFTC rate > 60%, ORR > 40%, and PFS > 13 months. These results indicate that anti-PD1/PDL1 ICI therapy has excellent outfield tumor control. The excellent systemic tumor control combined with sustained infield control produced by PBT can contribute to better ORR and overall PFS, and further lead to good OS. In addition, radiotherapy combined with anti-PD1/PDL1 ICI therapy may have a synergistic effect [28]. In our study, the outfield TTP of patients receiving palliative PBT can reach 15.9 months, which is much better than the results of the current systemic treatment of advanced HCC [5]. The excellent systemic anti-tumor effect comes from the synergistic enhancement of the anti-PD1/PDL1 ICI therapy itself and PBT irradiation [29,30].
Numerous preclinical studies have reported the immunologically synergistic effect between radiotherapy and ICI therapy [29]. Radiotherapy induces cell death and exposes tumor-specific antigens to activate tumor-specific T lymphocytes. Radiotherapy also can modulate the tumor microenvironment to facilitate the activated T-lymphocyte recruitment into the tumor. ICI therapy can act as a radiotherapy sensitizer to enhance the anti-tumor effect through the mechanisms by modulating PDL1 expression, regulating cytokine secretion, and remodeling tumor vasculature [29]. As for the proton therapy, an animal study has shown that PBT could induce intra-tumor immune cell infiltration, including CD8 + T cells, CD4 + T cells infiltration, and increased frequency of type 1 tumor associated-macrophage (TAM1) evaluated by flow cytometry and associated with activated transcriptomic antitumor immune response pathways by RNA sequencing analysis [31]. Although there are no data on the combination of proton therapy with immunotherapy in terms of immune cells in the tumor microenvironment, an interesting study explored the effects of high-energy carbon ion therapy in combination with immunotherapy in a murine model of osteosarcoma, especially focused on the abscopal effect. This study had shown that the combination could increase the infiltration of CD8-positive and CD11b-positive cells, especially in the abscopal tumor [32]. Therefore, the combination of proton therapy with immunotherapy for HCC treatment could follow the same phenomenon and is worthy of further exploration.
PBT irradiation combined with anti-PD1/PDL1 ICI therapy did not have unexpected AE. Although almost all patients experienced any-grade AE, most were self-limiting or well controlled with drugs or steroids. One recent preliminary study showed no increase of AE risk in the combination of photon radiotherapy with anti-PD1/PDL1 therapies [33]. In our study, severe hepatitis occurred in 3 patients (10%), immune-related in one and PBT-related in two. The risk of treatment-related hepatitis did not higher than the previous systemic therapies [5,34]. PBT has a limited liver injury risk compared with conventional photon radiotherapy [35]. RILD in our study was only 6.9%, which was comparable with the previous studies [36]. In addition, the liver reserve observed by changes in Child-Pugh scores or ALBI grades were minimal at 3-month and 6-month after PBT initiation. All observations suggested that PBT combined with anti-PD1/PDL1 is safe and tolerable. However, three deaths suggested the combination therapy should be used with caution, especially for a huge intrahepatic tumor with portal hypertension, previous photon experience, or repeated PBT administration. Repeating radiotherapy or unirradiated liver volume too small increases the risk of PBT-related complications [36,37]. Most patients can tolerate the regimens of ICI plus PBT, but caution should be exerted for patients receiving extensive irradiation or with previous radiation experience [36,38,39].
Small patient numbers from the same hospital and retrospective design without a control group for comparison limited the study’s evidence level. As a retrospective study, we cannot collect patients’ tissue or peripheral blood to provide the data supporting the synergistic effect between PBT and ICI therapy. However, we firstly reported that it is safe and effective for PBT irradiation combined with anti-PD1/PDL1 ICI therapies for advanced HCC. The PBT plus ICI therapy improves ORR and overall PFS and may overcome the unmet needs in the current recommendations. In addition, potential selection bias may exist in this small-scale retrospective study. Physicians are inclined to treat advanced HCC patients complicated with MVI by BPT [40]. In this study, the MVI patients had less EHS and small principal tumor size, and more MVI patients received PBT for curative purposes. This bias may lead to a better prognosis of patients with MVI. The heterogeneity of multiple ICI agents used in this retrospective study also limits the evaluation of the ICI dosage factor during combination therapy. However, anti-PD1 monotherapy might provide enough anti-tumor ability from immune cold to immune hot in combination with PBT [41]. Moreover, the anti-PD1 monotherapy probably has the benefits of lower AE risks and financial burden. Our small-scale study supported this possibility.
In conclusion, our retrospective cohort observation suggests that PBT combined with anti-PD1/PDL1 is safe and tolerable without unexpected AEs. The combination therapy provides a high rate and sustained local tumor control in the irradiation field and an excellent systemic therapeutic effect, resulting in overall tumor control and survival. Concurrent PBT and ICI can benefit the advanced HCC with or without extrahepatic tumors in the irradiation field for curative purposes. This combination therapy also provides advantages for PBT with palliative purpose because the unirradiated tumors probably could be effectively treated by anti-PD1/PDL1 by synergistic effect. A further prospective randomized trial is needed to confirm these conclusions.
Acknowledgements
The authors thank the HCC case manager Chingting Wang, Hsiuying Chai, and all members of the Cancer Center and Immune-Oncology Center of Chang Gung Memorial Hospital for their invaluable help.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 Investigators. Pembrolizumab As second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 4.Yau T, Park J, Finn R, Cheng AL, Mathurin P, Edeline J, Kudo M, Han KH, Harding J, Merle P. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30:v874–v875. [Google Scholar]
- 5.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 6.Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung DH, Kim KB, Lee DH, Han CJ, Kim J, Park SC, Kim YH. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 7.Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, Sykes J, Sherman M, Knox JJ, Dawson LA. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J. Clin. Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 8.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, Schipper MJ, Feng M. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J. Clin. Oncol. 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, Park JW. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial. J Hepatol. 2021;74:603–612. doi: 10.1016/j.jhep.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Goyal L, Murphy JE, Javle MM, Wolfgang JA, Drapek LC, Arellano RS, Mamon HJ, Mullen JT, Yoon SS, Tanabe KK, Ferrone CR, Ryan DP, DeLaney TF, Crane CH, Zhu AX. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2016;34:460–468. doi: 10.1200/JCO.2015.64.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Krishnan S, Zhang X, Dong L, Briere T, Crane CH, Martel M, Gillin M, Mohan R, Beddar S. Proton radiotherapy for liver tumors: dosimetric advantages over photon plans. Med Dosim. 2008;33:259–267. doi: 10.1016/j.meddos.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Hsu C, Chen BB, Chen CH, Ho MC, Cheng JC, Kokudo N, Murakami T, Yeo W, Seong J, Jia JD, Han KH, Cheng AL. Consensus development from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014) Liver Cancer. 2015;4:96–105. doi: 10.1159/000367732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 14.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M KEYNOTE-224 Investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 16.Keenan B, Griffith MJ, Bauer K, Bracci PM, Behr S, Umetsu SE, Gordan JD, Ngo Z, Iqbal S, Hanna DL, Venook AP, El-Khoueiry AB, Fong L, Kelley RK. Phase II multicenter pilot study of safety, efficacy, and immune cell profiling in advanced hepatocellular carcinoma (HCC) on combination of sorafenib (SOR) plus nivolumab (NIVO) J. Clin. Oncol. 2019;37:TPS464–TPS464. [Google Scholar]
- 17.Kudo M, Ikeda M, Motomura K, Okusaka T, Kato N, Dutcus CE, Hisai T, Suzuki M, Ikezawa H, Iwata T, Kumada H, Kobayashi M. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): study 117. J. Clin. Oncol. 2020;38:513–513. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee M, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, Hack S, Spahn J, Liu B, Abdullah H, He R, Lee KH. Randomised efficacy and safety results for atezolizumab (Atezo) + bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC) Ann Oncol. 2019;30:v875. [Google Scholar]
- 20.Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, Ten Haken RK. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(Suppl 4):S94–100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 23.Vogel A, Martinelli E ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org; ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32:801–805. doi: 10.1016/j.annonc.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117:3053–3059. doi: 10.1002/cncr.25809. [DOI] [PubMed] [Google Scholar]
- 25.Lee HL, Tsai JT, Chen CY, Lin YC, Ho CB, Ting LL, Kuo CC, Lai IC, Lin CY, Tang JH, Huang YM, Kao WY, Cheng SW, Shen CN, Chen SW, Chiou JF. Effectiveness of stereotactic ablative radiotherapy in patients with advanced hepatocellular carcinoma unsuitable for transarterial chemoembolization. Ther Adv Med Oncol. 2019;11:1758835919889002. doi: 10.1177/1758835919889002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, Lee HC, Lim YS. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong L, Wu D, Peng W, Sheng H, Xiao Y, Zhang X, Wang Y. Safety of PD-1/PD-L1 inhibitors combined with palliative radiotherapy and anti-angiogenic therapy in advanced hepatocellular carcinoma. Front Oncol. 2021;11:686621. doi: 10.3389/fonc.2021.686621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HJ Jr, Zeng J, Rengan R. Proton beam therapy and immunotherapy: an emerging partnership for immune activation in non-small cell lung cancer. Transl Lung Cancer Res. 2018;7:180–188. doi: 10.21037/tlcr.2018.03.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W, Lin SH. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol. 2018;9:185. doi: 10.3389/fphar.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: a potential synergistic treatment strategy. Front Oncol. 2019;9:1157. doi: 10.3389/fonc.2019.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirjolet C, Nicol A, Limagne E, Mura C, Richard C, Morgand V, Rousseau M, Boidot R, Ghiringhelli F, Noel G, Burckel H. Impact of proton therapy on antitumor immune response. Sci Rep. 2021;11:13444. doi: 10.1038/s41598-021-92942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helm A, Tinganelli W, Simoniello P, Kurosawa F, Fournier C, Shimokawa T, Durante M. Reduction of lung metastases in a mouse osteosarcoma model treated with carbon ions and immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys. 2021;109:594–602. doi: 10.1016/j.ijrobp.2020.09.041. [DOI] [PubMed] [Google Scholar]
- 33.Zhong L, Wu D, Peng W, Sheng H, Xiao Y, Zhang X, Wang Y. Safety of PD-1/PD-L1 inhibitors combined with palliative radiotherapy and anti-angiogenic therapy in advanced hepatocellular carcinoma. Front Oncol. 2021;11:686621–686621. doi: 10.3389/fonc.2021.686621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng JY, Liu CM, Wang YM, Hsu HC, Huang EY, Huang TT, Lee CH, Hung SP, Huang BS. Proton versus photon radiotherapy for primary hepatocellular carcinoma: a propensity-matched analysis. Radiat Oncol. 2020;15:159. doi: 10.1186/s13014-020-01605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh CE, Venkatesulu BP, Lee CH, Hung SP, Wong PF, Aithala SP, Kim BK, Rao A, Tung-Chieh Chang J, Tsang NM, Wang CC, Lee CC, Lin CC, Tseng JH, Chou WC, Wang YC, Krishnan S, Hong JH. Predictors of radiation-induced liver disease in Eastern and Western patients with hepatocellular carcinoma undergoing proton beam therapy. Int J Radiat Oncol Biol Phys. 2019;105:73–86. doi: 10.1016/j.ijrobp.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Chen SW, Fan CC, Ting LL, Kuo CC, Chiou JF. Clinical parameters for predicting radiation-induced liver disease after intrahepatic reirradiation for hepatocellular carcinoma. Radiat Oncol. 2016;11:89. doi: 10.1186/s13014-016-0663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo GS, Yu JI, Park HC, Hyun D, Jeong WK, Lim HY, Choi MS, Ha SY. Do biliary complications after proton beam therapy for perihilar hepatocellular carcinoma matter? Cancers. 2020;12:2395. doi: 10.3390/cancers12092395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shadad AK, Sullivan FJ, Martin JD, Egan LJ. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185–198. doi: 10.3748/wjg.v19.i2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Im JH, Yoon SM, Park HC, Kim JH, Yu JI, Kim TH, Kim JW, Nam TK, Kim K, Jang HS, Kim JH, Kim MS, Yoon WS, Jung I, Seong J. Radiotherapeutic strategies for hepatocellular carcinoma with portal vein tumour thrombosis in a hepatitis B endemic area. Liver Int. 2017;37:90–100. doi: 10.1111/liv.13191. [DOI] [PubMed] [Google Scholar]
- 41.Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in immunotherapy. Trends Cancer. 2016;2:286–294. doi: 10.1016/j.trecan.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


