Abstract
Triple-negative breast cancer (TNBC) is well-known as the most aggressive subtype of breast cancer. Because TNBC does not express Her2, estrogen receptor, and progesterone receptors, there had been no effective U.S. Food and Drug Administration-approved targeted therapy for it until PARP inhibitors and two PD-1/PD-L1 monoclonal antibodies were approved for treatment of TNBC. Most recently, an antibody-drug conjugate (ADC), called sacituzumab govitecan (SG), was approved for the treatment of TNBC patients previously received chemotherapy with advanced disease. SG consists of an anti-trophoblast cell-surface antigen 2 (Trop2) antibody conjugated with a topoisomerase I inhibitor, SN-38, which is diffused out of the targeted Trop2 positive cancer cells and induces the bystander killing effect on surrounding cells regardless of their Trop2 expression status. In the Phase III clinical trial, TNBC patients treated with SG showed significantly longer progression-free and overall survival compared to those who were received chemotherapy. In the present review, we summarized the cellular function and signaling of Trop2, the mechanism of action of SG, and the clinical trials of SG that led to its quick approval for TNBC. In addition, we introduced the current ongoing clinical trials of SG as well as another Trop2 ADC, which has potential to overcome some disadvantages of SG.
Keywords: Triple-negative breast cancer, sacituzumab govitecan, Trop2, SN-38, antibody-drug conjugates
Introduction
Antibody-drug conjugates (ADCs) are a category of drugs, possessing (1) a monoclonal antibody, (2) payload, and (3) linker, and have high specificity and affinity for the cell surface proteins recognized by the monoclonal antibody. ADCs bind to a membrane antigen, and then after the antigen internalization, deliver the payload (a toxic agent) into the cancer cell cytoplasm in response to low pH of lysosome [1]. The payload released into the cytoplasm is highly toxic and targets DNA structure, microtubule formation, or protein synthesis of the cancer cells; meanwhile, the payload can also be distributed to the neighboring cancer cells to induce the bystander effect. Although most tumors show heterogeneity and some neighboring cancer cells do not have the target antigen for ADCs, payloads of some ADCs could be distributed to the neighboring antigen-negative cells [2]. However, some ADCs such as ado-trastuzumab emtansine (T-DM1) that is used for human epidermal growth factor receptor 2 (Her2) positive breast cancer have a non-cleavable linker and do not support the bystander effect [3]. Therefore, some ADCs serve as a therapeutic vehicle delivering highly toxic chemotherapy to the tumor microenvironment possessing heterogeneous cancer cells and minimizing off-target drug delivery.
Sacituzumab govitecan (SG) (Trodelvy®) is an ADC targeting trophoblast cell-surface antigen 2 (Trop2) and smart therapy against triple-negative breast cancer (TNBC) with short history from the very beginning to the final approval [4]. Because Trop2 had not been paid much attention as a drug target for a long time, it would be a good example of the development of an effective drug for aggressive cancer with relatively few therapeutic options and has great lessons for cancer researchers who are interested in drug discovery. In the current review, we summarized the cellular function and signaling of Trop2, the clinical results, and the mechanism of action of SG, and then discussed the future direction including the new anti-Trop2 ADC (DS-1062a) that is currently under several clinical trials.
Therapeutic antibodies specifically approved for TNBC
TNBC is the most aggressive subtype of breast cancer that accounts for 15% to 20% of total breast cancer cases and lacks three prognostic indicators of breast cancer, namely estrogen receptor (ER), progesterone receptor, and Her2 [5,6]. TNBC is a heterogeneous disease and, based on the gene expression profile, subdivided into six categories; basal-like 1, basal-like 2, mesenchymal, immunomodulatory, mesenchymal stem-like, and luminal androgen receptor, among which basal-like subtypes show better responses to chemotherapy than the others [7,8]. Breast cancer patients are generally separated for treatment options based on their ER and Her2 expression status. Patients with ER-positive and Her2-postive breast cancer are treated with hormone therapy and Her2-targetd therapy, respectively, while chemotherapy is a major option for TNBC patients [9]. About 50% of TNBC patients respond to chemotherapy, while only 10-15% and 20% could be treated by PARP and programmed death-ligand 1 (PD-L1) inhibitors, respectively [10,11]. Therefore, a majority of TNBC patients are still treated with chemotherapy [7,12-14]. To develop effective treatment for TNBC, combination therapies have been extensively studied including the PARP inhibitors combined with anti-PD-1/PD-L1 [15,16], PARP inhibitors combined with tyrosine kinase inhibitors [17,18], and metformin combined with anti-CTLA4 [19]. However, such combination therapy has not been applied to clinic yet. There was no specific antibody therapy for TNBC until March 2019 when atezolizumab (Tecentriq®) in combination with nab-paclitaxel received approval by U.S. Food and Drug administration (FDA) for treatment of TNBC patients with PD-L1-positive tumors [20]. Based on reports from a phase III clinical trial, IMpassion130, atezolizumab combined with nab-paclitaxel was granted an accelerated approval to treat unresectable locally advanced or metastatic TNBC by FDA in 2019 [21,22]. The atezolizumab does not directly kill TNBC cells, but it enhances anti-tumor immunity by inhibiting the PD-1-mediated inhibitory signaling of T cells [20,23]. Unfortunately, the TNBC indication of atezolizumab was withdrawn in August 2021 due to a failure of a post-market Phase III clinical trial. TNBC cells have significantly higher levels of glycosylated PD-L1 [2], and we recently showed that a de-glycosylation of patient tissue samples can more accurately measure the level of PD-L1 by using immunohistochemical staining to stratify patients for atezolizumab treatment [24]. Therefore, it is worthy of proposing a clinical trial with a larger cohort to stratify TNBC patients with the de-glycosylation procedure, and then further validate clinical responses to atezolizumab. Moreover, it has been shown that glucose analog 2-deoxyglucose (2-DG) and metformin can reduce PD-L1 upregulation that is induced by PAPR inhibitors, and these drug would be combine with PARP inhibitors [25,26]. On the other hand, pembrolizumab (Keytruda®), a monoclonal antibody against PD-1, was approved for treatment of TNBC patients in July 2021 [27]. Before this approval, pembrolizumab have been used in clinic for treatment of various types of cancer such as non-small cell lung cancer (NSCLC), melanoma, bladder cancer, and Hodgkin lymphoma. It can be currently applied for high-risk, early-stage TNBC in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery [27,28].
SG was approved in April 2021 as the first ADC for treatment of TNBC patients. Treatment with SG showed a significant benefit (with 4% complete responding rate and 31% partial responding rate for 235 patients) in TNBC patients who do not respond to a wide range of chemotherapy [29]. SG contains the humanized RS7 antibody, which targets Trop2, encoded by the Tumor Associated Calcium Signal Transducer 2 (TACSTD2) gene. In addition, SG comprises an topoisomerase I inhibitor (SN-38, the active metabolite of irinotecan), which is conjugated to RS7 via a cleavable CL2A linker [30]. The phase I/II clinical trial of SG for treatment of various epithelial cancers was first started in 2012 (NCT01631552), and its first results were published in 2015 [31]. Following this trial, it was tested in patients with TNBC in the phase III trial between November 2017 and March 2020 (NCT02574455), and then SG was approved in April 2021 for treatment of TNBC patients with advanced disease that does not respond to chemotherapy [30].
Function and signaling of Trophoblast cell-surface antigen 2 (Trop2)
Trop2 is a transmembrane protein overexpressed in some cancer including breast, cervical, colorectal, gastric, prostate, lung, esophageal and oral cancers [32]. Trop2 mediates calcium signaling using the internal source of calcium, which leads to cell survival, proliferation, and self-renewal (Figure 1). Trop2 has a conserved binding motif for phosphatidylinositol 4,5-bisphosphate (PIP2) and a serine phosphorylation domain targeted by protein kinase C (PKC) [33,34]. PKC activated by calcium can phosphorylate the Trop2 intra-cellular domain (ICD), which is require for ADAM17-dependent cleavage of Trop2 [35]. Several reports showed that Trop2 also activates MAPK signaling (Figure 1) [36-38]. Moreover, the Trop2 ICD phosphorylated by Src and PKC is cleaved by γ-secretase and ADAM17 and translocates into the nucleus where it interacts with some nuclear proteins such as Cyclin D [35]. On the other hand, Trop2 activates the AP1 transcription factor via the activation of the MAPK pathway, which in turn leads to c-Myc and Cyclin D transcription [39]. Trop2 ICD nuclear accumulation requires Cyclin D and is induced by Src. Numb, which is a negative regulator of Trop2 by inhibiting its cleavage, is also inhibited by Cyclin D [39]. Therefore, Trop2 leads to Cyclin D expression, which not only results in cell proliferation, but also leads to inhibition of Numb through Trop2 ICD nuclear accumulation.
Figure 1.
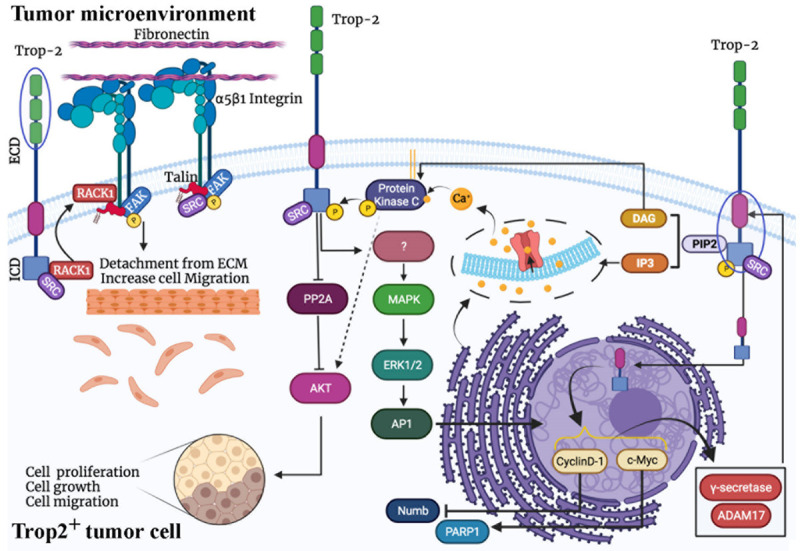
The Trop2-mediated signaling pathways. Trop2 activation is either dependent or independent on growth factors. Trop2 then increases internal Ca2+ level and enhances protein kinase C (PKC) signaling. On the other hand, Trop2 phosphorylation recruits RACK1 scaffold protein by which Trop2 interacts with other signaling pathways such as PI3K/Akt and MAPK pathways as well as FAK signaling. Furthermore, separation of transmembrane and intracellular parts of Trop2 by γ-secretase and ADAM-17, and its migration into nuclease interacts with other genes related to cancer progression. Overall, Trop2 can enhance cell proliferation, cell growth, and cell migration through several mechanisms. AP1: Activator protein 1; DAG: Diacylglycerol; ECD: Extracellular domain; ERK1/2: Extracellular signal-regulated kinase 1/2; FAK: Focal adhesion kinase; IP3: Inositol trisphosphate; ICD: Intracellular domain; MAPK: Mitogen-activated protein kinase; PARP1: poly (ADP-ribose) polymerase-1; PIP2: Phosphatidylinositol 4,5-bisphosphate; RACK1: receptor for activated C kinase 1; ADAM17: A disintegrin and metalloprotease 17.
Several studies have suggested that Trop2 also enhances epithelial-mesenchymal transition (EMT). A study showed that the expression of Trop2 is negatively correlated with the expression of E-cadherin, a mesenchymal cell marker, in breast cancer tissue samples [40]. Moreover, the levels of Trop2 upregulation and E-cadherin downregulation in TNBC patients are greater than patients with other breast cancer subtypes [40]. In addition, Trop2 facilitates cell migration when growth factors are absent [32]. Murine Trop2 can enhance tumor cell growth and cell proliferation in serum starvation condition [36]. α5β1 integrin and E-cadherin are well-known membrane proteins involved in cell-cell interaction, whose proteolysis leads to mesenchymal phenotype and tumor cell migration [41,42]. α5 integrin enhances focal adhesion kinase (FAK)/STAT/Akt signaling and leads to tumor progression and metastasis [43]. Furthermore, activated FAK/Src is associated with tumor cell metastasis. Catalytic activities of Src and FAK promote VEGF signaling and protease-associated tumor cell migration [44]. There are two essential ADAM proteases involved in tumor metastasis, ADAM10 and ADAM17 [45], both of which interact with Trop2 [35,46]. Trop2 binds to E-cadherin and α5β1 integrin, leading to ADAM10-dependent cleavage of E-cadherin and α5β1 integrin [46,47]. The receptor for activated C kinase 1 (RACK1) also serves as a connector between Trop2 and α5β1 integrin [48]. Therefore, Trop2 is a crucial promoter of cell motility when growth factors are downregulated in presence of TKIs. Furthermore, Trop2 as well as Integrin α5 may contribute to resistance to TKIs [49].
PI3K/Akt signaling is associated with resistance to chemotherapy [7]; however, only one PI3Kα inhibitor (alpelisib) has passed FDA-approval to date, which is for PIK3CA-mutated ER positive breast cancer, not for TNBC. Moreover, a majority of PI3K/Akt pathway inhibitors have failed during preclinical and clinical studies [23]. Interestingly, the association of Trop2 and Akt has been observed in xenograft animal models and breast cancer patients with activated Akt signaling [50]. Akt has two major phosphorylation sites, T308 and S473 [7]. In addition to phosphorylating Trop2, PKC also activates Akt through its phosphorylation at S473 [51]. In contrast, Trop2 activation results in phosphorylation of Akt at both phosphorylation sites, T308 and S473, through the downregulation of protein phosphatase 2A (PP2A) that is an Akt inhibitor [52]. Therefore, PKC and Trop2 collaboratively activate Akt [52]. Moreover, Trop2 enhances tumorigenesis and metastasis through EGFR and IGFR signaling pathways [37,53]. Therefore, Trop2 is suggested as the predicator of response to Akt pathway inhibitors as well as TKIs [49,52].
As mentioned earlier, Trop2 is upregulated in a wide range of cancer. Therefore, to treat cancer through targeting Trop2, at least three approaches are available: (1) Trop2/PD-L1 CAR-T cells, (2) Anti-Trop2 nano particle (ST-NPs), and (3) Trop2-targeting ADCs (SG and DS-1062a). Bispecific Trop2/PD-L1 CAR-T cells have been developed for treatment of gastric cancer [54]. They inhibit Trop2 signaling and enhance anti-cancer immunity. However, bispecific Trop2/PD-L1 CAR-T cells target Trop2 and PD-L1 positive cancer cells but not neighboring cancer cells that do not express them [54]. Moreover, anti-Trop2 antibody conjugated nanocarriers (ST NPs), which encapsulates doxorubicin, were also developed as a targeted drug delivery tool for Trop2 positive TNBC. ST-NPs are designed to release doxorubicin in response to reduced glutathione (GSH). In this approach, the nanoparticles, which carry anti-Trop2 antibody on their surfaces, can be internalized through the binding to Trop2, and then, they release doxorubicin into the cytoplasm when GSH level reaches 10 mM [55]. Lastly, SG is a second-generation ADC developed by Immunomedics that consists of a Trop2-targeting murine monoclonal antibody RS7-3G11 [56]. Breast, cervical, and ovarian cancer cells were shown to be sensitive to anti-Trop2 RS7-SG11 antibody [56,57].
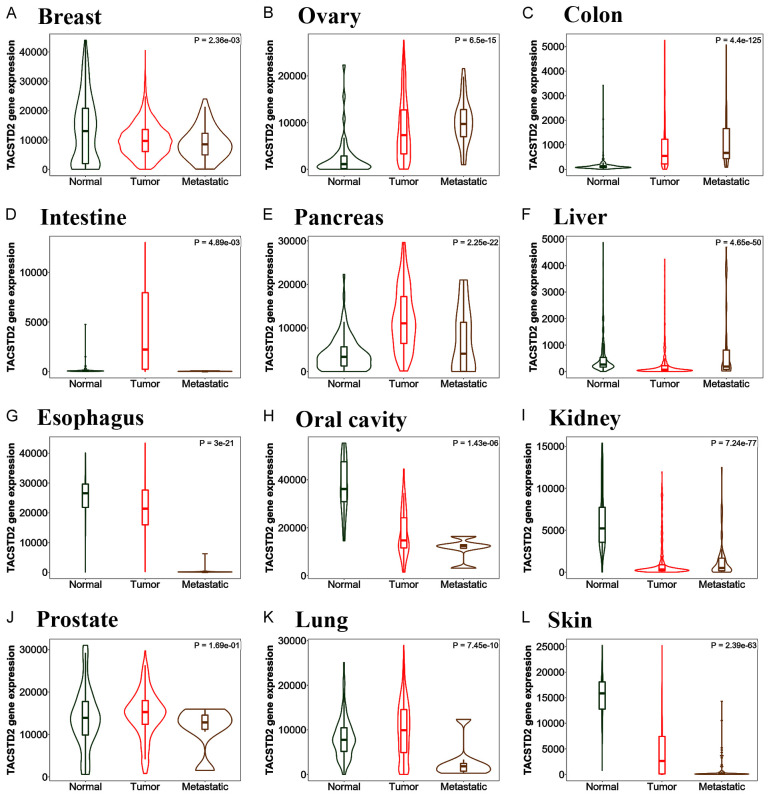
To figure out whether Trop2-targeted therapy may also be effective on other types of cancers, we analyzed TACSTD2 (Trop2 gene) expression in different cancer types. Figure 2 shows the TACSTD2 expression in different tissues and provides a detailed comparison among normal, tumor, and metastatic cases. Data analysis of TACSTD2 expression in breast demonstrates TACSTD2 is highly expressed in both normal and tumor cells, suggesting the potential toxicity of anti-Trop2 ADC against normal tissues (Figure 2A). Moreover, the analysis of TACSTD2 expression based on the breast cancer subtypes (Figure 3) shows TACSTD2 expression is significantly higher in TNBC compared with Her2 positive breast cancer but not with ER-positive breast cancer. Nonetheless, SG has been shown to have relatively low toxicity and high efficacy against TNBC patients in clinical studies. Thus, these clinical results may be partly due to the more specific toxicity of the payload to cancer cells. Moreover, the gene expression data (Figure 2) also suggests that other cancer types can be possibly treated with anti-Trop2 ADC in addition to breast cancer. Tissues where TACSTD2 is overexpressed in tumor and metastatic cases, but not normal cases, are suitable types for anti-Trop2 ADC therapy. Based on Figure 2, ovarian, pancreatic, and prostate cancers could be appropriate types of cancer for targeting Trop2 in the future research.
Figure 2.
Differential Trop2 gene (TACSTD2) expression analysis in different tumor, normal and metastatic tissues. TACSTD2 is highly expressed in ovary, pancreas, and prostate as well as breast, for which Trop2-targeted therapy may be effective; however, oral, kidney, and skin cancers are not good examples for targeting Trop2 because TACSTD2 is overexpressed in normal tissues. Although normal breast and lung cases expressed TACSTD2 as well as tumor breast and lung cases, breast and lung tumors are highly heterogenous and the use of Trop2-targeted therapy is highly dependent on the subtypes for breast and lung cancers. Data was analyzed using TNMplot.com [80].
Figure 3.
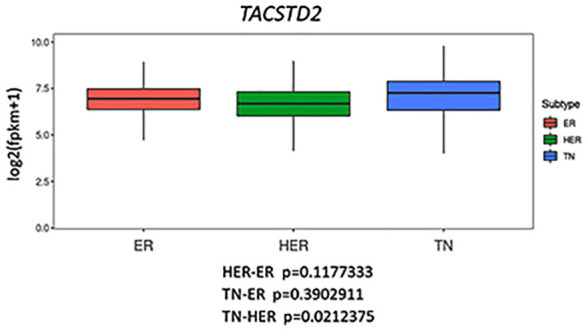
TACSTD2 expression in the different subtypes of breast cancer. TGCA breast cancer RNAseq data (FPKM) and phenotype data were downloaded from UCSC Xena (https://xena.ucsc.edu/), and the expression of TACSTD2 was analyzed using R, according to the result of IHC result of ER, PR and HER2. The data shows there is a significance difference between TNBC and Her2 positive breast cancer, but not between TNBC and ER positive breast cancer (One way ANOVA with post-hoc Tukey HSD test).
SN-38, topoisomerase I inhibitor payload for ADC
Several clinical trials have been conducted for the development of ADCs with topoisomerase inhibitors [58]. SN-38 is a cytotoxic payload derived from the active metabolite of irinotecan topoisomerase I inhibitor, which induces double-stranded breaks in DNA (Figure 4). Irinotecan is not commonly used in clinical practices but has shown a response rate of up to 23% in the patients with metastatic breast cancers who have previously been progressed on anthracyclines and taxanes [59]. The inhibition of topoisomerase I causes double-stranded DNA breaks and intrinsic apoptosis in both TNBC and Her2 positive breast cancer cells [60]. SG is interesting for three reasons. In contrast with ultra-toxic payloads of other ADCs, SG is benefited from a (1) moderately toxic payload (SN-38) that permitted (2) higher drug-to-antibody ratio (DAR) of approximately 8, compared with DAR of 2-4 of previous ADCs. In addition, most ADCs have stable linkers, whereas (3) SG possesses a cleavable carbonate linker, allowing it to have the stronger bystander killing effect; however, SG may also affect normal cells during its circulation [61].
Figure 4.
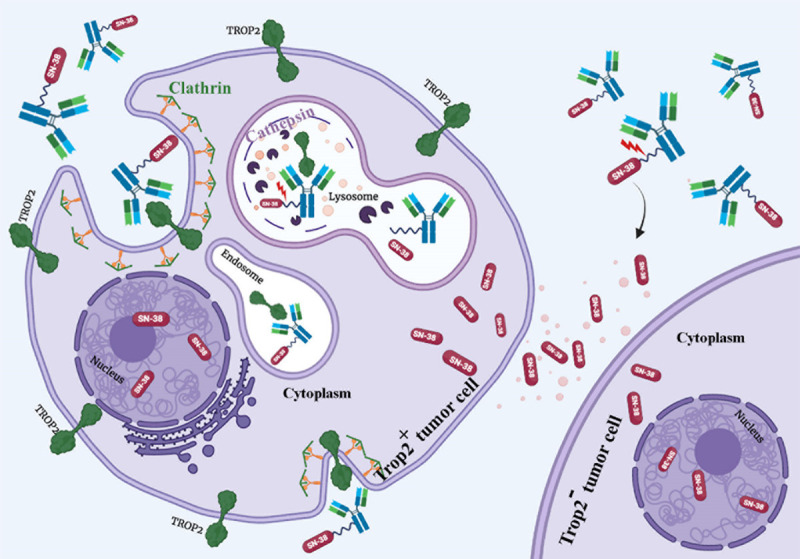
The mechanism of action of sacituzumab govitecan. Sacituzumab govitecan (SG) is the first ADC specifically approved for TNBC. There are three compartments in its structure: an anti-Trop2 antibody, a cleavable linker, and a topoisomerase I inhibitor payload (SN-38). The antibody part targets the drug on Trop2 positive tumor cells and enhances Trop2 internalization. After the internalization of the ADC, the linker is cleaved by cathepsin and other proteolytic enzymes located at lysosome. SN-38 can then distribute into the nucleus of Trop2 positive and/or Trop2- neighboring tumor cells through the bystander killing effect. Therefore, SG is effective against heterogeneous cancer.
Higher DAR of 7.6 for SG showed the optimum pharmacokinetics [57]. In addition, SN-38 can be released in both extracellular spaces and cytoplasm. Based on the result obtained from a clinical study, SG has a half-life of 11-14 hours in human plasma [62]. Similarly, the half-life of SG is 14 hours in a mouse and 50% of SN-38 is released from SG in 17.5 hours in mouse serum in vitro [63]. The SN-38 possesses the property of membrane permeability and thus diffuses out of the target cancer cells and induces its cytotoxic effects on surrounding cells regardless of their Trop-2 expression status (the bystander effect) [64]. As SN-38 is a moderately toxic agent with IC50 in nanomolar range, its off-target toxicity is lower than other payloads, which usually have IC50 in picomolar range. However, the FDA-approval of SG had postponed, and it needed the second Biologics License Application (BLA). In April 2021, SG was finally approved for TNBC patients previously showed resistance to conventional chemotherapy [65].
The linkers are a crucial part of ADCs as they (1) link antibody to the cytotoxic drug, (2) affect pharmacokinetic properties such as carrying capacity of a cytotoxic molecule by each antibody (DAR), and (3) determine the stability of the cytotoxic drug in the bloodstream and level of the bystander effect observed in surrounding cells [66]. SG possesses a cleavable maleimide linker with a short pegylated unit, which links cytotoxic payload SN-38 to humanized anti-Trop2 hRS7.
Clinical studies for evaluating sacituzumab govitecan (IMMU-132)
The efficacy and safety of SG were investigated in a phase I/II multicenter clinical trial (NCT01631552) [29,62,67-71]. In this trial, 495 patients with various advanced solid tumors such as breast, lung, colorectal, esophageal cancer received SG at the doses of 8, 10, 12, or 18 mg/kg and the adverse events were evaluated [67]. Overall, SG has a tolerable and predictable toxicity profile. The adverse events of SG include nausea (62.6%), diarrhea (56.2%), fatigue (48.3%), alopecia (40.4%) and neutropenia (57.8%). Grade >3 neutropenia and febrile neutropenia were observed in 42.4% and 5.3% of patients, respectively [67].
Moreover, the efficacy of SG was also evaluated in patients with various cancer types in this trial. Breast cancer patients show the better response rate than patients with other cancer types. In patients with TNBC, overall response rate (ORR) was 33.3% and the median duration of response (MDR) was 7.7 months [29]. Similarly, in patients with hormone receptor (HR)-positive/Her2-negative breast cancer, ORR was 31.5% and MDR was 8.7 months [71]. On the contrary, in non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) patients, ORR was 19% and 14%, respectively [68,69].
Following the phase I/II study, the efficacy of SG was further evaluated in the ASCENT phase III clinical trial (NCT02574455) [72]. In this trial, 468 TNBC patients, who had previously received taxanes and had no brain metastases, were assigned to receive either 10 mg/kg of SG or chemotherapy and subjected to comparison of survival benefit. The median progression-free survival of the patients treated SG and chemotherapy is 5.6 and 1.7 months, respectively. The median overall survival of patients treated with SG and chemotherapy is 12.1 and 6.7 months, respectively. The objective response rate of SG was 35% while that of chemotherapy was 5%. This result clearly shows a significant clinical benefit of SG compared with standard chemotherapy [72].
Furthermore, the predictive value of Trop2 expression for the responses to SG was also analyzed in the ASCENT trial [73]. The ORR of TNBC patients with high, medium, and low Trop2 expression to SG was 44%, 38%, and 22%, respectively. In contrast, the ORR of Trop-2 high, medium, and low patients to chemotherapy was 1%, 11%, and 6%, respectively [73]. These results indicate that Trop-2 expression level is associated with the response rate to SG but not chemotherapy, and it would serve as a predictive biomarker for SG therapy.
Based on the ASCENT trial, SG was approved for the treatment of TNBC patients. Currently, multiple clinical trials are ongoing for several cancer types in various settings, which are summarized in Table 1. In particular, the efficacy of SG on HR positive breast cancer patient is currently investigated in a randomized phase III trial (NCT03901339), which may lead the approval of SG on ER positive breast cancer in the near future.
Table 1.
Ongoing clinical studies investigating the effects of SG alone or combined with other drugs in patients with various cancer
| Clinical trial | Starting date | Sponsor | Disease | Drug (s) | Phase | Number of patients |
|---|---|---|---|---|---|---|
| NCT04039230 | 2019-10 | Massachusetts General Hospital | Breast Cancer | SG | 1/2 | 75 |
| Talazoparib | ||||||
| NCT05113966 | 2021-11 | G1 Therapeutics, Inc. | TNBC | SG | 2 | 45 |
| Trilaciclib | ||||||
| NCT05101096 | 2021-10 | Gilead Sciences | Advanced solid tumors | SG | 1/2 | 61 |
| TNBC | ||||||
| NCT03901339 | 2019-5 | Gilead Sciences | Metastatic breast cancer | SG | 3 | 543 |
| Eribulin | ||||||
| Capecitabine | ||||||
| Gemcitabine | ||||||
| Vinorelbine | ||||||
| NCT04468061 | 2020-7 | Dana-Farber Cancer Institute | PD-L1-negative TNBC | SG Pembrolizumab | 2 | 110 |
| NCT03964727 | 2019-10 | Gilead Sciences | Metastatic solid tumor | SG | 2 | 165 |
| NCT04319198 | 2020-8 | Gilead Sciences | Metastatic solid tumor | SG | 4 | 200 |
| NCT04639986 | 2020-11 | Everest Medicines | HR+/Her2 metastatic BC | SG | 3 | 330 |
| Eribulin mesylate | ||||||
| Capecitabine | ||||||
| Gemcitabine | ||||||
| Vinorelbine | ||||||
| NCT04448886 | 2020-9 | Sara Tolaney | Invasive BC | SG | 2 | 110 |
| Metastatic BC | Pembrolizumab | |||||
| HR+ BC | ||||||
| Her2- BC | ||||||
| NCT05119907 | 2021-9 | Everest Medicines | Solid tumors | SG | 2 | 180 |
| NCT04454437 | 2020-10 | Everest Medicines | Metastatic TNBC | SG | 2 | 80 |
| NCT04647916 | 2020-12 | Southwest Oncology Group | Her2- BC with brain metastasis | SG | 2 | 44 |
| NCT04230109 | 2020-7 | Aditya Bardia | TNBC | Pembrolizumab | 2 | 51 |
| SG | ||||||
| NCT04434040 | 2020-7 | Dana-Farber Cancer Institute | TNBC | SG | 2 | 40 |
| Atezolizumab | ||||||
| NCT03971409 | 2019-7 | Hope Rugo, MD | TNBC | SG | 2 | 150 |
| PF-04518600 | ||||||
| Avelumab | ||||||
| Binimetinib | ||||||
| Utomilumab | ||||||
| Liposomal Doxorubicin | ||||||
| NCT05006794 | 2021-9 | Gilead Sciences | Advanced solid malignancies | SG | 1 | 205 |
| GS-9716 | ||||||
| Docetaxel | ||||||
| Gemcitabine | ||||||
| NCT03992131 | 2019-6 | Clovis Oncology, Inc. | Ovarian cancer | SG | 1/2 | 329 |
| TNBC | Rucaparib | |||||
| Urothelial carcinoma | Lucitanib | |||||
| Solid tumor | ||||||
| NCT04986579 | 2021-11 | Dana-Farber Cancer Institute | Metastatic BC | SG | 2 | 120 |
| Eribulin | ||||||
| Trastuzumab deruxtecan | ||||||
| NCT03424005 | 2018-8 | Hoffmann-La Roche | TNBC | SG | 1/2 | 280 |
| Capecitabine | ||||||
| Atezolizumab | ||||||
| Ipatasertib | ||||||
| SGN-LIV1A | ||||||
| Bevacizumab | ||||||
| Gemcitabine + Carboplatin or Eribulin | ||||||
| Selicrelumab | ||||||
| Tocilizumab | ||||||
| Nab-Paclitaxel |
TNBC: Triple-negative breast cancer; HR: Hormone receptor; BC: Breast cancer; SG: Sacituzumab govitecan.
ADCs with deruxtecan, a novel payload developed by Daiichi Sankyo
The main disadvantage of SG is a short half-life and its instability in plasma [62]. A new Trop2-ADC developed by Daiichi Sankyo solves this issue by replacing the payload and linker. This company has previously developed deruxtecan (Dxd), which is a derivative of a topoisomerase inhibitor exatecan and 10 times more potent than SN-38. Dxd was then applied in development of an Her2-tageting ADC called fam-trastuzumab deruxtecan-nxki (DS-8201a) [74]. In the preclinical study with Her2 positive breast cancer cell lines as well as patient-derived xenograft models, the efficacy of DS-8201a and T-DM1 was compared. DS-8201a inhibits Her2-expression-dependent tumor growth and Akt phosphorylation. The safety range for DS-8201a was determined, which is between 1 and 30 mg/kg. Three topoisomerase I inhibitor payloads, SN-38, DX-8951f, and Dxd, were tested and their IC50s were 2.78, 0.25, and 0.35 μM, respectively. Therefore, Dxd and DX-8951f are more effective than SN-38; however, Dxd was used in generation of DS-8201a [74]. In DS-8201a, Dxd and trastuzumab are linked together with the maleimide-based linker that is different from the linker of SG. Interestingly, this maleimide linker has low off-target effects and high stability in plasma. DS-8201a has DAR of 7.7 and the linker can be specifically hydrolyzed by lysosomal proteases. The release rates of DXd from DS-8201a is only 2% in human plasma in 3 weeks, which makes it safer compared with T-DM1 [74]. Therefore, Dxd can be delivered into tumor microenvironment and have the bystander killing effect after delivery to its target cells [75]. Ultimately, DS-8201a was granted approval for treatment of patients with Her2 positive breast cancer who did not respond to previous anti-Her2-based regimens in December 2019 [76]. Indeed, Her2-targeted ADCs are effective on breast cancer patients who did not respond to other Her2 positive-targeted therapies [77].
Following DS-8201, Daiichi Sankyo developed a Trop2-targeting ADC called datopotamab deruxtecan (DS-1062a). The company has also used Dxd as a cytotoxic agent for DS-1062a. Antitumor activity and safety profile of DS-1062a were investigated in preclinical studies [78]. Currently, DS-1062a is being tested in the phase I clinical trials for NSCLC and breast cancer (Table 2). DS-1062a in combination with anti-PD-1 pembrolizumab is also being tested in the phase I clinical trial for advanced/metastatic NSCLC (NCT04526691) (Table 2) [79]. As these clinical trials of DS-1062a have recently started, these clinical data has not been available yet; however, the preclinical study of DS-1062a was recently published [78]. The efficacy and safety of DS-1062a were evaluated in various cancer cell lines. DS-1062a binds to Trop2 and is internalized into Trop2 positive cancer cells. However, the differences in efficacy and safety profile between DS-1602a and SG is still under investigation. For DS-1062a, DAR of 4 had the maximum therapeutic windows, whereas DAR of 7 had narrower therapeutic window. The safety profile of DS-1062a was also evaluated in rats and cynomolgus monkeys. DNA damage and apoptosis were observed in the Trop2 positive tumor cells treated with DS-1602a [78]. Obviously, this is the promising drug that would have better clinical effects and safety profile than SG.
Table 2.
Ongoing clinical studies of DS-1062a in patients with several cancer types
| Clinical trial | Starting date | Sponsor | Disease | ADC | Payload | Phase | Status |
|---|---|---|---|---|---|---|---|
| NCT03401385 | Jan. 2018 | DS/AZ | NSCLC | DS-1062a | Dxd | 1 | Recruiting |
| TNBC | |||||||
| HR+ BC | |||||||
| NCT03742102 | Dec. 2018 | AZ | TNBC | DS-1062a | Dxd | 1/2 | Recruiting |
| NCT04526691 | Sept. 2020 | DS/AZ/MSD | NSCLC | DS-1062a | Dxd | 1 | Recruiting |
| NCT04484142 | March 2021 | DS/AZ | NSCLC | DS-1062a | Dxd | 2 | Recruiting |
| NCT04940325 | June 2021 | DS/GR | NSCLC | DS-1062a | Dxd | 2 | Recruiting |
| NCT04656652 | Oct. 2021 | DS/AZ | NSCLC | DS-1062a | Dxd | 3 | Recruiting |
| NCT05104866 | Nov. 2021 | DS/AZ | BC | DS-1062a | Dxd | 3 | Recruiting |
| NCT04612751 | Nov. 2021 | DS/AZ | NSCLC | DS-1062a | Dxd | 1 | Recruiting |
NSCLC: Non-small cell lung cancer; TNBC: Triple-negative breast cancer; HR+ BC: Hormone receptor positive breast cancer; BC: Breast cancer; DS: Daiichi Sankyo, Inc.; AZ: AstraZeneca; MSD: Merck Sharp & Dohme Corp.; GR: Gustave Roussy, Cancer Campus, Grand Paris; DS-1062a: Datopotamab deruxtecan; Dxd: deruxtecan.
Conclusion
Trop2 is a new drug target for cancer patients who do not respond to conventional chemotherapy. SG reached approval in April 2021 because it shows significant efficacy against TNBC refractory to chemotherapy. The encouraging clinical trial data certainly shed light on this new direction to treat various cancers. Indeed, several clinical trials of SG as well as another Trop2-targeting ADC, DS-1062a, are ongoing in patients with several types of cancer. The advantages of DS-1062a compared with SG are the longer half-life in serum and more potent payload, which may reduce off-target toxicity on normal cells. However, there are still some rooms to be improved for the efficacy and safety by replacing new payloads and linkers. Nevertheless, the prospects of ADC against Trop2 positive are brightening.
Acknowledgements
We thank the Ministry of Science and Technology (MOST), Taiwan, for grants MOST 110-2639-B-039-001-ASP (to M-C.H.) and MOST 110-2314-B-039-060 (to H.Y.) and China Medical University for Yingtsai Scholar Award (CMU109-YT-06 to H.Y.).
Disclosure of conflict of interest
None.
References
- 1.Vankemmelbeke M, Durrant L. Third-generation antibody drug conjugates for cancer therapy--a balancing act. Ther Deliv. 2016;7:141–144. doi: 10.4155/tde-2016-0002. [DOI] [PubMed] [Google Scholar]
- 2.Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J, Cha JH, Xia W, Chan LC, Kim T, Chang SS, Lee HH, Chou CK, Liu YL, Yeh HC, Perillo EP, Dunn AK, Kuo CW, Khoo KH, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Huang TH, Sahin AA, Hortobagyi GN, Yoo SS, Hung MC. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33:187–201. e110. doi: 10.1016/j.ccell.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barroso-Sousa R, Tolaney SM. Clinical development of new antibody-drug conjugates in breast cancer: to infinity and beyond. BioDrugs. 2021;35:159–174. doi: 10.1007/s40259-021-00472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarantino P, Carmagnani Pestana R, Corti C, Modi S, Bardia A, Tolaney SM, Cortes J, Soria JC, Curigliano G. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022;72:165–182. doi: 10.3322/caac.21705. [DOI] [PubMed] [Google Scholar]
- 5.Meric-Bernstam F, Hung MC. Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin Cancer Res. 2006;12:6326–6330. doi: 10.1158/1078-0432.CCR-06-1732. [DOI] [PubMed] [Google Scholar]
- 6.Hsu JL, Hung MC. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016;35:575–588. doi: 10.1007/s10555-016-9649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabbarzadeh Kaboli P, Salimian F, Aghapour S, Xiang S, Zhao Q, Li M, Wu X, Du F, Zhao Y, Shen J, Cho CH, Xiao Z. Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer - a comprehensive review from chemotherapy to immunotherapy. Pharmacol Res. 2020;156:104806. doi: 10.1016/j.phrs.2020.104806. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hon JD, Singh B, Sahin A, Du G, Wang J, Wang VY, Deng FM, Zhang DY, Monaco ME, Lee P. Breast cancer molecular subtypes: from TNBC to QNBC. Am J Cancer Res. 2016;6:1864–1872. [PMC free article] [PubMed] [Google Scholar]
- 10.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A, Curran M, Hwu P, Sharma P, Litton JK, Molldrem JJ, Alatrash G. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beniey M, Haque T, Hassan S. Translating the role of PARP inhibitors in triple-negative breast cancer. Oncoscience. 2019;6:287–288. doi: 10.18632/oncoscience.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vagia E, Mahalingam D, Cristofanilli M. The Landscape of Targeted Therapies in TNBC. Cancers (Basel) 2020;12:916. doi: 10.3390/cancers12040916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cyprian FS, Akhtar S, Gatalica Z, Vranic S. Targeted immunotherapy with a checkpoint inhibitor in combination with chemotherapy: a new clinical paradigm in the treatment of triple-negative breast cancer. Bosn J Basic Med Sci. 2019;19:227–233. doi: 10.17305/bjbms.2019.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayan P, Wahby S, Gao JJ, Amiri-Kordestani L, Ibrahim A, Bloomquist E, Tang S, Xu Y, Liu J, Fu W, Song P, King-Kallimanis BL, Hou S, Gong Y, Kalavar S, Ghosh S, Philip R, Goldberg KB, Theoret MR, Blumenthal GM, Kluetz PG, Sridhara R, Pazdur R, Beaver JA. FDA approval summary: atezolizumab plus paclitaxel protein-bound for the treatment of patients with advanced or metastatic TNBC whose tumors express PD-L1. Clin Cancer Res. 2020;26:2284–2289. doi: 10.1158/1078-0432.CCR-19-3545. [DOI] [PubMed] [Google Scholar]
- 15.McCann KE, Hurvitz SA. Advances in the use of PARP inhibitor therapy for breast cancer. Drugs Context. 2018;7:212540. doi: 10.7573/dic.212540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH, Li CW, Chou CK, Lim SO, Chang SS, Litton J, Arun B, Hortobagyi GN, Hung MC. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23:3711–3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Y, Yamaguchi H, Wei Y, Hsu JL, Wang HL, Hsu YH, Lin WC, Yu WH, Leonard PG, Lee GR 4th, Chen MK, Nakai K, Hsu MC, Chen CT, Sun Y, Wu Y, Chang WC, Huang WC, Liu CL, Chang YC, Chen CH, Park M, Jones P, Hortobagyi GN, Hung MC. Blocking c-Met-mediated PARP1 phosphorylation enhances anti-tumor effects of PARP inhibitors. Nat Med. 2016;22:194–201. doi: 10.1038/nm.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu YY, Yam C, Chen MK, Chan LC, Xiao M, Wei YK, Yamaguchi H, Lee PC, Han Y, Nie L, Sun X, Moulder SL, Hess KR, Wang B, Hsu JL, Hortobagyi GN, Litton J, Chang JT, Hung MC. Blocking c-Met and EGFR reverses acquired resistance of PARP inhibitors in triple-negative breast cancer. Am J Cancer Res. 2020;10:648–661. [PMC free article] [PubMed] [Google Scholar]
- 19.Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, Li CW, Kim T, Chang SS, Lee HH, Hsu JL, Wang HL, Kuo CW, Chang WC, Hadad S, Purdie CA, McCoy AM, Cai S, Tu Y, Litton JK, Mittendorf EA, Moulder SL, Symmans WF, Thompson AM, Piwnica-Worms H, Chen CH, Khoo KH, Hung MC. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell. 2018;71:606–620. e607. doi: 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortes J, Andre F, Goncalves A, Kummel S, Martin M, Schmid P, Schuetz F, Swain SM, Easton V, Pollex E, Deurloo R, Dent R. IMpassion132 Phase III trial: atezolizumab and chemotherapy in early relapsing metastatic triple-negative breast cancer. Future Oncol. 2019;15:1951–1961. doi: 10.2217/fon-2019-0059. [DOI] [PubMed] [Google Scholar]
- 21.Emens LA, Adams S, Barrios CH, Dieras V, Iwata H, Loi S, Rugo HS, Schneeweiss A, Winer EP, Patel S, Henschel V, Swat A, Kaul M, Molinero L, Patel S, Chui SY, Schmid P. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol. 2021;32:983–993. doi: 10.1016/j.annonc.2021.05.355. [DOI] [PubMed] [Google Scholar]
- 22.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA IMpassion130 Trial Investigators. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 23.Kaboli PJ, Imani S, Jomhori M, Ling KH. Chemoresistance in breast cancer: PI3K/Akt pathway inhibitors vs the current chemotherapy. Am J Cancer Res. 2021;11:5155–5183. [PMC free article] [PubMed] [Google Scholar]
- 24.Ou-Yang F, Li CL, Chen CC, Shen YC, Moi SH, Luo CW, Xia WY, Wang YN, Lee HH, Wang LH, Wang SC, Pan MR, Hou MF, Hung MC. De-glycosylated membrane PD-L1 in tumor tissues as a biomarker for responsiveness to atezolizumab (Tecentriq) in advanced breast cancer patients. Am J Cancer Res. 2022;12:123–137. [PMC free article] [PubMed] [Google Scholar]
- 25.Shao B, Li CW, Lim SO, Sun L, Lai YJ, Hou J, Liu C, Chang CW, Qiu Y, Hsu JM, Chan LC, Zha Z, Li H, Hung MC. Deglycosylation of PD-L1 by 2-deoxyglucose reverses PARP inhibitor-induced immunosuppression in triple-negative breast cancer. Am J Cancer Res. 2018;8:1837–1846. [PMC free article] [PubMed] [Google Scholar]
- 26.Han Y, Li CW, Hsu JM, Hsu JL, Chan LC, Tan X, He GJ. Metformin reverses PARP inhibitors-induced epithelial-mesenchymal transition and PD-L1 upregulation in triple-negative breast cancer. Am J Cancer Res. 2019;9:800–815. [PMC free article] [PubMed] [Google Scholar]
- 27.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O’Shaughnessy J KEYNOTE-522 Investigators. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber AR, Kagihara JA, Weiss JA, Nicklawsky A, Gao D, Borges VF, Kabos P, Diamond JR. Clinical outcomes for patients with metastatic breast cancer treated with immunotherapy agents in phase I clinical trials. Front Oncol. 2021;11:640690. doi: 10.3389/fonc.2021.640690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, O’Shaughnessy J, Moroose RL, Santin AD, Abramson VG, Shah NC, Rugo HS, Goldenberg DM, Sweidan AM, Iannone R, Washkowitz S, Sharkey RM, Wegener WA, Kalinsky K. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380:741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 30.Corti C, Giugliano F, Nicolo E, Ascione L, Curigliano G. Antibody-drug conjugates for the treatment of breast cancer. Cancers (Basel) 2021;13:2898. doi: 10.3390/cancers13122898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starodub AN, Ocean AJ, Shah MA, Guarino MJ, Picozzi VJ Jr, Vahdat LT, Thomas SS, Govindan SV, Maliakal PP, Wegener WA, Hamburger SA, Sharkey RM, Goldenberg DM. First-in-human trial of a novel anti-Trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res. 2015;21:3870–3878. doi: 10.1158/1078-0432.CCR-14-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer. 2015;6:84–105. doi: 10.18632/genesandcancer.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cubas R, Li M, Chen C, Yao Q. Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009;1796:309–314. doi: 10.1016/j.bbcan.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 34.El Sewedy T, Fornaro M, Alberti S. Cloning of the murine TROP2 gene: conservation of a PIP2-binding sequence in the cytoplasmic domain of TROP-2. Int J Cancer. 1998;75:324–330. doi: 10.1002/(sici)1097-0215(19980119)75:2<324::aid-ijc24>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 35.Wanger TM, Dewitt S, Collins A, Maitland NJ, Poghosyan Z, Knauper V. Differential regulation of TROP2 release by PKC isoforms through vesicles and ADAM17. Cell Signal. 2015;27:1325–1335. doi: 10.1016/j.cellsig.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Cubas R, Zhang S, Li M, Chen C, Yao Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol Cancer. 2010;9:253. doi: 10.1186/1476-4598-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redlich N, Robinson AM, Nickel KP, Stein AP, Wheeler DL, Adkins DR, Uppaluri R, Kimple RJ, Van Tine BA, Michel LS. Anti-Trop2 blockade enhances the therapeutic efficacy of ErbB3 inhibition in head and neck squamous cell carcinoma. Cell Death Dis. 2018;9:5. doi: 10.1038/s41419-017-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan H, Guo Z, Liang W, Li H, Wei G, Xu L, Xiao H, Li Y. Trop2 enhances invasion of thyroid cancer by inducing MMP2 through ERK and JNK pathways. BMC Cancer. 2017;17:486. doi: 10.1186/s12885-017-3475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ju X, Jiao X, Ertel A, Casimiro MC, Di Sante G, Deng S, Li Z, Di Rocco A, Zhan T, Hawkins A, Stoyanova T, Ando S, Fatatis A, Lisanti MP, Gomella LG, Languino LR, Pestell RG. v-Src oncogene induces Trop2 proteolytic activation via cyclin D1. Cancer Res. 2016;76:6723–6734. doi: 10.1158/0008-5472.CAN-15-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao W, Kuai X, Zhou X, Jia L, Wang J, Yang X, Tian Z, Wang X, Lv Q, Wang B, Zhao Y, Huang W. Trop2 is a potential biomarker for the promotion of EMT in human breast cancer. Oncol Rep. 2018;40:759–766. doi: 10.3892/or.2018.6496. [DOI] [PubMed] [Google Scholar]
- 41.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu QP, Kuang JY, Yang QK, Bian XW, Yu SC. Beyond a tumor suppressor: soluble E-cadherin promotes the progression of cancer. Int J Cancer. 2016;138:2804–2812. doi: 10.1002/ijc.29982. [DOI] [PubMed] [Google Scholar]
- 43.Hsu SL, Cheng CC, Shi YR, Chiang CW. Proteolysis of integrin alpha5 and beta1 subunits involved in retinoic acid-induced apoptosis in human hepatoma Hep3B cells. Cancer Lett. 2001;167:193–204. doi: 10.1016/s0304-3835(01)00479-7. [DOI] [PubMed] [Google Scholar]
- 44.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Conrad C, Benzel J, Dorzweiler K, Cook L, Schlomann U, Zarbock A, Slater EP, Nimsky C, Bartsch JW. ADAM8 in invasive cancers: links to tumor progression, metastasis, and chemoresistance. Clin Sci (Lond) 2019;133:83–99. doi: 10.1042/CS20180906. [DOI] [PubMed] [Google Scholar]
- 46.Guerra E, Trerotola M, Relli V, Lattanzio R, Tripaldi R, Vacca G, Ceci M, Boujnah K, Garbo V, Moschella A, Zappacosta R, Simeone P, de Lange R, Weidle UH, Rotelli MT, Picciariello A, Depalo R, Querzoli P, Pedriali M, Bianchini E, Angelucci D, Pizzicannella G, Di Loreto C, Piantelli M, Antolini L, Sun XF, Altomare DF, Alberti S. Trop-2 induces ADAM10-mediated cleavage of E-cadherin and drives EMT-less metastasis in colon cancer. Neoplasia. 2021;23:898–911. doi: 10.1016/j.neo.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trerotola M, Jernigan DL, Liu Q, Siddiqui J, Fatatis A, Languino LR. Trop-2 promotes prostate cancer metastasis by modulating beta(1) integrin functions. Cancer Res. 2013;73:3155–3167. doi: 10.1158/0008-5472.CAN-12-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trerotola M, Li J, Alberti S, Languino LR. Trop-2 inhibits prostate cancer cell adhesion to fibronectin through the beta1 integrin-RACK1 axis. J Cell Physiol. 2012;227:3670–3677. doi: 10.1002/jcp.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Wang Y, Che X, Hou K, Wu J, Zheng C, Cheng Y, Liu Y, Hu X, Zhang J. Integrin alpha5 promotes migration and invasion through the FAK/STAT3/AKT signaling pathway in icotinib-resistant non-small cell lung cancer cells. Oncol Lett. 2021;22:556. doi: 10.3892/ol.2021.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Teng S, Zhang Y, Zhang W, Zhang X, Xu K, Yao H, Yao J, Wang H, Liang X, Hu Z. TROP2 promotes proliferation, migration and metastasis of gallbladder cancer cells by regulating PI3K/AKT pathway and inducing EMT. Oncotarget. 2017;8:47052–47063. doi: 10.18632/oncotarget.16789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, Qiao G, Ying H, Zhang J, Yin F. TCR-induced Akt serine 473 phosphorylation is regulated by protein kinase C-alpha. Biochem Biophys Res Commun. 2010;400:16–20. doi: 10.1016/j.bbrc.2010.07.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerra E, Trerotola M, Tripaldi R, Aloisi AL, Simeone P, Sacchetti A, Relli V, D’Amore A, La Sorda R, Lattanzio R, Piantelli M, Alberti S. Trop-2 induces tumor growth through AKT and determines sensitivity to AKT inhibitors. Clin Cancer Res. 2016;22:4197–4205. doi: 10.1158/1078-0432.CCR-15-1701. [DOI] [PubMed] [Google Scholar]
- 53.Tang G, Tang Q, Jia L, Chen Y, Lin L, Kuai X, Gong A, Feng Z. TROP2 increases growth and metastasis of human oral squamous cell carcinoma through activation of the PI3K/Akt signaling pathway. Int J Mol Med. 2019;44:2161–2170. doi: 10.3892/ijmm.2019.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao W, Jia L, Zhang M, Huang X, Qian P, Tang Q, Zhu J, Feng Z. The killing effect of novel bi-specific Trop2/PD-L1 CAR-T cell targeted gastric cancer. Am J Cancer Res. 2019;9:1846–1856. [PMC free article] [PubMed] [Google Scholar]
- 55.Son S, Shin S, Rao NV, Um W, Jeon J, Ko H, Deepagan VG, Kwon S, Lee JY, Park JH. Anti-Trop2 antibody-conjugated bioreducible nanoparticles for targeted triple negative breast cancer therapy. Int J Biol Macromol. 2018;110:406–415. doi: 10.1016/j.ijbiomac.2017.10.113. [DOI] [PubMed] [Google Scholar]
- 56.Varughese J, Cocco E, Bellone S, Ratner E, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Buza N, Pecorelli S, Santin AD. Cervical carcinomas overexpress human trophoblast cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized monoclonal anti-Trop-2 antibody. Am J Obstet Gynecol. 2011;205:567, e561–567. doi: 10.1016/j.ajog.2011.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC) Oncotarget. 2015;6:22496–22512. doi: 10.18632/oncotarget.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park IH, Im SA, Jung KH, Sohn JH, Park YH, Lee KS, Sim SH, Park KH, Kim JH, Nam BH, Kim HJ, Kim TY, Lee KH, Kim SB, Ahn JH, Lee S, Ro J. Randomized open label phase III trial of irinotecan plus capecitabine versus capecitabine monotherapy in patients with metastatic breast cancer previously treated with anthracycline and taxane: PROCEED trial (KCSG BR 11-01) Cancer Res Treat. 2019;51:43–52. doi: 10.4143/crt.2017.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumler I, Brunner N, Stenvang J, Balslev E, Nielsen DL. A systematic review on topoisomerase 1 inhibition in the treatment of metastatic breast cancer. Breast Cancer Res Treat. 2013;138:347–358. doi: 10.1007/s10549-013-2476-3. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez Stewart RM, Berry JTL, Berger AK, Yoon SB, Hirsch AL, Guberman JA, Patel NB, Tharp GK, Bosinger SE, Mainou BA. Enhanced killing of triple-negative breast cancer cells by reassortant reovirus and topoisomerase inhibitors. J Virol. 2019;93:e01411–19. doi: 10.1128/JVI.01411-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldenberg DM, Sharkey RM. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: a case study of anti-TROP-2 sacituzumab govitecan. MAbs. 2019;11:987–995. doi: 10.1080/19420862.2019.1632115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ocean AJ, Starodub AN, Bardia A, Vahdat LT, Isakoff SJ, Guarino M, Messersmith WA, Picozzi VJ, Mayer IA, Wegener WA, Maliakal P, Govindan SV, Sharkey RM, Goldenberg DM. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: safety and pharmacokinetics. Cancer. 2017;123:3843–3854. doi: 10.1002/cncr.30789. [DOI] [PubMed] [Google Scholar]
- 63.Sharkey RM, McBride WJ, Cardillo TM, Govindan SV, Wang Y, Rossi EA, Chang CH, Goldenberg DM. Enhanced delivery of SN-38 to human tumor xenografts with an anti-Trop-2-SN-38 antibody Conjugate (Sacituzumab Govitecan) Clin Cancer Res. 2015;21:5131–5138. doi: 10.1158/1078-0432.CCR-15-0670. [DOI] [PubMed] [Google Scholar]
- 64.Perrone E, Lopez S, Zeybek B, Bellone S, Bonazzoli E, Pelligra S, Zammataro L, Manzano A, Manara P, Bianchi A, Buza N, Tymon-Rosario J, Altwerger G, Han C, Menderes G, Ratner E, Silasi DA, Azodi M, Hui P, Schwartz PE, Scambia G, Santin AD. Preclinical activity of sacituzumab govitecan, an antibody-drug conjugate targeting trophoblast cell-surface antigen 2 (Trop-2) linked to the active metabolite of irinotecan (SN-38), in ovarian cancer. Front Oncol. 2020;10:118. doi: 10.3389/fonc.2020.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Syed YY. Sacituzumab govitecan: first approval. Drugs. 2020;80:1019–1025. doi: 10.1007/s40265-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eiger D, Agostinetto E, Saude-Conde R, de Azambuja E. The exciting new field of HER2-low breast cancer treatment. Cancers (Basel) 2021;13:1015. doi: 10.3390/cancers13051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, Moroose R, Santin AD, Kalinsky K, Picozzi V, O’Shaughnessy J, Gray JE, Komiya T, Lang JM, Chang JC, Starodub A, Goldenberg DM, Sharkey RM, Maliakal P, Hong Q, Wegener WA, Goswami T, Ocean AJ. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32:746–756. doi: 10.1016/j.annonc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Heist RS, Guarino MJ, Masters G, Purcell WT, Starodub AN, Horn L, Scheff RJ, Bardia A, Messersmith WA, Berlin J, Ocean AJ, Govindan SV, Maliakal P, Mudenda B, Wegener WA, Sharkey RM, Goldenberg DM, Camidge DR. Therapy of advanced non-small-cell lung cancer with an SN-38-Anti-Trop-2 drug conjugate, sacituzumab govitecan. J. Clin. Oncol. 2017;35:2790–2797. doi: 10.1200/JCO.2016.72.1894. [DOI] [PubMed] [Google Scholar]
- 69.Gray JE, Heist RS, Starodub AN, Camidge DR, Kio EA, Masters GA, Purcell WT, Guarino MJ, Misleh J, Schneider CJ, Schneider BJ, Ocean A, Johnson T, Gandhi L, Kalinsky K, Scheff R, Messersmith WA, Govindan SV, Maliakal PP, Mudenda B, Wegener WA, Sharkey RM, Goldenberg DM. Therapy of small cell lung cancer (SCLC) with a topoisomerase-I-inhibiting antibody-drug conjugate (ADC) targeting Trop-2, sacituzumab govitecan. Clin Cancer Res. 2017;23:5711–5719. doi: 10.1158/1078-0432.CCR-17-0933. [DOI] [PubMed] [Google Scholar]
- 70.Bardia A, Mayer IA, Diamond JR, Moroose RL, Isakoff SJ, Starodub AN, Shah NC, O’Shaughnessy J, Kalinsky K, Guarino M, Abramson V, Juric D, Tolaney SM, Berlin J, Messersmith WA, Ocean AJ, Wegener WA, Maliakal P, Sharkey RM, Govindan SV, Goldenberg DM, Vahdat LT. Efficacy and safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 2017;35:2141–2148. doi: 10.1200/JCO.2016.70.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalinsky K, Diamond JR, Vahdat LT, Tolaney SM, Juric D, O’Shaughnessy J, Moroose RL, Mayer IA, Abramson VG, Goldenberg DM, Sharkey RM, Maliakal P, Hong Q, Goswami T, Wegener WA, Bardia A. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020;31:1709–1718. doi: 10.1016/j.annonc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, Brufsky A, Sardesai SD, Kalinsky K, Zelnak AB, Weaver R, Traina T, Dalenc F, Aftimos P, Lynce F, Diab S, Cortés J, O’Shaughnessy J, Diéras V, Ferrario C, Schmid P, Carey LA, Gianni L, Piccart MJ, Loibl S, Goldenberg DM, Hong Q, Olivo MS, Itri LM, Rugo HS ASCENT Clinical Trial Investigators. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 73.Bardia A, Tolaney SM, Punie K, Loirat D, Oliveira M, Kalinsky K, Zelnak A, Aftimos P, Dalenc F, Sardesai S, Hamilton E, Sharma P, Recalde S, Gil EC, Traina T, O’Shaughnessy J, Cortes J, Tsai M, Vahdat L, Dieras V, Carey LA, Rugo HS, Goldenberg DM, Hong Q, Olivo M, Itri LM, Hurvitz SA. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32:1148–1156. doi: 10.1016/j.annonc.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 74.Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, Hirai T, Atsumi R, Nakada T, Hayakawa I, Abe Y, Agatsuma T. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 75.Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J. Clin. Oncol. 2020;38:1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Narayan P, Osgood CL, Singh H, Chiu HJ, Ricks TK, Chiu Yuen Chow E, Qiu J, Song P, Yu J, Namuswe F, Guiterrez-Lugo M, Hou S, Pierce WF, Goldberg KB, Tang S, Amiri-Kordestani L, Theoret MR, Pazdur R, Beaver JA. FDA approval summary: fam-trastuzumab deruxtecan-Nxki for the treatment of unresectable or metastatic HER2-positive breast cancer. Clin Cancer Res. 2021;27:4478–4485. doi: 10.1158/1078-0432.CCR-20-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mukohara T, Hosono A, Mimaki S, Nakayama A, Kusuhara S, Funasaka C, Nakao T, Fukasawa Y, Kondoh C, Harano K, Naito Y, Matsubara N, Tsuchihara K, Kuwata T. Effects of ado-trastuzumab emtansine and fam-trastuzumab deruxtecan on metastatic breast cancer harboring HER2 amplification and the L755S mutation. Oncologist. 2021;26:635–639. doi: 10.1002/onco.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okajima D, Yasuda S, Maejima T, Karibe T, Sakurai K, Aida T, Toki T, Yamaguchi J, Kitamura M, Kamei R, Fujitani T, Honda T, Shibutani T, Muramatsu S, Nakada T, Goto R, Takahashi S, Yamaguchi M, Hamada H, Noguchi Y, Murakami M, Abe Y, Agatsuma T. Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. 2021;20:2329–2340. doi: 10.1158/1535-7163.MCT-21-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shen M, Liu S, Stoyanova T. The role of Trop2 in prostate cancer: an oncogene, biomarker, and therapeutic target. Am J Clin Exp Urol. 2021;9:73–87. [PMC free article] [PubMed] [Google Scholar]
- 80.Bartha Á, Győrffy B. TNMplot. com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int J Mol Sci. 2021;22:2622. doi: 10.3390/ijms22052622. [DOI] [PMC free article] [PubMed] [Google Scholar]