Abstract
In the United States, combined stimulant/opioid overdose mortality has risen dramatically over the last decade. These increases may particularly affect non-Hispanic Black and Hispanic populations. We used death certificate data from the US National Center for Health Statistics (2007–2019) to compare state-level trends in overdose mortality due to opioids in combination with 1) cocaine and 2) methamphetamine and other stimulants (MOS) across racial/ethnic groups (non-Hispanic White, non-Hispanic Black, Hispanic, and non-Hispanic Asian American/Pacific Islander). To avoid unstable estimates from small samples, we employed principles of small area estimation and a Bayesian hierarchical model, enabling information-sharing across groups. Black Americans experienced severe and worsening mortality due to opioids in combination with both cocaine and MOS, particularly in eastern states. Cocaine/opioid mortality increased 575% among Black people versus 184% in White people (Black, 0.60 to 4.05 per 100,000; White, 0.49 to 1.39 per 100,000). MOS/opioid mortality rose 16,200% in Black people versus 3,200% in White people (Black, 0.01 to 1.63 per 100,000; White, 0.09 to 2.97 per 100,000). Cocaine/opioid overdose mortality rose sharply among Hispanic and Asian Americans. State-group heterogeneity highlighted the importance of data disaggregation and methods to address small sample sizes. Research to understand the drivers of these trends and expanded efforts to address them are needed, particularly in minoritized groups.
Keywords: drug overdose, harm reduction, opioids, psychostimulants, racial disparities, substance use
Abbreviation
- AAPI
Asian American and Pacific Islander
- MOS
methamphetamine and other stimulants
- NCHS
National Center for Health Statistics
Overdose mortality involving stimulant drugs, such as cocaine and methamphetamine, in combination with opioids has risen dramatically in recent years—more sharply than mortality involving stimulants alone (1–3). For example, mortality rates involving cocaine and any opioid rose by nearly 450% between 2009 and 2019 (4). By 2019, more than three-quarters of deaths involving cocaine and half of those involving methamphetamine or other stimulants also involved opioids (4). These trends vary by geographical region (5, 6) and, despite the predominant framing of the ongoing overdose crisis as a “White” epidemic (7), may be increasingly severe among Black and other non-White Americans (4), indicating a need for community-specific and racial justice–informed response and prevention efforts (8, 9). However, geography- and race/ethnicity-specific trends in combined stimulant/opioid overdose have not been well characterized.
Growing contamination of nonopioid drugs by illicitly manufactured fentanyl, an extremely potent synthetic opioid, is largely responsible for the increase in stimulant/opioid overdose mortality (2, 6). People who use drugs (PWUD) may be unaware of fentanyl’s presence in their stimulant supply, increasing the risk of overdose (10, 11). In addition, a growing proportion of PWUD may be intentionally using stimulants and opioids concomitantly and/or in combination (1, 12). Some PWUD describe synergistic effects of concomitant and/or combination stimulant/opioid use, including enhancing their high, counterbalancing unwanted effects of the other drug, and/or helping to manage withdrawal symptoms (1, 13). Among people who inject drugs in Seattle, for example, use of “goofballs” (i.e., simultaneous injection of methamphetamine and heroin) as a primary drug doubled in frequency between 2017 and 2019 (14).
Combined stimulant/opioid use and overdose represent a distinct public health challenge. For example, stimulant use may mask opioid overdose symptoms, decreasing the likelihood that a bystander might intervene and administer naloxone and in turn increasing the risk of fatal overdose (15). People who primarily use or intend to use stimulants may not identify as people who use opioids and therefore may less frequently obtain opioid-related harm reduction services, such as fentanyl test strips and take-home naloxone for opioid overdose reversal (16). Beyond overdose, people who use multiple substances are more likely to engage in high-risk injection and sexual practices and to experience more frequent comorbidities and poorer mental health and treatment outcomes (17–23). Finally, insufficient access to effective treatments for stimulant use disorder constrains efforts to stem harms related to combined stimulant/opioid use. While availability of evidence-based medication for opioid use disorder has grown (24), large treatment gaps remain (25, 26). Moreover, there are no approved medications available for stimulant use disorder, and the evidence-based treatment contingency management is infrequently available to those in need (27, 28). Importantly, there is a lack of evidence-based treatments for polysubstance use (29).
Rising stimulant/opioid deaths may particularly affect non-White Americans. Between 2018 and 2019, non-Hispanic Black and Hispanic Americans experienced a sharper increase in mortality due to opioids in combination with both cocaine and other stimulants, compared with White Americans (30). For example, the estimated 2018–2019 increase in the cocaine/opioid mortality rate was 29.6% in non-Hispanic Black people, 13.6% in Hispanic people, and 0.0% in non-Hispanic White people (30). Similarly, the estimated 2018–2019 increase in mortality due to opioids combined with noncocaine stimulants was 55.6% in non-Hispanic Black people, 40.0% in Hispanic people, and 32.1% in White people (30). Rates and trends for substance-specific mortality are frequently not disaggregated for the Asian American and Pacific Islander (AAPI) population, but recent research suggests this group may be experiencing increasing stimulant-related mortality as well (3, 30). However, it is not known where these rapid racially/ethnically patterned increases are concentrated geographically, preventing an effectively targeted public health and policy response. Cocaine-related mortality tends to be higher in the eastern United States, while mortality due to methamphetamine and other stimulants (e.g., amphetamines and prescription stimulants) is generally higher in the western part of the country. Given these regional differences, it is likely that race/ethnicity-specific trends in combined stimulant/opioid overdose death also vary geographically.
To fill this gap, we used 2007–2019 National Center for Health Statistics (NCHS) death certificate data to investigate whether, compared with non-Hispanic White Americans, non-Hispanic Black Americans have experienced sharper increases in overdose mortality related to a combination of opioids and stimulants, which we categorized as: 1) cocaine, and 2) methamphetamine and other stimulants (MOS). We also examined these trends in Hispanic and AAPI populations, among whom overdose trends are less well-explored but who may also be experiencing rising combined stimulant/opioid mortality (8, 31). We examined race-specific overdose trends by state to document geographic variation in these phenomena. However, disaggregating by both state and racial/ethnic group raises a key methodological challenge: Small counts for some state-group pairs produce unstable estimates. To address this, we used principles of small area estimation and a Bayesian hierarchical model to help stabilize estimates for smaller states and racial groups. In this approach, information about overdose mortality is shared: 1) between racial/ethnic groups within states, and 2) between states within a Census region. Importantly, this method is flexible and allows reasonable structure to be built into the model to aid in estimation for states and groups with smaller counts.
METHODS
We examined cocaine/opioid and MOS/opioid overdose mortality by state and over time for non-Hispanic White, non-Hispanic Black, Hispanic, and non-Hispanic Asian American and Pacific Islander people in the United States. We used 2007–2019 NCHS death certificate data to identify overdose deaths. To estimate overdose mortality per population, we used American Community Survey 1-year estimates to estimate the size of each racial/ethnic group in each state and year. To address undercounting of specific overdose-involved substances, which likely varies by state, group, and over time (32–34), we used imputation to predict whether each overdose death involved the substances of interest. We used principles of small area estimation and a Bayesian hierarchical model to enable estimation of mortality in small state-group pairs.
Data
We obtained individual death certificate data from the 2007–2019 NCHS restricted-use vital statistics data for all deaths coded as overdose in the United States (35). We categorized decedent race/ethnicity as non-Hispanic White, non-Hispanic Black, Hispanic, or non-Hispanic AAPI. In death certificate data, race/ethnicity information is typically collected by the funeral director, who is expected to obtain it from the decedent’s next of kin but may at times rely purely on observation (36). However, concordance with self-reported race is nearly 100% for Black and White people and more than 90% among AAPI people; concordance with self-reported Hispanic origin is nearly 90% (36). While variation in concordance by US Census region is minimal for Black and White race and limited for Hispanic origin, among AAPI people concordance varies with population size: In that group, misclassification is least frequent in the West, where the AAPI population is greatest (36). In our data set, NCHS imputed race information for approximately 2% of records; Hispanic origin was categorized as “unknown” for less than 1% of records across years.
In addition to race/ethnicity, deaths were grouped by state; states were grouped by Census region (Northeast, Midwest, South, and West). Analysis was performed at the state level according to racial/ethnic group. Total population estimates for each state and racial/ethnic group were obtained from American Community Survey 1-year estimates for each year included in the study.
Figure 1.
Levels and trends in combined cocaine/opioid mortality, by state and racial/ethnic group, United States, 2007–2019. Posterior mean estimates of the combined cocaine/opioid overdose mortality rates per 100,000 people in 2007 for White (A), Black (D), Hispanic (G), and Asian American and Pacific Islander (AAPI) (J) Americans and for 2019 for White (B), Black (E), Hispanic (H), and AAPI (K) Americans. Posterior mean estimates of the annual percent change (APC) in the mortality rate from 2007 to 2019 for White (C), Black (F), Hispanic (I), and AAPI (L) Americans.
We identified stimulant overdose deaths using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, underlying cause-of-death codes for unintentional overdose (codes X40–X44), suicide by drug self-poisoning (codes X60–X64), homicide by drug poisoning (code X85), and deaths of undetermined intent (codes Y10–Y14) involving a combination of opioids (T40.0–T40.4, T40.6) with either cocaine (T40.5) or other stimulants, primarily methamphetamine (T43.6). A death was classified as involving both cocaine and opioids if both cocaine and an opioid were recorded in the death record. Likewise, a death was classified as MOS and opioids if both a noncocaine stimulant and opioid were recorded in the death record. If opioids, cocaine, and a noncocaine stimulant were recorded in the death record, the death was classified in both categories.
Statistical considerations
The specific substances involved in an overdose death are often unidentified, resulting in undercounting that varies by substance, state, and race/ethnicity, as well as over time (32–34). If a death did not have both substances recorded but involved an unclassified substance (T50.9), then the status of death was considered to be missing, since the unclassified substance may have included the combination of an opioid, cocaine, and/or methamphetamines and other stimulants. In such cases, we used imputation to predict whether the overdose involved the substances of interest, employing the method demonstrated by Boslett et al. (32) to maximize predictive accuracy when imputing opioid involvement in an overdose death. To inform the imputation procedure, we used individual-level variables describing the contributing causes of death (Web Table 1, available at https://doi.org/10.1093/aje/kwab290) and decedent demographics (32). In addition to race/ethnicity, state, and year, individual characteristics included educational level (less than high school, high school degree, some college, at least a bachelor’s degree, and unknown), age at death, month of death, place of death (inpatient, outpatient or emergency department, dead on arrival to health-care facility, decedent’s home, hospice facility, nursing home, other, and unknown), marital status (single, married, widowed, divorced, and unknown), sex, and day of the week of death.
To conduct a single imputation for deaths that were considered unclassified, we built a logistic regression model for predicting combined cocaine/opioid deaths and a separate model for predicting combined MOS/opioid deaths. Using each fitted model to estimate probabilities for each unclassified death, we assigned the death to one of the categories of interest if the probability was greater than 0.5. The observed and imputed death classifications were then aggregated by race/ethnicity, year, and state to form the data set for the main analysis models. We also constructed a data set using only fully observed data (i.e., only included deaths that recorded an opioid and cocaine or an opioid and methamphetamine and other stimulant); results from that analysis are discussed below.
The analytical model aimed to describe state-level trends in overdose deaths involving 1) cocaine and opioids (d = 1) and 2) MOS and opioids (d = 2) in the United States. Let s = 1,…,51 index states and the District of Columbia, r = 1,…,4 index region, and t = 0,…,12 index year for the years 2007 to 2019. We considered trends within g = 1,…,4 racial/ethnic groups. We fitted a hierarchical model to exploit structure and correlations in the data to stabilize estimates for smaller states and racial/ethnic groups. The models for the 2 drug combinations were independent and identical and so will be described in general in what follows.
Data model.
We first specified the data model describing the observed annual count of overdose deaths for each racial/ethnic group in each state, Y(d)stg. We assumed the following generalized linear model:
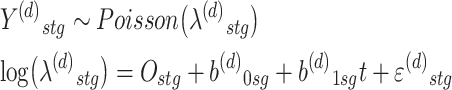 |
where b(d)0sg and b(d)1sg were state- and race/ethnicity-specific intercepts and slopes. We let Ostg = log(Pstg) be an offset where Pstg was the population in year t for racial/ethnic group g in state s. We also let ε(d)stg ~ N(0,σ2d) to account for overdispersion and error.
Process model.
In the process model, we added levels of hierarchy to enable borrowing of strength across states within regions and then across regions within the country. In addition, we allowed race/ethnicity-specific parameters within the same state and within the same region to be correlated, which enabled borrowing strength across racial/ethnic groups within the same geography. We believed this was a reasonable assumption as racial/ethnic groups within a state or region exist within the same social and policy environment, likely leading to correlation in the model parameters. Let b(d)ks = (b(d)ks1,…,b(d)ks4)′ for k = 0,1 and r[s] denote the region for state s. To capture state variation around regional averages, we assumed.
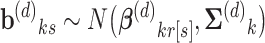 |
where β(d)kr[s] was a region-specific vector with elements for each race/ethnicity and 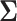 (d)k was a 4 × 4 covariance matrix.
(d)k was a 4 × 4 covariance matrix.
Similarly, there was likely regional variation around national averages. This level of the model enabled borrowing strength across regions, which was reasonable as all regions were within the United States. We assumed
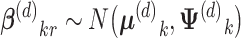 |
where μ(d)k was a vector with elements for each race/ethnicity and Ψ(d)k was a 4 × 4 covariance matrix. Thus, μ(d)k reflected a national average effect.
Prior model and computation.
Since we fitted the model in the Bayesian paradigm, we needed to specify prior distributions for all parameters. Let μ(d)kg be the gth element of μ(d)k. We assumed μ(d)kg ~ N(0, 1002) for all d, k, and g. We also assumed 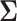 (d)k ~ Inverse Wishart(I4, 5) and Ψ(d)k ~ Inverse Wishart(I4, 5) where I4 was a 4 × 4 identity matrix for all d and k. Variance parameters were assigned inverse gamma distributions with shape and scale parameters of 0.1. We used NIMBLE in R (R Foundation for Statistical Computing, Vienna, Austria) to implement a Markov chain Monte Carlo (MCMC) algorithm (37). The algorithm was run for 500,000 iterations, discarding the first 300,000 as burn-in and thinning the remaining samples by keeping every 20th sample. Convergence was visually assessed using trace plots.
(d)k ~ Inverse Wishart(I4, 5) and Ψ(d)k ~ Inverse Wishart(I4, 5) where I4 was a 4 × 4 identity matrix for all d and k. Variance parameters were assigned inverse gamma distributions with shape and scale parameters of 0.1. We used NIMBLE in R (R Foundation for Statistical Computing, Vienna, Austria) to implement a Markov chain Monte Carlo (MCMC) algorithm (37). The algorithm was run for 500,000 iterations, discarding the first 300,000 as burn-in and thinning the remaining samples by keeping every 20th sample. Convergence was visually assessed using trace plots.
Results were computed by summarizing the posterior distribution. Slope terms were transformed to reflect rate ratios and annual percent change. The slope for each non-White racial group was compared with the slope for the White group to characterize differences in the trends over time. Posterior probabilities were computed to help characterize uncertainty around each point estimate.
This research was deemed exempt from oversight by the New York University Grossman School of Medicine Institutional Review Board (38).
RESULTS
Cocaine and opioids
Figure 1 shows the estimated posterior mean cocaine/opioid death rates per 100,000 people for 2007 and 2019 for each state and racial/ethnic group. The average population across the study period for each racial/ethnic group in each state is shown in Web Figure 1. In 2007, the estimated national cocaine/opioid overdose death rate per 100,000 was 0.49 for non-Hispanic White, 0.60 for non-Hispanic Black, 0.14 for Hispanic, and 0.03 for AAPI people. In 2019, the estimated national rate per 100,000 was 1.39 for non-Hispanic White people (184% increase), 4.05 for non-Hispanic Black people (575% increase), 1.16 for Hispanic people (729% increase), and 0.32 for AAPI people (967% increase). Figure 1 shows that most states experienced increased death rates, 2007–2019. In particular, rates appeared higher across the northeast and southwest for both White and Black people. We also noted elevated rates for the Black population in 2019 across the Midwest.
Figure 1 .
Continues
The center column of Figure 1 characterizes the change over time by the annual percent change from 2007 to 2019, as derived from the posterior mean slope of the annual log death rate. Web Figure 2a shows the posterior probability that the slope was positive (i.e., increasing death rate over time). In general, across racial/ethnic groups, we observed increases in the cocaine/opioid overdose death rate, particularly in the eastern United States, with smaller increases and some decreases across the upper Midwest and western United States
Figure 2 compares the estimated change for non-Hispanic Black, Hispanic, and AAPI people with the change among non-Hispanic White people and shows the posterior probability that mortality in the non-White group increased faster. In general, rates increased faster in each of the non-White groups than in the White group but particularly so across the south and southeast in non-Hispanic Black and Hispanic people. This can also be seen in Table 1, which shows the posterior mean estimated rate ratio for each region and racial/ethnic group per 1-year increase in time. For example, in the south, the rate increased 26% per year in Black people and 27% per year in Hispanic people, respectively, compared with 12% per year in non-Hispanic White people. Similar patterns are shown in Web Figure 3a, where only completely recorded deaths were included. Annual percent change estimates for each state and racial/ethnic group are displayed numerically in Web Table 2.
Figure 2.
Posterior mean estimates of the difference in 2007–2019 slope of combined cocaine/opioid overdose mortality rates, United States, for Black (A) Hispanic (C), and Asian American and Pacific Islander (AAPI) (E) Americans compared with the White population (e.g., Black minus White, and so on), and the posterior probability that the slope is greater than that of the White population for Black (B), Hispanic (D), and AAPI (F) Americans.
Table 1.
Estimated Annual Change in Mortality (Rate Ratioa) According to Drug Combination, Racial/Ethnic Group, and Census Region, United States, 2007–2019
| Drug Combination | Northeast | Midwest | South | West | ||||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CrI | RR | 95% CrI | RR | 95% CrI | RR | 95% CrI | |
| Cocaine and opioids | ||||||||
| Non-Hispanic White | 1.21 | 1.08, 1.35 | 1.08 | 0.98, 1.19 | 1.12 | 1.03, 1.21 | 0.99 | 0.90, 1.09 |
| Non-Hispanic Black | 1.25 | 1.11, 1.40 | 1.16 | 1.05, 1.30 | 1.26 | 1.16, 1.37 | 1.02 | 0.91, 1.14 |
| Hispanic | 1.25 | 1.10, 1.43 | 1.23 | 1.09, 1.39 | 1.27 | 1.15, 1.41 | 1.03 | 0.92, 1.16 |
| Non-Hispanic AAPI | 1.33 | 1.15, 1.54 | 1.26 | 1.09, 1.45 | 1.16 | 1.02, 1.31 | 1.08 | 0.95, 1.23 |
| MOS and opioids | ||||||||
| Non-Hispanic White | 1.39 | 1.24, 1.55 | 1.35 | 1.22, 1.49 | 1.37 | 1.26, 1.49 | 1.25 | 1.13, 1.37 |
| Non-Hispanic Black | 1.66 | 1.42, 1.93 | 1.72 | 1.51, 1.95 | 1.57 | 1.42, 1.75 | 1.33 | 1.19, 1.49 |
| Hispanic | 1.47 | 1.28, 1.68 | 1.40 | 1.24, 1.58 | 1.37 | 1.25, 1.52 | 1.28 | 1.16, 1.41 |
| Non-Hispanic AAPI | 1.42 | 1.19, 1.74 | 1.35 | 1.16, 1.59 | 1.25 | 1.09, 1.43 | 1.26 | 1.11, 1.44 |
Abbreviations: AAPI, Asian American and Pacific Islander; CrI, credible interval; MOS, methamphetamine and other substances; RR, rate ratio.
a Estimates refer to the posterior mean of the RR per 1-year change for each region, according to racial/ethnic group, from 2007 to 2019.
Methamphetamines and other stimulants and opioids
In Figure 3, we show the posterior mean death rates for each racial/ethnic group and state in 2007 and 2019 for MOS and opioids. At the national level in 2007, the posterior mean estimated rate per 100,000 people was 0.09 for non-Hispanic White, 0.01 for non-Hispanic Black, 0.02 for Hispanic, and 0.01 for AAPI people. In 2019, the estimated rate per 100,000 was 2.97 for non-Hispanic White people (3,200% increase), 1.63 for Black people (16,200% increase), 0.94 for Hispanic people (4,600% increase), and 0.25 for AAPI people (2,400% increase). Looking by state, we see higher rates in 2007 primarily among White people in the West. By 2019, rates were higher across the country, with the highest rates across all racial/ethnic groups in the West and in Midwest.
Figure 3 .
Continues
Examining the slopes in Figure 3, we observe that death rates have been increasing across the entire country for all racial/ethnic groups from 2007–2019. The fastest increases occurred across the eastern portion of the Midwest. This is particularly notable among Black people. In Figure 4, we show that death rates increased faster among non-Hispanic Black people than non-Hispanic White people across the entire country, with the largest difference in the Midwest. Among Hispanic people, we see that rates increased faster than among non-Hispanic White people in the West, Northeast, and upper Midwest. Rates among AAPI increased faster than among White people primarily along the West and Northeast. These trends can also be seen in the regional estimates in Table 1. We note that rates among non-Hispanic Black people increased 66%, 72%, and 57% per year in the Northeast, Midwest, and South, respectively, and were the fastest increasing rates among all racial/ethnic groups and regions. Annual percent change estimates for each state and racial/ethnic group are displayed numerically in Web Table 2. In Web Figure 3a, we show similar patterns when only completely observed deaths are included in the analysis.
Figure 4.
Posterior mean estimates of the difference in 2007–2019 slope of combined MOS/opioid overdose mortality rates, United States, for Black (A), Hispanic (C), and Asian American and Pacific Islander (AAPI) (E) Americans compared with the White population (e.g., Black minus White, and so on), and the posterior probability that the slope is greater than that of the White population for Black (B), Hispanic (D), and AAPI (F) Americans.
DISCUSSION
To our knowledge, this study is the first to estimate race/ethnicity-specific trends in combined stimulant/opioid overdose mortality by geography. Using a Bayesian hierarchical model to borrow strength and leverage common structure across groups and states, we were able to overcome challenges posed by small counts for Hispanic and AAPI people in many states. Our findings suggest that, between 2007 and 2019, non-Hispanic Black Americans in many states experienced sharper increases in both cocaine/opioid and MOS/opioid mortality than non-Hispanic White Americans. These increases have been particularly notable in eastern states; indeed, the largest estimated Black/White disparity in trends occurred in combined MOS/opioid mortality in the Midwest, although absolute rates of MOS/opioid mortality in non-Hispanic Black people remain higher in many western states than in the eastern United States. We also observed sharply increasing stimulant/opioid overdose rates in Hispanic and AAPI populations, up from comparatively low levels in 2007; for cocaine/opioid overdose, these increases were concentrated in eastern and southern states. While non-Hispanic White Americans in 12 states experienced stable or declining cocaine/opioid mortality over 2007–2019, this was true for non-Hispanic Black, Hispanic, and AAPI populations in only a few states.
The heterogeneity in results by race/ethnicity and state underscores the need for disaggregated analysis of overdose trends, which was enabled by our innovative methodological approach. For example, among most groups, trends in MOS/opioid mortality varied relatively little across geographical regions; among non-Hispanic Black Americans, in contrast, these upward trends were particularly steep in the Midwest and northern and central Appalachia. That is, states that were among the hardest hit by the first wave of the opioid overdose crisis (39) are now experiencing a new wave of increased combined stimulant/opioid mortality concentrated in non-Hispanic Black people; however, corresponding treatment capacity is sorely lacking in many of these settings and barriers to harm reduction programs are numerous (28, 40–43). Moreover, while regional differences were evident, our results revealed a number of outlier states. Across groups and substances, for example, combined stimulant/opioid mortality was estimated to be consistently more severe in New Mexico than in other southwestern states. Similarly, Hispanic people in Louisiana, Indiana, and Maine experienced sharp increases in cocaine/opioid mortality, while non-Hispanic White and Black people in those states did not. Our method of enabling information-sharing between groups to allow estimates for small samples may be useful for future research in substance use epidemiology—for example, when examining the intersection of multiple marginalized identities or, given hyperlocal variation in the overdose crisis and its evolution (44, 45), when obtaining geographically granular results.
Our findings provide further evidence that the opioid overdose crisis is not limited to non-Hispanic White Americans and that, in many states, it may be worsening more rapidly among non-Hispanic Black, Hispanic, and in some cases AAPI populations compared with the non-Hispanic White population (30). Scholarship, policies, and media narratives should reflect this reality (46–48). Locally informed efforts to address rising stimulant/opioid mortality across groups, and particularly among non-Hispanic Black and Hispanic Americans, are urgently needed. Moreover, overdose prevention efforts should target not only people who use opioids but also those who primarily use cocaine, methamphetamine, and/or other stimulants, who may not recognize a need for naloxone or fentanyl test strips. Due to pernicious effects of structural and systemic racism, access to harm reduction and evidence-based substance use disorder treatment services in predominantly Black and Hispanic neighborhoods is lacking; increased state and federal funding for these programs is needed (28, 49, 50). We recommend that states and other jurisdictions consider implementing supervised consumption facilities (i.e., overdose prevention sites), which White, Black, and Hispanic people who use drugs have shown a high degree of willingness to use (51, 52). Culturally informed approaches, including offering referrals to harm reduction and treatment programs in trusted settings like Black churches, may improve engagement amid low access to and trust in health care among Black Americans (53). Finally, information and services should be provided in the language(s) relevant to a given community to ensure access (8).
Figure 3.
Levels and trends in combined methamphetamine and other stimulants (MOS)/opioid mortality, by state and racial/ethnic group, United States, 2007–2019. Posterior mean estimates of the combined MOS/opioid overdose mortality rates per 100,000 people in 2007 for White (A), Black (D), Hispanic (G), and Asian American and Pacific Islander (AAPI) (J) Americans and for 2019 for White (B), Black (E), Hispanic (H), and AAPI (K) Americans. Posterior mean estimates of the annual percent change (APC) in the mortality rate from 2007 to 2019 for White (C), Black (F), Hispanic (I), and AAPI (L) Americans.
A better understanding of the causes of rising stimulant/opioid overdose mortality, and its particular prominence among Black and Hispanic Americans, is needed. Changing use or use disorder patterns may partly explain the racially disparate trends. While methamphetamine use has historically been lower among non-Hispanic Black people compared with non-Hispanic White people, prevalence of methamphetamine use disorder and substance use treatment admissions related to methamphetamine use have increased disproportionately among Black Americans in recent years (54, 55). It is not clear whether this explanation applies to disparate increases in cocaine/opioid mortality among non-Hispanic Black and Hispanic Americans. While prevalence of any cocaine use does not appear to have increased more rapidly in non-Hispanic Black or Hispanic populations compared with non-Hispanic White people nationally (56), it is not clear whether temporal changes in frequency of cocaine use have varied by racial/ethnic group.
It is unknown, moreover, what proportion of the observed deaths are due to inadvertent fentanyl exposure versus intentional concomitant and/or combined stimulant/opioid use and how this varies by race/ethnicity and geography. Attention to individuals’ underlying reasons for co-use is likely necessary to reduce it and related harms. For example, emerging evidence suggests that people, including those already using opioids, may initiate methamphetamine use in response to homelessness to remain alert and mobile amid continuous vulnerability and local policies against dwelling in public spaces (17, 57, 58). These findings highlight the need to address upstream determinants of health, including poverty and homelessness. Similarly, evidence that methamphetamine is often used to manage symptoms of opioid withdrawal when heroin is unavailable underscores the need to reduce incidence of withdrawal, for instance via improved access to low-threshold buprenorphine services and methadone, and to a safe opioid supply (1, 49, 59).
This study has several limitations. First, we were unable to provide further levels of disaggregation, although they may reveal important heterogeneity. For example, a recent study revealed variation in national overdose mortality by Hispanic subgroup: In 2017, Hispanic people of Puerto Rican heritage, but not other groups (i.e., Dominican, Cuban, Mexican, South American, Central American), experienced higher age-adjusted overdose mortality than non-Hispanic White Americans (8). Second, heterogeneity in race/ethnicity-specific trends by gender, sexual identity, and at more granular geographical levels is also likely (60, 61), but further disaggregation amplifies sample size constraints. However, many policies with impacts on substance use and overdose are state-level, making it a meaningful level of analysis. Third, due to small counts, we were unable to examine state-specific trends in combined stimulant/opioid use among the American Indian and Alaska Native population; yet mortality due to MOS is particularly high in this group and may be increasing rapidly (3). Fourth, misclassification of AAPI identity in vital statistics data is greater in the Midwest, Northeast, and South than in the West (36); estimates in the former regions may therefore understate the frequency of combined stimulant/opioid overdose. Fifth, while our imputation procedure aimed to account for potential racial/ethnic differences in the frequency with which overdose-involved substances are unspecified in death certificate data (62), misclassification of the underlying cause of death as overdose (or not) may also occur and could potentially vary by race/ethnicity. This has not to our knowledge been studied, but differential misclassification by race/ethnicity has been observed in documentation of suicide (63). Finally, variability in the imputed counts is underestimated due to the use of single imputation, which cannot fully propagate variability due to underreporting. Nonetheless, our method offers a promising approach for substance use epidemiologists encountering small counts in disaggregated analyses.
In conclusion, combined stimulant/opioid use and overdose represent a growing problem, likely due to both increased fentanyl contamination of nonopioid drugs and intentional co-use. Between 2007 and 2019, rising stimulant/opioid overdose mortality was particularly consistent and severe among non-Hispanic Black Americans, especially in the Midwest and in northern and central Appalachia. By 2019, rates of cocaine/opioid mortality were considerably higher in 47 states among non-Hispanic Black Americans than among non-Hispanic White Americans. In addition, Hispanic people and AAPI people experienced sharp increases in stimulant/opioid mortality, serving as a potential early warning of worsening outcomes in these groups. Expanded policies and programs to reduce harms related to combined stimulant/opioid use are urgently needed, including access to evidence-based treatment for substance use disorder, widespread provision of harm reduction services, including to people who do not intentionally use opioids, and policies to address upstream social determinants of stimulant and opioid use.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Center for Opioid Epidemiology and Policy, Division of Epidemiology, Department of Population Health, New York University Grossman School of Medicine, New York, New York, United States (Tarlise Townsend, Ariadne Rivera-Aguirre, Amanda M. Bunting, Magdalena Cerdá); Rory Meyers College of Nursing, New York University, New York, New York, United States (Tarlise Townsend); Center for Biostatistics, Department of Biomedical Informatics, The Ohio State University, Columbus, Ohio, United States (David Kline); Department of Biostatistics and Data Science, Wake Forest University School of Medicine, Winston-Salem, North Carolina, United States (David Kline); Section on Tobacco, Alcohol, and Drug Use, Department of Population Health, New York University Grossman School of Medicine, New York, New York, United States (Amanda M. Bunting); Department of Epidemiology, Columbia University Mailman School of Public Health, New York, New York, United States (Pia M. Mauro, Silvia Martins); and Department of Epidemiology, Brown University School of Public Health, Providence, Rhode Island, United States (Brandon D. L. Marshall).
T.T. and D.K. contributed equally to this study as first authors.
This work was supported by the National Institute on Drug Abuse (grants 5T32DA007233-37, K01DA045224, K01DA053435, R01-DA046620, R21DA045236, and R25DA037190) and the New York University Center for Opioid Epidemiology and Policy.
The authors do not have permission to share restricted-use mortality data used in this study. The data are available upon request at the National Center for Health Statistics website (https://www.cdc.gov/nchs/nvss/nvss-restricted-data.htm).
The authors thank Dr. Nicole Kravitz-Wirtz and William Ponicki for their contributions to this research.
Conflict of interest: none declared.
REFERENCES
- 1. Ellis MS, Kasper ZA, Cicero TJ. Twin epidemics: the surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend. 2018;193:14–20. [DOI] [PubMed] [Google Scholar]
- 2. Gladden RM, O’Donnell J, Mattson CL, et al. Changes in opioid-involved overdose deaths by opioid type and presence of benzodiazepines, cocaine, and methamphetamine—25 States, July–December 2017 to January–June 2018. MMWR Morb Mortal Wkly Rep. 2019;68(34):737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kariisa M, Scholl L, Wilson N, et al. Drug overdose deaths involving cocaine and psychostimulants with abuse potential—United States, 2003–2017. MMWR Morb Mortal Wkly Rep. 2019;68(17):388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Center for Health Statistics . Co-Involvement of Opioids in Drug Overdose Deaths Involving Cocaine and Psychostimulants. Hyattsville, MD: National Center for Health Statistics; 2021. (Data Brief No. 406). https://pubmed.ncbi.nlm.nih.gov/33814035/. Accessed December 2, 2021. [Google Scholar]
- 5. National Center for Health Statistics . Drug Overdose Deaths in the United States, 1999–2018. Hyattsville, MD: National Center for Health Statistics; 2020. (Data Brief No. 356). https://stacks.cdc.gov/view/cdc/84647. Accessed December 2, 2021. [Google Scholar]
- 6. Hoots B, Vivolo-Kantor A, Seth P. The rise in non-fatal and fatal overdoses involving stimulants with and without opioids in the United States. Addiction. 2020;115(5):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hansen H, Parker C, Netherland J. Race as a ghost variable in (White) opioid research. Sci Technol Hum Val. 2020;45(5):848–876. [Google Scholar]
- 8. Cano M. Drug overdose deaths among US Hispanics: trends (2000–2017) and recent patterns. Subst Use Misuse. 2020;55(13):2138–2147. [DOI] [PubMed] [Google Scholar]
- 9. Cano M. Racial/ethnic differences in US drug overdose mortality, 2017–2018. Addict Behav. 2021;112:106625. [DOI] [PubMed] [Google Scholar]
- 10. Amlani A, McKee G, Khamis N, et al. Why the FUSS (fentanyl urine screen study)? A cross-sectional survey to characterize an emerging threat to people who use drugs in British Columbia, Canada. Harm Reduct J. 2015;12(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Macmadu A, Carroll JJ, Hadland SE, et al. Prevalence and correlates of fentanyl-contaminated heroin exposure among young adults who use prescription opioids non-medically. Addict Behav. 2017;68:35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodwin RD, Moeller SJ, Zhu J, et al. The potential role of cocaine and heroin co-use in the opioid epidemic in the United States. Addict Behav. 2021;113:106680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez AM, Dhatt Z, Howe M, et al. Co-use of methamphetamine and opioids among people in treatment in Oregon: a qualitative examination of interrelated structural, community, and individual-level factors. Int J Drug Policy. 2021;91:103098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glick SN, Klein KS, Tinsley J, et al. Increasing heroin-methamphetamine (goofball) use and related morbidity among Seattle area people who inject drugs. Am J Addict. 2021;30(2):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nolan ML, Shamasunder S, Colon-Berezin C, et al. Increased presence of fentanyl in cocaine-involved fatal overdoses: implications for prevention. J Urban Health. 2019;96(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schneider KE, O’Rourke A, White RH, et al. Polysubstance use in rural West Virginia: associations between latent classes of drug use, overdose, and take-home naloxone. Int J Drug Policy. 2020;76:102642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al-Tayyib A, Koester S, Langegger S, et al. Heroin and methamphetamine injection: an emerging drug use pattern. Subst Use Misuse. 2017;52(8):1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herbeck DM, Brecht ML, Lovinger K, et al. Poly-drug and marijuana use among adults who primarily used methamphetamine. J Psychoactive Drugs. 2013;45(2):132–140. [DOI] [PubMed] [Google Scholar]
- 19. Jones CM, Underwood N, Compton WM. Increases in methamphetamine use among heroin treatment admissions in the United States, 2008–17. Addiction. 2020;115(2):347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meacham MC, Rudolph AE, Strathdee SA, et al. Polydrug use and HIV risk among people who inject heroin in Tijuana, Mexico: a latent class analysis. Subst Use Misuse. 2015;50(10):1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meacham MC, Strathdee SA, Rangel G, et al. Prevalence and correlates of heroin-methamphetamine co-injection among persons who inject drugs in San Diego, California, and Tijuana, Baja California, Mexico. J Stud Alcohol Drugs. 2016;77(5):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meacham MC, Roesch SC, Strathdee SA, et al. Perceived treatment need and latent transitions in heroin and methamphetamine polydrug use among people who inject drugs in Tijuana. J Psychoactive Drugs. 2018;50(1):62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L, Min JE, Krebs E, et al. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int J Drug Policy. 2017;49:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mojtabai R, Mauro C, Wall MM, et al. Medication treatment for opioid use disorders in substance use treatment facilities. Health Aff. 2019;38(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hadland SE, Jent VA, Alinsky RH, et al. Opioid use disorder treatment facilities with programs for special populations. Am J Prev Med. 2020;59(3):e125–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones CM, Campopiano M, Baldwin G, et al. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carroll KM. Lost in translation? Moving contingency management and cognitive behavioral therapy into clinical practice. Ann N Y Acad Sci. 2014;1327(1):94–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ronsley C, Nolan S, Knight R, et al. Treatment of stimulant use disorder: a systematic review of reviews. PLoS One. 2020;15(6):e0234809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Compton WM, Volkow ND, Throckmorton DC, et al. Expanded access to opioid overdose intervention: research, practice, and policy needs. Ann Intern Med. 2013;158(1):65–66. [DOI] [PubMed] [Google Scholar]
- 30. Kariisa M, Seth P, Scholl L, et al. Drug overdose deaths involving cocaine and psychostimulants with abuse potential among racial and ethnic groups—United States, 2004–2019. Drug Alcohol Depend. 2021;227:109001. [DOI] [PubMed] [Google Scholar]
- 31. Shiels MS, Freedman ND, Thomas D, et al. Trends in U.S. drug overdose deaths in non-Hispanic Black, Hispanic, and non-Hispanic White persons, 2000–2015. Ann Intern Med. 2018;168(6):453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boslett AJ, Denham A, Hill EL. Using contributing causes of death improves prediction of opioid involvement in unclassified drug overdoses in US death records. Addiction. 2020;115(7):1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buchanich JM, Balmert LC, Williams KE, et al. The effect of incomplete death certificates on estimates of unintentional opioid-related overdose deaths in the United States, 1999–2015. Public Health Rep. 2018;133(4):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruhm CJ. Geographic variation in opioid and heroin involved drug poisoning mortality rates. Am J Prev Med. 2017;53(6):745–753. [DOI] [PubMed] [Google Scholar]
- 35. National Center for Health Statistics . Restricted use—vital statistics data. 2021. https://www.cdc.gov/nchs/nvss/nvss-restricted-data.htm. Accessed January 11, 2021.
- 36. National Center for Health Statistics . The Validity of Race and Hispanic-Origin Reporting on Death Certificates in the United States: An Update. Hyattsville, MD: National Center for Health Statistics; 2016. (DHHS Publication No. 2016-1372). https://pubmed.ncbi.nlm.nih.gov/19024798/. Accessed December 2, 2021. [PubMed] [Google Scholar]
- 37. de Valpine P, Turek D, Paciorek CJ, et al. Programming with models: writing statistical algorithms for general model structures with NIMBLE. J Comput Graph Stat. 2017;26(2):403–413. [Google Scholar]
- 38. NYU Langone Health . Getting started with the institutional review board submission process. 2021. https://med.nyu.edu/research/office-science-research/clinical-research/resources-researchers-study-teams/institutional-review-board-operations/getting-started-the-irb-submission-process#determine-if-your-project-is-human-subjects-research. Accessed December 2, 2021.
- 39. Monnat S, Rigg K. The opioid crisis in rural and small town America. University of New Hampshire Carsey School of Public Policy; 2018. Report No. 135. https://scholars.unh.edu/carsey/343. Accessed July 29, 2021. [Google Scholar]
- 40. Richard EL, Schalkoff CA, Piscalko HM, et al. “You are not clean until you’re not on anything”: perceptions of medication-assisted treatment in rural Appalachia. Int J Drug Policy. 2020;85:102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dombrowski K, Crawford D, Khan B, et al. Current rural drug use in the US Midwest. J Drug Abuse. 2016;2(3):22. [PMC free article] [PubMed] [Google Scholar]
- 42. Baker LS, Smith W, Gulley T, et al. Community perceptions of comprehensive harm reduction programs and stigma towards people who inject drugs in rural Virginia. J Community Health. 2020;45(2):239–244. [DOI] [PubMed] [Google Scholar]
- 43. Allen ST, O’Rourke A, White RH, et al. Perspectives on fentanyl test strip use among people who inject drugs in rural Appalachia. Subst Use Misuse. 2020;55(10):1594–1600. [DOI] [PubMed] [Google Scholar]
- 44. Wagner J, Neitzke-Spruill L, O’Connell D, et al. Understanding geographic and neighborhood variations in overdose death rates. J Community Health. 2019;44(2):272–283. [DOI] [PubMed] [Google Scholar]
- 45. Cobert J, Lantos PM, Janko MM, et al. Geospatial variations and neighborhood deprivation in drug-related admissions and overdoses. J Urban Health. 2020;97(6):814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. James K, Jordan A. The opioid crisis in Black communities. J Law Med Ethics. 2018;46(2):404–421. [DOI] [PubMed] [Google Scholar]
- 47. Netherland J, Hansen H. White opioids: pharmaceutical race and the war on drugs that wasn’t. BioSocieties. 2017;12(2):217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Netherland J, Hansen HB. The war on drugs that wasn't: wasted whiteness, "dirty doctors," and race in media coverage of prescription opioid misuse. Cult Med Psychiatry. 2016;40(4):664–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goedel WC, Shapiro A, Cerdá M, et al. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lagisetty PA, Ross R, Bohnert A, et al. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiat. 2019;76(9):979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Klein KS, Glick SN, Mauro PM. Anticipated use of a supervised drug consumption site among syringe services program clients in King County, Washington: assessing the role of opioid overdose and injection behavior. Drug Alcohol Depend. 2020;213:108121. [DOI] [PubMed] [Google Scholar]
- 52. Park JN, Sherman SG, Rouhani S, et al. Willingness to use safe consumption spaces among opioid users at high risk of fentanyl overdose in Baltimore, Providence, and Boston. J Urban Health. 2019;96(3):353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jordan A, Babuscio T, Nich C, et al. A feasibility study providing substance use treatment in the Black church. J Subst Abuse Treat. 2021;124:108218. [DOI] [PubMed] [Google Scholar]
- 54. Jones CM, Olsen EO, O’Donnell J, et al. Resurgent methamphetamine use at treatment admission in the United States, 2008–2017. Am J Public Health. 2020;110(4):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Han B, Compton WM, Jones CM, et al. Methamphetamine use, methamphetamine use disorder, and associated overdose deaths among US adults. JAMA psychiatry. 2021;78(12):1329–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mustaquim D, Jones CM, Compton WM. Trends and correlates of cocaine use among adults in the United States, 2006–2019. Addict Behav. 2021;120:106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Damon W, McNeil R, Milloy MJ, et al. Residential eviction predicts initiation of or relapse into crystal methamphetamine use among people who inject drugs: a prospective cohort study. J Public Health. 2019;41(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Daniulaityte R, Silverstein SM, Crawford TN, et al. Methamphetamine use and its correlates among individuals with opioid use disorder in a Midwestern U.S. Subst Use Misuse. 2020;55(11):1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ivsins A, Boyd J, Beletsky L, et al. Tackling the overdose crisis: the role of safe supply. Int J Drug Policy. 2020;80:102769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Philbin MM, Greene ER, Martins SS, et al. Medical, nonmedical, and illegal stimulant use by sexual identity and gender. Am J Prev Med. 2020;59(5):686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Althoff KN, Leifheit KM, Park JN, et al. Opioid-related overdose mortality in the era of fentanyl: monitoring a shifting epidemic by person, place, and time. Drug Alcohol Depend. 2020;216:108321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Milam AJ, Furr-Holden D, Wang L, et al. Health data disparities in opioid-involved overdose deaths from 1999 to 2018 in the United States. Am J Public Health. 2021;111(9):1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rockett IR, Wang S, Stack S, et al. Race/ethnicity and potential suicide misclassification: window on a minority suicide paradox? BMC Psychiatry. 2010;10(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








