Abstract
Nevoid and myxoid melanoma are rare variants of melanoma; association of the two is a unique finding. Nevoid melanoma is characterized by morphologic resemblance to a nevus, whereas myxoid melanoma demonstrates a basophilic mucinous matrix. We present an atypical case of a melanoma progressing from a nevoid melanocytoma with myxoid changes. A 78-year-old female presented with a pigmented growth on her right thigh. Biopsy demonstrated a biphenotypic melanocytic proliferation composed of a nodule showing epithelioid melanocytes with enlarged nuclei, prominent nucleoli, lack of maturation, and abundant amphophilic cytoplasm with a rare mitotic figure. These findings were suggestive of melanoma along with a nevoid dermal component and myxoid stroma. FISH testing revealed a homozygous loss of 9p21 in the atypical component. SNP-microarray from the nevoid component demonstrated three abnormalities including a gain of whole chromosome 8, as well as loss of a copy of nearly an entire chromosome 9 and 16q most consistent with a melanocytoma.
Keywords: Myxoid melanoma, nevoid melanoma, malignant melanoma, melanocytoma
Introduction
Despite accounting for less than 5% of all cutaneous malignancies, melanoma ranks as the number one cause of skin cancer-related deaths with over 70,000 cases diagnosed in the US each year [1,2]. German Central Malignant Melanoma Registry data reveals that superficial spreading melanoma is the most common clinico-histopathologic subtype, followed by nodular melanoma, lentigo melanoma, acral melanoma, and other less common variants. Nevoid and myxoid types represent less than 1% of all melanoma cases [3,4]. Current literature only documented four reported cases of melanoma with nevoid cytology in the superficial dermal layer and mucin accumulation with spindle-shaped melanocytes in the deep dermis; these cases were found to harbor a kinase fusion [5]. Myxoid change within a melanocytic tumor poses additional diagnostic challenges and has a yet unknown prognostic impact.
Case presentation
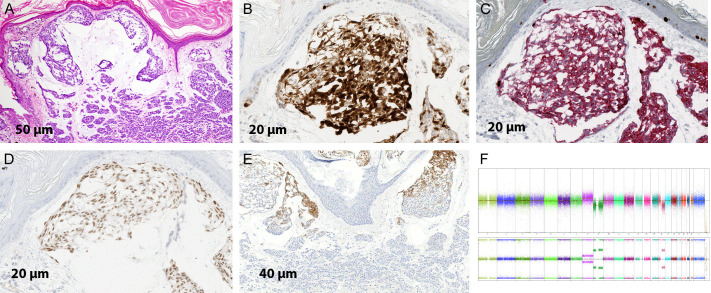
A 78-year-old female with remote history of nonmelanoma skin cancer presented for a nodular growth on the right thigh of unknown duration (Figure 1). Shave biopsy revealed an asymmetric, poorly circumscribed compound melanocytic tumor. The epidermis demonstrated an atypical melanocytic proliferation with lentiginous growth consistent with melanoma in situ. In the dermis the tumor exhibited a biphenotypic morphology with both typical and atypical melanocytes present (Figure 2A-C). The majority of the tumor showed nests of bland melanocytes without atypia or mitotic figures associated with areas of mucin deposition and demonstrating signs of maturation with dermal descent (Figure 3A, 3B). Immunohistochemical stains showed stratification of staining with HMB-45, uniform expression of p16, a low labeling index with Ki-67, and moderate expression of PRAME in 50-75% of the tumor cells (Figure 3C-E). SNP-microarray analysis was performed on this component and demonstrated a gain of whole chromosome 8, loss of a copy of nearly entire chromosome 9 (except 9p33.3-p34.3), and loss of one copy of 16q arm (Figure 3F). One of the tissue profiles revealed a nodular proliferation composed of large epithelioid melanocytes with pleomorphic nuclei, hyperchromasia, and few mitotic figures (Figure 4A). Immunohistochemical stains showed diffuse loss of p16, strong nuclear positivity for PRAME in almost all the cells, increased labeling index for Ki-67 and diffuse expression of HMB-45 (Figure 4B-E). Fluorescence in situ hybridization (FISH) demonstrated homozygous loss of 9p21, while only heterozygous loss was detected in the nevoid component consistent with the SNP array results (Figure 4F).
Figure 1.
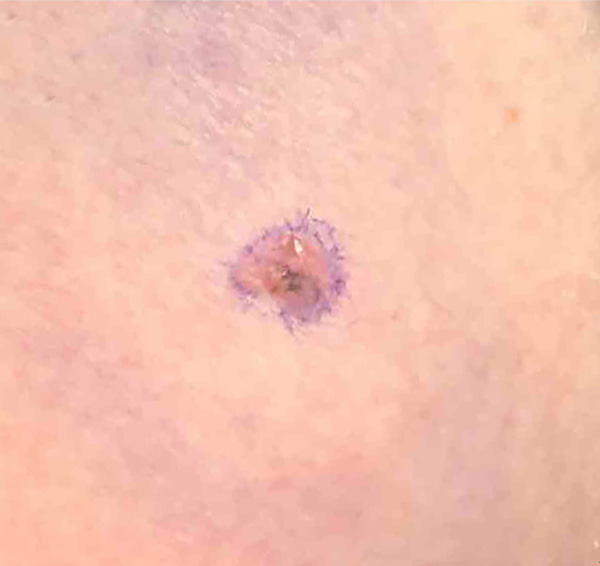
Clinical presentation. This tumor presented clinically as a pink irregular plaque with asymmetric hyperpigmentation.
Figure 2.
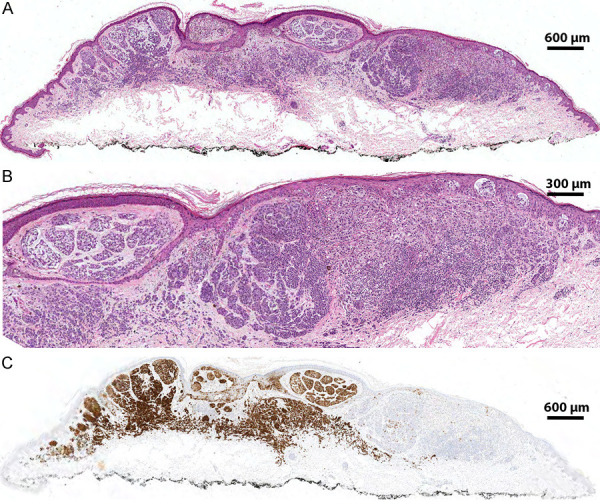
Biphenotypic melanocytic proliferation. A. Low power image showing a bi-phenotypic melanocytic lesion; on the left there is a nevoid component showing maturation with dermal descent while on the right there is a nodule of melanocytes without nesting (H&E; 2×). B. Medium magnification demonstrating on the left nests of bland melanocytes with areas of mucin deposition and maturation. On the right is a nodule of atypical melanocytes without maturation (H&E; 10×). C. Immunohistochemical stain for p16 demonstrating retained staining in the nevoid component with diffuse loss in the atypical nodule (IHC; 2×).
Figure 3.
Nevoid component of the lesion. A. Nevoid component showing nests of melanocytes with bland morphology and mucin deposition. In the deeper dermis there are smaller nests of nevoid melanocytes consistent with maturation. (H&E; 20×). B. Retained staining in the nevoid component. (IHC; 40×). C. Duplex immunohistochemical stain for Ki-67/Mart-1 demonstrating a low labeling index for Ki-67 in the melanocytes (IHC; 40×). D. Immunohistochemical stain for PRAME demonstrating moderate intensity expression in 50%-75% of the tumor cells (IHC; 40×). E. Immunohistochemical stain for HMB-45 demonstrating stratification of staining (IHC; 20×). F. SNP-microarray profile showing gain of whole chromosome 8 (red arrow) and losses of a copy of nearly entire chromosome 9 and 16q (green arrow).
Figure 4.
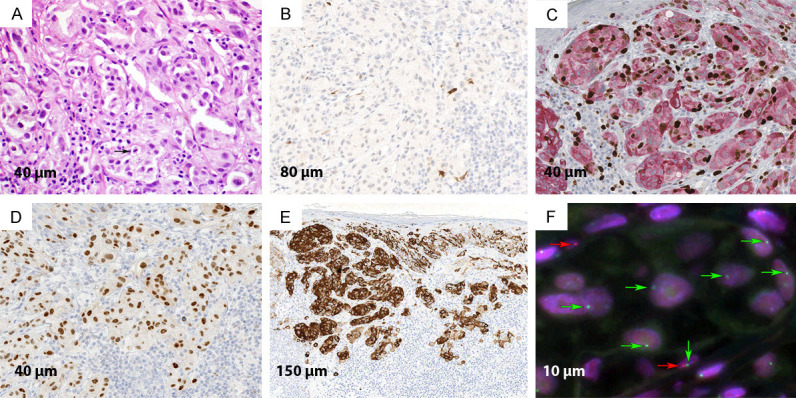
Malignant component of the lesion. A. Atypical nodule demonstrating compact growth of large epithelioid melanocytes with pleomorphic nuclei, hyperchromasia, and few mitotic figures (arrow) (H&E; 40×). B. Immunohistochemical stain for p16 demonstrating diffuse loss of staining (IHC; 20×). C. Duplex immunohistochemical stain for Ki-67/Mart-1 demonstrating increased labeling index for Ki-67 in the atypical melanocytes (IHC; 40×). D. Immunohistochemical stain for PRAME demonstrating strong expression almost all tumor cells (IHC; 40×). E. Immunohistochemical stain for HMB-45 demonstrating abnormal diffuse staining (IHC; 10×). F. FISH with probes for 9p21 (red) and centromere 9 (green). In the center there are few large nuclei with one copy of centromere 9 (green arrow) and no red signals consistent with homozygous loss of 9p21. Two normal cells are also seen with one copy of 9p21 (red arrow).
Based on the atypical nodule, degree of cytologic atypia, immunohistochemical profile including diffuse expression of PRAME, loss of p16, increased Ki-67 labeling index, lack of stratification with HMB-45, and homozygous loss of 9p21 that spans the locus of CDKN2A gene encoding for p16, are all with the findings were suggestive of melanoma (Figure 4B-F). The nevoid component demonstrated a relatively bland morphology and some reassuring immunohistochemical features including uniform p16 expression, low Ki-67 labeling index, and stratification with HMB-45 (Figure 3C-E). There were, however, some concerning features including increased expression of PRAME and few abnormalities detected by SNP microarray. In a recent study, the presence of three or less abnormalities by SNP-array was found to be indicative of benign or low-grade melanocytic tumor and not melanoma [6]. This array of findings was most consistent with a diagnosis of melanocytoma with nevoid morphology.
Discussion
We present here a skin lesion with progression from an intermediate grade melanocytic tumor or melanocytoma with nevoid morphology and myxoid changes to melanoma. In the current WHO classification of skin tumors, melanocytoma is defined as a melanocytic tumor of intermediate malignancy characterized by the presence of an initiating alteration. It is similar to nevi, such as having BRAF gene mutation and one or few additional alterations which are responsible for the atypical morphology but are not sufficient for complete malignant transformation (i.e. melanoma) [7]. Most melanocytomas are characterized by an indolent biologic behavior; however, there is a small risk of progression to melanoma from acquiring additional abnormalities, as documented in this case.
Nevoid melanoma is characterized by clinical and histologic features resembling those of benign nevi while exhibiting invasive characteristics typical of a dermatologic malignancy [8,9]. It was first described by Schmoeckel in 1985 and typically presents as a brown or black dome-shaped or verrucous nodule, usually on the trunk or extremities of male adults [10]. The nevoid variant accounts for less than 1% of all melanoma cases but confers a high mortality rate of 15-37% in three years [3]. With an average reported Breslow thickness of 3.2 mm (range 0.4-12), nevoid melanoma may be classified into the following subcategories: nevus-like (48%), amelanotic (30%), tumors of multi-component pattern (15%), and unclassified mixed pattern (7%) [8,10]. Immunohistochemical analysis is often used as a supplemental diagnostic tool. HMB-45 stains dermal melanocytes in a stratified pattern in benign nevi with a diffuse or random pattern in melanoma, while Ki-67 shows increased labeling in dermal melanocytes in nevoid melanoma and low labeling in benign nevi [11]. The 9p21 region is identified as the site of two downstream tumor suppressor genes CDKN2A (p16) and CDKN2B (p15) [12]. These genes act as kinase inhibitors that block cell cycle transit. In our case, SNP-microarray and FISH showed a heterozygous loss in the melanocytoma component with homozygous loss of 9p21 in the melanoma.
Myxoid change in melanocytic tumors, characterized by melanocytes embedded in basophilic mucinous matrix comprised of excess glycosaminoglycans, primarily hyaluronic acid of mesenchymal origin, is rare [7,13]. Myxoid change has been reported in both benign nevi and melanoma [5]. Microscopically, proliferation of spindle-shape or stellate cells with prominent nuclei in a myxoid stroma can be identified. For melanomas with this morphology the mean Breslow thickness is similar to other variants of malignant melanoma [4,14]. In four reported cases of nevoid melanoma with myxoid changes, immunohistochemical analysis demonstrated partial or complete loss of Melan-A and HMB45 expression [5]. FISH indicated the presence of Anaplastic Lymphoma Kinase gene rearrangement in these melanocytes and mucin accumulation was detected in the deeper dermis [5]. The etiology of such a variant is still unclear and it remains difficult to categorize this unique association into a specific nevoid melanoma subtype based on genetic and morphologic presentation.
Differential diagnosis of our case is broad. For myxoid melanoma, it includes soft tissue malignancies such as myxoid liposarcoma, myxoid malignant fibrous histiocytoma, low-grade fibromyxoid sarcoma, myxoid chondrosarcoma, or myxoid dermatofibrosarcoma protuberans. The differential diagnosis of nevoid melanoma includes minimal deviation melanoma, nodular melanoma, and melanoma in a dermal nevus [8].
Our case of myxoid changes in a nevoid melanocytoma which evolved to melanoma demonstrates features of both rare variants. Melanocytomas with myxoid change have not been reported, and the impact on our patient’s prognosis is unclear. This finding may pose the additional diagnostic challenge of nevoid melanoma as mucin deposition can be seen in benign or malignant melanocytic tumors [15]. The paucity of literature describing this entity highlights the need for dermatologists and dermatopathologists to recognize this unique association. Additional research and cases are needed to elucidate further the implications on prognosis of this rare melanoma subtype.
Acknowledgements
We would like to thank Dr. Nathan Nartker for editing the figures.
A signed informed consent was obtained from the patient.
Disclosure of conflict of interest
None.
References
- 1.Centers for Disease Control and Prevention. USCS Data Brief, No. 9. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. Melanoma Incidence and Mortality, United States-2012-2016. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm. [Google Scholar]
- 2.Matthews NH, Li WQ, Qureshi AA, Weinstock MA, Cho E. Cutaneous melanoma: etiology and therapy [Internet] Brisbane (AU): Codon Publications; 2017. Epidemiology of melanoma. [PubMed] [Google Scholar]
- 3.Zembowicz A, McCusker M, Chiarelli C, Dei Tos AP, Granter SR, Calonje E, McKee PH. Morphological analysis of nevoid melanoma: a study of 20 cases with a review of the literature. Am J Dermatopathol. 2001;23:167–175. doi: 10.1097/00000372-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Patel P, Levin K, Waltz K, Helm KF. Myxoid melanoma: immunohistochemical studies and a review of the literature. J Am Acad Dermatol. 2002;46:264–270. doi: 10.1067/mjd.2002.119650. [DOI] [PubMed] [Google Scholar]
- 5.Perron E, Pissaloux DP, Barra CC, Karanian M, Lamant L, Parfait S, Alberti L, de la Fouchardiere A. Melanocytic myxoid spindle cell tumor with ALK rearrangement (MMySTAR): report of 4 cases of a nevus variant with potential diagnostic challenge. Am J Surg Pathol. 2018;42:595–603. doi: 10.1097/PAS.0000000000000973. [DOI] [PubMed] [Google Scholar]
- 6.Alomari AK, Miedema JR, Carter MD, Harms PW, Lowe L, Durham AB, Fullen DR, Patel RM, Hristov AC, Chan MP, Wang M, Andea AA. DNA copy number changes correlate with clinical behavior in melanocytic neoplasms: proposal of an algorithmic approach. Mod Pathol. 2020;33:1307–1317. doi: 10.1038/s41379-020-0499-y. [DOI] [PubMed] [Google Scholar]
- 7.Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med. 2020;144:500–522. doi: 10.5858/arpa.2019-0561-RA. [DOI] [PubMed] [Google Scholar]
- 8.Magro CM, Crowson AN, Mihm MC. Unusual variants of malignant melanoma. Mod Pathol. 2006;19(Suppl 2):S41–S70. doi: 10.1038/modpathol.3800516. [DOI] [PubMed] [Google Scholar]
- 9.Schmoeckel C, Castro CE, Braun-Falco O. Nevoid malignant melanoma. Arch Derm Res. 1985;277:362–369. doi: 10.1007/BF00509234. [DOI] [PubMed] [Google Scholar]
- 10.Longo C, Piana S, Marghoob A, Cavicchini S, Rubegni P, Cota C, Ferrara G, Cesinaro AM, Baade A, Bencini PL, Mantoux F, Mijuskovic ZP, Pizzichetta MA, Stanganelli I, Carrera C, Giovene GL, Ranasinghe S, Zalaudek I, Lallas A, Moscarella E, Specchio F, Pepe P, Pellacani G, Argenziano G. Morphological features of naevoid melanoma: results of a multicentre study of the International Dermoscopy Society. Br J Dermatol. 2015;172:961–967. doi: 10.1111/bjd.13524. [DOI] [PubMed] [Google Scholar]
- 11.McKee PH. Melanoma variants - wolves in sheep’s clothing. Elson B. Helwig Memorial Lecture, The American Society of Dermatopathology. 2003 [Google Scholar]
- 12.Naylor MF, Brown S, Quinlan C, Pitha JV, Evertt MA. 9p21 deletions in primary melanoma. Dermatol Online J. 1997;3:1. [PubMed] [Google Scholar]
- 13.Cinotti E, Rongioletti F. Myxoid melanoma. Rare Malignant Skin Tumors. 2015:215–217. [Google Scholar]
- 14.Smoller BR. Diagnosing myxoid melanoma. NEJM Journal Watch. 2002 [Google Scholar]
- 15.Ulamec M, Soldo-Belić A, Vucić M, Buljan M, Kruslin B, Tomas D. Melanoma with second myxoid stromal changes after personally applied prolonged phototherapy. Am J Dermatopathol. 2008;30:185–187. doi: 10.1097/DAD.0b013e31816112cd. [DOI] [PubMed] [Google Scholar]