Abstract
Background & aims
There is no “gold standard” tool for the assessment of frailty in cirrhosis. This study compares Liver Frailty Index (LFI), Short Physical Performance Battery (SPPB), Fried Frailty Criteria (FFC), and Clinical Frailty Scale (CFS) for frailty assessment and ascertains its impact on predicting mortality and hospitalizations in a cohort of outpatients with cirrhosis.
Methods
116 patients were enrolled in this prospective observational cohort study. Frailty assessment was done using LFI, SPPB, FFC, and CFS. All patients were followed up for 6 months. The primary outcome was the first of either all-cause unplanned hospitalization or all-cause mortality occurring within 6 months of the study period.
Results
100 (86.2%) males and 16 (13.8%) females with a mean age of 50.2 (48.4–51.9, 95% CI) years were included. The most common cause of cirrhosis was alcoholic liver disease (47.4%) followed by hepatitis C (12.9%) and Nonalcoholic steatohepatitis (NASH) (10.3%). There was no significant difference in prevalence of frailty based on LFI (43.1%), FFC (36.2%), CFS (44%), and SPPB (47.4%) (P > 0.05). Frail patients had worse outcomes compared to the Not frail group. At 6 months, the mortality rate in Frail patients was 42% versus 1.5% for the Not frail; hospitalization in Frail patients occurred in 92% versus 6% in the Not frail. On multivariable analysis, independent predictors of mortality were Frailty [OR 14 (1.4–54.2)], alcohol-related cirrhosis [OR 4.2 (1.1–16.3)], Child-Turcotte-Pugh (CTP) [OR 2.1 (1.4–2.9)] and Chronic liver disease questionnaire (CLDQ) [OR 0.1 (0.1–0.4)] scores.
Conclusions
LFI, SPPB, FFC, and CFS are comparable in frailty assessment in patients with cirrhosis. Importantly, comparability of the commonly used scores for frailty assessment and prediction of hospitalization and mortality allows flexibility for clinical application.
Keywords: frailty, cirrhosis, mortality, hospitalization
Abbreviations: AKI, Acute Kidney Injury; ANOVA, Analysis Of Variance; AUC, Area Under the Curve; CFS, Clinical Frailty Scale; CI, Confidence Interval; CLDQ, Chronic liver disease questionnaire; CT, Computerized Tomography; CTP, Child-Turcotte-Pugh; FFC, Fried Frailty Criteria; FSS, Fatigue severity scale; HCC, Hepatocellular Carcinoma; HE, Hepatic Encephalopathy; HU, Hounsfield Units; IBM, International Business Machines; LFI, Liver Frailty Index; MELD, Model for End-Stage Liver Disease; MELDNa, Model for End-Stage Liver Disease with Sodium; MMSE, Mini-Mental State Examination; NASH, Nonalcoholic Steatohepatitis; NPV, Negative Predictive Value; PGIMER, Post Graduate Institute of Medical Education and Research; PPV, Positive Predictive Value; ROC, Receiver Operating Characteristic Curve; SBP, Spontaneous Bacterial Peritonitis; SPPB, Short Physical Performance Battery; SPSS, Statistical Package for Social Sciences; UTI, Urinary Tract infection
1.
Frailty is a multidimensional construct with its origin in geriatrics.1 Defined as a “loss of strength, endurance, physical ability, and cognitive function”, frailty increases vulnerability to disease, dependence, and death.2 Most frailty studies in cirrhosis have focused on the evaluation of physical frailty with prevalence across North American series ranging from 17 to 49%.3, 4, 5, 6, 7 Across studies, physical frailty has been identified as a robust, independent predictor of morbidity, hospitalizations, and mortality.3,4,8,9 There is growing data to support the value of assessing cognitive dysfunction in addition to physical frailty in cirrhosis, but to date, both of these dimensions of frailty have only been evaluated in a few studies.10,11
In 2019 the American Society of Transplantation Liver and Intestinal Community of Practice described the tools available for the evaluation of physical frailty in patients with cirrhosis.12 These tools included the Short Physical Performance Battery (SPPB),8 Fried Frailty Criteria (FFC),4 Clinical Frailty Scale (CFS),4 Liver Frailty Index (LFI),13 6-minute walk test (6MWT),14 Activities of Daily Living (ADL),3 cardiopulmonary exercise testing (CPET),14 gait speed,9 grip strength,12 Instrumental Activities of Daily Living (IADL)12 and Karnofsky Performance Status (KPS).15 Varying in their test characteristics, subjectivity, predictive validity for outcomes, reliability, responsiveness to change over time, time to administer, and whether specialized equipment or highly trained personnel would be required for testing,12 each tool has advantages and disadvantages depending upon the setting. For example, the CFS is easy and quick to perform but is subjective. The FFC is lengthy and has some subjective components but is a reliable predictor of outcomes. The LFI is objective but requires specialized equipment. The SPPB is objective without the need for equipment, but like the LFI includes three tests, and therefore, requires more time to be performed than a single measure.12 As evidence for these tools in cirrhosis evolves, knowledge gaps are becoming apparent. First, limited studies have reported a head-to-head comparison of various tools used for physical frailty assessment. Second, few studies have evaluated the presence of cognitive dysfunction in these patients and its independent contribution to outcomes (7,10,16). And last, there have been no studies from the Indian subcontinent in this space. This is relevant as muscle mass and function are known to vary considerably by ethnicity,17, 18, 19, 20 limiting the generalizability of North American studies to the Indian population.
Accordingly, in an outpatient cohort of patients from the Indian subcontinent, this study was designed to address these knowledge gaps.
2. Patients and methods
This was a prospective observational cohort study carried out at a tertiary care hospital. The Ethics Committee of the institute approved the study protocol (NK/4739/DM/515). Each subject provided written informed consent before inclusion in the study. The guidelines laid down by the Helsinki declarations (modified 1989) were adhered to in all patients in the study.
2.1. Patient Selection
Patients were consecutively enrolled from the outpatient Liver Clinic of the Department of Hepatology, between July 2018 and June 2019.
2.2. Inclusion Criteria
All patients with cirrhosis aged ≥18 years who attended the outpatient liver clinic and were willing to participate in the study were eligible for inclusion. The diagnosis of cirrhosis was based on clinical, biochemical, and ultrasonography, and/or liver histological data.
2.3. Exclusion Criteria
Those excluded were patients with hepatocellular carcinoma (HCC) or other active malignancy, Acute-on-Chronic Liver Failure (ACLF), overt HE at the time of testing, end-stage renal disease, pregnancy, history of recent (<6 weeks) use of drugs affecting psychometric performances that could influence performance in frailty testing, prior enrolment in another conflicting study, and those who could not provide informed written consent.
3. Assessments
3.1. Frailty Assessments
All enrolled patients were evaluated at the beginning of the study. Frailty assessment was carried out using Fried Frailty Criteria (FFC),21 Clinical Frailty Scale (CFS),22 Short Physical Performance Battery (SPPB),23 and Liver frailty index (LFI)13 by a single research scholar who administered the four frailty scores during the study.
3.1.1. Fried Frailty Criteria (FFC)
By using the FFC,21 diagnosis of frailty was established when ≥ 3 of the following 5 criteria were satisfied(i) weight loss of ≥4.5 kg or > 5% of the total weight of the body in the past year; (ii) low handgrip strength for that particular gender (iii) presence of exhaustion; (iv) slowness of the gait speed; and (v) low activity level. Handgrip strength was measured in the dominant hand using a handheld hydraulic dynamometer (JAMAR®).24 Three consecutive values were recorded, and the highest value in kg was taken as the final value of handgrip strength. The western cut-off values of 27 kg and 16 kg were taken for males and females respectively to detect low handgrip strength.25 Exhaustion was assessed using two questions from the CES-D scale.26 The gait speed was calculated by telling the patient to walk as fast as possible for a distance of 4 m, and the time taken was recorded using a stopwatch. The leisure time activity level was assessed using the physical activity scale for the elderly (PASE); the western cut-off of 90 was used to detect low activity levels.27 All the patients were assessed for frailty using FFC at the start of the study. The patients were diagnosed to have frailty when the FFC score was ≥3. It took around 15 min for the calculation of FFC in each patient.
3.1.2. Clinical Frailty Scale (CFS)
The CFS22 has nine categories starting from “very fit/robust health”, “well”, “well, with the treated comorbid disease”, “apparently vulnerable”, “mildly frail”, and “moderately frail” to “severely frail and functionally completely dependent on others”, and from “very severely frail”,’ and lastly, to “terminally ill”. Patients with a score of >4 were considered frail.
3.1.3. Short Physical Performance Battery (SPPB)
Short Physical Performance Battery (SPPB)23 includes tests for (i) balance, (ii) gait speed, and (iii) 5 chair stands. Balance testing has three parts. First, the patient was asked to stand with feet together side by side for 10 s. If the patient could successfully perform it, the patient got one point and proceeded to the next step; if the patient was unable to do it, then 0 points were given. In the second step, the patient was asked to stand with the heel of one foot against the side of the big toe of the other for 10 s. If completed successfully, the patient got one point and proceeded to the next step. In the third step, the patient was told to align his feet heel to toe and asked to stand for 10 s. If the patient stood for 10 s, 2 points were awarded; if the duration of tandem standing was between 3 and 9.99 s, one point was scored, and if less than 3 s, then 0 points were given.
For gait speed testing, the patient was asked to walk at normal speed for a distance of 4 m two times, and the time taken was noted. The lower value of time taken while walking was taken as final. If the patient took <4.82 s, then 4 points were given; if between 4.82 and 6.2 s, then 3 points were awarded; for 6.21–8.70 s, 2 points were scored, and for value >8.7 s, 1 point was scored; 0 point was awarded in case the patient was unable to complete a 4-m walk.
For the chair stand test, the patients folded their arms across their chest and stood up once from the chair. If they were unable to stand up, the test was stopped and 0 points were awarded. Otherwise, the patients were told to stand up as fast as possible from the chair five times without using their arms. If the time taken was ≤11.19 s, then 4 points were awarded. For the time between 11.2 and 13.69 s, 3 points were scored. For time taken between 13.7 and 16.69 s, 2 points were awarded. If the time taken was >16.7 s, 1 point was given, and if the time taken is >60 s, the patient got 0 points. The SPPB was done in all the patients at baseline, and the score was calculated. It took around 15–20 min for conducting SPPB in each patient. If the SPPB score was <10, then the patients were diagnosed with frailty.
3.1.4. Liver Frailty Index (LFI)
The LFI includes handgrip strength, chair stand test, and balance testing.13 A patient is said to be frail if LFI >4.5, prefrail if 3.2–4.5, and robust if LFI<3.2. The LFI was calculated in all patients at the start of the study using an online calculator available at http://liverfrailtyindex.ucsf.edu(28).
4. Other measurements
4.1. Sociodemographic Assessment
Sociodemographic data were collected for all patients and included age, gender, education, marital status, socioeconomic status, body mass index (BMI), presence of comorbidities, smoking history, alcohol consumption, etiology, and severity of the liver disease as assessed by CTP and MELD Na scores. The dry weight was used to calculate BMI. The dry weight was calculated by subtracting a correction for the grade of ascites and/or edema from the weight that was depicted on the weighing machine.29
4.2. Cognition and Health-related Quality of Life (HRQOL) Assessment
For the assessment of cognition, the mini-mental score examination (MMSE)30 was used with a cut-off of <24 to reflect cognitive dysfunction.31 For the assessment of HRQOL, the chronic liver disease questionnaire (CLDQ),32 and fatigue severity scale (FSS)33 questionnaires were used.
5. Outcome Measures
All patients were followed up for 6 months from the day of enrolment in the study or until death. The primary outcome of the study was the first of either all-cause unplanned hospitalization or all-cause mortality occurring within 6 months of the study period. Hospital admission was defined as any admission to an acute care hospital that was not planned to carry out an elective treatment.
6. Sample Size Estimation and Statistical Analysis
The prevalence of frailty was 21% in liver cirrhosis patients in the study published by Lai et al in the year 2018.34 Assuming a level of confidence as 95%, with a margin of error of 8% and power of study kept at 80%, a total of 100 patients were planned for enrolment, but to further increase the power of the study, a total of 116 patients were enrolled.
Statistical analysis was carried out using IBM® SPSS® Statistics version 23 for Mac. Data were expressed as mean with 95% confidence interval (CI) and proportions with 95% CI where appropriate. Statistical analysis for the categorical data was performed using the Chi-square test or Fisher’s exact test, and continuous data were checked for normalcy. For normally distributed data, ANOVA was used, and for skewed data, the Kruskal–Wallis test followed by the Mann–Whitney test was used. All the patients were divided into two groups- Frail and Not frail based on an LFI cut-off of >4.5. The clinical characteristics, hematological and biochemical parameters, and frailty-related parameters were compared between the Frail and Not frail group, and P-values were calculated. The odds ratios for different variables were investigated by univariate regression analysis as a possible predictor of death and/or hospitalization. The prognostic significance of the variables was analyzed using a Cox regression model. All the variables attaining a P-value of <0.05 in this univariate analysis were introduced as covariates in a multivariate analysis (stepwise Cox’s multiple regression procedure) to identify variables that were independent predictors of mortality. Survival curves were constructed to study the effect of frailty on survival using Kaplan–Meier analysis. The difference between the two survival curves was analyzed using the log-rank test. A probability level of P < 0.05 was set for statistical significance. ROC curves were drawn for CFS, LFI, FFC, and SPPB as predictors of hospitalization and/or mortality. The correlation between two variables was studied using Spearman’s correlation coefficient.
7. Results
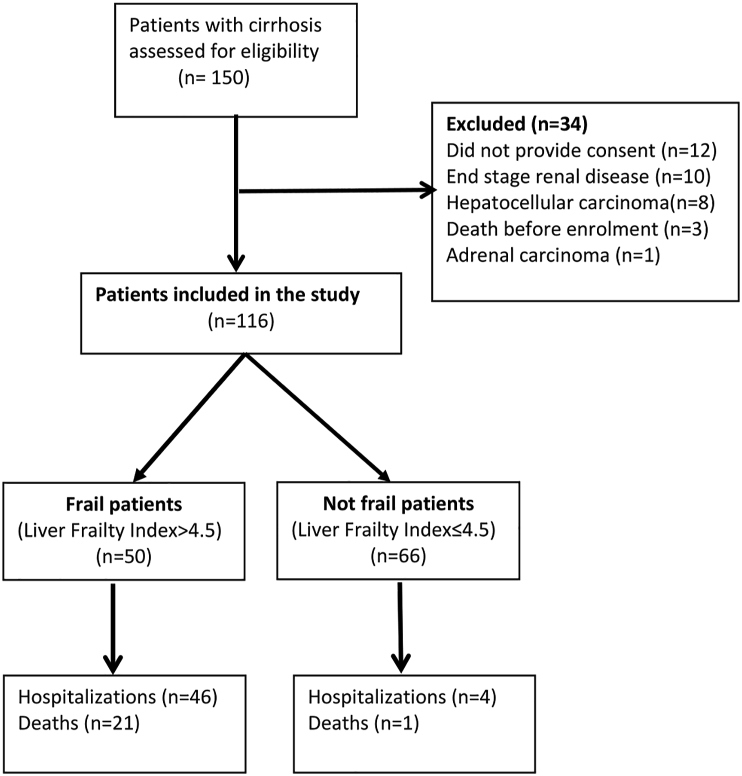
One hundred and fifty outpatients with cirrhosis were screened, out of which 34 patients were excluded. A total of 116 patients were included in the study, as shown in Figure 1. The mean age was 50.2 years (48.4–51.9, 95% CI). 16 (13.8%) of 116 patients were females. Alcohol (47.4%) was the most common etiology of cirrhosis, followed by Hepatitis C (12.9%) and NASH (10.3%); 32 patients with alcoholic cirrhosis were abstaining for more than 6 months; 13 patients with Hepatitis C received directly acting antivirals (DAA) therapy, and all of them achieved sustained virological response (SVR) at 12 weeks. The mean CTP (9.5 vs 7.1, P = 0.01) and MELD Na scores (19.4 vs 12.9, P = 0.01)) were significantly higher in the Frail patients as compared to Not frail ones. The baseline characteristics of the study population are described in Table 1.
Figure 1.
Flowchart of the patients included in the study.
Table 1.
Baseline Characteristics of the Patients in the Study Based on Liver Frailty Index (LFI).
| Parameters | Overall (n = 116) | Frail by LFI (n = 50) | Not frail by LFI(n = 66) | P-value |
|---|---|---|---|---|
| Agea(years) | 50.2 (48.4–51.9) | 51.4 (48.5–54.2) | 49.2 (47.1–51.4) | 0.35 |
| Sex | ||||
| Female | 16 (13.8%) | 13 (26%) | 3 (4.5%) | 0.01 |
| Male | 100 (86.2%) | 37 (74%) | 63 (95.5%) | |
| Obesityb | 34 (29.3%) | 5 (10%) | 29 (43.9%) | 0.01 |
| Diabetes Mellitus | 25 (21.6%) | 13 (26%) | 12 (18.2%) | 0.31 |
| Hypertension | 15 (12.9%) | 8 (16%) | 7 (10.6%) | 0.39 |
| Etiology of Cirrhosis | ||||
| Alcohol | 55 (47.4%) | 25 (50%) | 30 (45.5%) | 0.63 |
| Hepatitis C | 15 (12.9%) | 7 (14%) | 8 (12.1%) | 0.76 |
| Hepatitis B | 7 (6%) | 2 (4%) | 5 (7.6%) | 0.69 |
| NASH | 12 (10.3%) | 5 (10%) | 7 (10.6%) | 0.92 |
| Alcohol plus NASH | 8 (6.9%) | 2 (4%) | 6 (9.1%) | 0.46 |
| Alcohol plus Hepatitis C | 4 (3.4%) | 3 (6%) | 1 (1.5%) | 0.31 |
| Alcohol plus Hepatitis B | 3 (2.6%) | 2 (4%) | 1 (1.5%) | 0.58 |
| Autoimmune Hepatitis | 3 (2.6%) | 1 (2%) | 2 (3%) | 1.000 |
| Cryptogenic | 8 (6.9%) | 3 (6%) | 5 (7.6%) | 1.000 |
| Celiac disease | 1 (0.9%) | 0 | 1 (1.5%) | 1.000 |
| CTP Scorea | 8.2 (7.8–8.5) | 9.5 (8.9–10.0) | 7.1 (6.7–7.6) | 0.01 |
| CTP class | ||||
| A | 30 (25.9%) | 2 (4%) | 28 (42.4%) | |
| B | 52 (44.8%) | 21 (42%) | 31 (47%) | 0.01 |
| C | 34 (29.3%) | 27 (54%) | 7 (10.6%) | |
| MELD Naa | 16 (14.9–17) | 19.4 (17.9–20.9) | 12.9 (11.7–14.1) | 0.01 |
| Ascites | 86 (74.1%) | 46 (92%) | 40 (60.6%) | 0.01 |
| Hepatic Encephalopathy | 40 (34.5%) | 26 (52%) | 14 (21.2%) | 0.01 |
| Variceal bleed | 35 (30.2%) | 19 (38%) | 16 (24.2%) | 0.11 |
| MMSE | 25.2 (24.8–25.6) | 23.6 (23.2–23.9) | 26.6 (26.3–26.9) | 0.01 |
| CLDQ | 4.3 (3.9–4.6) | 2.6 (2.4–2.8) | 5.8 (5.5–6.3) | 0.01 |
| FSS | 4.5 (4.1–4.9) | 6.4 (6.2–6.7) | 2.8 (2.4–3.2) | 0.01 |
Abbreviations: NASH, Nonalcoholic steatohepatitis; CTP, Child-Turcotte-Pugh; MELD Na, Model of end-stage liver disease-Sodium; MMSE, Mini-mental state examination; CLDQ, Chronic liver disease questionnaire; FSS, Fatigue severity scale.
Values expressed as Mean (95% Confidence Interval, CI).
Obesity -BMI≥25 kg/m2.50
7.1. Prevalence of Frailty
There was no significant difference in the prevalence of frailty based on LFI, FFC, CFS, and SPPB (P > 0.05). 50 (43.1%) patients were frail using the Liver Frailty Index (LFI), 42 (36.2%) by Fried Frailty Criteria (FFC), 51 (44%) by the Clinical Frailty Scale (CFS), and 55 (47.4%) by the Short Physical Performance Battery (SPPB). Table 2 provides the frailty results for each of the frailty tools.
Table 2.
Frailty Assessment Results for Each of the Frailty Tools.
| Parameters | Frail by LFI(n = 50) Mean (95% CI) |
Not frail by LFI (n = 66) Mean (95% CI) |
P-value |
|---|---|---|---|
| Handgrip (kg) | |||
| Females | 11.7 (10.2–13.2) | 17.3 (6.9–27.7) | 0.02 |
| Males | 15.4 (14.4–16.6) | 30.8 (29.2–32.3) | 0.01 |
| Time to walk 4-m (sec) | 8.8 (8.2–9.4) | 5.2 (4.9–5.4) | 0.01 |
| Time for 5 chair stands (sec) | 20.8 (18.4–23.3) | 10.4 (10.0–10.7) | 0.01 |
| Gait speed (m/sec) | 0.45 (0.41–0.53) | 0.82 (0.7–0.89) | 0.01 |
| Balance score | 1.8 (1.6–2) | 3.9 (3.8–4.0) | 0.01 |
| CFS | 6.2 (5.9–6.5) | 2.3 (2.0–2.5) | 0.01 |
| FFC | 3.7 (3.4–4.0) | 0.7 (0.4–0.9) | 0.01 |
| LFI | 5.2 (5.0–5.3) | 3.7 (3.6–3.8) | 0.01 |
| SPPB | 4.8 (0.4–9.2) | 11.2 (8.9–13.2) | 0.01 |
Abbreviations: CI, Confidence interval; CFS, Clinical frailty scale; FFC, Fried frailty criteria; LFI, Liver frailty index; SPPB, Short physical performance battery.
7.2. Impact of Various Factors on the Presence of Frailty
Among the several variables analyzed on univariate analysis, female sex, BMI, CTP score, and MELD Na were found to be predictive of frailty. On subsequent multivariable analysis, the CTP score was the only independent predictor of frailty. Table 3 shows the various predictive factors of frailty in the study population.
Table 3.
Odds Ratios for the Different Variables Investigated by Univariate and Multivariable Analysis as Possible Predictive Factors of Frailty.
| Parameters | Univariate OR Mean (95% CI) |
P-value | Multivariate OR Mean (95% CI) |
P-value |
|---|---|---|---|---|
| Etiology of Liver disease | ||||
| Alcohol | 1.2 (0.6–2.5) | 0.63 | ||
| Hepatitis C | 1.2 (0.4–3.5) | 0.77 | ||
| NASH | 0.9 (0.3–3.1) | 0.92 | ||
| Alcohol + Hepatitis C | 4.1 (0.4–41.1) | 0.22 | ||
| Age | 1.1 (0.9–1.1) | 0.22 | ||
| Female sex | 7.3 (1.9–27.6) | 0.01 | 5 (0.2–130) | 0.33 |
| BMI | 0.7 (0.6–0.8) | 0.01 | 1.1 (0.7–1.6) | 0.62 |
| Diabetes mellitus | 1.6 (0.6–3.8) | 0.31 | ||
| Hypertension | 1.6 (0.5–4.7) | 0.39 | ||
| CTP | 1.9 (1.5–2.5) | 0.01 | 1.8 (1.2–2.7) | 0.01 |
| MELD Na | 1.2 (1.1–1.3) | 0.01 | ||
Abbreviations: CI-Confidence interval; NASH-Nonalcoholic steatohepatitis; CTP, Child-Turcotte-Pugh; MELD Na, Model of end-stage liver disease-sodium; INR, International normalization ratio.
7.3. Impact of Frailty on the Combined Outcome Measure of Hospitalization and/or Mortality
Frail patients had worse outcomes compared to the Not-frail group (at 6 months, the mortality rate was 42% versus 1.5% for the not-frail; hospitalization occurred in 92% versus 6% in the not-frail) (Table 4).
Table 4.
Outcome Measures in Frail and Not Frail Patients in the Study.
| Parameters | Frail by LFI (n = 50) |
Not frail by LFI (n = 66) |
Total (n = 116) |
P-value |
|---|---|---|---|---|
| No. of patients hospitalized within 6 months | 46 (92%) | 4 (6%) | 50 | 0.01 |
| No. of deaths within 6 months | 21 (42%) | 1 (1.5%) | 22 | 0.01 |
Abbreviations: LFI, Liver frailty index.
7.4. Impact of Frailty on Hospitalization
During the follow-up period of six months, a total of 50 patients were hospitalized, and the cumulative number of hospitalizations was 81 times (28 patients once, 13 patients twice, and 9 patients thrice). Most of the hospitalizations occurred in the Frail group 46 (92%), and 4 (8%) patients were hospitalized in the Not frail group (P = 0.001). The common causes of hospitalization were sepsis in 32 (39%) patients followed by HE in 25 (31%) patients, AKI in 12 (15%), variceal bleed in 6 (7%) patients, and refractory ascites in 6 (7%) patients (Supplementary Table 1).
7.5. Predictors of Hospitalization
On univariate analysis, BMI, SBP, CTP, MELD Na, Frailty (LFI), MMSE, CLDQ, and FSS were significantly associated with hospitalization. When analyzed by multivariable analysis, CTP (OR 1.9 (1.1–3.2, 95% CI), Frailty (LFI) (OR 5.6 (1.4–79.2, 95% CI), and CLDQ (OR 0.2 (0.04–0.6, 95% CI) emerged as independent predictors of hospitalization (Table 5).
Table 5.
Odds Ratios for the Different Variables Investigated by Univariate and Multivariable Analysis as Possible Predictive Factors of Hospitalization.
| Parameters | Univariate OR Mean (95% CI) |
P-value | Multivariate OR Mean (95% CI) |
P-value |
|---|---|---|---|---|
| BMI | 0.8 (0.7–0.8) | 0.01 | 0.9 (0.8–1.0) | 0.12 |
| Etiology of Liver disease | ||||
| • Alcohol | 1.2 (0.6–2.5) | 0.63 | ||
| • Hepatitis C | 0.86 (0.3–2.6) | 0.79 | ||
| • Hepatitis B | 0.5 (0.1–2.7) | 0.35 | ||
| • NASH | 0.9 (0.3–3.1) | 1 | ||
| • Alcohol plus NASH | 0.42 (0.1–2.1) | 0.46 | ||
| • Alcohol plus Hepatitis C | 4.1 (0.4–41.1) | 0.31 | ||
| • Alcohol plus Hepatitis B | 2.7 (0.2–30.7) | 0.58 | ||
| Spontaneous bacterial peritonitis | 40.8 (11.1–149.2) | 0.01 | 7.6 (0.5–114) | 0.14 |
| CTP | 2.4 (1.8–3.3) | 0.01 | 1.9 (1.1–3.2) | 0.02 |
| MELD Na | 1.3 (1.2–1.4) | 0.01 | ||
| Frailty (LFI) | 17.8 (4.2–75) | 0.01 | 5.6 (1.4–79.2) | 0.01 |
| MMSE | 0.1 (0.1–0.3) | 0.01 | 0.4 (0.2–1) | 0.06 |
| CLDQ | 0.1 (0.03–02) | 0.01 | 0.2 (0.04–0.6) | 0.01 |
| FSS | 4.6 (2.6–8.3) | 0.01 | 1.1 (0.4–2.9) | 0.83 |
Abbreviations: CI, Confidence interval; NASH, Nonalcoholic steatohepatitis; CTP, Child-Turcotte-Pugh; MELD Na, Model of end-stage liver disease-sodium; INR, International normalization ratio; LFI, Liver frailty index; MMSE, Mini-mental state examination; CLDQ, Chronic liver disease questionnaire; FSS, Fatigue severity scale.
7.6. Concordance Amongst Different Frailty Criteria
The concordance of LFI, FFC, CFS and SPPB with each other was assessed using Pearson correlation coefficient. All the four criteria were found to have significant correlation with each other (LFI with FFC, r = 0.9, P < 0.01, LFI with CFS, r = 0.92, P < 0.01, LFI with SPPB, r = 0.97, P < 0.01, FFC with CFS, r = 0.9, P,0.01, FFC with SPPB, r = 0.91, P < 0.01, CFS with SPPB < r = 0.92, P < 0.01).
7.7. Comparison of LFI, FFC, CFS, and SPPB for Predicting Hospitalization
The ability of LFI, FFC, CFS, and SPPB to predict hospitalization in the study population was evaluated using ROC curves. LFI had the maximum AUC of 0.97 (95% CI-0.94–0.99) with a sensitivity of 92%, a specificity of 93.9%, NPV-93.9%, PPV-92%, and diagnostic accuracy of 93.1% at a cut off of 4.5 for predicting hospitalization. There was no significant difference between the AUC of the LFI and the AUC of the other three frailty scores (P > 0.05) (Supplementary Table 2, Supplementary Figures 1 and 2). Using Youden’s index, the optimal LFI cut-off for predicting hospitalization was 4.3 (Youden’s index of 0.84).
7.8. Impact of Frailty on Survival
There was a significant difference in the survival between the Frail and Not frail group (P = 0.01). The mean overall survival was 165.2 (159.3–171.2, 95% CI) days. The mean survival was 147.3 (135.5–159.1) days in the Frail group and 178.9 (176.6–181.1) days in the Not frail group (Supplementary Figure 3). On multivariable analysis four factors were independent predictors of mortality: alcohol-related cirrhosis (OR 4.2 (1.1–16.3)), CTP score (OR 2.1 (1.4–2.9)), Frailty (OR 14 (1.4–54.2)) and CLDQ (OR 0.1 (0.1–0.4)) (Table 6).
Table 6.
Odds Ratios for the Different Variables Investigated by Univariate and Multivariable Analysis as Possible Predictive Factors of Mortality.
| Parameters | Univariate OR (Mean, 95% CI) |
P-value | Multivariate OR (Mean, 95% CI) |
P-value |
|---|---|---|---|---|
| Etiology of Liver disease | ||||
| Alcohol | 2.9 (1.1–7.8) | 0.03 | 4.2 (1.1–16.3) | 0.04 |
| Hepatitis C | 0.3 (0.03–2.2) | 0.29 | ||
| NASH | 0.36 (0.04–2.9) | 0.46 | ||
| Alcohol plus Hepatitis B | 2.2 (0.2–25.3) | 0.47 | ||
| Spontaneous bacterial peritonitis | 13.4 (4.4–41.0) | 0.01 | 2.7 (0.5–14.6) | 0.26 |
| CTP | 2.3 (1.1–4.8) | 0.03 | 2.1 (1.4–2.9) | 0.01 |
| MELD Na | 1.3 (1.1–1.4) | 0.01 | ||
| Body Mass Index | 0.73 (0.5–1.0) | 0.06 | ||
| Frailty (LFI) | 27.7 (3.8–69.2) | 0.01 | 14 (1.4–54.2) | 0.03 |
| MMSE | 0.3 (0.2–0.5) | 0.01 | 0.6 (0.3–1.2) | 0.19 |
| CLDQ | 0.04 (0.01–0.2) | 0.01 | 0.1 (0.1–0.4) | 0.01 |
| FSS | 4.6 (1.7–12.1) | 0.01 | 0.7 (0.1–4) | 0.7 |
Abbreviations: CI-Confidence interval; NASH-Nonalcoholic steatohepatitis; CTP, Child-Turcotte-Pugh; MELD Na, Model of end-stage liver disease-sodium; AST, Aspartate transaminase; ALT, Alanine transaminase; INR, International normalization ratio; LFI, Liver frailty index; MMSE, Mini-mental state examination; CLDQ, Chronic liver disease questionnaire; FSS, Fatigue severity scale.
7.9. Comparison of LFI, FFC, CFS, and SPPB in Predicting Mortality
Mortality prediction by LFI, FFC, CFS, and SPPB, taking LFI as the standard, were compared using ROC curves. LFI had the maximum AUC of 0.89 (95% CI - 0.84–0.96) with a sensitivity of 95.4%, a specificity of 69.1%, PPV of 42%, NPV 98.4% with a diagnostic accuracy of 74% at a cut-off value of 4.5. There was no significant difference in the AUC of LFI and the other three frailty scores (P > 0.05) (Supplementary Table 3, Supplementary Figures 4 and 5).
8. Discussion
This study highlights the high prevalence of frailty in outpatients with cirrhosis from the Indian subcontinent. Importantly, it supports the comparability of the commonly used scores for frailty assessment, including LFI, SPPB, CFS, and FFC, to predict hospitalization and mortality. Consistent with the North American literature, frailty was a robust predictor of hospitalization and death in Indian patients. Unique from North American studies, our patient group had very high rates of hospitalization (92% at 6 months) and death (42% at 6 months), virtually all of these outcomes occurring in frail patients. Although cognitive dysfunction did not add to the prediction of mortality, CLDQ emerged as an independent predictor of both hospitalization and mortality. These novel learnings are explored in more detail below.
This is the first study from the Indian subcontinent, which has evaluated the prevalence of frailty in outpatients with cirrhosis using four different tools. The prevalence of frailty in our study ranged from 36.2% to 47.4%, higher than the previous reports of (31%–38%) from North American studies (3,4). This higher prevalence may be due to the low socioeconomic status, poor nutrition, and late referral of patients to the hospital in developing countries compared to the West. Although the prevalence of frailty was highest with SPPB (47.4%) followed by CFS (44%), LFI (43.1%), and FFC (36.2%), this difference was not statistically significant.
Health-related quality of life (HRQOL) is an independent predictor of hospitalization and death. In this study, frail patients had lower CDLQ scores and higher FSS scores, indicating poor HRQOL and a higher degree of fatigue in patients with cirrhosis. CLDQ has been used as a predictor of HRQOL in patients with cirrhosis.35 Consistent with published work from Kok et al,36 in the current series, CLDQ was also found to be an independent predictor of all-cause unplanned hospitalization and deaths. Although uncommonly evaluated, this finding highlights the importance of measuring patient-reported outcomes.
Cognitive dysfunction does not add to the prediction of hospitalization or death. MMSE is the most commonly used brief global cognitive screen in India.37 Frail patients had significantly lower MMSE scores. Despite this, a lower MMSE score was not a predictor of patient outcomes in our study population. This is in contrast to the studies from North America, where cognitive dysfunction has been associated with poor patient outcomes (7,10). The reason for this lack of association remains unclear. A larger sample size may be needed to better evaluate the impact of cognitive dysfunction on patient outcomes in this population.
In our study, frailty emerged as an independent predictor of hospitalization and mortality, all outcomes virtually happening in the frail group. These higher rates of hospitalizations and deaths are not surprising given the poor physiological reserve and nutritional status of the frail patients. These findings are also in concordance with previous studies from the West (4, 5, 6,8,9,28). Infection and hepatic encephalopathy were the two most common indications for hospitalization (70% of patients). Both of these complications have been pathophysiologically linked to low muscle mass and frailty, including the potential for reduced ammonia detoxification and reduced immune function (7,16,38, 39, 40).
In our study, alcohol-related cirrhosis, CTP score, and CLDQ scores were independent predictors of mortality apart from frailty. CTP is well-recognized as an independent predictor of mortality in cirrhosis (41, 42, 43). Our study included 47.4% of patients with alcohol-related liver disease. The low social support and a tendency to seek late medical advice may be the reason for poor outcomes in this population. As noted above for the combined outcome of hospitalization or death, the relevance of CLDQ for the prediction of mortality in this population again brings up the relevance of measuring patient-reported outcomes.
LFI, FFC, CFS, and SPPB are similar in their performance in predicting hospitalization and mortality. LFI has been recognized as a preferred tool as it is objective, relatively easy to perform, and has good interobserver reproducibility. However, in a resource-limited setting, alternate tools, such as the CFS, can be used, which takes <1 min and perform similarly in the prediction of hospitalization and mortality.
Cirrhosis is a state of continuous catabolism in which there is dysfunctional protein synthesis, alteration of signaling pathways in the skeletal muscles, and impaired usage of various substrates.44 Recent data suggests that by increasing the protein intake and giving late evening snacks and branch chain amino acid supplements, improvement in muscle mass and function can occur.45 However, there is scarce literature on improvement in frailty by giving adequate nutritional support.
The role of pharmacotherapy in frailty is doubtful as none of the available pharmacological agents have shown a significant improvement in frailty.46 Sinclair et al published the results of the RCT on the effect of testosterone therapy in male patients with cirrhosis.47 They concluded that testosterone therapy causes an increase in muscle mass and reduces body fat, as well as HbA1c levels; however, there is a risk of adverse effects with testosterone, and more studies are needed before recommending its use in clinical practice.45,47,48 Initial studies on myostatin antagonists and IGF-1 have shown promising results; however these drugs are still in the experimental stage (45,47,48).
Moderate to high-intensity exercise, in the form of combined resistance and/or aerobic training, can improve the physical frailty in patients with cirrhosis. However, a small number of heterogeneous studies available lack applicability of these exercise regimens in clinical practice, especially in decompensated cirrhosis.49
There are a few limitations that must be acknowledged for this study. The results can only be generalized to outpatients. The high prevalence of frailty may not necessarily translate to the entire population as the study was done at a tertiary care center and was susceptible to referral bias. The follow-up period of six months was relatively short, but many adverse clinical outcomes happened over that time. The long-term consequences of frailty on outcomes will need to be evaluated in studies with a longer follow-up period. We did not perform dynamic frailty assessments in this study, so the impact of changes in various parameters over time could not be assessed. In the FFC, one of the components is unintentional weight loss. However, as a large number of patients in the study population had ascites and were on diuretics, the cause of weight loss could be multifactorial. The cut-offs used for handgrip strength, gait speed, and chair stands were taken from the studies from the West. Interestingly, our internal optimal cut-off for the LFI in males for the outcome of hospitalization or death was lower than the Western cut-off, suggesting that there may be distinct cut-points for frailty in non-Western patients. Further studies with established normative data from the eastern hemisphere and comparative studies for the available frailty assessment scores can help to establish a universal gold standard for diagnosis.
9. Conclusions
This study highlights the high prevalence and the prognostic relevance of frailty in patients with cirrhosis. Frailty assessment should no longer be limited to the research setting, and incorporation into routine clinical practice is the way forward. Importantly, comparability of the commonly used scores for frailty assessment and prediction of hospitalization and mortality allows flexibility for clinical application.
Patient consent statement
Consent taken
Data sharing policy
Data available on request from authors.
Credit authorship contribution statement
ST-conceptualized study design, interpreted data, drafted manuscript, critical revisions, PT-conceptualized study design, interpreted data, drafted manuscript, critical revisions, SS-acquisition, analysis, and interpretation of data, drafted manuscript, critical revisions, AB- acquisition, and interpretation of data, UG-interpreted the data and critical revisions, AR-acquisition of data and critical revisions, ADe-acquisition of data and critical revisions, NV- acquisition of data and critical revisions, MPK- acquisition of data and critical revisions, AD-acquisition of data and critical revisions, RKD-acquisition of data and critical revisions, VS- acquisition of data, critical revisions and administrative support.
Conflicts of interest
The authors have none to declare
Acknowledgement
None.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2021.07.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pritchard J.M., Kennedy C.C., Karampatos S., et al. Measuring frailty in clinical practice: a comparison of physical frailty assessment methods in a geriatric out-patient clinic. BMC Geriatr. 2017 Nov 13;17 doi: 10.1186/s12877-017-0623-0. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5683585/ [Internet] [cited 2019 Nov 18]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried L.P., Ferrucci L., Darer J., Williamson J.D., Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004 Mar;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 3.Lai J.C., Feng S., Terrault N.A., Lizaola B., Hayssen H., Covinsky K. Frailty predicts wait-list mortality in liver transplant candidates. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2014 Aug;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tandon P., Tangri N., Thomas L., et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol. 2016 Dec;111:1759–1767. doi: 10.1038/ajg.2016.303. [DOI] [PubMed] [Google Scholar]
- 5.Bhanji R.A., Narayanan P., Moynagh M.R., et al. Differing impact of sarcopenia and frailty in nonalcoholic steatohepatitis and alcoholic liver disease. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2019;25:14–24. doi: 10.1002/lt.25346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinclair M., Poltavskiy E., Dodge J.L., Lai J.C. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol. 2017;23:899. doi: 10.3748/wjg.v23.i5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapper E.B., Baki J., Parikh N.D., Lok A.S. Frailty, psychoactive medications, and cognitive dysfunction are associated with poor patient-reported outcomes in cirrhosis. Hepatol Baltim Md. 2019 Apr;69:1676–1685. doi: 10.1002/hep.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essam Behiry M., Mogawer S., Yamany A., et al. Ability of the short physical performance battery frailty index to predict mortality and hospital readmission in patients with liver cirrhosis. Int J Hepatol. 2019 May 2;2019:1–6. doi: 10.1155/2019/8092865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn M.A., Josbeno D.A., Tevar A.D., et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol. 2016 Dec;111:1768–1775. doi: 10.1038/ajg.2016.336. [DOI] [PubMed] [Google Scholar]
- 10.Ney M., Tangri N., Dobbs B., et al. Predicting hepatic encephalopathy-related hospitalizations using a composite assessment of cognitive impairment and frailty in 355 patients with cirrhosis. Am J Gastroenterol. 2018;113:1506–1515. doi: 10.1038/s41395-018-0243-0. [DOI] [PubMed] [Google Scholar]
- 11.Tapper E.B., Konerman M., Murphy S., Sonnenday C.J. Hepatic encephalopathy impacts the predictive value of the Fried Frailty Index. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2018;18:2566–2570. doi: 10.1111/ajt.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai J.C., Sonnenday C.J., Tapper E.B., et al. Frailty in liver transplantation: an expert opinion statement from the American society of transplantation liver and intestinal community of practice. Am J Transplant. 2019, Jul:1896–1906. doi: 10.1111/ajt.15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai J.C., Covinsky K.E., Dodge J.L., et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatol Baltim Md. 2017;66:564–574. doi: 10.1002/hep.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey E.J., Steidley D.E., Aqel B.A., et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transplant. 2010 Dec;16:1373–1378. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 15.Orman E.S., Ghabril M., Chalasani N. Poor performance status is associated with increased mortality in patients with cirrhosis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2016;14:1189–1195. doi: 10.1016/j.cgh.2016.03.036. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapper E.B., Derstine B., Baki J., Su G.L. Bedside measures of frailty and cognitive function correlate with sarcopenia in patients with cirrhosis. Dig Dis Sci. 2019;64:3652–3659. doi: 10.1007/s10620-019-05713-4. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin J., Shasthry V., Kaal C.R., et al. Characterization of body composition and definition of sarcopenia in patients with alcoholic cirrhosis: a computed tomography based study. Liver Int. 2017;37:1668–1674. doi: 10.1111/liv.13509. [DOI] [PubMed] [Google Scholar]
- 18.Du K., Goates S., Arensberg M.B., Pereira S., Gaillard T., Hegazi R. Ethnic variations in the prevalence of sarcopenia and sarcopenic obesity in older adults. Faseb J. 2017 Apr 1;31 lb317–lb317. [Google Scholar]
- 19.Shafiee G., Keshtkar A., Soltani A., Ahadi Z., Larijani B., Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. 2017 May 16;16 doi: 10.1186/s40200-017-0302-x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5434551/ [Internet] [cited 2020 Jun 7]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar V., Benjamin J., Shasthry V., et al. Sarcopenia in cirrhosis: fallout on liver transplantation. J Clin Exp Hepatol. 2020 Sep–Oct:467–476. doi: 10.1016/j.jceh.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar 1;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ Can Med Assoc J. 2005 Aug 30;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik J.M., Simonsick E.M., Ferrucci L., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 24.Frederiksen H., Hjelmborg J., Mortensen J., McGue M., Vaupel J.W., Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol. 2006 Jul;16:554–562. doi: 10.1016/j.annepidem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Cruz-Jentoft A.J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 01;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farmer M.E., Locke B.Z., Mościcki E.K., Dannenberg A.L., Larson D.B., Radloff L.S. Physical activity and depressive symptoms: the NHANES I epidemiologic follow-up study. Am J Epidemiol. 1988 Dec;128:1340–1351. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- 27.Washburn R.A., Smith K.W., Jette A.M., Janney C.A. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993 Feb;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 28.Lai J.C., Covinsky K.E., McCulloch C.E., Feng S. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol. 2018 Feb;113:235–242. doi: 10.1038/ajg.2017.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tandon P., Ney M., Irwin I., et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transplant. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 30.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Tombaugh T.N., McIntyre N.J. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 32.Younossi Z., Guyatt G., Kiwi M., Boparai N., King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999 Aug;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krupp L.B., LaRocca N.G., Muir-Nash J., Steinberg A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989 Oct;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 34.Lai J.C., Segev D.L., McCulloch C.E., Covinsky K.E., Dodge J.L., Feng S. Physical frailty after liver transplantation. Am J Transplant. 2018 Aug;18:1986–1994. doi: 10.1111/ajt.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labenz C., Toenges G., Schattenberg J.M., et al. Health-related quality of life in patients with compensated and decompensated liver cirrhosis. Eur J Intern Med. 2019 Dec;70:54–59. doi: 10.1016/j.ejim.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Kok B., Whitlock R., Ferguson T., et al. Health-related quality of life: a rapid predictor of hospitalization in patients with cirrhosis. Am J Gastroenterol. 2020;115:575–583. doi: 10.14309/ajg.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 37.Porrselvi A.P., Shankar V. Status of cognitive testing of adults in India. Ann Indian Acad Neurol. 2017 Dec;20:334–340. doi: 10.4103/aian.AIAN_107_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanai T., Shiraki M., Watanabe S., et al. Sarcopenia predicts minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol Res Off J Jpn Soc Hepatol. 2017 Dec;47:1359–1367. doi: 10.1111/hepr.12873. [DOI] [PubMed] [Google Scholar]
- 39.Kalaitzakis E., Josefsson A., Castedal M., et al. Hepatic encephalopathy is related to anemia and fat-free mass depletion in liver transplant candidates with cirrhosis. Scand J Gastroenterol. 2013 May;48:577–584. doi: 10.3109/00365521.2013.777468. [DOI] [PubMed] [Google Scholar]
- 40.Merli M., Giusto M., Lucidi C., et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013 Jun;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 41.Acharya G., Kaushik R.M., Gupta R., Kaushik R. Child-turcotte-pugh score, MELD score and MELD-Na score as predictors of short-term mortality among patients with end-stage liver disease in northern India. Inflamm Intest Dis. 2020;5:1–10. doi: 10.1159/000503921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales B.P., Planas R., Bartoli R., et al. Early hospital readmission in decompensated cirrhosis: incidence, impact on mortality, and predictive factors. Dig Liver Dis. 2017 Aug 1;49:903–909. doi: 10.1016/j.dld.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Patel R., Poddar P., Choksi D., et al. Predictors of 1-month and 3-months hospital readmissions in decompensated cirrhosis: a prospective study in a large asian cohort. Ann Hepatol. 2019 Jan 1;18:30–39. doi: 10.5604/01.3001.0012.7859. [DOI] [PubMed] [Google Scholar]
- 44.Lucero C., Verna E.C. The role of sarcopenia and frailty in hepatic encephalopathy management. Clin Liver Dis. 2015 Aug;19:507–528. doi: 10.1016/j.cld.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Duarte-Rojo A., Ruiz-Margáin A., Montaño-Loza A.J., Macías-Rodríguez R.U., Ferrando A., Kim W.R. Exercise and physical activity for patients with end-stage liver disease: improving functional status and sarcopenia while on the transplant waiting list. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2018;24:122–139. doi: 10.1002/lt.24958. [DOI] [PubMed] [Google Scholar]
- 46.Exterkate L., Slegtenhorst B.R., Kelm M., et al. Frailty and transplantation. Transplantation. 2016 Apr;100:727–733. doi: 10.1097/TP.0000000000001003. [DOI] [PubMed] [Google Scholar]
- 47.Sinclair M., Grossmann M., Hoermann R., Angus P.W., Gow P.J. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol. 2016;65:906–913. doi: 10.1016/j.jhep.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Sinclair M., Gow P.J., Grossmann M., Angus P.W. Review article: sarcopenia in cirrhosis - aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016 Apr;43:765–777. doi: 10.1111/apt.13549. [DOI] [PubMed] [Google Scholar]
- 49.Williams F.R., Berzigotti A., Lord J.M., Lai J.C., Armstrong M.J. Review article: impact of exercise on physical frailty in patients with chronic liver disease. Aliment Pharmacol Ther. 2019 Nov;50:988–1000. doi: 10.1111/apt.15491. [DOI] [PubMed] [Google Scholar]
- 50.Misra A. Ethnic-specific criteria for classification of body mass index: a perspective for asian Indians and American diabetes association position statement. Diabetes Technol Therapeut. 2015 Sep 1;17:667–671. doi: 10.1089/dia.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.