Abstract
Background & objectives
There are reports of worsening renal functions with sofosbuvir, but there are no comparative data of different direct-acting antivirals (DAAs) on serum creatinine. In this retrospective cohort analysis, we examined the treatment effect of two commonly used regimens, sofosbuvir/ledipasvir (SOF/LDV) and glecaprevir/pibrentasvir (GLE/PIB), on serum creatinine.
Methods
We included all patients treated with SOF/LDV (n = 825) and GLE/PIB (n = 116) between December 1, 2014, and December 31, 2018. An increase of serum creatinine ≥0.3 mg/dL was considered clinically significant. The change of creatinine values from pretreatment to posttreatment between two treatment groups was tested in unadjusted and adjusted generalized linear model, and risk factors associated with creatinine change were assessed. In addition, GLE/PIB-treated patients were matched 1:2 to SOF/LDV-treated patients using propensity scores, and then serum creatinine changes were compared.
Results
The mean baseline creatinine was higher in the GLE/PIB group vs. SOF/LDV group (1.39 ± 1.86 vs. 0.91 ± 0.24, P = 0.007). When compared to baseline, serum creatinine at posttreatment week 4 was significantly higher in SOF/LDV group (0.97 ± 0.4 vs.0.91 ± 0.24, P < 0.001), but there was no significant change in the GLE/PIB group (1.41 ± 1.73 vs. 1.39 ± 1.86, P = 0.52). Overall, there was no significant change in serum creatinine between posttreatment week 4 and week 24 (P = 0.6). Clinically significant increase in serum creatinine was seen in 6% (46/825) of SOF/LDV and 7% (8/116) of GLE/PIB (P = 0.6). The unadjusted and adjusted models indicated that the changes in creatinine from baseline to posttreatment week 4 and week 24 were not associated with the type of DAA combination.
Conclusion
Treatment of chronic hepatitis C infection with both SOF/LDV and GLE/PIB regimens may result in an increase of creatinine, and 6–7% will have an increase in serum creatinine of ≥0.3 mg/dL. The increase in creatinine, however, is unrelated to the type of DAA combination.
Keywords: direct antiviral agents, DAA, hepatitis C infection: serum creatinine
Abbreviations: AKI, acute kidney injury; Cr, creatinine; DAA, direct acting antivirals; GLE/PIB, glecaprevir/pibrentasvir; GFR, glomerular filtration rate; HAART, highly active antiretroviral therapy; HCV, hepatitis C; IFN, interferon; SOF/LDV, sofosbuvir/ledipasvir; SVR, sustained virological response; TLV/BOC, telaprevir/boceprevir
The prevalence of renal dysfunction is more common in those with hepatitis C infection (HCV) than in the general population.1,2 The prevalence of HCV is 9.5% in renal failure patients and 1.6% in the general population.3 The introduction of direct acting antivirals (DAAs) revolutionized the treatment of HCV with cure rate above 95% with minimal side effects.4 The first-generation protease inhibitors such as telaprevir (TLV) and boceprevir (BOC) had high cure rates when used in combination with interferon and ribavirin, but these combinations were associated with significant side effects including an increased incidence of renal impairment.5,6
Sofosbuvir (SOF) metabolites, the backbone of SOF-based combination treatment, are renally cleared which can be a concern in those patients with renal impairment.7 In one study, SOF-based therapy resulted in acute kidney injury (AKI) in 11% of patients, but AKI was reversible, and the risk was lower than that of AKI seen with TLV/BOC (18%)-based combination.8 There are only limited data regarding the safety of SOF in patients with advanced kidney disease, and studies have reported discordant results. Although some studies had suggested that SOF-based treatments in patients with glomerular filtration rate (GFR) ≤ 30 mL/min, including those on dialysis, are safe, there are also reports suggesting that the incidence of anemia, worsening renal functions, and adverse reactions are more common in patients with renal dysfunction.9, 10, 11, 12 Nevertheless, in November 2019, the Food and Drug Administration (FDA) approved the use of SOF-based regimens in individuals with GFR ≤ 30 mL/min and those on dialysis based on limited data.13 Glecaprevir/pibrentasvir (GLE/PIB) is metabolized and cleared mainly through biliary system, and only negligible amount is eliminated via the renal system.14 There are limited comparative data of the impact of different DAA on serum creatinine (Cr). The aim of this study was to identify and compare the risk of renal impairment with SOF/ledipasvir (LDV) and GLE/PIB treatment.
Patients and methods
We included all HCV-infected patients treated with SOF/LDV and GLE/PIB between December 1, 2014, and December 31, 2018. Patients with no available posttreatment serum Cr were excluded. In addition to serum Cr at baseline, and at various time intervals up to 24 weeks after treatment, we collected demographic information, genotype, comorbidities (diabetes mellitus, hypertension), concomitant medications, and treatment duration and response. Stage of liver fibrosis was identified using results of liver biopsy, FibroSURE, or FibroScan (Echosens™, Paris, France). Sustained virological response (SVR) was defined as undetectable HCV RNA 12 weeks after treatment.
Baseline Cr was determined by the mean of two Cr readings within 6 months before the initiation of the treatment. Chronic kidney disease (CKD) was defined as GFR < 90 mL/min/1.73 m2 calculated at 2 separate occasions, and CKD was graded based on the National Kidney Foundation criteria.15 An increase in serum Cr ≥ 0.3 mg/d at week 4 when compared to baseline was considered clinically significant. The primary outcome was change in Cr at week 4 and 24 after treatment.
Statistical Analysis
Descriptive statistics for characteristics of patients were presented as means and standard deviations for continuous variables and frequencies for categorical variables. The patients’ characteristics difference between two treatment groups was assessed by using Chi-square test for categorical variables and T test or Wilcoxon test as appropriate for continuous variables, a variable with P value ≤ 0.05 indicated a statistically significant difference between two groups.
Paired T test was used to test the differences in serum Cr between pretreatment and posttreatment week 4, pretreatment and posttreatment week 24, and posttreatment week 4 and week 24. To compare serum Cr change in pretreatment and posttreatment between two treatment arms, generalized linear model was performed. An unadjusted model was developed first, followed by an adjusted model. The final model retained variables that significantly (P ≤ 0.05) contributed to differentiation of serum Cr change.
As baseline serum Cr were significantly different between two treatment groups, as a sensitivity analysis, two-stage propensity score matching was implemented. First, the propensity score was calculated based on logistic regression with treatment group as the outcome variable and Cr before treatment, age, race, treatment duration, liver fibrosis stage, genotype, diabetes, diuretics, and CKD as covariates. GLE/PIB-treated patients were then matched 1:2 to SOF/LDV-treated patients using propensity scores. One-way ANOVA was then used to compare serum Cr change between the two groups. All analyses were performed using SAS version 9.4.
Results
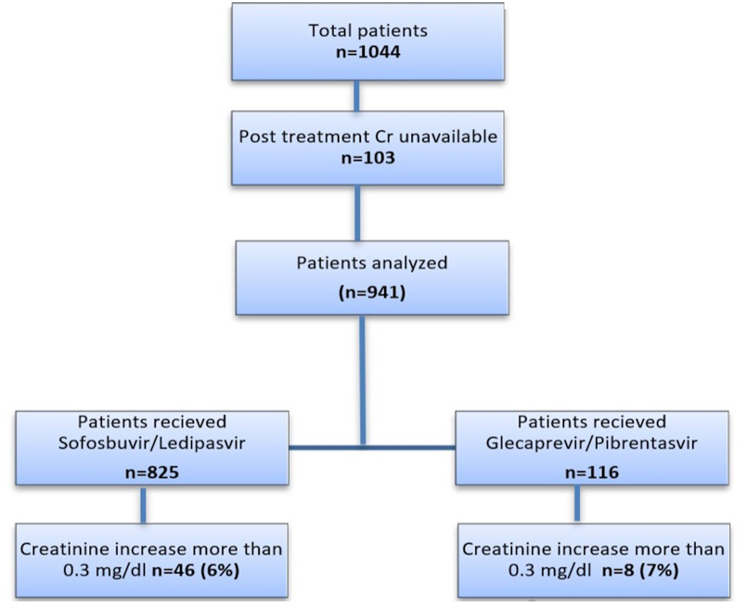
A total of 1044 patients had HCV treatment with DAA during the study period. Of these, 103 patients had no posttreatment Cr available, and they were excluded. Of the remaining 941 patients, 825 were treated with SOF/LDV and 116 with GLE/PIB. Among 941 patients, 673 had 24-week posttreatment Cr available (Figure 1). The demographics differences between the 2 groups and individual effects of clinical variables on Cr changes are summarized in Table 1. There were differences in age, race, genotype, prevalence of diabetes mellitus, CKD, and diuretic use between both groups. Most patients were men (60%) or black (55%). Genotype 1a was the most common genotype in both groups (Table 1). Treatment duration was longer in SOV/LDV, 11.5 ± 3.7 weeks, than that in GLE/PIB, 9.6 ± 2.3 weeks (P < 0.001). Advanced fibrosis (stage 3 or 4) was present in 46% of patients. SVR was achieved in 98% of SOF/LDV and 100% of GLE/PIB.
Figure 1.
Flowchart showing the patient population. Cr, creatinine.
Table 1.
Baseline Characteristics and Comparison of Sofosbuvir/Ledipasvir (SOD/LDV) With Glecaprevir/Pibrentasvir (GLE/PIB) Treatment.
| Variable | All (N = 941) | SOF/LDV (N = 825) | GLE/PIB (N = 116) | Difference Between 2 Groupsa | Individual Effect on Creatinine Before-After Changeb | |
|---|---|---|---|---|---|---|
| Age | 58.1 ± 9.1 | 58.5 ± 8.6 | 55.7 ± 11.7 | 0.02 | 0.15 | |
| Males | 561 (60%) | 491 (60%) | 70 (60%) | 0.87 | 0.21 | |
| Race | Black | 522 (55%) | 467 (57%) | 55 (47%) | <0.001 | 0.74 |
| White | 390 (41%) | 339 (41%) | 51 (44%) | |||
| Genotype | 1A | 732 (78%) | 659 (80%) | 73 (63%) | <0.001 | 0.01 |
| 1B | 185 (20%) | 160 (19%) | 25 (22%) | |||
| 2 | 11 (1%) | 0 (0%) | 11 (9%) | |||
| 3–6 | 11 (1%) | 4 (0.5%) | 7 (6%) | |||
| Duration of treatment (mean ± SD) | 11.3 ± 3.6 | 11.5 ± 3.7 | 9.6 ± 2.3 | <0.001 | 0.43 | |
| Fibrosis stage | F0-1 | 196 (21%) | 179 (22%) | 17 (15%) | 0.46 | 0.30 |
| F2 | 292 (31%) | 254 (31%) | 38 (33%) | |||
| F3 | 119 (13%) | 105 (13%) | 14 (12%) | |||
| F4 | 309 (33%) | 266 (32%) | 43 (37%) | |||
| SVR12 | 689 (98%) | 615 (98%) | 74 (100%) | 0.2 | 0.38 | |
| Diabetes mellitus | 212 (23%) | 196 (24%) | 16 (14%) | 0.02 | 0.78 | |
| Hypertension | 551 (59%) | 490 (59%) | 61 (53%) | 0.16 | 0.19 | |
| Diuretics | 270 (29%) | 253 (31%) | 17 (15%) | <0.001 | 0.01 | |
| ACEi/ARBs | 355 (38%) | 312 (38%) | 43 (37%) | 0.88 | 0.73 | |
| HAART | 5 (1%) | 5 (1%) | 0 (0%) | 0.40 | <0.001 | |
| Metformin | 104 (11%) | 97 (12%) | 7 (6%) | 0.07 | 0.59 | |
| NSAIDs | 134 (14%) | 118 (14%) | 16 (14%) | 0.88 | 0.69 | |
| Other nephrotoxic drugs | 42 (4%) | 40 (5%) | 2 (2%) | 0.13 | 0.21 | |
| CKD stage | 2 | 99 (11%) | 92 (11%) | 7 (6%) | <0.001 | <0.001 |
| 3 | 47 (5%) | 40 (5%) | 7 (6%) | |||
| 4 | 4 (0.004%) | 1 (0.001%) | 3 (3%) | |||
| 5 | 5 (1%) | 0 (0%) | 5 (4%) | |||
| Creatinine pre-treatment (mean ± SD) | 0.97 ± 0.71 | 0.91 ± 0.24 | 1.39 ± 1.86 | 0.01 | ||
| Creatinine post-treatment (mean ± SD) | 1.03 ± 0.73 | 0.97 ± 0.40 | 1.41 ± 1.73 | 0.01 | ||
| Creatinine at month 6 (mean ± SD) | 1.01 ± 0.58 | 0.97 ± 0.30 | 1.42 ± 1.76 | 0.07 | ||
| Creatinine increased ≥0.1 after treatment | 288 (31%) | 258 (31%) | 30 (26%) | 0.24 | ||
| Creatinine increased ≥0.2 after treatment | 112 (12%) | 97 (12%) | 15 (13%) | 0.72 | ||
| Creatinine increased ≥0.3 after treatment | 54 (6%) | 46 (6%) | 8 (7%) | 0.57 | ||
Abbreviations:SD, standard deviation; SVR12, sustained virologic response at 12 weeks after treatment; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HAART, highly active antiretroviral therapy; NSAIDs, nonsteroidal anti-inflammatory drugs; CKD, chronic kidney disease; Cr, creatinine.
Chi square test for categorical variables, T test or Wilcoxon test as appropriate for continuous variables.
Wald Statistics for type 3 analysis.
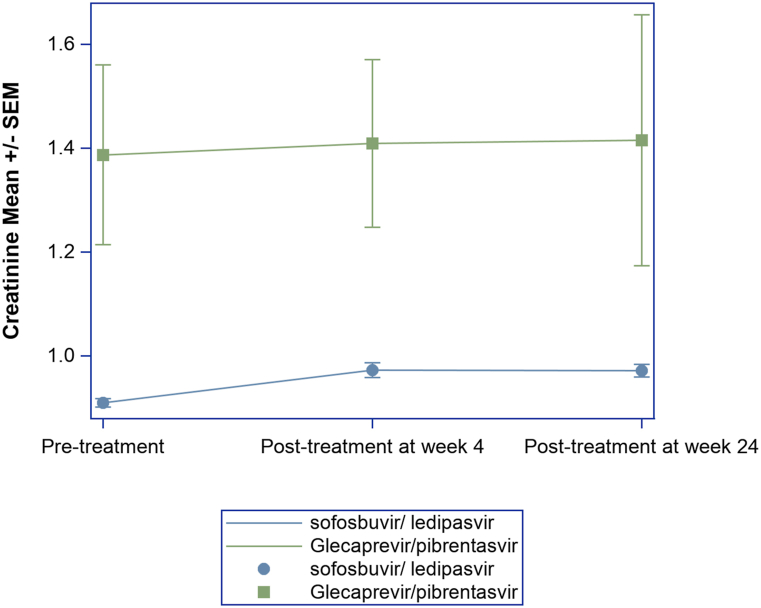
Baseline Cr was higher in the GLE/PIB treatment group (1.39 ± 1.86 mg/dL vs. 0.91 ± 0.24 mg/dL, P = 0.01) than that in the SOF/LDV group (Table 1). Mean and standard error overtime plot was generated to show serum Cr trend at pretreatment and post treatment week 4 and week 24 (Figure 2). The paired T test showed that, in the combined groups, serum Cr increased at posttreatment week 4 (P < 0.0001) and posttreatment week 24 (P < 0.0001), but when stratified by the treatment received, the change of serum Cr was significant only in the SOF/LDV group (P < 0.0001), but not in the GLE/PIB group (P = 0.52). In both groups, the mean changes were less than 0.3 mg/dL. However, serum Cr elevation of ≥0.3 mg/dL (posttreatment from baseline) was seen in 6% of SOF/LDV and 7% of GLE/PIB group (P = 0.6) (Table 1). There was no significant change in serum Cr values between posttreatment week 4 and week 24 (P = 0.6) (Table 2).
Figure 2.
Mean serum creatinine levels at baseline and posttreatment week 4 and week 24 in patients who received sofosbuvir/ledipasvir or glecaprevir/pibrentasvir treatments.
Table 2.
Creatinine Change Between Different Time Point and Paired T Test.
| Group | Comparison | N | Mean Difference (Group – Comparison) | Paired T test, P value |
|---|---|---|---|---|
| Overall | ||||
| Posttreatment week 4 | Pretreatment | 941 | 0.06 | <0.001 |
| Posttreatment week 24 | Pretreatment | 673 | 0.05 | <0.001 |
| Posttreatment week 24 | Posttreatment week 4 | 673 | −0.006 | 0.6 |
| Sofosbuvir/Ledipasvir-Treated Group | ||||
| Posttreatment week 4 | Pretreatment | 825 | 0.06 | <0.0001 |
| Posttreatment week 24 | Pretreatment | 620 | 0.06 | <0.0001 |
| Posttreatment week 24 | Posttreatment week 4 | 620 | −0.005 | 0.7 |
| Glecaprevir/Pibrentasvir-Treated Group | ||||
| Posttreatment week 4 | Pretreatment | 116 | 0.02 | 0.5 |
| Posttreatment week 24 | Pretreatment | 53 | −0.03 | 0.6 |
| Posttreatment week 24 | Posttreatment week 4 | 53 | −0.01 | 0.8 |
When the serum Cr changes were adjusted for age, race, genotype, and baseline serum Cr, the analysis showed that pretreatment and posttreatment Cr changes in both groups were not statistically significant (unadjusted model, P = 0.22, and adjusted model, P = 0.60). The multivariable analysis showed that baseline CKD, diuretics use, and highly active antiretroviral therapy (HAART) therapy were associated with increase in Cr while on treatment with DAA (Table 3). Although HAART therapy was significant, only 5 patients were on HAART therapy.
Table 3.
Risk Factors Associated With Creatinine Change in Multivariable Model.
| Parameter | Estimate | Standard Error | P Value | Type 3 Test, P Value | |
|---|---|---|---|---|---|
| Intercept | 0.075 | 0.035 | 0.03 | ||
| Treatment | SOF/LDV | −0.019 | 0.036 | 0.60 | 0.60 |
| GLE/PIB | 0.000 | 0.000 | . | ||
| Genotype | 1A | 0.000 | 0.000 | . | 0.03 |
| 1B | −0.024 | 0.027 | 0.39 | ||
| 2 | −0.109 | 0.105 | 0.30 | ||
| 3 | −0.494 | 0.139 | 0.00 | ||
| 4 | −0.064 | 0.165 | 0.70 | ||
| 5 | −0.181 | 0.331 | 0.59 | ||
| Unknown | 0.051 | 0.233 | 0.83 | ||
| Diuretics | No | 0.000 | 0.000 | . | 0.007 |
| Yes | 0.066 | 0.024 | 0.007 | ||
| HAART | No | 0.000 | 0.000 | . | <0.0001 |
| Yes | 0.622 | 0.153 | <0.0001 | ||
| CKD | 0 | 0.000 | 0.000 | . | <0.0001 |
| 2 | −0.073 | 0.035 | 0.04 | ||
| 3 | −0.076 | 0.050 | 0.13 | ||
| 4 | 0.386 | 0.171 | 0.02 | ||
| 5 | −0.593 | 0.153 | 0.000 | ||
Abbreviations:HAART, highly active antiretroviral therapy; CKD, chronic kidney disease.
In our sensitivity analysis, baseline covariates were balanced in two treatment groups in matched cohort (Table 4). The conclusion was consistent. There was no significant difference in serum Cr between SOF/LDV and GLE/PIB groups at posttreatment week 4 (P = 0.58) and posttreatment week 24 (P = 0.85).
Table 4.
Patients’ Characteristics in Matched Cohort.
| Variable | Sofosbuvir/Ledipasvir (N = 146) | Glecaprevir/Pibrentasvir (N = 73) | P Value | |
|---|---|---|---|---|
| Age (mean ± SD) | 55.9 ± 10.7 | 56.2 ± 10.5 | 0.9 | |
| Male | 87 (60%) | 44 (60%) | 0.9 | |
| Race | Black | 77 (53%) | 39 (53%) | 0.8 |
| White | 65 (45%) | 33 (45%) | ||
| Genotype | 1a | 109 (75%) | 56 (77%) | 0.8 |
| 1b | 36 (25%) | 17 (23%) | ||
| 4 | 1 (1%) | 0 (0%) | ||
| Duration | 9.9 ± 2.0 | 9.9 ± 2.0 | 0.9 | |
| SVR12 | 110 (96%) | 43 (100%) | 0.2 | |
| Liver stage | F0-1 | 21 (14%) | 9 (12%) | 0.9 |
| F2 | 52 (36%) | 25 (34%) | ||
| F3 | 12 (8%) | 8 (11%) | ||
| F4 | 55 (38%) | 29 (40%) | ||
| Diabetes mellitus | 19 (13%) | 9 (12%) | 0.9 | |
| Hypertension | 64 (44%) | 39 (53%) | 0.2 | |
| Diuretics | 26 (18%) | 12 (16%) | 0.8 | |
| ACEi/ARB | 36 (25%) | 27 (37%) | 0.06 | |
| Metformin | 8 (5%) | 4 (5%) | >0.9 | |
| NSAIDs | 29 (20%) | 11 (15%) | 0.4 | |
| Other nephrotoxic | 2 (1%) | 1 (1%) | >0.9 | |
| Creatinine pre-treatment (Mean ± SD) | 0.90 ± 0.22 | 0.91 ± 0.27 | 0.7 | |
| CKD | 2 | 134 (92%) | 62 (85%) | 0.2 |
| 3 | 9 (6%) | 7 (10%) | ||
| 4 | 3 (2%) | 4 (5%) | ||
Abbreviations:SD, standard deviation; SVR12, sustained virologic response at 12 weeks after treatment; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HAART, highly active antiretroviral therapy; NSAIDs, nonsteroidal anti-inflammatory drugs; CKD, chronic kidney disease; Cr, creatinine.
Discussion
In this study, we found that increase in serum Cr ≥ 0.03 mg/dL from baseline occurred in 6% of patients who were treated with DAA, and it was seen equally with SOF/LDV and GLE/PIB regimens. There was no improvement in serum Cr from posttreatment week 4 and posttreatment week 24. The overall effect of both medications on serum Cr was similar. Although patients treated with GLE/PIB had a higher baseline serum Cr of 1.4 mg/dL and 19% patients had CKD before treatment, serum Cr stayed clinically stable during and after the treatment in most patients.
After approval of SOF/LDV, there were few published case reports and studies which showed new or worsening renal failure.8,16, 17, 18, 19 These retrospective studies showed that AKI and worsening of baseline CKD after SOF/LDV treatment were reversible. The exact mechanism of nephrotoxicity with SOF containing DAA is not known. The renal biopsies in isolated case reports suggested acute tubular necrosis or acute interstitial nephritis as possible mechanisms. The SOF inhibits the carboxylesterase in the liver and kidney, and it can enhance the blood levels and nephrotoxicity with medications as tenofovir.20 This could be a possible explanation of worsening of renal functions in subjects on HAART, but our sample size was too small to make any conclusions. These observations may suggest that those who had HAART should have serum Cr monitored during HCV treatment with SOF-based regimens.
A recent nonrandomized prospective study had compared the SOF-based treatment with SOF-free treatment. This study showed decline in renal function during SOF-based DAA treatment as compared to SOF-free treatment. In both groups, the GFR improved 12 and 24 weeks after treatment as compared to baseline GFR levels. This was attributed to the beneficial effects of hepatitis C treatments on renal functions. The aforementioned study lacked propensity matching and had excluded patients with HIV or stage 4–5 CKD.21 There are only few studies which had included patients with advanced renal failure with or without dialysis who received the SOF-based treatments, and some of these studies had shown that the SOF treatment is safe in those renal dysfunction as compared to those with normal renal function.9, 10, 11 However, these studies had only a small number of patients. The TARGET-HCV trial had shown that the SOF-based treatment in renal failure cohort was effective in terms of achieving SVR, but serious side effects and worsening of renal failure were more frequent in patients with GFR ≤ 45 mL/min. However, the outcomes were similar for GFR 30–45 mL/min and ≤30 mL/min. The effect of SOF on renal functions was not followed after the treatment which was one of the limitations of this study.12 Another study showed that the presence of CKD stage 3 or worse increased the risk of worsening renal function while on SOF/LDV treatment.19 A veterans study had shown that patients treated with SOF/LDV had a low incidence of renal failure, but when seen, it was predominantly in those with cirrhosis.22 Another retrospective study from Japan showed that renal function stayed stable in stage 3 CKD as compared to stage 1 and 2.23 The hepatitis C cohort from Argentina who had mild to moderate kidney disease and treated predominantly with SOF-based combination showed no change in serum Cr and GFR at the end of treatment. Irreversible kidney injury was observed only in 1.7% of patients, and all these patients had CKD stage 3 at baseline.24 Studies among patients with end-stage renal disease with half-dose SOF showed that it was well tolerated in patients with severe renal impairment with good outcomes.25,26
Our study showed that a small sub-group may develop clinically meaningful worsening of creatine during treatment, but there was no class effect of drugs as it was seen equally with SOF/LDV and GLE/PIB regimens. The univariate and multivariate analysis showed that the presence of baseline CKD, diuretics use, and HAART therapy were risk factors for increase in Cr while on treatment with DAA. Although SOF-based treatments were approved by the FDA recently for this with renal failure, renal functions should be monitored carefully during and after treatment in those with risk factors and underlying CKD. For theoretical reasons alone, GLE/PIB may be preferred for those with pre-existing CKD despite our observations.
The novelty of our study is the comparison of two commonly used DAA on the renal functions using a relatively large sample size. However, there were few limitations including the retrospective observational nature of the study and few missing data. There could also be bias in the treatment selection as GLE/PIB was used more commonly in those with renal dysfunction. In summary, we showed that serum Cr increased with DAA treatment, but in most patients, the increase was not clinically meaningful. The incidence of AKI (Cr ≥ 0.3 mg/dL) was seen in only 6% of patients, and this was unrelated to type of DAA. We believe that serum Cr should be monitored during DAA treatment especially in those with risk factors for renal disease.
CRediT authorship contribution statement
P.J.T. and W.A. contributed to the conception and design; acquisition, analysis, and interpretation of the data; and drafting of the article. P.J.T. and A.M. were responsible for critical revision for important intellectual content. T.Z. did the statistical analysis, and all authors approved the final version and agree to be accountable for all aspects of the work.
Conflicts of interest
The authors have none to declare.
Funding
None.
References
- 1.Cacoub P., Comarmond C., Domont F., Savey L., Desbois A.C., Saadoun D. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther Adv Infect Dis. 2016;3:3–14. doi: 10.1177/2049936115585942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai T.-S., Lee M.-H., Yang H.-I., et al. Hepatitis C viral load, genotype, and increased risk of developing end-stage renal disease: REVEAL-HCV study. Hepatology. 2017;66:784–793. doi: 10.1002/hep.29192. [DOI] [PubMed] [Google Scholar]
- 3.Lens S., Rodriguez-Tajes S., Llovet L.-P., Maduell F., Londono M.-C. Treating hepatitis C in patients with renal failure. Dig Dis. 2017;35:339–346. doi: 10.1159/000456585. [DOI] [PubMed] [Google Scholar]
- 4.Webster D.P., Klenerman P., Dusheiko G.M. Hepatitis C. Lancet (London, England) 2015;385:1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virlogeux V., Pradat P., Bailly F., et al. Boceprevir and telaprevir-based triple therapy for chronic hepatitis C: virological efficacy and impact on kidney function and model for end-stage liver disease score. J Viral Hepat. 2014;21:e98–e107. doi: 10.1111/jvh.12237. [DOI] [PubMed] [Google Scholar]
- 6.Mauss S., Hueppe D., Alshuth U. Renal impairment is frequent in chronic hepatitis C patients under triple therapy with telaprevir or boceprevir. Hepatology. 2014;59:46–48. doi: 10.1002/hep.26602. [DOI] [PubMed] [Google Scholar]
- 7.Cha A., Budovich A. Sofosbuvir: a new oral once-daily agent for the treatment of hepatitis C virus infection. Pharm Ther. 2014;39:345–352. [PMC free article] [PubMed] [Google Scholar]
- 8.Maan R., Al Marzooqi S.H., Klair J.S., et al. The frequency of acute kidney injury in patients with chronic hepatitis C virus infection treated with sofosbuvir-based regimens. Aliment Pharmacol Ther. 2017;46:46–55. doi: 10.1111/apt.14117. [DOI] [PubMed] [Google Scholar]
- 9.Desnoyer A., Pospai D., Lê M.P., et al. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol. 2016;65:40–47. doi: 10.1016/j.jhep.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 10.Nazario H.E., Ndungu M., Modi A.A. Sofosbuvir and simeprevir in hepatitis C genotype 1-patients with end-stage renal disease on haemodialysis or GFR <30 ml/min. Liver Int. 2016;36:798–801. doi: 10.1111/liv.13025. [DOI] [PubMed] [Google Scholar]
- 11.Borgia S.M., Dearden J., Yoshida E.M., et al. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J Hepatol. 2019;71:660–665. doi: 10.1016/j.jhep.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Saxena V., Koraishy F.M., Sise M.E., et al. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016;36:807–816. doi: 10.1111/liv.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patients with Renal Impairment Recommendation for Patients with CKD Stage a. 2020. pp. 1–4.https://www.hcvguidelines.org/unique-populations/renal-impairment [Google Scholar]
- 14.Gane E., Lawitz E., Pugatch D., et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. 2017;377:1448–1455. doi: 10.1056/NEJMoa1704053. [DOI] [PubMed] [Google Scholar]
- 15.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashraf T., Majoni W. Acute interstitial nephritis associated with sofosbuvir and daclatasvir. ACG Case Reports J. 2017;4 doi: 10.14309/crj.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanchoo R., Thakkar J., Schwartz D., Jhaveri K.D. Harvoni (ledipasvir with sofosbuvir)-induced renal injury. Am J Gastroenterol. 2016;111:148–149. doi: 10.1038/ajg.2015.391. [DOI] [PubMed] [Google Scholar]
- 18.Bunnell K.L., Vibhakar S., Glowacki R.C., Gallagher M.A., Osei A.M., Huhn G. Nephrotoxicity associated with concomitant use of ledipasvir-sofosbuvir and tenofovir in a patient with hepatitis C virus and human immunodeficiency virus coinfection. Pharmacotherapy. 2016;36:e148–e153. doi: 10.1002/phar.1803. [DOI] [PubMed] [Google Scholar]
- 19.Brown P.R., Sadiq O., Weick A., et al. Acute kidney injury in patients undergoing chronic hepatitis C virus treatment with ledipasvir/sofosbuvir. Hepatol Commun. 2018;2:1172–1178. doi: 10.1002/hep4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Y., Yan B. Covalent inhibition of carboxylesterase-2 by sofosbuvir and its effect on the hydrolytic activation of tenofovir disoproxil. J Hepatol. 2017;66:660–661. doi: 10.1016/j.jhep.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C.-H., Lee M.-H., Lin J.-W., et al. Evolution of eGFR in chronic HCV patients receiving sofosbuvir-based or sofosbuvir-free direct-acting antivirals. J Hepatol. 2020;72:839–846. doi: 10.1016/j.jhep.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Butt A.A., Ren Y., Marks K., Shaikh O.S., Sherman K.E. Do directly acting antiviral agents for HCV increase the risk of hepatic decompensation and decline in renal function? Results from ERCHIVES. Aliment Pharmacol Ther. 2017;45:150–159. doi: 10.1111/apt.13837. [DOI] [PubMed] [Google Scholar]
- 23.Okubo T., Atsukawa M., Tsubota A., et al. Efficacy and safety of ledipasvir/sofosbuvir for genotype 1b chronic hepatitis C patients with moderate renal impairment. Hepatol Int. 2018;12:133–142. doi: 10.1007/s12072-018-9859-9. [DOI] [PubMed] [Google Scholar]
- 24.Ridruejo E., Garcia-Agudo R., Mendizabal M., et al. Efficacy and safety of direct-acting antiviral agents for HCV in mild-to-moderate chronic kidney disease. Nefrologia. 2020;40:46–52. doi: 10.1016/j.nefro.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Bhamidimarri K.R., Czul F., Peyton A., et al. Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end stage renal disease. J Hepatol. 2015;63:763–765. doi: 10.1016/j.jhep.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Perumpail R.B., Wong R.J., Ha L.D., et al. Sofosbuvir and simeprevir combination therapy in the setting of liver transplantation and hemodialysis. Transpl Infect Dis. 2015;17:275–278. doi: 10.1111/tid.12348. [DOI] [PubMed] [Google Scholar]