Abstract
Magnetic resonance imaging (MRI) is widely used in meningeal lesions due to rapid and accurate diagnosis and prevention of serious complications. The aim of the present study was to compare these two sequences after injection of a contrast agent into meningeal lesions. This is a descriptive-analytical study that was performed in 2018-2020 on patients referred to the radiology ward with detection of any meningeal involvements in the MRI images. In addition to T1-W, FLAIR sequence imaging was also performed. Images were initially evaluated by two expert radiologists and a neurologist. The diagnostic values of the sequences were compared. Overall, a total number of 147 patients with meningeal lesions in their brain MRI entered the study. 57.1% of cases (84 patients) had an infectious etiology and 42.9% (63 patients) had a tumoral etiology. T1-W images without contrast were able to diagnose 78 cases of meningitis (92.8% of them), and FLAIR sequences could diagnose 82 patients (97.6% of them). Without contrast injection on MRI, the diagnostic value of T1-W sequence was higher than FLAIR sequence for tumoral lesions (P < 0.01). The enhancement degree of T1-W was higher for tumoral findings (P < 0.01). In contrast, the enhancement degree of the FLAIR sequence was higher for infectious findings, which was also statistically significant (P = 0.015). FLAIR sequences had 92% sensitivity and 85% specificity for diagnosis of brain inflammatory diseases. Similar analysis showed that T1 sequence had 82% sensitivity and 73% specificity for diagnosis of brain inflammatory diseases.
Keywords: MRI, meningeal lesions, T1-W, FLAIR
Introduction
Meningitis is one of the infectious diseases in the central nervous system (CNS) associated with high rates of mortality. According to epidemiologic studies, the prevalence of meningitis in the Middle East is estimated to be 2.23 percent of the population [1]. Meningeal involvement with tumors is an increasing form in cancer patients that also leads to high mortality rates and complications. About 5-8% of cancer patients develop meningeal involvement [2,3]. Meningioma is the most common type of meningeal tumor, originating from the leptomeninges and accounting for 20-25% of primary intracranial tumors, with a prevalence of 7.8% per thousand population [4,5]. It has also been shown that the prevalence rate of meningioma increases within the fourth and fifth decades of life. Complications of meningeal involvement include seizures, cranial nerve palsy, focal brain lesions, hydrocephalus, and personality changes [6,7].
Diagnosis of meningitis is based on clinical signs and a cerebrospinal fluid (CSF) test. However, many patients with clinical symptoms have normal CSF [8]. Therefore, magnetic resonance imaging (MRI) is a method that can diagnose the disease in the early stages, which is widely used in the diagnosis of brain diseases due to its high sensitivity and specificity. MRI imaging is an important diagnostic method for meningeal tumor lesions because CSF cytological examinations could have false-negative results and sometimes patients are asymptomatic or have unexpected symptoms [9,10].
Nowadays, MRI is widely used in meningeal lesions due to rapid and accurate diagnosis and prevention of serious complications. An important point is to use an appropriate sequence for multiple brain lesions along with the injection of intravenous contrast that increases the accuracy of diagnosis and is also more sensitive than images without contrast. This procedure could be useful in detecting lesions, their number, and location [11]. Many studies have evaluated the diagnostic value of the fluid-attenuated inversion recovery (FLAIR) and contrast-enhanced T1-weighted (T1-W) sequences in intracranial lesions [12,13]. Tissue can be characterized by two different relaxation times - T1 and T2. T1 (longitudinal relaxation time) is the time constant which determines the rate at which excited protons return to equilibrium. It is a measure of the time taken for spinning protons to realign with the external magnetic field. Fluid attenuated inversion recovery (FLAIR) is a special inversion recovery sequence with a long inversion time. This removes signal from the cerebrospinal fluid in the resulting images.
Studies have shown that the T1-W sequence is a better technique in the assessment of intra-axial diseases and the FLAIR sequence is more effective for detecting extra-axial lesions such as meningeal lesions [14]. The results of studies have shown that FLAIR sequence is more effective than T1-W sequence in the detection of meningeal infection, inflammation, and metastases [15].
According to the fact that after injection of intravenous contrast agent, normal meninges on T1-W sequence can be enhanced to some degree, this may cause confusion, especially in children. Therefore, the FLAIR sequence can be a valuable adjunct in detecting meningeal lesions [16].
Due to the diagnostic importance of brain lesions, especially meningeal lesions, in terms of prevalence, increasing prevalence, and permanent neurological complications, the use of MRI imaging seems to be necessary. Due to the specificity and sensitivity of the contrast-enhanced weighted-T1 and FLAIR sequences, along with injection into intracranial lesions, it is necessary to make a comparison between these two sequences in meningeal lesions. Obviously, using the appropriate sequence will help in a better, faster, and more accurate diagnosis. The aim of the present study was to compare these two sequences after injection of a contrast agent into meningeal lesions.
Material and methods
Study design
This is a descriptive-analytical study that was performed in 2018-2020 at Firoozgar Hospital, affiliated with Iran University of Medical Sciences. The study population consisted of patients referred to the radiology ward (MRI unit) of Firoozgar hospital in 2018-2020. The study protocol was approved by the Research Committee of Iran University of Medical Sciences and the Ethics committee has confirmed it (Code: IR.IUMS.MED.REC.1390.253.12).
Inclusion and exclusion criteria
The inclusion criteria were age more than 18 years, undergoing brain MRI with contrast for any reason in 2018-2020, availability of T1-W, T2-W, and FLAIR sequences, detection of any meningeal involvement in the MRI images, and signing the written informed consent to participate in this study. The exclusion criteria were low quality of brain MRI images, absence of meningeal involvement, patients with vital organ injury, patients with traumatic events, patients with previous histories of brain surgeries or abscesses, patients that required emergent action, and patients who would exit the study.
Sample size calculation
We used the sequential sampling method and among patients who underwent MRI imaging with contrast, patients with meningeal involvements were selected. The sample size volume was calculated based on the formula of comparing two ratios with 95% confidence and 90% power and assuming a sensitivity of 34% and 66% in two sequences according to previous studies, and finally, the sample size of 147 patients was selected.
N = (Z 1-α/2 + Z 1-β )2 S 2 / (μ - μ 0)2
MRI apparatus
We have used the Philips model MRI machine and the intensity of 1 Tesla field. Ethically, the MRI was requested by the relevant specialist with indication, and performing the additional FLAIR sequence did not have any additional costs or side effects on the patient.
Data collection
In the first step, we selected the patients who met the inclusion criteria and those patients with meningeal involvements (infection or tumor) were included. Demographic data of all included patients, including age, gender, and primary diagnosis was recorded. In addition to T1-W and T2-W sequences, FLAIR sequence imaging was also performed. FLAIR and weighted-T1 images were initially evaluated by two expert radiologists and a neurologist with at least 8 years of experience in CNS imaging or CNS lesions. The diagnosis of meningeal lesions was made by the two experts and was confirmed by another radiologist. FLAIR and weighted-T1 images were compared in each patient in terms of the enhancement degree of the lesions based on the signal intensity. Further comparisons were made based on CSF analysis by the time of CNS infection, and also between infectious or tumoral meningeal lesions. The diagnosis of the lesions were made by radiologists and neurologists. We should note that the gold standard diagnostic method is lumbar puncture and CSF analysis.
Data analysis
After collecting the study data, it was entered into SPSS software (version 25, IBM Corporation, Armonk, NY) and analyzed. Qualitative variables were compared using X2 and quantitative variables were compared using a Paired T-test. Quantitative variables had a normal distribution and a standard deviation was presented. In all tests, values of P < 0.05 were considered as a significant level.
Results
Study population
Overall, a total number of 147 patients with meningeal lesions in the brain MRI entered the study. The mean age of the patients was 41.38 ± 10.28 with a range of 20-87 years. Primary analysis of our data showed that the most of our patients had 30-40 years and 60-70 years of age, and patients with 70-80 years had the lowest frequency distribution. This data is illustrated in Table 1. We also showed that 55.1% of our patients were males and 44.9% were females.
Table 1.
Frequency distribution of patients according to age groups
| Age (years) | 20-30 | 30-40 | 40-50 | 50-60 | 60-70 | 70-80 | 80-90 |
|---|---|---|---|---|---|---|---|
| Frequency | 13 | 28 | 21 | 24 | 32 | 25 | 4 |
| Percent | 8.8% | 19% | 14.3% | 16.3% | 21.7% | 17% | 2.9% |
MRI findings with contrast
In total, 57.1% of cases (84 patients) had an infectious etiology and 42.9% (63 patients) had a tumoral etiology. Among patients with infectious etiology, CSF analysis of 17 patients (20.2%) showed bacterial meningitis. T1-W images without contrast were able to diagnose 78 cases of meningitis (92.8% of them), and FLAIR sequences could diagnose 82 patients (97.6% of them). After contrast injection, T1 and FLAIR sequences showed higher contrast enhancement degrees in 95.2% and 98.8% of patients, respectively. Overall, diagnostic quality of the FLAIR and T1-W sequences without contrast injection was 51% and postcontrast FLAIR MRI was 42.9%. In terms of culture-positive (46 cases), a significant difference was observed between the two groups of tumoral and inflammatory lesions. (P < 0.01) (Table 2).
Table 2.
Comparison of infection frequency based on pathologic results
| Type of lesion | Tumoral | Inflammatory/infectious | P-value |
|---|---|---|---|
| Non-bacterial infection | 21 (45.6%) | 2 (4.4%) | < 0.001 |
| Bacterial infection | 0 | 10 (21.7%) | |
| Non-infectious | 0 | 15 (28.3%) |
MRI findings without contrast
Without contrast injection on MRI, the diagnostic value of T1-W sequence was higher than FLAIR sequence for tumoral lesions (P < 0.01). Comparison of FLAIR and T1 sequences quality without contrast injection showed that there is no statistical difference between these two sequences based on pathological diagnosis in the present study (Table 3).
Table 3.
Comparison of FLAIR and T1 sequences quality without contrast injection
| FLAIR sequence in comparison to T1-W sequence | Tumoral | Inflammatory/infectious | P-value |
|---|---|---|---|
| Higher compared to T1-W | 4 (8.2%) | 13 (26.5%) | 0.082 |
| Lower compared to T1-W | 5 (10.2%) | 2 (4.1%) | |
| Same compared to T1-W | 12 (24.5%) | 13 (26.5%) |
Enhancement degree
In MRI with contrast injection, the enhancement degree of T1-W was higher for tumoral findings (P < 0.01). In contrast, the enhancement degree of the FLAIR sequence was higher for infectious findings, which was also statistically significant (P = 0.015).
In general, a comparison of precontrast FLAIR and T1 sequences showed that the FLAIR sequence was more useful for infectious causes and less useful for tumoral lesions and this difference was also significant (P < 0.001). After contrast injection, comparison of FLAIR sequence with T1-W based on CSF culture (Table 4) also showed that the FLAIR sequence was more diagnostic for infection, that was also statistically significant (P = 0.006). Figures 1 and 2 show the brain MRI findings of tumor lesions.
Table 4.
Comparison of FLAIR sequence with T1-W based on CSF culture after contrast injection
| Comparison of FLAIR sequence compared to T1-W | Non-bacterial | Bacterial | Non-infectious | P-value |
|---|---|---|---|---|
| Higher compared to T1-W | 8 (16.3%) | 7 (14.3%) | 6 (12.2%) | 0.006 |
| Lower compared to T1-W | 3 (6.1%) | 0 | 15 (30.7%) | |
| Same compared to T1-W | 4 (8.2%) | 3 (6.1%) | 3 (6.1%) |
Figure 1.
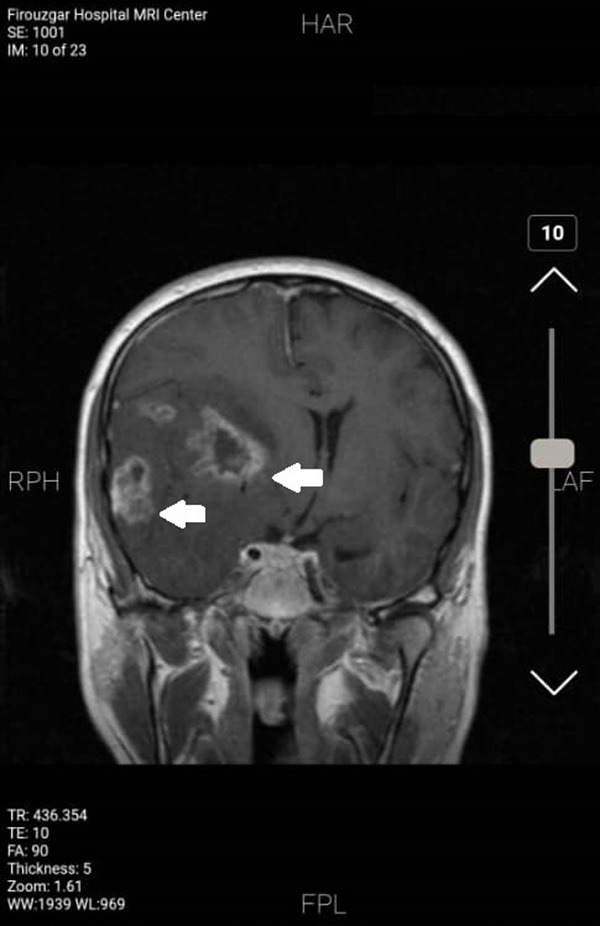
Brain MRI showing a tumoral lesion. The points indicate large tumor lesions (glioblastoma multiform (GBM)) in T1-weighted MRI. Heterogeneous lesions containing central necrotic and severe vasogenic edema in the environment in the right fronto-temporal lobe could be observed. The midline shift due to severe increased intracranial pressure is also observed.
Figure 2.
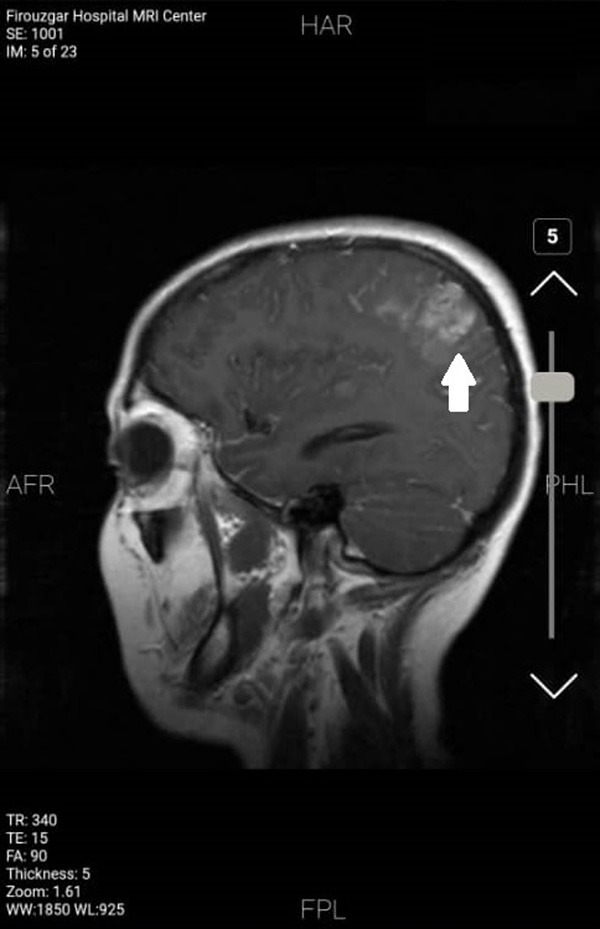
Brain metastasis demonstrated by T1-wighted MRI. Brain metastasis due to breast cancer indicated by T1-weighted MRI. Homogenous enhancement in the left parietal lobe could be observed.
Assessments of ROC and area under the curve (AUC) indicated that FLAIR sequences had 92% sensitivity and 85% specificity for diagnosis of brain inflammatory diseases. Similar analysis showed that T1 sequence had 82% sensitivity and 73% specificity for diagnosis of brain inflammatory diseases. These data are shown in Figure 3.
Figure 3.
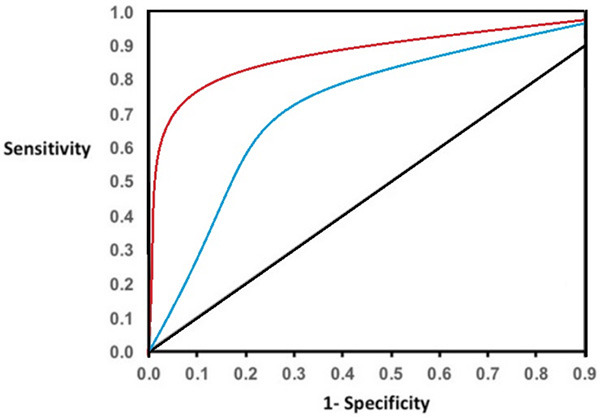
Evaluation of sensitivity and specificity of FLAIR and T1 sequences for diagnosis of brain inflammatory diseases. Right line: FLAIR sequence; blue line: T1 sequence.
Discussion
The present study was performed to compare FLAIR and weighted-T1 sequences with an injection of contrast agent in meningeal lesions. The results of the study showed that FLAIR sequences are more useful in infectious lesions and weighted-T1 performs better in non-infectious lesions.
T1-W images without contrast were able to diagnose 92.8% of cases with meningitis, and FLAIR sequences could diagnose 97.6% of these patients. After contrast injection, T1 and FLAIR sequences showed higher contrast enhancement degrees in 95.2% and 98.8% of patients, respectively. We also showed that without contrast injection on MRI, the diagnostic value of T1-W sequence was higher than the FLAIR sequence for tumoral lesions but in MRI with contrast injection, the enhancement degree of the T1-W was higher for tumoral findings. Based on our results, the enhancement degree of the FLAIR sequence was higher for infectious findings, which was also statistically significant. Overall, the contrast-enhanced FLAIR sequences showed improved diagnostic accuracy compared to the contrast-enhanced T1-W sequences.
In 2020, Kamr and colleagues performed a cross-sectional and prospective study of 55 cases to compare contrast-enhanced T1-W and contrast-enhanced FLAIR results in diagnosing meningeal abnormalities in cases of suspected meningitis. They observed that the sensitivity and specificity of the contrast-enhanced FLAIR sequences were 91.9% and 100%, respectively. These results are approximately similar to our study. In this study, they concluded that the accuracy of the contrast-enhanced FLAIR sequence was more than the contrast-enhanced T1-W. Therefore, contrast-enhanced FLAIR should be used for non-invasive diagnosis in cases of suspected meningitis [17].
In 2018, Mubashar and others conducted a similar study on 55 patients for a period of 4 months. In this study, similar results have been obtained so that the sensitivity of contrast-enhanced FLAIR and contrast-enhanced T1-W sequences were 95.3% and 76.7%, respectively. Therefore, post-contrast FLAIR sequences can be useful for the non-invasive diagnosis of meningitis and should be added as a regular sequence in the MRI protocol. Overall, this study is in line with our study as well as Azad and colleagues who reported that the contrast-enhanced FLAIR sequences showed improved diagnostic accuracy and sensitivity compared to the contrast-enhanced T1-W sequences [18,19].
Also, Vaswani and colleagues reported that the postcontrast FLAIR sequence had a specificity of 85.71% and a sensitivity of 96%, whereas the postcontrast T1-W sequence had a sensitivity of 68% and similar specificity (85.71%) in the diagnosis of meningitis. Considering that the sensitivity and specificity of the contrast-enhanced FLAIR sequence in this study was more than the contrast-enhanced T1-W sequence in the detection of meningitis, they suggested that the contrast-enhanced FLAIR sequence should be routinely used in suspected cases of meningitis [20]. Zheng and colleagues in a study of 104 patients found that more lesions were seen on contrast-enhancement T1-W than those on the contrast-enhancement FLAIR sequence. However, at first glance, it seems different from what we have achieved, but with some reflection on the details of the results and conditions of the samples in this study, we will find that there are many similarities between the two studies. In this study, only 4 lesions were revealed on the FLAIR sequence and only 7 lesions were found on the T1-W sequence. Zheng and others found that lesions located in the cerebellar hemispheres or the fourth ventricle and generally in the deep parts were revealed better on the T1-W sequence, and those that are more superficial, such as lateral ventricular and meningeal lesions were highly found on the FLAIR sequence. In this respect, our study has similar results to Zheng’s study, but due to the fact that his study samples were selected from patients with tumoral lesions, the final results of the two studies are different [21].
Parmar and others also found that the postcontrast FLAIR sequence has a higher specificity compared to contrast-enhanced T1-W in diagnosing infectious lesions. This point can also confirm the findings of our study, but for better investigation, the sensitivity and specificity of the FLAIR sequence and T1-W sequence after contrast injection should be considered in more studies [22]. In a study conducted by Keremer and colleagues, they found that the contrast-enhanced FLAIR sequence was better than the contrast-enhanced T1-W sequence in diagnosing infectious leptomeningeal lesions. This result was similar to what we obtained, but they also found that the FLAIR sequence was better for tumoral lesions. However, this difference can be due to the small sample size (10 patients). In their study, they also examined the difference between two sequences in both early and delayed enhanced, but in the present study, it is not clear which sequence was used. Therefore, we are not able to explain the main reason for the difference in the results because our results are similar to this study on early enhanced sequences and different in delayed enhanced sequences [23].
Splendiani and others noted that although contrast-enhanced T1-W sequences are used more in the diagnosis of pathological conditions of the CNS. However, contrast-enhanced FLAIR imaging had higher sensitivity for the early detection of both viral and bacterial meningitis [24]. The results obtained in our study is also well consistent with the above studies, but all of these studies in contrast with our study, included only a small number of patients.
In the present study, a review of the data shows that T1-W and FLAIR sequences after contrast injection showed higher contrast enhancement degrees. We also found that without contrast injection on MRI, the diagnostic value of the T1-W sequence was higher than the FLAIR sequence for tumoral lesions, but on MRI with contrast injection, the enhancement degree of the FLAIR sequence was higher for infectious lesions. Overall, the contrast-enhanced FLAIR sequences showed improved diagnostic accuracy compared to the contrast-enhanced T1-W sequences. In this study, a larger statistical population has been used than other studies, which can have a significant impact on the validation of the results. However, the limitations of the present study were the restricted study population and restricted centers in which we evaluated our results. We recommend that multi-centric studies be conducted on this issue.
Conclusion
On MRI with contrast injection, the degree of contrast enhancement was higher in T1-W for tumoral findings. In contrast, the degree of contrast enhancement was higher in the FLAIR sequence for infectious findings. Overall, after contrast injection, a comparison of FLAIR and T1 sequences with each other showed that the FLAIR sequence is better for infection and worse for tumoral lesions. As a final result, the use of contrast-enhanced FLAIR sequence is helpful in the early diagnosis of meningeal infections, but contrast-enhanced T1-W sequence is more enhanced in tumoral lesions.
Disclosure of conflict of interest
None.
References
- 1.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi SJ, Park YH, Kim JA, Han JH, Choe G, Kim S. Pearls & oy-sters: asymmetric meningeal involvement is a common feature of rheumatoid meningitis. Neurology. 2017;88:e108–e110. doi: 10.1212/WNL.0000000000003744. [DOI] [PubMed] [Google Scholar]
- 3.Rafiee Zadeh A, Ghadimi K, Ataei A, Askari M, Sheikhinia N, Tavoosi N, Falahatian M. Mechanism and adverse effects of multiple sclerosis drugs: a review article. Part 2. Int J Physiol Pathophysiol Pharmacol. 2019;11:105–114. [PMC free article] [PubMed] [Google Scholar]
- 4.Magill ST, Young JS, Chae R, Aghi MK, Theodosopoulos PV, McDermott MW. Relationship between tumor location, size, and WHO grade in meningioma. Neurosurg Focus. 2018;44:E4. doi: 10.3171/2018.1.FOCUS17752. [DOI] [PubMed] [Google Scholar]
- 5.Apra C, Peyre M, Kalamarides M. Current treatment options for meningioma. Expert Rev Neurother. 2018;18:241–249. doi: 10.1080/14737175.2018.1429920. [DOI] [PubMed] [Google Scholar]
- 6.Fahim M, Rafiee Zadeh A, Shoureshi P, Ghadimi K, Cheshmavar M, Sheikhinia N, Afzali M. Alcohol and multiple sclerosis: an immune system-based review. Int J Physiol Pathophysiol Pharmacol. 2020;12:58–69. [PMC free article] [PubMed] [Google Scholar]
- 7.Rafiee Zadeh A, Askari M, Azadani NN, Ataei A, Ghadimi K, Tavoosi N, Falahatian M. Mechanism and adverse effects of multiple sclerosis drugs: a review article. Part 1. Int J Physiol Pathophysiol Pharmacol. 2019;11:95–104. [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson MR, Sample HA, Zorn KC, Arevalo S, Yu G, Neuhaus J, Federman S, Stryke D, Briggs B, Langelier C. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Eng J Med. 2019;380:2327–2340. doi: 10.1056/NEJMoa1803396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dian S, Hermawan R, van Laarhoven A, Immaculata S, Achmad TH, Ruslami R, Anwary F, Soetikno RD, Ganiem AR, van Crevel R. Brain MRI findings in relation to clinical characteristics and outcome of tuberculous meningitis. PLoS One. 2020;15:e0241974. doi: 10.1371/journal.pone.0241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafiee Zadeh A, Ghadimi K, Mohammadi B, Hatamian H, Naghibi SN, Danaeiniya A. Effects of estrogen and progesterone on different immune cells related to multiple sclerosis. Casp J Neurol Sci. 2018;4:83–90. [Google Scholar]
- 11.Absinta M, Sati P, Reich DS. Advanced MRI and staging of multiple sclerosis lesions. Nat Rev Neurol. 2016;12:358–368. doi: 10.1038/nrneurol.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millward JM, de Schellenberger AA, Berndt D, Hanke-Vela L, Schellenberger E, Waiczies S, Taupitz M, Kobayashi Y, Wagner S, Infante-Duarte C. Application of europium-doped very small iron oxide nanoparticles to visualize neuroinflammation with MRI and fluorescence microscopy. Neuroscience. 2019;403:136–144. doi: 10.1016/j.neuroscience.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Kamath AA, Friedman DD, Hacker CD, Smyth MD, Limbrick DD Jr, Kim AH, Hawasli AH, Leuthardt EC. MRI-guided interstitial laser ablation for intracranial lesions: a large single-institution experience of 133 cases. Stereotact Funct Neurosurg. 2017;95:417–428. doi: 10.1159/000485387. [DOI] [PubMed] [Google Scholar]
- 14.Rafiee Zadeh A, Falahatian M, Alsahebfosoul F. Serum levels of histamine and diamine oxidase in multiple sclerosis. Am J Clin Exp Immunol. 2018;7:100–105. [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Kumar S, Surya M, Mahajan A, Sharma S. To compare diagnostic ability of contrast-enhanced three-dimensional T1-SPACE with three-dimensional fluid-attenuated inversion recovery and three-dimensional T1-magnetization prepared rapid gradient echo magnetic resonance sequences in patients of meningitis. J Neurosci Rural Pract. 2019;10:48. doi: 10.4103/jnrp.jnrp_157_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayaraman K, Rangasami R, Chandrasekharan A. Magnetic resonance imaging findings in viral encephalitis: a pictorial essay. J Neurosci Rural Pract. 2018;9:556. doi: 10.4103/jnrp.jnrp_120_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamr WH, Eissawy MG, Saadawy A. The value of contrast-enhanced FLAIR magnetic resonance imaging in detecting meningeal abnormalities in suspected cases of meningitis compared to conventional contrast-enhanced T1WI sequences. Egypt J Radiol Nucl Med. 2020;51:1–6. [Google Scholar]
- 18.Babak A, Rouzbahani R, Khalili Nejad R, Rafiee Zadeh A. Comparison of nutritional behaviors and physical activities between overweight/obese and normal-weight adults. Adv Biomed Res. 2019;8:62. doi: 10.4103/abr.abr_134_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azad R, Tayal M, Azad S, Sharma G, Srivastava RK. Qualitative and quantitative comparison of contrast-enhanced fluid-attenuated inversion recovery, magnetization transfer spin echo, and fat-saturation T1-weighted sequences in infectious meningitis. Korean J Radiol. 2017;18:973. doi: 10.3348/kjr.2017.18.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaswani AK, Nizamani WM, Ali M, Aneel G, Shahani BK, Hussain S. Diagnostic accuracy of contrast-enhanced FLAIR magnetic resonance imaging in diagnosis of meningitis correlated with CSF analysis. ISRN Radiol. 2014;2014:578986. doi: 10.1155/2014/578986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou ZR, Shen TZ, Chen XR, Peng WJ. Diagnostic value of contrast-enhanced fluid-attenuated inversion-recovery MRI for intracranial tumors in comparison with post-contrast T1W spin-echo MRI. Chin Med J. 2006;119:467–473. [PubMed] [Google Scholar]
- 22.Parmar H, Sitoh YY, Anand P, Chua V, Hui F. Contrast-enhanced flair imaging in the evaluation of infectious leptomeningeal diseases. Eur J Radiol. 2006;58:89–95. doi: 10.1016/j.ejrad.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Kremer S, Eid MA, Bierry G, Bogorin A, Koob M, Dietemann J, Fruehlich S. Accuracy of delayed post-contrast FLAIR MR imaging for the diagnosis of leptomeningeal infectious or tumoral diseases. J Neuroradiol. 2006;33:285–291. doi: 10.1016/s0150-9861(06)77286-8. [DOI] [PubMed] [Google Scholar]
- 24.Splendiani A, Puglielli E, De Amicis R, Necozione S, Masciocchi C, Gallucci M. Contrast-enhanced FLAIR in the early diagnosis of infectious meningitis. Neuroradiology. 2005;47:591–598. doi: 10.1007/s00234-005-1383-7. [DOI] [PubMed] [Google Scholar]


