Abstract
Immune checkpoint inhibitors (ICIs) with atezolizumab plus bevacizumab are promising agents for unresectable hepatocellular carcinoma (HCC). We tried to guide the treatment based on recent developed CRAFITY score combining with on-treatment AFP response. Eighty-nine patients who received atezolizumab plus bevacizumab regardless of as a first-line therapy or not for unresectable HCC were enrolled for analyses. Radiologic evaluation was based on modified Response Evaluation Criteria in Solid Tumors (mRECIST). The objective response rate (ORR) and disease control rate (DCR) were 25.0% and 65.5%, respectively. Multivariate analysis showed that low CRAFITY score (AFP<100 ng/ml or CRP<10 mg/l) and satisfactory AFP response at 6 weeks (≥75% decrease or ≤10% increase from baseline) were independent factors determining good overall survival (OS) (hazard ratio [HR]=0.143, P=0.002 & HR=0.337, P=0.031), progression-free survival (PFS) (HR=0.419, P=0.022 & HR=0.429, P=0.025) and good responder (odds ratio [OR]=1.763, P=0.044 & OR=3.881, P=0.011). Patients were further divided into three classes by combination of CRAFITY score and AFP response at 6 weeks [The CAR (CRAFITY score and AFP-Response) classification)]: low CRAFITY score with satisfactory AFP response at 6 weeks (class I), either high CRAFITY score or unsatisfactory AFP response at 6 weeks (class II) and high CRAFITY score together with unsatisfactory AFP response at 6 weeks (class III). ORR was 35.0%, 18.2%, and 0% in class I, II and III patients, respectively (overall P=0.034). Patients in the class I had the best OS and PFS, followed by class II and class III (median OS: not reached vs. 11.1 vs. 4.3 months, log-rank P<0.001; median PFS: 7.9 vs. 6.6 vs. 2.6 months, log-rank P=0.001). Combination CRAFITY score and AFP response at 6 weeks with AUROC predicts OS and tumor response to be 0.809 and 0.798, respectively, better than either CRAFITY score (0.771 & 0.750) or AFP response at 6 weeks (0.725 & 0.680) alone. In conclusions, the CAR classification which combining CRAFITY score and AFP response at 6 weeks provides a practical guidance for atezolizumab plus bevacizumab therapy in unresectable HCC patients.
Keywords: CAR classification, hepatocellular carcinoma, atezolizumab plus bevacizumab, prognosis
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide [1] that patients are often diagnosed at an advanced stage without the opportunity to receive curative treatments [2,3]. The atezolizumab plus bevacizumab combination therapy was approved in 2020 as the first immune-combined therapy for HCC and demonstrated better overall survival (OS), progression-free survival (PFS) as well as response rate than sorafenib [4,5], that is now replacing the previously recommended therapy and widely used worldwide.
Alpha-fetoprotein (AFP) is widely used not only for the diagnosis of HCC but also as adjunctive diagnostic tests to evaluate treatment effect in several studies. A decline of AFP level after therapy is associated with therapeutic efficacy and prognosis in patients treated with systemic chemotherapy [6-8], antiangiogenic therapy [9] and molecular-targeted therapy [10,11]. Early changes in AFP within 4 weeks is also useful for predicting the efficacy of immune checkpoint inhibitor (ICI) [12-14]. However, the correlation between changes in AFP level at which definite timepoint during treatment and the therapeutic efficacy of atezolizumab plus bevacizumab is still not clear. Recently, data from group A of the phase 1b GO30140 trial had been recently reported by Zhu et al [15] demonstrated that, compared with baseline AFP levels, a decrease in AFP levels of 75% or greater or increases of 10% or less at 6 weeks was significantly associated with OS and response versus those without AFP change from baseline. On the other hand, a new CRAFITY score composed of baseline AFP≥100 ng/ml and C-reactive protein (CRP) ≥1 mg/dl level (0 point: AFP<100 ng/ml and CRP<10 mg/l, 1 point: either AFP≥100 ng/ml or CRP≥10 mg/l, or 2 points: AFP≥100 ng/ml and CRP≥10 mg/l) is developed and validated to predict likelihood of immunotherapy success as well as improved survival in patients with advanced HCC who received ICI [16].
In the present study, we tried to investigate the outcome of atezolizumab plus bevacizumab based on baseline CRAFITY score combining with on-treatment AFP response at 6 weeks after the initiation of therapy to early estimate the prognosis and guide the therapy for unresectable HCC patients.
Patients and methods
Patient recruitment
A total of 116 unresectable HCC patients who received atezolizumab plus bevacizumab as first or beyond a first-line systemic therapy because their tumor burden fulfilled with Barcelona Clinic Liver Cancer (BCLC) stage C or not suitable for locoregional therapy in BCLC stage B from September 2020 to January 2022 were retrospectively enrolled. Patients who received only one cycle due to poor liver function, economic problem, lost to follow-up, had ICI as an adjuvant therapy after curative ablation, and had double malignancies were all excluded. Finally, 89 patients were included for analysis (Supplementary Figure 1). All patients received atezolizumab 1,200 mg and bevacizumab 5-7.5 mg/kg intravenously every 3 weeks according to the Patient Support Program (PSP) in Taiwan. Adverse events (AEs) were evaluated based on the Common Terminology Criteria for Adverse Events version 5.0 (NCI CTCAE; version 5.0).
Diagnosis of hepatocellular carcinoma and follow-up protocol
HCC was diagnosed according to the European Association for the Study of the Liver/European Organization for Research and Treatment of Cancer (EASL/EORTC) diagnostic guidelines [2]. Radiological antitumor responses were evaluated by two independent radiologists according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [17] and modified RECIST (mRECIST) [18]. Objective response rate (ORR) was assessed as complete response (CR) plus partial response (PR) and disease control rate (DCR) was assessed as ORR plus stable disease (SD). Image study with dynamic computerized tomography (CT) or Magnetic Resonance Imaging (MRI) were monitored every 9-12 weeks and serum AFP levels were measured every 3 weeks post therapy. Baseline AFP level was measured within 14 days before initiation of therapy.
Statistical analysis and definitions
Categorical variables between the two groups were calculated by Chi-square. Descriptive data with normal distribution are presented as mean ± standard deviation (SD), otherwise as median (interquartile range, IQR). The independent Student’s test and Mann-Whitney U test were used to assess differences between groups in normal distributed and non-normal distributed groups separately. Stepwise Cox regression models were used to determine the predictors of OS and PFS. Logistic regression models were used to determine the predictors of tumor response. A two-tailed P value <0.05 was considered as statistically significant.
PFS was defined as the time from the date of the first atezolizumab plus bevacizumab administration until radiological disease progression or death, whichever came first. Patients were censored at the date of the last contact or data cutoff for patients who were still alive without radiologically confirmed progression. OS was calculated from the start of atezolizumab plus bevacizumab until the date of death. Survival curves were calculated using the Kaplan-Meier method and compared using log-rank test. Receiver operating characteristic (ROC) curves were plotted with 1-specificity and sensitivity measured along the horizontal and vertical axes, respectively. Statistical analyses were performed using the SAS version 9.4 and SPSS software, version 20.0 (SPSS, Inc., Chicago, IL).
Results
Clinical characteristics of enrolled patients
A total of 89 patients were enrolled for analysis and the baseline characteristics are shown in Table 1. The median age was 61.3 years old (IQR 56.4-67.8) and 75 (84.3%) patients were male gender. The major etiology was hepatitis B or C virus infection (88.9%). Child-Pugh class A liver function at the time of initiation of therapy was observed in 79 patients (88.8%). When regarding to tumor characteristics, the median target tumor size was 8.0 cm (IQR 4.3-11.0) and 85.4% patients was beyond the up-to-seven criteria (seven as the sum of the size of the largest tumor (in cm) and the number of tumors) [19] while BCLC stage B and C was 23 and 66 patients, respectively. Portal vein thrombosis was noted in 45 patients (50.6%) and thirty-five patients (39.3%) had extrahepatic metastasis, with the most common metastatic site being the lungs (n=21, 60.0%), followed by lymph nodes (n=15, 42.9%) and bone (n=10, 28.6%). The median AFP level was 354 ng/mL (IQR 18-3596), and 48.3% of the patients had AFP levels ≥400 ng/mL at baseline. Fifty-three patients (59.6%) underwent esophagogastroduodenoscopy before treatment, and esophageal varices were found in 22 patients (41.5%). Atezolizumab and bevacizumab were administered as 1st-line and beyond 1st-line treatments in 49 (55.1%) and 40 (44.9%) patients, respectively. The median treatment cycles were 4 (IQR 3-6) with median observation duration was 7.2 months.
Table 1.
Baseline characteristics of enrolled patients
| Variables | No. of patients (N=89) | % |
|---|---|---|
| Age (years-old) | 61.3 (IQR 56.4-67.8) | 100 |
| Male gender | 75 | 84.3 |
| BW (kg) | 66.3 (IQR 57.0-74.9) | 100 |
| Etiology | ||
| HBV/HCV/NBNC | 69/10/10 | 77.6/11.2/11.2 |
| ECOG PS 0/1/2 | 35/49/5 | 39.3/55.1/5.6 |
| Child-Pugh A/B | 76/13 | 85.4/14.6 |
| ALBI grade I/II/III* | 34/50/1 | 40.0/58.8/1.2 |
| Portal vein thrombosis | 45 | 50.6 |
| Vp3/4 | 26/16 | 57.8/35.6 |
| Esophageal varices | 22 | 24.7 |
| Extra-hepatic metastasis | 35 | 39.3 |
| AFP (ng/ml) | 354 (IQR 18-3596) | 100 |
| ≥400 | 43 | 48.3 |
| BCLC stage B/C | 23/66 | 25.8/74.2 |
| Tumor size, maximum (cm) | 8.0 (IQR 4.3-11.0) | 100 |
| Beyond up-to-seven | 76 | 85.4 |
| Lines of systemic therapy | ||
| 1/≥2 | 49/40 | 55.1/44.9 |
| Prior resection history | 12 | 13.5 |
| Prior locoregional therapy | 54 | 60.7 |
| TACE/RFA/HAIC/RT | 48/18/4/11 | 88.9/33.3/7.4/20.4 |
| Previous TKI therapy | 33 | 37.1 |
| Sorafenib/Regorafenib/Lenvatinib/cabozantinib | 19/7/14/1 | 57.6/21.2/42.4/3.0 |
| Observation period (months) | 7.2 (IQR 4.0-11.0) | 100 |
| Treatment cycles | 4 (IQR 3-6) | 100 |
| Mortality | 29 | 32.6 |
Abbreviations: AFP, Alpha-fetoprotein; ALBI, Albumin-bilirubin index; BCLC, Barcelona Clinic Liver Cancer; BW, Body weight; ECOG, Eastern Cooperative Oncology Group; HAIC, Hepatic artery chemotherapy infusion; HBV, Hepatitis B virus; HCV, hepatitis C virus; NBNC, Non-hepatitis B and C virus; RFA, Radiofrequency ablation; RT, Radiotherapy; TACE, Transarterial chemoembolization; TKI, Tyrosine kinase inhibitor.
missing data.
Overall therapeutic outcomes of atezolizumab plus bevacizumab
The median OS did not reach the median time during the observation period (Supplementary Figure 2A) while PFS were 5.7 months (Supplementary Figure 2B). The initial radiological therapeutic response to atezolizumab plus bevacizumab was shown in Table 2. Eighty-four patients received initial tumor image assessment results within 12 weeks after initiation of therapy and no patients achieved CR. The overall ORR and DCR were 20.2% (17/84), 65.5% (55/84) and 25.0% (21/84), 65.5% (55/84) when in assessment using RECIST v1.1 and mRECIST, respectively. There was no statistically significant difference in ORR between the first-line group and beyond 1st-line group whether assessment by RECIST (P=0.115) or mRECIST (P=0.058). Twenty-nine (32.6%) patients died and 51 patients (58.6%) had disease progression or died at the clinical data cut-off (March 1, 2022).
Table 2.
Tumor response
| Variables | RECIST v1.1 | mRECIST | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Overall (n=89) | 1st line (n=49) | ≥2nd line (n=40) | P-value | Overall (n=89) | 1st line (n=49) | ≥2nd line (n=40) | P-value | |
| Initial response | ||||||||
| Complete response | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Partial response | 17 (20.2) | 12 (26.7) | 5 (12.8) | 21 (25.0) | 15 (33.3) | 6 (15.4) | ||
| Stable disease | 38 (45.3) | 18 (40.0) | 20 (51.3) | 34 (40.5) | 15 (33.3) | 19 (48.7) | ||
| Progressive disease | 29 (34.5) | 15 (33.3) | 14 (35.9) | 29 (34.5) | 15 (33.3) | 14 (35.9) | ||
| Not evaluable | 5 | 4 | 1 | 5 | 4 | 1 | ||
| Objective response | 17 (20.2) | 12 (26.7) | 5 (12.8) | 0.115 | 21 (25.0) | 15 (33.3) | 6 (15.4) | 0.058 |
| Disease control rate | 55 (65.5) | 30 (66.7) | 25 (64.1) | 0.805 | 55 (65.5) | 30 (66.7) | 25 (64.1) | 0.805 |
The predictor of OS, PFS and tumor response
The factors predicting the OS, PFS and response at first evaluation were examined separately. Univariate analysis showed that ALBI grade, portal vein thrombosis, CRAFITY score and AFP response at 6 weeks were associated with OS whereas CRAFITY score and AFP response at 6 weeks were both associated with PFS and tumor response. Multivariate analysis all showed that CRAFITY score and AFP response at 6 weeks were significant and independent predictor of OS (HR=0.143, 95% CI: 0.042-0.481; P=0.002; HR=0.337, 95% CI: 0.108-0.850; P=0.031, respectively) (Table 3), PFS (HR=0.419, 95% CI: 0.199-0.884; P=0.022; HR=0.429, 95% CI: 0.205-0.898; P=0.025, respectively) (Table 4) and the early tumor response (OR=1.763, 95% CI: 1.387-8.037; P=0.044; OR=3.881, 95% CI: 1.732-20.57; P=0.011, respectively) (Table 5). Overall, patients encountered AFP response at 6 weeks had prolonged OS (median: not reached vs. 13.0 months, log-rank P=0.040), PFS (median: 7.9 vs. 4.3 months, log-rank P=0.016) as well as higher initial tumor response rate compared to those AFP non-responders (37.3 vs. 6.2%, P=0.002). On the other hand, patients with low CRAFIRY score also had prolonged OS (median: not reached vs. 7.2 months, log-rank P=0.001), PFS (median: 7.9 vs. 4.3 months, log-rank P=0.036) as well as better initial tumor response rate with target tumor size shrinkage or decreased tumor numbers (26.7 vs. 15.0%, P=0.049) compared to those high CRAFITY score among 53 patients with complete CRAFITY score.
Table 3.
Cox’s proportional hazards model for predictors of overall survival
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age ≥65 y/o (vs. <65 y/o) | 0.664 | 0.299-1.473 | 0.314 | |||
| Male (vs. female) | 1.313 | 0.460-3.745 | 0.610 | |||
| ECOG 0 (vs. 1/2) | 0.449 | 0.202-1.007 | 0.059 | |||
| Viral infection (vs. others) | 0.669 | 0.257-1.742 | 0.411 | |||
| Child-Pugh grade A (vs. B) | 0.518 | 0.223-1.206 | 0.127 | |||
| ALBI grade I (vs. II/III) | 0.521 | 0.236-0.750 | 0.047 | 0.942 | 0.294-3.016 | 0.920 |
| Portal vein thrombosis (vs. No) | 2.365 | 1.160-4.825 | 0.018 | 1.397 | 0.455-4.286 | 0.559 |
| Extrahepatic metastasis (vs. No) | 1.114 | 0.558-2.225 | 0.759 | |||
| AFP≥400 ng/ml (vs. <400 ng/ml) | 1.445 | 0.724-2.886 | 0.296 | |||
| BCLC stage B (vs. C) | 0.474 | 0.195-1.151 | 0.099 | |||
| Beyond up-to-7 criteria (vs. within) | 1.082 | 0.417-2.808 | 0.872 | |||
| First line (vs. second line or later) | 1.454 | 0.725-2.917 | 0.292 | |||
| CRAFITY score low (vs. high) | 0.144 | 0.046-0.454 | 0.001 | 0.143 | 0.042-0.481 | 0.002 |
| ≥75% AFP decrease or ≤10% AFP increase at 6 weeks (vs. No) | 0.633 | 0.309-0.896 | 0.033 | 0.337 | 0.108-0.850 | 0.031 |
| Prior loco-regional therapy (vs. No) | 0.525 | 0.263-1.047 | 0.067 | |||
| IrAE (vs. No) | 1.184 | 0.560-2.505 | 0.659 | |||
Abbreviations: AFP, Alpha-fetoprotein; ALBI, Albumin-bilirubin index; BCLC, Barcelona Clinic Liver Cancer; CRAFITY, CRP and AFP in Immunotherapy; ECOG, Eastern Cooperative Oncology Group; IrAE, Immunotherapy related adverse events.
Table 4.
Cox’s proportional hazards model for predictors of progression-free survival
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age ≥65 y/o (vs. <65 y/o) | 1.101 | 0.603-2.012 | 0.754 | |||
| Male (vs. female) | 1.024 | 0.498-2.106 | 0.948 | |||
| ECOG 0 (vs. 1/2) | 0.633 | 0.353-1.135 | 0.125 | |||
| Viral infection (vs. others) | 0.903 | 0.385-2.119 | 0.814 | |||
| Child-Pugh grade A (vs. B) | 0.709 | 0.344-1.460 | 0.351 | |||
| ALBI grade I (vs. II/III) | 0.908 | 0.509-1.623 | 0.746 | |||
| Portal vein thrombosis (vs. No) | 1.679 | 0.959-2.938 | 0.070 | |||
| Extrahepatic metastasis (vs. No) | 1.173 | 0.671-2.051 | 0.575 | |||
| AFP≥400 ng/ml (vs. <400 ng/ml) | 1.083 | 0.625-1.879 | 0.776 | |||
| BCLC stage B (vs. C) | 0.651 | 0.334-1.270 | 0.208 | |||
| Beyond up-to-7 criteria (vs. within) | 1.432 | 0.610-3.360 | 0.409 | |||
| First line (vs. second line or later) | 1.012 | 0.584-1.756 | 0.965 | |||
| CRAFITY score low (vs. high) | 0.463 | 0.223-0.959 | 0.038 | 0.419 | 0.199-0.884 | 0.022 |
| ≥75% AFP decrease or ≤10% AFP increase at 6 weeks (vs. No) | 0.525 | 0.298-0.927 | 0.026 | 0.429 | 0.205-0.898 | 0.025 |
| Prior loco-regional therapy (vs. No) | 0.926 | 0.524-1.635 | 0.790 | |||
| IrAE (vs. No) | 0.924 | 0.522-1.633 | 0.784 | |||
Abbreviations: AFP, Alpha-fetoprotein; ALBI, Albumin-bilirubin index; BCLC, Barcelona Clinic Liver Cancer; CRAFITY, CRP and AFP in Immunotherapy; ECOG, Eastern Cooperative Oncology Group; IrAE, Immunotherapy related adverse events.
Table 5.
Logistic regression model for predictors of tumor responder
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age ≥65 y/o (vs. <65 y/o) | 0.407 | 0.146-1.129 | 0.084 | |||
| Male (vs. female) | 0.400 | 0.112-1.432 | 0.159 | |||
| ECOG 0 (vs. 1/2) | 2.050 | 0.754-5.577 | 0.160 | |||
| Viral infection (vs. others) | 2.500 | 0.289-21.60 | 0.405 | |||
| Child-Pugh grade A (vs. B) | 1.792 | 0.360-8.932 | 0.476 | |||
| ALBI grade I (vs. II/III) | 1.423 | 0.522-3.882 | 0.491 | |||
| Portal vein thrombosis (vs. No) | 0.525 | 0.191-1.442 | 0.211 | |||
| Extrahepatic metastasis (vs. No) | 0.712 | 0.252-2.006 | 0.520 | |||
| AFP≥400 ng/ml (vs. <400 ng/ml) | 0.475 | 0.172-1.308 | 0.150 | |||
| BCLC stage B (vs. C) | 2.154 | 0.745-6.231 | 0.157 | |||
| Beyond up-to-7 criteria (vs. within) | 0.618 | 0.166-2.309 | 0.474 | |||
| First line (vs. second line or later) | 2.750 | 0.945-8.002 | 0.063 | |||
| CRAFITY score low (vs. high) | 1.939 | 1.444-8.476 | 0.039 | 1.763 | 1.387-8.037 | 0.044 |
| ≥75% AFP decrease or ≤10% AFP increase at 6 weeks (vs. No) | 9.194 | 1.969-42.93 | 0.005 | 3.881 | 1.732-20.57 | 0.011 |
| Prior loco-regional therapy (vs. No) | 1.581 | 0.583-4.290 | 0.368 | |||
| IrAE (vs. No) | 1.316 | 0.466-3.716 | 0.604 | |||
Abbreviations: AFP, Alpha-fetoprotein; ALBI, Albumin-bilirubin index; BCLC, Barcelona Clinic Liver Cancer; CRAFITY, CRP and AFP in Immunotherapy; ECOG, Eastern Cooperative Oncology Group; IrAE, Immunotherapy related adverse events.
The CAR (CRAFITY score and AFP-Response) classification predicts OS/PFS/tumor response
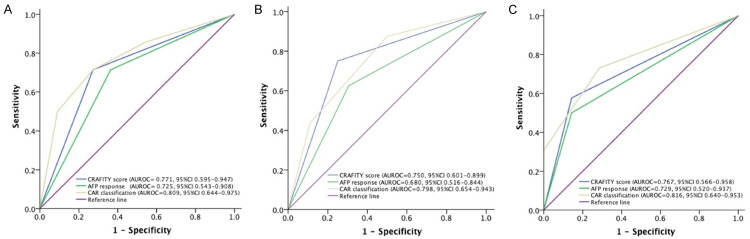
We then combined baseline CRAFITY score and on-treatment AFP response at 6 weeks and defined a novel CAR (CRAFITY score and AFP-Response) classification, which consisted of three classes: low CRAFITY score (AFP<100 ng/ml or CRP<10 mg/l) with satisfactory AFP response at 6 weeks (≥75% decrease or ≤10% increase from baseline) (class I), either high CRAFITY score (AFP≥100 ng/ml and CRP≥10 mg/l) or unsatisfactory AFP response at 6 weeks (<75% decrease or >10% increase from baseline) (class II) and high CRAFITY score and unsatisfactory AFP response at 6 weeks (class III) (Figure 1). Among 53 patients with complete CRAFITY score, there were 20 (37.7%), 22 (41.5%), and 11 (20.8%) patients in class I to III respectively according to the AFP response at 6 weeks (Figure 1). Patients in class I had the best ORR and DCR, followed by class II, and III (35.0% vs. 18.2% vs. 0%; 80.0% vs. 72.7% vs. 36.4%; all P<0.05 between each group) as well as longer median OS compared to those in class II and III (median: not reached vs. 11.1 and 4.3 months, overall log-rank P<0.001, Figure 2A). Similarly, patients in class I also had longer PFS than those in class II and III (median: 7.9 vs. 6.6 and 2.6 months, overall log-rank P=0.001, Figure 2B). Combination CRAFITY score and AFP response at 6 weeks with AUROC predicts OS (Figure 3A), PFS (Figure 3B) and tumor response (Figure 3C) to be 0.809, 0.798 and 0.816, respectively, superior to either using CRAFITY score (0.771, 0.750 and 0.767) or AFP response at 6 weeks (0.725, 0.680 and 0.729) alone. Comparing the predictive value for OS with CRAFITY score and AFP response at 6 weeks, CAR classification had the best sensitivity (76.7% vs. 71.4% vs. 53.8%) and specificity (89.5% vs. 69.2% vs. 66.7%). For predicting PFS, CAR classification had the best sensitivity (71.0% vs. 50.0% vs. 50.0%) and specificity (95.2% vs. 71.4% vs. 75.0%) compared to either CRAFITY score or AFP response at 6 weeks. When regarding to tumor response, CAR classification also had superior sensitivity (100% vs. 72.7% vs. 90.5%) and specificity (62.5% vs. 43.6% vs. 48.4%) to either CRAFITY score and AFP response at 6 weeks alone (Supplementary Table 1).
Figure 1.
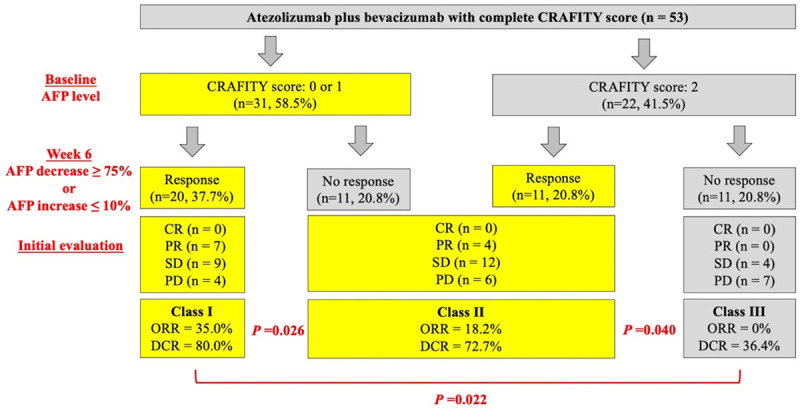
Three classifications by the CAR classification. Class I: low CRAFITY score with satisfactory AFP response at 6 weeks; Class II: either high CRAFITY score or unsatisfactory AFP response at 6 weeks; Class III: neither low CRAFITY score nor satisfactory AFP response at 6 weeks. Abbreviations: AFP, Alpha-fetoprotein; CR, Complete response; PD, Progressive disease; PR, Partial response; SD, Stable disease.
Figure 2.
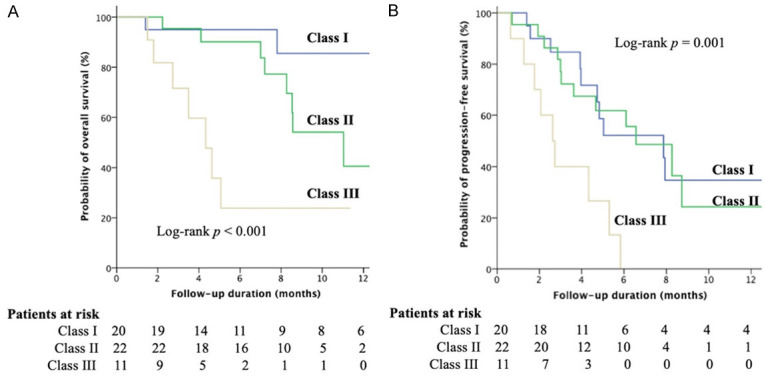
Kaplan-Meier curves. Comparison of (A) overall survival (OS) and (B) progression-free survival (PFS) among the patients stratified by the CAR classification. Patients in class I showed significantly the longest median OS and PFS among overall patients followed by class II and patients in class III had the worst outcome.
Figure 3.
The risk prediction models of comparison among CAR classification, CRAFITY score and AFP response at 6 weeks with ROC curves for predicting (A) overall survival (OS) (B) progression-free survival (PFS) and (C) tumor response Combination CRAFITY score and AFP response at 6 weeks had the best discriminative ability in predicting OS, PFS and tumor response, superior to either using CRAFITY score or AFP response at 6 weeks alone.
Overall safety outcomes
The treatment related adverse events (AEs) are shown in Supplementary Table 2. The overall incident rate of AEs at any grade and over grade 3 were 61.0% (n=54) and 10.0% (n=9), respectively. The most frequent AE at any grade was hypertension (21%), followed by aspartate aminotransferase (AST) elevation (19%) or alanine aminotransferase (ALT) elevation (17%), fatigue (13%), anorexia (12%), proteinuria (10%), and dermatitis (9%) in order. The most frequent AE over grade 3 was upper gastrointestinal bleeding (4%), followed by ALT elevation (2%) and hypertension (2%). Among the gastrointestinal bleeding events, three were variceal bleeding and the rest one was associated with ulcer.
Discussion
To the best of our knowledge, this is the first study not only to validate but also strengthen the CRAFITY score by proposing a novel CAR (CRAFITY score and AFP-Response) classification based on CRAFITY score and on-treatment AFP response at 6 weeks that predicts the outcome of unresectable HCC patients treated with atezolizumab plus bevacizumab. Accordingly, in contrast to patients with a high baseline CRACITY score with unsatisfactory AFP response at 6 weeks, patients with low CRAFITY score together with satisfactory AFP response at 6 weeks had the best tumor response and overall survival rate, even though patients who fulfilled only one criterion such as high CRAFITY score but satisfactory AFP response at 6 weeks still had a better outcome.
Despite the improvements of prognosis under current available ICIs [20-22], less than one-third of patients could achieve an ORR and median survival for patients with advanced-stage HCC remains below two years. In clinical trials, it is common to perform imaging assessments every 6-8 weeks, however, such a high frequency of imaging assessments may be rare in clinical practice. Therefore, one of the most relevant unsolved problems is to identify a treatment response biomarker that can help select patients with a higher probability of response to ICIs. However, there are now still lack of reliable biomarkers to predict the outcome of HCC patients treated with ICI therapy [20-22]. Although programmed cell death ligand 1 (PD-L1) expression in immune or tumor cells has good correlation with tumor response for several other cancers [23,24], the predictive quality of response targeting PD-L1 expression in hepatoma cells is restricted to certain variants [20,25]. In addition to baseline AFP level [26,27], on-treatment early AFP response decline within 4 week is reported to be associated with ORR and survival in patients who received 2 cycles nivolumab treatment administered every 2 weeks [13,14]. Besides, some patients with delayed AFP response beyond 4 weeks were still beneficial from ICI therapy [14].Therefore, it is a good rationale to define early AFP response at 6 weeks in patients who received 2 cycles atezolizumab plus bevacizumab administered every 3 weeks according to recent Japanese study [28]. In addition, Zhu et al [15] further demonstrated that reduction in AFP levels at 6 weeks with a AFP decrease ≥75% or increases ≤10% was significantly associated with OS and response versus those without AFP change from phase 1b trial. We further developed the CAR classification combining the pre-treatment AFP level and on-treatment AFP at 6 weeks to increase the sensitivity and specificity in the selection of patients and support decision-making in daily clinical practice. Patients were subdivided into 3 classes to assist physicians more precisely in identifying patients who are less likely to respond to atezolizumab plus bevacizumab treatment, and these patients may require more frequent imaging assessments or shift to other therapy as early as possible. The CAR classification greatly improved the discriminative ability of OS than using CRAFITY score alone in current study (AUROC: 0.81 vs. 0.77) and original study [16] (AUROC: 0.71). It is simple to use, low medical cost and no need for invasive histologic assessment.
AFP is a glycoprotein expressed and secreted by hepatoma cells in approximately 70% of patients with HCC. Apart from the indicator of high tumor aggressiveness such as vascular invasion, AFP plays an important role in the promoting proliferation of human hepatoma cells including mediated by the binding of AFP receptors (AFPR), leading to the intracellular Ca2+ increases [29], then the intracellular CAMP correspondingly rises dependent on the CAMP-PKA pathway and the activation of PI3K/AKT signal pathway [30], the stimulation of oncogene protein, and the dysfunction of PTEN antioncogene protein. AFP could promote tumor invasion and metastasis via upregulating expression of metastasis related proteins, such as keratin 19 (K19), epithelial cell adhesion molecule (EpCAM), matrix metalloproteinase 2/9 (MMP2/9), and CXC chemokine receptor 4 (CXCR4) [31]. Besides, AFP promoted the expression of FasL and TRAIL in hepatoma cells and Fas and TRAILR in lymphocytes that induced the escape of hepatoma cells from the host’s lymphocytes immune surveillance [32]. AFP also promote the tumor angiogenesis by increasing expression of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor 2 (VEGFR-2) [33]. In summary, AFP promotes the proliferation and metastasis of tumor cells as well as prevents apoptosis and escaping of HCC from immune surveillance. Therefore, it is reasonable for AFP responders to have better outcome than those AFP non-responders.
High CRP levels have been associated with increased cancer risk and also poor clinical outcome in different malignancies including hepatoma [34]. Recently, it has been shown in small studies that high CRP level was associated with a poor outcome when treated with the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blocking antibody tremelimumab for patients with melanoma [35] and PD-1 immune checkpoint blockade in patients with advanced non-small-cell lung cancer [36], yet the role of CRP in modulating the antitumor immune response has not been explored in detail. More recent evidence [37] showed that CRP has a profound suppressive effect on adaptive immunity in patients with melanoma. Up to 40% of CD8 T cells bound and internalized CRP, and high levels of CRP diminished the proliferation of CD4+ and CD8+ T cells from patients with melanoma, and it downregulated dendritic cell (DC) function and altered T-cell and DC phenotypes [38,39]. In non-small cell lung cancer patients, high CRP was associated with PD-L1 positivity [40] that CRP may impair the efficacy of immunotherapy. More recently, tissue samples from the Checkmate040 Trial demonstrated an inflammatory 4-gene signature, including CD274, CD8A, LAG3, and STAT1, was associated with objective responses and OS among patients treated with Nivolumab [41]. This observation was not only highlighted the importance of inflammation in ICI treatment but also point out the possible role of CRP, a marker that strongly correlated with inflammation, in ICI treatment [42]. The recently developed CRAFITY score including baseline AFP and CRP level that provides a good predictor for the outcome of immunotherapy [16] re-emphasized the importance of CRP. In the present study, we combined both CRAFITY score and on-treatment AFP response to create the CAR score. The benefit of this scoring system is to include the inflammation related CRP and tumor-related AFP together with the tumor response (on-treatment AFP response). This reasoning is confirmed by the good predictive ability of CAR score as shown in this study.
However, this study still has several limitations. First, this is a retrospective study with a relative small sample size. Second, most patients (77.5%) were hepatitis B virus infection and the predictive model should be interpreted cautiously when applying to other populations. Third, bevacizumab was given with dosage 5-7.5 mg/kg under patient support program in Taiwan instead of 15 mg/kg followed by the IMbrave150 trial, a dosage at the upper limit of the dosage range used in other cancers. Data from previous trials of bevacizumab indicated that doses of 5 or 10 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks were well tolerated in patients with HCC, while pharmacokinetics (PK) analyses showed that 10 mg/kg every 2 weeks and 15 mg/kg every 3 weeks were comparable with respect to maximum, minimum and mean serum concentrations [43]. In previous phase II studies in HCC, different doses and schedules of bevacizumab showed evidence of activity, although this finding has not been validated in the phase III randomized trial setting [44,45]. In current study, patients treated in the first-line although enrolled patients with Child-Pugh class B still had similar ORR compatible with IMbrave150 trial whether assessed by using RECIST (26.7% vs. 27.3%) or mRECIST (32.6% vs. 33.2%).
In conclusion, the novel CAR (CRAFITY score and AFP-Response) classification which combining CRAFITY score and AFP response at 6 weeks provides a practical guidance for atezolizumab plus bevacizumab therapy in unresectable HCC patients. Patients with low CRAFITY score concomitant with satisfactory AFP response at 6 weeks had the best disease control and survival rate, even though patients who fulfilled only one criterion such as high CRAFITY score but satisfactory AFP response at 6 weeks still had a good outcome. Patients with a high baseline CRACITY score with unsatisfactory AFP response at 6 weeks should shift early to other therapy if feasible. We still need a larger population to validate our recommendation.
Acknowledgements
The authors appreciate Ms. Ka-Ian Lei for her assistance in data acquisition and cleaning. This study was supported by grants from Chang Gung Medical Research Fund (CMRPG3J1341, CORPG3G0871, CORPG3H0641, CORPG3H0651, CORPG3H0661, CORPG3H0671, CMRPVVK0092, CMRPVVL0021), National Science Council, Taiwan (NMRPG3H0471, NMRPG3L0561).
Disclosure of conflict of interest
None.
Abbreviations
- AEs
Adverse events
- AFP
Alpha-fetoprotein
- AST
Aminotransferase
- ALT
Alanine aminotransferase
- BCLC
Barcelona Clinic Liver Cancer
- CR
Complete response
- CRAFITY
CRP and AFP in Immunotherapy
- CRP
C-reactive protein
- CT
Computerized tomography
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- DC
Dendritic cell
- DCR
disease control rate
- EASL/EORTC
European Association for the Study of the Liver/European Organization for Research and Treatment of Cancer
- HCC
Hepatocellular carcinoma
- ICI
Immune checkpoint inhibitor
- IQR
Interquartile range
- MRI
Magnetic Resonance Imaging
- ORR
Objective response rate
- OS
Overall survival
- PD-L1
Programmed cell death ligand 1
- PR
Partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- ROC
Receiver operating characteristic
- SD
Stable disease
Supporting Information
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Vogel A, Martinelli E ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32:801–805. doi: 10.1016/j.annonc.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, Kudo M, Breder V, Merle P, Kaseb A, Li D, Mulla S, Verret W, Xu DZ, Hernandez S, Ding B, Liu J, Huang C, Lim HY, Cheng AL, Ducreux M. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:991–1001. doi: 10.1016/S1470-2045(21)00151-0. [DOI] [PubMed] [Google Scholar]
- 5.Salem R, Li D, Sommer N, Hernandez S, Verret W, Ding B, Lencioni R. Characterization of response to atezolizumab + bevacizumab versus sorafenib for hepatocellular carcinoma: results from the IMbrave150 trial. Cancer Med. 2021;10:5437–5447. doi: 10.1002/cam4.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, Lam KC, Chan AT, Mok TS, Yeo W. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J. Clin. Oncol. 2009;27:446–452. doi: 10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 7.Chen LT, Liu TW, Chao Y, Shiah HS, Chang JY, Juang SH, Chen SC, Chuang TR, Chin YH, Whang-Peng J. Alpha-fetoprotein response predicts survival benefits of thalidomide in advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2005;22:217–226. doi: 10.1111/j.1365-2036.2005.02547.x. [DOI] [PubMed] [Google Scholar]
- 8.Chou WC, Lee CL, Yang TS, Huang CY, Teng W, Tseng YT, Chen JS, Lin YC, Hou MM, Chang HH, Chia-Hsun Hsieh J. Changes in serum alpha-fetoprotein level predicts treatment response and survival in hepatocellular carcinoma patients and literature review. J Formos Med Assoc. 2018;117:153–163. doi: 10.1016/j.jfma.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH, Cheng AL. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010;116:4590–4596. doi: 10.1002/cncr.25257. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Kim BK, Kim SU, Park JY, Kim do Y, Ahn SH, Han KH. Early alpha-fetoprotein response predicts survival in patients with advanced hepatocellular carcinoma treated with sorafenib. J Hepatocell Carcinoma. 2015;2:39–47. doi: 10.2147/JHC.S79353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeki I, Yamasaki T, Yamashita S, Hanazono T, Urata Y, Furutani T, Yokoyama Y, Oishi T, Maeda M, Kimura T, Kotoh Y, Sasaki R, Miyaji T, Oono T, Aibe Y, Hisanaga T, Iwamoto T, Matsumoto T, Hidaka I, Ishikawa T, Takami T, Sakaida I. Early predictors of objective response in patients with hepatocellular carcinoma undergoing lenvatinib treatment. Cancers (Basel) 2020;12:779. doi: 10.3390/cancers12040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao YY, Liu TH, Hsu C, Lu LC, Shen YC, Lin ZZ, Cheng AL, Hsu CH. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019;39:2184–2189. doi: 10.1111/liv.14210. [DOI] [PubMed] [Google Scholar]
- 13.Lee PC, Chao Y, Chen MH, Lan KH, Lee CJ, Lee IC, Chen SC, Hou MC, Huang YH. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers (Basel) 2020;12:182. doi: 10.3390/cancers12010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teng W, Lin CC, Ho MM, Lui KW, Wang SF, Hsu CW, Lin SM. Alpha-fetoprotein response at different time-points is associated with efficacy of nivolumab monotherapy for unresectable hepatocellular carcinoma. Am J Cancer Res. 2021;11:2319–2330. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu AX, Dayyani F, Yen CJ, Ren Z, Bai Y, Meng Z, Pan H, Dillon P, Mhatre SK, Gaillard VE, Hernandez S, Kelley RK, Sangro B. Alpha-fetoprotein as a potential surrogate biomarker for atezolizumab + bevacizumab treatment of hepatocellular carcinoma. Clin Cancer Res. 2022 doi: 10.1158/1078-0432.CCR-21-3275. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, Himmelsbach V, Schulze K, von Felden J, Frundt TW, Stadler M, Heinzl H, Shmanko K, Spahn S, Radu P, Siebenhuner AR, Mertens JC, Rahbari NN, Kutting F, Waldschmidt DT, Ebert MP, Teufel A, De Dosso S, Pinato DJ, Pressiani T, Meischl T, Balcar L, Muller C, Mandorfer M, Reiberger T, Trauner M, Personeni N, Rimassa L, Bitzer M, Trojan J, Weinmann A, Wege H, Dufour JF, Peck-Radosavljevic M, Vogel A, Pinter M. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol. 2021;76:353–363. doi: 10.1016/j.jhep.2021.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 20.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in keynote-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 22.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 23.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feun LG, Li YY, Wu C, Wangpaichitr M, Jones PD, Richman SP, Madrazo B, Kwon D, Garcia-Buitrago M, Martin P, Hosein PJ, Savaraj N. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer. 2019;125:3603–3614. doi: 10.1002/cncr.32339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 27.Snowberger N, Chinnakotla S, Lepe RM, Peattie J, Goldstein R, Klintmalm GB, Davis GL. Alpha fetoprotein, ultrasound, computerized tomography and magnetic resonance imaging for detection of hepatocellular carcinoma in patients with advanced cirrhosis. Aliment Pharmacol Ther. 2007;26:1187–1194. doi: 10.1111/j.1365-2036.2007.03498.x. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa Y, Tsuchiya K, Kurosaki M, Yasui Y, Kaneko S, Tanaka Y, Ishido S, Inada K, Kirino S, Yamashita K, Nobusawa T, Matsumoto H, Kakegawa T, Higuchi M, Takaura K, Tanaka S, Maeyashiki C, Tamaki N, Nakanishi H, Itakura J, Takahashi Y, Asahina Y, Okamoto R, Izumi N. Early experience of atezolizumab plus bevacizumab therapy in Japanese patients with unresectable hepatocellular carcinoma in real-world practice. Invest New Drugs. 2022;40:392–402. doi: 10.1007/s10637-021-01185-4. [DOI] [PubMed] [Google Scholar]
- 29.Li MS, Li PF, He SP, Du GG, Li G. The promoting molecular mechanism of alpha-fetoprotein on the growth of human hepatoma Bel7402 cell line. World J Gastroenterol. 2002;8:469–475. doi: 10.3748/wjg.v8.i3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Li H, Li C, Wang S, Jiang W, Liu Z, Zhou S, Liu X, McNutt MA, Li G. Alpha-fetoprotein: a new member of intracellular signal molecules in regulation of the PI3K/AKT signaling in human hepatoma cell lines. Int J Cancer. 2011;128:524–532. doi: 10.1002/ijc.25373. [DOI] [PubMed] [Google Scholar]
- 31.Zhu M, Guo J, Xia H, Li W, Lu Y, Dong X, Chen Y, Xie X, Fu S, Li M. Alpha-fetoprotein activates AKT/mTOR signaling to promote CXCR4 expression and migration of hepatoma cells. Oncoscience. 2015;2:59–70. doi: 10.18632/oncoscience.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Wang YR, Ding GH, Yang TS, Yao L, Hua J, He ZG, Qian MP. JAK2 inhibitor combined with DC-activated AFP-specific T-cells enhances antitumor function in a Fas/FasL signal-independent pathway. Onco Targets Ther. 2016;9:4425–4433. doi: 10.2147/OTT.S97941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng W, Li X, Bai Z, Li Y, Yuan J, Liu T, Yan J, Zhou W, Zhu K, Zhang H, Li Y. Silencing alpha-fetoprotein inhibits VEGF and MMP-2/9 production in human hepatocellular carcinoma cell. PLoS One. 2014;9:e90660. doi: 10.1371/journal.pone.0090660. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Shin JH, Kim CJ, Jeon EJ, Sung CO, Shin HJ, Choi J, Yu E. Overexpression of C-reactive protein as a poor prognostic marker of resectable hepatocellular carcinomas. J Pathol Transl Med. 2015;49:105–111. doi: 10.4132/jptm.2015.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarhini AA, Cherian J, Moschos SJ, Tawbi HA, Shuai Y, Gooding WE, Sander C, Kirkwood JM. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J. Clin. Oncol. 2012;30:322–328. doi: 10.1200/JCO.2011.37.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oya Y, Yoshida T, Kuroda H, Mikubo M, Kondo C, Shimizu J, Horio Y, Sakao Y, Hida T, Yatabe Y. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget. 2017;8:103117–103128. doi: 10.18632/oncotarget.21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida T, Ichikawa J, Giuroiu I, Laino AS, Hao Y, Krogsgaard M, Vassallo M, Woods DM, Stephen Hodi F, Weber J. C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J Immunother Cancer. 2020;8:e000234. doi: 10.1136/jitc-2019-000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang R, Becnel L, Li M, Chen C, Yao Q. C-reactive protein impairs human CD14+ monocyte-derived dendritic cell differentiation, maturation and function. Eur J Immunol. 2006;36:2993–3006. doi: 10.1002/eji.200635207. [DOI] [PubMed] [Google Scholar]
- 39.Jimenez RV, Wright TT, Jones NR, Wu J, Gibson AW, Szalai AJ. C-reactive protein impairs dendritic cell development, maturation, and function: implications for peripheral tolerance. Front Immunol. 2018;9:372. doi: 10.3389/fimmu.2018.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akamine T, Takada K, Toyokawa G, Kinoshita F, Matsubara T, Kozuma Y, Haratake N, Takamori S, Hirai F, Tagawa T, Okamoto T, Yoneshima Y, Okamoto I, Shimokawa M, Oda Y, Nakanishi Y, Maehara Y. Association of preoperative serum CRP with PD-L1 expression in 508 patients with non-small cell lung cancer: a comprehensive analysis of systemic inflammatory markers. Surg Oncol. 2018;27:88–94. doi: 10.1016/j.suronc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Sangro B, Melero I, Wadhawan S, Finn RS, Abou-Alfa GK, Cheng AL, Yau T, Furuse J, Park JW, Boyd Z, Tang HT, Shen Y, Tschaika M, Neely J, El-Khoueiry A. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol. 2020;73:1460–1469. doi: 10.1016/j.jhep.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pepys MB. C-reactive protein and the acute phase response. Nature. 1982;296:12. doi: 10.1038/296012a0. [DOI] [PubMed] [Google Scholar]
- 43.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF 3rd, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, Kaseb A, Glover K, Davila M, Abbruzzese J. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J. Clin. Oncol. 2009;27:843–850. doi: 10.1200/JCO.2008.18.3301. [DOI] [PubMed] [Google Scholar]
- 45.Kaseb AO, Morris JS, Iwasaki M, Al-Shamsi HO, Raghav KP, Girard L, Cheung S, Nguyen V, Elsayes KM, Xiao L, Abdel-Wahab R, Shalaby AS, Hassan M, Hassabo HM, Wolff RA, Yao JC. Phase II trial of bevacizumab and erlotinib as a second-line therapy for advanced hepatocellular carcinoma. Onco Targets Ther. 2016;9:773–780. doi: 10.2147/OTT.S91977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.