Abstract
Objectives
To assess the applicability of Electronic Frailty Index (eFI) and Hospital Frailty Risk Score (HFRS) algorithms to Japanese administrative claims data and to evaluate their association with long-term outcomes.
Study Design and Setting
A cohort study using a regional government administrative healthcare and long-term care (LTC) claims database in Japan 2014–18.
Participants
Plan enrollees aged ≥50 years.
Methods
We applied the two algorithms to the cohort and assessed the scores’ distributions alongside enrollees’ 4-year mortality and initiation of government-supported LTC. Using Cox regression and Fine–Gray models, we evaluated the association between frailty scores and outcomes as well as the models’ discriminatory ability.
Results
Among 827,744 enrollees, 42.8% were categorised by eFI as fit, 31.2% mild, 17.5% moderate and 8.5% severe. For HFRS, 73.0% were low, 24.3% intermediate and 2.7% high risk; 35 of 36 predictors for eFI, and 92 of 109 codes originally used for HFRS were available in the Japanese system. Relative to the lowest frailty group, the highest frailty group had hazard ratios [95% confidence interval (CI)] of 2.09 (1.98–2.21) for mortality and 2.45 (2.28–2.63) for LTC for eFI; those for HFRS were 3.79 (3.56–4.03) and 3.31 (2.87–3.82), respectively. The area under the receiver operating characteristics curves for the unadjusted model at 48 months was 0.68 for death and 0.68 for LTC for eFI, and 0.73 and 0.70, respectively, for HFRS.
Conclusions
The frailty algorithms were applicable to the Japanese system and could contribute to the identifications of enrollees at risk of long-term mortality or LTC use.
Keywords: Electronic Frailty Index, Hospital Frailty Risk Score, long-term care, administrative claims data, frailty, older people
Key Points
Evaluation of the transportability of Electronic Frailty Index (eFI) and Hospital Frailty Risk Score (HFRS) to health systems outside the UK is needed.
The usefulness of eFI and HFRS in predicting long-term clinical outcomes is understudied.
In our study, we were able to apply eFI and HFRS algorithms to the Japanese administrative claims database.
eFI and HFRS were able to discriminate those with or without incidences of death or long-term care (LTC) initiation within four years.
eFI and HFRS could contribute to identify older people at increased risk of long-term mortality or in need of LTC.
Introduction
Frailty is an age-related accumulation of physiological debilities, resulting in vulnerability, especially when triggered by clinical events. Previous studies have demonstrated that frail subjects have an increased risk of poor clinical outcomes, such as the requirement of long-term care (LTC) or mortality [1, 2]. With increasing life expectancy, the number of frail older people has increased substantially [3, 4] and has become a major issue [5]. The guideline published by the International Conference of Frailty and Sarcopenia Research task force strongly recommends that older people (aged ≥65 years) be screened with suitable frailty instruments [6]. Identification of frailty can be used to direct older persons towards appropriate interventions and assist them in the maintenance of a healthy and independent lifestyle.
To facilitate the early identification of frail individuals in community settings and subsequent intervention, a validated screening tool, ideally associated with long-term outcomes and the use of healthcare resources, is required. Large-scale healthcare data (e.g. administrative claims data and electronic health records) currently provide information on patients’ clinical encounters, diagnoses and resource use. These data are increasingly used in clinical research, and provide evidence to facilitate clinical and policy decisions, complementing the evidence from clinical trials. To date, several algorithms have been developed to identify and measure frailty using these data, including the Electronic Frailty Index (eFI) [7] and the Hospital Frailty Risk Score (HFRS) [8].
HFRS has been used and largely validated in acute care settings [9–11], whereas eFI has been widely used in general practice in England [12] and converted to other diagnostic codes [13, 14]. However, the international validity of these instruments and their association with long-term outcomes have been insufficiently studied. Therefore, we aimed to assess whether the eFI and HFRS, originally developed in the UK, can be implemented in the Japanese healthcare system. We also evaluated the association between frailty measured using these algorithms with long-term outcomes (i.e. mortality and the initiation for government-supported LTC).
Methods
Study design and data source
We conducted a cohort study using the Shizuoka Kokuho Database (SKDB), an administrative claims database of enrollees in the municipal government health insurance programme in Shizuoka Prefecture, Japan [15]. The SKDB contains health insurance claim data (e.g. monthly claims for patients’ diagnoses, procedures and medications) and LTC insurance claim data. Among residents aged <75 years, 22.3% are enrolled in the National Health Insurance (NHI) and all residents ≥75 are enrolled in the late-stage medical care system for the elderly. All residents aged ≥65 years or those aged 40–64 years with severe illness defined by the government (e.g. advanced cancer, rheumatic disease, amyotrophic lateral sclerosis, cerebrovascular disease) are eligible for LTC insurance. Details of the LTC system are explained in Appendix 1.
We accessed all data on procedures and diagnoses involved in health insurance (NHI and late-stage medical care system for the elderly) claims and the use of LTC insurance for all beneficiaries during April 2012–September 2018. The study was approved by the Institutional Review Board of Shizuoka General Hospital (Shizuoka, Japan) [SGHIRB #2020004].
Participants
We selected insurance enrollees aged ≥50 years on 1 April 2014, from the database. We included enrollees aged ≥50 years to compare the frailty score distributions among those aged 50–64 years with those who were older. We designated April 2014 as the ‘index month’, and the preceding 12-month period as the ‘look-back period’. We excluded any subjects who were ineligible for the health plan during the 24-month period preceding the index month to limit the target cohort to subjects with complete diagnostic records during the look-back period. We also excluded participants who had no recorded diagnosis during the look-back period. The assessment of frailty score distribution and its relationship with age was conducted using this full cohort. To reduce the required computation time, we conducted a time-to-event analysis in a randomly sampled 10% subset of the full analytical cohort. We sampled the enrollees using a simple random sampling method without replacement in SAS (PROC SURVEYSELECT). A longitudinal assessment of LTC service use was conducted among those from the subset of enrollees with no record of LTC service use during the look-back period and who were aged ≥65 years in April 2014 (LTC assessment cohort) (Figure 1). We deleted those aged <65 years because the requirement for the receipt of LTC services differed between participants aged 50–64 and ≥65 years.
Figure 1.
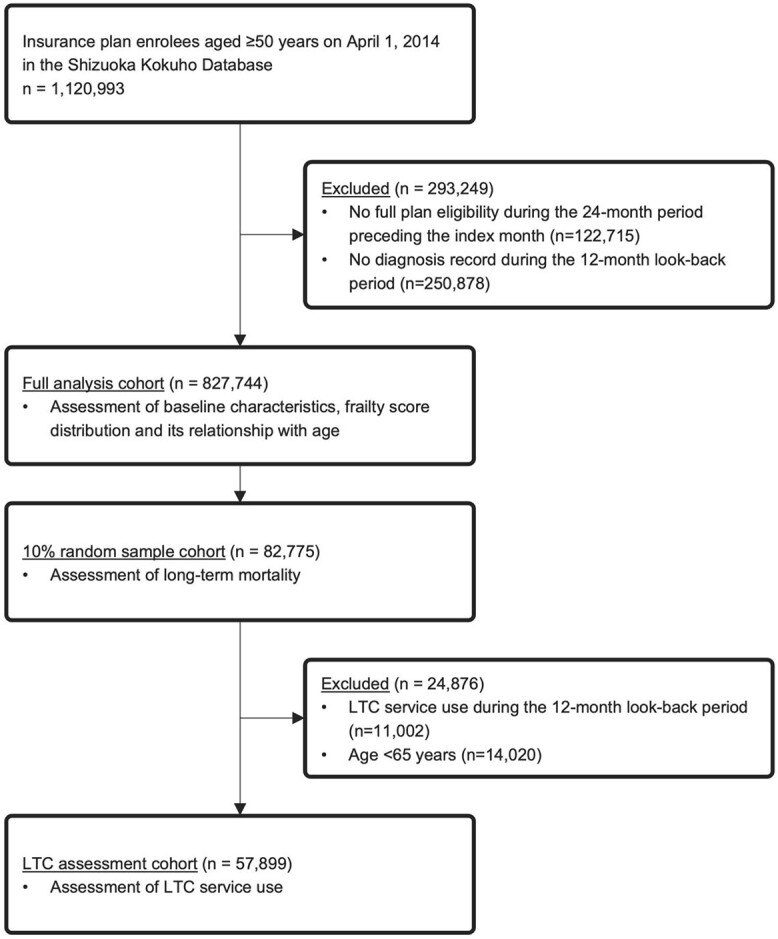
A flow diagram of the enrolment of the study cohort.
Member characteristics
Sex and age data were extracted from the subscriber list, with data on each patient’s diagnosis, medications and LTC service use during the 12-month look-back period obtained from the claims data. Diagnosis codes were extracted with the Japanese electronic claim codes, which are linked to the corresponding International Classification of Diseases 10th version (ICD-10) codes [16].
Frailty measurements
The eFI was developed by Clegg and colleagues based on the cumulative deficit model of frailty. The index is measured as the proportion of 36 equally weighted deficits present in an individual, captured with the Clinical Terms Version 3 Read codes in routinely collected primary care data in the United Kingdom [7, 17]. We used the converted ICD-10 list of deficits based on previous studies (Appendix 2) [13, 14]. We defined ‘polypharmacy’ as the prescription of ≥5 drugs over ≥6 months during the look-back period [18].
The HFRS is a score developed by Gilbert and colleagues from 109 ICD-10 codes. This score was developed to identify patients’ high-risk conditions using routinely collected secondary care hospital administrative data in the United Kingdom [8, 17]. Each ICD-10 code is given a weight of 0.1–7.1, corresponding to the strength of its association with being in the frail subgroup.
For each patient, we calculated eFI and HFRS based on the ICD-10 codes that were recorded in the inpatient and outpatient claims during the 12-month look-back period. We then grouped the members into categories, using the cut-off points described in the original studies [7, 8]: fit (0–0.12), mild frailty (>0.12–0.24), moderate frailty (>0.24–0.36) and severe frailty (>0.36) for eFI; low (<5), intermediate (5–15) and high (>15) risk for HFRS.
Outcomes
The main outcomes of interest were all-cause mortality and the use of government-supported LTC, which—accounting for death as a competing event—was assessed among those beneficiaries with no LTC service use records from the look-back period.
Statistical analysis
We tabulated the baseline characteristics of the plan enrollees. We assessed the distributions of the two frailty scores and assessed their categorisation. Each diagnosis code and polypharmacy’s prevalence was evaluated. Spearman’s correlation coefficient was used to assess the relationship between age and the continuous frailty scores.
We censored participants at the time when their insurance plan enrolment terminated or on 30 September 2018, whichever came first (maximum follow-up period of 53 months). We estimated overall survival for each frailty category using the Kaplan–Meier survival curves and compared them using the log-rank test. We constructed Cox proportional hazard models (with/without adjustment for age and sex) to determine the association between frailty score categories and mortality. We evaluated LTC initiation using cumulative incidence functions with Gray’s test and Fine–Gray’s subdistribution hazard models, with/without adjustment for age and sex. The discrimination was assessed from the time-dependent area under the receiver operating characteristic curves (area under the curve, AUC) using inverse probability weighting [19] for eFI and HFRS for outcomes at 12, 24, 36 and 48 months. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). R software version 3.5 (R Foundation for Statistical Computing, Vienna, Austria) was used to estimate the AUCs of the models with competing risks [20, 21].
Results
Participant characteristics
Among the 827,744 insurance enrollees, the mean (standard deviation, SD) age was 74.2 (10.4) years and 59.4% were female. Participants were followed up for a median (interquartile ranges, IQRs) of 4.4 (4.4–4.4) years. The median (IQRs) frailty measure was 0.14 (0.09–0.26) for eFI and 2.2 (0–5.3) for HFRS (Table 1). The distribution of each score was heavily right skewed, with maximum scores of 0.86 and 51.4, respectively (Appendix 3). Of the study population, 354,023 (42.8%) were categorised by eFI as fit, 258,549 (31.2%) as mildly frail, 145,091 (17.5%) as moderately frail and 70,081 (8.5%) as severely frail. Most participants were categorised by HFRS as low risk (604,211 [73.0%]), followed by intermediate (201,359 [24.3%]) and high (22,174 [2.7%]) risk. Among those aged <65 years (n = 140,774), frail patients were less frequently observed compared to the older patients, with the 2,610 (1.9%) patients categorised as severely frail using eFI and the 707 (0.5%) patients categorised as high risk using HFRS. Upon limiting the enrollees to those included in the original eFI study (i.e. aged ≥65 years), 255,668 (37.2%) were categorised as fit, 227,520 (33.1%) as mildly frail, 136,311 (19.8%) as moderately frail and 67,471 (9.8%) as severely frail. Among the participants aged ≥75 years with ≥1 admission record, the proportions of patients in the intermediate and high-risk groups based on HFRS were 54.1 and 16.7%, respectively (Appendix 5).
Table 1.
Baseline characteristics
| Characteristics | N = 827,744 | ||
|---|---|---|---|
| Age (years), mean (SD) | 74.2 | (10.4) | |
| Age group, N (%) | |||
| 50–64 | 140,774 | (17) | |
| 65–74 | 309,771 | (37.4) | |
| 75–84 | 228,628 | (27.6) | |
| ≥85 | 148,571 | (17.9) | |
| Female, N (%) | 491,497 | (59.4) | |
| Previous LTC service use, N (%) | 109,511 | (13.2) | |
| LTC level, N (%) | |||
| Support level | 21,465 | (2.6) | |
| Care level 1–3 | 63,597 | (7.7) | |
| Care level 4–5 | 24,449 | (3) | |
| Number of medications, median (IQR) | 13 | (6–22) | |
| eFI score, mean (SD) | 0.17 | (0.12) | |
| HFRS score, mean (SD) | 3.6 | (4.3) | |
| eFI frailty category, N (%) | |||
| Fit | 354,023 | (42.8) | |
| Mild | 258,549 | (31.2) | |
| Moderate | 145,091 | (17.5) | |
| Severe | 70,081 | (8.5) | |
| HFRS frailty category, N (%) | |||
| Low | 604,211 | (73.0) | |
| Intermediate | 201,359 | (24.3) | |
| High | 22,174 | (2.7) | |
Prevalence of predictors and relationships with age
We derived both HFRS and eFI using Japanese electronic claim codes linked to the corresponding ICD-10 codes. Appendix 6A and B list the variables and corresponding codes used for these measurements, together with their prevalence in the cohort. For HFRS, codes corresponding to 92 of the 109 ICD-10 diagnostic codes were available. The prevalence of each variable in the HFRS increased with age. Among the highly weighted diagnostic codes, Alzheimer’s disease and the sequelae of cerebrovascular disease were markedly more prevalent in people aged ≥85 years (15.2 and 12.9%, respectively) than in those aged 50–64 years (0.2 and 1.9%, respectively) (Appendix 6A). For eFI, which consists of 36 variables, 35 were identified in the Japanese coding system, the exception being the code for ‘falls’. Similar to the HFRS variables, many of the deficits had a much higher prevalence in people aged ≥85 years than in those aged 50–64 years (Appendix 6B). The prevalence of polypharmacy, cardiovascular diseases and osteoporosis was highest among people aged ≥85 years. The proportion of subjects classified as severely frail increased with age: from 5.6% among 50–64 years olds to 37.9% among those aged ≥85 years for eFI, and from 8.9 to 49.4%, respectively, for HFRS (Figure 1). Spearman’s correlation coefficients between two frailty scores and age were 0.43 [95% confidence interval (CI), 0.427–0.439] for eFI and 0.42 (95% CI, 0.415–0.427) for HFRS.
Risk of mortality and use of LTC service
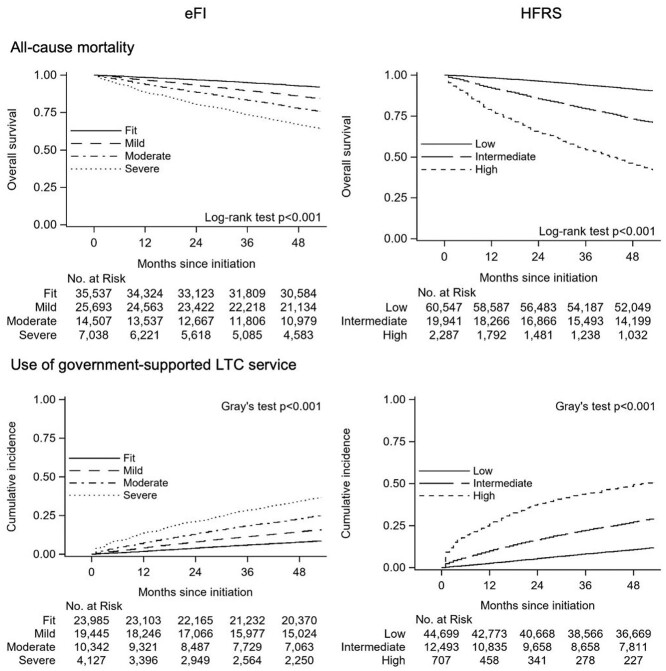
Among a 10% random sample of participants (n = 82,775), all-cause mortality per 100 person year was 3.9. The survival rate at 48 months decreased with increasing severity of the frailty category, from 92.8% (95% CI, 92.5–93.0%) in the fit group to 66.6% (95% CI, 65.5–67.7%) in the severely frail group according to eFI categories, and from 91.4% (95% CI, 91.2–91.6%) in the low-risk group to 45.6% (95% CI, 43.5–47.6%) in the high-risk group according to HFRS category (Figure 2). The hazard of death increased alongside the severity of frailty, with/without adjustment for age/sex: adjusted hazard ratio (HR) 2.09 (95% CI, 1.98–2.21) for severely frail subjects relative to fit subjects according to eFI, and HR 3.79 (95% CI, 3.56–4.03) for high-risk subjects relative to low-risk subjects according to HFRS (Table 2).
Figure 2.
Kaplan–Meier survival curves for mortality and cumulative incidence functions of government-supported LTC service use for up to 53 months according to frailty measures derived from healthcare claims data.
Table 2.
Results of Cox proportional hazard model regression for mortality and Fine–Gray proportional subdistribution hazard model regression for use of government-supported long-term care services among participants who used no long-term care services during the look-back period
| eFI | HFRS | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of events(*) | Unadjusted | Adjusted† | No. of events(*) | Unadjusted | Adjusted† | ||||||||||||
| Outcome | HR (95% CI)¶ | HR (95% CI)¶ | HR (95% CI)¶ | HR (95% CI)¶ | |||||||||||||
| Mortality‡ | 12,580 | (3.9) | 12,580 | (3.9) | |||||||||||||
| Fit | 2,714 | (1.9) | 1 | - | 1 | - | Low | 5,573 | (2.3) | 1 | – | 1 | – | ||||
| Mild | 3,920 | (3.8) | 2.05 | (1.95–2.15) | 1.22 | (1.16–1.28) | Intermediate | 5,695 | (7.8) | 3.45 | (3.33–3.58) | 1.87 | (1.80–1.95) | ||||
| Moderate | 3,460 | (6.3) | 3.35 | (3.19–3.53) | 1.52 | (1.45–1.60) | High | 1,312 | (20.1) | 8.99 | (8.47–9.55) | 3.79 | (3.56–4.03) | ||||
| Severe | 2,486 | (10.1) | 5.43 | (5.14–5.73) | 2.09 | (1.98–2.21) | |||||||||||
| Use of LTC services§ | 9,264 | (3.9) | 9,264 | (3.9) | |||||||||||||
| Fit | 2,073 | (2.1) | 1 | – | 1 | – | Low | 5,284 | (2.8) | 1 | – | 1 | – | ||||
| Mild | 3,100 | (3.9) | 1.9 | (1.80–2.01) | 1.3 | (1.23–1.38) | Intermediate | 3,623 | (7.4) | 2.74 | (2.63–2.86) | 1.75 | (1.67–1.83) | ||||
| Moderate | 2,584 | (6.2) | 3.14 | (2.97–3.33) | 1.7 | (1.60–1.80) | High | 357 | (15.2) | 5.99 | (5.32–6.74) | 3.31 | (2.87–3.82) | ||||
| Severe | 1,507 | (9.5) | 5.03 | (4.71–5.38) | 2.45 | (2.28–2.63) | |||||||||||
*Event rate per 100 person years.
†Adjusted for age and sex.
‡ n = 82,775.
§ n = 57,899; 24,876 participants aged ≤65 years or those with the records of the use of LTC service during the look-back period were excluded.
¶HRs for the use of LTC service were estimated using Fine–Gray’s subdistribution hazard models.
In participants aged ≥65 years with no previous LTC service use (n = 57,899), the event rate of the LTC service initiation was 3.9 per 100 person years. We observed a similar relationship between frailty severity and the 48-month cumulative incidence of LTC service use: from 7.9% (95% CI, 7.5–8.2%) in the fit group to 34.5% (95% CI, 33.0–35.9%) in the severely frail group for eFI, and from 10.8% (95% CI, 10.5–11.0%) in the low-risk group to 49.4% (95% CI, 45.6–53.0%) in the high-risk group for HFRS (Figure 2). We observed an increase in the incidence of LTC service initiation with increasing frailty severity; even after adjusting for age and sex. The subdistribution hazard ratio (sHR) for the severely frail compared with fit was 2.45 (95% CI, 2.28–2.63) on eFI, and the adjusted sHR for high risk compared with low-risk subjects was 3.31 (95% CI, 2.87–3.82) on HFRS (Table 2). Appendices 7 and 8 show the Kaplan–Meier survival curves and cumulative incidence functions stratified by age group, respectively. Mortality and incidence of LTC service use increased with age. Separation of the curves was most pronounced in the 75–84 years age group. Depicted separation was also more pronounced across the HFRS than eFI categories.
Model discrimination
The two algorithms discriminated the participants well at each time point. The AUC for mortality on HFRS (base model: 0.77 at 12 months, 0.73 at 48 months) was slightly superior to that on eFI (0.70 and 0.68, respectively). Among people aged ≥65 years with no history of LTC service use during the look-back period, the AUCs for LTC service use outcome at 12 and 48 months were 0.71 and 0.68, respectively, for eFI, and 0.75 and 0.70, respectively, for HFRS (Table 3). After adjustment for age and sex, the 48-month AUC for LTC service use was similar for eFI (48-month AUCs: mortality = 0.82, LTC service use = 0.84) and HFRS (0.84 and 0.85, respectively).
Table 3.
Time-dependent AUC estimates for mortality and government-supported long-term care service use
| Outcome | AUC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 12 months | 24 months | 36 months | 48 months | ||||||
| Base model | + Age, sex | Base model | + Age, sex | Base model | + Age, sex | Base model | + Age, sex | ||
| Mortality* | |||||||||
| eFI | 0.70 | 0.81 | 0.69 | 0.82 | 0.68 | 0.82 | 0.68 | 0.82 | |
| HFRS | 0.77 | 0.83 | 0.75 | 0.83 | 0.73 | 0.83 | 0.73 | 0.84 | |
| Use of LTC services† | |||||||||
| eFI | 0.71 | 0.82 | 0.68 | 0.83 | 0.68 | 0.84 | 0.68 | 0.84 | |
| HFRS | 0.75 | 0.84 | 0.72 | 0.84 | 0.70 | 0.84 | 0.70 | 0.85 | |
* n = 82,775.
† n = 57,899; 24,876 participants aged ≤65 years or those with the records of the use of LTC service during the look-back period were excluded.
Discussion
We applied two frailty scores, eFI and HFRS, to a Japanese health systems database. Although several diagnostic codes in the algorithms were unavailable in the Japanese coding system, the application of these algorithms resulted in frailty score distributions with good variation, similar to those reported in the original cohorts in the UK [7]. Frailty scores were strongly associated with the incidences of death and LTC service use, and showed acceptable discriminatory power (AUC > 0.68), supporting their transferability to the Japanese systems. The HFRS better discriminated the outcomes than the eFI at each time point.
The observed right-skewed distributions were similar to those reported in the original and other externally validated studies [7, 8, 22]. eFI was developed within an ambulatory population. eFI scores in our cohort were higher (fit, 43%; mild frailty, 31%; moderate frailty, 18%; severe frailty, 9%) than in the original study (fit, 50%; mild frailty, 35%; moderate frailty, 12%; severe frailty, 3%). Several deficits (e.g. cerebrovascular disease, diabetes mellitus, heart failure and peptic ulcer) were more prevalent in our cohort than in the original. The HFRSs in our cohort were lower (low risk, 73%; intermediate risk, 24%; high risk, 3%) than those in a previous study constructed with a 1-year look-back window (low risk, 41%; intermediate risk, 37%; high risk, 22%) [23]. This is probably attributable to the inclusion in our cohort of health plan enrollees without admission records. Upon restricting the participants to those aged ≥75 years with a history of one or more than one admissions, the prevalence of frailty became more similar to the previous study, and most patients were in the intermediate-risk category. Fewer participants were classified as low or high risk than in the previous study. This could be partly explained by our use of diagnostic records from both inpatient and outpatient claims.
Among the 36 deficits used in the algorithm to capture the original eFI using ICD-10 codes, one deficit, ‘fall’, was missing from the Japanese claim codes. Among the 109 codes used to define HFRS in the original study, 17 were missing. ‘Fall’, which is included in both eFI and HFRS, is one of the key factors used to measure frailty, given its high prevalence and strong association with frailty [24, 25]. Missing such a component from the algorithm to capture frailty possibly distorted the assessment of frailty relative to the originally intended concept. However, several diagnostic codes included in score measurements are strongly associated with falls [26]. In our study, codes for fragility fractures within the HFRS and eFI, such as fractures of the proximal humerus (included as part of ICD 10 code S42; fracture of shoulder and upper arm), distal radius (included as part of S52; fracture of forearm), and femoral neck and trochanteric fracture (included as part of S72; fracture of femur), were observed. These codes may have acted as proxies for ‘falls’ in our study, reducing the impact of missingness.
The long-term prognoses of frail patients have been evaluated with the eFI [7, 27]. HFRS is commonly used in studies evaluating clinical outcomes during hospitalisation or after discharge, [9, 11, 28] but the association with long-term outcomes is less studied. In our assessment, the discrimination of the HFRS model was better than that of the eFI model at each time point, supporting its potential utility in studies of long-term outcomes. At the same time, both frailty scores were associated with both outcomes in our study, even after stratification by age group. This supports the notion that these frailty scores identify frailty-specific conditions, independent of age.
The two frailty scores were clearly associated with mortality and LTC initiation, which is consistent with past prospective studies using clinically measured frailty scores [29–31]. The relationship between frailty and LTC resource utilisation is especially important in the context of LTC insurance system sustainability in ageing societies. In prior analyses, the need for help from others was considered as a proxy for frailty [32–34]. Another study developed a frailty score from healthcare claim data using activities of daily living dependency as an outcome [34]. Our study adds support to the longitudinal association between frailty and LTC requirement. The assessment of how frailty among older people increases the need for LTC services in the target population may help us to estimate resource needs in the near future.
Limitations
This study had several noteworthy limitations. First, the frailty scores of the participants admitted to geriatric health service facilities may have been underestimated because the payment for medical services (e.g. drugs prescribed for long-term disease management by the attached medical institution) is not covered by health insurance but by LTC insurance [35]. Second, the applicability of the algorithms to administrative claim data in this study does not ensure its transferability to other databases. Further studies are required to evaluate how generalisable these findings are to other health systems in epidemiological studies or for use in systematic frailty screening.
Conclusions
Our study shows that adapting two frailty algorithms with globally used diagnostic codes was useful for evaluating individuals’ frailty. The frailty scores can be measured in the Japanese claims database, and individuals at greater risk of death or more likely to use LTC services can be identified.
Supplementary Material
Acknowledgements
Based on a data use agreement with the regional insurers in the prefecture of Shizuoka, we are unable to make the analytical data accessible to readers.
Contributor Information
Shiori Nishimura, Department of Healthcare Quality Assessment, The University of Tokyo Graduate School of Medicine, Tokyo, Japan; Keio University Graduate School of Health Management, Kanagawa, Japan; Shizuoka Graduate University of Public Health, Shizuoka, Japan.
Hiraku Kumamaru, Department of Healthcare Quality Assessment, The University of Tokyo Graduate School of Medicine, Tokyo, Japan; Shizuoka Graduate University of Public Health, Shizuoka, Japan.
Satoshi Shoji, Shizuoka Graduate University of Public Health, Shizuoka, Japan; Department of Cardiology, Keio University School of Medicine, Tokyo, Japan.
Eiji Nakatani, Shizuoka Graduate University of Public Health, Shizuoka, Japan.
Hiroyuki Yamamoto, Department of Healthcare Quality Assessment, The University of Tokyo Graduate School of Medicine, Tokyo, Japan; Shizuoka Graduate University of Public Health, Shizuoka, Japan; Department of Health Policy and Management, Keio University School of Medicine, Tokyo, Japan.
Nao Ichihara, Department of Healthcare Quality Assessment, The University of Tokyo Graduate School of Medicine, Tokyo, Japan; Shizuoka Graduate University of Public Health, Shizuoka, Japan.
Yoshiki Miyachi, Shizuoka Graduate University of Public Health, Shizuoka, Japan.
Alexander T Sandhu, Division of Cardiovascular Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Paul A Heidenreich, Division of Cardiovascular Medicine, Stanford University School of Medicine, Stanford, CA, USA; Division of Cardiology, Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA.
Keita Yamauchi, Keio University Graduate School of Health Management, Kanagawa, Japan.
Michiko Watanabe, Department of Data Science, Rissho University, Saitama, Japan.
Hiroaki Miyata, Department of Healthcare Quality Assessment, The University of Tokyo Graduate School of Medicine, Tokyo, Japan; Shizuoka Graduate University of Public Health, Shizuoka, Japan; Department of Health Policy and Management, Keio University School of Medicine, Tokyo, Japan.
Shun Kohsaka, Department of Healthcare Quality Assessment, The University of Tokyo Graduate School of Medicine, Tokyo, Japan; Shizuoka Graduate University of Public Health, Shizuoka, Japan; Department of Cardiology, Keio University School of Medicine, Tokyo, Japan.
Declaration of Conflicts of Interest
Dr Kohsaka reports the receipt of investigator-initiated grant funding from Bayer and Daiichi Sankyo. Dr Kumamaru has received consultation fees from Mitsubishi Tanabe Pharma and speaker fees from Pfizer Japan Inc and Johnson & Johnson K.K. Dr Yamamoto has received consultation fees from Mitsubishi Tanabe Pharma, speaker fees from Chugai Pharmaceutical Co., Ltd and Ono Pharmaceutical Co., Ltd, and payment for a manuscript from Astellas Pharma Inc. Dr Miyata has received a research grant from AstraZeneca K.K. for an independent research project through the PeoPLe Consortium at Keio University. Drs Kohsaka, Kumamaru, Nishimura, Ichihara, Yamamoto and Miyata are affiliated with the Department of Healthcare Quality Assessment at The University of Tokyo. This department is a social collaboration department supported by the National Clinical Database, Johnson & Johnson K.K. and Nipro Corporation. Dr Sandhu receives research support from the National Heart, Lung and Blood Institute (1K23HL151672-01).
Declaration of Conflict of Interest
The other authors report no potentially conflicting interests.
Declaration of Sources of Funding
The Research Support Center at Shizuoka General Hospital and Shizuoka Graduate University of Public Health conducts contract research projects on public health in Shizuoka Prefecture, including this study.
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet 2019; 394: 1376–86. [DOI] [PubMed] [Google Scholar]
- 3. Matsubayashi K, Okumiya K, Wada T, Osaki Y, Doi Y, Ozawa T. Secular improvement in self-care independence of old people living in community in Kahoku, Japan. Lancet 1996; 347: 60. [DOI] [PubMed] [Google Scholar]
- 4. Fukutomi E, Kimura Y, Wada T, Okumiya K, Matsubayashi K. Long-term care prevention project in Japan. Lancet 2013; 381: 116. [DOI] [PubMed] [Google Scholar]
- 5. Nations U, of Economic D, Affairs S, Division P . World Population Ageing 2019: Highlights.
- 6. Dent E, Morley JE, Cruz-Jentoft AJ et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging 2019; 23: 771–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clegg A, Bates C, Young J et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016; 45: 353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gilbert T, Neuburger J, Kraindler J et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018; 391: 1775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McAlister FA, Savu A, Ezekowitz JA, Armstrong PW, Kaul P. The hospital frailty risk score in patients with heart failure is strongly associated with outcomes but less so with pharmacotherapy. J Intern Med 2020; 287: 322–32. [DOI] [PubMed] [Google Scholar]
- 10. Kundi H, Wadhera RK, Strom JB et al. Association of Frailty with 30-day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol 2019; 4: 1084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McAlister F, Van Walraven C. External validation of the hospital frailty risk score and comparison with the hospital-patient one-year mortality risk score to predict outcomes in elderly hospitalised patients: a retrospective cohort study. BMJ Qual Saf 2019; 28: 284–8. [DOI] [PubMed] [Google Scholar]
- 12. NHS . Electronic Frailty Index. https://www.england.nhs.uk/ourwork/clinical-policy/older-people/frailty/efi/#what-does-the-new-frailty-identification-requirement-in-the-gp-contract-mean-for-general-practice (22 February 2021, date last accessed).
- 13. Pajewski NM, Lenoir K, Wells BJ, Williamson JD, Callahan KE. Frailty screening using the electronic health record within a medicare accountable care organization. J Gerontol A Biol Sci Med Sci 2019; 74: 1771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jauhari Y, Gannon MR, Dodwell D et al. Construction of the secondary care administrative records frailty (SCARF) index and validation on older women with operable invasive breast cancer in England and Wales: a cohort study. BMJ Open 2020; 10: e035395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakatani E, Tabara Y, Sato Y, Tsuchiya A, Miyachi Y. Data resource profile of Shizuoka Kokuho Database (SKDB) using integrated health- and care-insurance claims and health checkups: the Shizuoka study. J Epidemiol 2021; 1–10. https://pubmed.ncbi.nlm.nih.gov/33518592/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ministry of Health L and W . Various Information of Medical Fee. https://shinryohoshu.mhlw.go.jp/shinryohoshu/ (26 March 2021, date last accessed).
- 17. Hollinghurst J, Housley G, Watkins A, Clegg A, Gilbert T, Conroy SP. A comparison of two national frailty scoring systems. Age Ageing 2021; 50: 1208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr 2017; 17: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uno H, Cai T, Tian L, Wei LJ. Evaluating prediction rules for t-year survivors with censored regression models. J Am Stat Assoc 2007; 102: 527–37. [Google Scholar]
- 20. Blanche MP. Package ‘timeROC’. 2019. https://cran.r-project.org/web/packages/timeROC/timeROC.pdf (31 May 2021, date last accessed).
- 21. Thomas A, Gerds A, Blanche P, Wright M, Muschelli J. Package ‘riskRegression’. 2020. https://cran.r-project.org/web/packages/riskRegression/riskRegression.pdf (31 May 2021, date last accessed).
- 22. Kundi H, Popma JJ, Reynolds MR et al. Frailty and related outcomes in patients undergoing transcatheter valve therapies in a nationwide cohort. Eur Heart J 2019; 40: 2231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Street A, Maynou L, Gilbert T, Stone T, Mason S, Conroy S. The use of linked routine data to optimise calculation of the hospital frailty risk score on the basis of previous hospital admissions: a retrospective observational cohort study. Lancet Heal Longev 2021; 2: e154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cummings SR, Nevitt MC. Non-skeletal determinants of fractures: the potential importance of the mechanics of falls. Osteoporos Int 1994; 4: 67–70. [DOI] [PubMed] [Google Scholar]
- 25. Berry SD, Miller RR. Falls: epidemiology, pathophysiology, and relationship to fracture. Curr Osteoporos Rep 2008; 6: 149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsuda T. Epidemiology of fragility fractures and fall prevention in the elderly. Curr Orthop Pract 2017; 28: 580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hollinghurst J, Fry R, Akbari A et al. External validation of the electronic frailty index using the population of Wales within the secure anonymised information linkage databank. Age Ageing 2019; 48: 922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eckart A, Hauser SI, Haubitz S et al. Validation of the hospital frailty risk score in a tertiary care hospital in Switzerland: results of a prospective, observational study. BMJ Open 2019; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–57. [DOI] [PubMed] [Google Scholar]
- 30. Bandeen-Roche K, Xue QL, Ferrucci L et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci 2006; 61: 262–6. [DOI] [PubMed] [Google Scholar]
- 31. Chen S, Honda T, Narazaki K, Chen T, Kishimoto H, Kumagai S. Physical frailty and risk of needing long-term care in community-dwelling older adults: a 6-year prospective study in Japan. J Nutr Health Aging 2019; 23: 856–61. [DOI] [PubMed] [Google Scholar]
- 32. Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J 2020; 23: 254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring frailty in administrative claims data: comparative performance of four claims-based frailty measures in the U.S. J Gerontol A Biol Sci Med Sci . 2019; XX: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faurot KR, Jonsson Funk M, Pate V et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf 2015; 24: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamada S, Kojima T, Sakata N et al. Drug costs in long-term care facilities under a per diem bundled payment scheme in Japan. Geriatr Gerontol Int 2019; 19: 667–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.