Abstract
Background
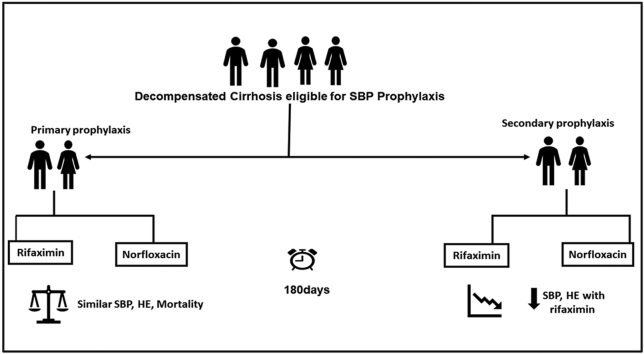
Spontaneous bacterial peritonitis (SBP) heralds increased mortality in cirrhosis, mandating strategies for prophylaxis. Norfloxacin has been the recommended choice for SBP prevention. However, its use has raised concerns about antibiotic resistance. Rifaximin has been suggested as an alternative. We investigated the efficacy of rifaximin against norfloxacin in primary and secondary prophylaxis of SBP.
Methods
In this open-labeled randomized trial, patients with either advanced cirrhosis having ascitic fluid protein levels (<1.5 g/l), Child-Pugh score ≥9 points, serum bilirubin ≥3 mg/dl or impaired renal function (primary prophylaxis group), or those with prior SBP (secondary prophylaxis group) received either norfloxacin (400 mg once daily) or rifaximin (550 mg twice daily). All patients were followed for six months, with the primary endpoint being the development of incident SBP.
Results
142 patients were assessed for eligibility, of which 132 met the enrolment criteria; 12 were lost to follow-up, while 4 discontinued treatment. In patients on primary prophylaxis, occurrence of SBP was similar (14.3% vs. 24.3%, P = 0.5), whereas in secondary prophylaxis SBP recurrence was lower with rifaximin (7% vs. 39% P = 0.004). Rifaximin significantly reduced the odds for SBP development in secondary prophylaxis [OR (95% CI0.14 (0.02–0.73; P = 0.02)]. Patients receiving rifaximin as secondary prophylaxis also had fewer episodes of hepatic encephalopathy (23.1% vs. 51.5%, P = 0.02). 180-day survival between the arms in either group was similar (P = 0.5, P = 0.2).
Conclusion
In comparison to norfloxacin, rifaximin significantly reduces incident events of SBP, as well as HE when used as a secondary prophylaxis, whereas for primary prophylaxis both have similar effects (NCT03695705).
Clinical trial registration
ClinicalTrials.gov number: NCT03695705.
Keywords: cirrhosis, ascites, SBP, infection, antibiotic, resistance, primary, secondary, hepatic encephalopathy, survival
Abbreviations: ACLF, Acute on chronic liver failure; AKI, Acute Kidney Injury; CONSORT, Consolidated Standards of Reporting Trials; CTP, Child-Turcotte-Pugh; HE, Hepatic encephalopathy; HRS, Hepatorenal syndrome; MELD, Model of end-stage liver disease; SAAG, Serum ascites albumin gradient; SBP, Spontaneous bacterial peritonitis; UGIB, Upper Gastrointestinal Bleed; VH, Variceal hemorrhage
Graphical abstract
Background
Spontaneous bacterial peritonitis (SBP) is a key complication of cirrhosis seen in around 7–30% of in-hospital patients.1 SBP is associated with high mortality with an estimated survival of 30–50% at one year and 25–30% at two years.2 The 1-year probability of recurrence of SBP is 40–70%, due to which secondary antibiotic prophylaxis is advocated.3 Additionally, primary prophylaxis is recommended in patients with a low ascitic fluid protein (<1.5 g/dL), Child-Pugh score ≥9, bilirubin level ≥3 mg/dl, renal dysfunction, and hyponatremia due to an increased risk of SBP in this group of patients.4
Multiple antibiotic regimens with differences in dosing schedule have been used both in primary and secondary prophylaxis. Norfloxacin (400 mg/day, orally) has been recommended as the first-line agent in both primary and secondary prophylaxis.4 However, emerging evidence suggests that rifaximin may be better or as efficacious as norfloxacin in SBP prophylaxis.5,6 Additionally, rifaximin being a nonabsorbable antibiotic, has a better safety profile with regard to emerging antibiotic resistance.7 However, the literature to support this evidence across studies remains inconclusive.1,5,6 In this background, this prospective randomized open-label trial was conducted to compare the efficacy of rifaximin with norfloxacin in both primary and secondary prophylaxis of SBP.
Methods
Trial Design and Setting
This is an open-label randomized controlled trial with a 1:1 allotment ratio conducted in a tertiary care teaching hospital with a specialized liver unit to compare the efficacy of rifaximin with norfloxacin in both primary and secondary prophylaxis of SBP. Approval was obtained before the commencement of the study from the institutional ethics committee, and the trial was registered prospectively (NCT03695705; ClinicalTrials.gov). The trial adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines, the Declaration of Helsinki provisions, and good clinical practice guidelines. Written informed consent was taken from all the subjects before being included in the study. All the study authors had access to the trial data and approved the final manuscript.
Participants
All the patients diagnosed to have cirrhosis with ascites, with or without prior SBP, who were admitted or on follow-up in a tertiary care teaching hospital with a specialized liver unit were screened for enrolment between January 1, 2016, to December 31, 2016. The diagnosis of cirrhosis was based on clinical, biochemical, radiological (ultrasonography), and endoscopic findings (presence of varices) or liver histology. Diagnosis of portal hypertensive ascites related to cirrhosis was evidenced by high serum ascites albumin gradient (SAAG) (>1.1 gm/dl) and low protein ascites in a patient with diagnosed cirrhosis.8 The diagnosis of SBP was based on a polymorphonuclear (PMN) cell count of 250 or more per cubic mm or culture positivity of ascitic fluid.4
Inclusion criteria
The inclusion criteria were (1) age >18 years (2) either gender (3) cirrhosis (of any etiology) with ascites (4) prior SBP or (5) need for primary prophylaxis as evidenced by low ascites protein levels (<15 g/l) with advanced liver failure (Child-Pugh score ≥9 points with serum bilirubin level ≥3 mg/dl) or impaired renal function (serum creatinine level ≥1.2 mg/dl, blood urea nitrogen level ≥25 mg/dl, or serum sodium level ≤130 mEq/L).4
Exclusion criteria
Patients with known allergy to norfloxacin or rifaximin, a recent history of upper gastrointestinal bleed (UGIB), patients with prior overt or recurrent hepatic encephalopathy (HE), patients with a history of multiple episodes of SBP, patients with hepatocellular carcinoma or other nonhepatic malignancy, patients on immunosuppression, those with human immunodeficiency virus infection, postliver transplant or those with recent (<six months) abdominal surgery, pregnant and lactating women, and patients with other associated causes of ascites like tubercular or malignant ascites were excluded.
Interventions
All eligible patients were randomized to receive either rifaximin (550 mg twice daily) or norfloxacin (400 mg once daily in the two groups on primary and secondary prophylaxis of SBP). All patients were subjected to detailed medical history, physical examination, and laboratory examinations. At baseline, routine laboratory measurements such as complete blood cell count, biochemistry, coagulation profile, urine analysis were performed together with ascetic fluid analysis (routine cell count, protein, albumin, glucose, bacterial culture, cytology, and adenosine deaminase), urine culture, and blood culture. In addition, plain chest and abdominal X-rays, as well as abdominal sonography were undertaken. Follow-up was done monthly in the liver clinic when all laboratory examinations were repeated for up to six months. Protocol-based diagnostic paracentesis was done at the third and sixth months or earlier if the patient became symptomatic.
Ascites was managed in these patients as per standard clinical practice guidelines.4 During an episode of active variceal hemorrhage (VH) or hepatic encephalopathy (HE) or any other systemic infection or SBP, oral prophylaxis was discontinued, and intravenous antibiotics were given as appropriate. During an episode of overt hepatic encephalopathy, the planned oral antibiotic prophylaxis was continued without change (via a nasogastric tube if necessary). In case any patient developed acute kidney injury (AKI) or acute-on-chronic failure (ACLF), management was continued as per standard recommendations for the management of AKI and ACLF, respectively.4,9,10 After recovery from these acute episodes, patients were continued on previously used antibiotic prophylaxis. Patients developing SBP on prophylaxis received intravenous antibiotics (ceftriaxone 1 gm q12h for five days along with intravenous 20% albumin on day 1 and day 3).9 In patients with nonimprovement of clinical symptoms, repeat paracentesis was done at 48 h of starting intravenous antibiotics.4 The second-line antibiotics for difficult-to-treat SBP were either piperacillin-tazobactam or carbapenem. These patients, after recovery from SBP, were switched over to alternate prophylactic antibiotics like cotrimoxazole. All patients were counseled for liver transplantation in view of shortened expected life span due to decompensation. Patients undergoing liver transplantation were excluded from follow-up.
Outcomes
The primary endpoint of the study was the development of SBP, while the secondary endpoints were the emergence of non-SBP complications (HE, AKI, VH) and all-cause mortality. The overall survival was determined at the end of six months. Follow-up was done monthly in the liver clinic when all laboratory examinations were repeated for up to six months. Patients who lost to follow-up or who discontinued prophylaxis without permission for more than seven days were designated as compliance failure on the day of last visit or the last dose.
Estimation of Sample Size
A previous study has shown that the recurrence of SBP was significantly lower in patients receiving rifaximin in comparison to those receiving norfloxacin (3.88 vs. 14.13%).11 Another study also showed similar results with patients on rifaximin developing fewer episodes of SBP than those on norfloxacin (4.7% vs. 14%).12 Hence, considering a probability of development of SBP in rifaximin arm as 4% and in norfloxacin arm as 14%, with a comparative hazard of 0.52, 40 patients are required in each group with the power of 80% and alpha-error of 5%. Adjusting for 20% dropouts, 50 patients in each arm was required, and we could recruit 66 patients in each arm with equal stratification into primary and secondary prophylaxis for SBP.
Randomization
In all the eligible patients, randomization was done using a computer random number generator with an equal allotment ratio (1:1) at discharge from the hospital or at the first visit to Liver Clinic. They were randomized to receive either rifaximin (550 mg twice daily) or norfloxacin (400 mg once daily in the two groups on primary and secondary prophylaxis of SBP). Randomization and assignment were done by an unrelated person using a computer-generated random number table. Allocation concealment was done using sequentially numbered opaque sealed envelopes.
Statistical Analysis
Discrete categorical data were presented as n (%); Continuous data were written in the form of its mean and standard deviation or the form of its median and interquartile range, as per the requirement. The normality of quantitative data was checked by Kolmogorov-Smirnov tests. For normally distributed t-test was applied for statistical analysis of two groups. For skewed data, a nonparametric Mann–Whitney U-test was used for statistical analysis of two groups. For categorical data, the Pearson Chi-square test or Fisher’s exact test as appropriate was applied. For time-related variables, the Wilcoxon signed-rank test was applied. Survival curves were constructed for up to 180 days by Kaplan–Meier analysis and compared by the log-rank method. All the statistical tests were two-sided and were performed at a significance level of α = 0.05. Analysis was conducted using IBM SPSS (version 22.0 Chicago Illinois, USA).
Results
Study Flow And Baseline Characteristics
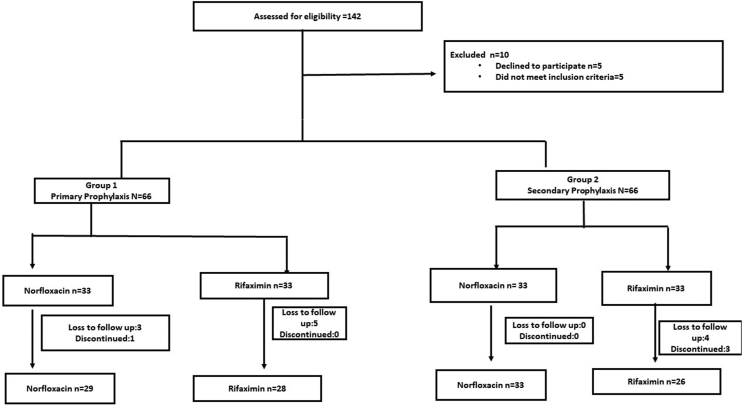
A total of 142 consecutive patients were screened. After satisfying the prespecified selection criteria, 132 patients were randomized into the study, with 33 patients in each of the four arms. Sixteen patients were excluded because of noncompliance to treatment or loss to follow-up. Finally, 116 patients were included in the final analysis on a per-protocol basis. The participant flow in the study is shown in Figure 1. The baseline characteristics of all the groups were similar except for serum albumin, which was low (P = 0.007) in patients on norfloxacin primary prophylaxis (Table 1). None of the patients were on fluoroquinolone prophylaxis at baseline.
Figure 1.
CONSORT flow diagram of the study. CONSORT, Consolidated standard of reporting trials.
Table 1.
Baseline Characteristics of Patients.
| Parameters | Primary prophylaxis | Secondary Prophylaxis | ||||
|---|---|---|---|---|---|---|
| Rifaximin (N = 28) | Norfloxacin (N = 29) | P-value | Rifaximin (N = 26) | Norfloxacin (N = 33) | P-value | |
| Age(years) | 48.3 ± 11.9a | 46.4 ± 11.2 | 0.5 | 47.5 ± 13.5 | 45.8 ± 11.6 | 0.5 |
| Sex (male) | 25 (89.3)b | 23 (79.3) | 0.4 | 16 (61.5) | 25 (75.8) | 0.2 |
| Serum albumin(gm/dL) | 3.0 ± 0.7 | 2.6 ± 0.3 | <0.05 | 2.6 ± 0.6 | 3.0 ± 0.8 | 0.1 |
| Bilirubin (mg/dL) | 3.1 ± 2.1 | 3.4 ± 2 | 0.2 | 4.2 ± 3.1 | 3.7 ± 3.1 | 0.6 |
| Serum sodium(mEq/L) | 133.3 ± 5.5 | 130.8 ± 6.81 | 0.1 | 134.3 ± 4.8 | 132.9 ± 6.2 | 0.3 |
| Serum creatinine(mg/dl) | 1.3 ± 1.1 | 1.0 ± 0.6 | 0.4 | 0.9 ± 0.8 | 1.1 ± 0.6 | 0.4 |
| INR | 1.4 ± 0.7 | 1.2 ± 0.7 | 0.3 | 1.4 ± 0.7 | 1.2 ± 0.7 | 0.4 |
| Platelet count(x109/L) | 136 ± 100 | 114 ± 657 | 0.3 | 163 ± 148 | 113 ± 67 | 0.3 |
| CTP score | 10.4 ± 1.5 | 10.0 ± 1.2 | 0.3 | 10.1 ± 1.1 | 10.1 ± 1.7 | 0.7 |
| MELD score | 19 ± 7.8 | 17 ± 7.7 | 0.4 | 16 ± 10 | 16 ± 10.1 | 0.8 |
| Variceal haemorrhage | 2 (7.1%) | 5 (17%) | 0.4 | 2 (7.7%) | 2 (6.1%) | 1 |
| Acute kidney injury | 0 (0.0%) | 3 (10.3%) | 0.2 | 1 (3.8%) | 0 (0.0%) | 0.4 |
| Ascitic fluid protein (gm/dL) | 1.2 ± 0.6 | 1.0 ± 0.6 | 0.3 | 1.3 ± 0.9 | 1.1 ± 0.6 | 0.8 |
CTP, Child Turcotte Pugh; MELD, Model for end-stage liver disease INR, International normalized ratio.
Mean±standard deviation
number of patients (%).
Development of Incident SBP
7 (24.3%) patients on norfloxacin and 4 (14.3%) on rifaximin developed SBP on primary prophylaxis (P = 0.500), whereas 13 (39%) patients on norfloxacin and 2 (7%) on rifaximin developed SBP on secondary prophylaxis (P = 0.004) (Table 2). In patients developing SBP, bacteriological cultures were positive for one patient in the rifaximin primary prophylaxis arm (Escherichia coli) and two patients in the norfloxacin secondary prophylaxis arm (Escherichia coli and Stenotrophomonas maltophilia).
Table 2.
Showing Follow-up Events of Patients on SBP Prophylaxis.
| Events on follow-up | Primary Prophylaxis | Secondary Prophylaxis | ||||
|---|---|---|---|---|---|---|
| Rifaximin n = 28 | Norfloxacin n = 29 | P-value | Rifaximin n = 26 | Norfloxacin n = 33 | P-value | |
| SBP | 4 (14%) | 7 (24%) | 0.55 | 2 (7%) | 13 (39%) | 0.004 |
| HE | 9 (32.1%) | 10 (34.5%) | 0.81 | 6 (23.1%) | 17 (51.5%) | 0.02 |
| AKI | 5 (17%) | 7 (24%) | 0.51 | 4 (15.4%) | 9 (27%) | 0.35 |
| VH | 3 (10.7%) | 3 (10.3%) | 1 | 4 (15.4%) | 1 (3%) | 0.15 |
| Mortality | 8 (28.6%) | 6 (20.74%) | 0.49 | 7 (28.6%) | 12 (26.9%) | 0.27 |
SBP, Spontaneous bacterial peritonitis; HE, Hepatic encephalopathy; AKI, Acute Kidney Injury; VH, Variceal hemorrhage.
Development of Non-SBP Incident Complications
The development of incident hepatic encephalopathy in patients on primary prophylaxis was similar between both groups (P = 0.800). However, in the secondary prophylaxis group, 51.5% of the patients on norfloxacin developed HE compared to 23.1% on rifaximin (P = 0.02). Development of incident AKI was similar between the groups irrespective of primary (P = 0.5) or secondary prophylaxis (P = 0.35). Similarly, there were no significant differences in the number of patients developing incident variceal hemorrhage in either group for primary and secondary prophylaxis, respectively (P = 1.0, P = 0.15). The results of the incident non-SBP complications stratified as per prophylaxis status have been shown in Table 2.
Survival Analysis
All the patients were followed till 180 days or death, whichever was earlier. The proportion of patients on primary prophylaxis that died in either group were similar (P = 0.490). Similarly, the proportion of patients on secondary prophylaxis that died in either group was also similar (P = 0.200) (Table 2). In patients on primary prophylaxis with norfloxacin, six patients died, of which two were in-hospital mortality (both due to sepsis), whereas out of the eight patients who died in the rifaximin primary prophylaxis, three had in-hospital mortality (all three due to sepsis). Among those on secondary prophylaxis, out of the 12 patients who died on norfloxacin, 4 had in-hospital mortality (one with acute kidney injury and three due to sepsis) out of the seven who died on rifaximin, 4 had in-hospital mortality (one due to refractory variceal hemorrhage, one due to HE and subsequent pneumonia, and two due to sepsis).
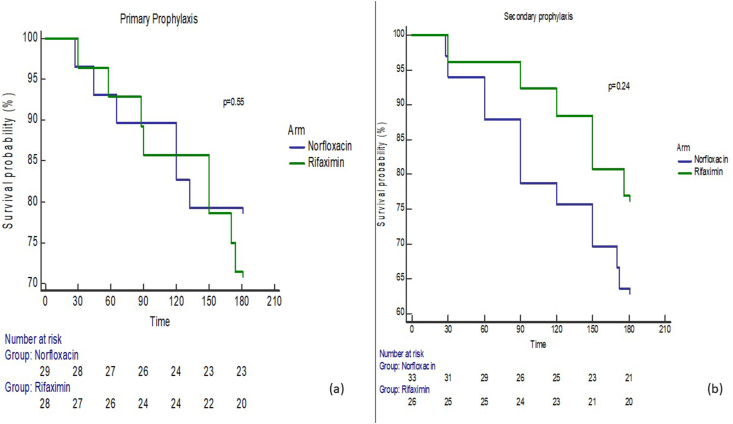
In patients on primary prophylaxis, the mean overall survival was 161.0 ± 8.03 days in the norfloxacin arm and 161.7 ± 7.7 days in the rifaximin arm. Using the log-rank (Mantel–Cox) test, there were no significant differences in survival between both arms (P = 0.55). In patients on secondary prophylaxis, the mean overall survival was 151.0 ± 8.4 days in the norfloxacin arm and 166.7 ± 6.8 days in the rifaximin arm. Using the log-rank (Mantel–Cox) test, there were no significant differences in survival between both arms (P = 0.24). The Kaplan–Meir survival analysis curves for overall survival at 180 days are shown in Figures 2a and 2b.
Figure 2.
(a) Kaplan–Meier curve showing survival in the patients on primary prophylaxis with rifaximin or norfloxacin (b) Kaplan–Meier curve showing survival in the patients on secondary prophylaxis with rifaximin or norfloxacin.
Predictors of SBP and Mortality
In patients on primary prophylaxis, MELD score [OR, (95% CI) 1.11 (1.01–1.21); P = 0.02) was the sole predictor for SBP recurrence while in those on secondary prophylaxis, receiving rifaximin significantly reduced the odds of developing SBP recurrence [OR (95% CI0.14 (0.02–0.73; P = 0.02)]. However, the allocation into either arm did not significantly influence six-month mortality. The analysis for predictors for incident SBP and mortality has been shown in Table 3 and Supplementary Table 1.
Table 3.
Predictors of Spontaneous Bacterial Peritonitis in Patients Receiving SBP Prophylaxis.
| Variables | Primary prophylaxis |
Secondary prophylaxis |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis OR (95% CI) |
P-value | Multivariate analysis OR (95% CI) |
P-value | Univariate analysis OR (95% CI) |
P-value | Multivariate analysis OR (95% CI) |
P-value | |
| Age | 1.01 (0.96–1.07) | 0.5 | 0.97 (0.92–1.01) | 0.2 | ||||
| Serum albumin | 0.72 (0.31–1.71) | 0.7 | 1.12 (0.50–2.41) | 0.7 | ||||
| Serum sodium | 0.97 (0.88–1.07) | 0.6 | 1.06 (0.95–1.1) | 0.2 | ||||
| Arm (Rifaximin vs. norfloxacin) | 0.52 (0.135–2.037) | 0.3 | 0.40 (0.09–1.75) | 0.2 | 0.14 (0.02–0.72) | 0.02 | 0.14 (0.02–0.73) | 0.02 |
| Ascitic fluid protein | 0.65 (0.20–2.08) | 0.4 | 1.32 (0.64–2.72) | 0.4 | ||||
| CTP | 0.7 (0.46–1.33) | 0.4 | 0.97 (0.65–1.46) | 0.8 | ||||
| MELD | 1.1 (1.01–1.19) | 0.02 | 1.11 (1.01–1.21) | 0.02 | 0.97 (0.87–1.085) | 0.6 | 0.97 (0.87–1.085) | 0.6 |
CTP, Child Turcotte Pugh; MELD, Model for end-stage liver disease; OR, Odds ratio.
Discussion
The occurrence of SBP in the natural history of cirrhosis marks an important event and is associated with a reduction in overall survival.13 Furthermore, the occurrence of an index event of SBP leads to a 70% increased risk of SBP recurrence at one year.14 In view of the high incidence of recurrence and associated mortality, prophylactic antibiotic strategies have been advocated with norfloxacin as the recommended first therapy choice.4 Rifaximin is a nonabsorbable antibiotic already recommended as a therapy for the prevention of recurrent HE.15 Given the strategic advantage of the high gut concentration of rifaximin, as well as risks of antibiotic resistance associated with norfloxacin, the use of rifaximin as an alternative to norfloxacin has been suggested especially in preliminary studies both in the setting of primary and secondary prophylaxis.11,16
In patients who received either rifaximin or norfloxacin as primary prophylaxis for the prevention of occurrence of SBP, we found no significant differences. Available literature specifically on the comparison of these two drugs in the setting of primary prophylaxis is limited. In a study with 86 patients with hepatitis C cirrhosis, of which 70% were on primary prophylaxis, patients on rifaximin developed fewer episodes of incident SBP (4.7% vs. 14%, P = 0.26) over a follow-up of six months. However, the differences were not statistically significant.12 Another prospective randomized trial failed to show any significant differences in SBP occurrence when rifaximin and norfloxacin were compared as primary prophylaxis (12.5% vs. 22.8%, P = 0.1).16
In contrast to primary prophylaxis, patients in our study who received rifaximin for secondary prophylaxis had significantly lesser episodes of recurrent SBP. Effert et al., in a previous randomized trial of 262 patients allocated to rifaximin or norfloxacin as secondary prophylaxis, showed a significantly lower rate of SBP in the rifaximin arm (3.88 vs. 14.13%). The pathophysiological basis that may confer the benefits to those receiving rifaximin is possibly diverse and includes its site of action and concentration in the gut lumen, broader antimicrobial spectrum, and lesser susceptibility to bacterial resistance.1,16,17 Hence, given the concerns of fluoroquinolone resistance, rifaximin does seem to be an attractive alternative, especially in the setting of secondary prophylaxis of SBP.
The occurrence of incident HE was similar in patients receiving primary prophylaxis for SBP. However, in those on secondary prophylaxis, follow-up events of HE were significantly higher in patients in the norfloxacin arm. The role of rifaximin in the prevention of HE recurrence has been widely studied. In our study, none of the patients had HE at baseline, and consequently, the results may indicate an additional benefit of rifaximin when used as a prophylaxis modality for SBP. Similar observations have been made by Shamseya et al., who also observed a lesser number of HE events in those on rifaximin prophylaxis for SBP, although the results did not reach statistical significance.12We did not observe any differences between follow-up events of VH or AKI. While VH has been reported to be similar in a previous study, literature from a recent meta-analysis does indicate a potential protective role of rifaximin in hepatorenal syndrome, albeit with very low-quality evidence.12,16,18
There were no differences in six-month survival between the arms, either in primary or secondary prophylaxis groups in our study. The available literature on survival is heterogeneous. While one study on primary prophylaxis shows no differences in survival, other studies spanning across both primary and secondary prophylaxis show improved survival with rifaximin.11,12,19 However, a major limitation of the available studies reporting survival is the intrinsic methodological issues of not addressing confounders and nonrandomized designs.12,19
Last, we observed that liver disease severity (MELD score) is the sole predictor of SBP occurrence in patients on primary prophylaxis irrespective of the drug of prophylaxis. Contrastingly, in patients on secondary prophylaxis, allocation to rifaximin significantly reduced the odds of having recurrent SBP. The results may have important implications in decision making in both choosing patients who need to have primary prophylaxis, as well as the choice of agent in secondary prophylaxis.
Limitations
Our study has some limitations, the most important of which being lack of generalizability, as it is a single-center study. Second, our study did not specifically look at non-SBP incident infections, which are also crucial determinants in overall outcomes. Third, we did not have patients who were already on primary prophylaxis with fluoroquinolones, which needs to be addressed in future studies to understand the impact of baseline fluoroquinolone resistance. Additionally, long-term follow-up and potential for emergent rifaximin resistance are areas that need to be studied in future studies. Furthermore, while our trial was designed and powered for SBP recurrence, for other crucial outcomes like mortality the study may be underpowered. Last, our results for incident events of HE were not adjusted for concomitant medication like lactulose, branched-chain amino acid, or l-ornithine l-aspartate.
In primary prophylaxis, rifaximin and norfloxacin are similar in the prevention of SBP occurrence. In secondary prophylaxis, rifaximin significantly reduces the odds of having recurrent SBP, as well as incident events of HE. Six-month survival is, however, similar between both groups in primary as well as secondary prophylaxis.
Statement of human rights
The study has been approved by the institutional ethics committee and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and revised in 2008.
Credit authorship contribution statement
DLP: Conceptualization, data curation, writing original draft; MPK: Project administration, resources, writing-review, and editing; NV: Visualization, writing-review, and editing; AR, Visualization, writing-review, and editing; ST Supervision, writing-review, and editing; AD Supervision, writing-review, and editing; RKD: Conceptualization, methodology, writing-review, and editing;
Conflicts of interest
The authors have none to declare.
Acknowledgement
Nil.
Funding
Nil.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2021.08.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Soni H., Kumar -M.P., Sharma V., et al. Antibiotics for prophylaxis of spontaneous bacterial peritonitis: systematic review & Bayesian network meta-analysis. Hepatol Int. 2020 Apr 7:1–5. doi: 10.1007/s12072-020-10025-1. [DOI] [PubMed] [Google Scholar]
- 2.Yim H.J., Suh S.J., Jung Y.K., et al. Daily norfloxacin vs. weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: a randomized controlled trial. Am J Gastroenterol. 2018 Aug 1;113:1167–1176. doi: 10.1038/s41395-018-0168-7. [DOI] [PubMed] [Google Scholar]
- 3.Wong F., Bernardi M., Balk R., et al. Sepsis in cirrhosis: report on the 7th meeting of the international ascites club. Gut. 2005 May 1;54:718–725. doi: 10.1136/gut.2004.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018 Aug 1;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Goel A., Rahim U., Nguyen L.H., Stave C., Nguyen M.H. Systematic review with meta-analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis. Aliment Pharmacol Ther. 2017 Dec;46:1029–1036. doi: 10.1111/apt.14361. [DOI] [PubMed] [Google Scholar]
- 6.Wang W., Yang J., Liu C., et al. Norfloxacin, ciprofloxacin, trimethoprim–sulfamethoxazole, and rifaximin for the prevention of spontaneous bacterial peritonitis: a network meta-analysis. Eur J Gastroenterol Hepatol. 2019 Aug 1;31:905–910. doi: 10.1097/MEG.0000000000001446. [DOI] [PubMed] [Google Scholar]
- 7.Koo H.L., DuPont H.L. Rifaximin: a unique gastrointestinalselective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010;26:17–25. doi: 10.1097/MOG.0b013e328333dc8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Runyon B.A., Montano A.A., Akriviadis E.A., Antillon M.R., Irving M.A., McHutchison J.G. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992 Aug 1;117:215–220. doi: 10.7326/0003-4819-117-3-215. [DOI] [PubMed] [Google Scholar]
- 9.European Association For The Study Of The Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010 Sep 1;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Sarin S.K., Choudhury A., Sharma M.K., et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019 Jul 1;13:353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elfert A., Abo Ali L., Soliman S., Ibrahim S., Abd-Elsalam S. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2016 Dec 1;28:1450–1454. doi: 10.1097/MEG.0000000000000724. [DOI] [PubMed] [Google Scholar]
- 12.Shamseya M.M., Madkour M.A. Rifaximin: a reasonable alternative for norfloxacin in the prevention of spontaneous bacterial peritonitis in patients with HCV-related liver cirrhosis. Alexandria J Med. 2016 Aug 16;52:219–226. [Google Scholar]
- 13.Singal A.K., Salameh H., Kamath P.S. Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: a nationwide study of hospitalised patients in the United States. Aliment Pharmacol Ther. 2014 Jul;40:105–112. doi: 10.1111/apt.12797. [DOI] [PubMed] [Google Scholar]
- 14.Titó L., Rimola A., Ginès P., Llach J., Arroyo V., Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology. 1988 Jan;8:27–31. doi: 10.1002/hep.1840080107. [DOI] [PubMed] [Google Scholar]
- 15.Kimer N., Krag A., Møller S., Bendtsen F., Gluud L.L. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014 Jul;40:123–132. doi: 10.1111/apt.12803. [DOI] [PubMed] [Google Scholar]
- 16.Assem M., Elsabaawy M., Abdelrashed M., et al. Efficacy and safety of alternating norfloxacin and rifaximin as primary prophylaxis for spontaneous bacterial peritonitis in cirrhotic ascites: a prospective randomized open-label comparative multicenter study. Hepatol Int. 2016 Mar 1;10:377–385. doi: 10.1007/s12072-015-9688-z. [DOI] [PubMed] [Google Scholar]
- 17.DuPont H.L., Jiang Z.D. Influence of rifaximin treatment on the susceptibility of intestinal Gram-negative flora and enterococci. Clin Microbiol Infect. 2004 Nov 1;10:1009–1011. doi: 10.1111/j.1469-0691.2004.00997.x. [DOI] [PubMed] [Google Scholar]
- 18.Kamal F., Khan M.A., Khan Z., et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017 Oct 1;29:1109–1117. doi: 10.1097/MEG.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 19.Hanouneh M.A., Hanouneh I.A., Hashash J.G., et al. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol. 2012 Sep 1;46:709–715. doi: 10.1097/MCG.0b013e3182506dbb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.