Abstract
Background
Spontaneous rupture of hepatocellular carcinoma (HCC) is a potentially fatal complication and the third leading cause of death in patients with HCC after tumor progression and liver failure. Previous studies suggested that improved HCC surveillance has decreased the incidence of rupture. This study aims to characterize patients with ruptured HCC over time and identify predictors of rupture.
Methods
We retrospectively reviewed a prospectively collected database of 1451 HCC patients to identify cases with rupture and predictors of rupture. Data were divided into three 9-year eras to compare and trend patient/tumor characteristics and rupture.
Results
Fifty-seven patients (3.9%) presented with spontaneous HCC rupture and the following characteristics: mean age 62.6 years, 73.7% males, 41% cirrhosis, and mean tumor size of 8.0 cm. On multivariate analyses, predictors of rupture included obesity, tumor >5 cm, and single tumors, whereas the presence of cirrhosis was a negative predictor for rupture.
Across three eras, there were changes in disease etiology and decreases in tumor size, and more HCCs were found with surveillance. However, more patients were noncirrhotic, and the incidence of spontaneous rupture was unchanged over time.
Conclusion
Despite improved early detection of HCC over time, the incidence of rupture has been unchanged. The persistent incidence of rupture may possibly be attributed to increasing proportion of fatty liver–related HCC patients who lack traditional risk factors for surveillance and may not have cirrhosis. Better identification of fatty liver disease and determining which patients need HCC surveillance may be needed in the future to prevent spontaneous rupture.
Keywords: cirrhosis, fatty liver diseases, liver resection, liver tumor
Abbreviations: AFP, alpha fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, Body Mass Index; HCC, hepatocellular carcinoma; INR, international normalized ratio; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; TACE, transarterial chemoembolization; TAE, transarterial embolization
Hepatocellular carcinoma (HCC) is one of the most common malignancies globally with an increasing incidence and a high case fatality rate. More than 800,000 new cases were diagnosed in 2018, making it the fifth most common cancer worldwide. HCC is also the fourth leading cause of cancer-related deaths, accounting for 700,000 deaths annually.1, 2, 3, 4, 5 The incidence rates of HCC in the United States have increased twofold to threefold in the last three decades. This can be attributed in part to the high prevalence of hepatitis C infection among people born between 1945 and 1965 as well to the progressive increase of HCC cases due to fatty liver disease over the past two decades.1
Spontaneous rupture is a life-threatening complication of HCC. The mortality due to HCC rupture in the acute phase can be as high as 25–75% and is the third most common cause of death due to HCC after tumor progression and liver failure.2,3,6 Recent studies have reported a decrease in overall mortality to about 23.5% and as low as 0.95% in patients who underwent successful surgical resection.2,7 The incidence of HCC rupture has distinct geographic variations. In the West, where the incidence of HCC is increasing, HCC rupture is relatively uncommon, occurring in less than 3%. However, HCC rupture is considerably higher in Asia and Africa ranging between 3% and 26%.2,3,6,8,9 Most patients present with acute abdominal pain (66%–100%), and 33–90% of patients can present with hemorrhagic shock.3,6,8,10,11
Although the mechanism of tumor rupture is not completely clear, several hypotheses have been suggested including vascular injury, venous congestion and “small room hypothesis” where the location of the tumor plays an important role.2,6,12,13 Tumors in the left lobe were at higher risk of rupture compared with tumors in the right lobe, likely due to decreased anatomical span of the left lobe compared with the right lobe.14 Reported risk factors for tumor rupture have included tumor size, hypertension, cirrhosis, extra-hepatic tumor involvement, and portal vein thrombosis.2,4,7,14 Many studies have suggested that the incidence of rupture has decreased with improved early detection, although the mortality remains high.2,4,6,8 In this study, we aimed to characterize 1451 patients diagnosed with HCC during the period between 1993 and 2020 and identify potential predictors of tumor rupture. We also explored how tumor rupture has changed over time (using three 9-year eras) with changes in HCC detection and better surveillance.
Methods
Patients
This was a retrospective study of 1451 HCC cases referred over a 27-year period (1993–2020) to a group of physicians who were associated with Hawaii's only liver transplant center and liver disease center in the State. It was also the tertiary referral center for the American territories of the Pacific Basin (including Samoa, Guam, Saipan, and the Marshall Islands). These patients also included foreign nationals from Asian countries who sought medical care in the United States. This liver center and transplant center were initially affiliated with St. Francis Medical Center (later renamed Hawaii Medical Center-East) and Queens Medical Center after 2012. These centers cared for approximately 60–70% of the HCC cases in Hawaii and included all patients who were referred to these centers during this timeframe. This study was approved by the Institutional Review Board of the University of Hawaii at Manoa. All data were deidentified prior to use for the study, thus exempt from requiring patient consent and in compliance with ethical regulations.
HCC was diagnosed histologically by percutaneous biopsy or at surgery. In the first decade and consistent with the previous United Network for Organ Sharing (UNOS) policy regarding transplant for HCC, patients without histologic confirmation were included if they had a history of chronic liver disease, a mass at least 2 cm seen on two imaging studies (ultrasound, CT scan, or MRI), and one of the following: (1) vascular blush evident on CT scan or MRI, (2) alpha fetoprotein (AFP) > 200 ng/ml, or (3) arteriogram confirming the tumor. More recently, the diagnosis of HCC was made with imaging alone if a contrast-enhanced study (dynamic CT or MRI) showed typical arterial enhancement with “washout” in the venous phase, as described by the American Association for the Study of Liver Disease (AASLD) guidelines.15,16
Data collected
Information on demographics, medical history, laboratory data, tumor characteristics, treatment, and survival was collected from clinical records. Demographic data included age, sex, birthplace, and the patient's self-reported ethnicity. Ethnicity was then categorized as “White,” “Asian” (East Asians, including Filipinos), “Pacific Islander,” “Mixed,” or “Other.” Medical history included diabetes mellitus, hyperlipidemia, smoking, and risk factors for HCC including viral hepatitis, significant alcohol use (defined as greater than two alcoholic beverages daily for at least 10 years), and other chronic liver diseases. Measured height and weight were used to determine body mass index (BMI). Obesity was defined as BMI ≥30.
The collected laboratory data included bilirubin, albumin, prothrombin time with international normalized ratio (INR), creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), platelet count, neutrophil count, lymphocyte count, and AFP. Hepatitis B and C serologies were obtained if this information was not available from pre-existing records. For hepatitis B, we also noted patients who had only hepatitis B core antibody positivity in the absence of hepatitis B surface antigen. Laboratory data that were used for the study had been obtained within 2 weeks of an initial visit or at the time of the visit. Tumors were stratified to largest tumor diameter ≥5 cm or <5 cm and also tumor diameter ≥10 cm or <10 cm. Normal AFP was defined as less than 20 ng/dL. We also categorized AFP into those that had values greater than 1000 ng/dL. Neutrophils and lymphocytes were used to calculate neutrophil/lymphocyte ratio.
Although our Liver Center recommends HCC surveillance in cirrhotics with AFP and liver ultrasound every 6 months, there was no uniform screening protocol used in the cohort. Referring physicians used a combination of AFP and/or imaging (ultrasound, CT scan, or MRI) at variable intervals. HCC was deemed to be found on “surveillance” if the patient had a previous imaging study from 3 to 12 months prior to the current study. HCC not found on screening was either found with symptoms (pain, abdominal mass, weight loss, jaundice) or incidentally with imaging done for unrelated reasons.
We identified those patients with spontaneous rupture characterized by presentation acutely to an emergency department with abdominal pain or hypotension with imaging evidence of free blood in the abdomen and a liver mass. Patients may have been seen at another institution but transferred to the care of this group of hepatobiliary surgeons.
Treatments
Treatments in the overall cohort included liver resection, transplantation, locoregional therapies (radiofrequency ablation, cryosurgery, percutaneous ethanol injection, transarterial chemoembolization [TACE] or Yttrium90 radioembolization), and systemic therapies. Liver resection was considered in Child's A patients and early Child's B patients (Child–Turcotte–Pugh score of 7, without any evidence of ascites or encephalopathy). Liver transplantation was considered in patients with unresectable HCC but met Milan criteria (single tumor less than 5 cm or 2 to 3 tumors, each less than 3 cm). All liver resections and transplantations were performed by a single surgical group practice. Patients who presented acute with tumor rupture underwent resuscitation and subsequent transarterial embolization (TAE) by an interventional radiologist or surgical intervention if embolization was not available or contraindicated. In some cases, patients did not undergo TAE because they were stable enough to wait for a more definitive therapy or they died before the TAE could be completed. Patients who presented with rupture were not considered candidates for liver transplantation.
Statistical analysis
All analyses were performed using Excel and SPSS statistical software (IBM, version 27). The cohort was divided into two groups of patients: those with spontaneous rupture of HCC and those who did not rupture. Categorical variables were analyzed using ANOVA and chi-square analysis. Odds-ratios with 95% confidence intervals were calculated using univariate and multivariate logistic regression. Predictors of recurrence were evaluated by unconditional logistic regression modeling recurrence/no recurrence. Variables significant at P = 0.10 in univariate analyses were included in the multivariate models along with age and sex. Significant variables to P = 0.100 were used to create a multivariate model. Variables significant at P < 0.05 were considered significant in the multivariate model. Overall survival was evaluated using Kaplan–Meier and Cox regression analyses. Variables significant on univariate analysis (P < 0.05) were included in multivariate models.
All HCC cases were then divided into three 9-year cohorts to determine trends in risk factors and tumor characteristics between the three eras. Categorical variables across three eras were compared using Chi-square analysis with cut off for significance at P = 0.05, whereas means of numerical values were compared using ANOVA analysis with cutoff significance at P = 0.05 as well. Main outcome measures included frequency of tumor rupture, cirrhosis, and if HCC was found with surveillance.
Results
Clinical and demographic characteristics of patients with ruptured hepatocellular carcinoma versus nonruptured hepatocellular carcinoma
In the cohort of 1451 patients with HCC, 57 (3.9%) had spontaneous rupture of HCC, whereas 1394 (96.1%) did not have rupture. Clinical characteristics of both groups are shown in Table 1, Table 2. There was no difference in mean age or proportion of patients who were 65 years or older. Most patients were male, and there was no difference in gender or ethnic distribution between the groups. Patients who ruptured were less likely to have hepatitis C, but there was no difference in hepatitis B, smoking, alcohol, diabetes, hyperlipidemia, hypertension, nonalcoholic steatohepatitis (NASH)/nonalcoholic fatty liver disease (NAFLD) or BMI between the groups.
Table 1.
Comparison of Risk Factors for Ruptured Versus Nonruptured Hepatocellular Carcinoma.
| Characteristic | Ruptured (n = 57) | Non-ruptured (n = 1394) | P value |
|---|---|---|---|
| Mean agein years(SD) | 62.6 (12.8) | 63.5 (11.3) | 0.55 |
| Males | 42 (73.7%) | 1038 (74.5%) | 0.87 |
| Age ≥ 65years | 24 (42.1%) | 625 (44.9%) | 0.79 |
| Ethnicity | |||
| Asian | 38 (66.7%) | 794 (57%) | 0.29 |
| White | 5 (8.8%) | 300 (21.5%) | |
| Pacific Islander | 11 (19.3%) | 208 (14.9%) | |
| Mixed | 1 (1.8%) | 48 (3.4%) | |
| Other | 2(3.6%) | 43(3.0%) | |
| Hepatitis Bsurface Ag+ | 15 (26.3%) | 344/1387 (24.8%) | 0.07 |
| Hepatitis B core Ab+ | 6 (10.5%) | 152 (11.0%) | 0.92 |
| Hepatitis C | 13 (22.8%) | 574/1387 (41.4%) | 0.005 |
| Significantalcoholuse | 20 (35.1%) | 606/1389 (43.6%) | 0.20 |
| NASH/NAFLD | 6 (10.5%) | 183/1389 (13.1%) | 0.56 |
| Mean BMI (SD) | 26.8 (5.56) | 27.0 (7.7) | 0.84 |
| BMI ≥ 30 | 15 (26.3%) | 288 (20.7%) | 0.32 |
| BMI ≥ 35 | 3 (5.3%) | 114 (8.2%) | 0.62 |
| Smoking history | 29/54 (53.7%) | 863/1377 (62.7%) | 0.18 |
| Diabetes mellitus | 18 (31.6%) | 488/1391 (35.1%) | 0.59 |
| Hyperlipidemia | 13/53 (24.5%) | 363/1370 (26.5%) | 0.75 |
| Childs class | 0.69 | ||
| Class A | 40/56 (71.4%) | 930/1382 (67.2%) | |
| Class B | 13/56 (23.2%) | 337/1382 (24.3%) | |
| Class C | 3/56 (5.3%) | 115/1382 (9.3%) | |
Table 2.
Comparison of Tumor characteristics and Laboratory Studies for Ruptured Versus Nonruptured Hepatocellular Carcinoma.
| Characteristic | Ruptured (n = 57) | Non-ruptured (n = 1394) | P value |
|---|---|---|---|
| Mean tumor sizein cm(SD) | 7.99 (3.93) | 5.73 (4.34) | <0.001 |
| Tumor ≥ 5 cm | 39 (88.4%) | 585 (42.0%) | <0.001 |
| Tumor ≥10 cm | 12 (21.1%) | 230 (16.5%) | 0.37 |
| Multiple tumors | 11 (19.3%) | 461 (33.1%) | 0.03 |
| Cirrhosis | 22/53 (41.5%) | 995/1375 (72.4%) | <0.001 |
| Mean bilirubinin mg/dL (SD) | 1.18 (1.16) | 1.59 (2.43) | 0.21 |
| Mean albuminin mg/dL (SD) | 3.29 (0.69) | 3.56 (0.70) | 0.004 |
| Mean INR | 1.19 (0.21) | 1.16 (0.27) | 0.45 |
| Mean platelet countx103/uL(SD) | 187.6 (93) | 166.9 (96.1) | 0.11 |
| Mean creatininein mg/dl(SD) | 1.24 (0.95) | 1.03 (0.80) | 0.69 |
| Mean ASTin IU/L (SD) | 94.4 (101.8) | 82.8 (77.4) | 0.39 |
| Mean ALTin IU/L (SD) | 66.2 (57.2) | 65 (57.5) | 0.87 |
| Mean AFP (SD) | 13,120 (50,890) | 11,754 (58,182) | 0.73 |
| Normal AFP<20 ng/dL | 21/56 (37.5%) | 559/1387 (40.3%) | 0.68 |
| AFP ≥ 1000 ng/dL | 15 (26.3%) | 289 (20.7%) | 0.32 |
| Mean neutrophil/lymphocyte ratio(SD) | 8.27 (8.71) | 3.26 (3.16) | <0.001 |
In terms of tumor characteristics, ruptured HCC patients had larger tumor size, a greater proportion with tumors 5 cm or larger and single tumors. The nonruptured HCC patients were significantly more likely to have cirrhosis in their underlying liver. Mean albumin was higher in the nonruptured HCC, and all other laboratory studies were similar (bilirubin, INR, platelet count, creatinine, AST, and ALT). There was no difference in the distribution of Child class between ruptured and nonruptured HCC groups. The median Child–Turcotte–Pugh score was also similar in both groups (6 vs. 5. P = 0.49). Patients with ruptured HCC had a significantly higher neutrophil/lymphocyte ratio.
In this cohort, 1017 patients had underlying cirrhosis, and 317 were non-cirrhotic. Patients with cirrhosis had a smaller mean tumor size of 4.7 cm (SD 3.4 cm) compared with noncirrhotics 8.5 cm (SD 5.1 cm) with P < 0.001. Cirrhotics were also less likely to present with tumor >5 cm compared with noncirrhotics (33.6% vs. 66.4%, P < 0.001). Furthermore, more patients who were cirrhotic had their HCC found with surveillance compared with noncirrhotics (30.2% vs. 16.4%, P < 0.001).
Treatment and outcome of patients with ruptured hepatocellular carcinoma
Of the 57 patients with ruptured HCC, 31 patients were treated initially with TAE (54.3%). Of these patients, 12 had subsequent liver resection, 15 patients did not receive any subsequent treatment, and 4 patients had other therapies (radiofrequency ablation, yttrium90 radioembolization, systemic therapy). Thirteen patients underwent immediate liver resection (22.8%), and six patients had other surgical interventions (10.5%). Three of these had exploratory laparotomy with control of bleeding including one who underwent unilateral hepatic artery ligation. Three patients had laparoscopic control of bleeding initially with one had a subsequent resection, one underwent embolization followed by RFA, and one had no other interventions. There were three patients who received locoregional therapy only (TACE, yttrium 90 radioembolization) and one patient who received only systemic therapy with sorafenib. Finally, there were three patients who did not receive any treatment (see Table 3).
Table 3.
Treatment Received by Patients with Ruptured Tumor.
| Type of treatment | Number of patients (%) |
|---|---|
| Embolization | 31 (54%) |
| Embolization alone | 15 |
| Embolization with subsequent liver resection | 12 |
| Embolization with other therapies (RFA, 90Y RE) | 4 |
| Immediate liver resection | 13 (22.8%) |
| Locoregional therapy | 3 (5.2%) |
| Systemic therapy (Sorafinib) | 1 (1.7%) |
| Other surgical interventions | 6 (17/7%) |
| Laparoscopy, control of bleeding | 3 (5.2%) |
| Hepatic artery ligation | 1 (1.7%) |
| Open surgery, control of bleeding | 2 (3.5%) |
| No treatment | 3 (5.2%) |
RFA: Radiofrequency ablation.
90Y RE: yttrium 90 radioembolization
In the ruptured HCC cohort, 5-year overall survival was 20.7% (median survival 21.6 months, 95% CI 9.5–33.8). Of the 26 patients who received liver resection, median overall survival was 28.5 months and 1-, 3-, and 5-year overall survival were 65.4%, 34.6%, and 26.9%, respectively. This is significantly worse than the 253 nonruptured HCC patients who underwent surgical resection who had median overall survival of 82.7 months and 1-, 3-, and 5-year OS of 82.4%, 68%, and 54.3% (log-rank P-value 0.019). The outcome for 31 ruptured HCC patients who did not undergo definitive liver resection was quite dismal with median survival of 13.5 months and 1-,3-, and 5-year overall survival of 47.6%, 9.6%, and 9.6%, respectively.
Predictors of spontaneous rupture of hepatocellular carcinoma
On both the univariate and multivariate analysis, the highest positive predictors of rupture included tumor ≥5 cm and obesity with BMI ≥30. Negative predictors included morbid obesity with BMI ≥35, the presence of multiple tumors and cirrhosis. Although the presence of smoking and large tumor size trended to being negative predictors on the univariate analysis, these were not significant factors on the multivariate analysis (see Table 4).
Table 4.
Univariate and Multivariate Analyses of Predictors of Spontaneous Tumor Rupture.
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95%CI) | P-value | |
| Age > 65 years | 0.65 (0.34–1.25) | 0.20 | 0.73 (0.40–1.33) | 0.31 |
| Male sex | 0.92 (0.43–1.93) | 0.82 | 0.91 (0.47–1.76) | 0.79 |
| Hepatitis B | 0.87 (0.28–2.65) | 0.13 | ||
| Hepatitis C | 0.40 (0.15–1.09) | 0.11 | ||
| Significant alcoholhistory | 0.61 (0.26–1.43) | 0.36 | ||
| Nonalcohol, HBV, or HCV | 0.51 (0.14–1.83) | 0.30 | ||
| NASH/NAFLD | 0.47 (0.14–1.60) | 0.38 | ||
| BMI≥30a | 2.77 (1.27–6.02) | 0.014 | 2.45 (1.21–4.97) | 0.02 |
| BMI≥35 | 0.26 (0.06–1.09) | 0.046 | 0.28 (0.07–1.09) | 0.045 |
| Smoking history | 0.66 (0.33–1.29) | 0.054 | 0.71 (0.39–1.27) | 0.08 |
| Diabetes mellitus | 0.87 (0.44–1.74) | 0.59 | ||
| Hyperlipidemia | 0.59 (0.27–1.28) | 0.06 | 0.67 (0.33–1.33) | 0.14 |
| Hypertension | 1.29 (0.62–2.68) | 0.56 | ||
| Normal AFP | 0.92 (0.49–1.71) | 0.82 | ||
| Tumor size ≥ 5 cm | 3.08 (1.52–6.21) | 0.001 | 3.16 (1.66–6.02) | <0.001 |
| Tumor size ≥ 10 cm | 0.52 (0.23–1.15) | 0.10 | 0.50 (0.23–1.07) | 0.07 |
| Single tumors | 2.22 (1.07–4.76) | 0.02 | 2.08 (1.03–4.16) | 0.03 |
| Cirrhosis | 0.35 (0.17–0.68) | 0.001 | 0.27 (0.15–0.51) | <0.001 |
Bold face variables are significant.
Comparison of characteristics over time
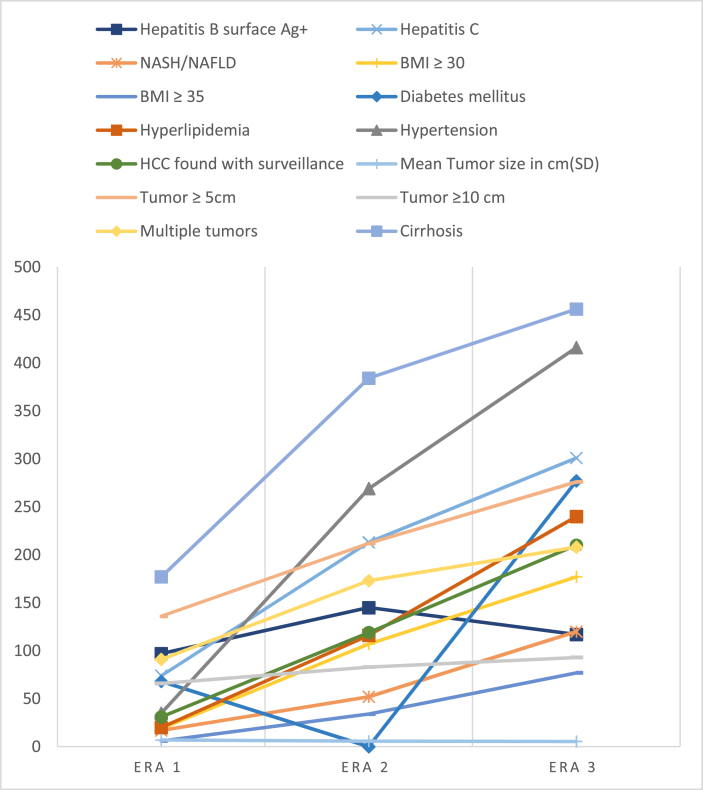
The 1451 patients with HCC were then separated into three 9-year eras (1993–2002, 2003–2012, 2013–2020), and there were increasing numbers of patients with successive eras. Patients were older with mean age increasing from 60.9 years to 65.6 years by the third era. There was also a larger proportion of patients who were older than 65 years in the most recent year. In all eras, there was a male predominance in the cases, and this did not change over time. The ethnic distribution did change over time with a progressively decreased proportion of cases in Asians and an increase in the other races. In addition, in all eras, the rate of ruptured HCC also did not change over time with P = 0.45. The mean BMI and tumor size differed between the eras. All other categorical variables were different between the three eras except for the presence of multiple tumors (see Table 5).
Table 5.
Comparison of Risk Factors, Tumor Characteristics by Era.
| Characteristic | Era 1 (1993–2002) (n = 244) | Era 2 (2003–2012) (n = 519) | Era 3 (2013–2020) (n = 688) | P value |
|---|---|---|---|---|
| Mean age in years (SD) | 60.9 (12.2) | 62.0 (11.5) | 65.6 (10.4) | <0.001 |
| Males | 172 (70.5%) | 398 (76.7%) | 510 (74.1%) | 0.185 |
| Age ≥ 65 years | 97 (39.8%) | 203 (39.1%) | 349 (50.7%) | <0.001 |
| Ethnicity | ||||
| Asian | 182 (74.6%) | 310 (59.7%) | 340 (49.4%) | <0.001 |
| White | 35 (14.3%) | 108 (20.8%) | 162 (23.5%) | |
| Pacific Islander | 24 (9.8%) | 85 (16.4%) | 110 (16%) | |
| Mixed | 0 | 7 (1.1%) | 42 (6.1%) | |
| Other | 3 (1.2%) | 9 (1.8%) | 33 (4.8%) | |
| Hepatitis B surface Ag+ | 97 (39.8%) | 145 (27.9%) | 117 (17%) | <0.001 |
| Hepatitis B core Ab + | 11 (4.5%) | 60 (11.6%) | 158 (10.9%) | <0.001 |
| Hepatitis C | 74 (30.3%) | 213 (41%) | 301 (43.8%) | <0.001 |
| Significant Alcohol use | 91 (37.3%) | 233 (44.9%) | 302 (43.9%) | 0.042 |
| NASH/NAFLD | 17 (7%) | 52 (10%) | 120 (17.4%) | <0.001 |
| Mean BMI (SD) | 25.5 (5.1) | 26.8 (5.3) | 27.4 (9.3) | 0.017 |
| BMI ≥ 30 | 19 (7.8%) | 107 (20.6%) | 177 (25.7%) | <0.001 |
| BMI ≥ 35 | 6 (2.5%) | 34 (6.6%) | 77 (11.2%) | <0.001 |
| Smoking history | 111 (45.5%) | 321 (61.8%) | 460 (66.9%) | <0.001 |
| Diabetes mellitus | 68 (27.9%) | 161 (31%) | 277 (40.3%) | 0.001 |
| Hyperlipidemia | 20 (8.2%) | 116 (22.4%) | 240 (34.9%) | <0.001 |
| Hypertension | 35 (14.3%) | 269 (51.8%) | 416 (60.5%) | <0.001 |
| Normal AFP | 76 (31.1%) | 193 (37.2%) | 311 (45.2%) | 0.001 |
| AFP ≥ 1000 ng/dL | 70 (28.7%) | 123 (23.7%) | 111(16.1%) | <0.001 |
| HCC found with surveillance | 31 (12.7%) | 119 (32.9%) | 210 (30.5%) | <0.001 |
| Mean Tumor size in cm(SD) | 7.04 (4.75) | 5.74 (4.35) | 5.48 (4.15) | <0.001 |
| Tumor ≥ 5 cm | 136 (55.7%) | 212 (40.9%) | 276 (40.1%) | <0.001 |
| Tumor ≥10 cm | 66 (27%) | 83 (16%) | 93 (16.7%) | <0.001 |
| Multiple tumors | 91 (37.3%) | 173 (33.3%) | 208 (30.2%) | 0.137 |
| Cirrhosis | 177 (72.5%) | 384 (74%) | 456 (66.3%) | 0.008 |
| Spontaneous rupture | 13 (5.3%) | 18 (3.5%) | 26 (3.9%) | 0.45 |
In terms of etiology of disease, there were increasing proportion of cases due to HCV and decreasing proportion due to HBV. There was increase in alcohol use in the last two eras compared with the first era, and there were more patients who had smoked in the most recent era. Overall, there was no difference in the proportion of patients who had no evidence of HBV, HCV, or significant alcohol use, but there was increasing proportion with NASH/NAFLD. There were also increasing proportions of patients with metabolic risk factors of diabetes, hyperlipidemia, and both obesity and morbid obesity.
With respect to tumor characteristics, the mean tumor size decreased over time with 7.04 cm in the earliest era and 5.48 cm in the latest era. There were also a smaller proportion of tumors >5 cm, tumors >10 cm, and multiple tumors. Although overall 70% of all patients were cirrhotic, this proportion decreased from 72.5% to 66.3% between the earliest and most recent era. Despite these significant changes in disease etiology, risk factors and tumor characteristics, the rate of spontaneous rupture was unchanged between the eras (see Table 5 and Figure 1).
Figure 1.
Changes of tumor and patient's characteristics over three different 9-year eras.
Discussion
Although spontaneous rupture of HCC can be fatal, inadequate liver function, portal vein thrombosis, and shock have been associated with poor survival, whereas smaller tumors and early Barcelona Clinic Liver Cancer stage were associated with better outcomes.1, 2, 3,17, 18, 19 Ruptured HCC has not typically been included in treatment algorithms, as management likely needs to be individualized depending on the severity of bleeding and available resources. Priorities include initial resuscitation and control of bleeding with TAE or TACE, which have success rates of 53–100%. Surgical control of bleeding is an alternative option if interventional radiology is unavailable or embolization fails, but this approach may have high in-hospital mortality. Stable patients can be monitored closely and prepared for definitive treatment with surgical resection or locoregional therapy.2,4,8,20,21 Although the laparoscopic approach to liver resection is well accepted in HCC, its use in ruptured cases has not been well defined.22 In our cohort, more than half of the patients were initially managed with TAE, and half of these underwent subsequent treatment either by staged liver resection or other therapies, whereas the remaining patients did not receive any subsequent treatments. One-third of our cohort was managed with immediate surgical intervention. These results differ from previous studies as we have more patients treated with TAE and surgical resection, and fewer patients were managed with other therapies.
Previous studies have suggested that patients who underwent liver resection after ruptured HCC had comparable outcomes with nonruptured cases. Specifically, Chua et al23 in a propensity matched comparison of 47 ruptured and 98 nonruptured cases who underwent liver resection, found no difference in 5-year overall and disease-free survival and hospital length of stay. However, these patients had large tumors with mean size of 8.5–9.5 cm. Another study with 221 advanced T4 HCC lesions, found that the 35 patients with ruptured HCC who presented with hemorrhagic shock had higher in-hospital mortality rates but significantly better long-term survival than hemodynamically stable patients with ruptured HCC and patients without rupture.19 Although smaller studies have not suggested differences in survival between ruptured and nonruptured HCC after resection, larger studies have demonstrated worse outcomes in ruptured HCC after resection.24, 25, 26 Our study confirms the findings of large studies who have included all tumor sizes and noted worse outcome with ruptured HCC. Although patients with ruptured HCC were often able to receive liver resections, these patients had poor long-term survival likely related to large tumor size and possibly the inability to ever qualify for liver transplantation. Patients with ruptured HCC were less likely to have hepatitis C, cirrhosis, and multiple tumors, but other risk factors were quite similar between the groups. Furthermore, our ruptured HCC patients had poor long-term survival whether they underwent resection or some other therapy. These findings highlight the poor prognosis that ruptured HCC carries even after liver resection and amplifies the importance of early detection of HCC to prevent this potentially fatal complication.
The mechanism of rupture has not been clearly delineated in HCC. Many hypotheses have been proposed including vascular injury, venous congestion, rapid tumor growth and necrosis, and “small room hypothesis” leading to rupture of overlying liver parenchyma and coagulopathy.2,3,6,12, 13, 14 Studies have also suggested that ischemic necrosis or vascular injury related to treatment can precipitate rupture.17,27, 28, 29, 30 Zhu et al31,32 suggested that tumor rupture maybe related to vascular dysfunction especially when blood vessels encounter high blood pressure or mechanical injury, although the hypothesis was not well investigated. Chearanai et al33 in a case series of 63 cases found that bleeding from a laceration in a superficial tumor, especially if located under the right diaphragm, secondary to minimal trauma, or even normal respiration can cause tumor rupture. Although our study did not suggest a definite mechanism, patients with larger tumor size were more likely to rupture, but there was no association with hypertension or coagulopathy. Contrary to previous studies, patients with cirrhosis in our cohort had less tendency to rupture.
It is important to understand risk factors for rupture as these are the patients who have eluded early detection. Zhu et al7 in a retrospective study of 4209 patients with HCC had 200 with rupture and found that hypertension, cirrhosis, tumor size >5 cm, tumor protrusion outside the surface, and extra-hepatic invasion were predictive factors of rupture. Kerdsuknirun et al34 in 51 ruptured cases out of 333 HCC patients found that age, abdominal pain, anemic symptoms, and Child–Pugh score more than 6 were independent predictive factors of ruptured HCC. Many of the studies on ruptured HCC are from large cohorts in Asia, which are quite homogenous, with viral hepatitis B and C as the main etiology of chronic liver disease. However, etiology of cirrhosis was never reported as a negative predictive factor of tumor rupture.2,35 In our heterogenous cohort with wide variety of chronic liver diseases, we found that tumor size >5 cm and single tumors were predictive factors of rupture. Obesity (BMI ≥30) was a predictive factor of tumor rupture compared with morbid obesity (BMI ≥35), which suggests that the degree of obesity and potentially body habitus may influence tumor rupture. Contrary to previous studies, we found that patients with rupture were less likely to have cirrhosis. It is likely that those patients with cirrhosis in our cohort had earlier detection of their HCC with a smaller mean tumor size and more of their tumors found with HCC surveillance. The noncirrhotic patients frequently included those with recently diagnosed HBV or NAFL/NASH who did not have the benefit of chronic disease management. Less surveillance and larger tumor size likely contributed to the increased incidence of rupture in noncirrhotics. In addition, patients with rupture had a significantly higher neutrophil/lymphocyte ratio, which may suggest that inflammatory changes may play a role in the pathophysiology of ruptured HCC. The advantage of using a marker such as neutrophil/lymphocyte ratio is an easily measured laboratory test and may have some prognostic value. Although this value has not been used to direct therapy prospectively at our center, this may become more important as other centers validate the importance of this inflammatory marker.
Finally, although the incidence of HCC is increasing in the West, likely due to underlying NAFLD and NASH,1,3,21 the incidence of spontaneous rupture is still uncommon at less than 3% compared with Asia where the incidence has been as high as 26%.2,3,6,8,9 NAFLD represents the largest proportion of new HCC cases without advanced fibrosis or cirrhosis in the United States; however, there is no consensus on which patients with NAFLD would benefit from HCC surveillance.21 Although some studies have suggested that the incidence of ruptured HCC is decreasing with improved surveillance,2, 3, 4,6 our study did not show this. Despite more HCC found with surveillance and a smaller proportion with large tumors over time, we identified an increased proportion of HCC patients with NAFLD/NASH, as well as with metabolic risk factors such as diabetes, hyperlipidemia, obesity, and morbid obesity, and less with underlying cirrhosis. These factors may be contributing to the sustained incidence of ruptured HCC despite the overall improvement in early detection over time.
This study is limited in that it is a single-center study in a unique population with ethnic diversity and a wide range of risk factors. The patient population may seem somewhat skewed as these cases were referred to a group of surgeons. Finally, the diagnosis of cirrhosis was sometimes based on clinical factors and imaging characteristics as histology on a liver biopsy was not available in all patients.
Despite these limitations, this study represents a large cohort of HCC patients in a well-annotated database over a 27-year period. It also represents a large proportion of all the cases in a State that has the second highest incidence of liver cancer in the United States. Such data are generally not available in large administrative and cancer databases that may have detailed information on staging but lack details on whether HCC presented with rupture.
In conclusion, we have demonstrated that despite improved early detection of HCC with surveillance over time, there were still patients who present with the dramatic presentation of rupture. These patients are more likely to be obese and have large solitary tumors. They are also less likely to have cirrhosis. Current established surveillance strategies have emphasized cirrhosis especially due to viral hepatitis. Although we are aware that patients with diabetes, obesity, and metabolic risk factors are at risk for fat-related liver disease and HCC, many of these patients are not specifically aware of their liver disease and are not seen in specialized liver centers. As the characteristics of patients who develop HCC have changed over time, efforts will be needed to better identify the population at risk and determine which of these patients would most benefit from HCC surveillance so we can ultimately avoid the highly fatal complications of tumor rupture.
CRediT authorship contribution statement
Adham E. Obeidat: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Project administration. Linda L. Wong: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Conflicts of interest
The authors have none to declare.
Acknowledgement
This work was supported by the National Institutes of Health grant 1U01CA230690-01, 2018.
FUNDING
None.
References
- 1.Yang J.D., Hainaut P., Gores G.J., Amina A., Amelie P., Lewis R.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahu S.K., Chawla Y.K., Dhiman R.K., et al. Rupture of hepatocellular carcinoma: a review of literature. J Clin Exp Hepatol. 2019;9:245–256. doi: 10.1016/j.jceh.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida H., Mamada Y., Taniai N., Uchida E. Spontaneous ruptured hepatocellular carcinoma. Hepatol Res. 2016;46:13–21. doi: 10.1111/hepr.12498. [DOI] [PubMed] [Google Scholar]
- 4.Moris D., Chakedis J., Sun S.H., et al. Management, outcomes, and prognostic factors of ruptured hepatocellular carcinoma: a systematic review. J Surg Oncol. 2018;117:341–353. doi: 10.1002/jso.24869. [DOI] [PubMed] [Google Scholar]
- 5.Llovet J.M., Burroughs A., Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 6.Lai E.C.H., Lau W.Y. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141:191–198. doi: 10.1001/archsurg.141.2.191. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Q., Li J., Yan J.-J., Huang L., Wu M.-C., Yan Y.-Q. Predictors and clinical outcomes for spontaneous rupture of hepatocellular carcinoma. World J Gastroenterol. 2012;18:7302–7307. doi: 10.3748/wjg.v18.i48.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassi N., Caratozzolo E., Bonariol L., et al. Management of ruptured hepatocellular carcinoma: implications for therapy. World J Gastroenterol. 2010;16:1221–1225. doi: 10.3748/wjg.v16.i10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergara V., Muratore A., Bouzari H., et al. Spontaneous rupture of hepatocelluar carcinoma: surgical resection and long-term survival. Eur J Surg Oncol. 2000;26:770–772. doi: 10.1053/ejso.2000.1001. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto M., Sudo T., Kuyama T. Spontaneous rupture of hepatocellular carcinoma: a review of 172 Japanese cases. Am J Gastroenterol. 1991;86:67–71. [PubMed] [Google Scholar]
- 11.Xu H.S., Yan J.B. Conservative management of spontaneous ruptured hepatocellular carcinoma. Am Surg. 1994;60:629–633. [PubMed] [Google Scholar]
- 12.Tan N.P., Majeed A., Roberts S.K., et al. Survival of patients with ruptured and non-ruptured hepatocellular carcinoma. Med J Aust. 2020;212:277–278. doi: 10.5694/mja2.50483. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Huang L., Liu C.-F., et al. Risk factors and surgical outcomes for spontaneous rupture of BCLC stages A and B hepatocellular carcinoma: a case-control study. World J Gastroenterol. 2014;20:9121–9127. doi: 10.3748/wjg.v20.i27.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C.Y., Lin X.Z., Shin J.S., et al. Spontaneous rupture of hepatocellular carcinoma. A review of 141 Taiwanese cases and comparison with nonrupture cases. J Clin Gastroenterol. 1995;21:238–242. [PubMed] [Google Scholar]
- 15.Heimbach J.K., Kulik L.M., Finn R.S., et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 16.Roberts L.R., Sirlin C.B., Zaiem F., et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology. 2018;67:401–421. doi: 10.1002/hep.29487. [DOI] [PubMed] [Google Scholar]
- 17.Battula N., Madanur M., Priest O., et al. Spontaneous rupture of hepatocellular carcinoma: a western experience. Am J Surg. 2009;197:164–167. doi: 10.1016/j.amjsurg.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Han X.-J., Su H.-Y., Shao H.-B., Xu K. Prognostic factors of spontaneously ruptured hepatocellular carcinoma. World J Gastroenterol. 2015;21:7488–7494. doi: 10.3748/wjg.v21.i24.7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan W., Hung C., Pan K., et al. Impact of spontaneous tumor rupture on prognosis of patients with T4 hepatocellular carcinoma. J Surg Oncol. 2016;113:789–795. doi: 10.1002/jso.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang B., Lu Y., Zhang X., Yu L., Pan C., Wu Z. Management of spontaneous rupture of hepatocellular carcinoma. ANZ J Surg. 2008;78:501–503. doi: 10.1111/j.1445-2197.2008.04543.x. [DOI] [PubMed] [Google Scholar]
- 21.Nuño-Guzmán C.M., Marín-Contreras M.E. Ruptured hepatocellular carcinoma and non-alcoholic fatty liver disease, a potentially life-threatening complication in a population at increased risk. Ann Hepatol. 2020;19:3–4. doi: 10.1016/j.aohep.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Khairuddin A., Ong G.H., Tan J.S., et al. Emergency laparoscopic resection of spontaneous rupture of hepatocellular carcinoma: a case report. Int J Surg Case Rep. 2019;66:104–106. doi: 10.1016/j.ijscr.2019.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chua D.W., Koh Y.-X., Allen J.C., et al. Impact of spontaneous rupture on the survival outcomes after liver resection for hepatocellular carcinoma: a propensity matched analysis comparing ruptured versus non-ruptured tumors. Eur J Surg Oncol. 2019;45:1652–1659. doi: 10.1016/j.ejso.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 24.Joliat G.-R., Labgaa I., Uldry E., Demartines N., Halkic N. Recurrence rate and overall survival of operated ruptured hepatocellular carcinomas. Eur J Gastroenterol Hepatol. 2018;30:792–796. doi: 10.1097/MEG.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 25.Yang T., Sun Y.F., Zhang J., et al. Partial hepatectomy for ruptured hepatocellular carcinoma. BJS (British Journal of Surgery) 2013;100:1071–1079. doi: 10.1002/bjs.9167. [DOI] [PubMed] [Google Scholar]
- 26.Chan A.C.Y., Dai J.W.C., Chok K.S.H., Cheung T.T., Lo C.M. Prognostic influence of spontaneous tumor rupture on hepatocellular carcinoma after interval hepatectomy. Surgery. 2016;159:409–417. doi: 10.1016/j.surg.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Bruls S., Joskin J., Chauveau R., Delwaide J., Meunier P. Ruptured hepatocellular carcinoma following transcatheter arterial chemoembolization. J Belg Radiol. 2011;94:68–70. [PubMed] [Google Scholar]
- 28.Rombolà F., Caravetta A., Mollo F., Spinoso A., Peluso L., Guarino R. Sorafenib, risk of bleeding and spontaneous rupture of hepatocellular carcinoma. A clinical case. Acta Med. 2011;54:177–179. doi: 10.14712/18059694.2016.46. [DOI] [PubMed] [Google Scholar]
- 29.Tsuboi R., Asano T., Matsuura K., Asabe S., Mashima H. Rupture of a small hepatocellular carcinoma in a stable disease state in a patient receiving sorafenib treatment. Chin Med J. 2018;131:999–1000. doi: 10.4103/0366-6999.229907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung J.W., Park J.H., Han J.K., et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996;198:33–40. doi: 10.1148/radiology.198.1.8539401. [DOI] [PubMed] [Google Scholar]
- 31.Zhu L.X., Geng X.P., Fan S.T. Spontaneous rupture of hepatocellular carcinoma and vascular injury. Arch Surg. 2001;136:682–687. doi: 10.1001/archsurg.136.6.682. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L.X., Meng X.L., Fan S.T. Elasticity of small artery in patient with spontaneous rupture of hepatocellular carcinoma. Hepatol Res. 2004;29:13–17. doi: 10.1016/j.hepres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Chearanai O., Plengvanit U., Asavanich C., Damrongsak D., Sindhvananda K., Boonyapisit S. Spontaneous rupture of primary hepatoma: report of 63 cases with particular reference to the pathogenesis and rationale treatment by hepatic artery ligation. Cancer. 1983;51:1532–1536. doi: 10.1002/1097-0142(19830415)51:8<1532::aid-cncr2820510829>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Kerdsuknirun J., Vilaichone V., Vilaichone R.-K. Risk factors and prognosis of spontaneously ruptured hepatocellular carcinoma in Thailand. Asian Pac J Cancer Prev. 2018;19:3629–3634. doi: 10.31557/APJCP.2018.19.12.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoki T., Kokudo N., Matsuyama Y., et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg. 2014;259:532–542. doi: 10.1097/SLA.0b013e31828846de. [DOI] [PubMed] [Google Scholar]